Regulatory Role of lncRNA MEG3 Silencing on PI3K/GSK3β/Tau Pathway in a High-Glucose-Induced Cell Model
Abstract
1. Introduction
2. Results
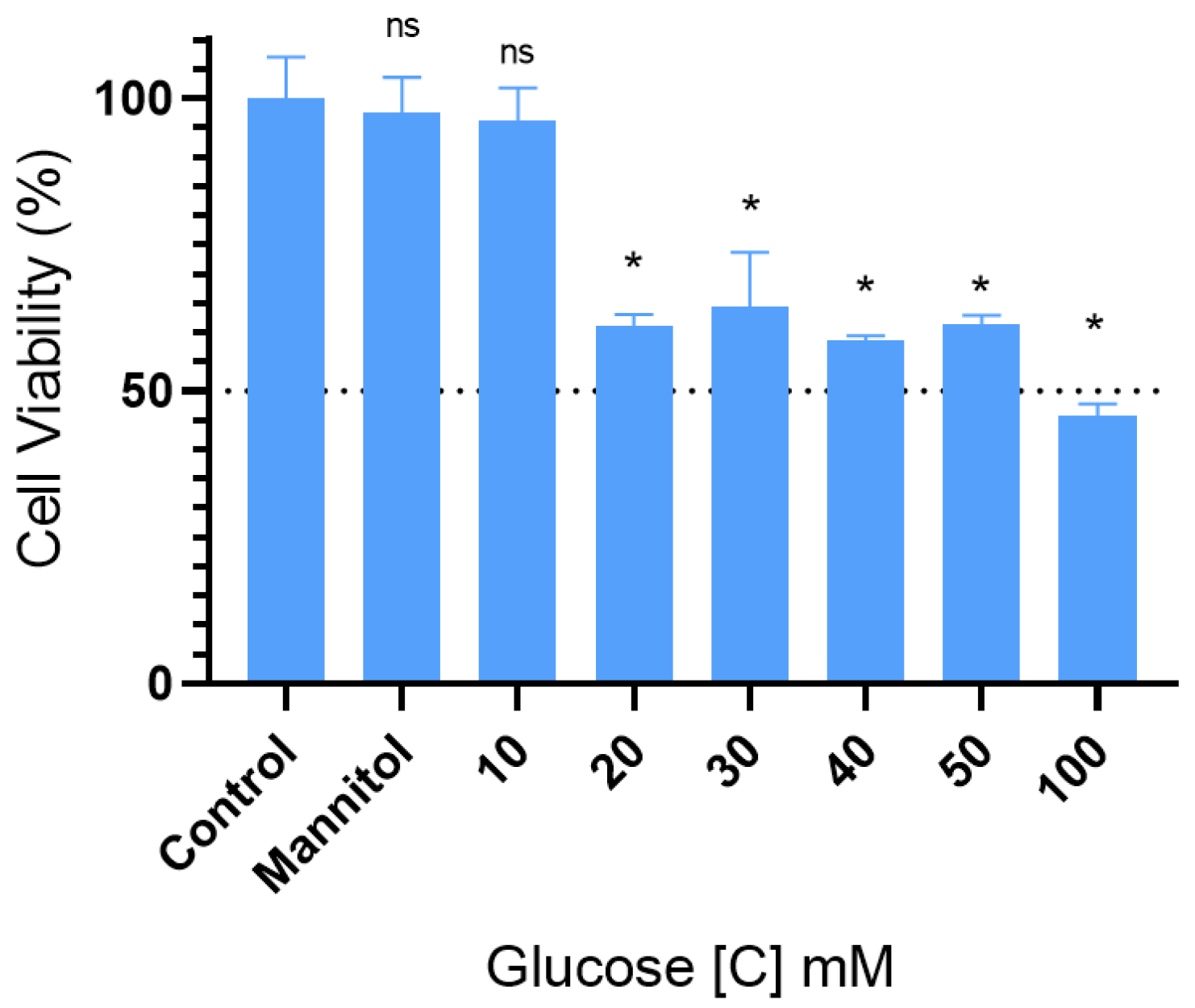
2.1. High Doses of Glucose Induced Neurotoxicity on the Viability of SH-SY5Y Cells
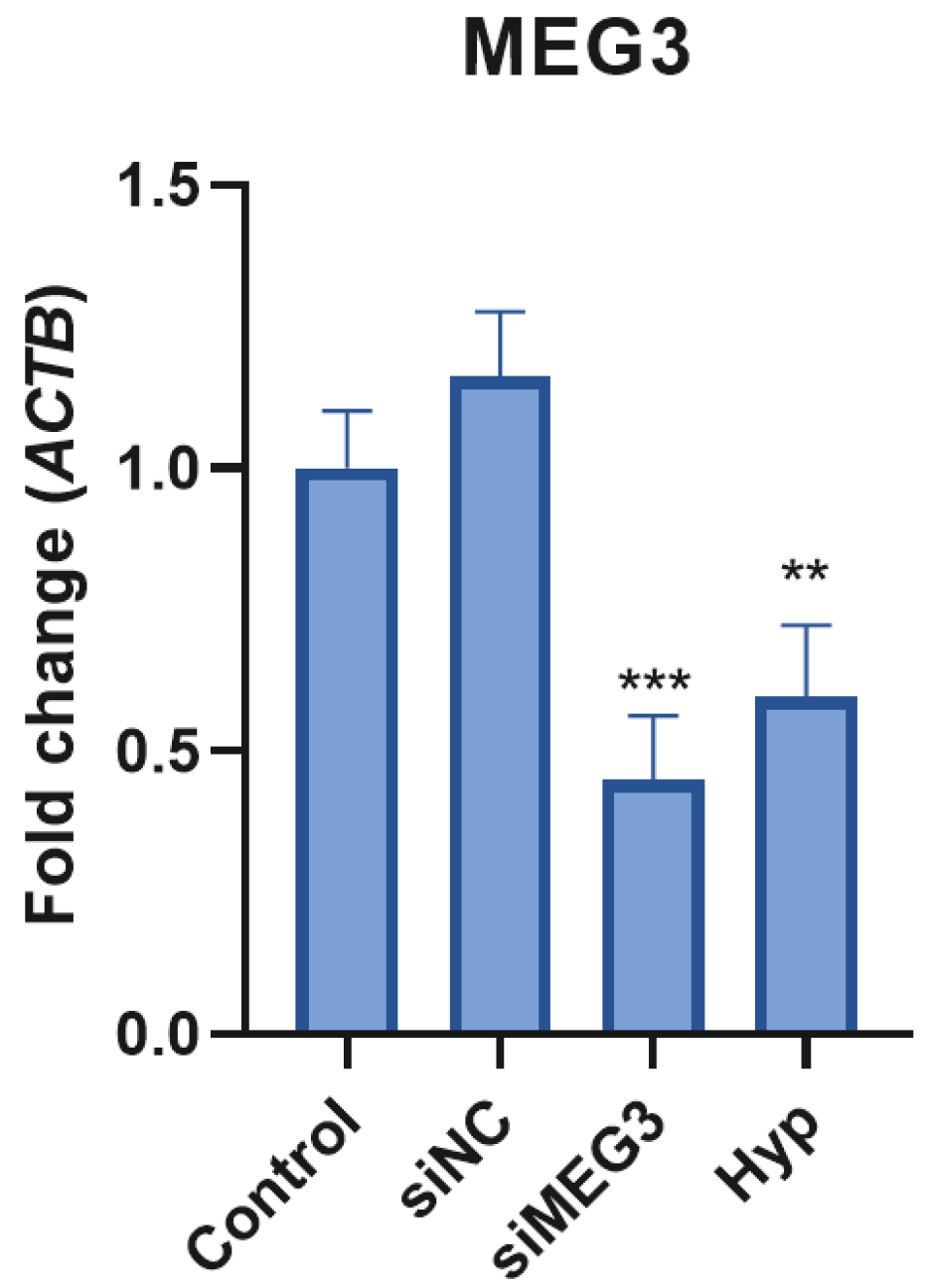
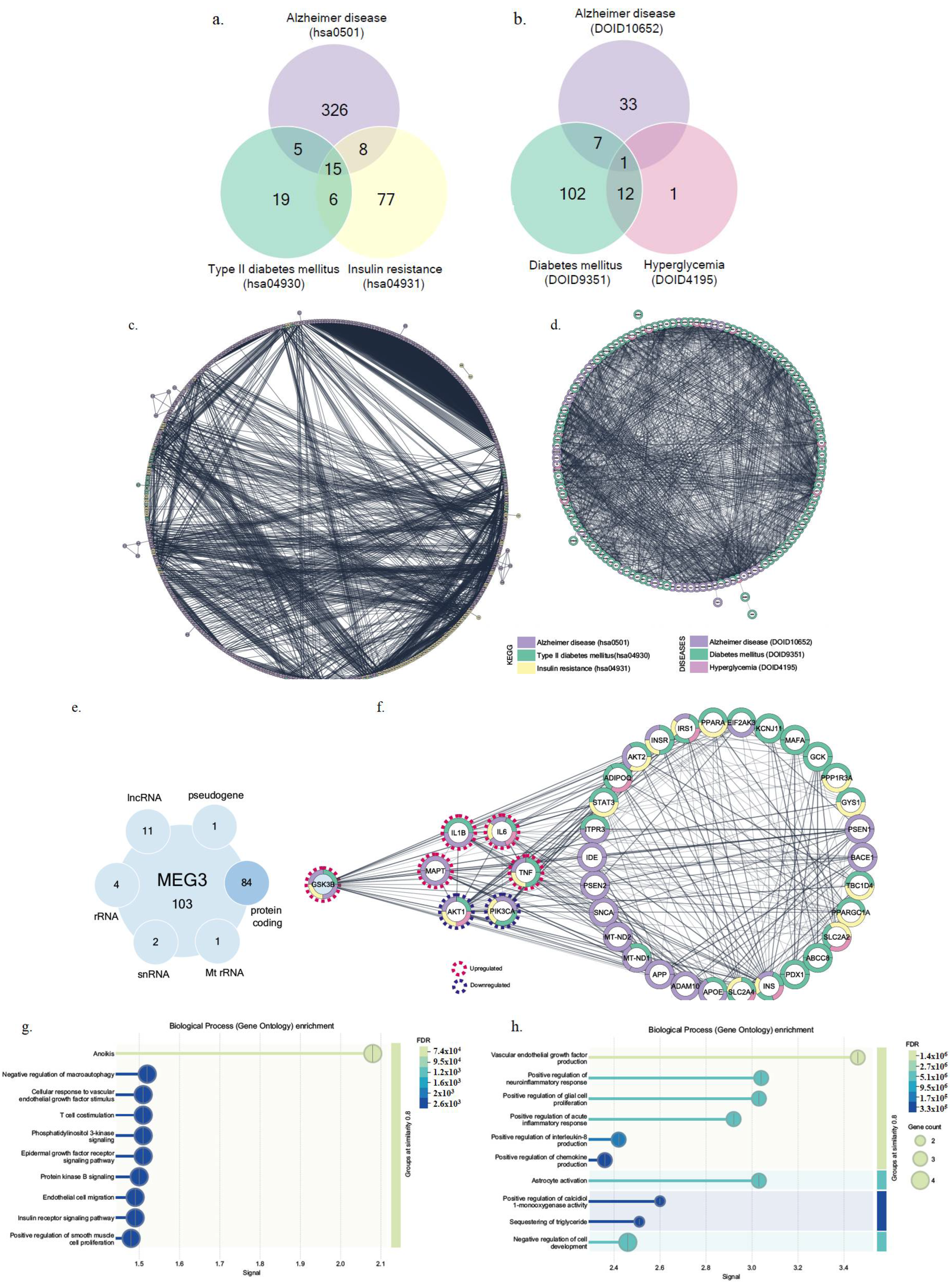
2.2. MEG3 siRNA Transfection Altered Insulin Signaling Pathway-Related Gene Expression in Glycemic and Hyperglycemic Model SH-SY5Y Cells
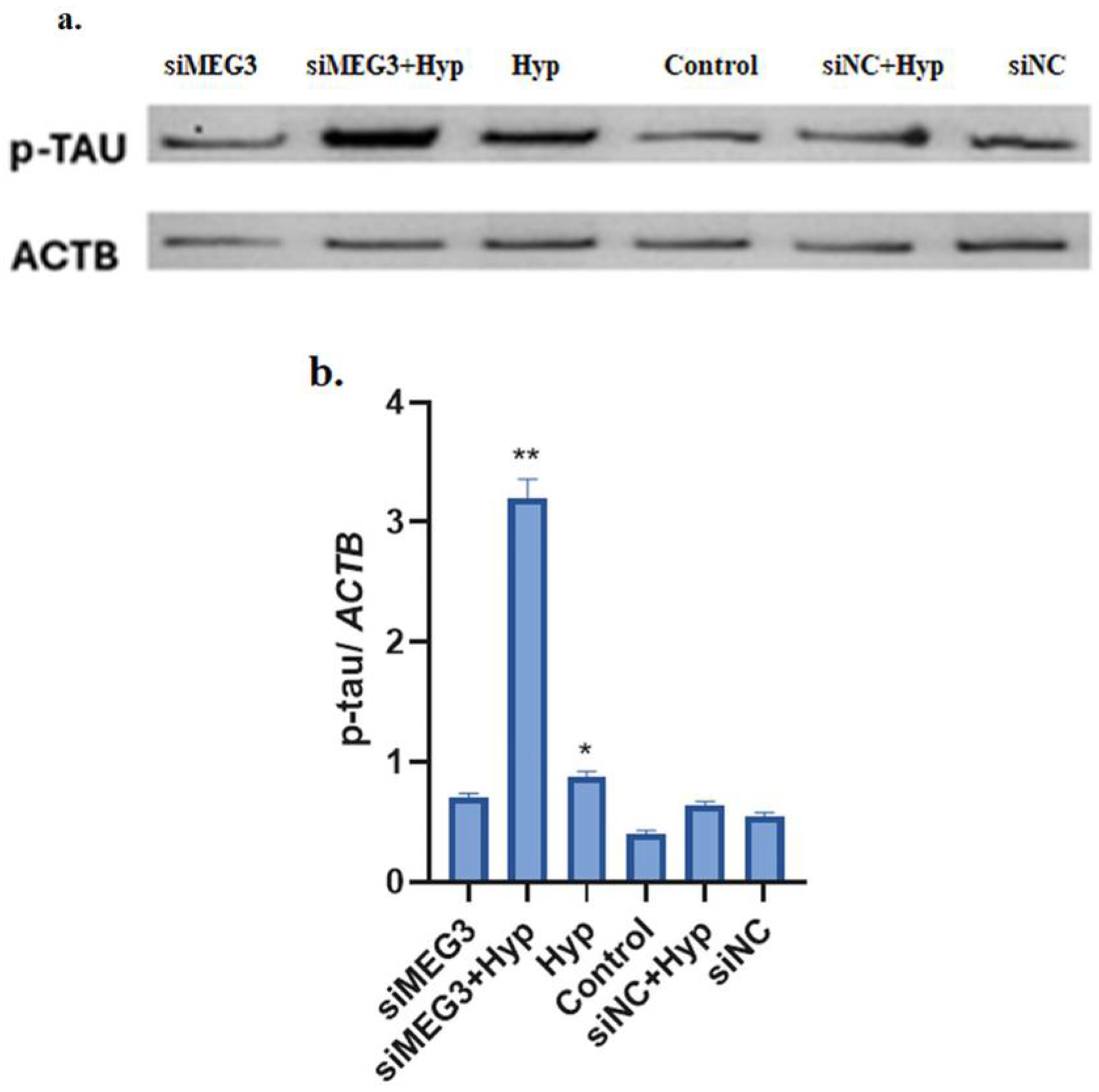
2.3. Knockdown of lncRNA MEG3 in Hyperglycemic SH-SY5Y Cells Mimicked Alzheimer’s Pathology Through Increased p-Tau Protein Level
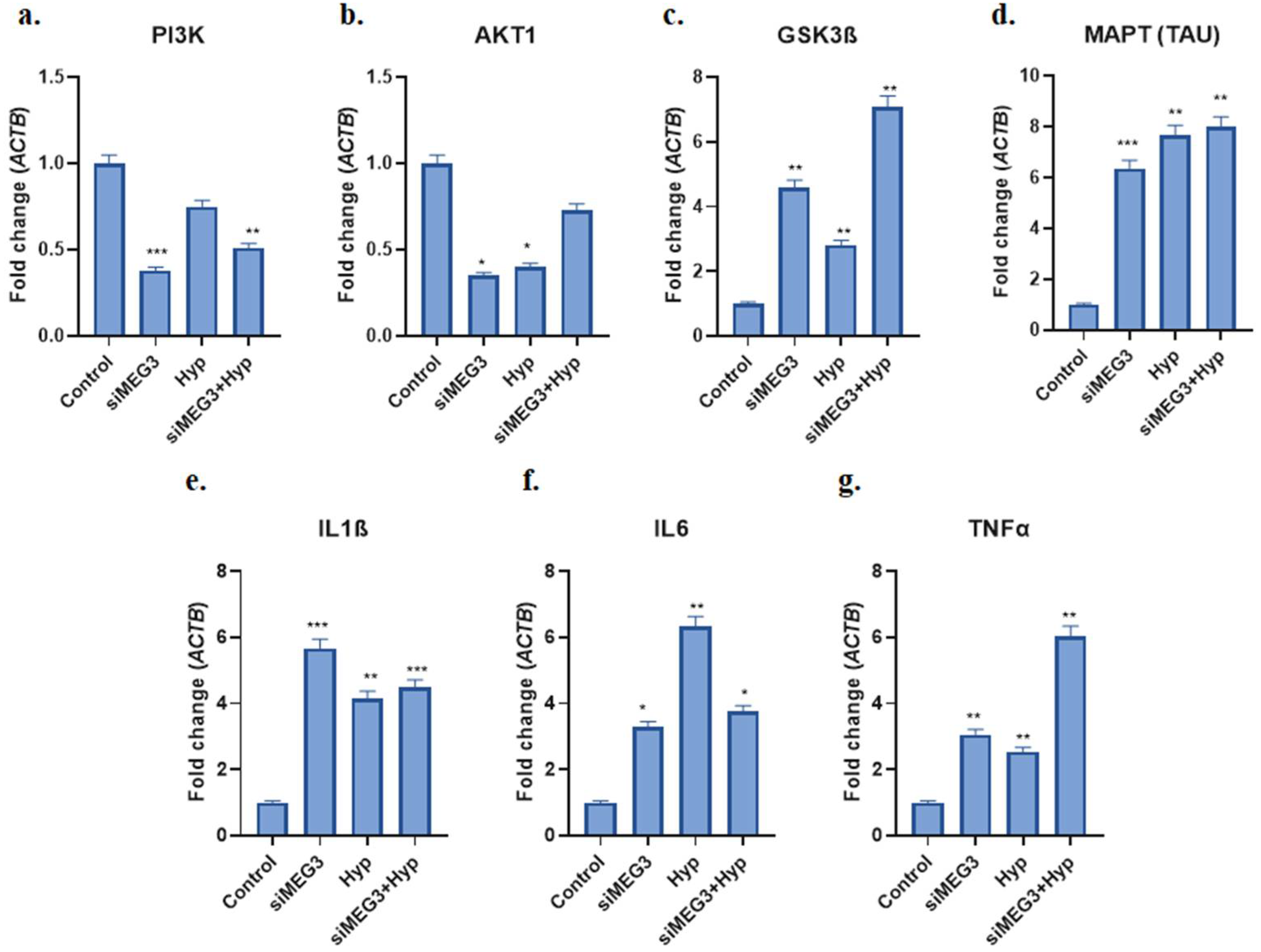
2.4. MEG3 Was Linked to GSK3β and Regulated PI3K Signaling and Neuroinflammation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. SH-SY5Y Cell Culture and High Glucose Treatment
4.3. Transfection of SH-SY5Y with lncRNA MEG3 siRNA
4.4. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
4.5. Western Blotting
4.6. Network Analyses
4.7. Statistics
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| LncRNA MEG3 | long non-coding RNA maternally expressed gene 3 |
| T3D | type 3 diabetes |
| T2D | type 2 diabetes |
| PI3K | phosphatidylinositol-3-kinase |
| AKT | protein kinase B |
| GSK3β | glycogen synthase kinase 3-beta |
| HG | high glucose |
| Hyp | hyperglycemic model |
| siRNA | small interfering RNA |
| siMEG3 | small interfering MEG3 |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| MAPT, TAU | microtubule-associated protein TAU |
| IL1β | interleukin 1 beta |
| IL6 | interleukin 6 |
| TNFα | tumor necrosis factor-α |
| AGEs | advanced glycation end products |
| ICA | icariin |
| Aβ 25-35 | amyloid beta 25-35 |
| IRS1 | insulin receptor substrate 1 |
| hs-CRP | high-sensitivity C-reactive protein |
| DMEM | Dulbecco’s modified eagle’s medium |
| FBS | fetal bovine serum |
| Opti-MEM | reduced serum medium |
| CSF | cerebrospinal fluid |
| DMSO | dimethyl sulfoxide |
| PBS | phosphate-buffered saline |
| MTT | thiazolyl blue tetrazolium bromide |
| Malat1 | metastasis-associated lung adenocarcinoma transcript 1 |
| ACTB | beta-actin |
| p-tau | phosphorylated tau |
| PPI | protein–protein interaction |
| SDS-PAGE | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| HRP | horseradish peroxidase |
References
- Ngolab, J.; Honma, P.; Rissman, R.A. Reflections on the utility of the retina as a biomarker for Alzheimer’s disease: A literature review. Neurol. Ther. 2019, 8, 57–72. [Google Scholar] [CrossRef]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef]
- Sousa, R.A.L.D.; Harmer, A.R.; Freitas, D.A.; Mendonça, V.A.; Lacerda, A.C.R.; Leite, H.R. An update on potential links between type 2 diabetes mellitus and Alzheimer’s disease. Mol. Biol. Rep. 2020, 47, 6347–6356. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.T.D.; Le, T.T.; Vo, V.G. Role of insulin resistance in the Alzheimer’s disease progression. Neurochem. Res. 2020, 45, 1481–1491. [Google Scholar] [CrossRef]
- Nguyen, T.T. Type 3 diabetes and its role implications in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef]
- Kroner, Z. The relationship between Alzheimer’s disease and diabetes: Type 3 diabetes? Altern. Med. Rev. 2009, 14, 373. [Google Scholar]
- Ferreiro, E.; Lanzillo, M.; Canhoto, D.; da Silva, A.M.C.; Mota, S.I.; Dias, I.S.; Ferreira, I.L.; Fontes, A.R.; Mastrella, G.; Pinheiro, P.; et al. Chronic hyperglycemia impairs hippocampal neurogenesis and memory in an Alzheimer’s disease mouse model. Neurobiol. Aging 2020, 92, 98–113. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Clemente, C.F.; Macías-Pérez, M.E.; Rodríguez-Fonseca, R.A.; Vázquez, M.I.N.; Martínez, J.; Ruvalcaba, R.M.; Rosas, M.M.; Jiménez, E.M. Contribution of hyperglycemia-induced changes in microglia to Alzheimer’s disease pathology. Pharmacol. Rep. 2022, 74, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Nisar, O.; Pervez, H.; Mandalia, B.; Waqas, M.; Sra, H.K. Type 3 diabetes mellitus: A link between Alzheimer’s disease and type 2 diabetes mellitus. Cureus 2020, 12, e11703. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Hwang, J.; Son, S.U.; Choi, J.; You, S.-W.; Park, H.; Cha, S.-Y.; Maeng, S. How can insulin resistance cause Alzheimer’s disease? Int. J. Mol. Sci. 2023, 24, 3506. [Google Scholar] [CrossRef]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered insulin signaling in Alzheimer’s disease brain: Special emphasis on PI3K-Akt pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Imran, I.; Zahra, Z.; Shah, J.; Aleem, A. Hyperglycemia-associated Alzheimer’s-like symptoms and other behavioral effects attenuated by Plumeria obtusa L. extract in alloxan-induced diabetic rats. Front. Pharmacol. 2022, 13, 1077570. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, H.; Liu, X.; Guo, X. Hypoglycemic medicines in the treatment of Alzheimer’s disease: Pathophysiological links between AD and glucose metabolism. Front. Pharmacol. 2023, 14, 1138499. [Google Scholar] [CrossRef]
- Baazaoui, N.; Alfaifi, M.Y.; Ben Saad, R.; Garzoli, S. Potential role of long noncoding RNA maternally expressed gene 3 (MEG3) in the process of neurodegeneration. Neuroscience 2025, 565, 487–498. [Google Scholar] [CrossRef]
- Balusu, S.; Horré, K.; Thrupp, N.; Craessaerts, K.; Snellinx, A.; Serneels, L.; T’sYen, D.; Chrysidou, I.; Arranz, A.M.; Sierksma, A.; et al. MEG3 activates necroptosis in human neuron xenografts modeling Alzheimer’s disease. Science 2023, 381, 1176–1182. [Google Scholar] [CrossRef]
- Yi, J.; Chen, B.; Yao, X.; Lei, Y.; Ou, F.; Huang, F. Upregulation of the lncRNA MEG3 improves cognitive impairment, alleviates neuronal damage, and inhibits activation of astrocytes in hippocampus tissues in Alzheimer’s disease through inactivating the PI3K/Akt signaling pathway. J. Cell. Biochem. 2019, 120, 18053–18065. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Chen, Y.; Jin, J.; Xu, Y.; Zhu, X. Long non-coding RNA: Insight into mechanisms of Alzheimer’s disease. Front. Mol. Neurosci. 2022, 14, 821002. [Google Scholar] [CrossRef]
- Nopparat, C.; Chaopae, W.; Boontem, P.; Sopha, P.; Wongchitrat, P.; Govitrapong, P. Melatonin attenuates high glucose-induced changes in beta amyloid precursor protein processing in human neuroblastoma cells. Neurochem. Res. 2022, 47, 2568–2579. [Google Scholar] [CrossRef]
- Kamsrijai, U.; Wongchitrat, P.; Nopparat, C.; Satayavivad, J.; Govitrapong, P. Melatonin attenuates streptozotocin-induced Alzheimer-like features in hyperglycemic rats. Neurochem. Int. 2020, 132, 104601. [Google Scholar] [CrossRef]
- Zeinali, F.; Zarch, S.M.A.; Jahan-Mihan, A.; Kalantar, S.M.; Mehrjardi, M.Y.V.; Fallahzadeh, H.; Hosseinzadeh, M.; Rahmanian, M.; Mozaffari-Khosravi, H.; Rom, S. Circulating microRNA-122, microRNA-126-3p and microRNA-146a are associated with inflammation in patients with pre-diabetes and type 2 diabetes mellitus: A case control study. PLoS ONE 2021, 6, e0251697. [Google Scholar] [CrossRef]
- Majumder, S.; Hadden, M.J.; Thieme, K.; Batchu, S.N.; Niveditha, D.; Chowdhury, S.; Yerra, V.G.; Advani, S.L.; Bowskill, B.B.; Liu, Y.; et al. Dysregulated expression but redundant function of the long non-coding RNA HOTAIR in diabetic kidney disease. Diabetologia 2019, 62, 2129–2142. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Tao, Y.; Chen, C.; Zhang, Q.; Liu, Z.; Li, L.; Xia, P.; Ye, Z. LncRNA-MEG3 attenuates hyperglycemia-induced damage by enhancing mitochondrial translocation of HSP90A in the primary hippocampal neurons. Exp. Cell Res. 2022, 419, 113320. [Google Scholar] [CrossRef]
- Yoo, J.; Park, J.E.; Han, J.S. HMC ameliorates hyperglycemia via acting on the PI3K/AKT pathway and improving the FOXO1 pathway in ob/ob mice. Nutrients 2023, 15, 2023. [Google Scholar] [CrossRef]
- John, C.M.; Mohamed Yusof, N.I.S.; Abdul Aziz, S.H.; Mohd Fauzi, F. Maternal cognitive impairment associated with gestational diabetes mellitus: A review of potential contributing mechanisms. Int. J. Mol. Sci. 2018, 19, 3894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Long non-coding RNAs in Alzheimer’s disease. Curr. Top. Med. Chem. 2016, 16, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimers Res. Ther. 2014, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Razani, E.; Pourbagheri-Sigaroodi, A.; Safaroghli-Azar, A.; Zoghi, A.; Shanaki-Bavarsad, M.; Bashash, D. The PI3K/Akt signaling axis in Alzheimer’s disease: A valuable target to stimulate or suppress? Cell Stress Chaperones 2021, 26, 871–887. [Google Scholar] [CrossRef]
- Wu, S.; Zheng, L.; Huang, J.; Wang, S.; Huang, Q.; Guo, S.; Qiu, T.; Shen, Q.; Li, C.; Huh, S.-O.; et al. Icariin, a natural flavonoid glucoside, inhibits neuroinflammation in mice with triple-transgenic Alzheimer’s disease by regulating the Akt/GSK-3β signaling pathway. J. Funct. Foods. 2024, 118, 106263. [Google Scholar] [CrossRef]
- Meng, J.; Ding, T.; Chen, Y.; Long, T.; Xu, Q.; Lian, W.; Liu, W. LncRNA-Meg3 promotes Nlrp3-mediated microglial inflammation by targeting miR-7a-5p. Int. Immunopharmacol. 2021, 90, 107141. [Google Scholar] [CrossRef]
- Zhou, S.; Yu, X.; Wang, M.; Meng, Y.; Song, D.; Yang, H.; Wang, D.; Bi, J.; Xu, S. Long non-coding RNAs in pathogenesis of neurodegenerative diseases. Front. Cell Dev. Biol. 2021, 9, 719247. [Google Scholar] [CrossRef]
- Cho, S.J.; Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Fernando, P.D.S.M.; Zhen, A.X.; Hyun, Y.J.; Ahn, M.J.; Kang, H.K.; Hyun, J.W. 7,8-Dihydroxyflavone protects high glucose-damaged neuronal cells against oxidative stress. Biomol. Ther. 2019, 27, 85–91. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, C.; Wang, B.; Ma, Z.; Wang, D.; Gong, B.; Di, S.; Jiang, S.; Li, Y.; Li, T.; et al. Pterostilbene attenuates high glucose-induced oxidative injury in hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 827–837. [Google Scholar] [CrossRef]
- Zhang, S.; Xue, R.; Geng, Y.; Wang, H.; Li, W. Fisetin Prevents HT22 Cells From High Glucose-Induced Neurotoxicity via PI3K/Akt/CREB Signaling Pathway. Front Neurosci. 2020, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network analysis and visualization of proteomics data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozpak, L. Regulatory Role of lncRNA MEG3 Silencing on PI3K/GSK3β/Tau Pathway in a High-Glucose-Induced Cell Model. Int. J. Mol. Sci. 2025, 26, 7944. https://doi.org/10.3390/ijms26167944
Ozpak L. Regulatory Role of lncRNA MEG3 Silencing on PI3K/GSK3β/Tau Pathway in a High-Glucose-Induced Cell Model. International Journal of Molecular Sciences. 2025; 26(16):7944. https://doi.org/10.3390/ijms26167944
Chicago/Turabian StyleOzpak, Lütfiye. 2025. "Regulatory Role of lncRNA MEG3 Silencing on PI3K/GSK3β/Tau Pathway in a High-Glucose-Induced Cell Model" International Journal of Molecular Sciences 26, no. 16: 7944. https://doi.org/10.3390/ijms26167944
APA StyleOzpak, L. (2025). Regulatory Role of lncRNA MEG3 Silencing on PI3K/GSK3β/Tau Pathway in a High-Glucose-Induced Cell Model. International Journal of Molecular Sciences, 26(16), 7944. https://doi.org/10.3390/ijms26167944





