Novel Carbazole–Thiazole Conjugates: Synthesis and Biophysical Characterization
Abstract
1. Introduction
2. Results and Discussion
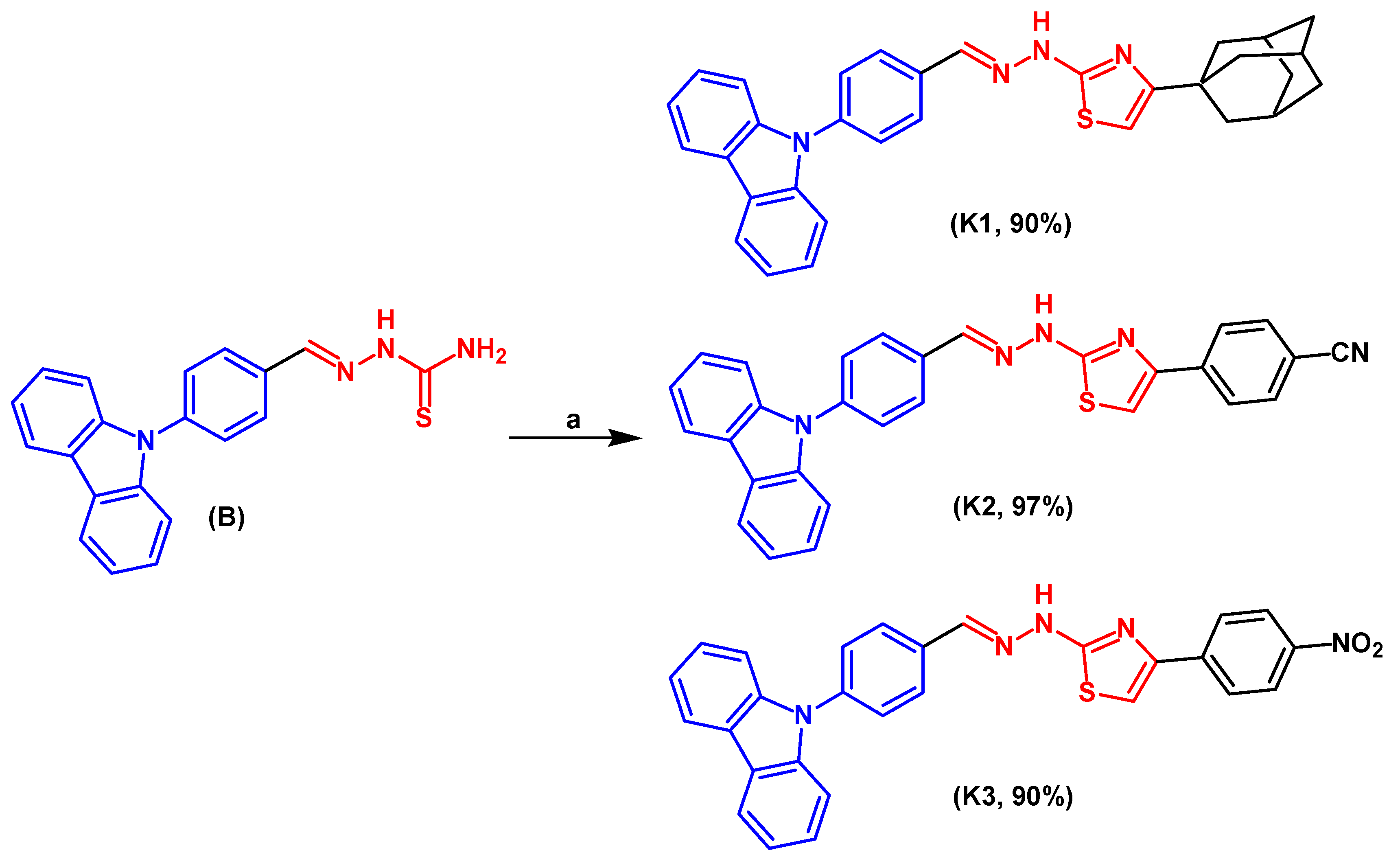
2.1. Chemical Synthesis
2.2. Mushroom Tyrosinase Inhibition
Kinetic Analysis of the Inhibition of Tyrosinase
2.3. Physicochemical Properties
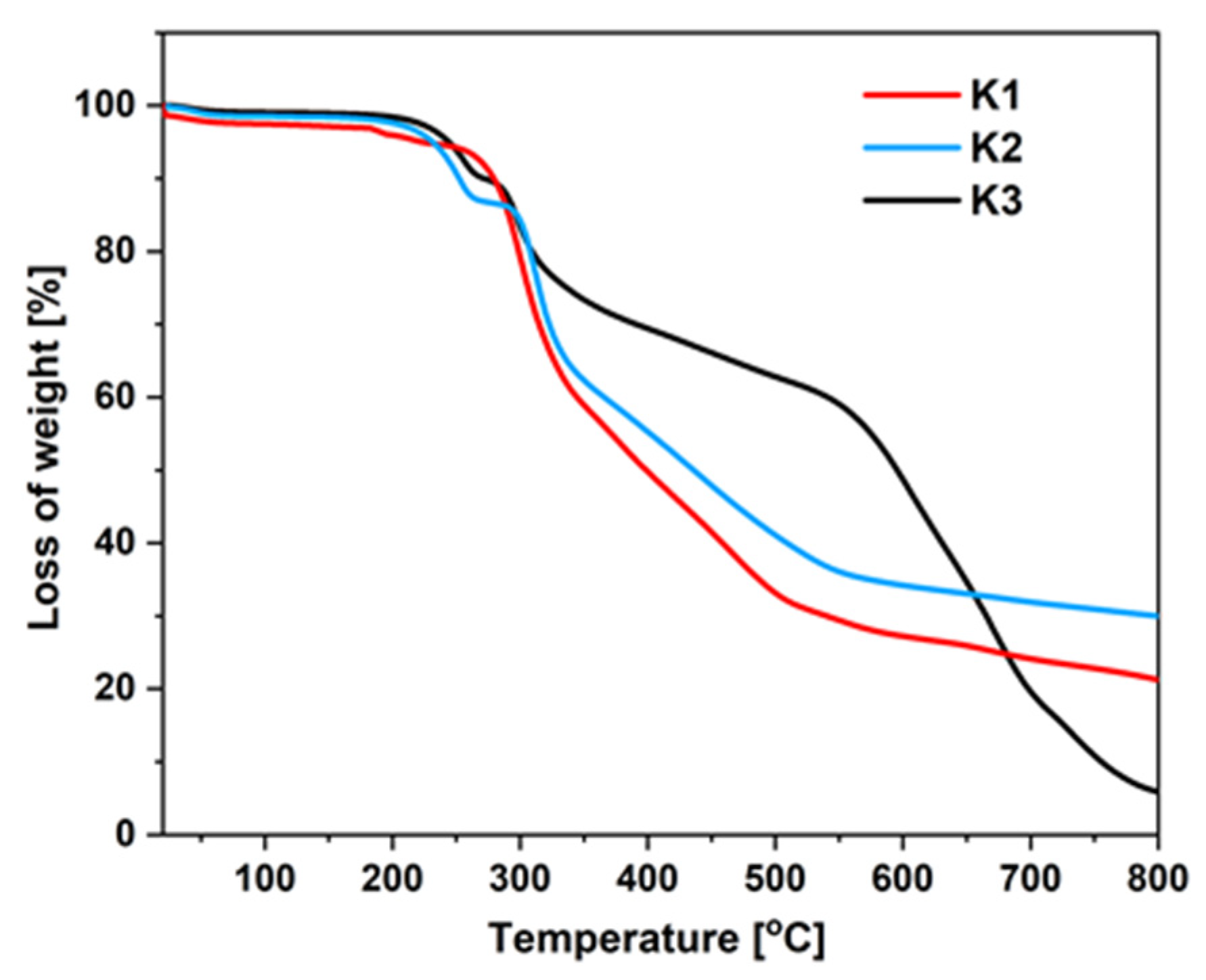
2.3.1. Thermal Stability
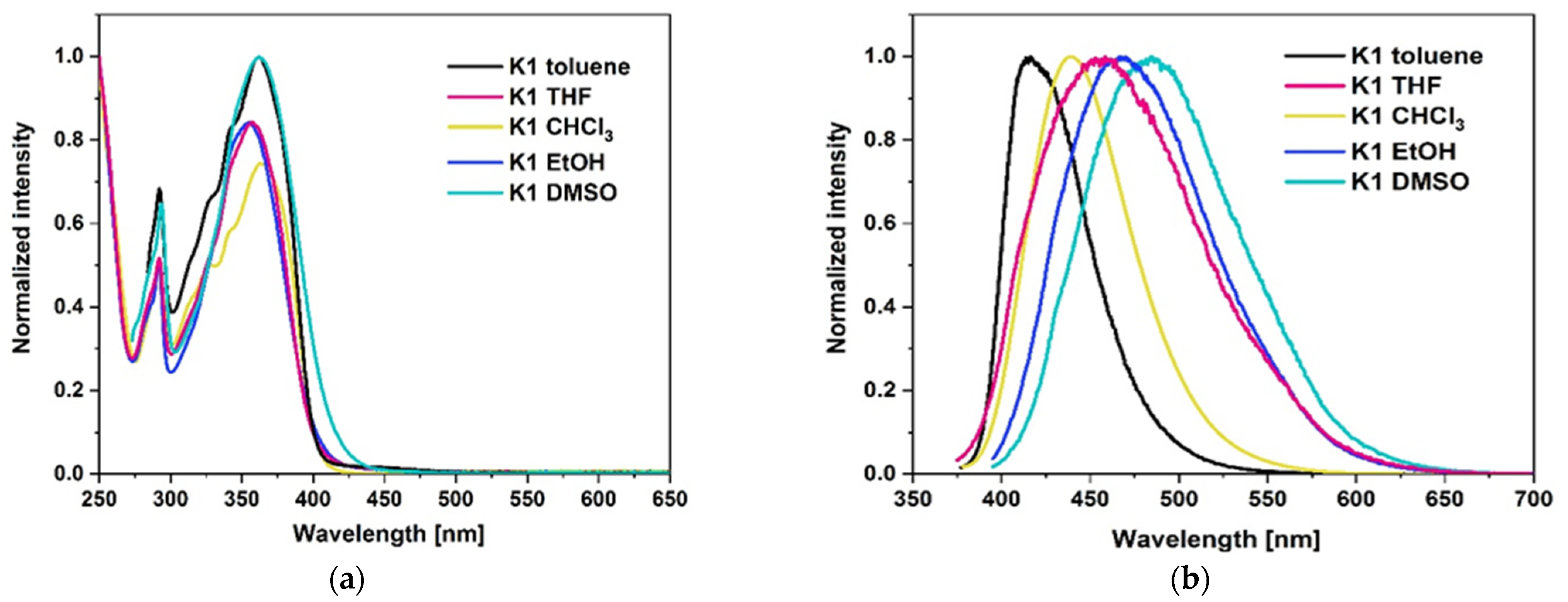
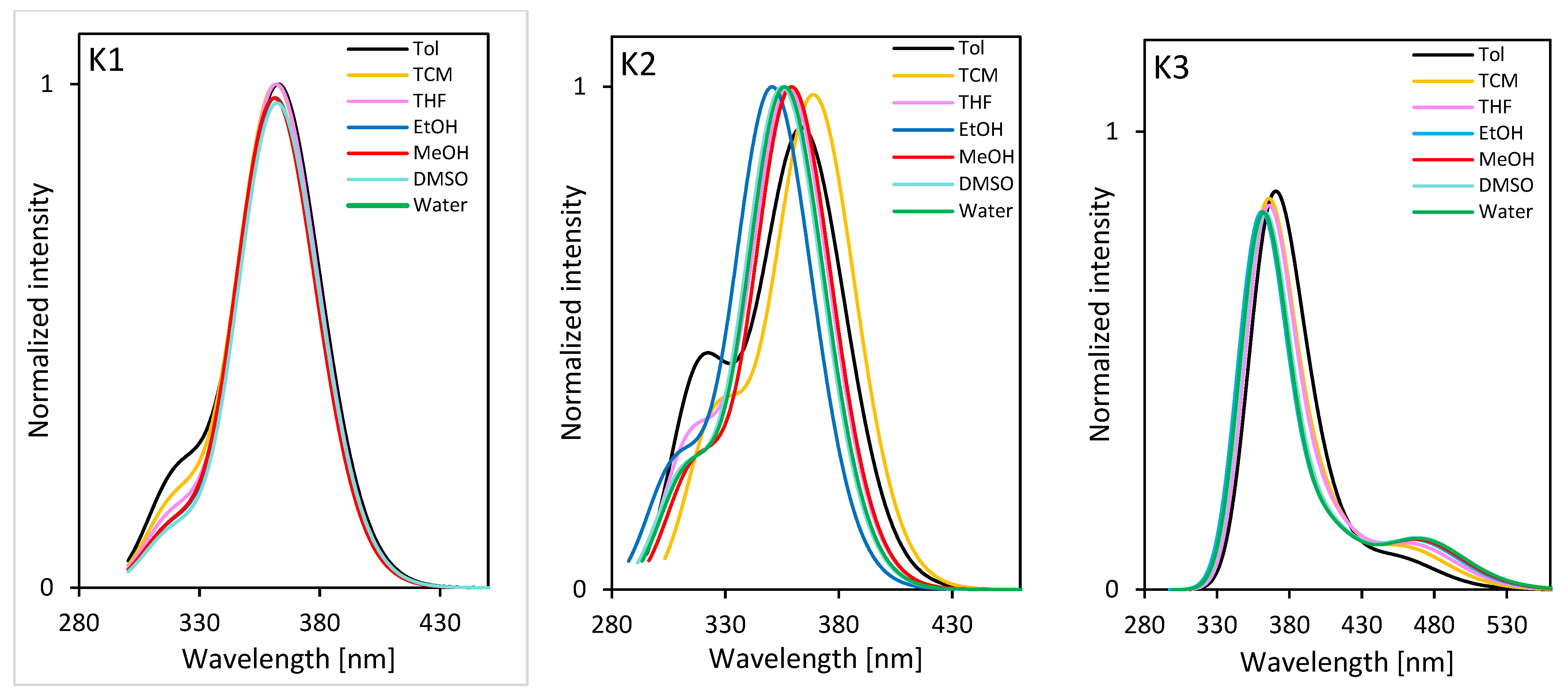
2.3.2. Photoluminescence Properties
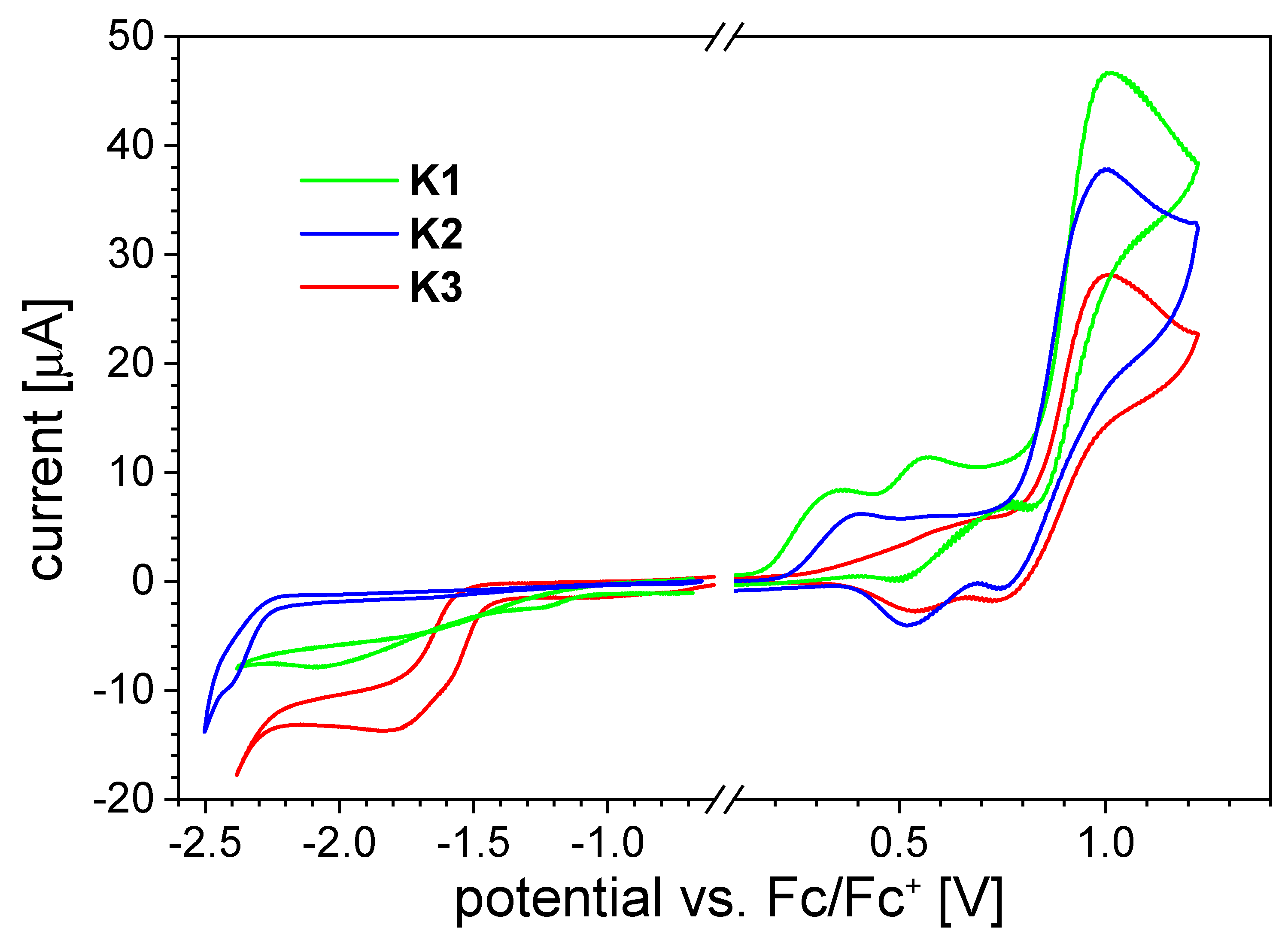
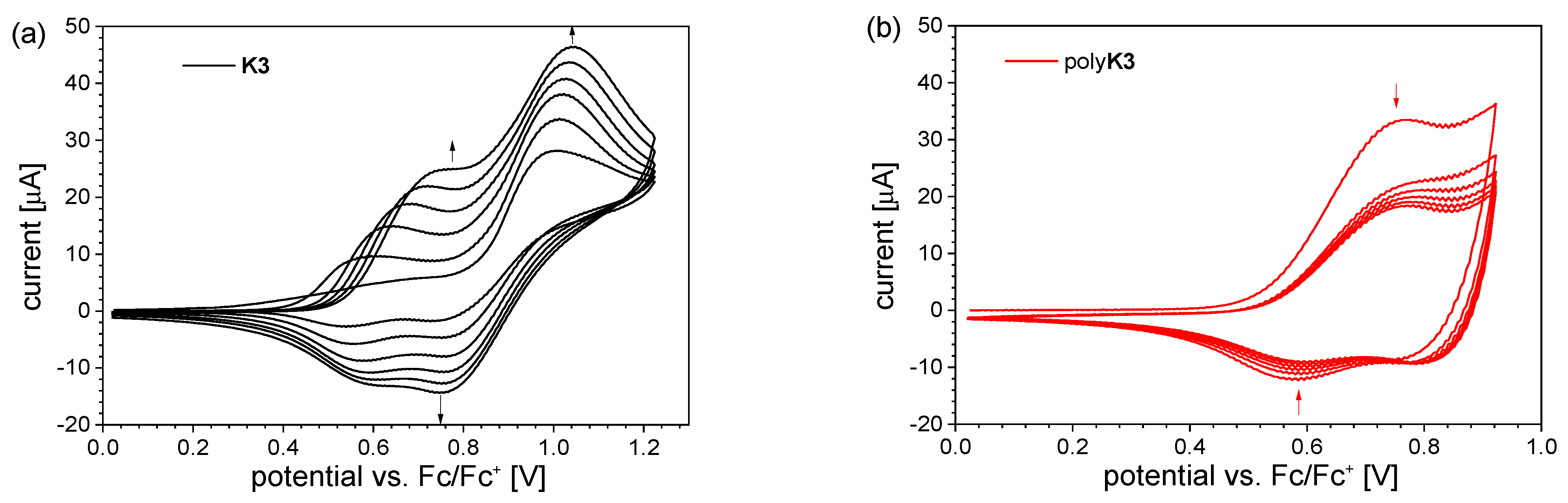
2.3.3. Cyclic Voltammetry Measurements
2.4. Computational Evaluation of Molecular Behavior
2.4.1. Molecular and Spectroscopic Characteristics
2.4.2. Delivery of Compounds
3. Materials and Methods
3.1. Measurement
General Experimental Procedure for Synthesis of Carbazole K1–K3
3.2. Mushroom Tyrosinase Inhibition Assay
3.3. Kinetic Analysis of the Inhibition of Tyrosinase
3.4. Thermogravimetric Analysis
3.5. Cyclic Voltammetry Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| ESI | electrospray ionization |
| HRMS | high-resolution mass spectrometry |
| IC50 | Half-maximal inhibitory concentration |
| SD | standard deviation |
| Vmax | maximum velocity |
| Km | inhibition constant |
| TGA | thermogravimetric analysis |
| THF | tetrahydrofuran |
| EtOH | ethanol |
| DMSO | dimethyl sulfoxide |
| CHCl3 | chloroform |
| CT | charge transfer |
| NBO | natural bond orbital |
| LUMO | lowest unoccupied molecular orbital |
| HOMO | highest occupied molecular orbital |
| MEP | molecular electrostatic potential |
| NLO | nonlinear optical materials |
| ConA | concanavalin A |
| HSA | human serum albumin |
| TDDFT | time-dependent density-functional theory |
| PBE0 | Perdew–Burke–Ernzerhof hybrid functional |
References
- Xu, Z.; Wu, D.; Fang, C.; Li, Y. Mini-Review on the Novel Synthesis and Potential Applications of Carbazole and Its Derivatives. Des. Monomers Polym. 2023, 26, 90–105. [Google Scholar] [CrossRef]
- Niedziałkowska, K.; Felczak, A.; Głowacka, I.E.; Piotrowska, D.G.; Lisowska, K. Antimicrobial Activity and Toxicity of Newly Synthesized 4-[4-(Benzylamino)butoxy]-9H-Carbazole Derivatives. Int. J. Mol. Sci. 2023, 24, 13722. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Hu, S.; Liu, Y.-Y.; Zhang, M.-M.; Zhang, W.-H.; Qiang, L.; Fu, Y.-H. Anti-Inflammatory and Antiproliferative Prenylated Carbazole Alkaloids from Clausena vestita. Bioorg. Chem. 2019, 91, 103107. [Google Scholar] [CrossRef] [PubMed]
- Issa, S.; Prandina, A.; Bedel, N.; Rongved, P.; Yous, S.; Le Borgne, M.; Bouaziz, Z. Carbazole Scaffolds in Cancer Therapy: A Review from 2012 to 2018. J. Enzyme Inhib. Med. Chem. 2019, 34, 1321–1346. [Google Scholar] [CrossRef]
- Çapan, İ.; Hawash, M.; Jaradat, N.; Sert, Y.; Servi, R.; Koca, I. Design, Synthesis, Molecular Docking and Biological Evaluation of New Carbazole Derivatives as Anticancer and Antioxidant Agents. BMC Chem. 2023, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xu, X.L.; Huang, L.J.; Zhang, Z.Y.; Zhou, Z.N.; Wang, J.Y.; Ouyang, X.; Xin, S.Y.; Zhang, Z.Y.; Xiong, Y.; et al. Bioactivities and Action Mechanisms of Ellipticine Derivatives Reported Prior to 2023. Chem. Biodivers. 2024, 21, e202400210. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Qu, G.; Wang, B. Murrayanine Suppresses the Proliferation and Metastasis of Human Breast Cancer Cells via Induction of Apoptosis and Inhibition of RANK/RANKL Signaling Pathway. Appl. Biol. Chem. 2021, 64, 32. [Google Scholar] [CrossRef]
- Ghani, U. Carbazole and Hydrazone Derivatives as New Competitive Inhibitors of Tyrosinase: Experimental Clues to Binuclear Copper Active Site Binding. Bioorg. Chem. 2019, 83, 235–241. [Google Scholar] [CrossRef]
- Cytarska, J.; Szulc, J.; Kołodziej-Sobczak, D.; Nunes, J.A.; da Silva-Júnior, E.F.; Łączkowski, K.Z. Cyrene™ as a Tyrosinase Inhibitor and Anti-Browning Agent. Food Chem. 2024, 442, 138430. [Google Scholar] [CrossRef]
- Yin, J.; Ma, Y.; Li, G.; Peng, M.; Lin, W. A Versatile Small-Molecule Fluorescence Scaffold: Carbazole Derivatives for Bioimaging. Coord. Chem. Rev. 2020, 412, 213257. [Google Scholar] [CrossRef]
- Li, D.; Tu, S.; Le, Y.; Zhou, Y.; Yang, L.; Ding, Y.; Huang, L.; Liu, L. Development of Carbazole-Based Fluorescent Probe for Highly Sensitive Application in Fluoride Ion Detection. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121816. [Google Scholar] [CrossRef]
- Yang, C.; Yu, C.; Zhang, M.; Yang, X.; Dong, H.; Dong, Q.; Zhang, H.; Li, L.; Guo, X.; Zang, H. Investigation of Protective Effect of Ethanol on the Natural Structure of Protein with Infrared Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 271, 120935. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, M.C.; Ferreira, J.L.M.; de Souza, A.P.N.; Tomaso, L.P.d.S.; da Silva, G.F.S.; Marques, A.d.S.; de Campos, J.B.; Malta, L.F.B.; Senra, J.D. Organic Electronics: Basic Fundamentals and Recent Applications Involving Carbazole-Based Compounds. Processes 2024, 12, 2945. [Google Scholar] [CrossRef]
- Tavgeniene, D.; Blazevicius, D.; Skuodis, E.; Grigalevicius, S.; Huang, C.-H.; Chen, Y.-H.; Chang, C.-H. High-Performance OLED Host Materials: Photophysical Properties and Device Optimization of Carbazole-Benzocarbazole Derivatives. Dyes Pigm. 2025, 242, 112965. [Google Scholar] [CrossRef]
- Krawczyk, P.; Kula, S.; Seklecka, K.; Łączkowski, K.Z. Synthesis, Electrochemical, Optical and Biological Properties of New Carbazole Derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120497. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, P.; Jędrzejewska, B.; Seklecka, K.; Cytarska, J.; Łączkowski, K.Z. Effect of the Chloro-Substitution on Electrochemical and Optical Properties of New Carbazole Dyes. Materials 2021, 14, 3091. [Google Scholar] [CrossRef] [PubMed]
- Suppan, P. Invited Review: Solvatochromic Shifts: The Influence of the Medium on the Energy of Electronic States. J. Photochem. Photobiol. A Chem. 1990, 50, 293–330. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Ledwon, P.; Lapkowski, M. The Role of Electrochemical and Spectroelectrochemical Techniques in the Preparation and Characterization of Conjugated Polymers: From Polyaniline to Modern Organic Semiconductors. Polymers 2022, 14, 4173. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lin, S.-W. Electrochemical Synthesis of Electrochromic Polycarbazole Films from N-Phenyl-3,6-Bis(N-Carbazolyl)Carbazoles. Polym. Chem. 2016, 7, 198–211. [Google Scholar] [CrossRef]
- Karon, K.; Lapkowski, M. Carbazole Electrochemistry: A Short Review. J. Solid. State Electrochem. 2015, 19, 2601–2610. [Google Scholar] [CrossRef]
- Chen, X.-C.; Tao, T.; Wang, Y.-G.; Peng, Y.-X.; Huang, W.; Qian, H.-F. Azo-Hydrazone Tautomerism Observed from UV-Vis Spectra by pH Control and Metal-Ion Complexation for Two Heterocyclic Disperse Yellow Dyes. Dalt. Trans. 2012, 41, 11107–11115. [Google Scholar] [CrossRef]
- Bártová, K.; Císařová, I.; Lyčka, A.; Dračínský, M. Tautomerism of Azo Dyes in the Solid State Studied by 15N, 14N, 13C and 1H NMR Spectroscopy, X-Ray Diffraction and Quantum-Chemical Calculations. Dye. Pigment. 2020, 178, 108342. [Google Scholar] [CrossRef]
- Yu, X.; Li, C.; Wang, B.; Ding, X.; Wang, N.; Xing, B.; Zhang, Z. Protein-Mediated Fluorescent Probes for Bioimaging and Biosensing: From Fundamentals to Applications. TrAC Trends Anal. Chem. 2024, 170, 117462. [Google Scholar] [CrossRef]
- Reeder, W.J.; Ekstedt, R.D. Study of the Interaction of Concanavalin A with Staphylococcal Teichoic Acids. J. Immunol. 1971, 106, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Mussini, A.; Delcanale, P.; Berni, M.; Pongolini, S.; Jordà-Redondo, M.; Agut, M.; Steinbach, P.J.; Nonell, S.; Abbruzzetti, S.; Viappiani, C. Concanavalin A Delivers a Photoactive Protein to the Bacterial Wall. Int. J. Mol. Sci. 2024, 25, 5751. [Google Scholar] [CrossRef]
- Olmsted, J., III. Calorimetric Determinations of Absolute Fluorescence Quantum Yields. J. Phys. Chem. 1979, 83, 2581–2684. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, G.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Adamo, C.; Scuseria, G.E.; Barone, V. Accurate Excitation Energies from Time-Dependent Density Functional Theory: Assessing the PBE0 Model. J. Chem. Phys. 1999, 111, 2889–2899. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868, Erratum in Phys. Rev. Lett. 1997, 78, 1396. https://doi.org/10.1103/PhysRevLett.78.1396. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, P.; Bratkowska, M.; Wybranowski, T.; Hołyńska-Iwan, I.; Cysewski, P.; Jędrzejewska, B. Experimental and Theoretical Insight into Spectroscopic Properties and Bioactivity of 4-(4-Formylbenzylidene)-2-Phenyloxazol-5(4H)-One Dye for Future Applications in Biochemistry. J. Mol. Liq. 2020, 314, 113632. [Google Scholar] [CrossRef]
- Krawczyk, P.; Wybranowski, T.; Kaźmierski, Ł.; Hołyńska-Iwan, I.; Bartkowska, M.; Cysewski, P.; Jędrzejewska, B. 2’(1H-Phenanthro[9,10-d]imidazol-2-yl-4-carboxyclic Acid N-Hydroxysuccimimide Ester: A New Phenanthroimidazole Derivative as a Fluorescent Probe for Medical Applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117757. [Google Scholar] [CrossRef]
- Krawczyk, P.; Jędrzejewska, B.; Pietrzak, M.; Janek, T. Synthesis, Photophysical Properties and Systematic Evaluations of New Phenanthroimidazole Fluorescent Probe for Bioimaging: Experimental and Theoretical Study. J. Photochem. Photobiol. B-Biol. 2017, 166, 74–85. [Google Scholar] [CrossRef]
- Szukalski, A.; Jędrzejewska, B.; Krawczyk, P.; Bajorek, A. An Optical Modulator on the Pyrazolone-Based Bi-Component System. Dye. Pigment. 2020, 172, 107805. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Krawczyk, P.; Józefowicz, M. Experimental and Theoretical Studies of the Influence of Solvent Polarity on the Spectral Properties of Two Push-Pull Oxazol-5-(4H)-One Compounds. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 171, 258–267. [Google Scholar] [CrossRef]
- Minezawa, N. State-Specific Solvation Effect on the Intramolecular Charge Transfer Reaction in Solution: A Linear-Response Free Energy TDDFT Method. Chem. Phys. Lett. 2014, 608, 140–144. [Google Scholar] [CrossRef]
- Ming Tong, G.S.; Chan, K.T.; Chang, X.; Che, C.-M. Theoretical Studies on the Photophysical Properties of Luminescent Pincer Gold(III) Arylacetylide Complexes: The Role of π-Conjugation at the C-Deprotonated [C^N^C] Ligand. Chem. Sci. 2015, 6, 3026–3037. [Google Scholar] [CrossRef]
- Kurtz, H.A.; Stewart, J.J.P.; Dieter, K.M. Calculation of the Nonlinear Optical Properties of Molecules. J. Comput. Chem. 1990, 11, 82–87. [Google Scholar] [CrossRef]
- Le Bahers, T.; Adamo, C.; Ciofini, I. A Qualitative Index of Spatial Extent in Charge-Transfer Excitations. J. Chem. Theory Comput. 2011, 7, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Cancés, M.T.; Mennucci, B.; Tomasi, J. A New Integral Equation Formalism for the Polarizable Continuum Model: Theoretical Background and Applications to Isotropic and Anisotropic Dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Arivazhagan, M.; Muniappan, P.; Meenakshi, R.; Rajavel, G. PCM/TD-DFT Analysis of 1-Bromo-2,3-Dichlorobenzene—A Combined Study of Experimental (FT-IR and FT-Raman) and Theoretical Calculations. Spectrochim. Acta Part. A 2013, 105, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.W. Nonlinear Optics, 2nd ed.; Academic Press: London, UK, 2003; p. 521. [Google Scholar]
- Craig, D.P.; Thirunamachandran, T. Theoretical Study of the Two-Photon Absorption Properties of Several Asymmetrically Substituted Stilbenoid Molecules. J. Chem. Phys. 2007, 127, 084504–084515. [Google Scholar] [CrossRef]
- Zaleśny, R.; Bartkowiak, W.; Styrcz, S.; Leszczynski, J. Solvent Effects on Conformationally Induced Enhancement of the Two-Photon Absorption Cross Section of a Pyridinium-N-Phenolate Betaine Dye: A Quantum Chemical Study. J. Phys. Chem. A 2002, 106, 4032–4037. [Google Scholar] [CrossRef]
- Olsen, J.; Jorgensen, P. Linear and Nonlinear Response Functions for an Exact State and for an MCSCF State. J. Chem. Phys. 1985, 82, 3235–3264. [Google Scholar] [CrossRef]
- Sałek, P.; Vahtras, O.; Guo, J.D.; Luo, Y.; Helgaker, T.; Ågren, H. Calculations of Two-Photon Absorption Cross Sections by Means of Density-Functional Theory. Chem. Phys. Lett. 2003, 374, 446–452. [Google Scholar] [CrossRef]
- DALTON, A Molecular Electronic Structure Program, Release Dalton 2011. Available online: http://daltonprogram.org (accessed on 27 June 2025).
- LSDALTON, A Linear Scaling Molecular Electronic Structure Program, Release Dalton 2011. Available online: http://daltonprogram.org (accessed on 27 June 2025).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Panjikar, C.S.; Tucker, P.A.; Weiss, M.S. On the Routine Use of Soft X-Rays in Macromolecular Crystallography, Part III—The Optimal Data Collection Wavelength. Acta Crystallogr. D Biol. Crystallogr. 2005, 61, 1263–1272. [Google Scholar] [CrossRef]
- Potemkin, V.; Grishina, M. A New Paradigm for Pattern Recognition of Drugs. J. Comput. Aided Mol. Des. 2008, 22, 489–505. [Google Scholar] [CrossRef]
- Potemkin, V.; Grishina, M. Principles for 3D/4D QSAR Classification of Drugs. Drug Discov. Today 2008, 13, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Mahesar, P.A.; Channar, P.A.; Abbas, Q.; Larik, F.A.; Hassan, M.; Raza, H.; Seo, S.-Y. Synthesis, molecular docking studies of coumarinyl-pyrazolinyl substituted thiazoles as non-competitive inhibitors of mushroom tyrosinase. Bioorg. Chem. 2017, 74, 187–196. [Google Scholar]
- Rosada, B.; Bekier, A.; Cytarska, J.; Płaziński, W.; Zavyalova, O.; Sikora, A.; Dzitko, K.; Łączkowski, K.Z. Benzo[b]thiophene-Thiazoles as Potent Anti-Toxoplasma gondii Agents: Design, Synthesis, Tyrosinase/Tyrosine Hydroxylase Inhibitors, Molecular Docking Study, and Antioxidant Activity. Eur. J. Med. Chem. 2019, 184, 111765. [Google Scholar] [CrossRef]
- Shin, N.H.; Ryu, S.Y.; Choi, E.J.; Kang, S.H.; Chang, I.M.; Min, K.R.; Kim, Y. Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 1998, 243, 801–803. [Google Scholar] [CrossRef] [PubMed]
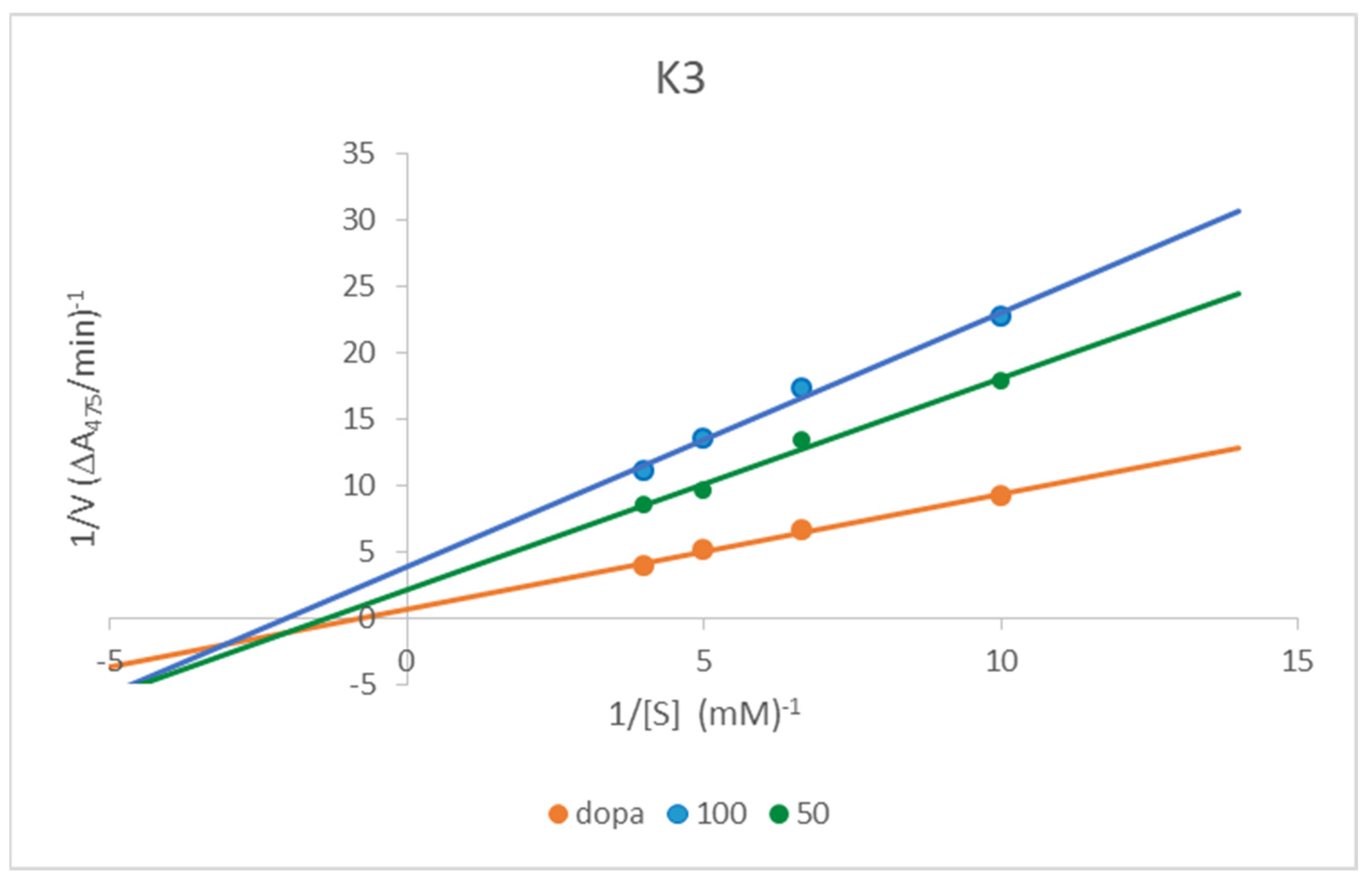
| Compound | IC50 ± SD [µM] | Inhibitory Mechanism | Vmax [mM/min] | Km [mM/min] |
|---|---|---|---|---|
| K1 | 59.36 ± 1.427 | Mixed | 0.2608 | 0.655 |
| K2 | 81.99 ± 3.387 | Mixed | 0.4078 | 0.314 |
| K3 | 45.95 ± 3.152 | Mixed | 0.4718 | 0.751 |
| Ascorbic acid | 386.50 ± 11.958 | - | - | - |
| Kojic acid | 72.27 ± 3.145 | - | - | - |
| Compound | T5 a [°C] | T10 a [°C] | Tmax b [°C] |
|---|---|---|---|
| K1 | 222 | 279 | 301, 468 |
| K2 | 231 | 252 | 313 |
| K3 | 244 | 271 | 298, 668 |
| Compound | Absorption (λabs) [nm] | ε [M−1 cm−1] | Emission (λem) [nm] | Stokes Shift a [cm−1] | |
|---|---|---|---|---|---|
| K1 | Toluene | 362 340 326 291 | 18,949 15,630 12,532 12,910 | 416 | 54 |
| CHCl3 | 363 340 326 291 | 16,942 13,099 11,245 10,984 | 439 | 75 | |
| THF | 357 291 | 20,853 12,831 | 456 | 100 | |
| EtOH | 355 291 | 26,895 16,018 | 468 | 115 | |
| DMSO | 362 293 | 22,354 14,292 | 485 | 123 | |
| K2 | Toluene | 362 340 328 293 | 10,289 10,811 10,289 9859 | 409; 432 | 47; 70 |
| CHCl3 | 367 326 291 258 | 20,657 20,988 21,829 33,111 | 451 | 84 | |
| THF | 342 291 281 257 | 21,634 14,393 16,167 24,112 | 439 | 97 | |
| EtOH | 342 291 254 | 28,198 18,266 30,235 | 441 | 99 | |
| DMSO | 345 293 281 | 28,389 19,278 21,595 | 462 | 117 | |
| K3 | Toluene | - | - | - | - |
| CHCl3 | 361 291 | 26,666 17,688 | 444 | 83 | |
| THF | 358 291 283 | 27,103 16,416 14,577 | nd | - | |
| EtOH | 356 291 281 | 14,913 9712 8671 | 447 | 92 | |
| DMSO | 362 293 | 35,033 22,045 | 475 | 113 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donarska, B.; Seklecka, K.; Cytarska, J.; Piechowska, K.; Ledwon, P.; Kula, S.; Krawczyk, P.; Baranowska-Łączkowska, A.; Łączkowski, K.Z. Novel Carbazole–Thiazole Conjugates: Synthesis and Biophysical Characterization. Int. J. Mol. Sci. 2025, 26, 7945. https://doi.org/10.3390/ijms26167945
Donarska B, Seklecka K, Cytarska J, Piechowska K, Ledwon P, Kula S, Krawczyk P, Baranowska-Łączkowska A, Łączkowski KZ. Novel Carbazole–Thiazole Conjugates: Synthesis and Biophysical Characterization. International Journal of Molecular Sciences. 2025; 26(16):7945. https://doi.org/10.3390/ijms26167945
Chicago/Turabian StyleDonarska, Beata, Klaudia Seklecka, Joanna Cytarska, Katarzyna Piechowska, Przemyslaw Ledwon, Sławomir Kula, Przemysław Krawczyk, Angelika Baranowska-Łączkowska, and Krzysztof Z. Łączkowski. 2025. "Novel Carbazole–Thiazole Conjugates: Synthesis and Biophysical Characterization" International Journal of Molecular Sciences 26, no. 16: 7945. https://doi.org/10.3390/ijms26167945
APA StyleDonarska, B., Seklecka, K., Cytarska, J., Piechowska, K., Ledwon, P., Kula, S., Krawczyk, P., Baranowska-Łączkowska, A., & Łączkowski, K. Z. (2025). Novel Carbazole–Thiazole Conjugates: Synthesis and Biophysical Characterization. International Journal of Molecular Sciences, 26(16), 7945. https://doi.org/10.3390/ijms26167945








