Gold Nanoparticles as Targeted Drug Delivery Systems for Liver Cancer: A Systematic Review of Tumor Targeting Efficiency and Toxicity Profiles
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Information Sources
2.2. Inclusion and Eligibility Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Risk of Bias
2.5. Synthesis of the Results
3. Results
3.1. Mechanisms of Action
3.2. Safety and Toxicity
Interpretation of the Results
4. Conclusions
Funding
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primer 2021, 7, 6. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Song, T. Conversion Therapy and Maintenance Therapy for Primary Hepatocellular Carcinoma. Biosci. Trends 2021, 15, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef]
- Marin, J.J.G.; Herraez, E.; Lozano, E.; Macias, R.I.R.; Briz, O. Models for Understanding Resistance to Chemotherapy in Liver Cancer. Cancers 2019, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.A.; Thomas, M.R.; Szijj, P.A.; Foote, J.; Chen, Y.; Nogueira, J.C.F.; Chudasama, V.; Stevens, M.M. Employing Defined Bioconjugates to Generate Chemically Functionalised Gold Nanoparticles for in Vitro Diagnostic Applications. Nanoscale 2021, 13, 11921–11931. [Google Scholar] [CrossRef] [PubMed]
- Goddard, Z.R.; Beekman, A.M.; Cominetti, M.M.D.; O’Connell, M.A.; Chambrier, I.; Cook, M.J.; Marín, M.J.; Russell, D.A.; Searcey, M. Peptide Directed Phthalocyanine-Gold Nanoparticles for Selective Photodynamic Therapy of EGFR Overexpressing Cancers. RSC Med. Chem. 2020, 12, 288–292. [Google Scholar] [CrossRef]
- Mocan, L.; Matea, C.; Tabaran, F.A.; Mosteanu, O.; Pop, T.; Mocan, T.; Iancu, C. Photothermal Treatment of Liver Cancer with Albumin-Conjugated Gold Nanoparticles Initiates Golgi Apparatus-ER Dysfunction and Caspase-3 Apoptotic Pathway Activation by Selective Targeting of Gp60 Receptor. Int. J. Nanomed. 2015, 10, 5435–5445. [Google Scholar] [CrossRef]
- Nasir, A.; Khan, A.; Li, J.; Naeem, M.; Khalil, A.A.K.; Khan, K.; Qasim, M. Nanotechnology, A Tool for Diagnostics and Treatment of Cancer. Curr. Top. Med. Chem. 2021, 21, 1360–1376. [Google Scholar] [CrossRef]
- Lopalco, A.; Iacobazzi, R.M.; Lopedota, A.A.; Denora, N. Recent Advances in Nanodrug Delivery Systems Production, Efficacy, Safety, and Toxicity. In Computational Toxicology; Methods in Molecular Biology; Humana: New York, NY, USA, 2025; Volume 2834, pp. 303–332. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in Cancer Theragnostic and Drug Delivery: A Comprehensive Review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Mir, M.; Ishtiaq, S.; Rabia, S.; Khatoon, M.; Zeb, A.; Khan, G.M.; Ur Rehman, A.; Ud Din, F. Nanotechnology: From In Vivo Imaging System to Controlled Drug Delivery. Nanoscale Res. Lett. 2017, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.F.; In, L.L.A.; Vijayaraj Kumar, P. Surface Functionalization of Gold Nanoparticles for Targeting the Tumor Microenvironment to Improve Antitumor Efficiency. ACS Appl. Bio Mater. 2023, 6, 2944–2981. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.-R.; Heo, J.H. Simultaneous Stabilization and Functionalization of Gold Nanoparticles via Biomolecule Conjugation: Progress and Perspectives. ACS Appl. Mater. Interfaces 2021, 13, 42311–42328. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef]
- Yao, L.; Bojic, D.; Liu, M. Applications and Safety of Gold Nanoparticles as Therapeutic Devices in Clinical Trials. J. Pharm. Anal. 2023, 13, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Ma, R.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.-S.; Zhou, Y. Gold Nanoparticles and Gold Nanorods in the Landscape of Cancer Therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef]
- Erfanian, S.S.; Ansari, H.; Javanmard, S.H.; Amini, Z.; Hajigholami, A. The Hepatorenal Protective Effects of Silymarin in Cancer Patients Receiving Chemotherapy: A Randomized, Placebo-Controlled Trial. BMC Complement. Med. Ther. 2024, 24, 329. [Google Scholar] [CrossRef]
- Cappuyns, S.; Corbett, V.; Yarchoan, M.; Finn, R.S.; Llovet, J.M. Critical Appraisal of Guideline Recommendations on Systemic Therapies for Advanced Hepatocellular Carcinoma: A Review. JAMA Oncol. 2024, 10, 395–404. [Google Scholar] [CrossRef]
- Mioc, A.; Mioc, M.; Ghiulai, R.; Voicu, M.; Racoviceanu, R.; Trandafirescu, C.; Dehelean, C.; Coricovac, D.; Soica, C. Gold Nanoparticles as Targeted Delivery Systems and Theranostic Agents in Cancer Therapy. Curr. Med. Chem. 2019, 26, 6493–6513. [Google Scholar] [CrossRef]
- Rosyidah, A.; Kerdtoob, S.; Yudhistyra, W.I.; Munfadlila, A.W. Gold Nanoparticle-Based Drug Nanocarriers as a Targeted Drug Delivery System Platform for Cancer Therapeutics: A Systematic Review. Gold Bull. 2023, 56, 121–134. [Google Scholar] [CrossRef]
- Zenze, M.; Singh, M. Receptor Targeting Using Copolymer-Modified Gold Nanoparticles for pCMV-Luc Gene Delivery to Liver Cancer Cells In Vitro. Int. J. Mol. Sci. 2024, 25, 5016. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Mahmoud, G.A. pH-Responsive Chitosan/Acrylamide/Gold/Nanocomposite Supported with Silver Nanoparticles for Controlled Release of Anticancer Drug. Sci. Rep. 2023, 13, 7818. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Kamel, A.G.; Hussein, A.H.; Azzam, M.; Makky, S.; Rezk, N.; Essam, K.; Agwa, M.M.; El-Shibiny, A. The Promising Antibacterial and Anticancer Activity of Green Synthesized Zinc Nanoparticles in Combination with Silver and Gold Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1868–1881. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; Zhong, C.; Zhang, X.; Bao, Y. Green Synthesis of Gold Nanoparticles from Dendrobium Officinale and Its Anticancer Effect on Liver Cancer. Drug Deliv. 2021, 28, 985–994. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, H.; Xu, C.; Tong, Z.; Zhang, S.; Bai, Y.; Chen, Y.; Xu, Q.; Zhou, L.; Ding, H.; et al. A Nanomedicine Fabricated from Gold Nanoparticles-Decorated Metal-Organic Framework for Cascade Chemo/Chemodynamic Cancer Therapy. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2020, 7, 2001060. [Google Scholar] [CrossRef] [PubMed]
- Hassanen, E.I.; Korany, R.M.S.; Bakeer, A.M. Cisplatin-Conjugated Gold Nanoparticles-Based Drug Delivery System for Targeting Hepatic Tumors. J. Biochem. Mol. Toxicol. 2021, 35, e22722. [Google Scholar] [CrossRef]
- Nandhini, J.T.; Ezhilarasan, D.; Rajeshkumar, S. An Ecofriendly Synthesized Gold Nanoparticles Induces Cytotoxicity via Apoptosis in HepG2 Cells. Environ. Toxicol. 2021, 36, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, S.; Zhang, X.; Zhu, R.; Chen, S.; Chen, X.; Song, J.; Yang, H. An Ultrasound Activated Vesicle of Janus Au-MnO Nanoparticles for Promoted Tumor Penetration and Sono-Chemodynamic Therapy of Orthotopic Liver Cancer. Angew. Chem. 2020, 59, 1682–1688. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C.; Al Farraj, D.A.; Soliman Elshikh, M.; Mohana Roopan, S. Employing Sulphated Polysaccharide (Fucoidan) as Medium for Gold Nanoparticles Preparation and Its Anticancer Study against HepG2 Cell Lines. Mater. Today Commun. 2021, 26, 101975. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, W.; Ahrari, S.; Yu, M.; Zheng, J. Crosstalk between Hepatic Glutathione Efflux and Tumor Targeting Efficiency of Indocyanine Green-Conjugated Gold Nanoparticles. Angew. Chem. 2023, 62, e202308909. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Pereira, A.C.; Resende, M.A.C.; Ferreira, L.F. Gold Nanoparticles: A Didactic Step-by-Step of the Synthesis Using the Turkevich Method, Mechanisms, and Characterizations. Analytica 2023, 4, 250–263. [Google Scholar] [CrossRef]
- Awashra, M.; Młynarz, P. The Toxicity of Nanoparticles and Their Interaction with Cells: An in Vitro Metabolomic Perspective. Nanoscale Adv. 2023, 5, 2674–2723. [Google Scholar] [CrossRef]
- Adewale, O.B.; Davids, H.; Cairncross, L.; Roux, S. Toxicological Behavior of Gold Nanoparticles on Various Models: Influence of Physicochemical Properties and Other Factors. Int. J. Toxicol. 2019, 38, 357–384. [Google Scholar] [CrossRef]
- Ouyang, R.; Cao, P.; Jia, P.; Wang, H.; Zong, T.; Dai, C.; Yuan, J.; Li, Y.; Sun, D.; Guo, N.; et al. Bistratal Au@Bi2S3 Nanobones for Excellent NIR-Triggered/Multimodal Imaging-Guided Synergistic Therapy for Liver Cancer. Bioact. Mater. 2021, 6, 386–403. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of Gold Nanoparticles (AuNPs): A Review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, K.; Zhao, G. Gold Nanoparticles: From Synthesis, Properties to Their Potential Application as Colorimetric Sensors in Food Safety Screening. Trends Food Sci. Technol. 2018, 78, 83–94. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Liu, K.; Luo, X.; Yin, Z.; Lin, H.; Gao, J. Recent Advances of Nanomedicines for Liver Cancer Therapy. J. Mater. Chem. B 2020, 8, 3747–3771. [Google Scholar] [CrossRef]
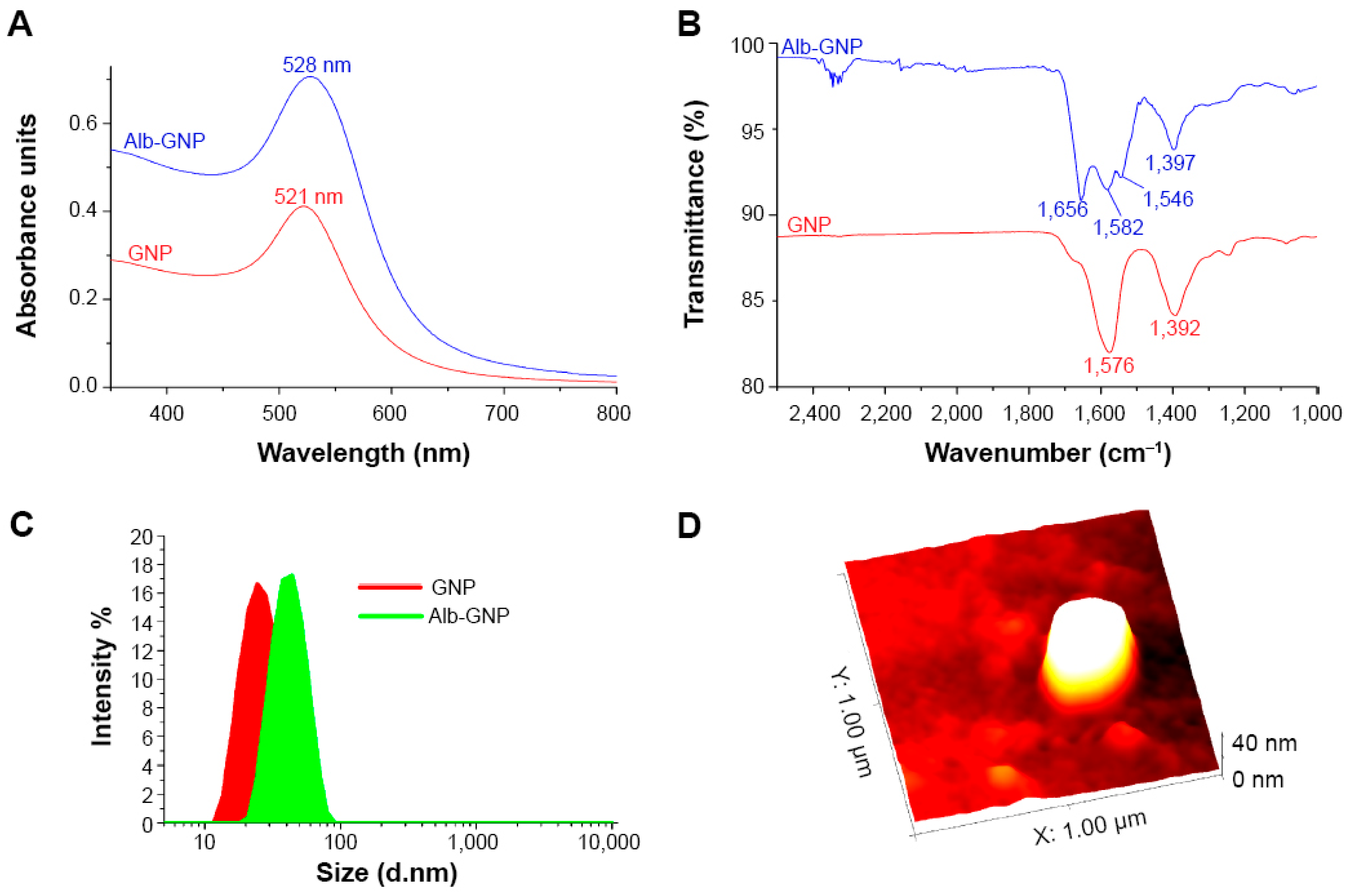
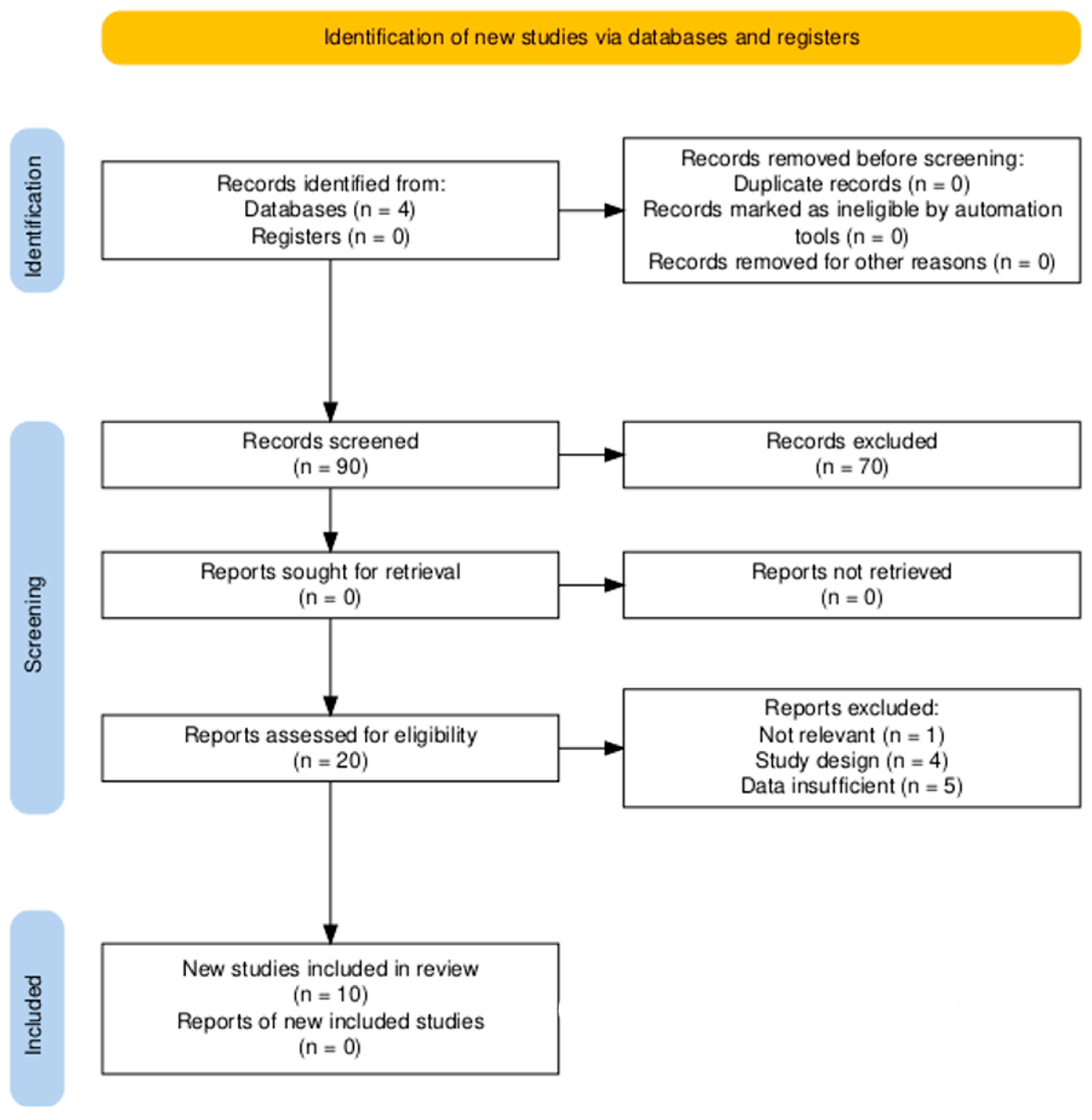

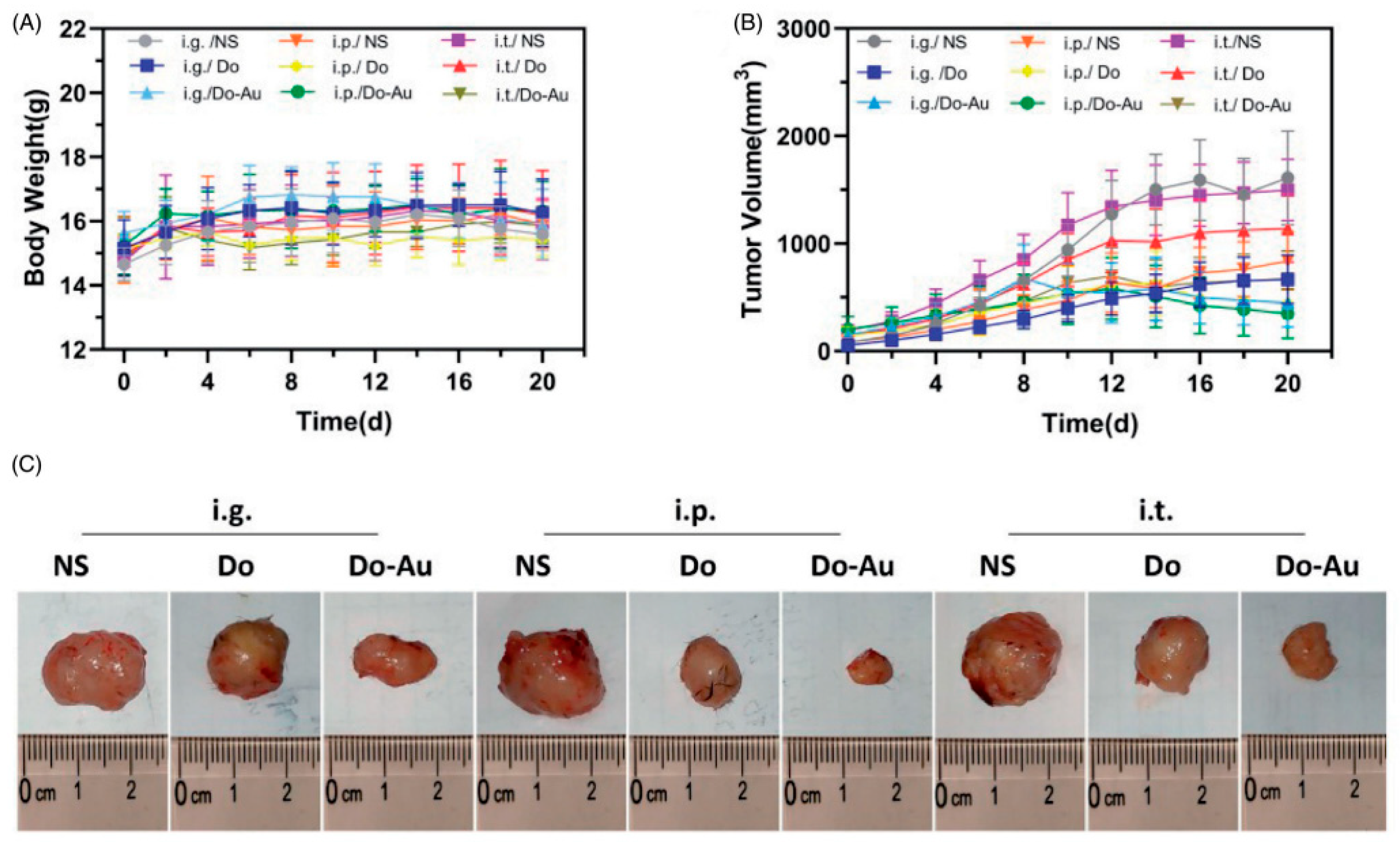
| Study, Year | Type of Study (In Vitro/In Vivo/Clinical) | Gold Nanoparticle Size (nm) | Drug Delivered | Dosage of Drug | Administration Route | Target Mechanism (e.g., Passive Targeting, Active Targeting) | Tumor Type (Hepatocellular Carcinoma, etc.) |
|---|---|---|---|---|---|---|---|
| Zenze & Singh, 2024 [22] | In vitro | 70–250 nm | pCMV-Luc DNA | Not specified | In vitro (cellular uptake via incubation) | Active targeting using lactobionic acid (LA) for receptor-mediated endocytosis | Hepatocellular carcinoma (HepG2 cells) |
| Nasef, Khozemy, & Mahmoud, 2023 [23] | In vitro | 41.7 nm (AuNPs), 66.7 nm (AAuNPs) | Fluorouracil | 95 mg/g loading | Oral delivery (targeting the intestines via pH) | pH-responsive (acidic to neutral pH environment) | Hepatocellular carcinoma (HepG2 cells) |
| Abdelsattar et al., 2023 [24] | In vitro | 22–73 nm (AuNPs@ZnO) | Not applicable (no drug, focus on nanoparticles) | Not applicable | Not specified | Active targeting through nanoparticle-mediated cytotoxicity | Breast cancer (MCF-7), Liver cancer (HepG-2) |
| Zhao et al., 2021 [25] | In vitro & In vivo | 30 nm | Dendrobium officinale extract | 2.4 mg/mL | Intraperitoneal (i.p.), Intragastric (i.g.), Intratumoral (i.t.) | Active targeting via immune modulation | Hepatocellular carcinoma (Liver cancer) |
| Ding et al., 2020 [26] | In vitro & In vivo | 5 nm (AuNPs), 50 nm (FeMOF) | Camptothecin (CPT) | 7.7% loading content | Intravenous (i.v.) | Passive targeting via EPR effect, active targeting through glucose oxidation, and Fenton reaction | Hepatocellular carcinoma (Liver cancer) |
| Hassanen et al., 2021 [27] | In vivo | 10–17.5 nm | Cisplatin | 3 mg/kg (Cisplatin) | Intraperitoneal (ip) | Passive targeting through the EPR effect | Hepatocellular carcinoma (HCC) |
| Nandhini et al., 2021 [28] | In vitro | 5–10 nm | No drug, Enterococcus-mediated AuNPs | 10 µg/mL (IC50 for AuNPs) | Direct exposure to cells in vitro (cell culture) | Passive targeting via ROS generation | Hepatocellular carcinoma (HepG2 cells) |
| Lin et al., 2020 [29] | In vivo | 10 nm (Au NPs), 20 nm (Janus Au-MnO NPs) | No drug, Janus Au-MnO nanoparticles | 1 mg/mL (vesicles) | Intravenous injection in mice | Active targeting, Sono-chemodynamic therapy (SDT/CDT) | Orthotopic liver cancer (Hepatocellular carcinoma) |
| Rajeshkumar et al., 2021 [30] | In vitro | 21–44 nm | No specific drug, Gold nanoparticles synthesized using fucoidan | Various concentrations (1, 10, 25, 50, 100 μg/mL) | Direct application to HepG2 cells | Active targeting via fucoidan-mediated synthesis | HepG2 (Liver cancer) |
| Huang et al., 2023 [31] | In vivo | ~1.1 nm (Au25SG18 nanoclusters) | Indocyanine Green (ICG)-Conjugated Au NPs | ~40 μM | Intravenous Injection | Active targeting via GSH responsiveness | 4T1 Triple-Negative Breast Cancer |
| Study, Year | Key Outcome Measures (e.g., Tumor Size Reduction, Survival Rate) | Toxicity Profile (Impact on Liver, Kidney, etc.) | Bioavailability Improvement | Survival Improvement (%) | Mechanism of Action | Limitations | Duration of Study |
|---|---|---|---|---|---|---|---|
| Zenze & Singh, 2024 [22] | A five-fold increase in luciferase gene expression in HepG2 cells compared to non-targeted NPs | Well-tolerated in all cells with cell viability >70% | Yes, improved cellular uptake and transgene activity | Not applicable (in vitro) | Receptor-mediated endocytosis via the asialoglycoprotein receptor in HepG2 cells | Limited to in vitro studies; does not address in vivo applications or long-term safety | Not specified (cell viability assessed after 48 h) |
| Nasef, Khozemy, & Mahmoud, 2023 [23] | 97% drug release in 300 min at pH 7.4, strong antimicrobial activity | Reduced cytotoxicity of AAuNPs by combining with AuNPs | Yes, enhanced due to pH responsiveness and polymer matrix | Not applicable (in vitro study) | Controlled drug release through pH change | Limited to in vitro studies, no in vivo testing | Not specified |
| Abdelsattar et al., 2023 [24] | Reduced cell viability in cancer cells (MCF-7 and HepG-2) | Lower cytotoxicity of AuNPs@ZnO and AAuNPs@ZnO compared to ZnO-NPs in normal cells (HSF) | No drug bioavailability assessed, focus on nanoparticles | Not applicable (in vitro study) | Cytotoxicity through ROS generation and membrane damage | High concentration of NPs required, in vitro only | Not specified |
| Zhao et al., 2021 [25] | Significant tumor size reduction, reduced cell viability in HepG2 cells | No significant liver or kidney toxicity, ALT, AST, BUN, and CRE normal | Improved through the combination with AuNPs | Not specified | Immune modulation, apoptosis, and increased tumor inhibition | Non-uniform particle size, lower efficacy of the i.t. method | 3 weeks |
| Ding et al., 2020 [26] | Significant tumor suppression, 85.6% tumor growth inhibition | No major systemic toxicity (ALT, AST, BUN, CREA normal), no damage to liver and kidney tissues | Improved circulation time and tumor accumulation | Significantly improved, prolonged survival in tumor-bearing mice | Chemodynamic therapy via Fenton reaction, glucose oxidation by AuNPs to generate H2O2 and OH∙ for ROS production | Requires further testing in human clinical trials, the phosphate-triggered release system complexity | Not specified |
| Hassanen et al., 2021 [27] | Significant reduction in liver enzymes (ALT, AST), improved histopathology, reduced nephrotoxicity | Reduced kidney and liver toxicity with cisplatin-AUNP conjugates compared to free cisplatin | Increased targeting and reduced off-target toxicity | Not specified | Conjugated AUNPs improved cisplatin’s delivery to hepatic tumors while reducing renal toxicity | Requires further studies on long-term effects | 6 months |
| Nandhini et al., 2021 [28] | Significant ROS generation, apoptosis induction, and reduced PCNA levels | No in vivo toxicity profile was provided, as it was an in vitro study | Not applicable | Not applicable | ROS-mediated apoptosis, cytochrome c release, and PCNA inhibition | Limited to in vitro; no animal or clinical data | 24 h (in vitro) |
| Lin et al., 2020 [29] | Tumor growth inhibition, enhanced ROS production, and better tumor penetration | Minimal toxicity to healthy tissues, stable blood circulation | Enhanced tumor penetration and accumulation | Not specifically reported | Sono-chemodynamic therapy (SDT/CDT) via ROS generation and cavitation | Limited clinical data; primarily an animal model study | 21 days (in vivo) |
| Rajeshkumar et al., 2021 [30] | Dose-dependent cytotoxic activity against HepG2 cells | Not specified | Improved targeting and stability via fucoidan | Not specifically reported | Induces apoptosis in HepG2 cells through oxidative stress and reduced cell viability | No in vivo studies were conducted; limited to in vitro tests | 24 h |
| Huang et al., 2023 [31] | Enhanced tumor targeting through GSH depletion and prolonged blood retention | No pathological damage to liver, slower hepatobiliary clearance | Increased due to reduced hepatic clearance and prolonged blood circulation | Not reported | GSH depletion slowed the clearance of ICG, enhancing tumor targeting | Limited to animal models, unclear long-term effects on other organs | 24 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosma, M.; Mocan, T.; Delcea, C.; Pop, T.; Mosteanu, O.; Mocan, L. Gold Nanoparticles as Targeted Drug Delivery Systems for Liver Cancer: A Systematic Review of Tumor Targeting Efficiency and Toxicity Profiles. Int. J. Mol. Sci. 2025, 26, 7917. https://doi.org/10.3390/ijms26167917
Cosma M, Mocan T, Delcea C, Pop T, Mosteanu O, Mocan L. Gold Nanoparticles as Targeted Drug Delivery Systems for Liver Cancer: A Systematic Review of Tumor Targeting Efficiency and Toxicity Profiles. International Journal of Molecular Sciences. 2025; 26(16):7917. https://doi.org/10.3390/ijms26167917
Chicago/Turabian StyleCosma, Meda, Teodora Mocan, Cristian Delcea, Teodora Pop, Ofelia Mosteanu, and Lucian Mocan. 2025. "Gold Nanoparticles as Targeted Drug Delivery Systems for Liver Cancer: A Systematic Review of Tumor Targeting Efficiency and Toxicity Profiles" International Journal of Molecular Sciences 26, no. 16: 7917. https://doi.org/10.3390/ijms26167917
APA StyleCosma, M., Mocan, T., Delcea, C., Pop, T., Mosteanu, O., & Mocan, L. (2025). Gold Nanoparticles as Targeted Drug Delivery Systems for Liver Cancer: A Systematic Review of Tumor Targeting Efficiency and Toxicity Profiles. International Journal of Molecular Sciences, 26(16), 7917. https://doi.org/10.3390/ijms26167917







