Broad-Spectrum Antiviral Activity of Cyclophilin Inhibitors Against Coronaviruses: A Systematic Review
Abstract
1. Introduction
2. Methods
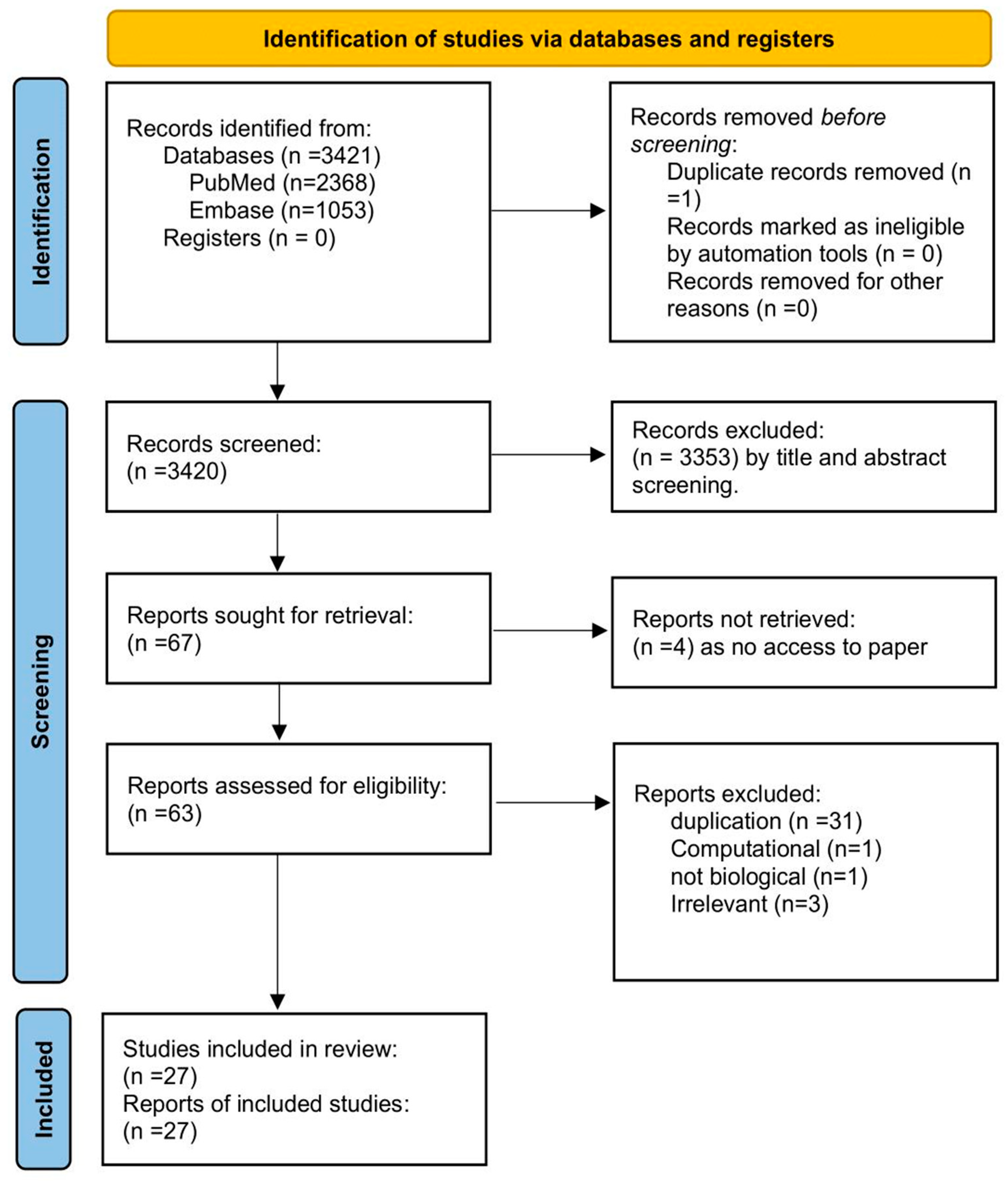
2.1. Search Strategy: (As Shown in Table 1)
| Database | Search Strategy | Results |
|---|---|---|
| PubMed | (“Viruses” [Mesh] OR “Virus Diseases” [Mesh] OR “Virus Replication” [Mesh] OR “Virology” [Mesh] OR “Virus Assembly” [All Fields] OR “Viral Assembly” [All Fields]) AND (“Cyclophilins” [MeSH Terms] OR “Peptidylprolyl Isomerase” [Mesh] OR “Cyclosporine” [Mesh] OR “Cyclosporins” [Mesh]) NOT “Review” [Publication Type] | 2368 |
| Embase | (‘viruses’ OR ‘virus diseases’ OR ‘virus replication’ OR ‘virology’ OR ‘virus assembly’ OR ‘viral assembly’) AND (‘cyclophilins’ OR ‘peptidylprolyl isomerase’ OR ‘cyclosporine’ OR ‘cyclosporins’) | 1053 |
2.2. Study Selection
2.2.1. Duplication Check
2.2.2. Screening Titles
2.2.3. Screening Abstracts
2.3. Inclusion Criteria
Types of Studies
2.4. Exclusion Criteria
2.4.1. Types of Publications
2.4.2. Study Focus
2.5. In-Depth Analysis
2.6. Data Extraction
3. Results and Discussion
3.1. Broad-Spectrum Antiviral Activity of Cyclophilin Inhibitors on Different Strains of Coronavirus (Supplementary Table S1, Table 2 and Table 3)
| Virus (Strain) | Cell Line | Compound | EC50 (µM) | CC50 (µM) | Reference |
|---|---|---|---|---|---|
| SARS-CoV-2 | Vero E6 | Alisporivir | 0.46 | - | [22] |
| MERS-CoV (EMC/2012) | Vero | Alisporivir | 3.6 ± 1.1 1/3.9 ± 1.7 2 | 26.4 | [20] |
| MERS-CoV (EMC/2012) | Huh7 | Alisporivir | 3.4 ± 1.0 1/2.8 ± 1.0 2 | 43.8 | [20] |
| MERS-CoV (EMC/2012) | LLC-MK2 | Alisporivir | 4.0 ± 1.1 2 | 14.3 ± 1.8 | [20] |
| MERS-CoV (N3/Jordan) | Vero | Alisporivir | 3.0 ± 1.0 1 | 26.4 ± 1.0 | [20] |
| MERS-CoV (N3/Jordan) | Huh7 | Alisporivir | 1.5 ± 1.0 1 | 43.8 | [20] |
| SARS-CoV (Frankfurt-1) | VeroE6 | Alisporivir | 8.3 ± 1.0 1 | >50 | [20] |
| SARS-CoV (Frankfurt-1) | VeroE6 | CsA | 3.3 | - | [23] |
| SARS-CoV (MA-15) | VeroE6 | Alisporivir | 1.3 ± 0.05 1 | >50 | [20] |
| HCoV-NL63 WT | Caco-2 | CsA | 0.9–2.0 | - | [24] |
| HCoV-NL63 WT | Caco-2 | Alisporivir | 0.8 | - | [24] |
| HCoV-NL63 WT | Caco-2 | NIM811 | 0.8 | - | [24] |
| HCoV-NL63 WT | Caco-2 | Compound 3 | 1.1 | - | [24] |
| HCoV-NL63 WT | Caco-2 | FK508 (Tacrolimus) | 6.6 | - | [24] |
| HCoV-229E-Luc | HuH-7.5 | CsA | 2.09/0.97 3 | - | [16] |
| HCoV-229E-Luc | HuH-7.5 | Alisporivir | 2.77/1.37 3 | - | [16] |
| HCoV-229E-Luc | HuH-7.5 | NIM811 | 3.11/1.19 3 | - | [16] |
| HCoV-229E-Luc | HuH-7.5 | Compound 3 | 2.05/0.92 3 | - | [16] |
| HCoV-229E | Huh7 | CsA | 2.3 | - | [23] |
| HCoV-NL63 | Caco-2 | CsA | 2.3 | - | [23] |
| Feline CoV | FCW | CsA | 2.7 | - | [23] |
| Virus | Animal Model | Intervention | Key Findings | Reference |
|---|---|---|---|---|
| MERS-CoV | Mouse (Ad-hDPP4) | (CsA) administered orally at 50 mg/kg/day for 6 days. | CsA treatment significantly induced the production of mouse IFNλ (mIFNλ) in bronchoalveolar lavage fluid (BALF). | [25] |
| MERS-CoV | Mouse (Ad-hDPP4) | CsA at 50 mg/kg/day given orally starting 3 days post-adenoviral transduction for human DPP4 receptor expression, with MERS-CoV infection introduced intranasally on day 5 post-adenoviral infection (1.5 × 105 TCID50·mL−1 MERS-CoV); 7 days post-infection, mice were killed, and lung tissue was used. | CsA treatment led to - significant elevation in IFNL2/3 mRNA. - decreased MERS-CoV viral load. - improved expression of the epithelial integrity marker SCNN1B. - There was also a significant reduction in lung pathology and interstitial inflammation compared to DMSO control. - Upon analysis on day seven post-infection, an inverse correlation was noted between IFNL expression and MERS-CoV levels in the lung homogenates. - The comprehensive data illustrate that the oral administration of CsA stimulates IFNλ synthesis in the pulmonary system of mice, thereby exerting significant anti-viral properties. | |
| SARS-CoV-2 WT | Balb/c mice expressing human ACE2 receptor. | Treatment with CsA (50 mg/kg/day) or DMSO orally for 6 days. Intranasal infection of SARS-COV-2 (1.5 × 104 TCID50/mL) on day 3 after CsA treatment started. Mice were sacrificed on day 4 post-infection, and viral RNA was isolated from lung homogenate. | On day 4 post-infection, a significant decrease in SARS-CoV-2 E gene was detected by qPCR in mice treated with CsA compared to DMSO (p < 0.001). | [19] |
| Treatment with CsA or DMSO (50 mg/kg/d) was initiated on the same day as infection. CsA or DMSO was applied orally for 6 days. Mice were sacrificed on day 7 post-infection, where the left lung lobe was extracted, embedded in paraffin, and stained. | Reduced infiltration of bone marrow-derived macrophages (p = 0.11) but unaffected recruitment of T-cells (p = 0.88) and neutrophils (p = 0.68). |
3.2. Clinical Trials on the Effect of Cyclophilin Inhibitors on Coronavirus Replication (Table 4)
| Virus | Population | Intervention | Key Findings | Reference |
|---|---|---|---|---|
| SARS-CoV-2 (COVID-19) | The study population consisted of 34 patients, with 18 in the CsA-SOC group (1 patient excluded due to ICU admission prior to trial initiation) and 16 in the SOC group. The mean age was 56.7 ± 11.8 years, 33.3% were female, and 69.7% were of Caucasian ethnicity. | CsA dosing was weight-based. At 0 h, patients received 100 mg/day for those weighing < 60 kg, 150 mg/day for those between 60 and 80 kg, and 200 mg/day for those >80 kg. At 48 h, the doses were adjusted to 150 mg/day for <60 kg, 200 mg/day for 60–80 kg, and 300 mg/day for >80 kg. The treatment duration was 1 month, with assessments conducted at 1 day, 4 days, 8 days, 30 days, and 90 days. | A higher proportion of patients in the CsA-SOC group achieved a clinical response without requiring invasive mechanical ventilation (IMV) compared to the SOC group. However, this did not reach statistical significance (p = 0.121). | [29] |
| SARS-CoV-2 (COVID-19) | A study was conducted involving 209 adult patients, with 105 assigned to the CsA group and 104 to the control group. | Oral CsA was administered at a dosage of 1–2 mg/kg/day, divided into two doses daily, for a duration of 7 days. Additionally, clarithromycin was given at 500 mg orally twice daily for 14 days, along with enoxaparin at 0.5 mg/kg/day for 14 days. Methylprednisolone was administered intravenously at 0.5 mg/kg once daily, or prednisone was given orally at 25 mg once daily, both for a period of 7 days. | Out of 149 discharged patients, 82 (55%) received CsA plus steroids, while 67 (45%) received steroids alone. Among the 60 deceased patients, 23 (38.3%) were treated with CsA plus steroids, and 37 (61.7%) received steroids alone. Data shows that patients in the CsA group had a better outcome than those with pneumonia in the progression phase. | [32] |
| SARS-CoV-2 WT | A total of 20 hospitalized patients (age 55.8 ± 12.9) with confirmed COVID-19 infection. Oxygen saturation ≤ 93% despite appropriate standard care for 72 h of admission, bilateral chest involvement, and unresponsiveness to dexamethasone therapy. Based in Iran. No control. | Cyclosporine (NEORAL®) was administered to patients who did not respond to dexamethasone therapy through saline injection over 6–8 h. Dose: 10 mg/kg followed by 5 mg/kg 24 h later. | Out of the 20 patients who received the intervention, 1. A total of 10 died. 2. A total of 10 required mechanical ventilation. 3. A total of 14 were admitted to the ICU, with a mean length of stay of 8.13 ± 6.81 days. 4. A total of 7 patients had improved lung appearance on CT scan. 5. No adverse reactions observed after cyclosporine treatment. 6. Significant (p < 0.05) increase in ferritin levels and WBC count after two doses of cyclosporine compared to before treatment. | [27] |
| SARS-CoV-2 WT | A total of 29 kidney transplant patients (median age 66.26) with PCR-confirmed SARS-CoV-2 infection. Patients were symptomatic upon admission, and all had at least one cardiovascular risk factor. 18 received supplementary oxygen during their hospital stay. | Patients were separated into two groups: 6 were randomized to minimized immunosuppressive therapy and 23 to cyclosporine-based therapy. CsA was continued in low doses in patients already on CsA. Patients on tacrolimus or mTOR inhibitors were switched to CsA. Target concentration of CsA: 50–100 ng/mL. In the minimized immunosuppressive therapy group, patients were given a reduced dose of calcineurin inhibitor. | The median CsA concentration was 60 ng/mL (IQR: 40–82.5 ng/mL). Among the patients, 3 out of 6 (50%) in the non-CsA group and 3 out of 26 (11.5%) in the CsA group died due to ARDS resulting from COVID-19. Mechanical ventilation was required for 1 out of 6 patients in the non-CsA group (16.6%) compared to 4 out of 23 (17.3%) in the CsA group. Acute kidney injury was observed in 13 patients in the CsA group upon admission, with 9 of these patients subsequently recovering. No cases of acute organ rejection or deterioration in renal function were reported in the CsA group. In non-surviving patients, inflammatory markers—such as RCP, PCT, D-dimer, ferritin, LDH, and IL-6—were significantly elevated upon admission and peaked later in the course of the disease. | [28] |
| SARS-CoV-2 (COVID-19) | A total of 10 hospitalized patients who required oxygen but were not in a critical condition. (median age 57.5 years) | CsA was administered at an initial dosage of 9 mg/kg/d. Median treatment duration of 4 days (range, 2–6 days), median doses received were 8 (range, 3–11 doses) | Five patients experienced adverse effects. Two discontinued treatments due to adverse events. Significant reductions (p ≤ 0.05) in pro-inflammatory cytokines (CXCL10, IL-10, IL-7, IL-8) were observed on day 3 post- CsA administration. Gene expression profiles in PBMCs showed downregulation of genes associated with Type 1 IFN response and innate immune cell activation post- CsA treatment. | [26] |
3.3. Mechanism of Action of Cyclophilins and Cyclophilin Inhibitors in Coronavirus Replication (Supplementary Table S2 and Table 5)
| Mechanism of Action | Details | References |
|---|---|---|
| Modulation of host immune response | - Enhanced the expression of interferon-stimulated genes (ISGs) and interferon-beta, boosting the innate anti-viral response. | [2,25,33] |
| - Suppressed inflammatory signaling pathways (STAT1, AKT, and p38) and reduced pro-inflammatory cytokines such as IL-6. | [2] | |
| Interferon signaling and IRF1 activation | - Stimulated type I and III interferons (notably IFNλ), reducing viral load and improving lung pathology in MERS-CoV-infected models. | [25] |
| - Activates IRF1-dependent anti-viral pathways, including the induction of anti-viral genes such as MX1, by promoting nuclear translocation of IRF1 independent of changes in its mRNA or protein levels. | [25,33] | |
| - Significant increase in IRF1 expression in response to CsA treatment. | [25] | |
| Anti-Inflammatory and tissue-protective effects | - Decreases pro-inflammatory cytokine release and macrophage infiltration in lung tissues, reducing inflammation. | [19] |
| - Significant reductions in pro-inflammatory cytokines (CXCL10, IL-10, IL-7, IL-8) observed on day 3 post-CsA administration. | [25] | |
| - CsA inhibits Nsp1-induced expression of IL-2 and IL-8. | [23] | |
| - Significantly reduced IL-6 levels by CsA | [31] | |
| - High concentrations of CsA significantly reduced cytokine RNA levels. | [34] | |
| - Maintained epithelial integrity and enhanced CFTR expression, potentially preserving lung function during infection | [25] | |
| NFAT signaling and apoptosis modulation | - Antiviral effects are independent of both the interferon-stimulated gene response (ISRE Luciferase reporter) and the calcineurin-NFAT pathway | [35] |
| - Reduced SARS-CoV Nsp1-mediated enhancement of NFAT activity, which may contribute to anti-viral effects. | [23] | |
| - Mitigated virus-induced apoptosis by inhibiting cyclophilin D, disrupting mitochondrial apoptotic pathways, and providing cryoprotection during coronavirus infection. | [36] | |
| Inhibition of cytopathic effects | - CsA eliminated the cytopathic effect induced by viral infection and reduced retraction of dendrites and axons. - (CsA) and cyclophilin D (CyPD) inhibition significantly reduce viral-induced cytopathic effects (CPE) and neuronal cell death by modulating mitochondrial pathways (retaining AIF/CytC in mitochondria). | [37] |
| - Protected cells from MERS-CoV-induced cytopathic effects and foci formation. - Transepithelial resistance measurements showed improved epithelial integrity in CsA-treated cells and appear to help preserve cell barrier function. | [19] | |
| Restoring cell function | - Enhanced vectorial water transport ability in CsA-treated cells, returning to normal levels compared to infected controls. | [25] |
3.3.1. Role of Cyclophilins in Viral Replication
3.3.2. Modulation of Host Immune Response
3.3.3. Interferon Signaling and IRF1 Activation
3.3.4. Anti-Inflammatory and Tissue-Protective Effects
3.3.5. NFAT Signaling and Apoptosis Modulation
3.4. Limitations
- Limited Clinical Data: While preclinical studies have shown promising results, there is a need for more extensive clinical trials to evaluate the efficacy and safety of these compounds in human patients.
- Inconsistent Reporting of Antiviral Potency: Among the in vitro studies on cyclophilin inhibitors, some reported EC50 values, others included both EC50 and CC50, while several did not report cytotoxicity at all. This inconsistency makes cross-comparison of results challenging and may lead to differences in perceived efficacy and safety across studies [2,10,13,14,17].
- Nephrotoxicity and Metabolic Side Effects: Cyclosporine A is associated with dose-dependent nephrotoxicity, hypertension, and metabolic disturbances, which pose significant challenges for long-term clinical use. These adverse effects necessitate careful therapeutic drug monitoring and patient management, thereby limiting its utility as a broadly applicable anti-viral agent [43].
3.5. Future Directions
- Novel Cyclophilin Inhibitors: Since CsA and some of its derivatives cause immunosuppression, developing non-immunosuppressive cyclophilin inhibitors with retained anti-viral potency is a promising strategy to improve safety and broaden clinical use.
- Combination Therapy: Exploring combination therapies with other anti-viral agents or immunomodulatory drugs may enhance the therapeutic efficacy of cyclophilin inhibitors.
- Targeted Drug Delivery: Developing targeted drug delivery systems to deliver cyclophilin inhibitors specifically to infected cells could improve their efficacy and reduce side effects.
- Mechanistic Studies: Further research is needed to elucidate the precise mechanisms of action of cyclophilin inhibitors and their interactions with host factors and viral proteins.
- Clinical Trials: Large-scale clinical trials are necessary to evaluate the safety and efficacy of cyclophilin inhibitors in treating COVID-19 and other coronavirus infections.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuiken, T.; Fouchier, R.; Rimmelzwaan, G.; Osterhaus, A. Emerging viral infections in a rapidly changing world. Curr. Opin. Biotechnol. 2003, 14, 641–646. [Google Scholar] [CrossRef]
- Li, H.S.; Kuok, D.I.T.; Cheung, M.C.; Ng, M.M.T.; Ng, K.C.; Hui, K.P.Y.; Peiris, J.S.M.; Chan, M.C.W.; Nicholls, J.M. Effect of interferon alpha and cyclosporine treatment separately and in combination on Middle East Respiratory Syndrome Coronavirus (MERS-CoV) replication in a human in-vitro and ex-vivo culture model. Antivir. Res. 2018, 155, 89–96. [Google Scholar] [CrossRef]
- Schwartz, D.A. Prioritizing the continuing global challenges to emerging and reemerging viral infections. Front. Virol. 2021, 1, 701054. [Google Scholar] [CrossRef]
- Mallah, S.I.; Ghorab, O.K.; Al-Salmi, S.; Abdellatif, O.S.; Tharmaratnam, T.; Iskandar, M.A.; Sefen, J.A.N.; Sidhu, P.; Atallah, B.; El-Lababidi, R.; et al. COVID-19: Breaking down a global health crisis. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, P.; Rudnicka, L.; Warszawik-Hendzel, O.; Sikora, M.; Goldust, M.; Gajda, P.; Stochmal, A.; Blicharz, L.; Rakowska, A.; Olszewska, M. The antiviral properties of cyclosporine. Focus on coronavirus, hepatitis C virus, influenza virus, and human immunodeficiency virus infections. Biology 2020, 9, 192. [Google Scholar] [CrossRef]
- Davis, T.L.; Walker, J.R.; Campagna-Slater, V.; Finerty, P.J.; Paramanathan, R.; Bernstein, G.; MacKenzie, F.; Tempel, W.; Ouyang, H.; Lee, W.H.; et al. Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol. 2010, 8, e1000439. [Google Scholar] [CrossRef]
- Nigro, P.; Pompilio, G.; Capogrossi, M. Cyclophilin A: A key player for human disease. Cell Death Dis. 2013, 4, e888. [Google Scholar] [CrossRef]
- Elrod, J.W.; Molkentin, J.D. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ. J. 2013, 77, 1111–1122. [Google Scholar] [CrossRef]
- Nagy, P.D.; Wang, R.Y.; Pogany, J.; Hafren, A.; Makinen, K. Emerging picture of host chaperone and cyclophilin roles in RNA virus replication. Virology 2011, 411, 374–382. [Google Scholar] [CrossRef]
- Gaither, L.A.; Borawski, J.; Anderson, L.J.; Balabanis, K.A.; Devay, P.; Joberty, G.; Rau, C.; Schirle, M.; Bouwmeester, T.; Mickanin, C.; et al. Multiple cyclophilins involved in different cellular pathways mediate HCV replication. Virology 2010, 397, 43–55. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, M.B.; Lupberger, J.; Fofana, I.; Baumert, T.F. Host-targeting agents for prevention and treatment of chronic hepatitis C—Perspectives and challenges. J. Hepatol. 2013, 58, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Heitman, J. The cyclophilins. Genome Biol. 2005, 6, 226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hopkins, S.; Gallay, P. Cyclophilin inhibitors: An emerging class of therapeutics for the treatment of chronic hepatitis C infection. Viruses 2012, 4, 2558–2577. [Google Scholar] [CrossRef]
- Ma-Lauer, Y.; Zheng, Y.; Malešević, M.; von Brunn, B.; Fischer, G.; von Brunn, A. Influences of cyclosporin A and non-immunosuppressive derivatives on cellular cyclophilins and viral nucleocapsid protein during human coronavirus 229E replication. Antivir. Res. 2020, 173, 104620. [Google Scholar] [CrossRef]
- Berthold, E.J.; Ma-Lauer, Y.; Chakraborty, A.; von Brunn, B.; Hilgendorff, A.; Hatz, R.; Behr, J.; Hausch, F.; Staab-Weijnitz, C.A.; von Brunn, A. Effects of immunophilin inhibitors and non-immunosuppressive analogs on coronavirus replication in human infection models. Front. Cell Infect. Microbiol. 2022, 12, 958634. [Google Scholar] [CrossRef]
- de Wilde, A.H.; Zevenhoven-Dobbe, J.C.; van der Meer, Y.; Thiel, V.; Narayanan, K.; Makino, S.; Snijder, E.J.; van Hemert, M.J. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011, 92, 2542–2548. [Google Scholar] [CrossRef]
- Sauerhering, L.; Kuznetsova, I.; Kupke, A.; Meier, L.; Halwe, S.; Rohde, C.; Schmidt, J.; Morty, R.E.; Danov, O.; Braun, A.; et al. Cyclosporin A Reveals Potent Antiviral Effects in Preclinical Models of SARS-CoV-2 Infection. Am. J. Respir. Crit. Care Med. 2022, 205, 964–968. [Google Scholar] [CrossRef]
- de Wilde, A.H.; Falzarano, D.; Zevenhoven-Dobbe, J.C.; Beugeling, C.; Fett, C.; Martellaro, C.; Posthuma, C.C.; Feldmann, H.; Perlman, S.; Snijder, E.J. Alisporivir inhibits MERS- and SARS-coronavirus replication in cell culture, but not SARS-coronavirus infection in a mouse model. Virus Res. 2017, 228, 7–13. [Google Scholar] [CrossRef]
- de Wilde, A.H.; Raj, V.S.; Oudshoorn, D.; Bestebroer, T.M.; van Nieuwkoop, S.; Limpens, R.; Posthuma, C.C.; van der Meer, Y.; Bárcena, M.; Haagmans, B.L.; et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J. Gen. Virol. 2013, 94, 1749–1760. [Google Scholar] [CrossRef]
- Softic, L.; Brillet, R.; Berry, F.; Ahnou, N.; Nevers, Q.; Morin-Dewaele, M.; Hamadat, S.; Bruscella, P.; Fourati, S.; Pawlotsky, J.M.; et al. Inhibition of SARS-CoV-2 Infection by the Cyclophilin Inhibitor Alisporivir (Debio 025). Antimicrob. Agents Chemother. 2020, 64, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Pfefferle, S.; Schöpf, J.; Kögl, M.; Friedel, C.C.; Müller, M.A.; Carbajo-Lozoya, J.; Stellberger, T.; von Dall’Armi, E.; Herzog, P.; Kallies, S. The SARS-coronavirus-host interactome: Identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011, 7, e1002331. [Google Scholar] [CrossRef] [PubMed]
- Carbajo-Lozoya, J.; Ma-Lauer, Y.; Malešević, M.; Theuerkorn, M.; Kahlert, V.; Prell, E.; von Brunn, B.; Muth, D.; Baumert, T.F.; Drosten, C.; et al. Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including Alisporivir. Virus Res. 2014, 184, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sauerhering, L.; Kupke, A.; Meier, L.; Dietzel, E.; Hoppe, J.; Gruber, A.D.; Gattenloehner, S.; Witte, B.; Fink, L.; Hofmann, N.; et al. Cyclophilin inhibitors restrict Middle East respiratory syndrome coronavirus via interferon-λ in vitro and in mice. Eur. Respir. J. 2020, 56, 1901826. [Google Scholar] [CrossRef]
- Blumberg, E.A.; Noll, J.H.; Tebas, P.; Fraietta, J.A.; Frank, I.; Marshall, A.; Chew, A.; Veloso, E.A.; Carulli, A.; Rogal, W.; et al. A phase I trial of cyclosporine for hospitalized patients with COVID-19. JCI Insight 2022, 7, e155682. [Google Scholar] [CrossRef]
- Barati, S.; MohammadReza Hashemian, S.; Tabarsi, P.; Abedini, A.; Ashrafzadeh, M.; Haseli, S.; Abtahian, Z.; Yousefian, S.; Dastan, A.; Sobhanian, A.; et al. Combined Therapy of Ciclosporin Plus Favipiravir in the Management of Patients with Severe COVID-19, not Responding to Dexamethasone: A non-Controlled Prospective Trial. Int. Immunopharmacol. 2021, 99, 108043. [Google Scholar] [CrossRef]
- Rodriguez-Cubillo, B.; de la Higuera, M.A.M.; Lucena, R.; Franci, E.V.; Hurtado, M.; Romero, N.C.; Moreno, A.R.; Valencia, D.; Velo, M.; Fornie, I.S.; et al. Should cyclosporine be useful in renal transplant recipients affected by SARS-CoV-2? Am. J. Transplant. 2020, 20, 3173–3181. [Google Scholar] [CrossRef]
- Cobo-Ibáñez, T.; Mora Ortega, G.; Sánchez-Piedra, C.; Serralta-San Martín, G.; Thuissard-Vasallo, I.J.; Lores Gutiérrez, V.; Soler Rangel, L.; García Yubero, C.; Esteban-Vázquez, A.; López-Aspiroz, E.; et al. Cyclosporine A in hospitalized COVID-19 pneumonia patients to prevent the development of interstitial lung disease: A pilot randomized clinical trial. Sci. Rep. 2024, 14, 3789. [Google Scholar] [CrossRef]
- Guareschi, F.; Del Favero, E.; Ricci, C.; Cantù, L.; Brandolini, M.; Sambri, V.; Nicoli, S.; Pescina, S.; D’Angelo, D.; Rossi, I.; et al. Cyclosporine A micellar nasal spray characterization and antiviral action against SARS-CoV-2. Eur. J. Pharm. Sci. 2024, 193, 106673. [Google Scholar] [CrossRef]
- D’Angelo, D.; Quarta, E.; Glieca, S.; Varacca, G.; Flammini, L.; Bertoni, S.; Brandolini, M.; Sambri, V.; Grumiro, L.; Gatti, G.; et al. An Enhanced Dissolving Cyclosporin-A Inhalable Powder Efficiently Reduces SARS-CoV-2 Infection In Vitro. Pharmaceutics 2023, 15, 1023. [Google Scholar] [CrossRef]
- Gálvez-Romero, J.L.; Palmeros-Rojas, O.; Real-Ramírez, F.A.; Sánchez-Romero, S.; Tome-Maxil, R.; Ramírez-Sandoval, M.P.; Olivos-Rodríguez, R.; Flores-Encarnación, S.E.; Cabrera-Estrada, A.A.; Ávila-Morales, J.; et al. Cyclosporine A plus low-dose steroid treatment in COVID-19 improves clinical outcomes in patients with moderate to severe disease: A pilot study. J. Intern. Med. 2021, 289, 906–920. [Google Scholar] [CrossRef]
- Mamatis, J.E.; Gallardo-Flores, C.E.; Sangwan, U.; Tooley, T.H.; Walsh, T.; Colpitts, C.C. Induction of antiviral gene expression by cyclosporine A, but not inhibition of cyclophilin A or B, contributes to its restriction of human coronavirus 229E infection in a lung epithelial cell line. Antivir. Res. 2023, 219, 105730. [Google Scholar] [CrossRef]
- Fenizia, C.; Galbiati, S.; Vanetti, C.; Vago, R.; Clerici, M.; Tacchetti, C.; Daniele, T. Cyclosporine A Inhibits Viral Infection and Release as Well as Cytokine Production in Lung Cells by Three SARS-CoV-2 Variants. Microbiol. Spectr. 2022, 10, e0150421. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, Y.; Osawa, S.; Inoue, M.; Tanaka, S.; Sasaki, T. Suppression of feline coronavirus replication in vitro by cyclosporin A. Vet. Res. 2012, 43, 41. [Google Scholar] [CrossRef]
- Zhang, J.; Han, Y.; Shi, H.; Chen, J.; Zhang, X.; Wang, X.; Zhou, L.; Liu, J.; Zhang, J.; Ji, Z.; et al. Swine acute diarrhea syndrome coronavirus-induced apoptosis is caspase- and cyclophilin D- dependent. Emerg. Microbes Infect. 2020, 9, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Favreau, D.J.; Meessen-Pinard, M.; Desforges, M.; Talbot, P.J. Human Coronavirus-Induced Neuronal Programmed Cell Death Is Cyclophilin D Dependent and Potentially Caspase Dispensable. J. Virol. 2012, 86, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sato, Y.; Sasaki, T. Feline coronavirus replication is affected by both cyclophilin A and cyclophilin B. J. Gen. Virol. 2017, 98, 190–200. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, A.H.; Zevenhoven-Dobbe, J.C.; Beugeling, C.; Chatterji, U.; de Jong, D.; Gallay, P.; Szuhai, K.; Posthuma, C.C.; Snijder, E.J. Coronaviruses and arteriviruses display striking differences in their cyclophilin A-dependence during replication in cell culture. Virology 2018, 517, 148–156. [Google Scholar] [CrossRef]
- Chatterji, U.; Bobardt, M.; Selvarajah, S.; Yang, F.; Tang, H.; Sakamoto, N.; Vuagniaux, G.; Parkinson, T.; Gallay, P. The Isomerase Active Site of Cyclophilin A Is Critical for Hepatitis C Virus Replication. J. Biol. Chem. 2009, 284, 16998–17005. [Google Scholar] [CrossRef]
- Liu, X.; Sun, L.; Yu, M.; Wang, Z.; Xu, C.; Xue, Q.; Zhang, K.; Ye, X.; Kitamura, Y.; Liu, W. Cyclophilin A interacts with influenza A virus M1 protein and impairs the early stage of the viral replication. Cell. Microbiol. 2009, 11, 730–741. [Google Scholar] [CrossRef]
- Bai, X.; Yang, W.; Li, H.; Zhao, Y.; Fan, W.; Zhang, H.; Liu, W.; Sun, L. Cyclosporine A Regulates Influenza A Virus-induced Macrophages Polarization and Inflammatory Responses by Targeting Cyclophilin A. Front. Immunol. 2022, 13, 861292. [Google Scholar] [CrossRef]
- Colombo, D.; Chimenti, S.; Grossi, P.; Marchesoni, A.; Di Nuzzo, S.; Griseta, V.; Gargiulo, A.; Parodi, A.; Simoni, L.; Bellia, G. Prevalence of past and reactivated viral infections and efficacy of cyclosporine A as monotherapy or in combination in patients with psoriatic arthritis--synergy study: A longitudinal observational study. Biomed. Res. Int. 2014, 2014, 941767. [Google Scholar] [CrossRef]
- Ogando, N.S.; Metscher, E.; Moes, D.J.; Arends, E.J.; Tas, A.; Cross, J.; Snijder, E.J.; Teng, Y.O.; de Vries, A.P.; van Hemert, M.J. The Cyclophilin-Dependent Calcineurin Inhibitor Voclosporin Inhibits SARS-CoV-2 Replication in Cell Culture. Transpl. Int. 2022, 35, 10369. [Google Scholar] [CrossRef]
- Luo, C.; Luo, H.; Zheng, S.; Gui, C.; Yue, L.; Yu, C.; Sun, T.; He, P.; Chen, J.; Shen, J.; et al. Nucleocapsid protein of SARS coronavirus tightly binds to human cyclophilin A. Biochem. Biophys. Res. Commun. 2004, 321, 557–565. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhabyan, A.; Khan, M.U.S.; Elhabyan, A.; Abukhatwa, R.; Uzair, H.; Jimenez, C.; Elhabyan, A.; Chan, Y.L.; Shabana, B. Broad-Spectrum Antiviral Activity of Cyclophilin Inhibitors Against Coronaviruses: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 7900. https://doi.org/10.3390/ijms26167900
Elhabyan A, Khan MUS, Elhabyan A, Abukhatwa R, Uzair H, Jimenez C, Elhabyan A, Chan YL, Shabana B. Broad-Spectrum Antiviral Activity of Cyclophilin Inhibitors Against Coronaviruses: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(16):7900. https://doi.org/10.3390/ijms26167900
Chicago/Turabian StyleElhabyan, Abdelazeem, Muhammad Usman S. Khan, Aliaa Elhabyan, Rawan Abukhatwa, Hadia Uzair, Claudia Jimenez, Asmaa Elhabyan, Yee Lok Chan, and Basma Shabana. 2025. "Broad-Spectrum Antiviral Activity of Cyclophilin Inhibitors Against Coronaviruses: A Systematic Review" International Journal of Molecular Sciences 26, no. 16: 7900. https://doi.org/10.3390/ijms26167900
APA StyleElhabyan, A., Khan, M. U. S., Elhabyan, A., Abukhatwa, R., Uzair, H., Jimenez, C., Elhabyan, A., Chan, Y. L., & Shabana, B. (2025). Broad-Spectrum Antiviral Activity of Cyclophilin Inhibitors Against Coronaviruses: A Systematic Review. International Journal of Molecular Sciences, 26(16), 7900. https://doi.org/10.3390/ijms26167900






