CAR Cell-Derived Exosomes in Cancer Therapy: Biogenesis, Engineering Strategies and Antitumor Mechanisms
Abstract
1. Introduction
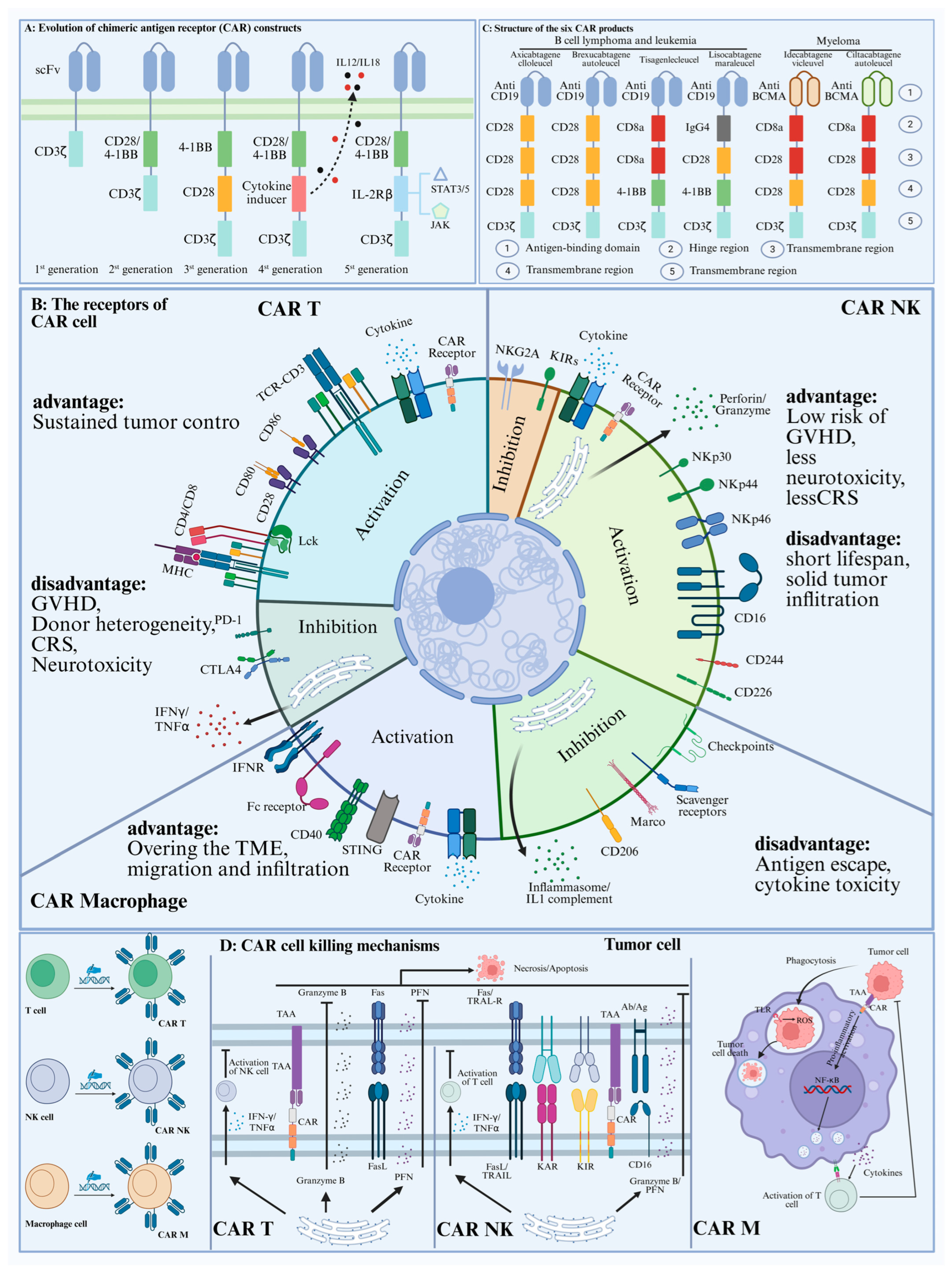
2. CAR-Cell Therapy in Cancer Progress
2.1. CAR-T Cell Therapy
2.2. Car-NK Cell Therapy
2.3. Car-Macrophage Cell Therapy
| Product | Clinical Trials ID | Study Phase | Edited Genes | Cancer | Target Antigens | Efficacy | Toxicities | Refs. |
|---|---|---|---|---|---|---|---|---|
| TT52CAR19 | NCT04557436 | I | CD52 | B-ALL | CD19 | A total of 6 evaluable patients, 4 of whom (66.7%) had CR, received allo-HSCT consolidation. | CRS: 100%; ICANS: 33.3%; GVHD: 33.3%; Cytopenias: 100%. | [48] |
| CTA101 | NCT04227015 | I | CD52 | B-ALL | CD19 and CD22 | A total of 6 evaluable patients, 5 of whom (66.7%) had CR/Cri. | CRS: 100%; ICANS: none; GVHD: none; Cytopenias: 50%. | [49] |
| UCART19 | NCT02640209 | I | CD52 | B-ALL | CD19 | 7 evaluable children, 25 evaluable adults. 6/7 children (85.7%) CR/CRi, 12/25 adults (48%) CR/Cri. | CRS: 90.5%; ICANS: 38.1%; GVHD: 9.5%; Cytopenias: 31.6%. | [50] |
| BE-CAR7 | NCT05397184 | I | CD52 and CD7 | T-ALL | CD7 | A total of 3 evaluable patients, 3 of whom (100%) had CR/Cri. | CRS: 100%; ICANS: 33%; GVHD: 33%; Cytopenias: 100%. | [51] |
| GC027 | NCT04264078 | I | CD7 | T-ALL | CD7 | A total of 12 evaluable patients, 11 of whom (91.7%) had CR/Cri. | CRS: 83.3%; ICANS: none; GVHD: NR; Cytopenias: NR. | [52] |
| CTX130 | NCT04502446 | I | CD70 | ccRCC | CD70 | A total of 16 evaluable patients, 1 of whom (6.2%) had CR/Cri. | CRS: 50%; ICANS: none; GVHD: none; Cytopenias: NR. | [53] |
3. Comparison of CAR Cell-Derived Exosomes and CAR Cells in Cancer Therapy
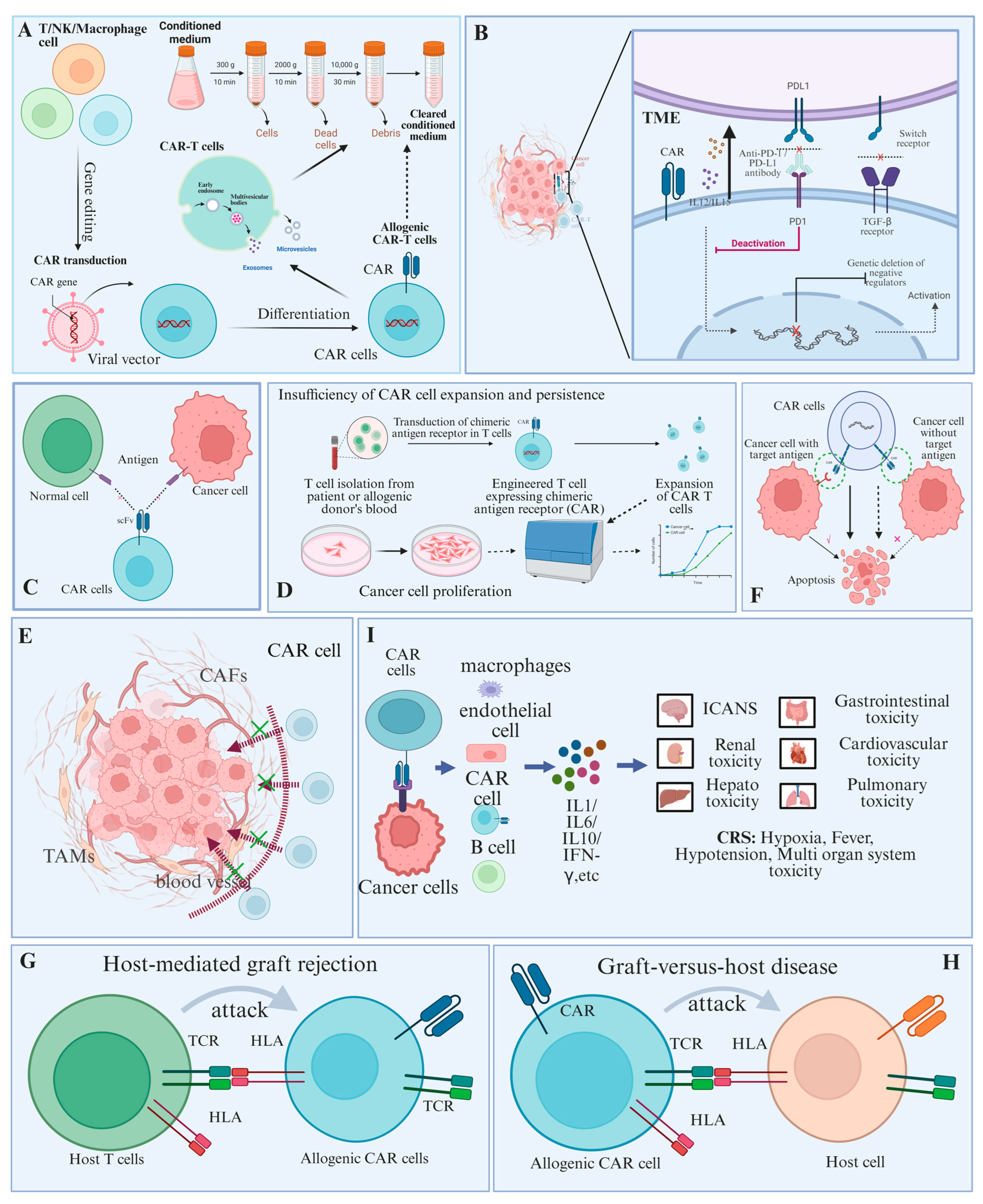
3.1. Limitations of CAR Cell Therapy
3.1.1. Tumor Microenvironment
3.1.2. Lack of Specific Targets
3.1.3. Insufficiency of CAR Cell Expansion and Persistence
3.1.4. Inefficiency of CAR Cell Trafficking and Infiltration
3.1.5. Down-Regulation or Absence of Target Antigen
3.1.6. Host-Mediated Graft Rejection
3.1.7. Graft-Versus-Host Disease
3.1.8. Systemic Toxicity
3.2. Advantages of CAR Cell-Derived Exosomes
3.2.1. Higher Security
3.2.2. Ability to Break the Blood-Brain Barrier
3.2.3. Multifunctional Loads and Efficient Delivery
3.2.4. Regulation of the Tumor Microenvironment
3.2.5. Participation in the Diagnosis and Treatment of Cancer
4. CAR Cell-Derived Exosomes Could Serve as a Drug and Delivery Platforms for Tumor Therapy
4.1. Evolution of the CAR Structure
4.2. CAR Cell-Derived Exosomes Are Spatially Targeted to Tumor Cells
4.3. CAR Cell-Derived Exosomes Target Tumor Sites Temporally
| Classification | Target | Subtypes | Research Progress | Refs. |
|---|---|---|---|---|
| CAR-T cell-derived exosomes | EGFR | TNBC | Cetuzumab transduction shows TGI dose-dependence in the MDAMB-231 mouse xenograft model. | [82] |
| MSLN | TNBC | Exosomes produced by CAR-T cells transduced with trastuzumab showed significant antitumor effects on the treatment of MCF-7 HER2 cells and SK-BR-3 cells. | [82] | |
| MSLN | Lung cancer | Delivery of PTX to tumor cells by continuous targeted delivery enhances antitumor effects and prolongs survival time of hormonal mice in CT-26 metastatic lung cancer model. | [120] | |
| HER2 | HER-2-positive | Exosomes from CAR-T cells targeting MSLN showed strong antitumor effects on MSLN-positive TNBC. | [121] | |
| CAR NK cell-derived exosomes | HER2 | HER-2-positive | Able to penetrate the blood-brain barrier and selectively exert antitumor effects on HER2-positive breast cancer cells in the brain. | [21] |
| CAR M cell-derived exosomes | CXCL10 | lymphoma | Significantly enhances the immune activation and migration of T lymphocytes and promotes their differentiation into CD8+ T cells. Meanwhile, it increases the proportion of M1 macrophages, which exerts excellent antitumor activity in vivo. | [122] |
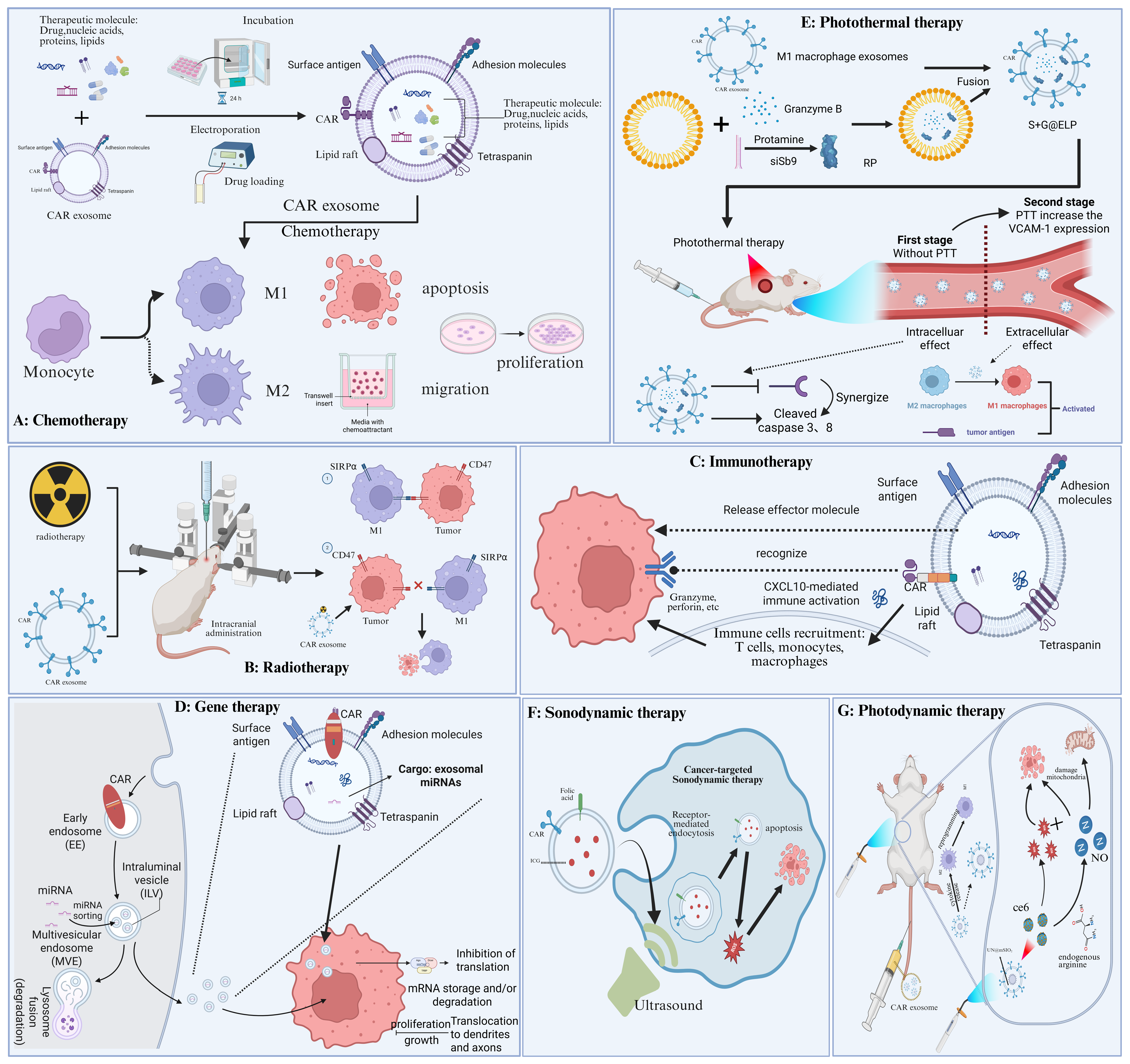
5. Combination Therapy Based on CAR Cell-Derived Exosomes
5.1. Chemotherapy
5.2. Radiotherapy
5.3. Immunotherapy
5.4. Gene Therapy
5.5. Photothermal Therapy
5.6. Sonodynamic Therapy
5.7. Photodynamic Therapy
5.8. Precision Therapy
6. Challenges and Outlook
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAR | Chimeric antigen receptor |
| BCMA | B cell mature antigen |
| NK | Natural killer |
| PDT | Photodynamic therapy |
| Ce6 | Chlorine e6 |
| GVHD | Graft-versus-host disease |
| ROS | Reactive oxygen species |
| PTT | Photothermal therapies |
| MVB | multivesicular bodies |
| TAA | tumor-associated antigen |
| TIL | tumor-infiltrating lymphocyte |
| ALL | acute lymphoblastic leukaemia |
| MM | multiple myeloma |
| CLL | chronic lymphocytic leukaemia |
| SOC | standard of care |
| CIML | cytokine-induced memory-like |
| TME | tumor microenvironment |
| ECM | extracellular matrix |
| CCL19 | C-C motif chemokine ligand 19 |
| MDSCs | myeloid-derived suppressor cells |
| TAMs | tumor-associated macrophages |
| MART1 | melanoma antigen 1 |
| gp100 | glycoprotein 100 |
| CAFs | cancer-associated fibroblast |
| MHCI | MHC class I |
| β2M | β2 microglobulin |
| PD1 | programmed cell death protein 1 |
| HER2 | human epidermal growth factor receptor 2 |
| ITAM | immunoreceptor tyrosine-based activation motif |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- GlobalSurg Collaborative and National Institute for Health Research Global Health Research Unit on Global Surgery. Global variation in postoperative mortality and complications after cancer surgery: A multicentre, prospective cohort study in 82 countries. Lancet 2021, 397, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Benenati, M.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Cozzi, D.; De Robertis, R.; Gentili, F.; Grazzini, I.; et al. Imaging side effects and complications of chemotherapy and radiation therapy: A pictorial review from head to toe. Insights Imaging 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Orzetti, S.; Tommasi, F.; Bertola, A.; Bortolin, G.; Caccin, E.; Cecco, S.; Ferrarin, E.; Giacomin, E.; Baldo, P. Genetic Therapy and Molecular Targeted Therapy in Oncology: Safety, Pharmacovigilance, and Perspectives for Research and Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3012. [Google Scholar] [CrossRef]
- Papaioannou, N.E.; Beniata, O.V.; Vitsos, P.; Tsitsilonis, O.; Samara, P. Harnessing the immune system to improve cancer therapy. Ann. Transl. Med. 2016, 4, 261. [Google Scholar] [CrossRef]
- Poorebrahim, M.; Abazari, M.F.; Sadeghi, S.; Mahmoudi, R.; Kheirollahi, A.; Askari, H.; Wickstrom, S.L.; Poortahmasebi, V.; Lundqvist, A.; Kiessling, R.; et al. Genetically modified immune cells targeting tumor antigens. Pharmacol. Ther. 2020, 214, 107603. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, X.; Yuan, X.; Wang, W.; Wang, Y. Chimeric antigen receptor-engineered NK cells: New weapons of cancer immunotherapy with great potential. Exp. Hematol. Oncol. 2022, 11, 85. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Wang, T.; He, T.; Ma, L.; Yang, Y.; Feng, R.; Ding, Y.; Shan, Y.; Bu, B.; Qi, F.; Wu, F.; et al. Clinical Outcomes of BCMA CAR-T Cells in a Multiple Myeloma Patient With Central Nervous System Invasion. Front. Oncol. 2022, 12, 854448. [Google Scholar] [CrossRef]
- Liu, G.; Rui, W.; Zhao, X.; Lin, X. Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cell Mol. Immunol. 2021, 18, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Basar, R.; Daher, M.; Rezvani, K. Next-generation cell therapies: The emerging role of CAR-NK cells. Hematol. Am. Soc. Hematol. Educ. Program. 2020, 2020, 570–578. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lasser, C.; Lotvall, J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat. Protoc. 2021, 16, 1548–1580. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Zafarani, A.; Taghavi-Farahabadi, M.; Razizadeh, M.H.; Amirzargar, M.R.; Mansouri, M.; Mahmoudi, M. The Role of NK Cells and Their Exosomes in Graft Versus Host Disease and Graft Versus Leukemia. Stem Cell Rev. Rep. 2023, 19, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Fiore, P.F.; Di Pace, A.L.; Conti, L.A.; Tumino, N.; Besi, F.; Scaglione, S.; Munari, E.; Moretta, L.; Vacca, P. Different effects of NK cells and NK-derived soluble factors on cell lines derived from primary or metastatic pancreatic cancers. Cancer Immunol. Immunother. 2023, 72, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, J.; Jia, C.; He, Y.; Deng, C. Therapeutic applications of adipose cell-free derivatives: A review. Stem Cell Res. Ther. 2020, 11, 312. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal 2021, 19, 47. [Google Scholar] [CrossRef]
- Zhang, P.F.; Gao, C.; Huang, X.Y.; Lu, J.C.; Guo, X.J.; Shi, G.M.; Cai, J.B.; Ke, A.W. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol. Cancer 2020, 19, 110. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- Tao, B.; Du, R.; Zhang, X.; Jia, B.; Gao, Y.; Zhao, Y.; Liu, Y. Engineering CAR-NK cell derived exosome disguised nano-bombs for enhanced HER2 positive breast cancer brain metastasis therapy. J. Control. Release 2023, 363, 692–706. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Oliveira, G.; Wu, C.J. Dynamics and specificities of T cells in cancer immunotherapy. Nat. Rev. Cancer 2023, 23, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Perica, K.; Klebanoff, C.A.; Wolchok, J.D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Wachsmann, T.L.A.; Wouters, A.K.; Remst, D.F.G.; Hagedoorn, R.S.; Meeuwsen, M.H.; van Diest, E.; Leusen, J.; Kuball, J.; Falkenburg, J.H.F.; Heemskerk, M.H.M. Comparing CAR and TCR engineered T cell performance as a function of tumor cell exposure. Oncoimmunology 2022, 11, 2033528. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Shimabukuro-Vornhagen, A.; Boll, B.; Schellongowski, P.; Valade, S.; Metaxa, V.; Azoulay, E.; von Bergwelt-Baildon, M. Critical care management of chimeric antigen receptor T-cell therapy recipients. CA Cancer J. Clin. 2022, 72, 78–93. [Google Scholar] [CrossRef]
- Brudno, J.N.; Maus, M.V.; Hinrichs, C.S. CAR T Cells and T-Cell Therapies for Cancer: A Translational Science Review. JAMA 2024, 332, 1924–1935. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Jacobs, M.T.; Fehniger, T.A. Allogeneic natural killer cell therapy. Blood 2023, 141, 856–868. [Google Scholar] [CrossRef]
- Mitra, A.; Barua, A.; Huang, L.; Ganguly, S.; Feng, Q.; He, B. From bench to bedside: The history and progress of CAR T cell therapy. Front. Immunol. 2023, 14, 1188049. [Google Scholar] [CrossRef] [PubMed]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK cell-cancer cycle: Advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Cozar, B.; Greppi, M.; Carpentier, S.; Narni-Mancinelli, E.; Chiossone, L.; Vivier, E. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2021, 11, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.S.; Zhang, J.Y.; Teng, Y.S.; Zhao, Y.L.; Wang, T.T.; Mao, F.Y.; Lv, Y.P.; Cheng, P.; Li, W.H.; Chen, N.; et al. Tumor-Associated Monocytes/Macrophages Impair NK-Cell Function via TGFbeta1 in Human Gastric Cancer. Cancer Immunol. Res. 2017, 5, 248–256. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Yin, W.W.; Xia, Y.; Yi, Y.Y.; He, Q.F.; Wang, X.; Ren, H.; Zhang, D.Z. Liver-infiltrating CD11b(-)CD27(-) NK subsets account for NK-cell dysfunction in patients with hepatocellular carcinoma and are associated with tumor progression. Cell Mol. Immunol. 2017, 14, 819–829. [Google Scholar] [CrossRef]
- Zheng, X.; Qian, Y.; Fu, B.; Jiao, D.; Jiang, Y.; Chen, P.; Shen, Y.; Zhang, H.; Sun, R.; Tian, Z.; et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat. Immunol. 2019, 20, 1656–1667. [Google Scholar] [CrossRef]
- Mamessier, E.; Sylvain, A.; Thibult, M.L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Goncalves, A.; Andre, P.; Romagne, F.; Thibault, G.; et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef]
- Terren, I.; Orrantia, A.; Vitalle, J.; Zenarruzabeitia, O.; Borrego, F. NK Cell Metabolism and Tumor Microenvironment. Front. Immunol. 2019, 10, 2278. [Google Scholar] [CrossRef]
- Menard, C.; Blay, J.Y.; Borg, C.; Michiels, S.; Ghiringhelli, F.; Robert, C.; Nonn, C.; Chaput, N.; Taieb, J.; Delahaye, N.F.; et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res. 2009, 69, 3563–3569. [Google Scholar] [CrossRef]
- Pernot, S.; Terme, M.; Radosevic-Robin, N.; Castan, F.; Badoual, C.; Marcheteau, E.; Penault-Llorca, F.; Bouche, O.; Bennouna, J.; Francois, E.; et al. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer 2020, 23, 73–81. [Google Scholar] [CrossRef]
- Semeraro, M.; Rusakiewicz, S.; Minard-Colin, V.; Delahaye, N.F.; Enot, D.; Vely, F.; Marabelle, A.; Papoular, B.; Piperoglou, C.; Ponzoni, M.; et al. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci. Transl. Med. 2015, 7, 283ra255. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Marin, D.; Li, Y.; Basar, R.; Rafei, H.; Daher, M.; Dou, J.; Mohanty, V.; Dede, M.; Nieto, Y.; Uprety, N.; et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19(+) B cell tumors: A phase 1/2 trial. Nat. Med. 2024, 30, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Trotta, R.; Dal Col, J.; Yu, J.; Ciarlariello, D.; Thomas, B.; Zhang, X.; Allard, J., 2nd; Wei, M.; Mao, H.; Byrd, J.C.; et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J. Immunol. 2008, 181, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- Friese, M.A.; Wischhusen, J.; Wick, W.; Weiler, M.; Eisele, G.; Steinle, A.; Weller, M. RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 2004, 64, 7596–7603. [Google Scholar] [CrossRef]
- Crane, C.A.; Han, S.J.; Barry, J.J.; Ahn, B.J.; Lanier, L.L.; Parsa, A.T. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol. 2010, 12, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Henze, A.T.; Mazzone, M. The impact of hypoxia on tumor-associated macrophages. J. Clin. Investig. 2016, 126, 3672–3679. [Google Scholar] [CrossRef]
- Ottaviano, G.; Georgiadis, C.; Gkazi, S.A.; Syed, F.; Zhan, H.; Etuk, A.; Preece, R.; Chu, J.; Kubat, A.; Adams, S.; et al. Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for treatment of children with refractory B cell leukemia. Sci. Transl. Med. 2022, 14, eabq3010. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Y.; Zhang, M.; Ge, W.; Li, Y.; Yang, L.; Wei, G.; Han, L.; Wang, H.; Yu, S.; et al. CRISPR/Cas9-Engineered Universal CD19/CD22 Dual-Targeted CAR-T Cell Therapy for Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2021, 27, 2764–2772. [Google Scholar] [CrossRef]
- Benjamin, R.; Jain, N.; Maus, M.V.; Boissel, N.; Graham, C.; Jozwik, A.; Yallop, D.; Konopleva, M.; Frigault, M.J.; Teshima, T.; et al. UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): A phase 1, dose-escalation trial. Lancet Haematol. 2022, 9, e833–e843. [Google Scholar] [CrossRef]
- Chiesa, R.; Georgiadis, C.; Syed, F.; Zhan, H.; Etuk, A.; Gkazi, S.A.; Preece, R.; Ottaviano, G.; Braybrook, T.; Chu, J.; et al. Base-Edited CAR7 T Cells for Relapsed T-Cell Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2023, 389, 899–910. [Google Scholar] [CrossRef]
- Lin, B.; Guan, C. Assessing consumption-based carbon footprint of China’s food industry in global supply chain. Sustain. Prod. Consum. 2023, 35, 365–375. [Google Scholar] [CrossRef]
- Pal, S.K.; Tran, B.; Haanen, J.; Hurwitz, M.E.; Sacher, A.; Tannir, N.M.; Budde, L.E.; Harrison, S.J.; Klobuch, S.; Patel, S.S.; et al. CD70-Targeted Allogeneic CAR T-Cell Therapy for Advanced Clear Cell Renal Cell Carcinoma. Cancer Discov. 2024, 14, 1176–1189. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Johnson, L.A.; Morgan, R.A.; Dudley, M.E.; Cassard, L.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Royal, R.E.; Sherry, R.M.; Wunderlich, J.R.; et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009, 114, 535–546. [Google Scholar] [CrossRef]
- Turtle, C.J.; Hanafi, L.A.; Berger, C.; Hudecek, M.; Pender, B.; Robinson, E.; Hawkins, R.; Chaney, C.; Cherian, S.; Chen, X.; et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016, 8, 355ra116. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.D.; Ali Hosseini Rad, S.M. Chimeric antigen receptor T cell persistence and memory cell formation. Immunol. Cell Biol. 2019, 97, 664–674. [Google Scholar] [CrossRef]
- van der Stegen, S.J.; Hamieh, M.; Sadelain, M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 2015, 14, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Condomines, M.; van der Stegen, S.J.C.; Perna, F.; Kloss, C.C.; Gunset, G.; Plotkin, J.; Sadelain, M. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell 2015, 28, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014, 6, 224ra225. [Google Scholar] [CrossRef]
- Ying, Z.; He, T.; Wang, X.; Zheng, W.; Lin, N.; Tu, M.; Xie, Y.; Ping, L.; Zhang, C.; Liu, W.; et al. Parallel Comparison of 4-1BB or CD28 Co-stimulated CD19-Targeted CAR-T Cells for B Cell Non-Hodgkin’s Lymphoma. Mol. Ther. Oncolytics 2019, 15, 60–68. [Google Scholar] [CrossRef]
- Kunkele, A.; Johnson, A.J.; Rolczynski, L.S.; Chang, C.A.; Hoglund, V.; Kelly-Spratt, K.S.; Jensen, M.C. Functional Tuning of CARs Reveals Signaling Threshold above Which CD8+ CTL Antitumor Potency Is Attenuated due to Cell Fas-FasL-Dependent AICD. Cancer Immunol. Res. 2015, 3, 368–379. [Google Scholar] [CrossRef]
- Wang, L.C.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014, 2, 154–166. [Google Scholar] [CrossRef]
- Craddock, J.A.; Lu, A.; Bear, A.; Pule, M.; Brenner, M.K.; Rooney, C.M.; Foster, A.E. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 2010, 33, 780–788. [Google Scholar] [CrossRef]
- Majzner, R.G.; Mackall, C.L. Tumor Antigen Escape from CAR T-cell Therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Ruella, M.; Kenderian, S.S. Next-Generation Chimeric Antigen Receptor T-Cell Therapy: Going off the Shelf. BioDrugs 2017, 31, 473–481. [Google Scholar] [CrossRef]
- Valton, J.; Guyot, V.; Marechal, A.; Filhol, J.M.; Juillerat, A.; Duclert, A.; Duchateau, P.; Poirot, L. A Multidrug-resistant Engineered CAR T Cell for Allogeneic Combination Immunotherapy. Mol. Ther. 2015, 23, 1507–1518. [Google Scholar] [CrossRef]
- Ren, J.; Liao, X.; Lewis, J.M.; Chang, J.; Qu, R.; Carlson, K.R.; Foss, F.; Girardi, M. Generation and optimization of off-the-shelf immunotherapeutics targeting TCR-Vbeta2+ T cell malignancy. Nat. Commun. 2024, 15, 519. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Watanabe, N.; McKenna, M.K.; Hicks, M.J.; Srinivasan, M.; Gomes-Silva, D.; Atilla, E.; Smith, T.; Ataca Atilla, P.; Ma, R.; et al. Engineered off-the-shelf therapeutic T cells resist host immune rejection. Nat. Biotechnol. 2021, 39, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Watanabe, N.; Omdahl, K.I.; Burkhardt, P.M.; Ding, X.; Hayase, E.; Panoskaltsis-Mortari, A.; Jenq, R.R.; Heslop, H.E.; Kean, L.S.; et al. Engineering T cells to suppress acute GVHD and leukemia relapse after allogeneic hematopoietic stem cell transplantation. Blood 2023, 141, 1194–1208. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef]
- Qasim, W. Allogeneic CAR T cell therapies for leukemia. Am. J. Hematol. 2019, 94, S50–S54. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, X.; Fang, C.; Jiang, S.; June, C.H.; Zhao, Y. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clin. Cancer Res. 2017, 23, 2255–2266. [Google Scholar] [CrossRef]
- Jo, S.; Das, S.; Williams, A.; Chretien, A.S.; Pagliardini, T.; Le Roy, A.; Fernandez, J.P.; Le Clerre, D.; Jahangiri, B.; Chion-Sotinel, I.; et al. Endowing universal CAR T-cell with immune-evasive properties using TALEN-gene editing. Nat. Commun. 2022, 13, 3453. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. Cancer Vaccines and Oncolytic Viruses Exert Profoundly Lower Side Effects in Cancer Patients than Other Systemic Therapies: A Comparative Analysis. Biomedicines 2020, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, O.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Park, J.H.; Riviere, I.; Gonen, M.; Wang, X.; Senechal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Si, C.; Gao, J.; Ma, X. Natural killer cell-derived exosome-based cancer therapy: From biological roles to clinical significance and implications. Mol. Cancer 2024, 23, 134. [Google Scholar] [CrossRef]
- Teachey, D.T.; Lacey, S.F.; Shaw, P.A.; Melenhorst, J.J.; Maude, S.L.; Frey, N.; Pequignot, E.; Gonzalez, V.E.; Chen, F.; Finklestein, J.; et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016, 6, 664–679. [Google Scholar] [CrossRef]
- Cherkassky, L.; Morello, A.; Villena-Vargas, J.; Feng, Y.; Dimitrov, D.S.; Jones, D.R.; Sadelain, M.; Adusumilli, P.S. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Investig. 2016, 126, 3130–3144. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Lei, C.; Liu, S.; Cui, Y.; Wang, C.; Qian, K.; Li, T.; Shen, Y.; Fan, X.; Lin, F.; et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat. Commun. 2019, 10, 4355. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, C.; Wen, X.; Liu, W.; Huang, X.; Li, Y.; Zang, J.; Weng, Z.; Lu, D.; Tsang, C.K.; et al. Circular RNA circ-FoxO3 attenuates blood-brain barrier damage by inducing autophagy during ischemia/reperfusion. Mol. Ther. 2022, 30, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- El-Hage, W.; Leman, S.; Camus, V.; Belzung, C. Mechanisms of antidepressant resistance. Front. Pharmacol. 2013, 4, 146. [Google Scholar] [CrossRef]
- Cazals, F.; Huguenot, D.; Crampon, M.; Colombano, S.; Betelu, S.; Galopin, N.; Perrault, A.; Simonnot, M.O.; Ignatiadis, I.; Rossano, S. Production of biosurfactant using the endemic bacterial community of a PAHs contaminated soil, and its potential use for PAHs remobilization. Sci. Total Environ. 2020, 709, 136143. [Google Scholar] [CrossRef]
- Chang, Y.; Jin, G.; Luo, W.; Luo, Q.; Jung, J.; Hummel, S.N.; Torregrosa-Allen, S.; Elzey, B.D.; Low, P.S.; Lian, X.L.; et al. Engineered human pluripotent stem cell-derived natural killer cells with PD-L1 responsive immunological memory for enhanced immunotherapeutic efficacy. Bioact. Mater. 2023, 27, 168–180. [Google Scholar] [CrossRef]
- Du, R.; Zhang, X.; Lu, X.; Ma, X.; Guo, X.; Shi, C.; Ren, X.; Ma, X.; He, Y.; Gao, Y.; et al. PDPN positive CAFs contribute to HER2 positive breast cancer resistance to trastuzumab by inhibiting antibody-dependent NK cell-mediated cytotoxicity. Drug Resist. Updat. 2023, 68, 100947. [Google Scholar] [CrossRef]
- Valiente, M.; Obenauf, A.C.; Jin, X.; Chen, Q.; Zhang, X.H.; Lee, D.J.; Chaft, J.E.; Kris, M.G.; Huse, J.T.; Brogi, E.; et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 2014, 156, 1002–1016. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192.e5. [Google Scholar] [CrossRef] [PubMed]
- Katila, N.; Duwa, R.; Bhurtel, S.; Khanal, S.; Maharjan, S.; Jeong, J.H.; Lee, S.; Choi, D.Y.; Yook, S. Enhancement of blood-brain barrier penetration and the neuroprotective effect of resveratrol. J. Control. Release 2022, 346, 1–19. [Google Scholar] [CrossRef]
- Li, J.; Sun, C.; Tao, W.; Cao, Z.; Qian, H.; Yang, X.; Wang, J. Photoinduced PEG deshielding from ROS-sensitive linkage-bridged block copolymer-based nanocarriers for on-demand drug delivery. Biomaterials 2018, 170, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Abedi, A.; Moosazadeh Moghaddam, M.; Kachuei, R.; Imani Fooladi, A.A. Exosomes as a Therapeutic Strategy in Cancer: Potential Roles as Drug Carriers and Immune Modulators. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189238. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Qu, Y.; Cheng, L.; Yoon, C.W.; He, P.; Monther, A.; Guo, T.; Chittle, S.; Wang, Y. Engineering chimeric antigen receptor T cells for solid tumour therapy. Clin. Transl. Med. 2022, 12, e1141. [Google Scholar] [CrossRef]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichon, T.; Kulach, N. Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.E10. [Google Scholar] [CrossRef]
- Chen, W.H.; Huang, Q.Y.; Wang, Z.Y.; Zhuang, X.X.; Lin, S.; Shi, Q.Y. Therapeutic potential of exosomes/miRNAs in polycystic ovary syndrome induced by the alteration of circadian rhythms. Front. Endocrinol. 2022, 13, 918805. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Dong, P.Y.; Yang, G.M.; Gurunathan, S. A comprehensive review on the composition, biogenesis, purification, and multifunctional role of exosome as delivery vehicles for cancer therapy. Biomed. Pharmacother. 2023, 165, 115087. [Google Scholar] [CrossRef]
- Li, C.; Xu, X.; Wei, S.; Jiang, P.; Xue, L.; Wang, J.; Senior, C. Tumor-associated macrophages: Potential therapeutic strategies and future prospects in cancer. J. Immunother. Cancer 2021, 9, e001341. [Google Scholar] [CrossRef]
- Rao, L.; He, Z.; Meng, Q.F.; Zhou, Z.; Bu, L.L.; Guo, S.S.; Liu, W.; Zhao, X.Z. Effective cancer targeting and imaging using macrophage membrane-camouflaged upconversion nanoparticles. J. Biomed. Mater. Res. A 2017, 105, 521–530. [Google Scholar] [CrossRef]
- Susek, K.H.; Karvouni, M.; Alici, E.; Lundqvist, A. The Role of CXC Chemokine Receptors 1-4 on Immune Cells in the Tumor Microenvironment. Front. Immunol. 2018, 9, 2159. [Google Scholar] [CrossRef]
- Weber, E.W.; Maus, M.V.; Mackall, C.L. The Emerging Landscape of Immune Cell Therapies. Cell 2020, 181, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Huang, Z.; Wang, Q.; Chen, W.; Huang, Y.; Sun, X.; Chen, J.; Feng, S. IAPP blocks anti-breast cancer function of CD8(+)T cells via targeting cuproptosis. Front. Immunol. 2024, 15, 1481129. [Google Scholar] [CrossRef]
- Ramalingam, P.S.; Premkumar, T.; Sundararajan, V.; Hussain, M.S.; Arumugam, S. Design and development of dual targeting CAR protein for the development of CAR T-cell therapy against KRAS mutated pancreatic ductal adenocarcinoma using computational approaches. Discov. Oncol. 2024, 15, 592. [Google Scholar] [CrossRef]
- Marotte, L.; Simon, S.; Vignard, V.; Dupre, E.; Gantier, M.; Cruard, J.; Alberge, J.B.; Hussong, M.; Deleine, C.; Heslan, J.M.; et al. Increased antitumor efficacy of PD-1-deficient melanoma-specific human lymphocytes. J. Immunother. Cancer 2020, 8, e000311. [Google Scholar] [CrossRef]
- Sommermeyer, D.; Hill, T.; Shamah, S.M.; Salter, A.I.; Chen, Y.; Mohler, K.M.; Riddell, S.R. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia 2017, 31, 2191–2199. [Google Scholar] [CrossRef]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef]
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724. [Google Scholar] [CrossRef]
- Kershaw, M.H.; Westwood, J.A.; Parker, L.L.; Wang, G.; Eshhar, Z.; Mavroukakis, S.A.; White, D.E.; Wunderlich, J.R.; Canevari, S.; Rogers-Freezer, L.; et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006, 12, 6106–6115. [Google Scholar] [CrossRef]
- Park, J.R.; Digiusto, D.L.; Slovak, M.; Wright, C.; Naranjo, A.; Wagner, J.; Meechoovet, H.B.; Bautista, C.; Chang, W.C.; Ostberg, J.R.; et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007, 15, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Rouce, R.; Robertson, C.S.; Reyna, A.; Narala, N.; Vyas, G.; Mehta, B.; Zhang, H.; Dakhova, O.; Carrum, G.; et al. In Vivo Fate and Activity of Second- versus Third-Generation CD19-Specific CAR-T Cells in B Cell Non-Hodgkin’s Lymphomas. Mol. Ther. 2018, 26, 2727–2737. [Google Scholar] [CrossRef]
- Ramello, M.C.; Benzaid, I.; Kuenzi, B.M.; Lienlaf-Moreno, M.; Kandell, W.M.; Santiago, D.N.; Pabon-Saldana, M.; Darville, L.; Fang, B.; Rix, U.; et al. An immunoproteomic approach to characterize the CAR interactome and signalosome. Sci. Signal 2019, 12, eaap9777. [Google Scholar] [CrossRef]
- Wagner, J.; Wickman, E.; DeRenzo, C.; Gottschalk, S. CAR T Cell Therapy for Solid Tumors: Bright Future or Dark Reality? Mol. Ther. 2020, 28, 2320–2339. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, M.; Yang, T.; Mo, Z.; Wei, G.; Jing, R.; Zhao, H.; Chen, R.; Zu, C.; Gu, T.; et al. Sequential CD7 CAR T-Cell Therapy and Allogeneic HSCT without GVHD Prophylaxis. N. Engl. J. Med. 2024, 390, 1467–1480. [Google Scholar] [CrossRef]
- Peng, J.J.; Wang, L.; Li, Z.; Ku, C.L.; Ho, P.C. Metabolic challenges and interventions in CAR T cell therapy. Sci. Immunol. 2023, 8, eabq3016. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, T.; Tang, L.; Li, Z.; Jiang, G.; Huang, X. Inhalable CAR-T cell-derived exosomes as paclitaxel carriers for treating lung cancer. J. Transl. Med. 2023, 21, 383. [Google Scholar] [CrossRef]
- Lv, Q.; Cheng, L.; Lu, Y.; Zhang, X.; Wang, Y.; Deng, J.; Zhou, J.; Liu, B.; Liu, J. Thermosensitive Exosome-Liposome Hybrid Nanoparticle-Mediated Chemoimmunotherapy for Improved Treatment of Metastatic Peritoneal Cancer. Adv. Sci. 2020, 7, 2000515. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, Z.; Jiang, G.; Huang, X. Sequential Targeting Hybrid Nanovesicles Composed of Chimeric Antigen Receptor T-Cell-Derived Exosomes and Liposomes for Enhanced Cancer Immunochemotherapy. ACS Nano 2023, 17, 16770–16786. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Cao, X.; Cai, H.; Feng, P.; Chen, X.; Zhu, Y.; Yang, Y.; An, W.; Yang, Y.; Jie, J. The exosomes derived from CAR-T cell efficiently target mesothelin and reduce triple-negative breast cancer growth. Cell Immunol. 2021, 360, 104262. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, X.; Fan, D.; Liu, P.; Zhou, M.; Cheng, M.; Huang, J.; Luo, Y.; Guo, Y.; Yang, T. Advancing Tumor-Targeted Chemo-Immunotherapy: Development of the CAR-M-derived Exosome-Drug Conjugate. J. Med. Chem. 2024, 67, 13959–13974. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.H.; Zhang, Y.; Pan, H.; Feng, J.; Zhang, T.; Liu, T.; Qin, Y.; Qin, S.; Yin, X.; Liu, B.; et al. Efficacy and safety of weekly paclitaxel with or without ramucirumab as second-line therapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma (RAINBOW-Asia): A randomised, multicentre, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; Francois, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouche, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Marupudi, N.I.; Han, J.E.; Li, K.W.; Renard, V.M.; Tyler, B.M.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert. Opin. Drug Saf. 2007, 6, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Ransom, D.; Wilson, K.; Fournier, M.; Simes, R.J.; Gebski, V.; Yip, D.; Tebbutt, N.; Karapetis, C.S.; Ferry, D.; Gordon, S.; et al. Final results of Australasian Gastrointestinal Trials Group ARCTIC study: An audit of raltitrexed for patients with cardiac toxicity induced by fluoropyrimidines. Ann. Oncol. 2014, 25, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Paulete, A.R.; Mateus-Tique, J.; Mollaoglu, G.; Nielsen, S.R.; Marks, A.; Lakshmi, A.; Khan, J.A.; Wilk, C.M.; Pia, L.; Baccarini, A.; et al. Targeting Macrophages with CAR T Cells Delays Solid Tumor Progression and Enhances Antitumor Immunity. Cancer Immunol. Res. 2022, 10, 1354–1369. [Google Scholar] [CrossRef]
- Zheng, W.; Ling, S.; Cao, Y.; Shao, C.; Sun, X. Combined use of NK cells and radiotherapy in the treatment of solid tumors. Front. Immunol. 2023, 14, 1306534. [Google Scholar] [CrossRef]
- Pedersen, H.; Schmiegelow, K.; Hamerlik, P. Radio-Resistance and DNA Repair in Pediatric Diffuse Midline Gliomas. Cancers 2020, 12, 2813. [Google Scholar] [CrossRef]
- Najafi, M.; Mortezaee, K.; Majidpoor, J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019, 234, 116781. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yao, M.; Gao, Y.; Yue, Y.; Li, Y.; Zhang, T.; Nie, G.; Zhao, X.; Liang, X. Functional Immune Cell-Derived Exosomes Engineered for the Trilogy of Radiotherapy Sensitization. Adv. Sci. 2022, 9, e2106031. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- High, K.A.; Roncarolo, M.G. Gene Therapy. N. Engl. J. Med. 2019, 381, 455–464. [Google Scholar] [CrossRef]
- Bose, R.J.; Kumar, U.S.; Garcia-Marques, F.; Zeng, Y.; Habte, F.; McCarthy, J.R.; Pitteri, S.; Massoud, T.F.; Paulmurugan, R. Engineered Cell-Derived Vesicles Displaying Targeting Peptide and Functionalized with Nanocarriers for Therapeutic microRNA Delivery to Triple-Negative Breast Cancer in Mice. Adv. Healthc. Mater. 2022, 11, e2101387. [Google Scholar] [CrossRef]
- Gulei, D.; Berindan-Neagoe, I. Activation of Necroptosis by Engineered Self Tumor-Derived Exosomes Loaded with CRISPR/Cas9. Mol. Ther. Nucleic Acids 2019, 17, 448–451. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Chen, L.; Bian, H.; Chen, X.; Zheng, H.; Yang, P.; Chen, Q.; Xu, H. Natural killer cell-derived exosomal miR-1249-3p attenuates insulin resistance and inflammation in mouse models of type 2 diabetes. Signal Transduct. Target. Ther. 2021, 6, 409. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Jin, X.; Hu, D.; Xia, C.; Xu, H.; Hu, J. NK cell-derived exosomes carry miR-207 and alleviate depression-like symptoms in mice. J. Neuroinflammation 2020, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Chen, C. Near-infrared light-mediated nanoplatforms for cancer thermo-chemotherapy and optical imaging. Adv. Mater. 2013, 25, 3869–3880. [Google Scholar] [CrossRef]
- Wu, G.; Mikhailovsky, A.; Khant, H.A.; Fu, C.; Chiu, W.; Zasadzinski, J.A. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. J. Am. Chem. Soc. 2008, 130, 8175–8177. [Google Scholar] [CrossRef]
- Timko, B.P.; Arruebo, M.; Shankarappa, S.A.; McAlvin, J.B.; Okonkwo, O.S.; Mizrahi, B.; Stefanescu, C.F.; Gomez, L.; Zhu, J.; Zhu, A.; et al. Near-infrared-actuated devices for remotely controlled drug delivery. Proc. Natl. Acad. Sci. USA 2014, 111, 1349–1354. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, W.; Chang, J.; Tan, Y.W.; Li, J.; Chen, M.; Sun, Y.; Li, F. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Commun. 2016, 7, 10437. [Google Scholar] [CrossRef] [PubMed]
- Bladergroen, B.A.; Meijer, C.J.; ten Berge, R.L.; Hack, C.E.; Muris, J.J.; Dukers, D.F.; Chott, A.; Kazama, Y.; Oudejans, J.J.; van Berkum, O.; et al. Expression of the granzyme B inhibitor, protease inhibitor 9, by tumor cells in patients with non-Hodgkin and Hodgkin lymphoma: A novel protective mechanism for tumor cells to circumvent the immune system? Blood 2002, 99, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Yu, L.; Zhao, C.; Li, Y.; Ma, Y.; Zhai, Y.; Qian, Z.; Gu, Y.; Li, S. Inhibition of SerpinB9 to enhance granzyme B-based tumor therapy by using a modified biomimetic nanoplatform with a cascade strategy. Biomaterials 2022, 288, 121723. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.E. High-intensity focused ultrasound in the treatment of solid tumours. Nat. Rev. Cancer 2005, 5, 321–327. [Google Scholar] [CrossRef]
- Furtado, D.; Bjornmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the Blood-Brain Barrier: The Role of Nanomaterials in Treating Neurological Diseases. Adv. Mater. 2018, 30, e1801362. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, N.; Wang, Z.; Wu, M.; Chen, Y.; Ma, M.; Chen, H.; Shi, J. Endogenous Catalytic Generation of O(2) Bubbles for In Situ Ultrasound-Guided High Intensity Focused Ultrasound Ablation. ACS Nano 2017, 11, 9093–9102. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Song, G.; Chen, Y.; Liang, C.; Yi, X.; Liu, J.; Sun, X.; Shen, S.; Yang, K.; Liu, Z. Catalase-Loaded TaOx Nanoshells as Bio-Nanoreactors Combining High-Z Element and Enzyme Delivery for Enhancing Radiotherapy. Adv. Mater. 2016, 28, 7143–7148. [Google Scholar] [CrossRef]
- Du, J.; Wan, Z.; Wang, C.; Lu, F.; Wei, M.; Wang, D.; Hao, Q. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics 2021, 11, 8185–8196. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, Y.; Choi, W.; Jangili, P.; Ge, Y.; Xu, Y.; Kang, J.; Liu, L.; Zhang, B.; Xie, Z.; et al. Overcoming barriers in photodynamic therapy harnessing nano-formulation strategies. Chem. Soc. Rev. 2021, 50, 9152–9201. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, C.; Li, Y.; Wang, Y.; Gao, J.; Ma, X. CAR Cell-Derived Exosomes in Cancer Therapy: Biogenesis, Engineering Strategies and Antitumor Mechanisms. Int. J. Mol. Sci. 2025, 26, 7890. https://doi.org/10.3390/ijms26167890
Si C, Li Y, Wang Y, Gao J, Ma X. CAR Cell-Derived Exosomes in Cancer Therapy: Biogenesis, Engineering Strategies and Antitumor Mechanisms. International Journal of Molecular Sciences. 2025; 26(16):7890. https://doi.org/10.3390/ijms26167890
Chicago/Turabian StyleSi, Chaohua, Yuanyuan Li, Yunwen Wang, Jianen Gao, and Xu Ma. 2025. "CAR Cell-Derived Exosomes in Cancer Therapy: Biogenesis, Engineering Strategies and Antitumor Mechanisms" International Journal of Molecular Sciences 26, no. 16: 7890. https://doi.org/10.3390/ijms26167890
APA StyleSi, C., Li, Y., Wang, Y., Gao, J., & Ma, X. (2025). CAR Cell-Derived Exosomes in Cancer Therapy: Biogenesis, Engineering Strategies and Antitumor Mechanisms. International Journal of Molecular Sciences, 26(16), 7890. https://doi.org/10.3390/ijms26167890





