Investigation of Pharmacological Mechanisms and Active Ingredients of Cichorium intybus L. in Alleviating Renal Urate Deposition via lncRNA H19/miR-21-3p Regulation to Enhance ABCG2 Expression
Abstract
1. Introduction
2. Results
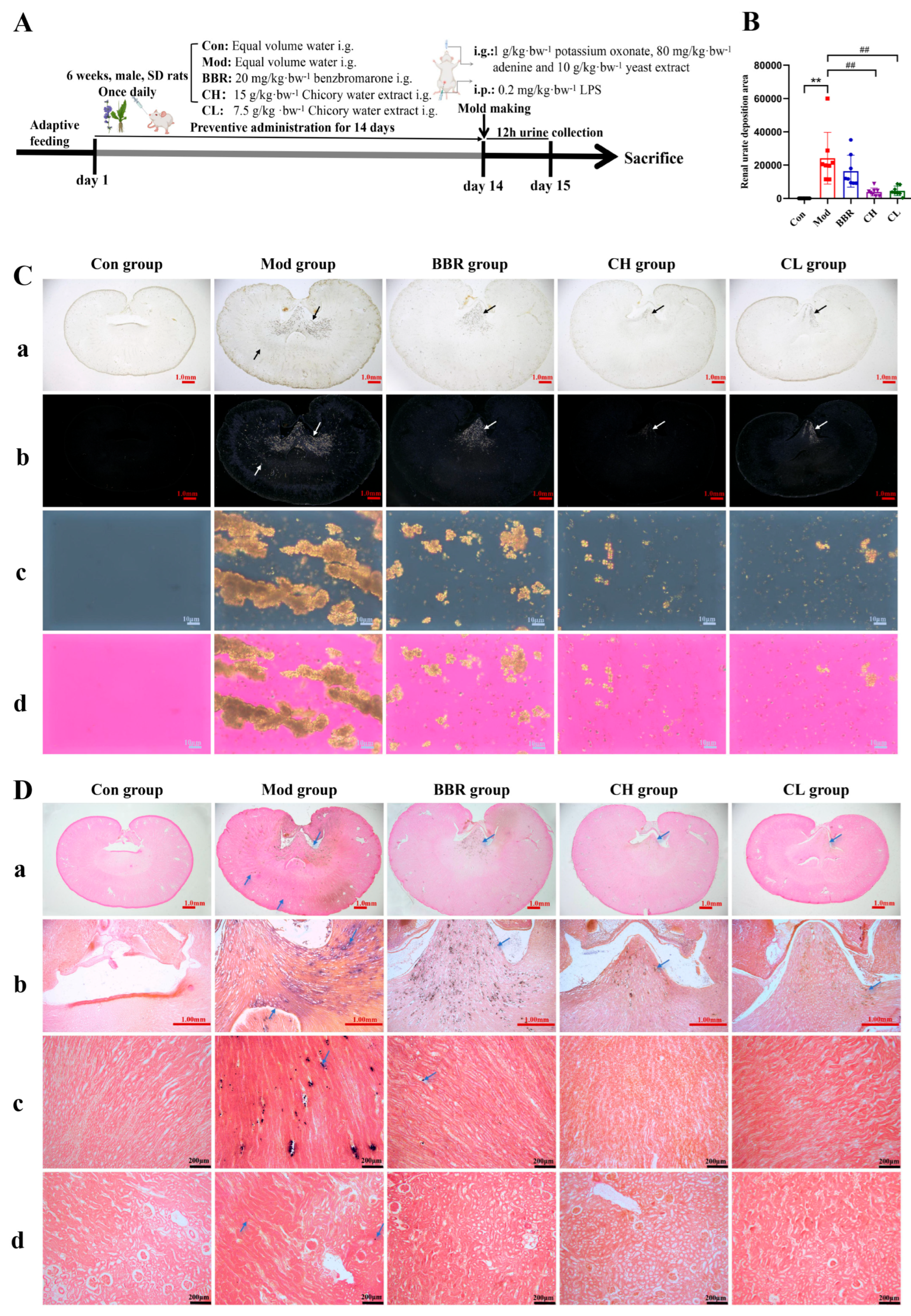
2.1. Chicory Ameliorates Renal Urate Deposition in Rats
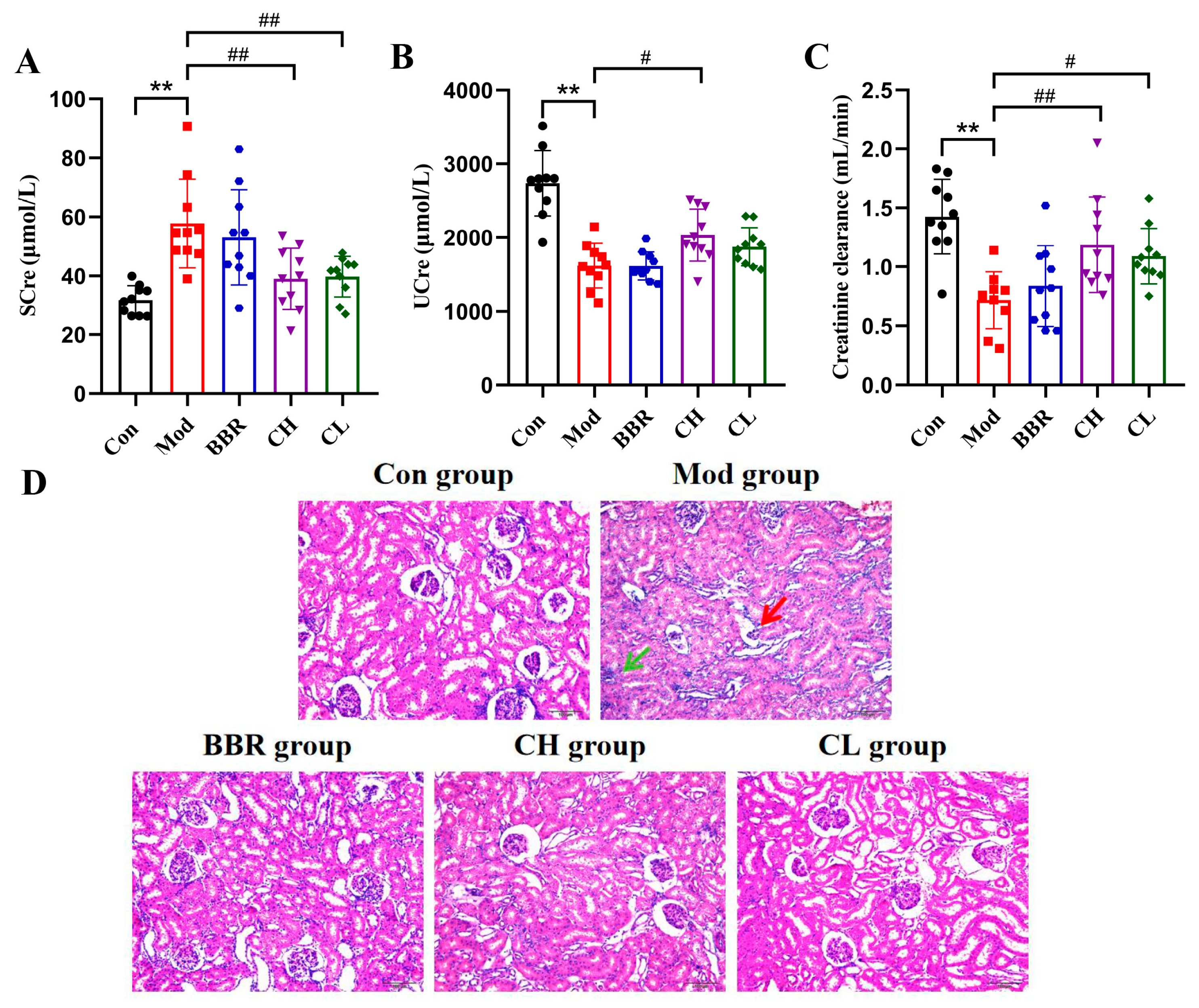
2.2. Chicory Improved Kidney Dysfunction and Attenuated Renal Histopathologic Injury in Rats with Renal Urate Deposition
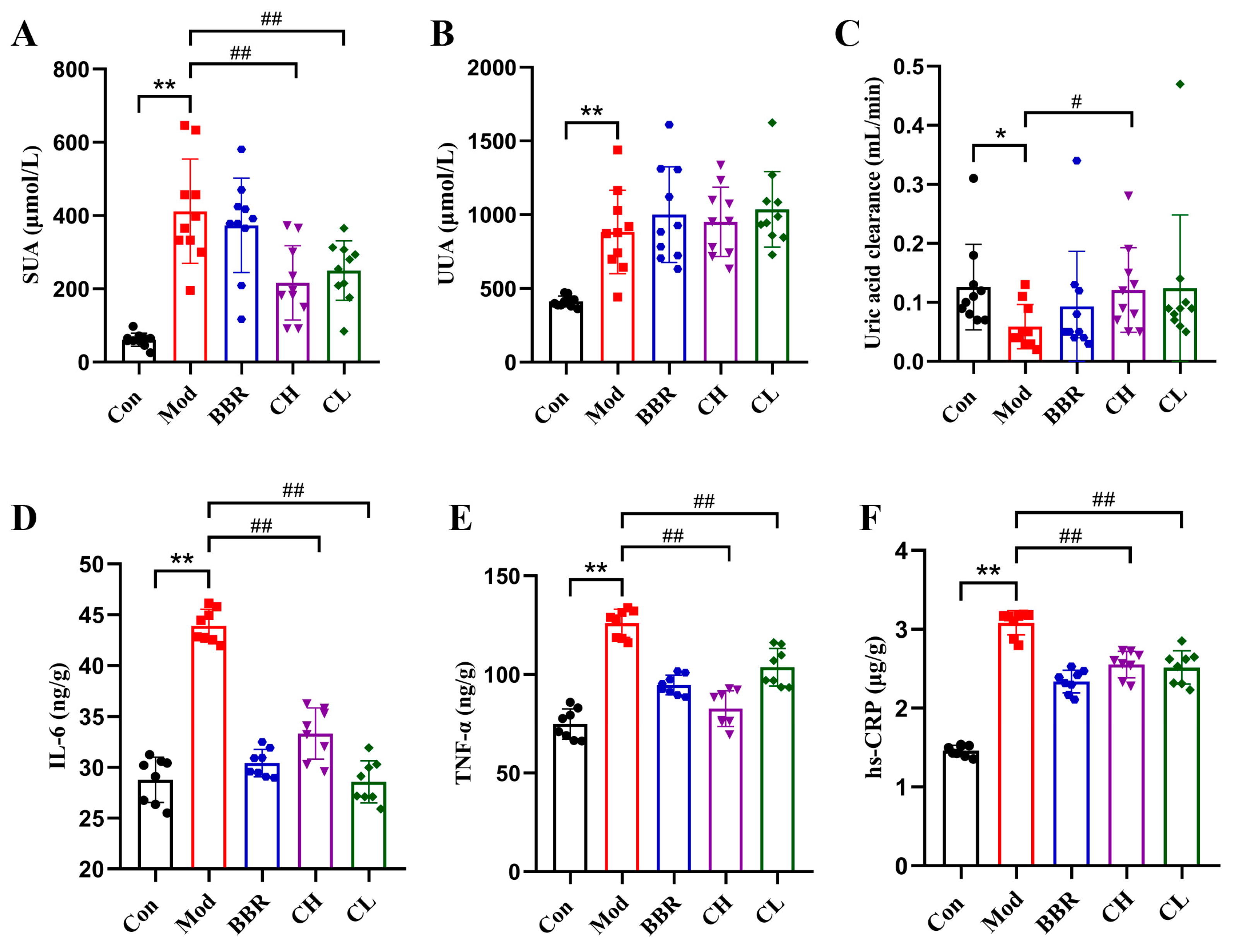
2.3. Chicory Reduced SUA Levels and Attenuated Low-Grade Renal Inflammation in Rats with Renal Urate Deposition
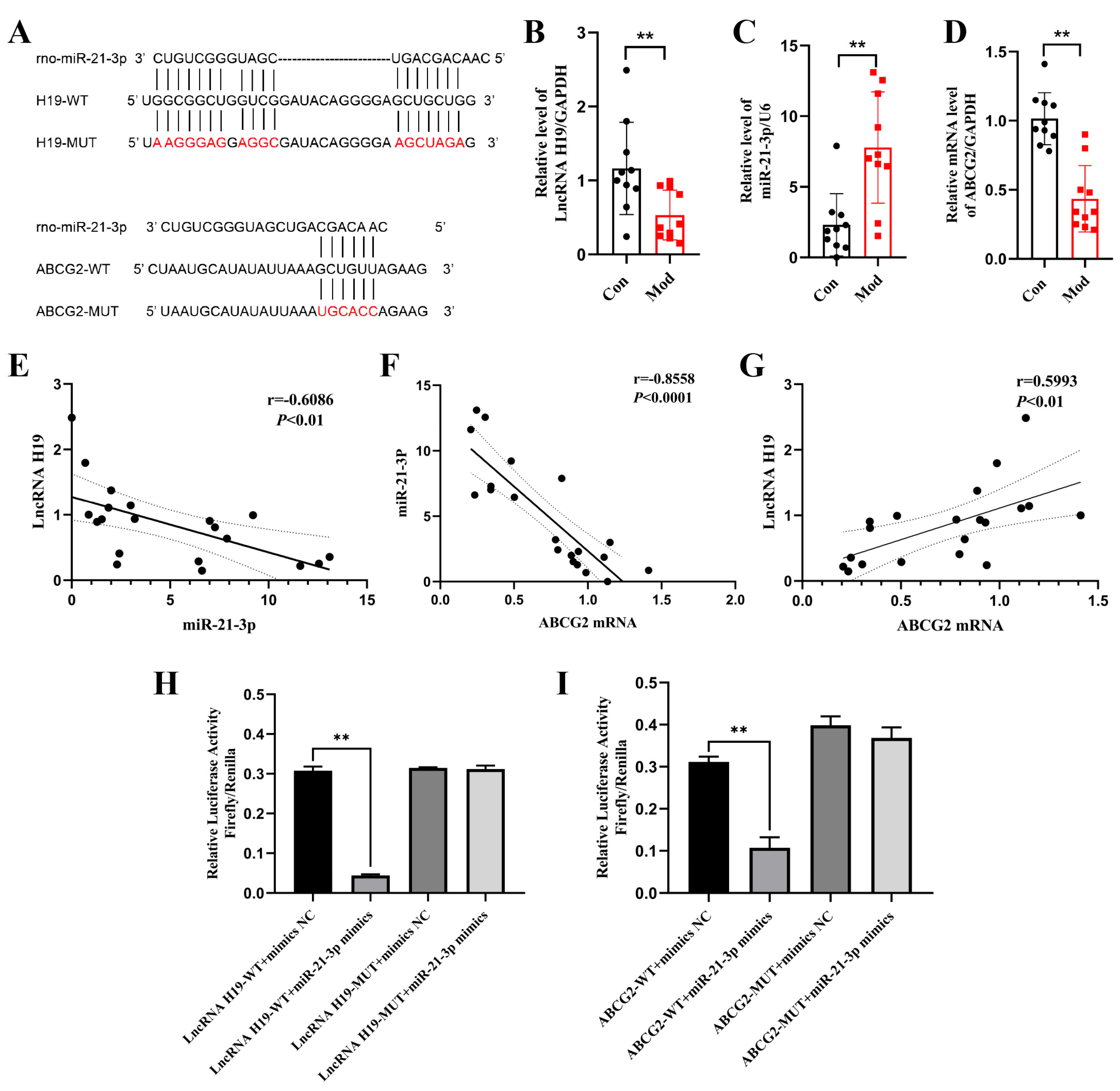
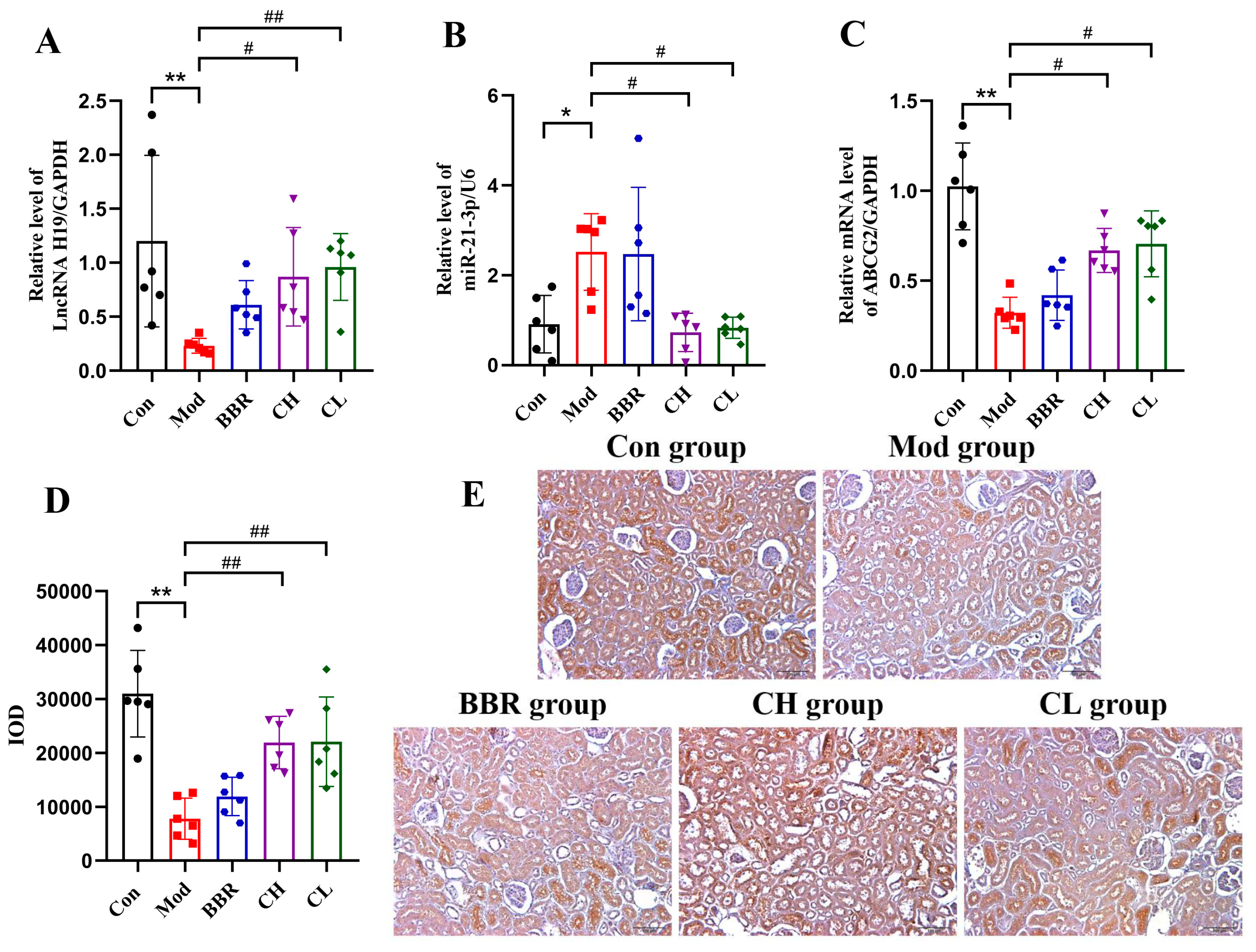
2.4. Chicory Increased ABCG2 Regulated by lncRNA H19/miR-21-3p in Rats with Renal Urate Deposition
2.4.1. Analysis of Targeting Regulatory Relations Between miR-21-3p, lncRNA H19, and ABCG2
2.4.2. Chicory Promotes ABCG2 Expression Regulated by lncRNA H19/miR-21-3p
2.5. Analysis of Active Ingredients in Chicory Extract to Alleviate Renal Urate Deposition
2.6. Analysis of Active Ingredients in Chicory Extract Targeting ABCG2 and the lncRNA H19/miR-21-3p Axis
Cell Experiments
3. Discussion
3.1. Establishment of In Vivo and In Vitro Models for Renal Urate Deposition
3.2. The Pharmacological Effect and Active Ingredients of Chicory in Renal Urate Deposition
4. Materials and Methods
4.1. Preparation of Chicory Solution
4.2. Animal Experiments
4.2.1. Drug Administration and Sample Collection
4.2.2. Histological Analysis
4.2.3. Immunohistochemical Analysis
4.2.4. RT-qPCR Analysis
4.3. Analysis of the lncRNA H19/miR-21-3p/ABCG2 in Renal Urate Deposition
4.3.1. Prediction of Targeting Regulatory Relations Between miR-21-3p, lncRNA H19, and ABCG2
4.3.2. Animal Experiment
4.3.3. Luciferase Reporter Assay
4.4. Active Ingredients Analysis
4.4.1. Sample Preparation
4.4.2. UPLC-LTQ-Orbitrap-MS Analysis
4.5. Cell Experiments
4.5.1. Cell Culture and Cell Viability Assay
4.5.2. Western Blotting Analysis
4.5.3. RT-qPCR Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- So, A.; Thorens, B. Uric Acid Transport and Disease. J. Clin. Investig. 2010, 120, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Nie, Y.; Chang, Y.; Zeng, S.; Liang, C.; Zheng, X.; Xiao, D.; Zhan, S.; Zheng, Q. Protective Effects of Rhizoma Smilacis Glabrae Extracts on Potassium Oxonate- and Monosodium Urate-Induced Hyperuricemia and Gout in Mice. Phytomedicine 2019, 59, 152772. [Google Scholar] [CrossRef] [PubMed]
- Pascual, E.; Addadi, L.; Andrés, M.; Sivera, F. Mechanisms of Crystal Formation in Gout—A Structural Approach. Nat. Rev. Rheumatol. 2015, 11, 725–730. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, C.; Zhang, X.; Wu, Z.; Wu, Y.; Li, D.; Gao, J. Shizhifang Ameliorates Pyroptosis of Renal Tubular Epithelial Cells in Hyperuricemia through Inhibiting NLRP3 Inflammasome. J. Ethnopharmacol. 2023, 317, 116777. [Google Scholar] [CrossRef]
- Gonçalves, D.L.N.; Moreira, T.R.; da Silva, L.S. A Systematic Review and Meta-Analysis of the Association between Uric Acid Levels and Chronic Kidney Disease. Sci. Rep. 2022, 12, 6251. [Google Scholar] [CrossRef]
- Su, H.; Yang, C.; Liang, D.; Liu, H. Research Advances in the Mechanisms of Hyperuricemia-Induced Renal Injury. Biomed Res. Int. 2020, 2020, 5817348. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, Y.; Bian, H.; Yang, H.; Wang, H.; Meng, X.; Jin, J. Function of Uric Acid Transporters and Their Inhibitors in Hyperuricaemia. Front. Pharmacol. 2021, 12, 667753. [Google Scholar] [CrossRef]
- Yang, L.; Liang, J.; Zheng, Q.; Zhou, L.; Xiong, Y.; Wang, H.; Yuan, J. A Comparative Study of Serum Pharmacochemistry of Kai-Xin-San in Normal and AD Rats Using UPLC-LTQ-Orbitrap-MS. Pharmaceuticals 2022, 16, 30. [Google Scholar] [CrossRef]
- Hyndman, D.; Liu, S.; Miner, J.N. Urate Handling in the Human Body. Curr. Rheumatol. Rep. 2016, 18, 34. [Google Scholar] [CrossRef]
- Du, L.; Zong, Y.; Li, H.; Wang, Q.; Xie, L.; Yang, B.; Pang, Y.; Zhang, C.; Zhong, Z.; Gao, J. Hyperuricemia and Its Related Diseases: Mechanisms and Advances in Therapy. Signal Transduct. Target. Ther. 2024, 9, 212. [Google Scholar] [CrossRef]
- Skoczyńska, M.; Chowaniec, M.; Szymczak, A.; Langner-Hetmańczuk, A.; Maciążek-Chyra, B.; Wiland, P. Pathophysiology of Hyperuricemia and Its Clinical Significance–a Narrative Review. Rheumatology 2020, 58, 312–323. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, Z.; Wang, Y.; Mao, Q. A Method for Establishing an Animal Model of Renal Urate Deposition. CN202210822311, 13 September 2023. [Google Scholar]
- Xu, J.; Tu, M.; Fan, X.; Guo, Y.; Zhang, T.; Zeng, X.; Cai, Z.; Wu, Z.; Pan, D. A Novel Strain of Levilactobacillus brevis PDD-5 Isolated from Salty Vegetables Has Beneficial Effects on Hyperuricemia through Anti-Inflammation and Improvement of Kidney Damage. Food Sci. Hum. Wellness 2024, 13, 898–908. [Google Scholar] [CrossRef]
- Kukal, S.; Guin, D.; Rawat, C.; Bora, S.; Mishra, M.K.; Sharma, P.; Paul, P.R.; Kanojia, N.; Grewal, G.K.; Kukreti, S.; et al. Multidrug Efflux Transporter ABCG2: Expression and Regulation. Cell. Mol. Life Sci. 2021, 78, 6887–6939. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Bian, H.; Liu, X.; Zhang, H.; Ying, J.; Yang, H.; Zu, T.; Cui, G.; Liao, Y.; Xu, M.; et al. GRP/GRPR Signaling Pathway Aggravates Hyperuricemia-Induced Renal Inflammation and Fibrosis via ABCG2-Dependent Mechanisms. Biochem. Pharmacol. 2023, 218, 115901. [Google Scholar] [CrossRef]
- Ying, S.; Zheng, T.; Chen, P.; Guo, H.; Xu, C. Research on the Structure, Function, and Related Inhibitors of BCRP/ABCG2. Chin. Med. Biotech. 2013, 8, 201–205. [Google Scholar]
- Eckenstaler, R.; Benndorf, R.A. The Role of ABCG2 in the Pathogenesis of Primary Hyperuricemia and Gout—An Update. Int. J. Mol. Sci. 2021, 22, 6678. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-Coding RNAs in Disease: From Mechanisms to Therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Heikkinen, L.; Wang, C.; Yang, Y.; Sun, H.; Wong, G. Trends in the Development of MiRNA Bioinformatics Tools. Brief. Bioinform. 2019, 20, 1836–1852. [Google Scholar] [CrossRef]
- Zhou, Y.; Yue, Y.; Fan, S.; Jia, Q.; Ding, X. Advances in Pathophysiology of Triple-Negative Breast Cancer: The Potential of LncRNAs for Clinical Diagnosis, Treatment, and Prognostic Monitoring. Mol. Biotechnol. 2021, 63, 1093–1102. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, F.; Hong, S.; Wang, J.; Chen, B.; Li, L.; Yang, J.; Yao, Y.; Yang, C.; Hu, Y.; et al. Role of MicroRNA Alternation in the Pathogenesis of Gouty Arthritis. Front. Endocrinol. 2022, 13, 967769. [Google Scholar] [CrossRef]
- Xie, J.; He, C.; Su, Y.; Ding, Y.; Zhu, X.; Xu, Y.; Ding, J.; Zhou, H.; Wang, H. Research Progress on MicroRNA in Gout. Front. Pharmacol. 2022, 13, 981799. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhu, M.; Li, J.; Zhang, X.; Liu, Y.; Wu, X.; Liu, Z. Effects of Xie-Zhuo-Chu-Bi-Fang on MiR-34a and URAT1 and Their Relationship in Hyperuricemic Mice. J. Ethnopharmacol. 2015, 161, 163–169. [Google Scholar] [CrossRef]
- Paraskevopoulou, M.D.; Hatzigeorgiou, A.G. Analyzing MiRNA–LncRNA Interactions; Springer: Berlin/Heidelberg, Germany, 2016; pp. 271–286. [Google Scholar] [CrossRef]
- Huang, Y. The Novel The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J. Cell Mol. Med. 2018, 22, 5768–5775. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Wang, Q. The Relationship between Long Chain Non Coding RNA H19 Gene Polymorphism and the Risk of Hyperuricemia. Shandong Med. J. 2018, 58, 80–83. [Google Scholar]
- Perović, J.; Šaponjac, V.T.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a Food Ingredient–Nutritional Composition, Bioactivity, Safety, and Health Claims: A Review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Li, G. Hepatoprotective Effect of Cichorium intybus L., a Traditional Uighur Medicine, against Carbon Tetrachloride-Induced Hepatic Fibrosis in Rats. World J. Gastroenterol. 2014, 20, 4753. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. The Pharmacopoeia of the People’s Republic of China 2020 Edition; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Satmbekova, D.; Srivedavyasasri, R.; Orazbekov, Y.; Omarova, R.; Datkhayev, U.; Ross, S.A. Chemical and Biological Studies on Cichorium intybus L. Nat. Prod. Res. 2018, 32, 1343–1347. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Zhang, B.; Jiang, Z.; Guo, F.; Yang, T. Cichorium intybus L. Extract Suppresses Experimental Gout by Inhibiting the NF-ΚB and NLRP3 Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 4921. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Mao, Q.; Hang, Z.; Lin, Z.; Zhang, B. Research Progress on Chemical Constituents and Its Prevention and Treatment of Uric Acid-Related Metabolic Diseases in Cichorium intybus L. World Chin. Med. 2021, 16, 35–40. [Google Scholar]
- Wang, Y.; Lin, Z.; Zhang, B.; Nie, A.; Bian, M. Cichorium intybus L. Promotes Intestinal Uric Acid Excretion by Modulating ABCG2 in Experimental Hyperuricemia. Nutr. Metab. 2017, 14, 38. [Google Scholar] [CrossRef]
- Yang, C.; Su, H.; An, N.; Wu, H.; Guo, X.; Li, Z.; Chen, X.; Zhu, S.; Wu, D.; Li, H.; et al. AMP-activated protein kinase α2 contributes to acute and chronic hyperuricemic nephropathy via renal urate deposition in a mouse model. Eur. J. Med. Res. 2022, 27, 176. [Google Scholar] [CrossRef]
- Bjornsson, T.D. Use of Serum Creatinine Concentrations to Determine Renal Function1. Clin. Pharmacokinet. 1979, 4, 200–222. [Google Scholar] [CrossRef]
- Perrone, R.D.; Madias, N.E.; Levey, A.S. Serum Creatinine as an Index of Renal Function: New Insights into Old Concepts. Clin. Chem. 1992, 38, 1933–1953. [Google Scholar] [CrossRef]
- Domazet, S.L.; Olesen, T.B.; Stidsen, J.V.; Svensson, C.K.; Nielsen, J.S.; Thomsen, R.W.; Jessen, N.; Vestergaard, P.; Andersen, M.K.; Hansen, T.; et al. Low-grade Inflammation in Persons with Recently Diagnosed Type 2 Diabetes: The Role of Abdominal Adiposity and Putative Mediators. Diabetes Obes. Metab. 2024, 26, 2092–2101. [Google Scholar] [CrossRef]
- Tian, W.; Tang, B.; Liu, L.; Yu, D.; Hua, H. Research Progress on Curcumin Improving Chronic Low-Grade Inflammation and Related Diseases. Zhongguo Zhong Yao Za Zhi 2024, 49, 2607–2618. [Google Scholar] [PubMed]
- Khanna, P.; Johnson, R.J.; Marder, B.; LaMoreaux, B.; Kumar, A. Systemic Urate Deposition: An Unrecognized Complication of Gout? J. Clin. Med. 2020, 9, 3204. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Shen, J.; Wei, Y.; Zhao, T.; Xiao, N.; Lv, X.; Qin, D.; Xu, Y.; Zhou, Y.; et al. Models of Gouty Nephropathy: Exploring Disease Mechanisms and Identifying Potential Therapeutic Targets. Front. Med. 2024, 11, 1305431. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, R.; Wu, K.; Mo, J.; Li, M.; Chen, Z.; Wang, G.; Zhou, P.; Lan, T. Establishment and Optimization of a Novel Mouse Model of Hyperuricemic Nephropathy. Ren. Fail. 2024, 46, 2427181. [Google Scholar] [CrossRef]
- Xue, L.; Tao, Q.; Chang, H.; Yan, S.; Wang, L.; Zhao, Z.; Tu, C. Tigulixostat Alleviates Hyperuricemic Nephropathy by Promoting M2 Macrophage Polarization. J. Inflamm. Res. 2025, 18, 17–30. [Google Scholar] [CrossRef]
- Riaz, M.; Al Kury, L.T.; Atzaz, N.; Alattar, A.; Alshaman, R.; Shah, F.A.; Li, S. Carvacrol Alleviates Hyperuricemia-Induced Oxidative Stress and Inflammation by Modulating the NLRP3/NF-ΚB Pathwayt. Drug Des. Devel Ther. 2022, 16, 1159–1170. [Google Scholar] [CrossRef]
- Cheng, X.; Lu, E.; Fan, M.; Pi, Z.; Zheng, Z.; Liu, S.; Song, F.; Liu, Z. A Comprehensive Strategy to Clarify the Pharmacodynamic Constituents and Mechanism of Wu-Tou Decoction Based on the Constituents Migrating to Blood and Their in Vivo Process under Pathological State. J. Ethnopharmacol. 2021, 275, 114172. [Google Scholar] [CrossRef]
- Ha, A.T.; Rahmawati, L.; You, L.; Hossain, M.A.; Kim, J.-H.; Cho, J.Y. Anti-Inflammatory, Antioxidant, Moisturizing, and Antimelanogenesis Effects of Quercetin 3-O-β-D-Glucuronide in Human Keratinocytes and Melanoma Cells via Activation of NF-ΚB and AP-1 Pathways. Int. J. Mol. Sci. 2021, 23, 433. [Google Scholar] [CrossRef]
- Granica, S.; Czerwińska, M.E.; Żyżyńska-Granica, B.; Kiss, A.K. Antioxidant and Anti-Inflammatory Flavonol Glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef]
- Khajuria, V.; Gupta, S.; Sharma, N.; Tiwari, H.; Bhardwaj, S.; Dutt, P.; Satti, N.; Nargotra, A.; Bhagat, A.; Ahmed, Z. Kaempferol-3-o-β- d -Glucuronate Exhibit Potential Anti-Inflammatory Effect in LPS Stimulated RAW 264.7 Cells and Mice Model. Int. Immunopharmacol. 2018, 57, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Prajapati, R.; Seong, S.H.; Jung, H.A.; Choi, J.S. Antioxidant and Antineuroinflammatory Mechanisms of Kaempferol-3-O-β-d-Glucuronate on Lipopolysaccharide-Stimulated BV2 Microglial Cells through the Nrf2/HO-1 Signaling Cascade and MAPK/NF-ΚB Pathway. ACS Omega 2023, 8, 6538–6549. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Yang, T.; Ye, Z.-Q.; Tian, J.; Fang, H.-R.; Han, J.-J.; Wang, Z.-Z.; Li, X. Scopoletin Suppresses Activation of Dendritic Cells and Pathogenesis of Experimental Autoimmune Encephalomyelitis by Inhibiting NF-ΚB Signaling. Front. Pharmacol. 2019, 10, 863. [Google Scholar] [CrossRef]
- Parama, D.; Girisa, S.; Khatoon, E.; Kumar, A.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. An Overview of the Pharmacological Activities of Scopoletin against Different Chronic Diseases. Pharmacol. Res. 2022, 179, 106202. [Google Scholar] [CrossRef] [PubMed]
- de Kraker, J.-W.; Franssen, M.C.R.; de Groot, A.; König, W.A.; Bouwmeester, H.J. (+)-Germacrene A Biosynthesis. Plant Physiol. 1998, 117, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; Marques, D.; Figueira, I.; Cankar, K.; Bosch, D.; Brito, M.A.; dos Santos, C.N. Costunolide and Parthenolide: Novel Blood-Brain Barrier Permeable Sesquiterpene Lactones to Improve Barrier Tightness. Biomed. Pharmacother. 2023, 167, 115413. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, J.; Liu, W.; Zhao, Y.; Chen, H. The Function and Regulation Mechanism of Non-Coding RNAs in Muscle Development. Int. J. Mol. Sci. 2023, 24, 14534. [Google Scholar] [CrossRef] [PubMed]
- Krek, A.; Grün, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial MicroRNA Target Predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Wang, J.; Wang, Y.; Nie, A.; Zhu, C.; Sun, Z.; Zhou, Z.; Zhang, B. Chicory Ameliorates Hyperuricemia via Modulating Gut Microbiota and Alleviating LPS/TLR4 Axis in Quail. Biomed. Pharmacother. 2020, 131, 110719. [Google Scholar] [CrossRef] [PubMed]

| Group | No. | Compound | tR/min | Formula | Ion Type | Theoretical Molecular Weight (m/z) | Mass Error (ppm) | MS/MS (m/z) |
|---|---|---|---|---|---|---|---|---|
| Normal rats | 1 a | quinic acid | 1.53 | C7H12O6 | [M-H]- | 191.05611 | 0.26 | 172.8746, 126.9849, 111.0423, 92.9883, 84.9185 |
| 2 | matrine | 4.47 | C15H24N2O | [M+H]+ | 249.19613 | −1.85 | 231.1492, 180.0513, 151.9155, 150.0055, 148.0275 | |
| 3 | isovanillic acid isomers | 8.40 | C8H8O4 | [M-H]- | 167.03498 | −3.53 | 151.9480, 122.9770, 107.8528 | |
| 4 | esculetin isomers | 11.22 | C9H6O4 | [M+H]+ | 179.03388 | −1.68 | 160.9808, 150.8389, 134.8390, 132.7981, 122.8295 | |
| 5 | caffeic acid isomer 2 | 17.48 | C9H8O4 | [M-H]- | 179.03498 | −3.46 | 134.8119, 106.5763 | |
| 6 | 11β,13-dihydrolactucin | 18.56 | C15H18O5 | [M+H]+ | 279.12270 | −2.65 | 261.0302, 243.0755, 215.0181, 187.0436 | |
| 7 | 8-deoxylactucin | 22.09 | C15H16O4 | [M+H]+ | 261.11213 | −2.07 | 243.0645, 224.9774, 215.0443, 197.0028, 186.9890 | |
| 8 a | scopoletin | 23.32 | C10H8O4 | [M+H]+ | 193.04953 | −1.92 | 177.9821, 165.0286, 160.9478, 148.9473, 136.9090, 132.8042, 104.9988 | |
| 9 a | quercetin-3-O-β-D-glucuronide | 31.72 | C21H18O13 | [M-H]- | 477.06746 | −2.26 | 433.2766, 408.7473, 301.0847, 257.1440, 179.0615 | |
| 10 a | kaempferol-3-O-β-D-glucuronide | 36.27 | C21H18O12 | [M-H]- | 461.07254 | −3.19 | 414.8183, 392.8910, 285.0891, 174.8812 | |
| 11 | isorhamnetin 7-O-glucuronide | 37.84 | C22H20O13 | [M-H]- | 491.08311 | −3.10 | 315.0623, 300.0404, 174.9728 | |
| 12 a | 11β,13-dihydrolactucopicrin | 43.08 | C23H24O7 | [M+H]+ | 413.15947 | −0.56 | 261.1538, 215.0710 | |
| 13 | santonin | 52.04 | C15H18O3 | [M+H]+ | 247.13287 | −2.10 | 229.0562, 201.0007, 183.0377, 172.9719, 159.0342 | |
| Rats with renal urate deposition | 1 | esculetin isomers | 11.27 | C9H6O4 | [M+H]+ | 179.03388 | 0.95 | 160.8696, 150.8482, 134.8097, 132.8424, 122.9272 |
| 2 | 11β,13-dihydrolactucin | 18.52 | C15H18O5 | [M+H]+ | 279.12270 | −1.15 | 261.0950, 243.0711, 233.1350, 214.9881 | |
| 3 | 8-deoxylactucin | 22.05 | C15H16O4 | [M+H]+ | 261.11213 | −2.30 | 243.1488, 225.0357, 215.0478, 197.0870, 168.9458 | |
| 4 a | scopoletin | 23.28 | C10H8O4 | [M+H]+ | 193.04953 | −2.38 | 177.9432, 164.8572, 160.8682, 136.8060, 132.8942, 104.9909 | |
| 5 a | quercetin-3-O-β-D-glucuronide | 31.70 | C21H18O13 | [M-H]- | 477.06746 | −1.89 | 301.0476, 178.9900 | |
| 6 a | kaempferol-3-O-β-D-glucuronide | 36.25 | C21H18O12 | [M-H]- | 461.07254 | −2.19 | 285.1616, 175.0296 | |
| 7 | isorhamnetin 7-O-glucuronide | 37.82 | C22H20O13 | [M-H]- | 491.08311 | −3.01 | 315.1833, 300.1340 | |
| 8 a | 11β,13-dihydrolactucopicrin | 43.09 | C23H24O7 | [M+H]+ | 413.15947 | −2.76 | 260.9975, 215.0058 | |
| 9 | santonin | 51.99 | C15H18O3 | [M+H]+ | 247.13287 | 0.04 | 229.0214, 201.0340, 183.0294, 172.9458, 158.8941 |
| Gene List | Sequence (5′-3′) |
|---|---|
| lncRNA H19 | Forward primer: CAGGTAGAGCGAGGTAAAGCA |
| Reverse primer: ACACCTGTCATCCTCGCCTT | |
| ABCG2 | Forward primer: GGCCTGGACAAAGTAGCAGA |
| Reverse primer: GTTGTGGGCTCATCCAGGAA | |
| GAPDH | Forward primer: GGTGGACCTCATGGCCTACA |
| Reverse primer: ATTGTGAGGGAGATCCTCAGTGT | |
| miR-21-3p | Stem-loop primer: GTCGTATCCAGTGCAGGGTCC GAGGTATTCGCACTGGATACGACGACAGC |
| Forward primer: CGCAACAGCAGTCGATGG | |
| U6 | Forward primer: CTCGCTTCGGCAGCACA |
| Reverse primer: AACGCTTCACGAATTTGCGT |
| Database | Website |
|---|---|
| BiBiServ2 | https://bibiserv.cebitec.uni-bielefeld.de/reputer, accessed on 15 February 2023 |
| lnclocater | http://www.csbio.sjtu.edu.cn/bioinf/lncLocator/, accessed on 15 February 2023 |
| LncATLAS | https://ngdc.cncb.ac.cn/databasecommons/database/id/6018, accessed on 15 February 2023 |
| TargetScan | https://www.targetscan.org/, accessed on 15 February 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, X.; Xu, Y.; Mao, Q.; Lu, C.; Yin, X.; Chen, S.; Zhang, B.; Lin, Z.; Wang, Y. Investigation of Pharmacological Mechanisms and Active Ingredients of Cichorium intybus L. in Alleviating Renal Urate Deposition via lncRNA H19/miR-21-3p Regulation to Enhance ABCG2 Expression. Int. J. Mol. Sci. 2025, 26, 7892. https://doi.org/10.3390/ijms26167892
An X, Xu Y, Mao Q, Lu C, Yin X, Chen S, Zhang B, Lin Z, Wang Y. Investigation of Pharmacological Mechanisms and Active Ingredients of Cichorium intybus L. in Alleviating Renal Urate Deposition via lncRNA H19/miR-21-3p Regulation to Enhance ABCG2 Expression. International Journal of Molecular Sciences. 2025; 26(16):7892. https://doi.org/10.3390/ijms26167892
Chicago/Turabian StyleAn, Xiaoye, Yi Xu, Qiuyue Mao, Chengjin Lu, Xiaoyang Yin, Siying Chen, Bing Zhang, Zhijian Lin, and Yu Wang. 2025. "Investigation of Pharmacological Mechanisms and Active Ingredients of Cichorium intybus L. in Alleviating Renal Urate Deposition via lncRNA H19/miR-21-3p Regulation to Enhance ABCG2 Expression" International Journal of Molecular Sciences 26, no. 16: 7892. https://doi.org/10.3390/ijms26167892
APA StyleAn, X., Xu, Y., Mao, Q., Lu, C., Yin, X., Chen, S., Zhang, B., Lin, Z., & Wang, Y. (2025). Investigation of Pharmacological Mechanisms and Active Ingredients of Cichorium intybus L. in Alleviating Renal Urate Deposition via lncRNA H19/miR-21-3p Regulation to Enhance ABCG2 Expression. International Journal of Molecular Sciences, 26(16), 7892. https://doi.org/10.3390/ijms26167892







