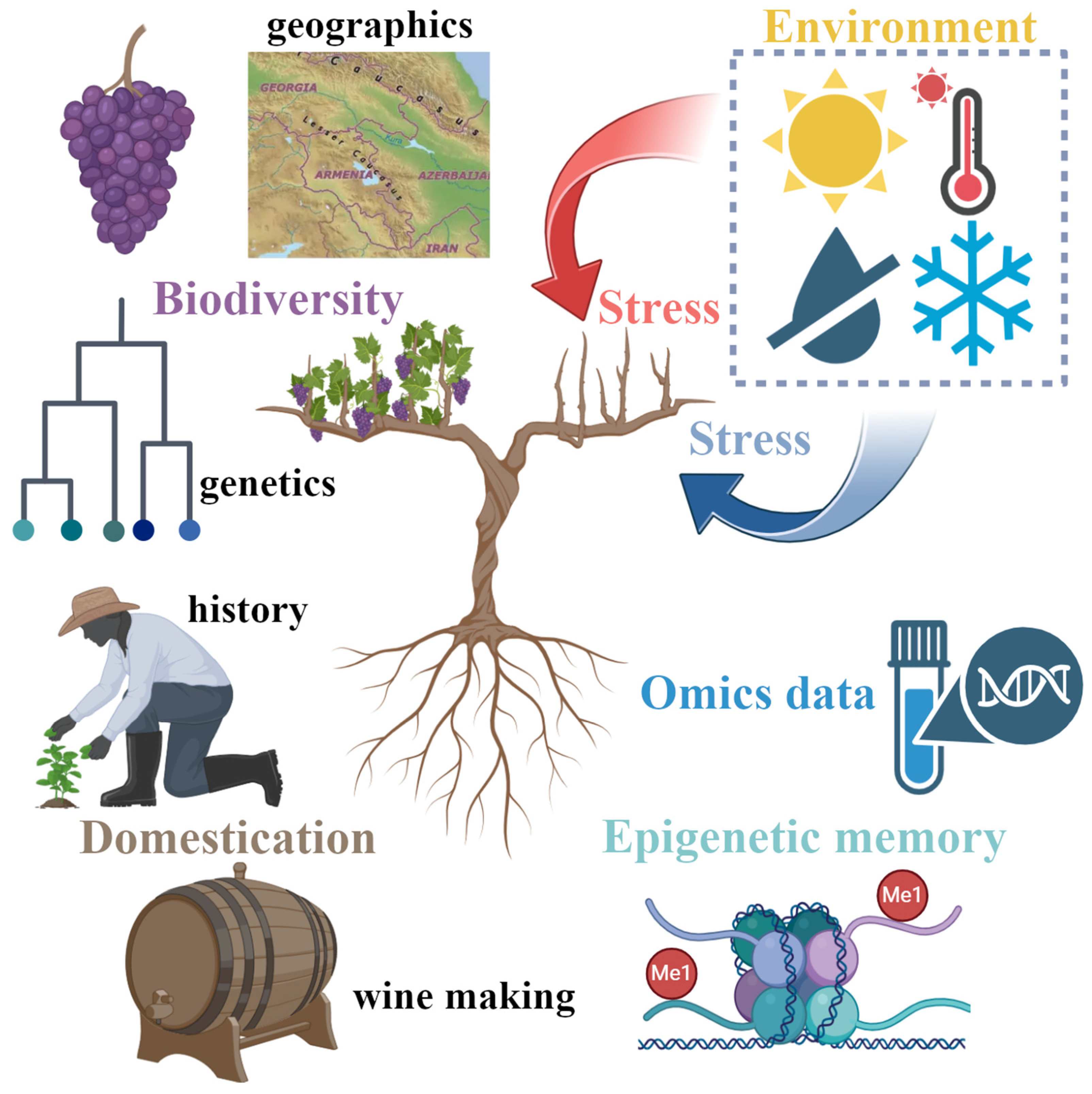
Responding to Stress: Diversity and Resilience of Grapevine in a Changing Climate Under the Perspective of Omics Research
Abstract
1. Introduction
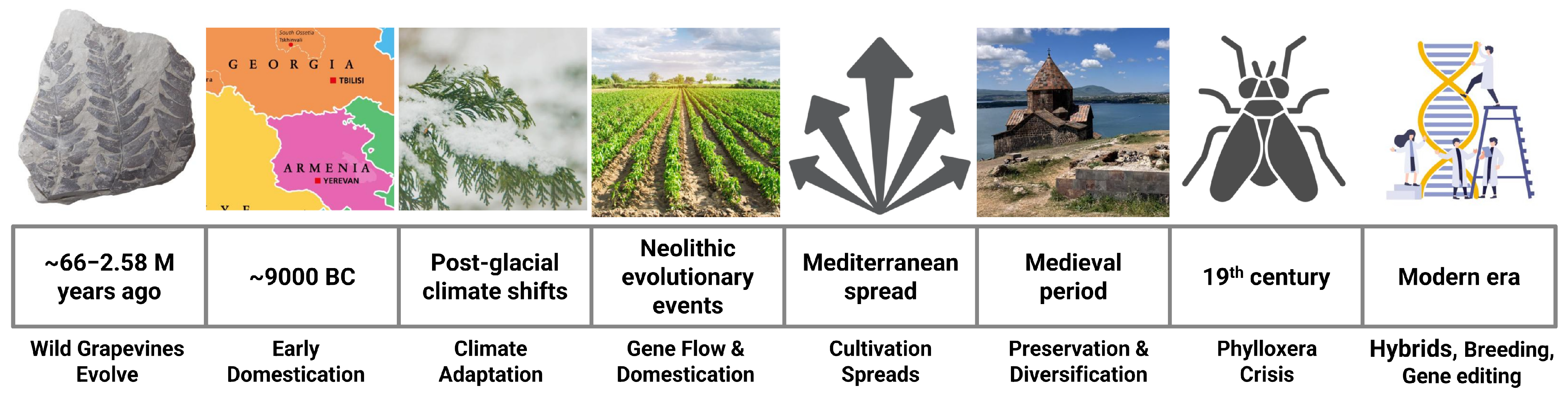
2. Grapevine Evolution, Domestication, and Diversity
2.1. Responses to Climate and Environmental Changes Across the Millennia
2.2. Genetic Diversity of Grapevines—A Reservoir for Stress Tolerance
2.3. Diversity Linked with Domestication Journey
2.4. Novel Perspectives: Caucasian Grapevines—Diverse but (Still) Understudied
3. Environmental Factors Causing Abiotic Stress in Grapevine
3.1. Heat and Drought
3.2. Cold and Freezing
3.3. UV Radiation and Soil Salinity
4. Omics Strategies for Abiotic Stress Resilience
4.1. Genetic Insights into Grapevine Stress Tolerance
4.2. Genome-Wide Association Studies and Beyond
4.3. Genomic and Transcriptomic Studies
4.4. Proteomics and Metabolomics
4.5. Integrative Omics and Meta-Analyses
5. Epigenetic Regulation in Grapevine
5.1. Epigenetic Mechanisms in Plants
5.2. Epigenetic Memory of Stress Responses
6. Viticulture in a Changing Climate: Challenges and Opportunities
6.1. Environmental Challenges and Local Adaptation Strategies
6.2. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABA | Abscisic Acid |
| Cas9 | CRISPR-associated protein 9 |
| CBF | C-repeat Binding Factor |
| COR | Cold-Responsive gene |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| GWASs | Genome-Wide Association Studies |
| HSP | Heat Shock Protein |
| ICE1 | Inducer of CBF Expression 1 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| RdDM | RNA-directed DNA Methylation |
| RNA-seq | RNA sequencing |
| ROS | Reactive Oxygen Species |
| SNP | Single-Nucleotide Polymorphism |
| TFs | Transcription Factors |
References
- Santos, R.B.; Figueiredo, A. Biotic and Abiotic Stress Management in Grapevine: Recent Advances and Major Breakthroughs. Agronomy 2023, 13, 1584. [Google Scholar] [CrossRef]
- Dai, Z.; Meddar, M.; Delrot, S.; Gomés, E. Development and Implementation of an in vitro Culture System for Intact Detached Grape Berries. Bio-Protocol 2015, 5, e1510. [Google Scholar] [CrossRef]
- Ren, C.; Mohamed, M.S.M.; Aini, N.; Kuang, Y.; Liang, Z. CRISPR/Cas in Grapevine Genome Editing: The Best Is Yet to Come. Horticulturae 2024, 10, 965. [Google Scholar] [CrossRef]
- Tyagi, A.; Mir, Z.A.; Almalki, M.A.; Deshmukh, R.; Ali, S. Genomics-Assisted Breeding: A Powerful Breeding Approach for Improving Plant Growth and Stress Resilience. Agronomy 2024, 14, 1128. [Google Scholar] [CrossRef]
- Bharati, R.; Sen, M.K.; Severová, L.; Svoboda, R.; Fernández-Cusimamani, E. Polyploidization and genomic selection integration for grapevine breeding: A perspective. Front. Plant Sci. 2023, 14, 1248978. [Google Scholar] [CrossRef]
- Savoi, S.; Santiago, A.; Orduña, L.; Matus, J.T. Transcriptomic and metabolomic integration as a resource in grapevine to study fruit metabolite quality traits. Front. Plant Sci. 2022, 13, 937927. [Google Scholar] [CrossRef]
- Daldoul, S.; Ben Amar, A.; Guillaumie, S.; Mliki, A. Integration of omics and system biology approaches to study grapevine (Vitis vinifera L.) response to salt stress: A perspective for functional genomics—A review. OENO One 2014, 48, 189. [Google Scholar] [CrossRef]
- Fabres, P.J.; Collins, C.; Cavagnaro, T.R.; Rodríguez López, C.M. A Concise Review on Multi-Omics Data Integration for Terroir Analysis in Vitis vinifera. Front. Plant Sci. 2017, 8, 1065. [Google Scholar] [CrossRef]
- Ghan, R.; Van Sluyter, S.C.; Hochberg, U.; Degu, A.; Hopper, D.W.; Tillet, R.L.; Schlauch, K.A.; Haynes, P.A.; Fait, A.; Cramer, G.R. Five omic technologies are concordant in differentiating the biochemical characteristics of the berries of five grapevine (Vitis vinifera L.) cultivars. BMC Genom. 2015, 16, 946. [Google Scholar] [CrossRef]
- Ren, C.; Li, Z.; Song, P.; Wang, Y.; Liu, W.; Zhang, L.; Li, X.; Li, W.; Han, D. Overexpression of a Grape MYB Transcription Factor Gene VhMYB2 Increases Salinity and Drought Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 10743. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, L.; Dai, J.; Li, Z.; Zhang, A.; Wang, T.; Liu, W.; Li, X.; Han, D. Overexpression of a Grape WRKY Transcription Factor VhWRKY44 Improves the Resistance to Cold and Salt of Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 7437. [Google Scholar] [CrossRef]
- Han, J.; Dai, J.; Chen, Z.; Li, W.; Li, X.; Zhang, L.; Yao, A.; Zhang, B.; Han, D. Overexpression of a ‘Beta’ MYB Factor Gene, VhMYB15, Increases Salinity and Drought Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 1534. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.; Wang, Y.; Liang, X.; Han, J.; Han, D. MbMYBC1, a M. baccata MYB transcription factor, contribute to cold and drought stress tolerance in transgenic Arabidopsis. Front. Plant Sci. 2023, 14, 1141446. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, P.; Chen, H.; Zhong, J.; Liang, X.; Wei, Y.; Zhang, L.; Wang, H.; Han, D. Overexpression of a Fragaria vesca 1R-MYB Transcription Factor Gene (FvMYB114) Increases Salt and Cold Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 5261. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wei, Y.; Zhang, L.; Wang, Y.; Song, P.; Li, X.; Han, D. FvMYB44, a Strawberry R2R3-MYB Transcription Factor, Improved Salt and Cold Stress Tolerance in Transgenic Arabidopsis. Agronomy 2023, 13, 1051. [Google Scholar] [CrossRef]
- Grassi, F.; De Lorenzis, G. Back to the Origins: Background and Perspectives of Grapevine Domestication. Int. J. Mol. Sci. 2021, 22, 4518. [Google Scholar] [CrossRef]
- Riaz, S.; De Lorenzis, G.; Velasco, D.; Koehmstedt, A.; Maghradze, D.; Bobokashvili, Z.; Musayev, M.; Zdunic, G.; Laucou, V.; Andrew Walker, M.; et al. Genetic diversity analysis of cultivated and wild grapevine (Vitis vinifera L.) accessions around the Mediterranean basin and Central Asia. BMC Plant Biol. 2018, 18, 137. [Google Scholar] [CrossRef]
- Guzmán-Ardiles, R.E.; Pegoraro, C.; Da Maia, L.C.; Costa De Oliveira, A. Genetic changes in the genus Vitis and the domestication of vine. Front. Plant Sci. 2023, 13, 1019311. [Google Scholar] [CrossRef]
- Dong, Y.; Duan, S.; Xia, Q.; Liang, Z.; Dong, X.; Margaryan, K.; Musayev, M.; Goryslavets, S.; Zdunić, G.; Bert, P.-F.; et al. Dual domestications and origin of traits in grapevine evolution. Science 2023, 379, 892–901. [Google Scholar] [CrossRef]
- Pambianchi, G.; Gentilucci, M. Hystory of viticulture in relation to climate change (from Neolithic to the fall of the Roman Empire). Catena 2024, 247, 108528. [Google Scholar] [CrossRef]
- Zhou, Y.; Massonnet, M.; Sanjak, J.S.; Cantu, D.; Gaut, B.S. Evolutionary genomics of grape (Vitis vinifera ssp. vinifera) domestication. Proc. Natl. Acad. Sci. USA 2017, 114, 11715–11720. [Google Scholar] [CrossRef]
- Ramos-Madrigal, J.; Runge, A.K.W.; Bouby, L.; Lacombe, T.; Samaniego Castruita, J.A.; Adam-Blondon, A.-F.; Figueiral, I.; Hallavant, C.; Martínez-Zapater, J.M.; Schaal, C.; et al. Palaeogenomic insights into the origins of French grapevine diversity. Nat. Plants 2019, 5, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Magris, G.; Jurman, I.; Fornasiero, A.; Paparelli, E.; Schwope, R.; Marroni, F.; Di Gaspero, G.; Morgante, M. The genomes of 204 Vitis vinifera accessions reveal the origin of European wine grapes. Nat. Commun. 2021, 12, 7240. [Google Scholar] [CrossRef] [PubMed]
- Terral, J.-F.; Tabard, E.; Bouby, L.; Ivorra, S.; Pastor, T.; Figueiral, I.; Picq, S.; Chevance, J.-B.; Jung, C.; Fabre, L.; et al. Evolution and history of grapevine (Vitis vinifera) under domestication: New morphometric perspectives to understand seed domestication syndrome and reveal origins of ancient European cultivars. Ann. Bot. 2010, 105, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Barnard, H.; Dooley, A.N.; Areshian, G.; Gasparyan, B.; Faull, K.F. Chemical evidence for wine production around 4000 BCE in the Late Chalcolithic Near Eastern highlands. J. Archaeol. Sci. 2011, 38, 977–984. [Google Scholar] [CrossRef]
- McGovern, P.; Jalabadze, M.; Batiuk, S.; Callahan, M.P.; Smith, K.E.; Hall, G.R.; Kvavadze, E.; Maghradze, D.; Rusishvili, N.; Bouby, L.; et al. Early Neolithic wine of Georgia in the South Caucasus. Proc. Natl. Acad. Sci. USA 2017, 114, E10309–E10318. [Google Scholar] [CrossRef]
- Bacilieri, R.; Lacombe, T.; Le Cunff, L.; Di Vecchi-Staraz, M.; Laucou, V.; Genna, B.; Péros, J.-P.; This, P.; Boursiquot, J.-M. Genetic structure in cultivated grapevines is linked to geography and human selection. BMC Plant Biol. 2013, 13, 25. [Google Scholar] [CrossRef]
- Granett, J.; Walker, M.A.; Kocsis, L.; Omer, A.D. Biology and Management of Grape Phylloxera. Annu. Rev. Entomol. 2001, 46, 387–412. [Google Scholar] [CrossRef]
- Yobrégat, O. Introduction to resistant vine types: A brief history and overview of the situation. OENO One 2018, 52, 241–246. [Google Scholar] [CrossRef]
- Klein, L.L.; Miller, A.J.; Ciotir, C.; Hyma, K.; Uribe-Convers, S.; Londo, J. High-throughput sequencing data clarify evolutionary relationships among North American Vitis species and improve identification in USDA Vitis germplasm collections. Am. J. Bot. 2018, 105, 215–226. [Google Scholar] [CrossRef]
- Roviello, V.; Caruso, U.; Dal Poggetto, G.; Naviglio, D. Hybrid Grapes for a Sustainable Viticulture in South Italy: Parentage Diagram Analysis and Metal Assessment in a Homemade Wine of Chambourcin Cultivar. Sustainability 2021, 13, 12472. [Google Scholar] [CrossRef]
- Wan, D.-Y.; Guo, Y.; Cheng, Y.; Hu, Y.; Xiao, S.; Wang, Y.; Wen, Y.-Q. CRISPR/Cas9-mediated mutagenesis of VvMLO3 results in enhanced resistance to powdery mildew in grapevine (Vitis vinifera). Hortic. Res. 2020, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; De Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Margaryan, K.; Töpfer, R.; Gasparyan, B.; Arakelyan, A.; Trapp, O.; Röckel, F.; Maul, E. Wild grapes of Armenia: Unexplored source of genetic diversity and disease resistance. Front. Plant Sci. 2023, 14, 1276764. [Google Scholar] [CrossRef]
- This, P.; Lacombe, T.; Thomas, M. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef]
- Fedosov, D.Y.; Korzhenkov, A.A.; Petrova, K.O.; Sapsay, A.O.; Sharko, F.S.; Toshchakov, S.V.; Kolosova, A.A.; Bakhmutova, E.D.; Patrushev, M.V. SNP-Based Analysis Reveals Authenticity and Genetic Similarity of Russian Indigenous V. vinifera Grape Cultivars. Plants 2021, 10, 2696. [Google Scholar] [CrossRef]
- Belkina, D.; Stepanov, I.; Makarkina, M.; Porotikova, E.; Lifanov, I.; Kozhevnikov, E.; Gorislavets, S.; Vinogradova, S. In-depth population genetic study of Vitis vinifera ssp. sylvestris from the Black Sea region and its virome. Front. Plant Sci. 2025, 16, 1536862. [Google Scholar] [CrossRef]
- Daldoul, S.; Khattab, I.M.; Hanzouli, F.; Bahlouli, I.; Nick, P.; Mliki, A.; Gargouri, M. Help from the past to cope with the future: Vitis sylvestris as a resource for abiotic stress resilience. Plants People Planet 2025, ppp3.70034. [Google Scholar] [CrossRef]
- Villano, C.; Aiese Cigliano, R.; Esposito, S.; D’Amelia, V.; Iovene, M.; Carputo, D.; Aversano, R. DNA-Based Technologies for Grapevine Biodiversity Exploitation: State of the Art and Future Perspectives. Agronomy 2022, 12, 491. [Google Scholar] [CrossRef]
- Buck, K.; Worthington, M. Genetic Diversity of Wild and Cultivated Muscadine Grapes (Vitis rotundifolia Michx.). Front. Plant Sci. 2022, 13, 852130. [Google Scholar] [CrossRef]
- Myles, S.; Boyko, A.R.; Owens, C.L.; Brown, P.J.; Grassi, F.; Aradhya, M.K.; Prins, B.; Reynolds, A.; Chia, J.-M.; Ware, D.; et al. Genetic structure and domestication history of the grape. Proc. Natl. Acad. Sci. USA 2011, 108, 3530–3535. [Google Scholar] [CrossRef]
- Lijavetzky, D.; Ruiz-García, L.; Cabezas, J.A.; De Andrés, M.T.; Bravo, G.; Ibáñez, A.; Carreño, J.; Cabello, F.; Ibáñez, J.; Martínez-Zapater, J.M. Molecular genetics of berry colour variation in table grape. Mol. Genet. Genom. 2006, 276, 427–435. [Google Scholar] [CrossRef]
- Myles, S. Improving fruit and wine: What does genomics have to offer? Trends Genet. 2013, 29, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Velt, A.; Frommer, B.; Blanc, S.; Holtgräwe, D.; Duchêne, É.; Dumas, V.; Grimplet, J.; Hugueney, P.; Kim, C.; Lahaye, M.; et al. An improved reference of the grapevine genome reasserts the origin of the PN40024 highly homozygous genotype. G3 Genes Genomes Genet. 2023, 13, jkad067. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Cao, S.; Wang, X.; Huang, S.; Wang, Y.; Liu, Z.; Liu, W.; Leng, X.; Peng, Y.; Wang, N.; et al. The complete reference genome for grapevine (Vitis vinifera L.) genetics and breeding. Hortic. Res. 2023, 10, uhad061. [Google Scholar] [CrossRef] [PubMed]
- Onetto, C.A.; Ward, C.M.; Borneman, A.R. The Genome Assembly of Vitis vinifera cv. Shiraz. Aust. J. Grape Wine Res. 2023, 2023, 6686706. [Google Scholar] [CrossRef]
- Cheng, G.; Wu, D.; Guo, R.; Li, H.; Wei, R.; Zhang, J.; Wei, Z.; Meng, X.; Yu, H.; Xie, L.; et al. Chromosome-scale genomics, metabolomics, and transcriptomics provide insight into the synthesis and regulation of phenols in Vitis adenoclada grapes. Front. Plant Sci. 2023, 14, 1124046. [Google Scholar] [CrossRef]
- Zdunić, G.; Lukšić, K.; Nagy, Z.A.; Mucalo, A.; Hančević, K.; Radić, T.; Butorac, L.; Jahnke, G.G.; Kiss, E.; Ledesma-Krist, G.; et al. Genetic Structure and Relationships among Wild and Cultivated Grapevines from Central Europe and Part of the Western Balkan Peninsula. Genes 2020, 11, 962. [Google Scholar] [CrossRef]
- Maghradze, D.; Aslanishvili, A.; Mdinaradze, I.; Tkemaladze, D.; Mekhuzla, L.; Lordkipanidze, D.; Jalabadze, M.; Kvavadze, E.; Rusishvili, N.; McGovern, P.; et al. Progress for research of grape and wine culture in Georgia, the South Caucasus. BIO Web Conf. 2019, 12, 03003. [Google Scholar] [CrossRef]
- Bouby, L.; Wales, N.; Jalabadze, M.; Rusishvili, N.; Bonhomme, V.; Ramos-Madrigal, J.; Evin, A.; Ivorra, S.; Lacombe, T.; Pagnoux, C.; et al. Tracking the history of grapevine cultivation in Georgia by combining geometric morphometrics and ancient DNA. Veget. Hist. Archaeobot. 2021, 30, 63–76. [Google Scholar] [CrossRef]
- Maghradze, D.; Kikilashvili, S.; Gotsiridze, O.; Maghradze, T.; Fracassetti, D.; Failla, O.; Rustioni, L. Comparison between the Grape Technological Characteristics of Vitis vinifera Subsp. sylvestris and Subsp. sativa. Agronomy 2021, 11, 472. [Google Scholar] [CrossRef]
- Sargolzaei, M.; Rustioni, L.; Cola, G.; Ricciardi, V.; Bianco, P.A.; Maghradze, D.; Failla, O.; Quaglino, F.; Toffolatti, S.L.; De Lorenzis, G. Georgian Grapevine Cultivars: Ancient Biodiversity for Future Viticulture. Front. Plant Sci. 2021, 12, 630122. [Google Scholar] [CrossRef]
- De Lorenzis, G.; Chipashvili, R.; Failla, O.; Maghradze, D. Study of genetic variability in Vitis vinifera L. germplasm by high-throughput Vitis18kSNP array: The case of Georgian genetic resources. BMC Plant Biol. 2015, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, M.; Esoyan, S.; Gasparyan, B.; Smith, A.; Hovhannisyan, N. Genetic diversity and traditional uses of aboriginal grape (Vitis vinifera L.) varieties from the main viticultural regions of Armenia. Genet. Resour. Crop Evol. 2020, 67, 999–1024. [Google Scholar] [CrossRef]
- Margaryan, K.; Gasparyan, B.; Prtrosyan, A.; Harutyunyan, F.; Töpfer, R.; Maul, E. Grapevine genetic resources of Armenia: Molecular fingerprinting and phylogenetic relationship among wild and cultivated grapevine. VITIS J. Grapevine Res. 2023, 62, 11–22. [Google Scholar] [CrossRef]
- Magaryan, K.; Nikogհosyan, M.; Baloyan, A.; Gasoyan, H.; Hovhannisyan, E.; Galstyan, L.; Konecny, T.; Arakelyan, A.; Binder, H. Machine learned-based visualization of the diversity of grapevine genomes worldwide and in Armenia using SOMmelier. BIO Web Conf. 2023, 68, 01009. [Google Scholar] [CrossRef]
- Nikoghosyan, M.; Schmidt, M.; Margaryan, K.; Loeffler-Wirth, H.; Arakelyan, A.; Binder, H. SOMmelier—Intuitive Visualization of the Topology of Grapevine Genome Landscapes Using Artificial Neural Networks. Genes 2020, 11, 817. [Google Scholar] [CrossRef]
- Wirth, H.; Löffler, M.; von Bergen, M.; Binder, H. Expression cartography of human tissues using self organizing maps. BMC Bioinform. 2011, 12, 306. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Future scenarios for viticultural zoning in Europe: Ensemble projections and uncertainties. Int. J. Biometeorol. 2013, 57, 909–925. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef]
- Keller, M. Climate Change Impacts on Vineyards in Warm and Dry Areas: Challenges and Opportunities: From the ASEV Climate Change Symposium Part 1—Viticulture. Am. J. Enol. Vitic. 2023, 74, 0740033. [Google Scholar] [CrossRef]
- Naulleau, A.; Gary, C.; Prévot, L.; Hossard, L. Evaluating Strategies for Adaptation to Climate Change in Grapevine Production–A Systematic Review. Front. Plant Sci. 2021, 11, 607859. [Google Scholar] [CrossRef] [PubMed]
- de Orduña, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Chaves, M.M. How Plants Cope with Water Stress in the Field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Sharma, M.; Negi, S.; Kumar, P.; Srivastava, D.K.; Choudhary, M.K.; Irfan, M. Fruit ripening under heat stress: The intriguing role of ethylene-mediated signaling. Plant Sci. 2023, 335, 111820. [Google Scholar] [CrossRef]
- Rehman, S.; Ahmad, Z.; Ramakrishnan, M.; Kalendar, R.; Zhuge, Q. Regulation of plant epigenetic memory in response to cold and heat stress: Towards climate resilient agriculture. Funct. Integr. Genom. 2023, 23, 298. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Barril, C. Impact of short temperature exposure of Vitis vinifera L. cv. Shiraz grapevine bunches on berry development, primary metabolism and tannin accumulation. Environ. Exp. Bot. 2019, 168, 103866. [Google Scholar] [CrossRef]
- Hendrickson, L.; Ball, M.C.; Wood, J.T.; Chow, W.S.; Furbank, R.T. Low temperature effects on photosynthesis and growth of grapevine. Plant Cell Environ. 2004, 27, 795–809. [Google Scholar] [CrossRef]
- Schultz, H.R. Global Climate Change, Sustainability, and Some Challenges for Grape and Wine Production. J. Wine Econ. 2016, 11, 181–200. [Google Scholar] [CrossRef]
- Ren, C.; Fan, P.; Li, S.; Liang, Z. Advances in understanding cold tolerance in grapevine. Plant Physiol. 2023, 192, 1733–1746. [Google Scholar] [CrossRef]
- Londo, J.P.; Kovaleski, A.P.; Lillis, J.A. Divergence in the transcriptional landscape between low temperature and freeze shock in cultivated grapevine (Vitis vinifera). Hortic. Res. 2018, 5, 1–14. [Google Scholar] [CrossRef]
- Savoi, S.; Herrera, J.C.; Carlin, S.; Lotti, C.; Bucchetti, B.; Peterlunger, E.; Castellarin, S.D.; Mattivi, F. From grape berries to wines: Drought impacts on key secondary metabolites. OENO One 2020, 54, 569–582. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR Signaling Cascade and Its Regulation in Plants Responding to Cold Stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef]
- Lv, K.; Li, J.; Zhao, K.; Chen, S.; Nie, J.; Zhang, W.; Liu, G.; Wei, H. Overexpression of an AP2/ERF family gene, BpERF13, in birch enhances cold tolerance through upregulating CBF genes and mitigating reactive oxygen species. Plant Sci. 2020, 292, 110375. [Google Scholar] [CrossRef] [PubMed]
- Kovaleski, A.P.; Londo, J.P. Tempo of gene regulation in wild and cultivated Vitis species shows coordination between cold deacclimation and budbreak. Plant Sci. 2019, 287, 110178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple Functions of MYB Transcription Factors in Abiotic Stress Responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Zhong, H.; Patel, M.K.; Zhang, S.; Zhou, X.; Zhang, C.; Zhang, J.; Su, J.; Zhang, F.; Wu, X. Integrated omics-based exploration for temperature stress resilience: An approach to smart grape breeding strategies. Plant Stress 2024, 11, 100356. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Wilson, R.J.; Maclean, I.M.D. Climate Change and Crop Exposure to Adverse Weather: Changes to Frost Risk and Grapevine Flowering Conditions. PLoS ONE 2015, 10, e0141218. [Google Scholar] [CrossRef]
- Ferguson, J.C.; Tarara, J.M.; Mills, L.J.; Grove, G.G.; Keller, M. Dynamic thermal time model of cold hardiness for dormant grapevine buds. Ann. Bot. 2011, 107, 389–396. [Google Scholar] [CrossRef]
- Poni, S.; Sabbatini, P.; Palliotti, A. Facing Spring Frost Damage in Grapevine: Recent Developments and the Role of Delayed Winter Pruning—A Review. Am. J. Enol. Vitic. 2022, 73, 211–226. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Torres, N.; Hilbert, G.; Richard, T.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomès, E. Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries. Phytochemistry 2014, 102, 106–114. [Google Scholar] [CrossRef]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Correll, R.L. Rootstock effects on salt tolerance of irrigated field-grown grapevines (Vitis vinifera L. cv. Sultana): 1. Yield and vigour inter-relationships. Aust. J. Grape Wine Res. 2002, 8, 3–14. [Google Scholar] [CrossRef]
- Prinsi, B.; Simeoni, F.; Galbiati, M.; Meggio, F.; Tonelli, C.; Scienza, A.; Espen, L. Grapevine Rootstocks Differently Affect Physiological and Molecular Responses of the Scion under Water Deficit Condition. Agronomy 2021, 11, 289. [Google Scholar] [CrossRef]
- Keller, M. The Science of Grapevines: Anatomy and Physiology; Elsevier Science & Technology: Burlington, MA, USA, 2010; ISBN 978-0-08-089048-7. [Google Scholar]
- Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; García-Navarro, F.J.; Jiménez-Ballesta, R. Effects of Water Stress on Vegetative Growth and ‘Merlot’ Grapevine Yield in a Semi-Arid Mediterranean Climate. Horticulturae 2020, 6, 95. [Google Scholar] [CrossRef]
- Pastore, C.; Frioni, T.; Diago, M.P. Editorial: Resilience of grapevine to climate change: From plant physiology to adaptation strategies. Front. Plant Sci. 2022, 13, 994267. [Google Scholar] [CrossRef] [PubMed]
- Frioni, T.; Pastore, C.; Diago, M.P. Editorial: Resilience of grapevine to climate change: From plant physiology to adaptation strategies, volume II. Front. Plant Sci. 2023, 14, 1268158. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Keller, M.; Stoll, M.; Friedel, M. Wind speed, sun exposure and water status alter sunburn susceptibility of grape berries. Front. Plant Sci. 2023, 14, 1145274. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef]
- Liu, G.-T.; Wang, J.-F.; Cramer, G.; Dai, Z.-W.; Duan, W.; Xu, H.-G.; Wu, B.-H.; Fan, P.-G.; Wang, L.-J.; Li, S.-H. Transcriptomic analysis of grape (Vitis vinifera L.) leaves during and after recovery from heat stress. BMC Plant Biol. 2012, 12, 174. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2006, 58, 221–227. [Google Scholar] [CrossRef]
- Grimplet, J.; Wheatley, M.D.; Jouira, H.B.; Deluc, L.G.; Cramer, G.R.; Cushman, J.C. Proteomic and selected metabolite analysis of grape berry tissues under well-watered and water-deficit stress conditions. Proteomics 2009, 9, 2503–2528. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, S.; Fang, X.; Ren, Y.; You, Z.; Xia, J.; Hakeem, A.; Yang, Y.; Wang, L.; Fang, J.; et al. The physiology of drought stress in two grapevine cultivars: Photosynthesis, antioxidant system, and osmotic regulation responses. Physiol. Plant. 2023, 175, e14005. [Google Scholar] [CrossRef] [PubMed]
- Fennell, A. Freezing Tolerance and Injury in Grapevines. J. Crop Improv. 2004, 10, 201–235. [Google Scholar] [CrossRef]
- Nenko, N.I.; Ilyina, I.A.; Kiseleva, G.K.; Yablonskaya, E.K. Low-Temperature Stress Tolerance of Grapevine Varieties of Different Ecological and Geographical Origin. Proc. Latv. Acad. Sci. Sect. B. Nat. Exact Appl. Sci. 2019, 73, 56–65. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Dong, F.; Gao, J.; Galbraith, D.W.; Song, C.-P. Hydrogen Peroxide Is Involved in Abscisic Acid-Induced Stomatal Closure in Vicia faba. Plant Physiol. 2001, 126, 1438–1448. [Google Scholar] [CrossRef]
- Thomashow, M.F. Molecular Basis of Plant Cold Acclimation: Insights Gained from Studying the CBF Cold Response Pathway. Plant Physiol. 2010, 154, 571–577. [Google Scholar] [CrossRef]
- Henderson, S.W.; Baumann, U.; Blackmore, D.H.; Walker, A.R.; Walker, R.R.; Gilliham, M. Shoot chloride exclusion and salt tolerance in grapevine is associated with differential ion transporter expression in roots. BMC Plant Biol. 2014, 14, 273. [Google Scholar] [CrossRef]
- Lu, X.; Ma, L.; Zhang, C.; Yan, H.; Bao, J.; Gong, M.; Wang, W.; Li, S.; Ma, S.; Chen, B. Grapevine (Vitis vinifera) responses to salt stress and alkali stress: Transcriptional and metabolic profiling. BMC Plant Biol. 2022, 22, 528. [Google Scholar] [CrossRef]
- Lu, X.; Chen, G.; Ma, L.; Yan, H.; Zhang, C.; Nai, G.; Bao, J.; Liu, Y.; Lai, Y.; Li, S.; et al. Abscisic acid enhances alkaline stress tolerance in grapevines: Physiological and transcriptional profiling. Sci. Hortic. 2024, 336, 113368. [Google Scholar] [CrossRef]
- Bassil, E.; Coku, A.; Blumwald, E. Cellular ion homeostasis: Emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J. Exp. Bot. 2012, 63, 5727–5740. [Google Scholar] [CrossRef] [PubMed]
- Scholasch, T.; Rienth, M. Review of water deficit mediated changes in vine and berry physiology; Consequences for the optimization of irrigation strategies. OENO One 2019, 53, 423–444. [Google Scholar] [CrossRef]
- Dai, Z.W.; Meddar, M.; Renaud, C.; Merlin, I.; Hilbert, G.; Delrot, S.; Gomès, E. Long-term in vitro culture of grape berries and its application to assess the effects of sugar supply on anthocyanin accumulation. J. Exp. Bot. 2013, 65, 4665–4677. [Google Scholar] [CrossRef] [PubMed]
- Feechan, A.; Anderson, C.; Torregrosa, L.; Jermakow, A.; Mestre, P.; Wiedemann-Merdinoglu, S.; Merdinoglu, D.; Walker, A.R.; Cadle-Davidson, L.; Reisch, B.; et al. Genetic dissection of a TIR-NB-LRR locus from the wild North American grapevine species Muscadinia rotundifolia identifies paralogous genes conferring resistance to major fungal and oomycete pathogens in cultivated grapevine. Plant J. 2013, 76, 661–674. [Google Scholar] [CrossRef]
- Barker, C.L.; Donald, T.; Pauquet, J.; Ratnaparkhe, M.B.; Bouquet, A.; Adam-Blondon, A.-F.; Thomas, M.R.; Dry, I. Genetic and physical mapping of the grapevine powdery mildew resistance gene, Run1, using a bacterial artificial chromosome library. Theor. Appl. Genet. 2005, 111, 370–377. [Google Scholar] [CrossRef]
- The French–Italian Public Consortium for Grapevine Genome Characterization The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [CrossRef]
- Razalli, I.I.; Abdullah-Zawawi, M.-R.; Tamizi, A.-A.; Harun, S.; Zainal-Abidin, R.-A.; Jalal, M.I.A.; Ullah, M.A.; Zainal, Z. Accelerating crop improvement via integration of transcriptome-based network biology and genome editing. Planta 2025, 261, 92. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Luchaire, N.; Chatbanyong, R.; Lecourieux, D.; Kelly, M.T.; Romieu, C. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (Vitis vinifera) fruit. BMC Plant Biol. 2014, 14, 108. [Google Scholar] [CrossRef]
- Cramer, G.R.; Ergül, A.; Grimplet, J.; Tillett, R.L.; Tattersall, E.A.R.; Bohlman, M.C.; Vincent, D.; Sonderegger, J.; Evans, J.; Osborne, C.; et al. Water and salinity stress in grapevines: Early and late changes in transcript and metabolite profiles. Funct. Integr. Genom. 2007, 7, 111–134. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, N.; Su, Y.; Long, Q.; Peng, Y.; Shangguan, L.; Zhang, F.; Cao, S.; Wang, X.; Ge, M.; et al. Grapevine pangenome facilitates trait genetics and genomic breeding. Nat. Genet. 2024, 56, 2804–2814. [Google Scholar] [CrossRef]
- D’Onofrio, C. Introgression Among Cultivated and Wild Grapevine in Tuscany. Front. Plant Sci. 2020, 11, 202. [Google Scholar] [CrossRef]
- Flutre, T.; Le Cunff, L.; Fodor, A.; Launay, A.; Romieu, C.; Berger, G.; Bertrand, Y.; Terrier, N.; Beccavin, I.; Bouckenooghe, V.; et al. A genome-wide association and prediction study in grapevine deciphers the genetic architecture of multiple traits and identifies genes under many new QTLs. G3 Genes Genomes Genet. 2022, 12, jkac103. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Torres, N.; Tanner, J.D.; Kacur, S.M.; Marigliano, L.E.; Zumkeller, M.; Gilmer, J.C.; Gambetta, G.A.; Kurtural, S.K. Adapting wine grape production to climate change through canopy architecture manipulation and irrigation in warm climates. Front. Plant Sci. 2022, 13, 1015574. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, L.; Fang, X.; Chen, L.; Cui, L.; Fang, J. Genome-wide analysis of autophagy-related genes (ARGs) in grapevine and plant tolerance to copper stress. Planta 2018, 247, 1449–1463. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, C.; Xuan, W.; An, H.; Tian, Y.; Wang, B.; Chi, W.; Chen, G.; Ge, Y.; Li, J.; et al. Genome-wide association studies identify OsWRKY53 as a key regulator of salt tolerance in rice. Nat. Commun. 2023, 14, 3550. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Y.; Zhao, H.; Guo, L.; Guo, Y.; Liu, C.-M. Genome-wide association studies identified OsTMF as a gene regulating rice seed germination under salt stress. Front. Plant Sci. 2024, 15, 1384246. [Google Scholar] [CrossRef]
- Tello, J.; Ibáñez, J. Review: Status and prospects of association mapping in grapevine. Plant Sci. 2023, 327, 111539. [Google Scholar] [CrossRef]
- Butiuc-Keul, A.; Coste, A. Biotechnologies and Strategies for Grapevine Improvement. Horticulturae 2023, 9, 62. [Google Scholar] [CrossRef]
- Marrano, A.; Micheletti, D.; Lorenzi, S.; Neale, D.; Grando, M.S. Genomic signatures of different adaptations to environmental stimuli between wild and cultivated Vitis vinifera L. Hortic. Res. 2018, 5, 34. [Google Scholar] [CrossRef]
- Coupel-Ledru, A.; Westgeest, A.J.; Albasha, R.; Millan, M.; Pallas, B.; Doligez, A.; Flutre, T.; Segura, V.; This, P.; Torregrosa, L.; et al. Clusters of grapevine genes for a burning world. New Phytol. 2024, 242, 10–18. [Google Scholar] [CrossRef]
- Liang, G.; He, H.; Nai, G.; Feng, L.; Li, Y.; Zhou, Q.; Ma, Z.; Yue, Y.; Chen, B.; Mao, J. Genome-wide identification of BAM genes in grapevine (Vitis vinifera L.) and ectopic expression of VvBAM1 modulating soluble sugar levels to improve low-temperature tolerance in tomato. BMC Plant Biol. 2021, 21, 156. [Google Scholar] [CrossRef]
- Li, M.-Y.; Jiao, Y.-T.; Wang, Y.-T.; Zhang, N.; Wang, B.-B.; Liu, R.-Q.; Yin, X.; Xu, Y.; Liu, G.-T. CRISPR/Cas9-mediated VvPR4b editing decreases downy mildew resistance in grapevine (Vitis vinifera L.). Hortic. Res. 2020, 7, 149. [Google Scholar] [CrossRef]
- Luca, L.P.; Guardo, M.D.; Bennici, S.; Ferlito, F.; Nicolosi, E.; La Malfa, S.; Gentile, A.; Distefano, G. Development of an efficient molecular-marker assisted selection strategy for berry color in grapevine. Sci. Hortic. 2024, 337, 113522. [Google Scholar] [CrossRef]
- Possamai, T.; Scota, L.; Velasco, R.; Migliaro, D. A Sustainable Strategy for Marker-Assisted Selection (MAS) Applied in Grapevine (Vitis spp.) Breeding for Resistance to Downy (Plasmopara Viticola) and Powdery (Erysiphe Necator) Mildews. Plants 2024, 13, 2001. [Google Scholar] [CrossRef] [PubMed]
- Brault, C.; Segura, V.; Roques, M.; Lamblin, P.; Bouckenooghe, V.; Pouzalgues, N.; Cunty, C.; Breil, M.; Frouin, M.; Garcin, L.; et al. Enhancing grapevine breeding efficiency through genomic prediction and selection index. G3 Genes Genomes Genet. 2024, 14, jkae038. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Gaonkar, T.; Upadhyay, A.K.; Jogaiah, S.; Shinde, M.P.; Kadoo, N.Y.; Gupta, V.S. Global transcriptome analysis of grapevine (Vitis vinifera L.) leaves under salt stress reveals differential response at early and late stages of stress in table grape cv. Thompson Seedless. Plant Physiol. Biochem. 2018, 129, 168–179. [Google Scholar] [CrossRef]
- Vannozzi, A.; Wong, D.C.J.; Höll, J.; Hmmam, I.; Matus, J.T.; Bogs, J.; Ziegler, T.; Dry, I.; Barcaccia, G.; Lucchin, M. Combinatorial Regulation of Stilbene Synthase Genes by WRKY and MYB Transcription Factors in Grapevine (Vitis vinifera L.). Plant Cell Physiol. 2018, 59, 1043–1059. [Google Scholar] [CrossRef]
- Ogundipe, D.S. Developing drought and heat-resistant grapevine cultivars through marker-assisted breeding. Int. J. Sci. Res. Arch. 2024, 13, 1011–1023. [Google Scholar] [CrossRef]
- Delrot, S.; Grimplet, J.; Carbonell-Bejerano, P.; Schwandner, A.; Bert, P.-F.; Bavaresco, L.; Costa, L.D.; Di Gaspero, G.; Duchêne, E.; Hausmann, L.; et al. Genetic and Genomic Approaches for Adaptation of Grapevine to Climate Change. In Genomic Designing of Climate-Smart Fruit Crops; Kole, C., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 157–270. [Google Scholar] [CrossRef]
- Rurek, M.; Smolibowski, M. Variability of plant transcriptomic responses under stress acclimation: A review from high throughput studies. Acta Biochim. Pol. 2024, 71, 13585. [Google Scholar] [CrossRef]
- Dal Santo, S.; Palliotti, A.; Zenoni, S.; Tornielli, G.B.; Fasoli, M.; Paci, P.; Tombesi, S.; Frioni, T.; Silvestroni, O.; Bellincontro, A.; et al. Distinct transcriptome responses to water limitation in isohydric and anisohydric grapevine cultivars. BMC Genom. 2016, 17, 815. [Google Scholar] [CrossRef]
- Xu, X.; Fonseca De Lima, C.F.; Vu, L.D.; De Smet, I. When drought meets heat—A plant omics perspective. Front. Plant Sci. 2023, 14, 1250878. [Google Scholar] [CrossRef]
- Gomès, É.; Maillot, P.; Duchêne, É. Molecular Tools for Adapting Viticulture to Climate Change. Front. Plant Sci. 2021, 12, 633846. [Google Scholar] [CrossRef]
- Konecny, T.; Asatryan, A.; Nikoghosyan, M.; Binder, H. Unveiling Iso- and Aniso-Hydric Disparities in Grapevine—A Reanalysis by Transcriptome Portrayal Machine Learning. Plants 2024, 13, 2501. [Google Scholar] [CrossRef] [PubMed]
- Konecny, T.; Nikoghosyan, M.; Binder, H. Machine learning extracts marks of thiamine’s role in cold acclimation in the transcriptome of Vitis vinifera. Front. Plant Sci. 2023, 14, 1303542. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Lin, L.; Huang, G.; Shi, X.; Wei, R.; Han, J.; Zhou, S.; Zhang, Y.; Xie, T.; Bai, X.; et al. Transcriptome Analysis of ‘Kyoho’ Grapevine Leaves Identifies Heat Response Genes Involved in the Transcriptional Regulation of Photosynthesis and Abscisic Acid. Agronomy 2022, 12, 2591. [Google Scholar] [CrossRef]
- Tan, J.W.; Shinde, H.; Tesfamicael, K.; Hu, Y.; Fruzangohar, M.; Tricker, P.; Baumann, U.; Edwards, E.J.; Rodríguez López, C.M. Global transcriptome and gene co-expression network analyses reveal regulatory and non-additive effects of drought and heat stress in grapevine. Front. Plant Sci. 2023, 14, 1096225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xin, H.; Fan, P.; Zhang, J.; Liu, Y.; Dong, Y.; Wang, Z.; Yang, Y.; Zhang, Q.; Ming, R.; et al. The genome of Shanputao (Vitis amurensis) provides a new insight into cold tolerance of grapevine. Plant J. 2021, 105, 1495–1506. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.; Liu, C.; Liu, G.; Li, S.; Wang, L. Integrating Omics and Alternative Splicing Reveals Insights into Grape Response to High Temperature. Plant Physiol. 2017, 173, 1502–1518. [Google Scholar] [CrossRef]
- Sarry, J.; Sommerer, N.; Sauvage, F.; Bergoin, A.; Rossignol, M.; Albagnac, G.; Romieu, C. Grape berry biochemistry revisited upon proteomic analysis of the mesocarp. Proteomics 2004, 4, 201–215. [Google Scholar] [CrossRef]
- Giribaldi, M.; Giuffrida, M.G. Heard it through the grapevine: Proteomic perspective on grape and wine. J. Proteom. 2010, 73, 1647–1655. [Google Scholar] [CrossRef]
- George, I.S.; Haynes, P.A. Current perspectives in proteomic analysis of abiotic stress in Grapevines. Front. Plant Sci. 2014, 5, 686. [Google Scholar] [CrossRef] [PubMed]
- Lecourieux, D.; Kappel, C.; Claverol, S.; Pieri, P.; Feil, R.; Lunn, J.E.; Bonneu, M.; Wang, L.; Gomès, E.; Delrot, S.; et al. Proteomic and metabolomic profiling underlines the stage- and time-dependent effects of high temperature on grape berry metabolism. J. Integr. Plant Biol. 2020, 62, 1132–1158. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: A commonalty in the response of plants to dehydration and low temperature. Physiol. Plant. 1997, 100, 291–296. [Google Scholar] [CrossRef]
- Masocha, V.F.; Li, Q.; Zhu, Z.; Chai, F.; Sun, X.; Wang, Z.; Yang, L.; Wang, Q.; Xin, H. Proteomic variation in Vitis amurensis and V. vinifera buds during cold acclimation. Sci. Hortic. 2020, 263, 109143. [Google Scholar] [CrossRef]
- Jan, N.; Qazi, H.A.; Raja, V.; John, R. Proteomics: A tool to decipher cold tolerance. Theor. Exp. Plant Physiol. 2019, 31, 183–213. [Google Scholar] [CrossRef]
- Estêvão, C.; Rodrigues, L.; Rato, A.E.; Garcia, R.; Cardoso, H.; Campos, C. Applicability of metabolomics to improve sustainable grapevine production. Front. Mol. Biosci. 2024, 11, 1395677. [Google Scholar] [CrossRef]
- Conde, A.; Regalado, A.; Rodrigues, D.; Costa, J.M.; Blumwald, E.; Chaves, M.M.; Gerós, H. Polyols in grape berry: Transport and metabolic adjustments as a physiological strategy for water-deficit stress tolerance in grapevine. J. Exp. Bot. 2015, 66, 889–906. [Google Scholar] [CrossRef]
- Huang, F.; Lei, Y.; Duan, J.; Kang, Y.; Luo, Y.; Ding, D.; Chen, Y.; Li, S. Investigation of heat stress responses and adaptation mechanisms by integrative metabolome and transcriptome analysis in tea plants (Camellia sinensis). Sci. Rep. 2024, 14, 10023. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Wu, D.; Hui, M.; Wang, Y.; Han, X.; Yao, F.; Cao, X.; Li, Y.-H.; Li, H.; Wang, H. Screening of cold hardiness-related indexes and establishment of a comprehensive evaluation method for grapevines (V. vinifera). Front. Plant Sci. 2022, 13, 1014330. [Google Scholar] [CrossRef]
- Sawicki, M.; Rondeau, M.; Courteaux, B.; Rabenoelina, F.; Guerriero, G.; Gomès, E.; Soubigou-Taconnat, L.; Balzergue, S.; Clément, C.; Ait Barka, E.; et al. On a Cold Night: Transcriptomics of Grapevine Flower Unveils Signal Transduction and Impacted Metabolism. Int. J. Mol. Sci. 2019, 20, 1130. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, J.; Fang, X.; Jia, H.; Wang, X.; Lin, Y.; Liu, S.; Ge, M.; Pu, Y.; Fang, J.; et al. Drought stress in ‘Shine Muscat’ grapevine: Consequences and a novel mitigation strategy–5-aminolevulinic acid. Front. Plant Sci. 2023, 14, 1129114. [Google Scholar] [CrossRef]
- Dal Santo, S.; Zenoni, S.; Sandri, M.; De Lorenzis, G.; Magris, G.; De Paoli, E.; Di Gaspero, G.; Del Fabbro, C.; Morgante, M.; Brancadoro, L.; et al. Grapevine field experiments reveal the contribution of genotype, the influence of environment and the effect of their interaction (G×E) on the berry transcriptome. Plant J. 2018, 93, 1143–1159. [Google Scholar] [CrossRef]
- Valenzuela, A.V.; Navarro-Paya, D.; Santiago, A.; Sonego, P.; Gainza-Cortes, F.; Malnoy, M.; Matus, J.T. Developing the Grapevine Hydric Stress Atlas: A Meta-Analysis Resource for Exploring transcriptome Responses to Drought. bioRxiv 2025. [Google Scholar] [CrossRef]
- Guarino, F.; Cicatelli, A.; Castiglione, S.; Agius, D.R.; Orhun, G.E.; Fragkostefanakis, S.; Leclercq, J.; Dobránszki, J.; Kaiserli, E.; Lieberman-Lazarovich, M.; et al. An Epigenetic Alphabet of Crop Adaptation to Climate Change. Front. Genet. 2022, 13, 818727. [Google Scholar] [CrossRef] [PubMed]
- Venios, X.; Gkizi, D.; Nisiotou, A.; Korkas, E.; Tjamos, S.; Zamioudis, C.; Banilas, G. Emerging Roles of Epigenetics in Grapevine and Winegrowing. Plants 2024, 13, 515. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, A.S.; Kiselev, K.V.; Khristenko, V.S.; Aleynova, O.A. VaCPK20, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr., mediates cold and drought stress tolerance. J. Plant Physiol. 2015, 185, 1–12. [Google Scholar] [CrossRef]
- Bhadouriya, S.L.; Mehrotra, S.; Basantani, M.K.; Loake, G.J.; Mehrotra, R. Role of Chromatin Architecture in Plant Stress Responses: An Update. Front. Plant Sci. 2021, 11, 603380. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Aleksandrushkina, N.I.; Vanyushin, B.F. Epigenetic Mechanisms of Plant Adaptation to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2020, 21, 7457. [Google Scholar] [CrossRef]
- Lämke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017, 18, 124. [Google Scholar] [CrossRef]
- Rodriguez-Izquierdo, A.; Carrasco, D.; Anand, L.; Magnani, R.; Catarecha, P.; Arroyo-Garcia, R.; Rodriguez Lopez, C.M. Epigenetic differences between wild and cultivated grapevines highlight the contribution of DNA methylation during crop domestication. BMC Plant Biol. 2024, 24, 504. [Google Scholar] [CrossRef]
- Matzke, M.A.; Mosher, R.A. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef]
- Roy, S.; Soni, P. Unraveling the Epigenetic Landscape for Salt Tolerance in Plants. Int. J. Plant Biol. 2022, 13, 443–462. [Google Scholar] [CrossRef]
- Asensi-Fabado, M.-A.; Amtmann, A.; Perrella, G. Plant responses to abiotic stress: The chromatin context of transcriptional regulation. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2017, 1860, 106–122. [Google Scholar] [CrossRef]
- Berger, M.M.J.; Stammitti, L.; Carrillo, N.; Blancquaert, E.; Rubio, B.; Teyssier, E.; Gallusci, P. Epigenetics: An innovative lever for grapevine breeding in times of climatic changes: This article is published in cooperation with the 22nd GiESCO International Meeting, hosted by Cornell University in Ithaca, NY, July 17–21, 2023. OENO One 2023, 57, 265–282. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.-K. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009, 12, 133–139. [Google Scholar] [CrossRef]
- Kim, J.-M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 2015, 6, 114. [Google Scholar] [CrossRef]
- Dal Santo, S.; De Paoli, E.; Pagliarani, C.; Amato, A.; Celii, M.; Boccacci, P.; Zenoni, S.; Gambino, G.; Perrone, I. Stress responses and epigenomic instability mark the loss of somatic embryogenesis competence in grapevine. Plant Physiol. 2022, 188, 490–508. [Google Scholar] [CrossRef]
- Tan, J.W.; Rodríguez López, C.M. Epigenomics: A new tool for the generation of climate resilient grapevines. Front. Hortic. 2023, 2, 1116866. [Google Scholar] [CrossRef]
- Pereira, J.P.; Bevilacqua, I.; Santos, R.B.; Varotto, S.; Chitarra, W.; Nerva, L.; Figueiredo, A. Epigenetic regulation and beyond in grapevine-pathogen interactions: A biotechnological perspective. Physiol. Plant. 2025, 177, e70216. [Google Scholar] [CrossRef]
- Mladenov, V.; Fotopoulos, V.; Kaiserli, E.; Karalija, E.; Maury, S.; Baranek, M.; Segal, N.; Testillano, P.; Vassileva, V.; Pinto, G.; et al. Deciphering the Epigenetic Alphabet Involved in Transgenerational Stress Memory in Crops. Int. J. Mol. Sci. 2021, 22, 7118. [Google Scholar] [CrossRef]
- Roy, S.; Mishra, M.; Dhankher, O.P.; Singla-Pareek, S.L.; Pareek, A. Molecular Chaperones: Key Players of Abiotic Stress Response in Plants. In Genetic Enhancement of Crops for Tolerance to Abiotic Stress: Mechanisms and Approaches, Vol. I; Springer: Cham, Switzerland, 2019; pp. 125–165. [Google Scholar] [CrossRef]
- Xie, H.; Konate, M.; Sai, N.; Tesfamicael, K.G.; Cavagnaro, T.; Gilliham, M.; Breen, J.; Metcalfe, A.; Stephen, J.R.; De Bei, R.; et al. Global DNA Methylation Patterns Can Play a Role in Defining Terroir in Grapevine (Vitis vinifera cv. Shiraz). Front. Plant Sci. 2017, 8, 1860. [Google Scholar] [CrossRef] [PubMed]
- Schwope, R.; Magris, G.; Miculan, M.; Paparelli, E.; Celii, M.; Tocci, A.; Marroni, F.; Fornasiero, A.; De Paoli, E.; Morgante, M. Open chromatin in grapevine marks candidate CREs and with other chromatin features correlates with gene expression. Plant J. 2021, 107, 1631–1647. [Google Scholar] [CrossRef] [PubMed]
- Araujo, F.; Williams, L.E.; Matthews, M.A. A comparative study of young ‘Thompson Seedless’ grapevines (Vitis vinifera L.) under drip and furrow irrigation. II. Growth, water use efficiency and nitrogen partitioning. Sci. Hortic. 1995, 60, 251–265. [Google Scholar] [CrossRef]
- Ollat, N.; Bordenave, L.; Tandonnet, J.P.; Boursiquot, J.M.; Marguerit, E. Grapevine rootstocks: Origins and perspectives. Acta Hortic. 2016, 1136, 11–22. [Google Scholar] [CrossRef]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.). Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Droulia, F.; Charalampopoulos, I. Future Climate Change Impacts on European Viticulture: A Review on Recent Scientific Advances. Atmosphere 2021, 12, 495. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Escalona, J.-M.; Pou, A.; Fuentes, S.; Flexas, J.; Bota, J. Improving water use efficiency of vineyards in semi-arid regions. A review. Agron. Sustain. Dev. 2015, 35, 499–517. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Darriet, P. The Impact of Climate Change on Viticulture and Wine Quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- Hunter, J.J.; Volschenk, C.G.; Zorer, R. Vineyard row orientation of Vitis vinifera L. cv. Shiraz/101-14 Mgt: Climatic profiles and vine physiological status. Agric. For. Meteorol. 2016, 228, 104–119. [Google Scholar] [CrossRef]
- Cantu, D.; Massonnet, M.; Cochetel, N. The wild side of grape genomics. Trends Genet. 2024, 40, 601–612. [Google Scholar] [CrossRef]
| Stress Type | Affected Regions | Severity and Impact | Morphological Changes | Biochemical Responses | Molecular Mechanisms | Key Genes and Known Mechanisms |
|---|---|---|---|---|---|---|
| Heat stress | Mediterranean, California, Australia | Up to 30% yield loss in extreme heat years [84]. | Accelerated phenology, smaller berries, leaf senescence, reduced shoot growth [85,86,87,88] | Increased abscisic acid, elevated flavonoid synthesis [89] | Activation of heat shock proteins and MAP kinase pathways counteract protein denaturation [90] | Heat Shock Proteins (HSP70, HSP90, HSF1, APX1), MAP kinase pathways [90] |
| Drought | Southern Europe, Chile, South Africa | 20–40% reduction in grapevine vigor and berry size [91]. | Reduced shoot growth, smaller berries [85,86,87,88] | Increased ABA induces stomatal closure [89] | ABA-responsive elements trigger drought-responsive genes [92] | VvNCED1, VvDREB1/2—ABA synthesis, VvGRIK1, VvRFS2, VvLKR drought-responsive genes [92,93,94] |
| Cold stress | Canada, Eastern Europe | 50–100% bud mortality during severe frost events [95] | Bud necrosis, shoot dieback [95,96] | Accumulated proline and soluble sugars protecting cells against freezing [97] | ICE1-CBF-COR cascade regulates cold-responsive genes, enhancing freezing tolerance [98] | VvCBF4, VvICE1—ICE1-CBF-COR cascade, enhancing freezing tolerance [98] |
| UV radiation | Arid regions, coastal vineyards | Impaired photosynthesis, enhanced flavonoid synthesis [81] | Leaf bronzing, cuticle thickening [81] | Enhanced production of UV-absorbing compounds like anthocyanins and flavonols [81] | ROS scavenging pathways, involving superoxide dismutase and catalase, mitigate oxidative damage [81] | VvMYB4—UV shielding and antioxidant defenses [81] |
| Soil salinity | Coastal vineyards, irrigated areas | Disrupted ion homeostasis, osmotic stress [99] | Reduced growth, leaf chlorosis [100] | Increased ABA for stomatal regulation [101] | Ion homeostasis mechanisms reduce salt toxicity [100] | VvNHX1—Ion transport, maintaining osmotic balance [102] |
| Spring frost | Northern Europe, Canada, USA | Severe bud damage and yield loss in early-budding grapevines [78] | Bud necrosis, shoot dieback [79] | Increased soluble sugars to lower freezing point [79] | ICE1-CBF-COR pathway activation protects cellular structures [98] | VvCBF2, VvCBF3—Frost tolerance via cold-responsive gene activation [98] |
| Water deficit | Mediterranean, California, Australia | Reduction in yield by 20–50%, smaller berries, reduced canopy growth [103] | Decreased leaf area, reduced shoot growth, and smaller berries [85,86,87,88] | Increased ABA, enhanced flavonoid synthesis [91] | Upregulation of drought-responsive genes, enhanced expression of dehydration-responsive elements [92] | VvNCED1, VvDREB2—ABA synthesis, drought-responsive genes [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konecny, T.; Asatryan, A.; Binder, H. Responding to Stress: Diversity and Resilience of Grapevine in a Changing Climate Under the Perspective of Omics Research. Int. J. Mol. Sci. 2025, 26, 7877. https://doi.org/10.3390/ijms26167877
Konecny T, Asatryan A, Binder H. Responding to Stress: Diversity and Resilience of Grapevine in a Changing Climate Under the Perspective of Omics Research. International Journal of Molecular Sciences. 2025; 26(16):7877. https://doi.org/10.3390/ijms26167877
Chicago/Turabian StyleKonecny, Tomas, Armine Asatryan, and Hans Binder. 2025. "Responding to Stress: Diversity and Resilience of Grapevine in a Changing Climate Under the Perspective of Omics Research" International Journal of Molecular Sciences 26, no. 16: 7877. https://doi.org/10.3390/ijms26167877
APA StyleKonecny, T., Asatryan, A., & Binder, H. (2025). Responding to Stress: Diversity and Resilience of Grapevine in a Changing Climate Under the Perspective of Omics Research. International Journal of Molecular Sciences, 26(16), 7877. https://doi.org/10.3390/ijms26167877






