Salivary Protein Profile in Patients with Recurrent Aphthous Stomatitis: A Pilot Proteomic Study
Abstract
1. Introduction
2. Results
2.1. Patients
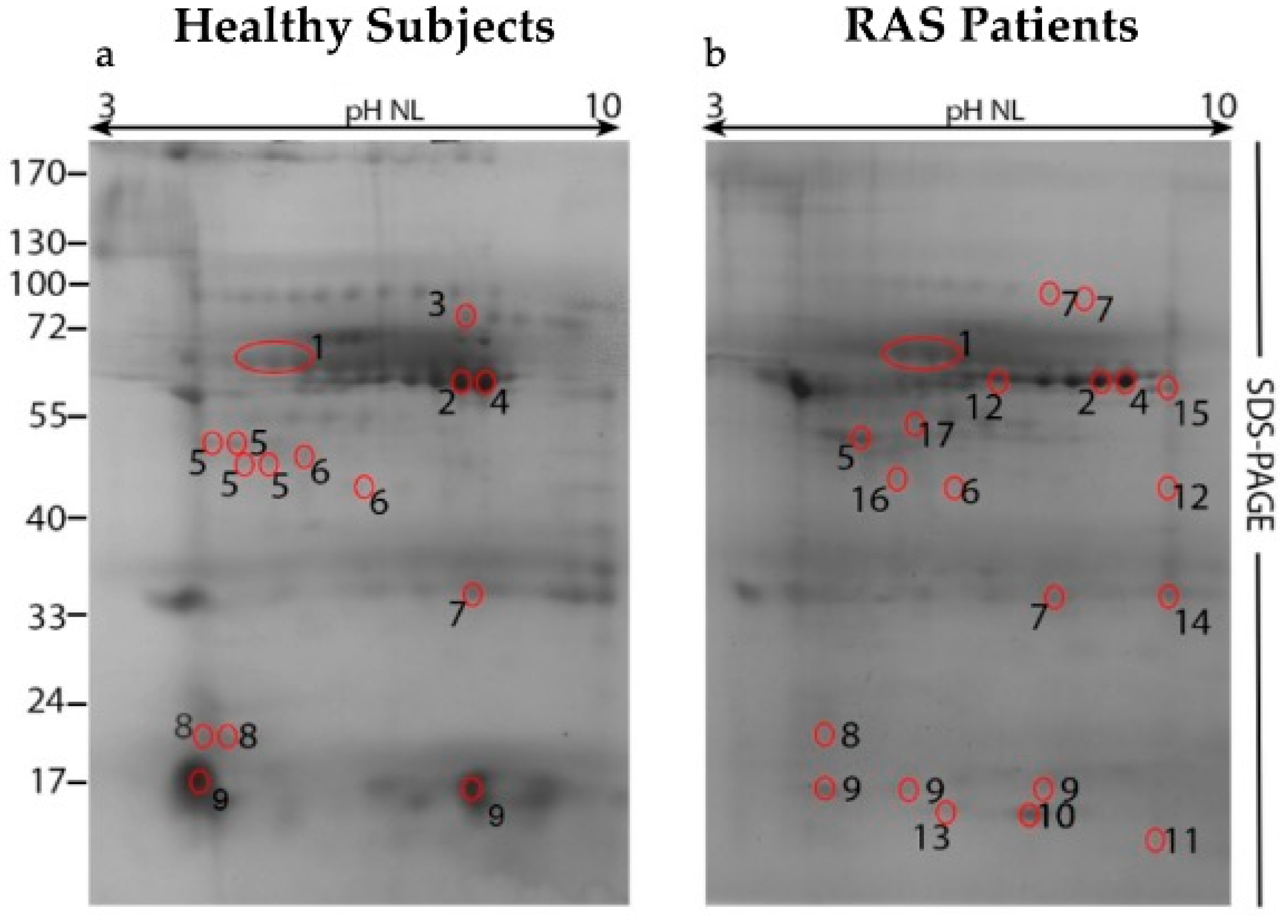
2.2. Salivary Proteomic Signature
2.3. Validation of Selected Protein by Western Blot Analysis
3. Discussion
4. Materials and Methods
4.1. Ethical Aspects and Patient Recruitment
4.2. Salivary Collection and Protein Sample Preparation
4.3. Two-Dimensional Gel Electrophoresis
4.4. Mass Spectrometry and Protein Identification
4.5. Western Blotting to Validate Selected Protein
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Porter, S.R.; Scully, C.; Pedersen, A. Recurrent aphthous stomatitis. Crit. Rev. Oral Biol. Med. 1998, 9, 306–321. [Google Scholar] [CrossRef]
- Askitopoulou, H.; Nyktari, V.; Papaioannou, A.; Stefanakis, G.; Konsolaki, E. The origins of oral medicine in the Hippocratic collected works. J. Oral Pathol. Med. 2017, 46, 689–694. [Google Scholar] [CrossRef]
- Jurge, S.; Kuffer, R.; Scully, C.; Porter, S.R. Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Dis. 2006, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bernal, J.; Conejero, C.; Conejero, R. Recurrent Aphthous Stomatitis. Actas Dermosifiliogr. 2020, 111, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Stanley, H.R. Aphthous lesions. Oral Surg. Oral Med. Oral Pathol. 1972, 3, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Saikaly, S.K.; Saikaly, T.S.; Saikaly, L.E. Recurrent aphthous ulceration: A review of potential causes and novel treatments. J. Dermatol. Treat. 2018, 29, 542–552. [Google Scholar] [CrossRef]
- Slebioda, Z.; Szponar, E.; Kowalska, A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: Literature review. Arch. Immunol. Ther. Exp. 2014, 62, 205–215. [Google Scholar] [CrossRef]
- Edgar, N.R.; Saleh, D.; Miller, R.A. Recurrent Aphthous Stomatitis: A Review. J. Clin. Aesthet. Dermatol. 2017, 10, 26–36. [Google Scholar] [PubMed]
- Pappa, E.; Vougas, K.; Zoidakis, J.; Vastardis, H. Proteomic advances in salivary diagnostics. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140494. [Google Scholar] [CrossRef]
- Aro, K.; Wei, F.; Wong, D.T.; Tu, M. Saliva Liquid Biopsy for Point-of-Care Applications. Front. Public Health 2017, 5, 77. [Google Scholar] [CrossRef]
- Spielmann, N.; Wong, D.T. Saliva: Diagnostics and therapeutic perspectives. Oral Dis. 2011, 17, 345–354. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; Pérez-Sayáns, M.; Bravo, S.B.; López-Jornet, P.; García-Vence, M.; Alonso-Sampedro, M.; Carballo, J.; García-García, A. Protein-Based Salivary Profiles as Novel Biomarkers for Oral Diseases. Dis. Markers 2018, 2018, 6141845. [Google Scholar] [CrossRef]
- Hallaji, Z.; Mortazavi, H.; Lajevardi, V.; Tamizifar, B.; AmirZangar, A.; Daneshpazhooh, M.; Chams-Davatchi, C. Serum and salivary desmoglein 1 and 3 enzyme-linked immunosorbent assay in pemphigus vulgaris: Correlation with phenotype and severity. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 275–280. [Google Scholar] [CrossRef]
- Esmaili, N.; Mortazavi, H.; Kamyab-Hesari, K.; Aghazadeh, N.; Daneshpazhooh, M.; Khani, S.; Chams-Davatchi, C. Diagnostic accuracy of BP180 NC16a and BP230-C3 ELISA in serum and saliva of patients with bullous pemphigoid. Clin. Exp. Dermatol. 2015, 40, 324–330. [Google Scholar] [CrossRef]
- Lopez-Jornet, P.; Cayuela, C.A.; Tvarijonaviciute, A.; Parra-Perez, F.; Escribano, D.; Ceron, J. Oral lichen planus: Saliva biomarkers cortisol, immunoglobulin A, adiponectin. J. Oral Pathol. Med. 2016, 45, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Mansourian, A.; Najafi, S.; Nojoumi, N.; Parhami, P.; Moosavi, M.S. Salivary cortisol and salivary flow rate in clinical types of oral lichen planus. Skinmed 2018, 16, 19–22. [Google Scholar] [PubMed]
- Girardi, C.; Luz, C.; Cherubini, K.; Figueiredo, M.A.Z.; Nunes, M.L.T.; Salum, F.G. Salivary cortisol and dehydroepiandrosterone (DHEA) levels, psychological factors in patients with oral lichen planus. Arch. Oral Biol. 2011, 56, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Camisasca, D.R.; da Rós Gonçalves, L.; Soares, M.R.; Sandim, V.; Nogueira, F.C.; Garcia, C.H.; Santana, R.; de Oliveira, S.P.; Buexm, L.A.; de Faria, P.A.; et al. A proteomic approach to compare saliva from individuals with and without oral leukoplakia. J. Proteom. 2017, 151, 43–52. [Google Scholar] [CrossRef]
- Ajona, D.; Pajares, M.J.; Chiara, M.D.; Rodrigo, J.P.; Jantus-Lewintre, E.; Camps, C.; Suarez, C.; Bagan, J.V.; Montuenga, L.M.; Pio, R. Complement activation product C4d in oral and oropharyngeal squamous cell carcinoma. Oral Dis. 2015, 21, 899–904. [Google Scholar] [CrossRef]
- Hoffmann, R.R.; Yurgel, L.S.; Campos, M.M. Evaluation of salivary endothelin-1 levels in oral squamous cell carcinoma and oral leukoplakia. Regul. Pept. 2011, 166, 55–58. [Google Scholar] [CrossRef]
- Gaba, F.I.; Sheth, C.C.; Veses, V. Salivary biomarkers and their efficacies as diagnostic tools for Oral Squamous Cell Carcinoma: Systematic review and meta-analysis. J. Oral Pathol. Med. 2021, 50, 299–307. [Google Scholar] [CrossRef]
- Shpitzer, T.; Hamzany, Y.; Bahar, G.; Feinmesser, R.; Savulescu, D.; Borovoi, I.; Gavish, M.; Nagler, R.M. Salivary analysis of oral cancer biomarkers. Br. J. Cancer 2009, 101, 1194–1198. [Google Scholar] [CrossRef]
- Eguia-del Valle, A.; Martínez-Conde-Llamosas, R.; López-Vicente, J.; Uribarri-Etxebarria, A.; Aguirre-Urizar, J.M. Salivary cortisol determination in patients from the Basque Country with recurrent aphthous stomatitis. A pilot study. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e207–e211. [Google Scholar] [CrossRef]
- Vandana, S.; Kavitha, B.; Sivapathasundharam, B. Salivary cortisol and dehydroepiandrosterone as oral biomarkers to determine stress in patients with recurrent aphthous stomatitis. J. Oral Maxillofac. Pathol. 2019, 23, 213–217. [Google Scholar] [CrossRef]
- Nadendla, L.K.; Meduri, V.; Paramkusam, G.; Pachava, K.R. Relationship of salivary cortisol and anxiety in recurrent aphthous stomatitis. Indian J. Endocrinol. Metab. 2015, 19, 56–59. [Google Scholar] [CrossRef]
- Kunikullaya, U.K.; Kumar, M.A.; Ananthakrishnan, V.; Jaisri, G. Stress as a Cause of Recurrent Aphthous Stomatitis and Its Correlation with Salivary Stress Markers. Chin. J. Physiol. 2017, 60, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Polat, C.; Düzer, S.; Ayyıldız, H.; Seç, S.; Aksoy, N.; Sakallıoğlu, Ö.; Akyiğit, A.; Çetiner, H. Association Between Anxiety, Depression, and Salivary Cortisol Levels in Patients with Recurrent Aphthous Stomatitis. Turk. Arch. Otorhinolaryngol. 2018, 56, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Cağlayan, F.; Miloglu, O.; Altun, O.; Erel, O.; Yilmaz, A.B. Oxidative stress and myeloperoxidase levels in saliva of patients with recurrent aphthous stomatitis. Oral Dis. 2008, 14, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Soltani, T. Evaluation and Comparison of Total Antioxidant Capacity of Saliva Between Patients with Recurrent Aphthous Stomatitis and Healthy Subjects. Open Dent. J. 2018, 12, 303–309. [Google Scholar] [CrossRef]
- Karincaoglu, Y.; Batcioglu, K.; Erdem, T.; Esrefoglu, M.; Genc, M. The levels of plasma and salivary antioxidants in the patient with recurrent aphthous stomatitis. J. Oral Pathol. Med. 2005, 34, 7–12. [Google Scholar] [CrossRef]
- Ziaudeen, S.; Ravindran, R. Assessment of Oxidant-Antioxidant Status and Stress Factor in Recurrent Aphthous Stomatitis Patients: Case Control Study. J. Clin. Diagn. Res. 2017, 11, ZC01–ZC04. [Google Scholar] [CrossRef]
- Boras, V.V.; Lukac, J.; Brailo, V.; Picek, P.; Kordić, D.; Zilić, I.A. Salivary interleukin-6 and tumor necrosis factor-alpha in patients with recurrent aphthous ulceration. J. Oral Pathol. Med. 2006, 35, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.; Nair, K.K.; Ashok, L. Salivary levels of TNF-α in patients with recurrent aphthous stomatitis: A cross-sectional study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2018, 12, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Ajila, V.; Babu, S.; Kumari, S.; Ullal, H.; Madiyal, A. Evaluation of salivary tumour necrosis factor-alpha in patients with recurrent aphthous stomatitis. Eur. Oral Res. 2018, 52, 157–161. [Google Scholar] [CrossRef]
- Mathur, S.; Sutton, J. Personalized medicine could transform healthcare. Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.B.; Smith, G.P. Recurrent Aphthous Stomatitis: A Comprehensive Review and Recommendations on Therapeutic Options. Dermatol. Ther. 2022, 35, e15500. [Google Scholar] [CrossRef]
- Karna, S.; Shin, Y.J.; Kim, S.; Kim, H.D. Salivary S100 proteins screen periodontitis among Korean adults. J. Clin. Periodontol. 2019, 46, 181–188. [Google Scholar] [CrossRef]
- Bobek, L.A.; Levine, M.J. Cystatins—Inhibitors of Cysteine Proteinases. Crit. Rev. Oral Biol. Med. 1992, 3, 307–332. [Google Scholar] [CrossRef]
- Manconi, B.; Liori, B.; Cabras, T.; Vincenzoni, F.; Iavarone, F.; Castagnola, M.; Messana, I.; Olianas, A. Salivary Cystatins: Exploring New Post-Translational Modifications and Polymorphisms by Top-Down High-Resolution Mass Spectrometry. J. Proteome Res. 2017, 16, 4196–4207. [Google Scholar] [CrossRef]
- Richards, R.; St Pierre, L.; Trabi, M.; Johnson, L.A.; de Jersey, J.; Masci, P.P.; Lavin, M.F. Cloning and characterisation of novel cystatins from elapid snake venom glands. Biochimie 2011, 93, 659–668. [Google Scholar] [CrossRef]
- Chmelař, J.; Kotál, J.; Langhansová, H.; Kotsyfakis, M. Protease Inhibitors in Tick Saliva: The Role of Serpins and Cystatins in Tick-host-Pathogen Interaction. Front. Cell. Infect. Microbiol. 2017, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, F.; Pan, W.; Wu, Q.; Wang, J.; Dai, J. An Immunosuppressive Tick Salivary Gland Protein DsCystatin Interferes with Toll-Like Receptor Signaling by Downregulating TRAF6. Front. Immunol. 2018, 9, 1245. [Google Scholar] [CrossRef]
- Otto, H.H.; Schirmeister, T. Cysteine Proteases and Their Inhibitors. Chem. Rev. 1997, 97, 133–172. [Google Scholar] [CrossRef]
- Fábián, T.F.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary Defense Proteins: Their Network and Role in Innate and Acquired Oral Immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef]
- Turk, V.; Stoka, V.; Turk, D. Cystatins: Biochemical and structural properties, and medical relevance. Front. Biosci. 2008, 13, 5406–5420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, J. Research progress of cystatin SN in cancer. Onco Targets Ther. 2019, 12, 3411–3419. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Imatani, T.; Minaguchi, K.; Saitoh, E.; Okuda, K. Salivary cystatins induce interleukin-6 expression via cell surface molecules in human gingival fibroblasts. Mol. Immunol. 2002, 39, 423–430. [Google Scholar] [CrossRef]
- Fukuoka, A.; Matsushita, K.; Morikawa, T.; Adachi, T.; Yasuda, K.; Kiyonari, H.; Fujieda, S.; Yoshimoto, T. Human cystatin SN is an endogenous protease inhibitor that prevents allergic rhinitis. J. Allergy Clin. Immunol. 2019, 143, 1153–1162. [Google Scholar] [CrossRef]
- Vray, B.; Hartmann, S.; Hoebeke, J. Immunomodulatory properties of cystatins. Cell. Mol. Life Sci. 2002, 59, 1503–1512. [Google Scholar] [CrossRef]
- Zavasnik-Bergant, T. Cystatin protease inhibitors and immune functions. Front. Biosci. 2008, 13, 4625–4637. [Google Scholar] [CrossRef]
- Oh, S.S.; Park, S.; Lee, K.; Madhi, H.; Park, S.G.; Lee, H.G.; Cho, Y.; Yoo, J.; Kim, K.D. Extracellular cystatin SN and cathepsin B prevent cellular senescence by inhibiting abnormal glycogen accumulation. Cell Death Dis. 2017, 8, e2729. [Google Scholar] [CrossRef]
- Teran, L.M.; Rüggeberg, S.; Santiago, J.; Fuentes-Arenas, F.; Hernández, J.L.; Montes-Vizuet, A.R.; Xinping, L.; Franz, T. Immune Response to Seasonal Influenza A Virus Infection: A Proteomic Approach. Arch. Med. Res. 2012, 43, 464–469. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Wang, Y.; Du, X.; Yao, J. Cystatin SN Affects Cell Proliferation by Regulating the ERα/PI3K/AKT/ERα Loopback Pathway in Breast Cancer. Onco Targets Ther. 2019, 12, 11359–11369. [Google Scholar] [CrossRef]
- Chen, Y.F.; Ma, G.; Cao, X.; Luo, R.Z.; He, L.R.; He, J.H.; Huang, Z.L.; Zeng, M.S.; Wen, Z.S. Overexpression of cystatin SN positively affects survival of patients with surgically resected esophageal squamous cell carcinoma. BMC Surg. 2013, 13, 15. [Google Scholar] [CrossRef]
- Baron, A.; DeCarlo, A.; Featherstone, J. Functional aspects of the human salivary cystatins in the oral environment. Oral Dis. 1999, 5, 234–240. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, H.L.; Tao, L.; Lin, X.Y.; Yang, Y.D.; Tan, S.W.; Wu, B. Let-7d inhibits colorectal cancer cell proliferation through the CST1/p65 pathway. Int. J. Oncol. 2018, 53, 781–790. [Google Scholar] [CrossRef]
- Douglas, S.A.; Haase, K.; Kamm, R.D.; Platt, M.O. Cysteine cathepsins are altered by flow within an engineered in vitro microvascular niche. APL Bioeng. 2020, 4, 046102. [Google Scholar] [CrossRef]
- Henskens, Y.M.; Veerman, E.C.; Mantel, M.S.; van der Velden, U.; Nieuw Amerongen, A.V. Cystatins S and C in human whole saliva and in glandular salivas in periodontal health and disease. J. Dent. Res. 1994, 73, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Henskens, Y.M.; van den Keijbus, P.A.; Veerman, E.C.; Van der Weijden, G.A.; Timmerman, M.F.; Snoek, C.M.; Van der Velden, U.; Nieuw Amerongen, A.V. Protein composition of whole and parotid saliva in healthy and periodontitis subjects. Determination of cystatins, albumin, amylase and IgA. J. Periodontal Res. 1996, 31, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hartenbach, F.A.R.R.; Velasquez, É.; Nogueira, F.C.S.; Domont, G.B.; Ferreira, E.; Colombo, A.P.V. Proteomic analysis of whole saliva in chronic periodontitis. J. Proteom. 2020, 213, 103602. [Google Scholar] [CrossRef]
- Romano, F.; Franco, F.; Corana, M.; Abbadessa, G.; Di Scipio, F.; Pergolizzi, B.; Castrignano, C.; Aimetti, M.; Berta, G.N. Cystatin SN (CST1) as a Novel Salivary Biomarker of Periodontitis. Int. J. Mol. Sci. 2023, 24, 13834. [Google Scholar] [CrossRef]
- Yang, Z.; Cui, Q.; An, R.; Wang, J.; Song, X.; Shen, Y.; Wang, M.; Xu, H. Comparison of microbiomes in ulcerative and normal mucosa of recurrent aphthous stomatitis (RAS)-affected patients. BMC Oral Health 2020, 20, 128. [Google Scholar] [CrossRef]
- Hietanen, J.; Häyrinen-Immonen, R.; Al-Samadi, A.; Trokovic, N.; Koskenpato, K.; Konttinen, Y.T. Recurrent aphthous ulcers--a Toll-like receptor-mediated disease? J. Oral Pathol. Med. 2012, 41, 158–164. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of recurrent aphthous stomatitis. Biomed. Rep. 2019, 11, 47–50. [Google Scholar] [CrossRef]
- Borilova Linhartova, P.; Janos, J.; Slezakova, S.; Bartova, J.; Petanova, J.; Kuklinek, P.; Fassmann, A.; Dusek, L.; Izakovicova Holla, L. Recurrent aphthous stomatitis and gene variability in selected interleukins: A case-control study. Eur. J. Oral Sci. 2018, 126, 485–492. [Google Scholar] [CrossRef]
- Kato, T.; Imatani, T.; Miura, T.; Minaguchi, K.; Saitoh, E.; Okuda, K. Cytokine-inducing activity of family 2 cystatins. Biol. Chem. 2000, 381, 1143–1147. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Curley, G.F.; Rose-John, S.; McElvaney, N.G. Interleukin-6: Obstacles to targeting a complex cytokine in critical illness. Lancet Respir. Med. 2021, 9, 643–654. [Google Scholar] [CrossRef]
- Loo, J.A.; Yan, W.; Ramachandran, P.; Wong, D.T. Comparative human salivary and plasma proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Meijer, J.; Ieong, S.; Xie, Y.; Yu, T.; Zhou, H.; Henry, S.; Vissink, A.; Pijpe, J.; et al. Salivary proteomic and genomic biomarkers for primary Sjögren’s syndrome. Arthritis Rheum. 2007, 56, 3588–3600. [Google Scholar] [CrossRef] [PubMed]
- Giusti, L.; Baldini, C.; Bazzichi, L.; Ciregia, F.; Tonazzini, I.; Mascia, G.; Giannaccini, G.; Bombardieri, S.; Lucacchini, A. Proteome analysis of whole saliva: A new tool for rheumatic diseases—The example of Sjögren’s syndrome. Proteomics 2007, 7, 1634–1643. [Google Scholar] [CrossRef]
- Kojima, T.; Andersen, E.; Sanchez, J.C.; Wilkins, M.R.; Hochstrasser, D.F.; Pralong, W.F.; Cimasoni, G. Human gingival crevicular fluid contains MRP8 (S100A8) and MRP14 (S100A9), two calcium-binding proteins of the S100 family. J. Dent. Res. 2000, 79, 740–747. [Google Scholar] [CrossRef]
- Navazesh, M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef]
- Pergolizzi, B.; Carriero, V.; Abbadessa, G.; Penna, C.; Berchialla, P.; De Francia, S.; Bracco, E.; Racca, S. Subchronic nandrolone administration reduces cardiac oxidative markers during restraint stress by modulating protein expression patterns. Mol. Cell. Biochem. 2017, 434, 51–60. [Google Scholar] [CrossRef]
- Abbadessa, G.; Maniscalco, E.; Grasso, L.; Popara, J.; Di Scipio, F.; Franco, F.; Mancardi, D.; Pigozzi, F.; Borrione, P.; Berta, G.N.; et al. Metformin Protects Rat Skeletal Muscle from Physical Exercise-Induced Injury. Biomedicines 2023, 11, 2334. [Google Scholar] [CrossRef]

| Functional Category | Spot Number | Protein | Protein ID * | Expect Value ** | pI | MW |
|---|---|---|---|---|---|---|
| Inflammatory, antimicrobial | 1 | ALB | P02768 | 1.8 × 10−5 | ~5.0 | 71.3 |
| 9 | CST1 | P01037 | 1.0 × 10−7 | 7.5 | 16.6 | |
| Hydrolases/Anti-hydrolases | 2 | AMY1A | P0DUB6 | 2.0 × 10−14 | ~6.5 | 58.4 |
| 3 | LTF | P02788 | 4.0 × 10−5 | ~8.0 | 80.14 | |
| 4 | AMY2B | P19961 | 7.2 × 10−5 | ~6.7 | 42.021 | |
| Cytoskeletal | 5 | ACTB | P60709 | 0.00097 | ~5.3 | 42.052 |
| 6 | K1C10 | P13645 | 0.0012 | ~5.13 | 58.827 | |
| Immune System | 7 | pIgR | P01833 | 0.0039 | ~7.0 | 84.4 |
| Other Proteins | 8 | PIP | P12273 | 5.1 × 10−10 | ~5.0 | 16.572 |
| Functional Category | Spot Number | Protein | Protein ID * | Expect Value ** | pI | MW |
|---|---|---|---|---|---|---|
| Inflammatory, antimicrobial | 1 | ALB | P02768 | 8.1 × 10−25 | ~5.0 | 71.3 |
| 9 | CST1 | P01037 | 1.0 × 10−7 | ~7.5 | 16.6 | |
| 10 | CST4 | P01036 | 0.00061 | ~7.5 | 16.4 | |
| 11 | S100A8 | P05109 | 0.0027 | ~5.0 | 10.8 | |
| Hydrolases/Anti-hydrolases | 2 | AMY1A | P0DUB6 | 5.1 × 10−14 | ~5.95 | 58.4 |
| 2 | AMY2B | P19961 | 2.0 × 10−10 | ~5.2 | 58.3 | |
| 12 | AMY2A | P04746 | 4.0 × 10−11 | ~5.5 | 58.3 | |
| Cytoskeletal | 5 | ACTB | P60709 | 0.0001 | ~5.3 | 42.0 |
| 6 | K1C9 | P35527 | 0.0021 | ~5.0 | 62.2 | |
| 13 | K1C10 | P13645 | 0.0016 | ~5.13 | 59.02 | |
| Immune System | 14 | IGKC | P01834 | 6.4 × 10−6 | ~5.5 | 11.9 |
| 15 | IGKCγ1 | P01834 | 0.0023 | ~5.5 | 36.5 | |
| 16 | IGKCα1 | P01834 | 4.8 × 10−5 | ~5.5 | 38.4 | |
| 17 | FGB | P02675 | 8.1 × 10−7 | ~5.5 | 56.5 | |
| 7 | pIgR | P01833 | 6.9 × 10−5 | ~7.0 | 84.4 | |
| Other Proteins | 8 | PIP | P12273 | 2.5 × 10−7 | ~5.0 | 16.5 |
| Healthy Subjects (H) | Level of CST1 Expression | RAS Patients (R) | Level of CST1 Expression |
|---|---|---|---|
| H1 | + | R1 | − |
| H2 | + | R2 | − |
| H3 | + | R3 | − |
| H4 | + | R4 | − |
| H5 | + | R5 | − |
| H6 | + | R6 | − |
| H7 | + | R7 | − |
| H8 | + | R8 | − |
| H9 | + | R9 | − |
| H10 | + | R10 | − |
| H11 | + | R11 | − |
| H12 | + | R12 | − |
| H13 | + | R13 | − |
| H14 | + | R14 | − |
| H15 | + | R15 | − |
| H16 | + | R16 | − |
| H17 | + | R17 | + |
| H18 | + | R18 | − |
| H19 | + | R19 | − |
| H20 | + | R20 | − |
| H21 | + | R21 | − |
| H22 | + | R22 | + |
| H23 | + | R23 | − |
| H24 | + | R24 | − |
| H25 | + | R25 | − |
| H26 | + | R26 | − |
| H27 | + | R27 | − |
| H28 | + | R28 | − |
| H29 | + | R29 | − |
| H30 | + | R30 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, F.; Namarvari, N.; Gambino, A.; Romano, F.; Pergolizzi, B.; Zhang, J.; Abbadessa, G.; Mognetti, B.; Ceccarelli, A.; Arduino, P.G.; et al. Salivary Protein Profile in Patients with Recurrent Aphthous Stomatitis: A Pilot Proteomic Study. Int. J. Mol. Sci. 2025, 26, 7878. https://doi.org/10.3390/ijms26167878
Franco F, Namarvari N, Gambino A, Romano F, Pergolizzi B, Zhang J, Abbadessa G, Mognetti B, Ceccarelli A, Arduino PG, et al. Salivary Protein Profile in Patients with Recurrent Aphthous Stomatitis: A Pilot Proteomic Study. International Journal of Molecular Sciences. 2025; 26(16):7878. https://doi.org/10.3390/ijms26167878
Chicago/Turabian StyleFranco, Francesco, Nima Namarvari, Alessio Gambino, Federica Romano, Barbara Pergolizzi, Jianjian Zhang, Giuliana Abbadessa, Barbara Mognetti, Adriano Ceccarelli, Paolo Giacomo Arduino, and et al. 2025. "Salivary Protein Profile in Patients with Recurrent Aphthous Stomatitis: A Pilot Proteomic Study" International Journal of Molecular Sciences 26, no. 16: 7878. https://doi.org/10.3390/ijms26167878
APA StyleFranco, F., Namarvari, N., Gambino, A., Romano, F., Pergolizzi, B., Zhang, J., Abbadessa, G., Mognetti, B., Ceccarelli, A., Arduino, P. G., & Berta, G. N. (2025). Salivary Protein Profile in Patients with Recurrent Aphthous Stomatitis: A Pilot Proteomic Study. International Journal of Molecular Sciences, 26(16), 7878. https://doi.org/10.3390/ijms26167878









