Molecular Mechanisms of Probiotic Action Against Gastrointestinal Cancers
Abstract
1. Introduction
2. Mechanisms of Probiotic Action Against Gastrointestinal Cancers
2.1. Antiproliferation and Cell Death Induction
2.2. Anticarcinogenic Compounds Production
2.3. Reduction in Chemotherapy-Related Toxicity
2.4. Gut Microbiota Modulation
2.5. Intestinal Barrier Improvement
2.6. Antioxidant Activity
2.7. Immunomodulatory and Anti-Inflammatory Effects
2.8. Carcinogen Detoxification
3. Challenges and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| 5-HT | 5-Hydroxytryptamine (Serotonin) |
| AAPH | 2,2′-Azobis (2-Amidinopropane) Dihydrochloride |
| ACP | S-Acetyltransferase |
| ALP | Alkaline Phosphatase |
| AMPs | Antimicrobial Peptides |
| AOM | Azoxymethane |
| Apc | Adenomatous polyposis coli |
| ARE | Antioxidant Response Element |
| Bcl-2 | B-cell Lymphoma-2 |
| Bcl-xL | B-cell Lymphoma-xL |
| CA | Cholic Acid |
| CAC | Colitis-Associated Colorectal Cancer |
| CAT | Catalase |
| CEA | Carcinoembryonic Antigen |
| CFS | Cell-free Supernatant |
| cIAP2 | Cellular Inhibitor of Apoptosis Protein 2 |
| CLA | Conjugated Linoleic Acid |
| CNS | Central Nervous System |
| COX-2 | Cyclooxygenase-2 |
| CRC | Colorectal Cancer |
| CREB | cAMP Response Element-Binding Protein |
| CTLs | Cytotoxic T Lymphocytes |
| CXCR-4 | C-X-C Chemokine Receptor type 4 |
| DAMPs | Damage-Associated Molecular Patterns |
| DEPs | Differentially Expressed Proteins |
| DMH | 1,2-Dimethylhydrazine |
| DSS | Dextran Sodium Sulfate |
| EcN | Escherichia coli Nissle 1917 |
| EFSA | European Food Safety Authority |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial–Mesenchymal Transition |
| ENS | Enteric Nervous System |
| EPS | Exopolysaccharides |
| ETEC | Enterotoxin-producing Escherichia coli |
| EVs | Extracellular Vesicles |
| FAO | Food and Agriculture Organization |
| GABA | Gamma-Aminobutyric Acid |
| GABAAR | Ionotropic GABAA Receptor |
| GABABR | Metabotropic GABAB Receptor |
| Gal | Galunisertib |
| GBA | Gut-Brain Axis |
| GI | Gastrointestinal |
| GIT | Gastrointestinal Tract |
| GPR | G-Protein Coupled Receptor |
| GSH-Px | Glutathione Peroxidase |
| GST | Glutathione S-Transferase |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| IBD | Inflammatory Bowel Disease |
| ICA | Indole-3-Carboxylic acid |
| ICD | Immunogenic Cell Death |
| IECs | Intestinal Epithelial Cells |
| IFN-γ | Interferon-γ |
| IGF-1 | Insulin-like Growth Factor-1 |
| IL | Interleukin |
| ILA | Indole-3-Lactic Acid |
| IM | Intestinal Mucositis |
| iNOS | inducible Nitric Oxide Synthase |
| IPA | Indole-3-Propionic Acid |
| ISAPP | International Scientific Association of Probiotics and Prebiotics |
| Keap1 | Kelchlike ECH-associated protein-1 |
| LAB | Lactic Acid Bacteria |
| LDH | Lactate Dehydrogenase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-Activated Protein Kinase |
| MDA | Malondialdehyde |
| METTL3 | Methyltransferase-like 3 |
| MHC | Major Histocompatibility Complex |
| MMP | Mitochondrial Membrane Potential |
| MNNG | N-Methyl-N’-nitro-N-nitrosoguanidine |
| MPTP | Mitochondrial Permeability Transition Pore |
| mTOR | Mammalian Target of Rapamycin |
| MyD88 | Myeloid Differentiation Factor 88 |
| NF-κB | Nuclear Factor-kappa B |
| NFKB1 | Nuclear Factor-kappa B subunit 1 |
| NK cells | Natural Killer cells |
| NLRP3 | NOD-like Receptor Protein 3 |
| NOD2 | Nucleotide-binding Oligomerization Domain-containing protein 2 |
| Nrf-2 | Nuclear Factor Erythroid 2-related factor 2 |
| ODC | Ornithine Decarboxylase |
| PD-L1 | Programmed Death-Ligand 1 |
| PI3K | Phosphatidylinositol 3-Kinase |
| PINK1 | PTEN Induced Kinase 1 |
| PKB | Protein Kinase B (Akt) |
| PTEN | Phosphatase and Tensin Homolog |
| PTGS2 | Prostaglandin-Endoperoxide Synthase 2 |
| ROS | Reactive Oxygen Species |
| RXR | Retinoid X Receptor |
| SCFAs | Short-Chain Fatty Acids |
| SOD | Superoxide Dismutase |
| SPF | Specific Pathogen-Free |
| TAA | Thioacetamide |
| TCA | Tricarboxylic Acid Cycle |
| TGF-β | Transforming Growth Factor-β |
| Th1 | Type 1 T helper cells |
| Th17 | Type 17 T helper cells |
| TJ | Tight Junction |
| TLR | Toll-like Receptor |
| TME | Tumor Microenvironment |
| TNBS | Trinitrobenzene Sulfonic Acid |
| TNF-α | Tumor Necrosis Factor-α |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| Treg | T regulatory cells |
| uPA | Urokinase Plasminogen Activator |
| uPAR | Urokinase Plasminogen Activator Receptor |
| VEGFR | Vascular Endothelial Growth Factor Receptor |
| VM | Vasculogenic mimicry |
| WHO | World Health Organization |
References
- Queen, J.; Shaikh, F.; Sears, C.L. Understanding the mechanisms and translational implications of the microbiome for cancer therapy innovation. Nat. Cancer. 2023, 4, 1083–1094. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Mei, J.X.; Yu, G.; Lei, L.; Zhang, W.H.; Liu, K.; Chen, X.L.; Kołat, D.; Yang, K.; Hu, J.K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Ciernikova, S.; Sevcikova, A.; Mladosievicova, B.; Mego, M. Microbiome in Cancer Development and Treatment. Microorganisms 2024, 12, 24. [Google Scholar] [CrossRef]
- Constantin, M.; Chifiriuc, M.C.; Mihaescu, G.; Corcionivoschi, N.; Burlibasa, L.; Bleotu, C.; Tudorache, S.; Mitache, M.M.; Filip, R.; Munteanu, S.G.; et al. Microbiome and cancer: From mechanistic implications in disease progression and treatment to development of novel antitumoral strategies. Front. Immunol. 2024, 15, 1373504. [Google Scholar] [CrossRef]
- Kumari, S.; Srilatha, M.; Nagaraju, G.P. Effect of Gut Dysbiosis on Onset of GI Cancers. Cancers 2025, 17, 90. [Google Scholar] [CrossRef]
- Mishra, Y.; Ranjan, A.; Mishra, V.; Chattaraj, A.; Aljabali, A.A.A.; El-Tanani, M.; Hromić-Jahjefendić, A.; Uversky, V.N.; Tambuwala, M.M. The role of the gut microbiome in gastrointestinal cancers. Cell Signal. 2024, 115, 111013. [Google Scholar] [CrossRef]
- Profir, M.; Roşu, O.A.; Creţoiu, S.M.; Gaspar, B.S. Friend or Foe: Exploring the Relationship between the Gut Microbiota and the Pathogenesis and Treatment of Digestive Cancers. Microorganisms 2024, 12, 955. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, A.; Afkhami, H. The role of microbiomes in gastrointestinal cancers: New insights. Front. Oncol. 2024, 13, 1344328. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Sharma, A.; Kaushik, H.; Sharma, L.; Nistha; Anwer, M.K.; Sachdeva, M.; Elossaily, G.M.; Zhang, Y.; Pillappan, R.; et al. Shaping the future of gastrointestinal cancers through metabolic interactions with host gut microbiota. Heliyon 2024, 10, e35336. [Google Scholar] [CrossRef] [PubMed]
- Adlakha, Y.K.; Chhabra, R. The human microbiome: Redefining cancer pathogenesis and therapy. Cancer Cell Int. 2025, 25, 165. [Google Scholar] [CrossRef]
- Alum, E.U.; Uti, D.E.; Ugwu, O.P.; Alum, B.N.; Edeh, F.O.; Ainebyoona, C. Unveiling the microbial orchestra: Exploring the role of microbiota in cancer development and treatment. Discov. Oncol. 2025, 16, 646. [Google Scholar] [CrossRef]
- Kim, K.; Lee, M.; Shin, Y.; Lee, Y.; Kim, T.-J. Optimizing Cancer Treatment Through Gut Microbiome Modulation. Cancers 2025, 17, 1252. [Google Scholar] [CrossRef]
- Neurath, M.F.; Artis, D.; Becker, C. The intestinal barrier: A pivotal role in health, inflammation, and cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef]
- Nobels, A.; van Marcke, C.; Jordan, B.F.; Van Hul, M.; Cani, P.D. The gut microbiome and cancer: From tumorigenesis to therapy. Nat. Metab. 2025, 7, 895–917. [Google Scholar] [CrossRef]
- Sun, J.; Song, S.; Liu, J.; Chen, F.; Li, X.; Wu, G. Gut microbiota as a new target for anticancer therapy: From mechanism to means of regulation. NPJ Biofilms Microbiomes 2025, 11, 43. [Google Scholar] [CrossRef]
- Zhang, X.; Fam, K.T.; Dai, T.; Hang, H.C. Microbiota mechanisms in cancer progression and therapy. Cell Chem. Biol. 2025, 32, 653–677. [Google Scholar] [CrossRef]
- Ullah, H.; Arbab, S.; Chang, C.; Bibi, S.; Muhammad, N.; Rehman, S.U.; Suleman; Ullah, I.; Hassan, I.U.; Tian, Y.; et al. Gut microbiota therapy in gastrointestinal diseases. Front. Cell Dev. Biol. 2025, 13, 1514636. [Google Scholar] [CrossRef]
- Sevcikova, A.; Mladosievicova, B.; Mego, M.; Ciernikova, S. Exploring the Role of the Gut and Intratumoral Microbiomes in Tumor Progression and Metastasis. Int. J. Mol. Sci. 2023, 24, 17199. [Google Scholar] [CrossRef]
- Cao, Y.; Xia, H.; Tan, X.; Shi, C.; Ma, Y.; Meng, D.; Zhou, M.; Lv, Z.; Wang, S.; Jin, Y. Intratumoural microbiota: A new frontier in cancer development and therapy. Signal Transduct. Target. Ther. 2024, 9, 15. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, P.; Wang, J.; Wei, X.; Wang, M. Unveiling intratumoral microbiota: An emerging force for colorectal cancer diagnosis and therapy. Pharmacol. Res. 2024, 203, 107185. [Google Scholar] [CrossRef]
- Wang, N.; Wu, S.; Huang, L.; Hu, Y.; He, X.; He, J.; Hu, B.; Xu, Y.; Rong, Y.; Yuan, C.; et al. Intratumoral microbiome: Implications for immune modulation and innovative therapeutic strategies in cancer. J. Biomed. Sci. 2025, 32, 23. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377–388. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.; Huang, W.; Zhou, H.; Zhang, W. Drug-microbiota interactions: An emerging priority for precision medicine. Signal Transduct. Target. Ther. 2023, 8, 386. [Google Scholar] [CrossRef]
- Mafe, A.N.; Büsselberg, D. Microbiome Integrity Enhances the Efficacy and Safety of Anticancer Drug. Biomedicines 2025, 13, 422. [Google Scholar] [CrossRef]
- He, Z.; Xie, H.; Xu, H.; Wu, J.; Zeng, W.; He, Q.; Jobin, C.; Jin, S.; Lan, P. Chemotherapy-induced microbiota exacerbates the toxicity of chemotherapy through the suppression of interleukin-10 from macrophages. Gut Microbes 2024, 16, 2319511. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Roy, R.; Singh, S.K. The Microbiome Modulates the Immune System to Influence Cancer Therapy. Cancers 2024, 16, 779. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Liu, Y.; Gao, Y.; Yang, R. Regulation of gut microbiota on immune cell ferroptosis: A novel insight for immunotherapy against tumor. Cancer Lett. 2024, 598, 217115. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, F. The role of the gut microbiota in tumor, immunity, and immunotherapy. Front. Immunol. 2024, 15, 1410928. [Google Scholar] [CrossRef]
- De Luca, R.; Arrè, V.; Nardone, S.; Incerpi, S.; Giannelli, G.; Trivedi, P.; Anastasiadou, E.; Negro, R. Gastrointestinal microbiota and inflammasomes interplay in health and disease: A gut feeling. Gut, 2025; advance online publication. [Google Scholar] [CrossRef]
- Ashique, S.; Bhowmick, M.; Pal, R.; Khatoon, H.; Kumar, P.; Sharma, H.; Garg, A.; Kumar, S.; Das, U. Multi drug resistance in Colorectal Cancer—approaches to overcome, advancements and future success. Adv. Cancer Biol. Metast. 2024, 10, 100114. [Google Scholar] [CrossRef]
- Chawrylak, K.; Leśniewska, M.; Mielniczek, K.; Sędłak, K.; Pelc, Z.; Pawlik, T.M.; Polkowski, W.P.; Rawicz-Pruszyński, K. Gut Microbiota—Adversary or Ally? Its Role and Significance in Colorectal Cancer Pathogenesis, Progression, and Treatment. Cancers 2024, 16, 2236. [Google Scholar] [CrossRef]
- Cheraghpour, M.; Fatemi, N.; Shadnoush, M.; Talebi, G.; Tierling, S.; Bermúdez-Humarán, L.G. Immunomodulation aspects of gut microbiome-related interventional strategies in colorectal cancer. Med. Oncol. 2024, 41, 231. [Google Scholar] [CrossRef]
- Garvey, M. Intestinal Dysbiosis: Microbial Imbalance Impacts on Colorectal Cancer Initiation, Progression and Disease Mitigation. Biomedicines 2024, 12, 740. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, P.; Deng, K.; Zhou, Y.; Hu, K. Targeting the gut microbiota: A new strategy for colorectal cancer treatment. J. Transl. Med. 2024, 22, 915. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Georgescu, T.F.; Buica, V.; Catanescu, M.-S.; Cercel, I.-A.; Budeanu, B.; Budan, M.; Bacalbasa, N.; Diaconu, C. Exploring the Role of the Gut Microbiota in Colorectal Cancer Development. Gastrointest. Disord. 2024, 6, 526–537. [Google Scholar] [CrossRef]
- Long, J.; Wang, J.; Xiao, C.; You, F.; Jiang, Y.; Li, X. Intratumoral microbiota in colorectal cancer: Focus on specific distribution and potential mechanisms. Cell Commun. Signal. 2024, 22, 455. [Google Scholar] [CrossRef]
- Torres-Galarza, A.; Toledo, Z.; Bailón-Moscoso, N. The role of human microbiota in the development of colorectal cancer: A literature review. Med. Microecol. 2024, 20, 100100. [Google Scholar] [CrossRef]
- White, M.T.; Sears, C.L. The microbial landscape of colorectal cancer. Nat. Rev. Microbiol. 2024, 22, 240–254. [Google Scholar] [CrossRef]
- Cui, X.; Li, C.; Zhong, J.; Liu, Y.; Xiao, P.; Liu, C.; Zhao, M.; Yang, W. Gut microbiota—bidirectional modulator: Role in inflammatory bowel disease and colorectal cancer. Front. Immunol. 2025, 16, 1523584. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Diaconu, C.C.; Gheorghe, G.; Mihai, M.-M.; Diaconu, C.C.; Bostan, M.; Bleotu, C. Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection. Int. J. Mol. Sci. 2025, 26, 3733. [Google Scholar] [CrossRef]
- Karam, F.; El Deghel, Y.; Iratni, R.; Dakroub, A.H.; Eid, A.H. The Gut Microbiome and Colorectal Cancer: An Integrative Review of the Underlying Mechanisms. Cell Biochem. Biophys. 2025; advance online publication. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Y.; Feng, X.; Yang, J.; Wang, B.; Fang, J.; Wang, Z.; Zhu, J.; Niu, G.; Guo, Y. The power of microbes: The key role of gut microbiota in the initiation and progression of colorectal cancer. Front. Oncol. 2025, 15, 1563886. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Dhieb, D.; Osman, H.A.; Mekideche, H. The Gut Microbiota and Colorectal Cancer: Understanding the Link and Exploring Therapeutic Interventions. Biology 2025, 14, 251. [Google Scholar] [CrossRef]
- Xing, G.; Cui, Y.; Guo, Z.; Han, B.; Zhao, G. Progress on the mechanism of intestinal microbiota against colorectal cancer. Front. Cell Infect. Microbiol. 2025, 15, 1565103. [Google Scholar] [CrossRef]
- Raoul, P.; Maccauro, V.; Cintoni, M.; Scarpellini, E.; Ianiro, G.; Gasbarrini, A.; Mele, M.C.; Rinninella, E. Microbiota–Gastric Cancer Interactions and the Potential Influence of Nutritional Therapies. Int. J. Mol. Sci. 2024, 25, 1679. [Google Scholar] [CrossRef]
- Zeng, R.; Gou, H.; Lau, H.C.H.; Yu, J. Stomach microbiota in gastric cancer development and clinical implications. Gut 2024, 73, 2062–2073. [Google Scholar] [CrossRef]
- Wu, M.; Tian, C.; Zou, Z.; Jin, M.; Liu, H. Gastrointestinal Microbiota in Gastric Cancer: Potential Mechanisms and Clinical Applications—A Literature Review. Cancers 2024, 16, 3547. [Google Scholar] [CrossRef]
- Yu, L.X.; Schwabe, R.F. The gut microbiome and liver cancer: Mechanisms and clinical translation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 527–539. [Google Scholar] [CrossRef]
- Rajapakse, J.; Khatiwada, S.; Akon, A.C.; Yu, K.L.; Shen, S.; Zekry, A. Unveiling the complex relationship between gut microbiota and liver cancer: Opportunities for novel therapeutic interventions. Gut Microbes. 2023, 15, 2240031. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Jiang, R. Microbiome interplays in the gut-liver axis: Implications for liver cancer pathogenesis and therapeutic insights. Front. Cell Infect. Microbiol. 2025, 15, 1467197. [Google Scholar] [CrossRef]
- Yao, J.; Ning, B.; Ding, J. The gut microbiota: An emerging modulator of drug resistance in hepatocellular carcinoma. Gut Microbes. 2025, 17, 2473504. [Google Scholar] [CrossRef]
- Sexton, R.E.; Uddin, M.H.; Bannoura, S.; Khan, H.Y.; Mzannar, Y.; Li, Y.; Aboukameel, A.; Al-Hallak, M.N.; Al-Share, B.; Mohamed, A.; et al. Connecting the Human Microbiome and Pancreatic Cancer. Cancer Metastasis Rev. 2022, 41, 317–331. [Google Scholar] [CrossRef]
- de Castilhos, J.; Tillmanns, K.; Blessing, J.; Laraño, A.; Borisov, V.; Stein-Thoeringer, C.K. Microbiome and pancreatic cancer: Time to think about chemotherapy. Gut Microbes. 2024, 16, 2374596. [Google Scholar] [CrossRef]
- Guo, X.; Wang, P.; Li, Y.; Chang, Y.; Wang, X. Microbiomes in pancreatic cancer can be an accomplice or a weapon. Crit. Rev. Oncol. Hematol. 2024, 194, 104262. [Google Scholar] [CrossRef]
- Pourali, G.; Kazemi, D.; Chadeganipour, A.S.; Arastonejad, M.; Kashani, S.N.; Pourali, R.; Maftooh, M.; Akbarzade, H.; Fiuji, H.; Hassanian, S.M.; et al. Microbiome as a biomarker and therapeutic target in pancreatic cancer. BMC Microbiol. 2024, 24, 16. [Google Scholar] [CrossRef]
- Liang, Y.; Du, M.; Li, X.; Gao, J.; Li, Q.; Li, H.; Li, J.; Gao, X.; Cong, H.; Huang, Y.; et al. Upregulation of Lactobacillus spp. in gut microbiota as a novel mechanism for environmental eustress-induced anti-pancreatic cancer effects. Gut Microbes. 2025, 17, 2470372. [Google Scholar] [CrossRef]
- Gou, H.; Zeng, R.; Lau, H.C.H.; Yu, J. Gut microbial metabolites: Shaping future diagnosis and treatment against gastrointestinal cancer. Pharmacol. Res. 2024, 208, 107373. [Google Scholar] [CrossRef]
- Wu, H.; Ma, W.; Wang, Y.; Wang, Y.; Sun, X.; Zheng, Q. Gut microbiome-metabolites axis: A friend or foe to colorectal cancer progression. Biomed. Pharmacother. 2024, 173, 116410. [Google Scholar] [CrossRef]
- Anwer, E.K.E.; Ajagbe, M.; Sherif, M.; Musaibah, A.S.; Mahmoud, S.; ElBanbi, A.; Abdelnaser, A. Gut Microbiota Secondary Metabolites: Key Roles in GI Tract Cancers and Infectious Diseases. Biomedicines 2025, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Mafe, A.N.; Büsselberg, D. The Effect of Microbiome-Derived Metabolites in Inflammation-Related Cancer Prevention and Treatment. Biomolecules 2025, 15, 688. [Google Scholar] [CrossRef]
- Wijesekara, T.; Abeyrathne, E.D.N.S.; Ahn, D.U. Effect of Bioactive Peptides on Gut Microbiota and Their Relations to Human Health. Foods 2024, 13, 1853. [Google Scholar] [CrossRef]
- Lawrence, G.W.; Garcia-Gutierrez, E.; O’Mahony, A.K.; Walsh, C.J.; O’Connor, P.M.; Begley, M.; Guinane, C.M.; Cotter, P.D. A gut-derived Streptococcus salivarius produces the novel nisin variant designated nisin G and inhibits Fusobacterium nucleatum in a model of the human distal colon microbiome. MBio 2025, 16, e01573-24. [Google Scholar] [CrossRef]
- Thulasinathan, B.; Suvilesh, K.N.; Maram, S.; Grossmann, E.; Ghouri, Y.; Teixeiro, E.P.; Chan, J.; Kaif, J.T.; Rachagani, S. The impact of gut microbial short-chain fatty acids on colorectal cancer development and prevention. Gut Microbes. 2025, 17, 2483780. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Zare, M.; Aghayari, M.; Afkhami, H.; Jafari, G.A. Therapeutic bacteria and viruses to combat cancer: Double-edged sword in cancer therapy: New insights for future. Cell Commun. Signal. 2024, 22, 239. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, D. Unveiling the microbial influence: Bacteria’s dual role in tumor metastasis. Front. Oncol. 2025, 15, 1524887. [Google Scholar] [CrossRef]
- Sehrawat, N.; Yadav, M.; Singh, M.; Kumar, V.; Sharma, V.R.; Sharma, A.K. Probiotics in microbiome ecological balance providing a therapeutic window against cancer. Semin. Cancer Biol. 2021, 70, 24–36. [Google Scholar] [CrossRef]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.Y.; Zhang, H.; Sun, Z. Targeting gut microbiota and metabolism as the major probiotic mechanism—An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Singh, A.; Alexander, S.G.; Martin, S. Gut microbiome homeostasis and the future of probiotics in cancer immunotherapy. Front. Immunol. 2023, 14, 1114499. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, W.; Ma, C.; Zhang, Z.; Zhang, W.; Zhang, J. An emerging strategy: Probiotics enhance the effectiveness of tumor immunotherapy via mediating the gut microbiome. Gut Microbes. 2024, 16, 2341717. [Google Scholar] [CrossRef]
- Kim, Y.T.; Mills, D.A. Exploring the gut microbiome: Probiotics, prebiotics, synbiotics, and postbiotics as key players in human health and disease improvement. Food Sci. Biotechnol. 2024, 33, 2065–2080. [Google Scholar] [CrossRef]
- Masheghati, F.; Asgharzadeh, M.R.; Jafari, A.; Masoudi, N.; Maleki-Kakelar, H. The role of gut microbiota and probiotics in preventing, treating, and boosting the immune system in colorectal cancer. Life Sci. 2024, 344, 122529. [Google Scholar] [CrossRef]
- Skoufou, M.; Tsigalou, C.; Vradelis, S.; Bezirtzoglou, E. The Networked Interaction between Probiotics and Intestine in Health and Disease: A Promising Success Story. Microorganisms 2024, 12, 194. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Z.; Gao, H.; Zhou, D.; Zhu, Z.; Tao, L.; Guan, W.; Gao, Y.; Song, Y.; Wang, M. Microfluidics-Derived Microparticles with Prebiotics and Probiotics for Enhanced In Situ Colonization and Immunoregulation of Colitis. Nano Lett. 2024, 24, 1081–1089. [Google Scholar] [CrossRef]
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef] [PubMed]
- Drago, L. Probiotics and Colon Cancer. Microorganisms 2019, 7, 66. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Agha, A.S.A.A.; Al-Remawi, M.; Al-Adham, I.S.I.; Daadoue, S.; Alsisan, A.; Khattab, D.; Malath, D.; Salameh, H.; Al-Betar, M.; et al. What We Know About the Actual Role of Traditional Probiotics in Health and Disease. Probiotics Antimicrob. Proteins 2024, 16, 1836–1856. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.W.; Khoo, S.C.; Paul, R.P.M.; Luang-In, V.; Lam, S.D.; Ma, N.L. Potential of Synbiotics and Probiotics as Chemopreventive Agent. Probiotics Antimicrob. Proteins 2024, 16, 2085–2101. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, J.; Dharmasiddhi, I.P.W.; Chen, S.; Liu, Y.; Liu, H. Review of the Potential of Probiotics in Disease Treatment: Mechanisms, Engineering, and Applications. Processes 2024, 12, 316. [Google Scholar] [CrossRef]
- Chakravarty, K.; Gaur, S.; Kumar, R.; Jha, N.K.; Gupta, P.K. Exploring the Multifaceted Therapeutic Potential of Probiotics: A Review of Current Insights and Applications. Probiotics Antimicrob. Proteins 2025, 17, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Karukuvelraja, R.; Saranya, N. Exploring the Multitude: A Review on Different Categorization of Probiotics. Probiotics Antimicrob. Proteins, 2025; advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.Q.; Luo, Q.; Wang, L.; Song, G.B.; Sheng, J.P.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, G.P.; Kuruvalli, G.; Syed, K.; Reddy, V.D. An Updated Review on Probiotic Production and Applications. Gastroenterol. Insights 2024, 15, 221–236. [Google Scholar] [CrossRef]
- Lu, K.; Dong, S.; Wu, X.; Jin, R.; Chen, H. Probiotics in Cancer. Front. Oncol. 2021, 11, 638148. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, A.; Nagpal, R.; Mohania, D.; Behare, P.; Verma, V.; Kumar, P.; Poddar, D.; Aggarwal, P.K.; Henry, C.J.; et al. Cancer-preventing attributes of probiotics: An update. Int. J. Food Sci. Nutr. 2010, 61, 473–496. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Dubey, S.K. Overview of the Mechanistic Potential of Probiotics and Prebiotics in Cancer Chemoprevention. Mol. Nutr. Food Res. 2023, 67, e2300221. [Google Scholar] [CrossRef]
- Sankarapandian, V.; Venmathi Maran, B.A.; Rajendran, R.L.; Jogalekar, M.P.; Gurunagarajan, S.; Krishnamoorthy, R.; Gangadaran, P.; Ahn, B.-C. An Update on the Effectiveness of Probiotics in the Prevention and Treatment of Cancer. Life 2022, 12, 59. [Google Scholar] [CrossRef]
- Naeem, H.; Hassan, H.U.; Shahbaz, M.; Imran, M.; Memon, A.G.; Hasnain, A.; Murtaza, S.; Alsagaby, S.A.; al Abdulmonem, W.; Hussain, M.; et al. Role of Probiotics against Human Cancers, Inflammatory Diseases, and Other Complex Malignancies. J. Food Biochem. 2024, 2024, 6632209. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, J.; Yu, P.; Qiu, T.; Jiang, S.; Yu, R. Unlocking the power of probiotics, postbiotics: Targeting apoptosis for the treatment and prevention of digestive diseases. Front. Nutr. 2025, 12, 1570268. [Google Scholar] [CrossRef]
- Ha, S.; Zhang, X.; Yu, J. Probiotics intervention in colorectal cancer: From traditional approaches to novel strategies. Chin. Med. J. 2024, 137, 8–20. [Google Scholar] [CrossRef]
- Tripathy, A.; Dash, J.; Kancharla, S.; Kolli, P.; Mahajan, D.; Senapati, S.; Jena, M.K. Probiotics: A Promising Candidate for Management of Colorectal Cancer. Cancers 2021, 13, 3178. [Google Scholar] [CrossRef]
- Pezeshki, B.; Abdulabbas, H.T.; Alturki, A.D.; Mansouri, P.; Zarenezhad, E.; Nasiri-Ghiri, M.; Ghasemian, A. Synergistic Interactions Between Probiotics and Anticancer Drugs: Mechanisms, Benefits, and Challenges. Probiotics Antimicrob. Proteins, 2025; advance online publication. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef] [PubMed]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef]
- Hamamah, S.; Lobiuc, A.; Covasa, M. Antioxidant Role of Probiotics in Inflammation-Induced Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 9026. [Google Scholar] [CrossRef] [PubMed]
- Thoda, C.; Touraki, M. Immunomodulatory Properties of Probiotics and Their Derived Bioactive Compounds. Appl. Sci. 2023, 13, 4726. [Google Scholar] [CrossRef]
- Li, W.; Zeng, Y.; Zhong, J.; Hu, Y.; Xiong, X.; Zhou, Y.; Fu, L. Probiotics Exert Gut Immunomodulatory Effects by Regulating the Expression of Host miRNAs. Probiotics Antimicrob. Proteins 2025, 17, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Legesse Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef]
- Virk, M.S.; Virk, M.A.; He, Y.; Tufail, T.; Gul, M.; Qayum, A.; Rehman, A.; Rashid, A.; Ekumah, J.-N.; Han, X.; et al. The Anti-Inflammatory and Curative Exponent of Probiotics: A Comprehensive and Authentic Ingredient for the Sustained Functioning of Major Human Organs. Nutrients 2024, 16, 546. [Google Scholar] [CrossRef]
- Beikmohammadi, M.; Halimi, S.; Fard, N.A.; Wen, W. Therapeutic Potential of Probiotics: A Review of Their Role in Modulating Inflammation. Probiotics Antimicrob. Proteins, 2025; advance online publication. [Google Scholar] [CrossRef]
- Tieu, M.V.; Pham, D.T.; Cho, S. Bacteria-based cancer therapy: Looking forward. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189112. [Google Scholar] [CrossRef]
- Thapa, D.; Kumar, V.; Naik, B.; Kumar, V.; Gupta, A.K.; Mohanta, Y.K.; Mishra, B.; Rustagi, S. Harnessing probiotic foods: Managing cancer through gut health. Food Sci. Biotechnol. 2024, 33, 2141–2160. [Google Scholar] [CrossRef]
- Dos Reis, S.A.; da Conceição, L.L.; Siqueira, N.P.; Rosa, D.D.; da Silva, L.L.; Peluzio, M.D. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr. Res. 2017, 37, 1–19. [Google Scholar] [CrossRef]
- Molska, M.; Reguła, J. Potential Mechanisms of Probiotics Action in the Prevention and Treatment of Colorectal Cancer. Nutrients. 2019, 11, 2453. [Google Scholar] [CrossRef]
- Ding, S.; Hu, C.; Fang, J.; Liu, G. The Protective Role of Probiotics against Colorectal Cancer. Oxid. Med. Cell Longev. 2020, 2020, 8884583. [Google Scholar] [CrossRef]
- Li, Q.; Liu, D.; Liang, M.; Zhu, Y.; Yousaf, M.; Wu, Y. Mechanism of probiotics in the intervention of colorectal cancer: A review. World J. Microbiol. Biotechnol. 2024, 40, 306. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Nema, V.; Khan, Z. Current status of probiotics for prevention and management of gastrointestinal cancers. Expert. Opin. Biol. Ther. 2021, 21, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Fukumoto, A.; Matsuda, S. Beneficial Probiotics with New Cancer Therapies for Improved Treatment of Hepatocellular Carcinoma. Diseases 2025, 13, 111. [Google Scholar] [CrossRef]
- Lee, T.S. Are Probiotics Beneficial or Harmful for Pancreatic Cancer Outcomes? Probiotics Antimicrob. Proteins, 2024; advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Orlando, A.; Linsalata, M.; Cavallini, A.; Messa, C. Effects of Lactobacillus rhamnosus GG on the cell growth and polyamine metabolism in HGC-27 human gastric cancer cells. Nutr. Cancer 2007, 59, 106–114. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, W.C.; Kong, M.S.; Shi, H.N.; Walker, W.A.; Lin, C.Y.; Huang, C.T.; Lin, Y.C.; Jung, S.M.; Lin, T.Y. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br. J. Nutr. 2012, 107, 1623–1634. [Google Scholar] [CrossRef]
- Orlando, A.; Refolo, M.G.; Messa, C.; Amati, L.; Lavermicocca, P.; Guerra, V.; Russo, F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 2012, 64, 1103–1111. [Google Scholar] [CrossRef]
- Gamallat, Y.; Meyiah, A.; Kuugbee, E.D.; Hago, A.M.; Chiwala, G.; Awadasseid, A.; Bamba, D.; Zhang, X.; Shang, X.; Luo, F.; et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed. Pharmacother. 2016, 83, 536–541. [Google Scholar] [CrossRef]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C.; et al. Lactobacillus casei Exerts Anti-Proliferative Effects Accompanied by Apoptotic Cell Death and Up-Regulation of TRAIL in Colon Carcinoma Cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Hsieh, Y.-M.; Huang, C.-C.; Tsai, C.-C. Inhibitory Effects of Probiotic Lactobacillus on the Growth of Human Colonic Carcinoma Cell Line HT-29. Molecules 2017, 22, 107. [Google Scholar] [CrossRef]
- Jacouton, E.; Chain, F.; Sokol, H.; Langella, P.; Bermúdez-Humarán, L.G. Probiotic Strain Lactobacillus casei BL23 Prevents Colitis-Associated Colorectal Cancer. Front. Immunol. 2017, 8, 1553. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, L.K.; Sharma, M.; Chandel, D.; Shukla, G. Prophylactic intervention of probiotics (L. acidophilus, L. rhamnosus GG) and celecoxib modulate Bax-mediated apoptosis in 1,2-dimethylhydrazine-induced experimental colon carcinogenesis. BMC Cancer 2018, 18, 1111. [Google Scholar] [CrossRef] [PubMed]
- Karimi Ardestani, S.; Tafvizi, F.; Tajabadi Ebrahimi, M. Heat-killed probiotic bacteria induce apoptosis of HT-29 human colon adenocarcinoma cell line via the regulation of Bax/Bcl2 and caspases pathway. Hum. Exp. Toxicol. 2019, 38, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Liu, S.; Hu, C.; Liu, C. Single probiotic supplement suppresses colitis-associated colorectal tumorigenesis by modulating inflammatory development and microbial homeostasis. J. Gastroenterol. Hepatol. 2019, 34, 1182–1192. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Gao, J.; Jiang, X.; Tao, M.; Zeng, X.; Wu, Z.; Pan, D. Lactobacillus acidophilus CICC 6074 inhibits growth and induces apoptosis in colorectal cancer cells in vitro and in HT-29 cells induced-mouse model. J. Funct. Foods 2020, 75, 104290. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, S.; Shi, J.; Xie, Q.; Li, N.; Guan, J.; Evivie, S.E.; Liu, F.; Li, B.; Huo, G. Effects of Lactobacillus acidophilus KLDS1.0901 on Proliferation and Apoptosis of Colon Cancer Cells. Front. Microbiol. 2022, 12, 788040. [Google Scholar] [CrossRef]
- Budu, O.; Banciu, C.D.; Soica, C.; Lighezan, D.F.; Milan, A.; Prodea, A.; Mioc, A.; Mioc, M.; Mardale, G.; Sima, L. Lacticaseibacillus rhamnosus—A Promising Tool for Colorectal Cancer Treatment. Processes 2023, 11, 781. [Google Scholar] [CrossRef]
- Chen, T.; Li, B.; Zheng, K.; Liu, Y.; Zhang, Z.; Hu, H.; Qian, G.; Jiang, J. Lactobacillus paracasei R3 Alleviates Tumor Progression in Mice with Colorectal Cancer. Curr. Microbiol. 2023, 81, 38. [Google Scholar] [CrossRef]
- Aindelis, G.; Glaros, V.; Fragkoulis, K.; Mouchtari, A.; Spyridopoulou, K.; Chlichlia, K. Colon Cancer Cells Treated with Lacticaseibacillus casei Undergo Apoptosis and Release DAMPs Indicative of Immunogenic Cell Death. Probiotics Antimicrob Proteins. 2024; advance online publication. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, T.; Wang, Y.; Yang, R.; Han, Y.; Li, S.; Liu, D.; Yue, Y.; Cao, Y.; Li, B.; et al. Effects of Viable and Heat-Inactivated Bifidobacterium longum D42 on Proliferation and Apoptosis of HT-29 Human Colon Cancer Cells. Foods 2024, 13, 958. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, D.; Kim, D.; Cho, J.; Yang, J.; Chung, M.; Kim, K.; Ha, N. Inhibition of proliferation in colon cancer cell lines and harmful enzyme activity of colon bacteria by Bifidobacterium adolescentis SPM0212. Arch. Pharm. Res. 2008, 31, 468–473. [Google Scholar] [CrossRef]
- Chae, J.M.; Heo, W.; Cho, H.T.; Lee, D.H.; Kim, J.H.; Rhee, M.S.; Park, T.S.; Kim, Y.K.; Lee, J.H.; Kim, Y.J. Effects of Orally-Administered Bifidobacterium animalis subsp. lactis Strain BB12 on Dextran Sodium Sulfate-Induced Colitis in Mice. J. Microbiol. Biotechnol. 2018, 28, 1800–1805. [Google Scholar] [CrossRef]
- Shang, F.; Jiang, X.; Wang, H.; Guo, S.; Kang, S.; Xu, B.; Wang, X.; Chen, S.; Li, N.; Liu, B.; et al. Bifidobacterium longum suppresses colorectal cancer through the modulation of intestinal microbes and immune function. Front. Microbiol. 2024, 15, 1327464. [Google Scholar] [CrossRef] [PubMed]
- Yue, F.; Zeng, X.; Wang, Y.; Fang, Y.; Yue, M.; Zhao, X.; Zhu, R.; Zeng, Q.; Wei, J.; Chen, T. Bifidobacterium longum SX-1326 ameliorates gastrointestinal toxicity after irinotecan chemotherapy via modulating the P53 signaling pathway and brain-gut axis. BMC Microbiol. 2024, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Son, S.H.; Jeon, E.B.; Jung, G.H.; Lee, J.Y.; Paik, H.D. The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J. Funct. Foods 2015, 14, 513–518. [Google Scholar] [CrossRef]
- Han, K.J.; Lee, N.K.; Park, H.; Paik, H.D. Anticancer and Anti-Inflammatory Activity of Probiotic Lactococcus lactis NK34. J. Microbiol. Biotechnol. 2015, 25, 1697–1701. [Google Scholar] [CrossRef]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; et al. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yuan, W.; Yang, B.; Pei, W.; Ma, J.; Feng, Q. Clostridium butyricum inhibits the progression of colorectal cancer and alleviates intestinal inflammation via the myeloid differentiation factor 88 (MyD88)-nuclear factor-kappa B (NF-κB) signaling pathway. Ann. Transl. Med. 2022, 10, 478. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, Y.; Li, W.; Zhu, M. Clostridium butyricum Regulates the Inflammatory and Immunoregulatory Pathway Through NFKB1 in Colorectal Cancer Treatment. Probiotics Antimicrob. Proteins, 2025; advance online publication. [Google Scholar] [CrossRef]
- Thirabunyanon, M.; Boonprasom, P.; Niamsup, P. Probiotic potential of lactic acid bacteria isolated from fermented dairy milks on antiproliferation of colon cancer cells. Biotechnol. Lett. 2009, 31, 571–576. [Google Scholar] [CrossRef]
- Li, Q.; Hu, W.; Liu, W.X.; Zhao, L.Y.; Huang, D.; Liu, X.D.; Chan, H.; Zhang, Y.; Zeng, J.D.; Coker, O.O.; et al. Streptococcus thermophilus Inhibits Colorectal Tumorigenesis Through Secreting β-Galactosidase. Gastroenterology 2021, 160, 1179–1193.e14. [Google Scholar] [CrossRef]
- Chen, X.; Fruehauf, J.; Goldsmith, J.D.; Xu, H.; Katchar, K.K.; Koon, H.W.; Zhao, D.; Kokkotou, E.G.; Pothoulakis, C.; Kelly, C.P. Saccharomyces boulardii inhibits EGF receptor signaling and intestinal tumor growth in Apc(min) mice. Gastroenterology 2009, 137, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Li, J.L.; Xie, Y.H.; Wang, Y.; Shen, X.N.; Qian, Y.; Han, J.X.; Chen, Y.X.; Fang, J.Y. Saccharomyces cerevisiae may serve as a probiotic in colorectal cancer by promoting cancer cell apoptosis. J. Dig. Dis. 2020, 21, 571–582. [Google Scholar] [CrossRef]
- Shamekhi, S.; Abdolalizadeh, J.; Ostadrahimi, A.; Mohammadi, S.A.; Barzegari, A.; Lotfi, H.; Bonabi, E.; Zarghami, N. Apoptotic Effect of Saccharomyces cerevisiae on Human Colon Cancer SW480 Cells by Regulation of Akt/NF-ĸB Signaling Pathway. Probiotics Antimicrob. Proteins 2020, 12, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kahouli, I.; Malhotra, M.; Westfall, S.; Alaoui-Jamali, M.A.; Prakash, S. Design and validation of an orally administrated active L. fermentum-L. acidophilus probiotic formulation using colorectal cancer Apc Min/+ mouse model. Appl. Microbiol. Biotechnol. 2017, 101, 1999–2019. [Google Scholar] [CrossRef]
- Asadollahi, P.; Ghanavati, R.; Rohani, M.; Razavi, S.; Esghaei, M.; Talebi, M. Anti-cancer effects of Bifidobacterium species in colon cancer cells and a mouse model of carcinogenesis. PLoS ONE 2020, 15, e0232930. [Google Scholar] [CrossRef]
- Ghanavati, R.; Asadollahi, P.; Shapourabadi, M.B.; Razavi, S.; Talebi, M.; Rohani, M. Inhibitory effects of Lactobacilli cocktail on HT-29 colon carcinoma cells growth and modulation of the Notch and Wnt/β-catenin signaling pathways. Microb. Pathog. 2020, 139, 103829. [Google Scholar] [CrossRef]
- Shang, J.; Liu, L.; Yang, S.; Duan, B.; Xie, S.; Meng, X. A New Combination of Bifidobacterium bifidum and Lactococcus lactis Strains with Synergistic Effects Alleviates Colitis-Associated Colorectal Cancer. Foods 2024, 13, 3054. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Su, X.; Niu, L.; Li, N.; Xu, C.; Sun, Z.; Guo, H.; Shen, S.; Yu, M. Non-pathogenic Trojan horse Nissle1917 triggers mitophagy through PINK1/Parkin pathway to discourage colon cancer. Mater. Today Bio 2024, 29, 101273. [Google Scholar] [CrossRef]
- Niu, L.; Liu, Y.; Li, N.; Wang, Y.; Kang, L.; Su, X.; Xu, C.; Sun, Z.; Sang, W.; Xu, J.; et al. Oral probiotics microgel plus Galunisertib reduced TGF-β blockade resistance and enhanced anti-tumor immune responses in colorectal cancer. Int. J. Pharm. 2024, 652, 123810. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Li, N.; Kataria, J.; Russell, M.; Neu, J. Live and ultraviolet-inactivated Lactobacillus rhamnosus GG decrease flagellin-induced interleukin-8 production in Caco-2 cells. J. Nutr. 2008, 138, 2264–2268. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.; Yi, R.; Long, X.; Zhao, X. Effect of Lactobacillus fermentum ZS40 on the NF-κB signaling pathway in an azomethane-dextran sulfate sodium-induced colon cancer mouse model. Front. Microbiol. 2022, 13, 953905. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, H.; Chen, H.; Liu, X.; Zhao, J.; Stanton, C.; Ross, R.P.; Chen, W.; Yang, B. Bifidobacterium inhibits the progression of colorectal tumorigenesis in mice through fatty acid isomerization and gut microbiota modulation. Gut Microbes. 2025, 17, 2464945. [Google Scholar] [CrossRef]
- Maleki-Kakelar, H.; Dehghani, J.; Barzegari, A.; Barar, J.; Shirmohamadi, M.; Sadeghi, J.; Omidi, Y. Lactobacillus plantarum induces apoptosis in gastric cancer cells via modulation of signaling pathways in Helicobacter pylori. Bioimpacts 2020, 10, 65–72. [Google Scholar] [CrossRef]
- Ali, M.S.; Hussein, R.M.; Gaber, Y.; Hammam, O.A.; Kandeil, M.A. Modulation of JNK-1/ β-catenin signaling by Lactobacillus casei, inulin and their combination in 1,2-dimethylhydrazine-induced colon cancer in mice. RSC Adv. 2019, 9, 29368–29383. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shan, Y.J.; He, C.X.; Ren, M.H.; Tian, P.J.; Song, W. Effects of L. paracasei subp. paracasei X12 on cell cycle of colon cancer HT-29 cells and regulation of mTOR signalling pathway. J. Funct. Foods 2016, 21, 431–439. [Google Scholar] [CrossRef]
- Saxami, G.; Karapetsas, A.; Lamprianidou, E.; Kotsianidis, I.; Chlichlia, A.; Tassou, C.; Zoumpourliset, V.; Galanis, A. Two potential probiotic lactobacillus strains isolated from olive microbiota exhibit adhesion and anti-proliferative effects in cancer cell lines. J. Funct. Foods 2016, 24, 461–471. [Google Scholar] [CrossRef]
- Walia, S.; Kamal, R.; Dhawan, D.K.; Kanwar, S.S. Chemoprevention by Probiotics During 1,2-Dimethylhydrazine-Induced Colon Carcinogenesis in Rats. Dig. Dis. Sci. 2018, 63, 900–909. [Google Scholar] [CrossRef]
- Heydari, Z.; Rahaie, M.; Alizadeh, A.M.; Agah, S.; Khalighfard, S.; Bahmani, S. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum Probiotics on the Expression of MicroRNAs 135b, 26b, 18a and 155, and Their Involving Genes in Mice Colon Cancer. Probiotics Antimicrob. Proteins 2019, 11, 1155–1162. [Google Scholar] [CrossRef]
- Zaylaa, M.; Alard, J.; Kassaa, I.A.; Peucelle, V.; Boutillier, D.; Desramaut, J.; Rosenstiel, P.; Nguyen, H.T.T.; Dabboussi, F.; Pot, B.; et al. Autophagy: A Novel Mechanism Involved in the Anti-Inflammatory Abilities of Probiotics. Cell Physiol. Biochem. 2019, 53, 774–793. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Omrani, G.R.; Ebrahimi, B.; Montazeri-Najafabady, N. The Beneficial Effects of Probiotics via Autophagy: A Systematic Review. Biomed. Res. Int. 2021, 2021, 2931580. [Google Scholar] [CrossRef]
- Garavaglia, B.; Vallino, L.; Ferraresi, A.; Visciglia, A.; Amoruso, A.; Pane, M.; Munteanu, C.; Isidoro, C. The Anti-Inflammatory, Immunomodulatory, and Pro-Autophagy Activities of Probiotics for Colorectal Cancer Prevention and Treatment: A Narrative Review. Biomedicines 2025, 13, 1554. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, S.; Lv, H.; Peng, L.; Yang, W.; Chen, J.; Wu, Z.; Wan, C. Effect of Bifidobacterium animalis subsp. lactis SF on enhancing the tumor suppression of irinotecan by regulating the intestinal flora. Pharmacol. Res. 2022, 184, 106406. [Google Scholar] [CrossRef]
- Jouriani, F.H.; Torkamaneh, M.; Torfeh, M.; Ashrafian, F.; Aghamohammad, S.; Rohani, M. Native Lactobacillus and Bifidobacterium probiotics modulate autophagy genes and exert anti-inflammatory effect. Sci. Rep. 2025, 15, 25006. [Google Scholar] [CrossRef]
- Liu, C.; Tan, M.; Zhao, L.; Gai, M.; Zhou, T.; Yu, C.; Zhao, Z. Anticancer activity of Weizmannia coagulans MZY531 on H22 tumor-bearing mice by regulating inflammation, autophagy-dependent apoptosis, and gut microbiota. Sci. Rep. 2025, 15, 8250. [Google Scholar] [CrossRef]
- Yang, W.; Li, T.; An, S.; Chen, R.; Zhao, Y.; Cui, J.; Zhang, M.; Lu, J.; Tian, Y.; Bao, L.; et al. Ligilactobacillus salivarius LZZAY01 accelerated autophagy and apoptosis in colon cancer cells and improved gut microbiota in CAC mice. Microbiol. Spectr. 2025, 13, e0186124. [Google Scholar] [CrossRef]
- Chen, X.; Yang, G.; Song, J.H.; Xu, H.; Li, D.; Goldsmith, J.; Zeng, H.; Parsons-Wingerter, P.A.; Reinecker, H.C.; Kelly, C.P. Probiotic yeast inhibits VEGFR signaling and angiogenesis in intestinal inflammation. PLoS ONE 2013, 8, e64227. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, Y.; Li, M.; Zhang, W.; Ding, Y.; Wang, X.; Chen, D.; Liu, T.; Wang, B.; Cao, H.; et al. Clostridium butyricum inhibits epithelial-mesenchymal transition of intestinal carcinogenesis through downregulating METTL3. Cancer Sci. 2023, 114, 3114–3127. [Google Scholar] [CrossRef]
- El-Baz, A.M.; El-Mahmoudy, A.A.; Saber, S.; ElRakaiby, M.T. The coadministration of Lactobacillus probiotic augments the antitumor effect of telmisartan in rats. AMB Express 2025, 15, 38. [Google Scholar] [CrossRef]
- Wang, M.; Gao, C.; Lessing, D.J.; Chu, W. Saccharomyces cerevisiae SC-2201 Attenuates AOM/DSS-Induced Colorectal Cancer by Modulating the Gut Microbiome and Blocking Proinflammatory Mediators. Probiotics Antimicrob. Proteins 2025, 17, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, R.; Graczyk, D.; Siedlecki, P. Molecular and Cellular Mechanisms Influenced by Postbiotics. Int. J. Mol. Sci. 2021, 22, 13475. [Google Scholar] [CrossRef] [PubMed]
- Thoda, C.; Touraki, M. Probiotic-Derived Bioactive Compounds in Colorectal Cancer Treatment. Microorganisms 2023, 11, 1898. [Google Scholar] [CrossRef]
- Da, M.; Sun, J.; Ma, C.; Li, D.; Dong, L.; Wang, L.S.; Chen, F. Postbiotics: Enhancing human health with a novel concept. eFood 2024, 5, e180. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, N.K.; Paik, H.D. A Narrative Review on the Advance of Probiotics to Metabiotics. J. Microbiol. Biotechnol. 2024, 34, 487–494. [Google Scholar] [CrossRef]
- Rad, A.H.; Aghebati-Maleki, L.; Kafil, H.S.; Abbasi, A. Molecular mechanisms of postbiotics in colorectal cancer prevention and treatment. Crit. Rev. Food Sci. Nutr. 2021, 61, 1787–1803. [Google Scholar] [CrossRef]
- Kudra, A.; Kaźmierczak-Siedlecka, K.; Sobocki, B.K.; Muszyński, D.; Połom, J.; Carbone, L.; Marano, L.; Roviello, F.; Kalinowski, L.; Stachowska, E. Postbiotics in oncology: Science or science fiction? Front. Microbiol. 2023, 14, 1182547. [Google Scholar] [CrossRef]
- Balendra, V.; Rosenfeld, R.; Amoroso, C.; Castagnone, C.; Rossino, M.G.; Garrone, O.; Ghidini, M. Postbiotics as Adjuvant Therapy in Cancer Care. Nutrients 2024, 16, 2400. [Google Scholar] [CrossRef] [PubMed]
- Sudaarsan, A.S.K.; Ghosh, A.R. Appraisal of postbiotics in cancer therapy. Front. Pharmacol. 2024, 15, 1436021. [Google Scholar] [CrossRef]
- Xie, W.; Zhong, Y.S.; Li, X.J.; Kang, Y.K.; Peng, Q.Y.; Ying, H.Z. Postbiotics in colorectal cancer: Intervention mechanisms and perspectives. Front. Microbiol. 2024, 15, 1360225. [Google Scholar] [CrossRef]
- D’Amore, T.; Zolfanelli, C.; Lauciello, V.; Di Ciancia, A.; Vagliasindi, A.; Smaoui, S.; Varzakas, T. Using Postbiotics from Functional Foods for Managing Colorectal Cancer: Mechanisms, Sources, Therapeutic Potential, and Clinical Perspectives. Microorganisms 2025, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, G.; Ali, S.A. Postbiotics Implication in the Microbiota-Host Intestinal Epithelial Cells Mutualism. Probiotics Antimicrob. Proteins 2024, 16, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Green, K.M.; Rawat, M. A Comprehensive Overview of Postbiotics with a Special Focus on Discovery Techniques and Clinical Applications. Foods 2024, 13, 2937. [Google Scholar] [CrossRef]
- Amin, M.; Navidifar, T.; Saeb, S.; Barzegari, E.; Jamalan, M. Tumor-targeted induction of intrinsic apoptosis in colon cancer cells by Lactobacillus plantarum and Lactobacillus rhamnosus strains. Mol. Biol. Rep. 2023, 50, 5345–5354. [Google Scholar] [CrossRef]
- Soraya, M.; Moazamian, E.; Shamsdin, S.A.; Dehghani, M. Bifidobacterium apoptosis induction by measuring bax and caspases on SW948 human colon cancer cell line. Int. J. Biochem. Cell Biol. 2025, 186, 106813. [Google Scholar] [CrossRef]
- Aimaier, R.; Li, H.; Cao, W.; Cao, X.; Zhang, H.; You, J.; Zhao, J.; Zhang, Q.; Yin, L.; Mei, Q.; et al. The Secondary Metabolites of Bacillus subtilis Strain Z15 Induce Apoptosis in Hepatocellular Carcinoma Cells. Probiotics Antimicrob. Proteins 2025, 17, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Salek, F.; Mirzaei, H.; Khandaghi, J.; Javadi, A.; Nami, Y. Apoptosis induction in cancer cell lines and anti-inflammatory and anti-pathogenic properties of proteinaceous metabolites secreted from potential probiotic Enterococcus faecalis KUMS-T48. Sci. Rep. 2023, 13, 7813. [Google Scholar] [CrossRef]
- Dubey, V.; Ghosh, A.R.; Bishayee, K.; Khuda-Bukhsh, A.R. Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: In vitro and in vivo approaches. J. Funct. Foods. 2016, 23, 66–79. [Google Scholar] [CrossRef]
- Pakbin, B.; Allahyari, S.; Dibazar, S.P.; Peymani, A.; Haghverdi, M.K.; Taherkhani, K.; Javadi, M.; Mahmoudi, R. Anticancer Properties of Saccharomyces boulardii Metabolite Against Colon Cancer Cells. Probiotics Antimicrob. Proteins 2024, 16, 224–232. [Google Scholar] [CrossRef]
- Konishi, H.; Isozaki, S.; Kashima, S.; Moriichi, K.; Ichikawa, S.; Yamamoto, K.; Yamamura, C.; Ando, K.; Ueno, N.; Akutsu, H.; et al. Probiotic Aspergillus oryzae produces anti-tumor mediator and exerts anti-tumor effects in pancreatic cancer through the p38 MAPK signaling pathway. Sci. Rep. 2021, 11, 11070. [Google Scholar] [CrossRef]
- Zhong, B.; Zhao, Y.; Gao, L.; Yang, G.; Gao, Y.; Li, F.; Li, S. Anticancer Effects of Weizmannia coagulans MZY531 Postbiotics in CT26 Colorectal Tumor-Bearing Mice by Regulating Apoptosis and Autophagy. Life 2024, 14, 1334. [Google Scholar] [CrossRef]
- Rezaie, N.; Aghamohammad, S.; Haj Agha Gholizadeh Khiavi, E.; Khatami, S.; Sohrabi, A.; Rohani, M. The comparative anti-oxidant and anti-inflammatory efficacy of postbiotics and probiotics through Nrf-2 and NF-kB pathways in DSS-induced colitis model. Sci. Rep. 2024, 14, 11560. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, N.; Aghamohammad, S.; Khiavi, E.H.A.G.; Talebi, M.; Pourshafie, M.R.; Rohani, M. The Analysis and Comparison of Anti-Inflammatory and Antioxidant Characteristics of Postbiotic and Paraprobiotic Derived From Novel Native Probiotic Cocktail in DSS-Induced Colitic Mice. Food Sci. Nutr. 2025, 13, e70034. [Google Scholar] [CrossRef]
- Jin, Y.; Xu, X.; Huang, K.; Liang, Z. Pre-Administration of Saccharomyces boulardii-Derived Postbiotics Effectively Prevents Dextran Sulfate Sodium-Induced Colitis in Mice. Foods 2025, 14, 1109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hong, T.; Yu, Q.; Nie, S.; Gong, D.; Xiong, T.; Xie, M. Exopolysaccharides from Lactobacillus plantarum NCU116 induce c-Jun dependent Fas/Fasl-mediated apoptosis via TLR2 in mouse intestinal epithelial cancer cells. Sci. Rep. 2017, 7, 14247. [Google Scholar] [CrossRef]
- Du, Y.; Liu, L.; Yan, W.; Li, Y.; Li, Y.; Cui, K.; Yu, P.; Gu, Z.; Zhang, W.; Feng, J.; et al. The anticancer mechanisms of exopolysaccharide from Weissella cibaria D-2 on colorectal cancer via apoptosis induction. Sci. Rep. 2023, 13, 21117. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, S.; Yun, H.S.; Oh, S.; Kim, S.H. Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells. Lett. Appl. Microbiol. 2010, 51, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Abdelnasser, S.M.; Abu-Shahba, N. Bacillus sonorinses derived exopolysaccharide enhances cell cycle arrest, apoptosis, necrosis, autophagy and COX-2 down regulation in liver cancer cells. Biotechnol. Rep. 2024, 43, e00848. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, H.; Xiong, S.; Chen, X.; Gao, X.; Huang, P.; Zou, J.; Cao, H. Lactobacillus plantarum SMUM211204 Exopolysaccharides Have Tumor-Suppressive Effects on Colorectal Cancer by Regulating Autophagy via the mTOR Pathway. J. Agric. Food Chem. 2025, 73, 5931–5946. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, M.; Yao, M.; Naseeb, J.; Sarwar, A.; Yang, Z.; Aziz, T.; Alhomrani, M.; Alsanie, W.F.; Alamri, A.S. Lactiplantibacillus plantarum NMGL2 exopolysaccharide ameliorates DSS-induced IBD in mice mainly by regulation of intestinal tight junction and NF-κB p65 protein expression. Front. Microbiol. 2024, 15, 1491727. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Sheng, K.; Wang, Y. Effect of probiotic extracellular vesicles and their applications on health and disease. J. Sci. Food Agric. 2025, 105, 3539–3549. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Debnath, N.; Pradhan, D.; Mehta, P.K.; Kumar, A.; Yadav, M.L.; Yadav, A.K. Probiotic Lactobacillus-Derived Extracellular Vesicles: Insights Into Disease Prevention and Management. Mol. Nutr. Food Res. 2025, e70013. [Google Scholar] [CrossRef]
- Yi, S.; Jung, E.; Kim, H.; Choi, J.; Kim, S.; Lim, E.K.; Kim, K.S.; Kang, T.; Jung, J. Harnessing Lactobacillus reuteri-Derived Extracellular Vesicles for Multifaceted Cancer Treatment. Small 2025, 21, e2406094. [Google Scholar] [CrossRef]
- Abedi, A.; Tafvizi, F.; Jafari, P.; Akbari, N. The inhibition effects of Lentilactobacillus buchneri-derived membrane vesicles on AGS and HT-29 cancer cells by inducing cell apoptosis. Sci. Rep. 2024, 14, 3100. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, C.; Cao, W.; Li, L.; Liu, K.; Zhu, H.; Balcha, F.; Fang, Y. Extracellular vesicles from Lacticaseibacillus paracasei PC-H1 inhibit HIF-1α-mediated glycolysis of colon cancer. Future Microbiol. 2024, 19, 227–239. [Google Scholar] [CrossRef]
- Salek Farrokhi, A.; Mohammadlou, M.; Abdollahi, M.; Eslami, M.; Yousefi, B. Histone Deacetylase Modifications by Probiotics in Colorectal Cancer. J. Gastrointest. Cancer 2020, 51, 754–764. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Viladomiu, M.; Pedragosa, M.; De Simone, C.; Carbo, A.; Shaykhutdinov, R.; Jobin, C.; Arthur, J.C.; Corl, B.A.; Vogel, H.; et al. Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR γ to suppress colitis. PLoS ONE 2012, 7, e31238. [Google Scholar] [CrossRef]
- Dubey, V.; Mishra, A.K.; Ghosh, A.R. Appraisal of the Possible Role of PPARγ Upregulation by CLA of Probiotic Pediococcus pentosaceus GS4 in Colon Cancer Mitigation. PPAR Res. 2023, 2023, 9458308. [Google Scholar] [CrossRef]
- Khaleel, S.M.; Shanshal, S.A.; Khalaf, M.M. The Role of Probiotics in Colorectal Cancer: A Review. J. Gastrointest. Cancer 2023, 54, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Fullaondo, A.; Rodríguez, J.; Tirnauca, C.; Odriozola, I.; Odriozola, A. Conjugated linoleic acid metabolite impact in colorectal cancer: A potential microbiome-based precision nutrition approach. Nutr. Rev. 2025, 83, e602–e614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, Q.; Li, T.; Lu, L.; Wang, F.; Zhang, H.; Liu, Z.; Ma, H.; Zhu, Q.; Wang, J.; et al. Lactobacillus plantarum-derived indole-3-lactic acid ameliorates colorectal tumorigenesis via epigenetic regulation of CD8+ T cell immunity. Cell Metab. 2023, 35, 943–960.e9. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Pathak, R.; Dayal, R.; Parihar, H.; Kathireshan, A.K.; Tirumalai, P.S. Colorectal Cancer Mitigation Through Probiotics: Current Evidence and Future Directions. Curr. Microbiol. 2025, 82, 339. [Google Scholar] [CrossRef]
- Tarrah, A.; de Castilhos, J.; Rossi, R.C.; Duarte, V.D.S.; Ziegler, D.R.; Corich, V.; Giacomini, A. In vitro Probiotic Potential and Anti-cancer Activity of Newly Isolated Folate-Producing Streptococcus thermophilus Strains. Front. Microbiol. 2018, 9, 2214. [Google Scholar] [CrossRef] [PubMed]
- Levit, R.; Savoy de Giori, G.; de Moreno de LeBlanc, A.; LeBlanc, J.G. Folate-producing lactic acid bacteria reduce inflammation in mice with induced intestinal mucositis. J. Appl. Microbiol. 2018, 125, 1494–1501. [Google Scholar] [CrossRef]
- Morsli, D.S.; Tbahriti, H.F.; Rahli, F.; Mahammi, F.Z.; Nagdalian, A.; Hemeg, H.A.; Imran, M.; Rauf, A.; Shariati, M.A. Probiotics in colorectal cancer prevention and therapy: Mechanisms, benefits, and challenges. Discov. Oncol. 2025, 16, 406. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Seok, H.; Ha, E.M. GABA-producing Lactobacillus plantarum inhibits metastatic properties and induces apoptosis of 5-FU-resistant colorectal cancer cells via GABAB receptor signaling. J. Microbiol. 2021, 59, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Hsu, L.J.; Lee, H.L.; Lin, C.P.; Huang, S.W.; Lai, C.J.; Lin, C.W.; Chen, W.T.; Chen, Y.J.; Lin, Y.C.; et al. Lactobacillus Attenuate the Progression of Pancreatic Cancer Promoted by Porphyromonas Gingivalis in K-rasG12D Transgenic Mice. Cancer 2020, 12, 3522. [Google Scholar] [CrossRef]
- Chen, S.M.; Chieng, W.W.; Huang, S.W.; Hsu, L.J.; Jan, M.S. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci. Rep. 2020, 10, 20319. [Google Scholar] [CrossRef]
- Mahdy, M.S.; Azmy, A.F.; Dishisha, T.; Mohamed, W.R.; Ahmed, K.A.; Hassan, A.; Aidy, S.E.; El-Gendy, A.O. Irinotecan-gut microbiota interactions and the capability of probiotics to mitigate Irinotecan-associated toxicity. BMC Microbiol. 2023, 23, 53. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Su, L.; Cao, M.; Sun, Y.; Dai, J.; He, Y.; Li, W.; Ge, W.; Lv, X.; Zhang, Q.; et al. Improvement and Recovery of Intestinal Flora Disorder Caused by Ciprofloxacin Using Lactic Acid Bacteria. Probiotics Antimicrob. Proteins, 2024; advance online publication. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; Babalghith, A.O.; Faidah, H.; Ahmed, F.; Khanam, A.; Mozaffar, B.; Kambal, N.; et al. Probiotics and Cancer: Mechanistic Insights and Organ-Specific Impact. Biomolecules 2025, 15, 879. [Google Scholar] [CrossRef]
- Kaeid Sharaf, L.; Shukla, G. Probiotics (Lactobacillus acidophilus and Lactobacillus rhamnosus GG) in Conjunction with Celecoxib (selective COX-2 inhibitor) Modulated DMH-Induced Early Experimental Colon Carcinogenesis. Nutr. Cancer 2018, 70, 946–955. [Google Scholar] [CrossRef]
- Nada, H.G.; Sudha, T.; Darwish, N.H.E.; Mousa, S.A. Lactobacillus acidophilus and Bifidobacterium longum exhibit antiproliferation, anti-angiogenesis of gastric and bladder cancer: Impact of COX2 inhibition. PharmaNutrition 2020, 14, 100219. [Google Scholar] [CrossRef]
- Baldwin, C.; Millette, M.; Oth, D.; Ruiz, M.T.; Luquet, F.M.; Lacroix, M. Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr. Cancer 2010, 62, 371–378. [Google Scholar] [CrossRef]
- Genaro, S.C.; Lima de Souza Reis, L.S.; Reis, S.K.; Rabelo Socca, E.A.; Fávaro, W.J. Probiotic supplementation attenuates the aggressiveness of chemically induced colorectal tumor in rats. Life Sci. 2019, 237, 116895. [Google Scholar] [CrossRef]
- Li, Y.; He, P.; Chen, Y.; Hu, J.; Deng, B.; Liu, C.; Yu, B.; Dong, W. Microbial metabolite sodium butyrate enhances the anti-tumor efficacy of 5-fluorouracil against colorectal cancer by modulating PINK1/Parkin signaling and intestinal flora. Sci. Rep. 2024, 14, 13063. [Google Scholar] [CrossRef]
- Salek, S.; Moazamian, E.; Mohammadi Bardbori, A.; Shamsdin, S.A. The anticancer effect of potential probiotic L. fermentum and L. plantarum in combination with 5-fluorouracil on colorectal cancer cells. World J. Microbiol. Biotechnol. 2024, 40, 139. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Liu, C.Y.; Lee, H.C.; Huang, Y.H.; Li, L.H.; Chiau, J.C.; Wang, T.E.; Chu, C.H.; Shih, S.C.; Tsai, T.H.; et al. Lactobacillus casei Variety rhamnosus Probiotic Preventively Attenuates 5-Fluorouracil/Oxaliplatin-Induced Intestinal Injury in a Syngeneic Colorectal Cancer Model. Front. Microbiol. 2018, 9, 983. [Google Scholar] [CrossRef]
- Kim, H.J.; An, J.; Ha, E.M. Lactobacillus plantarum-derived metabolites sensitize the tumor-suppressive effects of butyrate by regulating the functional expression of SMCT1 in 5-FU-resistant colorectal cancer cells. J. Microbiol. 2022, 60, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Dong, Y.; Zhang, B.; Wang, H.; Peter, C.C.K.; Gao, P.; Fu, H.; Gao, Y. Bifidobacterium Infantis Ameliorates Chemotherapy-Induced Intestinal Mucositis Via Regulating T Cell Immunity in Colorectal Cancer Rats. Cell Physiol. Biochem. 2017, 42, 2330–2341. [Google Scholar] [CrossRef]
- Badgeley, A.; Anwar, H.; Modi, K.; Murphy, P.; Lakshmikuttyamma, A. Effect of probiotics and gut microbiota on anti-cancer drugs: Mechanistic perspectives. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188494. [Google Scholar] [CrossRef]
- Ramesh, A.; Srinivasan, D.; Subbarayan, R.; Chauhan, A.; Krishnamoorthy, L.; Kumar, J.; Krishnan, M.; Shrestha, R. Enhancing Colorectal Cancer Treatment: The Role of Bifidobacterium in Modulating Gut Immunity and Mitigating Capecitabine-Induced Toxicity. Mol. Nutr. Food Res. 2025, 69, e70023. [Google Scholar] [CrossRef] [PubMed]
- Petrariu, O.A.; Barbu, I.C.; Niculescu, A.G.; Constantin, M.; Grigore, G.A.; Cristian, R.E.; Mihaescu, G.; Vrancianu, C.O. Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front Microbiol. 2024, 14, 1296447. [Google Scholar] [CrossRef]
- Lau, L.Y.J.; Quek, S.Y. Probiotics: Health benefits, food application, and colonization in the human gastrointestinal tract. Food Bioeng. 2024, 3, 41–64. [Google Scholar] [CrossRef]
- Appleyard, C.B.; Cruz, M.L.; Isidro, A.A.; Arthur, J.C.; Jobin, C.; De Simone, C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G1004–G1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fan, X.; Fang, B.; Zhu, C.; Zhu, J.; Ren, F. Effects of Lactobacillus salivarius Ren on cancer prevention and intestinal microbiota in 1,2-dimethylhydrazine-induced rat model. J. Microbiol. 2015, 53, 398–405. [Google Scholar] [CrossRef]
- Kuugbee, E.D.; Shang, X.; Gamallat, Y.; Bamba, D.; Awadasseid, A.; Suliman, M.A.; Zang, S.; Ma, Y.; Chiwala, G.; Xin, Y.; et al. Structural change in microbiota by a probiotic cocktail enhances the gut barier and reduces cancer via TLR2 signaling in a rat model of colon cancer. Dig. Dis. Sci. 2016, 61, 2908–2920. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sung, C.Y.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef]
- Chandel, D.; Sharma, M.; Chawla, V.; Sachdeva, N.; Shukla, G. Isolation, characterization and identification of antigenotoxic and anticancerous indigenous probiotics and their prophylactic potential in experimental colon carcinogenesis. Sci. Rep. 2019, 9, 14769. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, W.; Wang, H.; Ma, Y.; Zhao, X.; Zhang, X.; Yang, H.; Qian, J.; Li, J. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-α and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol. 2019, 19, 246. [Google Scholar] [CrossRef]
- Sugimura, N.; Li, Q.; Chu, E.S.H.; Lau, H.C.H.; Fong, W.; Liu, W.; Liang, C.; Nakatsu, G.; Su, A.C.Y.; Coker, O.O.; et al. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut 2021, 71, 2011–2021. [Google Scholar] [CrossRef]
- Rehman, A.U.; Iqbal khan, A.; Xin, Y.; Yousuf, W.; Ahmad; Liang, W. Lactobacillus acidophilus CGMCC 878 impacts colorectal cancer in Sprague-Dawley rats through changing the gut microbiota. Med. Microecol. 2022, 14, 100062. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, J.; Khan, A.; Hu, T.; Wang, Y.; Salama, E.S.; Su, S.; Han, H.; Jin, W.; Li, X. A probiotic Limosilactobacillus fermentum GR-3 mitigates colitis-associated tumorigenesis in mice via modulating gut microbiome. NPJ Sci. Food. 2024, 8, 61. [Google Scholar] [CrossRef]
- Niechcial, A.; Schwarzfischer, M.; Wawrzyniak, P.; Determann, M.; Pöhlmann, D.; Wawrzyniak, M.; Gueguen, E.; Walker, M.R.; Morsy, Y.; Atrott, K.; et al. Probiotic Administration Modulates Gut Microbiota and Suppresses Tumor Growth in Murine Models of Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 4404. [Google Scholar] [CrossRef]
- Zhao, F.; Hiraishi, K.; Li, X.; Hu, Y.; Kojima, D.; Sun, Z.; Zhang, H.; Kurahara, L.H. Long-Term Tracking of the Effects of Colostrum-Derived Lacticaseibacillus rhamnosus Probio-M9 on Gut Microbiota in Mice with Colitis-Associated Tumorigenesis. Biomedicines. 2024, 12, 531. [Google Scholar] [CrossRef]
- Yue, Y.; Ye, K.; Lu, J.; Wang, X.; Zhang, S.; Liu, L.; Yang, B.; Nassar, K.; Xu, X.; Pang, X.; et al. Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment. Biomed. Pharmacother. 2020, 127, 110159. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Wang, P.; Liu, Y.; Wang, G.; Shan, Y.; Yi, Y.; Zhou, Y.; Liu, B.; Wang, X.; et al. Lactobacillus coryniformis MXJ32 administration ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated colorectal cancer via reshaping intestinal microenvironment and alleviating inflammatory response. Eur. J. Nutr. 2022, 61, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.L.; Liao, J.C.; Li, T.L.; Wu, C.J.; Chiu, Y.H. Bifidobacterium lactis ameliorates AOM/DSS-induced inflammation, dysbiosis, and colonic precancerous lesions. Appl. Microbiol. Biotechnol. 2025, 109, 69. [Google Scholar] [CrossRef]
- Panebianco, C.; Pisati, F.; Villani, A.; Andolfo, A.; Ulaszewska, M.; Bellini, E.; Ferro, C.; Lombardi, R.; Orsenigo, F.; Latiano, T.P.; et al. Counteracting gemcitabine+nab-paclitaxel induced dysbiosis in KRAS wild type and KRASG12D mutated pancreatic cancer in vivo model. Cell Death Discov. 2023, 9, 116. [Google Scholar] [CrossRef]
- Thirabunyanon, M.; Hongwittayakorn, P. Potential probiotic lactic acid bacteria of human origin induce antiproliferation of colon cancer cells via synergic actions in adhesion to cancer cells and short-chain fatty acid bioproduction. Appl. Biochem. Biotechnol. 2013, 169, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, J.; Zhang, Y.; Chen, X.; Wang, C.; Suo, H.; Song, J. An Update on the Pivotal Roles of Probiotics, Their Components, and Metabolites in Preventing Colon Cancer. Foods 2023, 12, 3706. [Google Scholar] [CrossRef]
- Caballero-Franco, C.; Keller, K.; De Simone, C.; Chadee, K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J. Physiol. Gastrointest Liver Physiol. 2007, 292, G315–G322. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.K.; Socca, E.A.R.; de Souza, B.R.; Genaro, S.C.; Durán, N.; Fávaro, W.J. Effects of probiotic supplementation on chronic inflammatory process modulation in colorectal carcinogenesis. Tissue Cell 2024, 87, 102293. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kissoon-Singh, V.; Coria, A.L.; Moreau, F.; Chadee, K. Probiotic mixture VSL#3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G34–G45. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, L.; Dou, X.; Wang, C.; Zhang, W.; Gao, K.; Liu, J.; Wang, H. Lactobacillus reuteri ZJ617 maintains intestinal integrity via regulating tight junction, autophagy and apoptosis in mice challenged with lipopolysaccharide. Oncotarget 2017, 8, 77489–77499. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luk, B.; Chang-Graham, A.L.; Hall, A.; Herrmann, B.; Ruan, W.; Endres, B.T.; Shi, Z.; Garey, K.W.; Hyser, J.M.; et al. Bifidobacterium dentium Fortifies the Intestinal Mucus Layer via Autophagy and Calcium Signaling Pathways. mBio 2019, 10, e01087-19. [Google Scholar] [CrossRef]
- Liu, M.; Xie, W.; Wan, X.; Deng, T. Clostridium butyricum protects intestinal barrier function via upregulation of tight junction proteins and activation of the Akt/mTOR signaling pathway in a mouse model of dextran sodium sulfate-induced colitis. Exp. Ther. Med. 2020, 20, 10. [Google Scholar] [CrossRef]
- Lao, J.; Yan, S.; Yong, Y.; Li, Y.; Wen, Z.; Zhang, X.; Ju, X.; Li, Y. Lacticaseibacillus casei IB1 Alleviates DSS-Induced Inflammatory Bowel Disease by Regulating the Microbiota and Restoring the Intestinal Epithelial Barrier. Microorganisms 2024, 12, 1379. [Google Scholar] [CrossRef]
- Liu, S.; Cai, P.; You, W.; Yang, M.; Tu, Y.; Zhou, Y.; Valencak, T.G.; Xiao, Y.; Wang, Y.; Shan, T. Enhancement of gut barrier integrity by a Bacillus subtilis secreted metabolite through the GADD45A-Wnt/β-catenin pathway. iMeta 2025, 4, e70005. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, Y.; Guo, W.; Sun, Y.; Zhang, W.; Zhao, Q.; Zhang, Y.; Jiang, Y. Effects of Lactobacillus paracei JY062 Postbiotic on Intestinal Barrier, Immunity, and Gut Microbiota. Nutrients 2025, 17, 1272. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, B.; Xu, H.; Tang, L.; Li, Y.; Gong, L.; Wang, Y.; Li, W. Probiotic Bacillus Attenuates Oxidative Stress-Induced Intestinal Injury via p38-Mediated Autophagy. Front Microbiol. 2019, 10, 2185. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen, S.; de Moreno de LeBlanc, A.; Levit, R.; Azevedo, V.; Langella, P.; Bermúdez-Humarán, L.G.; LeBlanc, J.G. Anti-cancer effect of lactic acid bacteria expressing antioxidant enzymes or IL-10 in a colorectal cancer mouse model. Int. Immunopharmacol. 2017, 42, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Kavyani, B.; Ahmadi, S.; Nabizadeh, E.; Abdi, M. Anti-oxidative activity of probiotics; focused on cardiovascular disease, cancer, aging, and obesity. Microb. Pathog. 2024, 196, 107001. [Google Scholar] [CrossRef]
- Nowak, A.; Paliwoda, A.; Błasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 3456–3467. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kanmani, P.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Thirunavukkarasu, C.; Arul, V. Lactobacillus plantarum AS1 isolated from south Indian fermented food Kallappam suppress 1,2-dimethyl hydrazine (DMH)-induced colorectal cancer in male Wistar rats. Appl. Biochem. Biotechnol. 2012, 166, 620–631. [Google Scholar] [CrossRef]
- de Moreno de LeBlanc, A.; LeBlanc, J.G.; Perdigón, G.; Miyoshi, A.; Langella, P.; Azevedo, V.; Sesma, F. Oral administration of a catalase-producing Lactococcus lactis can prevent a chemically induced colon cancer in mice. J. Med. Microbiol. 2008, 57 Pt 1, 100–105. [Google Scholar] [CrossRef]
- Finamore, A.; Ambra, R.; Nobili, F.; Garaguso, I.; Raguzzini, A.; Serafini, M. Redox Role of Lactobacillus casei Shirota Against the Cellular Damage Induced by 2,2′-Azobis (2-Amidinopropane) Dihydrochloride-Induced Oxidative and Inflammatory Stress in Enterocytes-Like Epithelial Cells. Front. Immunol. 2018, 9, 1131. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Mu, G.; Xu, Y.; Wang, X.; Tuo, Y.; Qian, F. Protecting Effect of Bacillus coagulans T242 on HT-29 Cells Against AAPH-Induced Oxidative Damage. Probiotics Antimicrob. Proteins 2022, 14, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yi, B.; Tang, Z.; Chen, X.; Li, M.; Xu, T.; Zhao, Z.; Tang, C. Lactobacillus casei combined with Lactobacillus reuteri alleviate pancreatic cancer by inhibiting TLR4 to promote macrophage M1 polarization and regulate gut microbial homeostasis. BMC Cancer. 2023, 23, 1044. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, A.M.; El-Kharashi, O.A.; Hamam, G.G.; Nabih, E.S.; Magdy, Y.M.; Abd El Samad, A.A. Involvement of TLR4/CXCL9/PREX-2 pathway in the development of hepatocellular carcinoma (HCC) and the promising role of early administration of lactobacillus plantarum in Wistar rats. Tissue Cell 2019, 60, 38–47. [Google Scholar] [CrossRef]
- Dai, C.; Zheng, C.Q.; Meng, F.J.; Zhou, Z.; Sang, L.X.; Jiang, M. VSL#3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-κB pathway in rat model of DSS-induced colitis. Mol. Cell Biochem. 2013, 374, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, A.H.; Casolaro, V.; Bermúdez-Humarán, L.G.; Keyvani, H.; Taghinezhad, S.S. Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response. Gut Microbes 2021, 13, 1–17. [Google Scholar] [CrossRef]
- Aghamohammad, S.; Sepehr, A.; Miri, S.T.; Najafi, S.; Rohani, M.; Pourshafiea, M.R. The effects of the probiotic cocktail on modulation of the NF-kB and JAK/STAT signaling pathways involved in the inflammatory response in bowel disease model. BMC Immunol. 2022, 23, 8. [Google Scholar] [CrossRef]
- Liu, M.; Ding, J.; Zhang, H.; Shen, J.; Hao, Y.; Zhang, X.; Qi, W.; Luo, X.; Zhang, T.; Wang, N. Lactobacillus casei LH23 modulates the immune response and ameliorates DSS-induced colitis via suppressing JNK/p-38 signal pathways and enhancing histone H3K9 acetylation. Food Funct. 2020, 11, 5473–5485. [Google Scholar] [CrossRef]
- Agah, S.; Alizadeh, A.M.; Mosavi, M.; Ranji, P.; Khavari-Daneshvar, H.; Ghasemian, F.; Bahmani, S.; Tavassoli, A. More Protection of Lactobacillus acidophilus Than Bifidobacterium bifidum Probiotics on Azoxymethane-Induced Mouse Colon Cancer. Probiotics Antimicrob. Proteins 2019, 11, 857–864. [Google Scholar] [CrossRef]
- Owens, J.A.; Saeedi, B.J.; Naudin, C.R.; Hunter-Chang, S.; Barbian, M.E.; Eboka, R.U.; Askew, L.; Darby, T.M.; Robinson, B.S.; Jones, R.M. Lactobacillus rhamnosus GG Orchestrates an Antitumor Immune Response. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Shen, S.; Zhang, T.; Zhang, J.; Huang, S.; Sun, Z.; Zhang, H. Lacticaseibacillus rhamnosus Probio-M9 enhanced the antitumor response to anti-PD-1 therapy by modulating intestinal metabolites. EBioMedicine 2023, 91, 104533. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Y.; Fu, Z.J.; Wang, Y.Z.; Zhang, C.; Chen, Q.W.; An, J.X.; Zhang, X.Z. Probiotics functionalized with a gallium-polyphenol network modulate the intratumor microbiota and promote anti-tumor immune responses in pancreatic cancer. Nat. Commun. 2024, 15, 7096. [Google Scholar] [CrossRef]
- Hu, J.; Wang, C.; Ye, L.; Yang, W.; Huang, H.; Meng, F.; Shi, S.; Ding, Z. Anti-tumour immune effect of oral administration of Lactobacillus plantarum to CT26 tumourbearing mice. J. Biosci. 2015, 40, 269–279. [Google Scholar] [CrossRef]
- Mahooti, M.; Abdolalipour, E.; Sanami, S.; Zare, D. Inflammatory Modulation Effects of Probiotics: A Safe and Promising Modulator for Cancer Prevention. Curr. Microbiol. 2024, 81, 372. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, X.; Li, Y.; Liu, X.; Fang, L.; Jiang, Z. Probiotics and the Role of Dietary Substrates in Maintaining the Gut Health: Use of Live Microbes and Their Products for Anticancer Effects against Colorectal Cancer. J. Microbiol. Biotechnol. 2024, 34, 1933–1946. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Dong, S.; Ismael, M.; Shan, Y.; Wang, X.; Lü, X. Lacticaseibacillus rhamnosus LS8 Ameliorates Azoxymethane/Dextran Sulfate Sodium-Induced Colitis-Associated Tumorigenesis in Mice via Regulating Gut Microbiota and Inhibiting Inflammation. Probiotics Antimicrob. Proteins 2022, 14, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, P.; Li, S.; Yu, T.; Lai, X.; He, Y. Study on the effect and mechanism of Lacticaseibacillus rhamnosus AFY06 on inflammation-associated colorectal cancer induced by AOM/DSS in mice. Front. Microbiol. 2024, 15, 1382781. [Google Scholar] [CrossRef]
- Gryaznova, M.; Burakova, I.; Smirnova, Y.; Morozova, P.; Chirkin, E.; Gureev, A.; Mikhaylov, E.; Korneeva, O.; Syromyatnikov, M. Effect of Probiotic Bacteria on the Gut Microbiome of Mice with Lipopolysaccharide-Induced Inflammation. Microorganisms 2024, 12, 1341. [Google Scholar] [CrossRef]
- Gao, C.; Major, A.; Rendon, D.; Lugo, M.; Jackson, V.; Shi, Z.; Mori-Akiyama, Y.; Versalovic, J. Histamine H2 Receptor-Mediated Suppression of Intestinal Inflammation by Probiotic Lactobacillus reuteri. mBio 2015, 6, e01358-15. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; Bolivar, S.; Ávila-Román, J.; Alcaide, A.; Fiorucci, S.; Motilva, V. Inhibition of chronic ulcerative colitis-associated adenocarcinoma development in mice by VSL#3. Inflamm. Bowel Dis. 2015, 21, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Brandi, J.; Di Carlo, C.; Manfredi, M.; Federici, F.; Bazaj, A.; Rizzi, E.; Cornaglia, G.; Manna, L.; Marengo, E.; Cecconi, D. Investigating the Proteomic Profile of HT-29 Colon Cancer Cells After Lactobacillus kefiri SGL 13 Exposure Using the SWATH Method. J. Am. Soc. Mass. Spectrom. 2019, 30, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yuan, L.; Deng, J.; Yang, Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front. Cell Infect. Microbiol. 2015, 5, 26. [Google Scholar] [CrossRef]
- Nenu, I.; Baldea, I.; Coadă, C.A.; Crăciun, R.C.; Moldovan, R.; Tudor, D.; Petrushev, B.; Toma, V.A.; Ştefanescu, H.; Procopeţ, B.; et al. Lactobacillus rhamnosus probiotic treatment modulates gut and liver inflammatory pathways in a hepatocellular carcinoma murine model. A preliminary study. Food Chem. Toxicol. 2024, 183, 114314. [Google Scholar] [CrossRef]
- Chung, I.C.; OuYang, C.N.; Yuan, S.N.; Lin, H.C.; Huang, K.Y.; Wu, P.S.; Liu, C.Y.; Tsai, K.J.; Loi, L.K.; Chen, Y.J.; et al. Pretreatment with a Heat-Killed Probiotic Modulates the NLRP3 Inflammasome and Attenuates Colitis-Associated Colorectal Cancer in Mice. Nutrients 2019, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Jin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef]
- Gosai, V.; Ambalam, P.; Raman, M.; Kothari, C.R.; Kothari, R.K.; Vyas, B.R.; Sheth, N.R. Protective effect of Lactobacillus rhamnosus 231 against N-Methyl-N’-nitro-N-nitrosoguanidine in animal model. Gut Microbes 2011, 2, 319–325. [Google Scholar] [CrossRef][Green Version]
- Verma, A.; Shukla, G. Probiotics Lactobacillus rhamnosus GG, Lactobacillus acidophilus suppresses DMH-induced procarcinogenic fecal enzymes and preneoplastic aberrant crypt foci in early colon carcinogenesis in Sprague Dawley rats. Nutr. Cancer 2013, 65, 84–91. [Google Scholar] [CrossRef]
- Chang, J.H.; Shim, Y.Y.; Cha, S.K.; Reaney, M.J.T.; Chee, K.M. Effect of Lactobacillus acidophilus KFRI342 on the development of chemically induced precancerous growths in the rat colon. J. Med. Microbiol. 2012, 61 Pt 3, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Davoodvandi, A.; Fallahi, F.; Tamtaji, O.R.; Tajiknia, V.; Banikazemi, Z.; Fathizadeh, H.; Abbasi-Kolli, M.; Aschner, M.; Ghandali, M.; Sahebkar, A.; et al. An Update on the Effects of Probiotics on Gastrointestinal Cancers. Front. Pharmacol. 2021, 12, 680400. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, Z.; Sun, Y.; Sun, X.; Jin, Y.; Zhu, J.; Yu, J.; Wu, T. Enteric Delivery of Probiotics: Challenges, Techniques, and Activity Assays. Foods 2025, 14, 2318. [Google Scholar] [CrossRef]
- Zhong, H.; Jiang, J.; Hussain, M.; Zhang, H.; Chen, L.; Guan, R. The Encapsulation Strategies for Targeted Delivery of Probiotics in Preventing and Treating Colorectal Cancer: A Review. Adv. Sci. 2025, 12, e2500304. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Tarapoulouzi, M.; Varzakas, T.; Jafari, S.M. Application of Encapsulation Strategies for Probiotics: From Individual Loading to Co-Encapsulation. Microorganisms 2023, 11, 2896. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shan, X.; Zhu, B.; Cai, Y.; He, Z.; Zhou, L.; Yin, L.; Liu, Y.; Liu, K.; Zhang, T.; et al. 5-Fluorouracil Loaded Prebiotic-Probiotic Liposomes Modulating Gut Microbiota for Improving Colorectal Cancer Chemotherapy. Adv. Healthc. Mater. 2025, 14, e2403587. [Google Scholar] [CrossRef]
- Wang, J.-L.; Yeh, C.-H.; Huang, S.-H.; Wu, L.S.-H.; Chen, M.C.-M. Effects of Resistant-Starch-Encapsulated Probiotic Cocktail on Intestines Damaged by 5-Fluorouracil. Biomedicines 2024, 12, 1912. [Google Scholar] [CrossRef]
- Alkushi, A.G.; Abdelfattah-Hassan, A.; Eldoumani, H.; Elazab, S.T.; Mohamed, S.A.M.; Metwally, A.S.; El-Shetry, E.S.; Saleh, A.A.; ElSawy, N.A.; Ibrahim, D. Probiotics-loaded nanoparticles attenuated colon inflammation, oxidative stress, and apoptosis in colitis. Sci. Rep. 2022, 12, 5116. [Google Scholar] [CrossRef]
- Varada, V.V.; Kumar, S.; Balaga, S.; Thanippilly, A.J.; Pushpadass, H.A.; Rashmi, M.H.; Jangir, B.L.; Tyagi, N.; Samanta, A.K. Oral delivery of electrohydrodynamically encapsulated Lactiplantibacillus plantarum CRD7 modulates gut health, antioxidant activity, and cytokines-related inflammation and immunity in mice. Food Funct. 2024, 15, 10761–10781. [Google Scholar] [CrossRef]
- Elmetwalli, A.; Abdel-Monem, M.O.; El-Far, A.H.; Ghaith, G.S.; Albalawi, N.A.N.; Hassan, J.; Ismail, N.F.; El-Sewedy, T.; Alnamshan, M.M.; ALaqeel, N.K.; et al. Probiotic-derived silver nanoparticles target mTOR/MMP-9/BCL-2/dependent AMPK activation for hepatic cancer treatment. Med. Oncol. 2024, 41, 106. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, L.; Huang, W.; Gao, Z.; Jin, M. Current Advances of Nanomaterial-Based Oral Drug Delivery for Colorectal Cancer Treatment. Nanomaterials 2024, 14, 557. [Google Scholar] [CrossRef]
- Fareez, I.M.; Lim, S.M.; Ramasamy, K. Chemoprevention by Microencapsulated Lactiplantibacillus Plantarum LAB12 Against Orthotopic Colorectal Cancer Mice is Associated with Apoptosis and Anti-angiogenesis. Probiotics Antimicrob. Proteins 2024, 16, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.W.; Li, R.Q.; An, J.X.; Xie, T.Q.; Han, Z.Y.; Xu, R.; Fang, Y.; Zhang, X.Z. Prebiotics-Encapsulated Probiotic Spores Regulate Gut Microbiota and Suppress Colon Cancer. Adv. Mater. 2020, 32, e2004529. [Google Scholar] [CrossRef]
- Pandey, R.P.; Dhiman, R.; Chang, C.M. The potential of nanoencapsulated probiotics in the modulation of the gut microbiome. Nanomedicine 2025, 20, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.R.; Isabella, V.M.; Li, N.; Kurtz, C.B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 2020, 11, 1738. [Google Scholar] [CrossRef]
- Cho, Y.S.; Han, K.; Xu, J.; Moon, J.J. Novel strategies for modulating the gut microbiome for cancer therapy. Adv. Drug Deliv. Rev. 2024, 210, 115332. [Google Scholar] [CrossRef]
- Han, H.; Zhang, Y.; Tang, H.; Zhou, T.; Khan, A. A Review of the Use of Native and Engineered Probiotics for Colorectal Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 3896. [Google Scholar] [CrossRef]
- Parvin, T.; Sadras, S.R. Advanced probiotics: Bioengineering and their therapeutic application. Mol. Biol. Rep. 2024, 51, 361. [Google Scholar] [CrossRef]
- Zalatan, J.G.; Petrini, L.; Geiger, R. Engineering bacteria for cancer immunotherapy. Curr. Opin. Biotechnol. 2024, 85, 103061. [Google Scholar] [CrossRef] [PubMed]
- Zahedifard, Z.; Mahmoodi, S.; Ghasemian, A. Genetically Engineered Bacteria as a Promising Therapeutic Strategy Against Cancer: A Comprehensive Review. Biotechnol. Appl. Biochem. 2025; advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Liu, S.; Wu, X. Engineered probiotics as live biotherapeutics for diagnosis and treatment of human diseases. Crit. Rev. Microbiol. 2024, 50, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Rebeck, O.N.; Wallace, M.J.; Prusa, J.; Ning, J.; Evbuomwan, E.M.; Rengarajan, S.; Habimana-Griffin, L.; Kwak, S.; Zahrah, D.; Tung, J.; et al. A yeast-based oral therapeutic delivers immune checkpoint inhibitors to reduce intestinal tumor burden. Cell Chem. Biol. 2025, 32, 98–110.e7. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Yan, X.; Liu, X.Y.; Zhang, D.; Zhang, Y.D.; Li, Z.H.; Liu, X.; Wu, F.; Chen, G.Q. Construction of a sustainable 3-hydroxybutyrate-producing probiotic Escherichia coli for treatment of colitis. Cell Mol. Immunol. 2021, 18, 2344–2357. [Google Scholar] [CrossRef]
- Li, M.; Liu, N.; Zhu, J.; Wu, Y.; Niu, L.; Liu, Y.; Chen, L.; Bai, B.; Miao, Y.; Yang, Y.; et al. Engineered probiotics with sustained release of interleukin-2 for the treatment of inflammatory bowel disease after oral delivery. Biomaterials 2024, 309, 122584. [Google Scholar] [CrossRef]
- Gurbatri, C.R.; Radford, G.A.; Vrbanac, L.; Im, J.; Thomas, E.M.; Coker, C.; Taylor, S.R.; Jang, Y.U.; Sivan, A.; Rhee, K.; et al. Engineering tumor-colonizing E. coli Nissle 1917 for detection and treatment of colorectal neoplasia. Nat. Commun. 2024, 15, 646. [Google Scholar] [CrossRef]
- Yao, W.Q.; Song, W.F.; Deng, X.C.; Lin, Y.T.; Meng, R.; Wang, J.W.; Chen, W.H.; Zhang, X.Z. Harnessing the Engineered Probiotic-Nanosystem to Remodulate Tumor Extracellular Matrix and Regulate Tumor-Colonizing Bacteria for Improving Pancreatic Cancer Chemo-Immunotherapy. Small 2025, 21, e2406837. [Google Scholar] [CrossRef]
- Al-Fakhrany, O.M.; Elekhnawy, E. Next-generation probiotics: The upcoming biotherapeutics. Mol. Biol. Rep. 2024, 51, 505. [Google Scholar] [CrossRef] [PubMed]
- Jan, T.; Negi, R.; Sharma, B.; Kumar, S.; Singh, S.; Rai, A.K.; Shreaz, S.; Rustagi, S.; Chaudhary, N.; Kaur, T.; et al. Next generation probiotics for human health: An emerging perspective. Heliyon 2024, 10, e35980. [Google Scholar] [CrossRef] [PubMed]
- Vijayaganapathi, A.; Mohanasrinivasan, V. A Review of Next-Generation Probiotics-As a Gateway to Biotherapeutics. Probiotics Antimicrob. Proteins, 2025; advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Ika Krisnawati, D.; Susilowati, E.; Mutalik, C.; Kuo, T.R. Next-Generation Probiotics and Chronic Diseases: A Review of Current Research and Future Directions. J. Agric. Food Chem. 2024, 72, 27679–27700. [Google Scholar] [CrossRef]
- Naz, S.S.; Zafar, S. Analyzing potential of next-generation probiotics in cancer management. Mol. Biol. Rep. 2025, 52, 646. [Google Scholar] [CrossRef]
- Murali, S.K.; Mansell, T.J. Next generation probiotics: Engineering live biotherapeutics. Biotechnol. Adv. 2024, 72, 108336. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Lalowski, P.; Zielińska, D. The Most Promising Next-Generation Probiotic Candidates—Impact on Human Health and Potential Application in Food Technology. Fermentation 2024, 10, 444. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Hupp, T.; Duchnowska, R.; Marek-Trzonkowska, N.; Połom, K. Next-generation probiotics—do they open new therapeutic strategies for cancer patients? Gut Microbes 2022, 14, 2035659. [Google Scholar] [CrossRef] [PubMed]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Gao, J.; Zhao, R.; Li, H.; Cui, H.; Yuan, Z.; Ren, H.; Cao, B.; Wei, B. Akkermansia muciniphila-derived pentadecanoic acid enhances oxaliplatin sensitivity in gastric cancer by modulating glycolysis. Pharmacol. Res. 2024, 206, 107278. [Google Scholar] [CrossRef]
- Lee, A.H.; Rodriguez Jimenez, D.M.; Meisel, M. Limosilactobacillus reuteri—a probiotic gut commensal with contextual impact on immunity. Gut Microbes 2025, 17, 2451088. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yan, L.; Yu, X.; Guo, H.; He, Y.; Wen, S.; Yu, T.; Wang, W. The alleviating effect of Akkermansia muciniphila PROBIO on AOM/DSS-induced colorectal cancer in mice and its regulatory effect on gut microbiota. J. Funct. Foods 2024, 114, 106091. [Google Scholar] [CrossRef]
- Kang, X.; Liu, C.; Ding, Y.; Ni, Y.; Ji, F.; Lau, H.C.H.; Jiang, L.; Sung, J.J.; Wong, S.H.; Yu, J. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8+ T cells. Gut 2023, 72, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
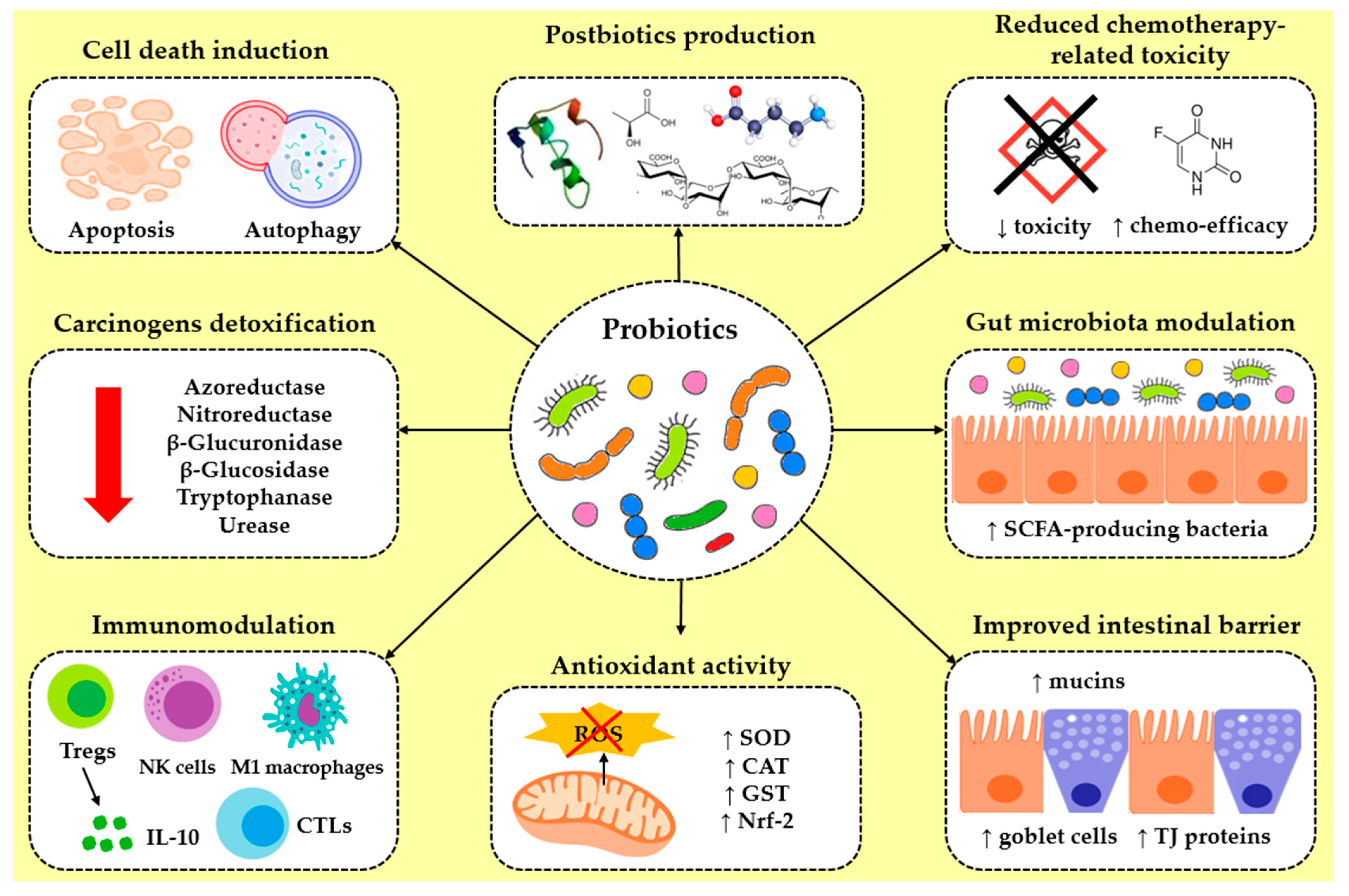
| Cancer Type | Probiotic | Cell Line | Effect/Mode of Action | Reference |
|---|---|---|---|---|
| Colorectal | Bacillus coagulans T242 | HT-29 | ↑ Nrf-2/Keap1 pathway-related protein expression, ↑ antioxidant enzymes (GSH, CAT, SOD), ↓ MDA, ↓ pro-inflammatory cytokines (IL-6, IL-8, TNF-α) | [264] |
| Bacillus polyfermenticus KU3 | LoVo, HT-29 | ↓ proliferation | [131] | |
| Bifidobacterium spp. | ||||
| B. adolescentis SPM0212 | HT-29, SW480, Caco-2 | ↓ proliferation, ↓ TNF-α, changes in cellular morphology | [127] | |
| B. longum | LoVo, SW480, SW1463 | ↓ proliferation, ↓ migration | [129] | |
| B. longum D42 | HT-29 | ↓ proliferation, ↑ LDH release, ↑ apoptosis, ↑ ROS, ↑ caspase-3, -9, ↑ Bax/↓ Bcl-2, ↓ MMP | [126] | |
| Clostridium butyricum | HCT-116 | 168 DEPs enriched in apoptosis and inflammatory pathways, ↓ NFKB1 protein level | [135] | |
| C. butyricum ATCC 19398 | HCT-116, Caco-2 | ↓ METTL3 expression, ↓ vimentin, ↓ VEGFR2, ↓ EMT, ↓ VM formation | [164] | |
| Enterococcus faecium FP51 | Caco-2 | ↓ proliferation, ↑ adherence to cancer cells, ↑ SCFAs bioproduction | [246] | |
| E. faecium RM11 | Caco-2 | ↓ proliferation | [136] | |
| Escherichia coli Nissle 1917 | CT26 | ↑ Bax/Bcl-2 ratio, ↑ caspase-3, synergistic enhancement of Gal anti-tumor efficacy by ICD induction | [146] | |
| ↑ apoptosis, ↓ MMP, PINK1/Parkin pathway activation, ↑ mitophagy, MPTP disruption, cytochrome C release | [145] | |||
| Lactobacillus spp. | ||||
| L. acidophilus CICC 6074 | HT-29 | ↓ proliferation in a dose-and time-dependent manner, ↑ apoptosis, ↓ MMP, cytochrome C release, ↑ caspase-3, -9, ↑ Bax/↓ Bcl-2 | [121] | |
| L. acidophilus KLDS1.0901 | HT-29, Caco-2 | ↓ proliferation in a dose-dependent manner, ↑ apoptosis, ↓ MMP, ↓ NF-κB and PI3K/AKT pathways | [122] | |
| L. casei ATCC 393 | HT-29 | ↓ proliferation in a dose-and time-dependent manner, ↑ apoptosis | [115] | |
| CT26, HT-29 | ↓ proliferation, ↑ caspase-3, ↑ ICD | [125] | ||
| L. casei Shirota | Caco-2/TC7 | prevention of membrane barrier disruption, ↓ ROS accumulation, ↑ GPX2 expression, ↓ p65 phosphorylation, ↑ Nrf-2 pathway | [263] | |
| L. fermentum RM28 | Caco-2 | ↓ proliferation | [136] | |
| L. fructosus C2 | Caco-2 | ↓ dextran permeability, ↓ IL-8, ↓ p-ERK and p-JNK after cells infection with ETEC or Salmonella typhimurium | [284] | |
| L. kefiri SGL 13 | HT-29 | ↓ proliferation correlated with the eIF2 and RXR activation pathways, ↑ Bax, ↓ IL-8 in cells stimulated with LPS | [283] | |
| L. paracasei, L. brevis | HT-29 | ↓ proliferation, ↑ apoptosis, ↑ Bax, ↑ caspase-3, -9, ↓ Bcl-2 | [119] | |
| L. paracasei IMPC2.1 | DLD-1 | ↓ proliferation, ↑ apoptosis | [113] | |
| L. paracasei subp. paracasei X12 | HT-29 | G1-phase arrest, ↓ cyclin E1, ↑ p27, ↓ mTOR/4EBP1 pathway | [152] | |
| L. rhamnosus (Probio-M9) | Caco-2 | prevention of LPS-induced damage of tight junction integrity | [241] | |
| L. rhamnosus GG | Caco-2 | ↓ IL-8 in cells stimulated by flagellin, ↓ NF-κB pathway | [147] | |
| HT-29, HCT-116 | apoptotic-related nuclear morphological changes, ↑ caspase-3, ↑ Bax, ↓ Bcl-2, ↓ cyclin D1, mitochondrial function impairment | [123] | ||
| L. salivarius FP25 | Caco-2 | ↓ proliferation, ↑ adherence to cancer cells, ↑ SCFAs bioproduction | [246] | |
| L. salivarius FP35 | ||||
| Lactococcus lactis NK34 | DLD-1, HT-29, LoVo | ↓ proliferation | [132] | |
| Ligilactobacillus salivarius LZZAY01 | CT26, HCT-116, SW620, NCM460 | ↓ proliferation, ↑ autophagy and apoptosis | [162] | |
| Saccharomyces boulardii | HT-29, SW480, HCT-116 | ↓ EGFR-Erk and EGFR-Akt pathways, ↑ apoptosis, ↓ HER-2, ↓ HER-3, ↓ IGF-1 receptor | [138] | |
| S. cerevisiae | SW480 | ↑ apoptosis, ↓ p-Akt1, ↓ Rel A, ↓ Bcl-XL, ↓ pro-caspase 3, -9, ↑ Bax, ↑ cleaved caspase-3, -9 | [140] | |
| Streptococcus thermophilus | HCT-116, HT-29, Caco-2 | ↓ proliferation, cell cycle arrest, ↑ apoptosis | [137] | |
| S. thermophilus CRL 808 | Caco-2 | ↑ cytotoxicity of 5-FU | [210] | |
| S. thermophilus M17PTZA496, S. thermophilus TH982 | HT-29 | anticancer activity via folate production | [209] | |
| Pediococcus pentosaceus FP3 | Caco-2 | ↓ proliferation, ↑ adherence to cancer cells, ↑ SCFAs bioproduction | [246] | |
| Various Lactobacillus strains | HT-29 | ↓ proliferation, ↑ NO secretion, ↑ Bax/Bcl-2 ratio, ↑ LDH | [116] | |
| Mixed formulations | ||||
| Bifidobacteria spp. cocktail | LS174T | ↑ apoptosis, ↓ EGFR, ↓ HER-2, ↓ PTGS-2 | [142] | |
| Bifidobacterium bifidum H3-R2 and L. lactis KLDS4.0325 | HT-29 | ↑ caspase-3, -9, ↑ Bax, ↓ Bcl-2 | [144] | |
| Lactobacillus spp. cocktail | HT-29 | ↓ proliferation, ↑ apoptosis, Notch and Wnt/β-catenin pathways modulation | [143] | |
| L. acidophilus ATCC 314 and L. fermentum NCIMB 5221 | Caco-2, CRL-1831 | ↓ proliferation and ↑ apoptosis towards cancer cells, significant protection of normal colon cells | [141] | |
| L. acidophilus CL1285 and L. casei LBC80R | CRL-2134 (LS513) | improved dose-dependent apoptotic efficacy of 5-FU, ↑ caspase-3, ↓ p21 | [220] | |
| L. pentosus B281 and L. plantarum B282 | Caco-2 | ↓ proliferation, G1-phase arrest, ↓ cyclins A, B1, B2, E | [153] | |
| Probiotic cocktail | HT-29 | ↓ JAK, ↓ TIRAP, ↓ IRAK4, ↓ NEMO, ↓ RIP, ↓ IL-6, ↓ IL-1β | [269] | |
| Probiotic cocktail | HT-29 | ↑ autophagy genes (PIK3C3, ATG14, Beclin, PIK3R4, ATG5, ATG16, ATG7, and ATG3), anti-inflammatory effects | [160] | |
| Gastric | B. polyfermenticus KU3 | AGS | ↓ proliferation | [131] |
| B. longum subsp. longum 35624 | AGS | ↓ proliferation, ↓ COX-2 expression in combination with celecoxib | [219] | |
| L. acidophilus La-14 SD-5212 | ||||
| L. paracasei IMPC2.1 | HGC-27 | ↓ proliferation, ↑ apoptosis | [113] | |
| L. plantarum | AGS, CRL-1739 | ↑ PTEN, ↓ AKT pathway | [150] | |
| L. rhamnosus GG | HGC-27 | ↓ proliferation, ↑ apoptosis, ↓ ODC activity | [111] | |
| Lactococcus lactis NK34 | AGS | ↓ proliferation | [132] | |
| Pancreatic | Aspergillus oryzae ATCC 42149 | SUIT2, Panc-1, MIA-PaCa-2 | ↓ proliferation via heptelidic acid production through the p38 MAPK pathway | [186] |
| L. casei ATCC 39392 and L. reuteri ATCC 23272 | PaCa-2, Panc-1, AsPC-1, BxPC-3 | ↓ proliferation, migration, and invasion via TLR4 suppression | [265] | |
| Cancer Type | Probiotic | Animal Model | Effect/Mode of Action | Reference |
|---|---|---|---|---|
| Colorectal | Bifidobacterium spp. | |||
| B. adolescentis SPM0212 | male Sprague Dawley rats | ↓ harmful fecal enzymes (β-glucuronidase, β-glucosidase, tryptophanase, and urease) | [127] | |
| B. animalis subsp. lactisBB12 | male C57BL/6J mice with DSS- induced cancer | colitis amelioration, ↓ TNF-α, ↑ apoptosis | [128] | |
| B. animalis subsp. lactis SF | male pathogen-free BALB/c tumor- bearing mice | enhancement of irinotecan’s antitumor effect, ↓ tumor growth and invasion, ↓ intestinal inflammation, gut microbiota modulation, ↓ TGF-β leakage, ↓ PI3K/AKT pathway, ↑ autophagy, ↑ CD4+ and CD8+ T cells differentiation in tumor tissue | [159] | |
| B. animalis subsp. lactis TCI604 | female C57BL/6 J mice with AOM/DSS- induced cancer | ↓ colonic polyps, ↓ pro-inflammatory cytokines, ↓ inflammatory immune cells, ↓ NF-κB pathway, dysbiosis reversion | [244] | |
| B. breve CCFM683 | male C57BL/6J ApcMin+ mice | ↑ CLA levels, ↓ NF-κB pathway, ↑ MUC2, ↑ Claudin-1, ↑ ZO-1, ↑ tumor cell apoptosis via the CLA-PPAR-γ axis | [149] | |
| B. infantis | Sprague Dawley rats with DMH-induced cancer | ↓ IL-6, ↓ IL-1β, ↓ TNF-α, ↓ Th1 and Th17 response, ↑ CD4+ CD25+ Foxp3+ Tregs response | [226] | |
| male BALB/c mice | ↓ tumor growth, gut microbiota composition regulation, immune function enhancement | [129] | ||
| B. longum SX-1326 | C57BL/6 mice with AOM/DSS-induced cancer | ↑ caspase-3, ↓ Bcl-2, ↑ p53 pathway, GBA regulation via restoration of damaged EC cells, ↓ release of 5-HT in brain tissue, dysbiosis reversion, ↓TLR4/MyD88/NF-κB pathway | [130] | |
| Clostridium butyricum | female Apcmin/+ mice | ↓ proliferation, ↑ apoptosis, ↓ Wnt/β-catenin pathway, ↓ pathogenic bacteria and bile acid-biotransforming bacteria, ↑ SCFA-producing bacteria, ↑ GPR43 and GPR109A | [133] | |
| male C57BL/6 mice with DSS-induced cancer | improved intestinal barrier function, ↑ TJ-related protein expression levels, ↓ TNF-α, ↓ IL-1β, ↓ IL-13, ↑ IL-10, ↓ oxidative stress, ↑ phosphorylation of Akt, mTOR and p70 ribosomal protein S6 kinase | [253] | ||
| male C57BL/6J with AOM/DSS-induced cancer | ↓ CRC incidence, ↓ inflammation, ↑ apoptotic cells in the tumor tissue, ↓ IL-6, ↑ IL-10, gut microbiota composition enrichment, ↓ MyD88 and NF-κB expression | [134] | ||
| C. butyricum ATCC 19398 | female BALB/c HCT-116 tumor- bearing mice | ↓ tumor metastasis, ↓ EMT, ↓ VM formation | [164] | |
| Enterococcus faecalis KH2 | C57BL/6 mice | ↓ NLRP3-mediated colitis and inflammation-associated CRC | [286] | |
| E. coli Nissle 1917 | CT26 tumor- bearing mice | antitumor effect via gut microbiota regulation, ↑ infiltration of CD8+ T cells into the ΤΜΕ | [146] | |
| ↑ antitumor efficacy in synergy with the autophagy activator rapamycin | [145] | |||
| Lactobacillus spp. | ||||
| Lactobacillus spp. | male Sprague Dawley rats with DMH- induced cancer | ↓ angiogenesis/↓ inflammation after coadministration with telmisartan, ↑ programmed cell death, dysbiosis reversion, ↓ CEA levels | [165] | |
| L. acidophilus | male BALB/c mice with AOM-induced cancer | ↓ colonic lesions, ↓ CEA and CA19-9 tumor markers, ↑ CD4+ and CD8+ cells number, ↑ IFN-γ and IL-10 serum levels | [271] | |
| L. acidophilus CGMCC 878 | male Sprague Dawley rats with DMH- induced cancer | ↓ tumor number, gut microbiota alteration, ↓ fecal β-glucuronidase | [238] | |
| L. acidophilus CICC 6074 | female HT-29 tumor- bearing BALB/c mice | ↑ apoptosis, cytochrome C release, ↑ Bax and Caspase-3, -9/↓ Bcl-2 | [121] | |
| L. acidophilus KFRI342 | male F344 rats with DMH-induced cancer | ↓ aberrant crypt foci, ↓ E. coli number in fecal samples, ↓ β-glucuronidase and β-glucosidase activities | [290] | |
| L. acidophilus NCFM | female CT26 tumor-bearing BALB/cByJ mice | ↓ tumor volume growth, ↓ colonic carcinogenesis, ↑ apoptosis, ↓ CXCR4 mRNA expression in the colon, ↓ MHC class I expression | [112] | |
| L. acidophilus, B. bifidum | male BALB/c mice with AOM-induced cancer | ↓ miR-135b, miR-155, and KRAS expression, ↑ miR-26b, miR-18a, APC, PU.1, and PTEN expression | [155] | |
| L. acidophilus, L. rhamnosus GG | Sprague Dawley rats with DMH-induced cancer | ↓ aberrant crypt foci, ↓ fecal nitroreductase activity, ↓ β-glucuronidase activity | [289] | |
| ↓ aberrant crypt foci, ↓ β-catenin, ↓ NF-κB, ↓ COX-2 in conjuction with celecoxib | [218] | |||
| L. acidophilus, L. rhamnosus GG | Sprague Dawley rats with DMH-induced cancer | ↓ tumor multiplicity, ↑ Bax/↓ Bcl-2, ↓ K-ras/↑ p53 expression | [118] | |
| L. casei ATCC 393 | female BALB/c mice | ↓ tumor volume by 80%, ↑ TRAIL, ↓ survivin | [115] | |
| male Swiss mice with DMH-induced cancer | ↓ CEA, ↓ aberrant crypt foci, ↑ p-JNK-1 expression, ↓ β-catenin, ↓ p-GSK3b, beneficial bacterial genera enrichment in the gut | [151] | ||
| L. casei BL23 | female C57BL/6 mice | antiproliferative and immunomodulatory effect, ↓ IL-22, ↑ caspase-7,-9, ↑ Bik, ↓ gut dysbiosis | [117] | |
| L. casei LH23 | female C57BL/6J with DSS-induced cancer | ↓ macrophages (CD11b+F4/80+) numbers, ↓ pro-inflammatory cytokines, ↓ MPO activity, ↑ Tregs, ↑ SCFAs, ↑ histone H3K9 acetylation in colon tissues | [271] | |
| L. casei subsp. rhamnosus (Lcr35) | CT26 tumor-bearing BALB/c mice | ↓ diarrhea severity and intestinal mucositis after FOLFOX treatment, ↓ NF-κB pathway, ↓ TNF-α, ↓ IL-6, gut microbiota modulation | [224] | |
| L. coryniformis MXJ32 | male C57BL/6 mice with AOM/DSS- induced cancer | ↓ tumor number, intestinal barrier damage prevention, ↑ TJ proteins (occludin, claudin-1, and ZO-1) expression, ↓ inflammation, ↓ pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-γ, and IL-17a), ↓ chemokines, ↑ SCFAs-producing bacteria/↓ pro-inflammatory bacteria abundance | [243] | |
| L. fermentum ZS40 | male C57BL/6 J mice with AOM/DSS- induced cancer | ↓ colonic lesions, ↓ TNF-α, ↓ IL-1β, ↓ NF-κB pathway, ↓ COX-2 expression | [148] | |
| L. gallinarum | male and female ApcMin/+ C57B/6 mice | ↓ tumor number and size, ILA enrichment in the gut | [237] | |
| L. helveticus NS8 | C57BL/6 mice with AOM/DSS- induced cancer | ↓ tumor number, ↑ apoptosis, ↓ NF-κB pathway, ↑ IL-10, ↓ IL-17-producing T cells, dysbiosis reversion | [120] | |
| L. paracasei R3 | male MC-38 tumor-bearing C57BL/6 SPF grade mice | tumor-suppressive activity | [124] | |
| L. plantarum AS1 | male albino Wistar with DMH-induced cancer | ↓ lipid peroxidation, ↑ antioxidant enzymes (SOD, GST, catalase) and marker enzymes (ALP, ACP) activities, ↓ total number of tumors in AS1 pre- and post-treated rats in a time-dependent manner | [261] | |
| L. plantarum L168 | C57BL/6 (B6) mice | ↓ tumor growth, enhancement of CD8+ T cells function due to ILA secretion | [207] | |
| L. plantarum YYC-3 | C57BL/6 APCMin/+ mice | mucosal damage prevention, dysbiosis restoration, ↓ NF-κB and Wnt pathways, ↓ pro-inflammatory cytokines (IL-6, IL-17, and IL-22), ↓ pro-inflammatory cells infiltration | [242] | |
| L. plantarum A, L. rhamnosus b | female CT26 tumor-bearing BALB/c mice | ↓ tumor cell growth, prolonged survival time, ↑ CD8+ T and NK cell infiltration into TME, ↑ IFN-γ production, ↑ Th1-type CD4+ T differentiation | [275] | |
| L. reuteri | female BALB/c mice with TNBS-induced colitis | ↓ intestinal inflammation mediated by Histamine H2 Receptor | [281] | |
| L. rhamnosus 231 | male Wistar rats with MNNG-induced cancer | ↓ fecal azoreductase and nitroreductase activity, ↓ GST, ↑ GSH, ↓ inflammation | [288] | |
| L. rhamnosus AFY06 | C57BL/6 mice with AOM/DSS- induced cancer | ↓ tumor incidence, ↓ pro-inflammatory cytokines, ↓ IkBb, p65, p50, p52, Bcl-2, and Bcl-xL expression, ↑ Bid and CASP-8 | [279] | |
| L. rhamnosus GG | Sprague Dawley rats | ↓ tumor incidence, ↑ apoptosis, ↓ NF-κB pathway | [114] | |
| C57BL/6 mice with AOM/DSS- induced cancer | ↑ colonic CD8+ T-cell responses dependent on dendritic cell activation mediated via TLR-2 | [272] | ||
| L. rhamnosus LS8 | C57BL/6 male mice with AOM/DSS- induced cancer | ↓ tumor formation, goblet cell loss prevention, ↑ TJ proteins (ZO-1, occludin, and claudin-1) expression, dysbiosis reversion, ↓ inflammation, ↓ TLR4/NF-κB, ↓ pro-inflammatory cytokines, ↓ chemokines | [278] | |
| L. rhamnosus (Probio-M9) | CT26 tumor-bearing SPF BALB/c mice | enhanced immunotherapy response, ↑ beneficial microbes and metabolites in the gut, ↑ CTLs infiltration in the TME | [273] | |
| female C57BL/6NCrSlc mice with AOM/DSS- induced cancer | accelerated recovery of the gut microbiota composition and function | [241] | ||
| L. salivarius Ren | male F344 rats | ↓ cancer incidence, gut microbiota modulation | [232] | |
| L. rhamnosus MD14, L. plantarum GMD | male Sprague Dawley rats with DMH- induced cancer | ↓ aberrant crypt foci, ↓ fecal pH, ↑ fecal LAB, altered fecal enzymes activities, gut microbiota modulation | [235] | |
| Lactococcus lactis | BALB/c mice with DMH-induced cancer | ↑ CAT activity, ↓ H2O2 levels, ↓ colonic damage, ↓ inflammation, ↓ tumor incidence | [262] | |
| Ligilactobacillus salivarius LZZAY01 | male C57BL/6J mice with AOM/DSS- induced cancer | ↑ autophagy, ↑ apoptosis, ↑ intestinal TJs, ↓ intestinal barrier degradation, gut microbiota abundance modification, ↓ inflammatory reactions | [162] | |
| Limosilactobacillus fermentum GR-3 | female C57BL/6J mice with AOM/DSS- induced cancer | ↓ intestinal barrier disruption, ↓ tumor incidence, ↓ oxidative stress, ↓ inflammation, ↑ apoptosis, gut microbiota modulation, ↑ beneficial metabolites (SCFAs, ICA, IPA, vitamin B12 and vitamin D3), ↓ harmful secondary bile acids | [239] | |
| Saccharomyces boulardii | C57BL/6J ApcMin/+ mice | ↓ intestinal tumor growth | [138] | |
| female C57BL6 mice with DSS-induced cancer | ↓ histological damage, mucosal recovery restoration, ↓ VEGF-induced angiogenesis | [163] | ||
| C57BL/6 mice with AOM/DSS- induced cancer | ↓ carcinogenesis, ↓ TNF-α, ↓ IL-6, gut microbiota alterations | [236] | ||
| S. cerevisiae | C57BL/6 mice with AOM-induced cancer and APCMin/+ mice | ↓ carcinogenesis, ↑ apoptosis, ↓ NF-κB pathway, gut microbiota and intestinal immunity modulation | [139] | |
| S. cerevisiae SC-2201 | male C57BL/6N mice with AOM/DSS- induced cancer | ↓ colonic shortening and histological damage, ↓ pro-inflammatory mediators (IL-1β, IL-6, COX-2, VEGF, NBD, LRR, and NLRP3) expression, gut microbiota modulation | [166] | |
| Streptococcus thermophilus | male C57BL/6 mice with AOM-induced cancer and male ApcMin/+ mice | protective effect against intestinal tumorigenesis via β-galactosidase secretion, gut microbiota modulation, ↓ Hippo oncogenic pathway, OXPHO activation | [137] | |
| Mixed formulations | ||||
| Mix I: lactobacilli and bifidobacteria Mix II: bifidobacteria | model 1: female C57BL/6 J mice with AOM/DSS- induced cancer model 2: female MC-38 tumor-bearing mice | Mix I: significant antitumor effects in the model 2, associated with microbiota-driven mechanisms Mix II: more effective in the model 1, ↓ colonic inflammation, tumor development prevention | [240] | |
| Bifidobacteria spp. cocktail | female BALB/c mice with AOM/DSS- induced cancer | colon length restoration, ↓ tumor incidence | [142] | |
| B. bifidum H3-R2 and Lactococcus lactis KLDS4.0325 | male C57 BL/6 J mice with AOM/DSS- induced cancer | tissue damage relief, ↓ pro-inflammatory cytokines, ↑ anti-inflammatory cytokines, ↓ MPO activity, ↓ HIF-1α level, ↑ colonic TJ proteins, ↓ NLRP3 inflammasome, gut microbiota imbalance improvement | [144] | |
| GM-LAB | BALB/c mice with DMH-induced cancer | ↓ intestinal damage, antioxidant enzyme activities modifications, ↑ anti-inflammatory cytokines | [258] | |
| L. acidophilus ATCC 314 and L. fermentum NCIMB 5221 | male wild-type C57BL/6J– ApcMin/+ mice | ↓ intestinal tumor multiplicity, ↓ proliferation markers (β-catenin and Ki-67) | [141] | |
| L. rhamnosus GG and L. plantarum AdF10 | female Sprague Dawley rats with DMH-induced cancer | ↑ antioxidant enzymes (GSH, GPx, GST, SOD, CAT) activities, ↑ p53-mediated apoptotic pathway | [154] | |
| Probiotic cocktail (lactobacilli and bifidobacteria) | Sprague Dawley rats with DMH-induced cancer | gut microbiota alteration, ↑ MUC2, ↑ ZO-1, ↑ occludin, ↑ TLR2, ↓ TLR4, ↓ COX-2, ↓ β-catenin | [233] | |
| Probiotic mixture VSL#3 | male Sprague Dawley rats with TNBS- induced cancer | ↓ intestinal damage, ↑ VDR expression, ↑ gut microbiota species richness and diversity, ↓ ALP levels, ↑ angiostatin expression in the colon | [231] | |
| male Wistar rats with DSS-induced cancer | ↓ MPO activity, ↓ iNOS, ↓ COX-2, ↓ NF-κB pathway, ↓ TNF-α, ↓ IL-6, ↓ p-Akt, ↑ IL-10 | [267] | ||
| female C57BL/6 mice with DSS-induced cancer | ↓ inflammation, ↓ colonic lesions, ↓ TNF-α, ↓ IL-6, ↓ IL-1β, ↓ COX-2, ↑ IL-10 | [282] | ||
| Probiótico 20 bi | male F344 rats with DMH-induced cancer | ↓ aberrant crypt foci, ↓ tumor malignancy progression, 5-FU antitumor effect enhancement | [221] | |
| male C57BL/6 J mice | ↓ NF-κB pathway, mitigation of mucin depletion, ↑ Ki-67 production | [249] | ||
| Liver | L. plantarum EMCC-1039 | male Wister rats with TAA-induced cancer | ↓ TLR4, CXCL9 and PREX-2 expression, liver function improvement | [266] |
| L. rhamnosus ATCC 53103 | male Swiss mice with induced HCC via DEN and CCl4 injection | gut leakage prevention, ↓ iNOS and IL-6 levels, ↓ intestinal and liver tissue inflammation | [285] | |
| Probiotic mix | male C57BL6/N mice | ↓ tumor size and weight, ↓ IL-17, ↑ IL-10, ↓ pro-angiogenic genes expression, gut microbiota modulation, ↓ Th17 differentiation in the gut | [234] | |
| Weizmannia coagulans MZY531 | female HT-22 tumor-bearing BALB/c mice | ↓ tumor weight and size, ↓ pro-inflammatory cytokines, ↑ caspase-3, gut microbiota remodeling, ↑ AMPK/mTOR autophagy-dependent pathway, TLR4/MyD88/TRAF-6/NF-κB and JAK2/STAT3 inflammatory pathways regulation | [161] | |
| Pancreatic | L. rhamnosus GG | female C57BL/6 Panc-02 tumor- bearing mice | ↓ intratumor-promoting Proteobacteria and microbiota-derived LPSs, ↓ tumoral TLRs activation, ↓ PD-L1 and IL-1β expression by tumor cells, improved cytotoxic T lymphocytes infiltration in tumors | [274] |
| Mixed formulations | ||||
| L. casei ATCC 39392 and L. reuteri ATCC 23272 | BxPC-3 tumor- bearing Balb/c mice | ↓ TLR4 leading to gut microbial and metabolic homeostasis regulation | [265] | |
| L. reuteri GMNL-89 and L. paracasei GMNL-133 | P. gingivalis-treated KC mice | ↓ carcinogenesis | [213] | |
| KC transgenic mice | ↓ PanIN formation following probiotics and gemcitabine combination, ↓ vimentin and Ki-67 expression, ↓ AST, ↓ ALT levels | [214] | ||
| Probiotic blend | female BxPC-3 tumor-bearing Balb/c mice | ↑ species richness and SCFAs producing-bacteria in fecal microbiota, ↑ phosphatidylcholine and phosphatidylethanolamine levels, ↓ amino acids (glutamic acid, aspartic acid, threonine and serine) levels | [245] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoda, C.; Touraki, M. Molecular Mechanisms of Probiotic Action Against Gastrointestinal Cancers. Int. J. Mol. Sci. 2025, 26, 7857. https://doi.org/10.3390/ijms26167857
Thoda C, Touraki M. Molecular Mechanisms of Probiotic Action Against Gastrointestinal Cancers. International Journal of Molecular Sciences. 2025; 26(16):7857. https://doi.org/10.3390/ijms26167857
Chicago/Turabian StyleThoda, Christina, and Maria Touraki. 2025. "Molecular Mechanisms of Probiotic Action Against Gastrointestinal Cancers" International Journal of Molecular Sciences 26, no. 16: 7857. https://doi.org/10.3390/ijms26167857
APA StyleThoda, C., & Touraki, M. (2025). Molecular Mechanisms of Probiotic Action Against Gastrointestinal Cancers. International Journal of Molecular Sciences, 26(16), 7857. https://doi.org/10.3390/ijms26167857







