Polyphenolic Profile and Biological Activities in HT29 Intestinal Epithelial Cells of Feijoa sellowiana Fruit Extract
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Compounds in Feijoa Fruit Pulp Extract
2.2. Scavenging Activity and Total Antioxidant Capacity
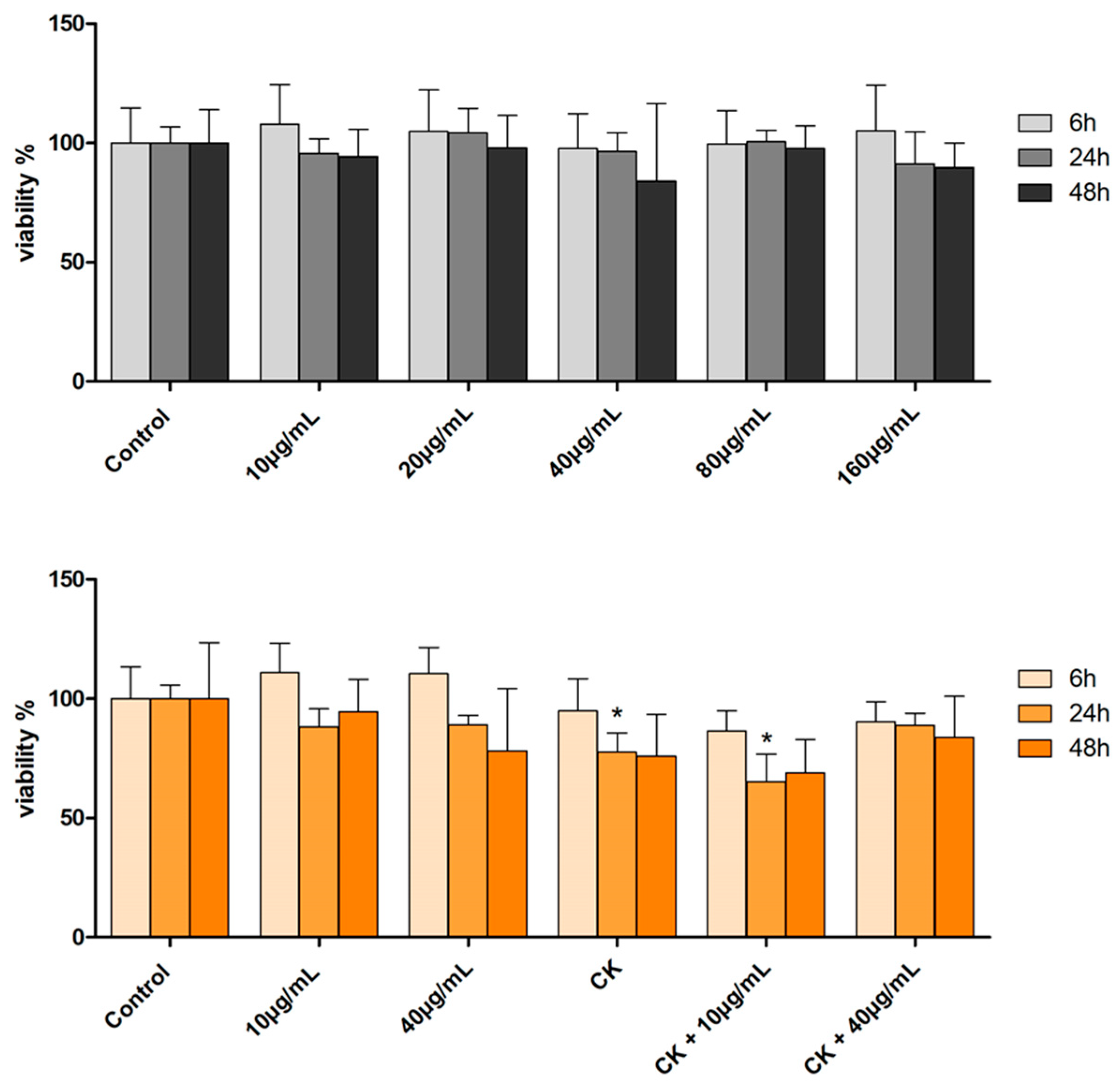
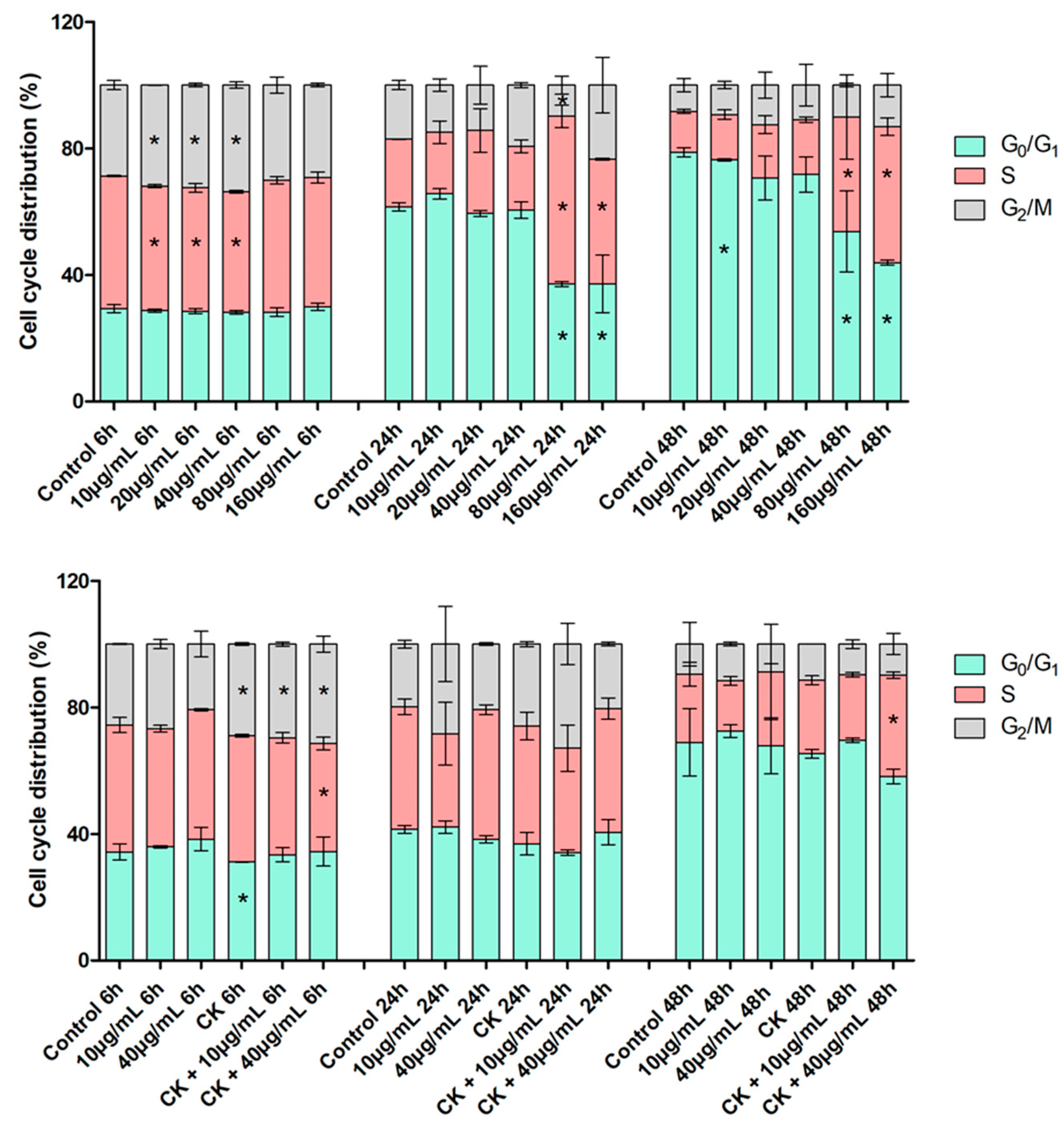
2.3. Cell Viability and Distribution in the Cell Cycle Phases by Cytofluorimetric Analysis
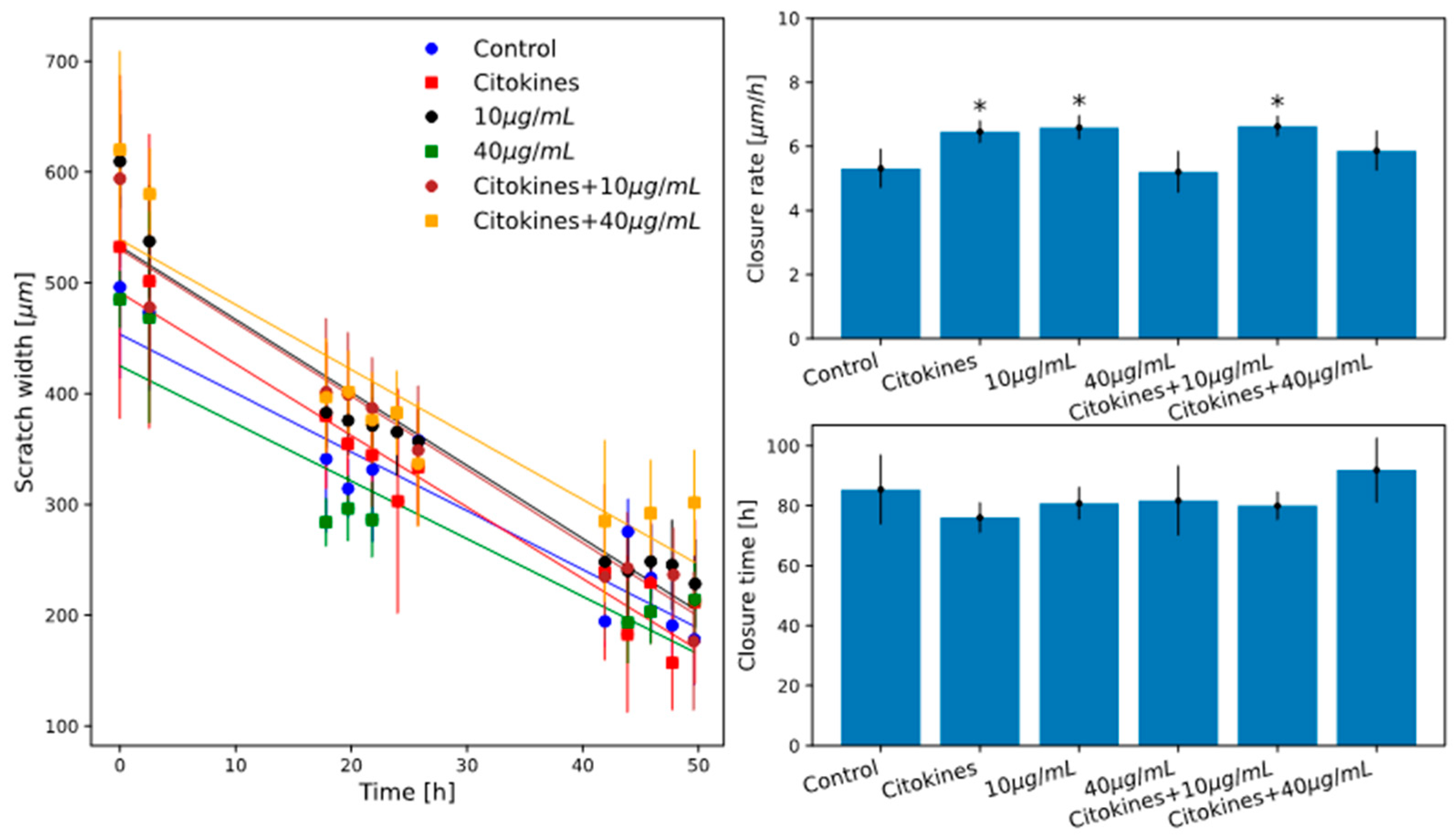
2.4. Scratch Assay
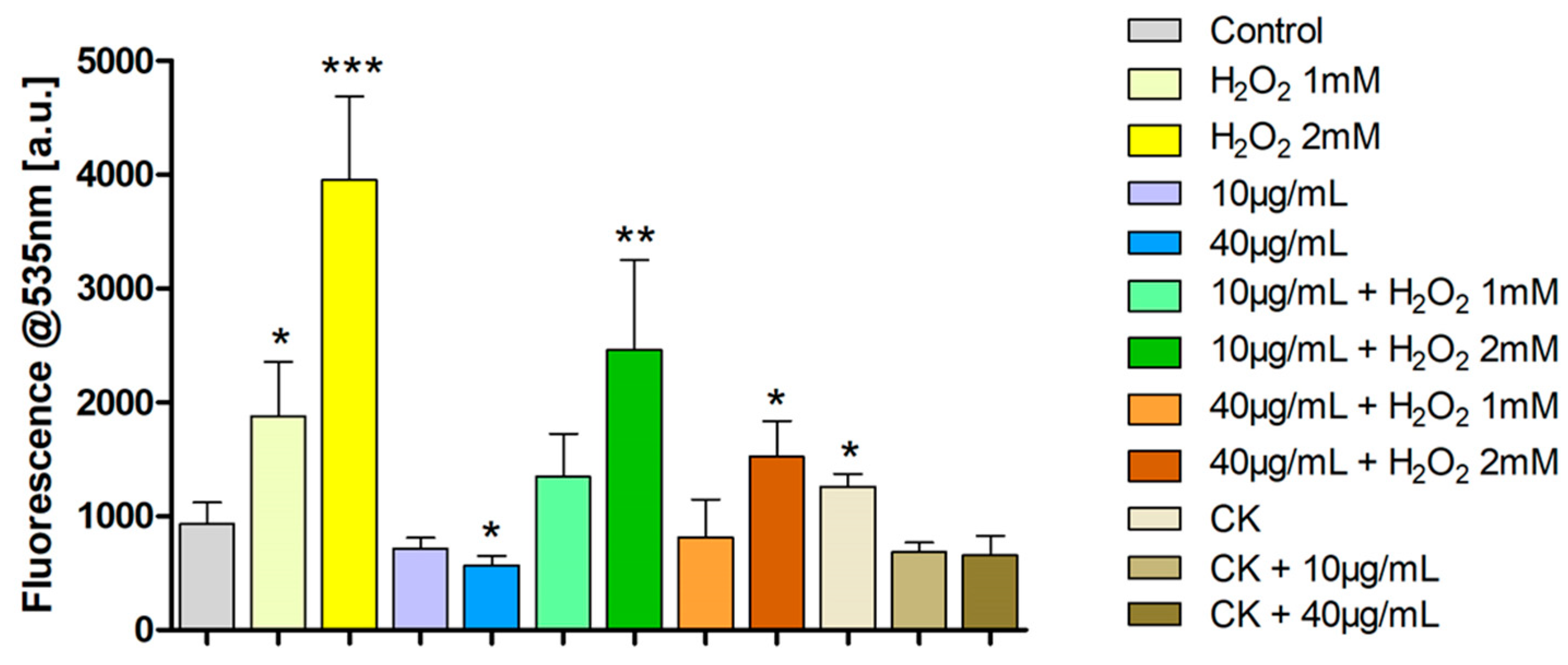
2.5. ROS Production Evaluation by DCFH-DA Assay
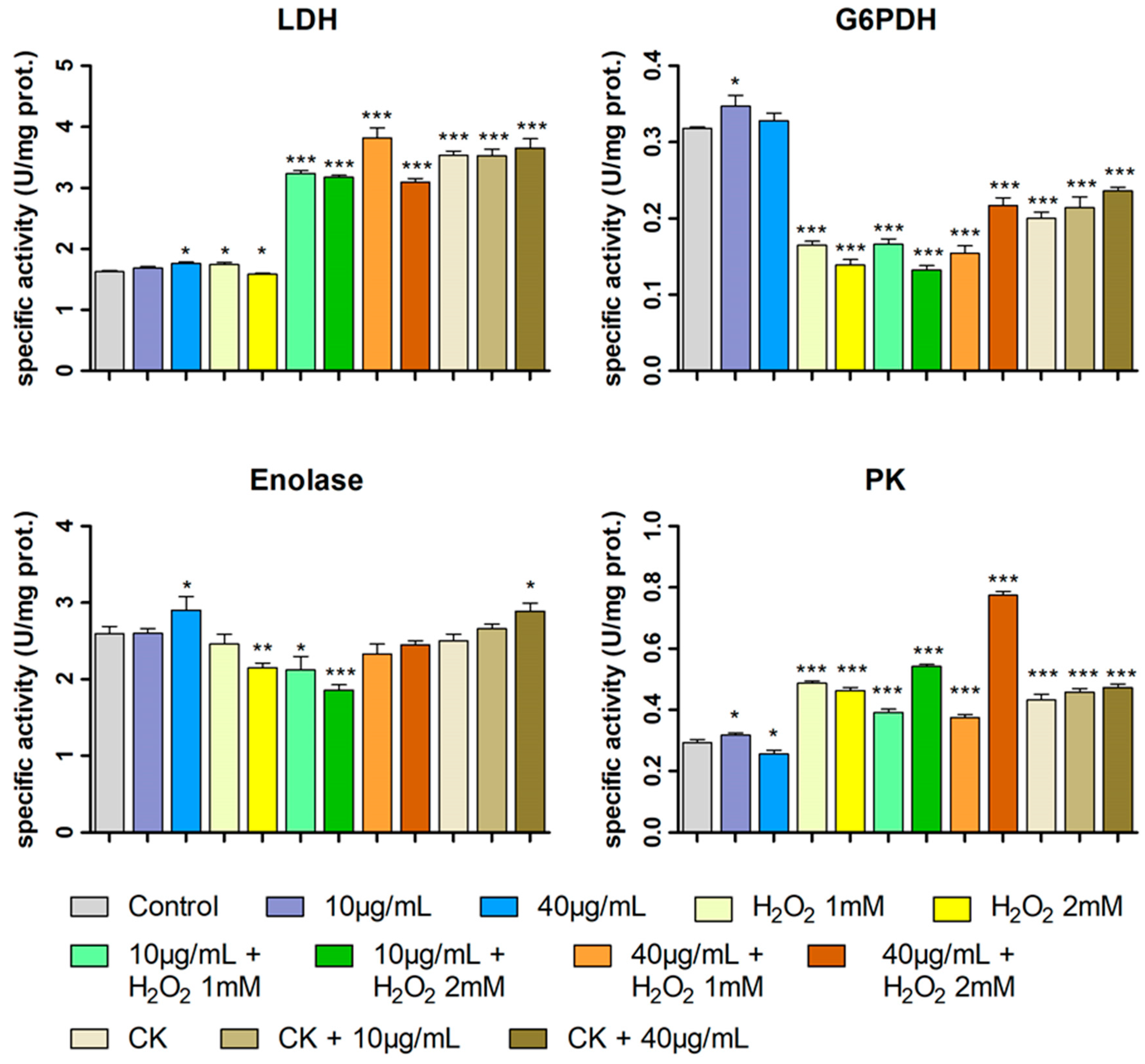
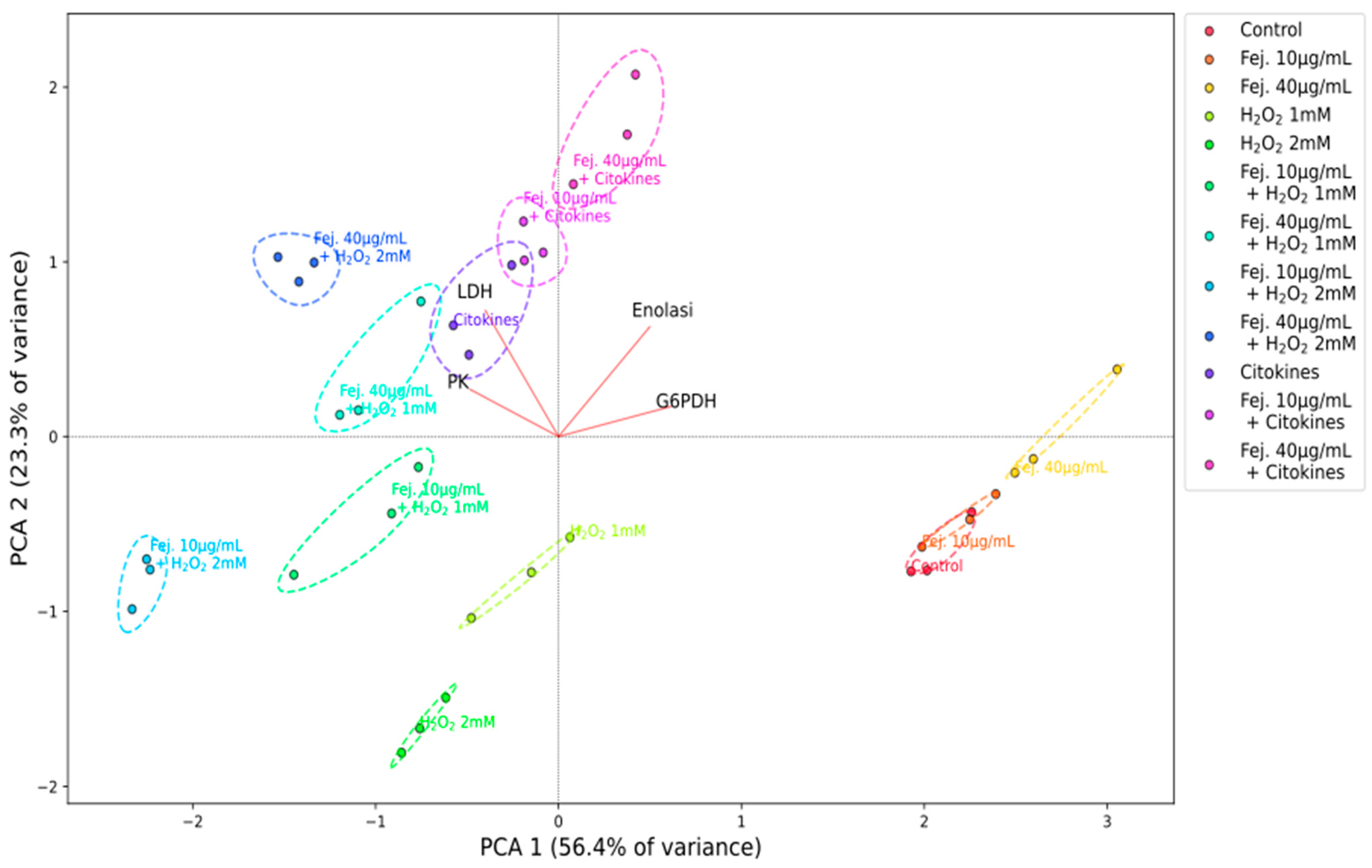
2.6. Enzymatic Assays, PCA and Pearson Correlation Analyses
3. Materials and Methods
3.1. Chemicals, Reagents, and Standards
3.2. Preparation of Extracts from Feijoa Sellowiana Fruit Pulp
3.3. Evaluation of Total Phenolic Content by Folin–Ciocalteu Assay
3.4. HPLC-DAD-MS Analysis
3.5. Determination of Scavenging Activity by DPPH Test
3.6. Determination of Total Antioxidant Capacity by Phosphomolybdenum Assay
3.7. HT29 Cell Line
3.8. Assessment of Cell Viability by MTT Test
3.9. Cell Distribution in the Cell Cycle Phases by Cytofluorimetric Analysis
3.10. Scratch Assay
3.11. Evaluation of Intracellular ROS Production
3.12. Enzymatic Assays
3.13. Principal Component Analysis (PCA) of Enzyme Activities
3.14. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HHDP-glucose | Hexahydroxydiphenoyl-glucose |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| PCA | Principal Component Analysis |
| ROS | Reactive Oxygen Species |
| GAE | Gallic Acid Equivalent |
| FDP | Freeze-Dried Pulp |
| FP | Fresh Pulp |
| CK | Cytokines |
| TNF-α | Tumour Necrosis Factor alpha |
| IL-1β | Interleukin 1 beta |
| DCFH-DA | 2′,7′-Dichlorofluorescein diacetate |
| PK | Pyruvate Kinase |
| ENO | Enolase |
| LDH | Lactic Dehydrogenase |
| G6PDH | Glucose-6 Phosphate Dehydrogenase |
| PBS | Phosphate-Buffered Solution |
| DMSO | Dimethyl sulphoxide |
References
- Grigorieva, E.; Livenets, A.; Stelmakh, E. Adaptation of Agriculture to Climate Change: A Scoping Review. Climate 2023, 11, 202. [Google Scholar] [CrossRef]
- Howden, S.M.; Soussana, J.; Tubiello, F.N.; Chhetri, N.; Dunlop, M.; Meinke, H. Adapting agriculture to climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19691–19696. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Georgakopoulos, P.; Travlos, I.S.; Kakabouki, I.; Kontopoulou, C.-K.; Pantelia, A.; Bilalis, D.J. Climate Change and Chances for the Cultivation of New Crops. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 347–353. [Google Scholar] [CrossRef]
- Kakabouki, I.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Roussis, I.; Katsenios, N.; Aspasia Efthimiadou, A.; Papastylianou, P. Introduction of alternative crops in the Mediterranean to satisfy EU Green Deal goals. A review. Agron. Sustain. Dev. 2021, 41, 71. [Google Scholar] [CrossRef]
- Fatehi, S.E.; Ater, M.; Hmimsa, Y. Alternative Crops for Adaptation to Climate Change: The Importance of Conserving the Diversity of Lathyrus cicera L. Landraces Adapted to the Morocco Mountains. Biol. Life Sci. Forum 2021, 3, 21. [Google Scholar] [CrossRef]
- Sloat, L.L.; Davis, S.J.; Gerber, J.S.; Moore, F.C.; Ray, D.K.; West, P.C.; Mueller, N.D. Climate adaptation by crop migration. Nat. Commun. 2020, 11, 1243. [Google Scholar] [CrossRef]
- Zhu, F. Chemical and biological properties of feijoa (Acca sellowiana). Trends Food Sci. Technol. 2018, 81, 121–131. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, X.; Yao, J.; Li, Z.; Jiang, Y.; Yu, Q.; Huang, Z.; Liu, H.; Xiao, Y.; Dai, F. Structural characteristics, functional properties, antioxidant and hypoglycemic activities of pectins from feijoa (Acca sellowiana) peel. Food Chem. 2023, 428, 136819. [Google Scholar] [CrossRef]
- Keles, H.; Ince, S.; Küçükkurt, I.; Tatli, I.I.; Akkol, E.K.; Kahraman, C.; Demirel, H.H. The effects of Feijoa sellowiana fruits on the antioxidant defense system, lipid peroxidation, and tissue morphology in rats. Pharm. Biol. 2012, 3, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.H.; Costa Kammers, J.; Silva, A.P.; Oliveira, J.V.; Hense, H. Antioxidant and antibacterial compounds from feijoa leaf extracts obtained by pressurized liquid extraction and supercritical fluid extraction. Food Chem. 2021, 344, 128620. [Google Scholar] [CrossRef]
- Guerra, M.P.; Cangahuala-Inocente, G.C.; Vesco, L.L.D.; Pescador, R.; Caprestano, C.A. Micropropagation Systems of Feijoa (Acca sellowiana (O. Berg) Burret). Methods Mol. Biol. 2013, 11013, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.C.; Da Silva Tognon, L.; Martins, Y.G.; Louise dos Santos, K.; Picolotto, L.; Araújo, L.; Haygert Lencina, K. Enraizamento de Estacas de Feijoa sellowiana Com Uso de Antioxidante. Agropecuária Catarin. 2023, 36, 42–47. [Google Scholar] [CrossRef]
- Álvarez-Herrera, J.G.; Tovar-Escobar, J.; Ruiz, H.D. Postharvest behavior of feijoa fruit (Acca sellowiana Berg) subjected to different 1-MCP doses and storage temperatures. Rev. Bras. Frutic. 2023, 45, e-000. [Google Scholar] [CrossRef]
- Rupavatharam, S.; East, A.R.; Heyes, J.A. Re-Evaluation of Harvest Timing in ‘Unique’Feijoa Using 1-MCP and Exogenous Ethylene Treatments. Postharvest Biol. Technol. 2015, 99, 152–159. [Google Scholar] [CrossRef]
- Oseko, J.; East, A.; Heyes, J. Recent Advances in the Postharvest Technology of Feijoa. Sci. Hortic. 2022, 297, 110969. [Google Scholar] [CrossRef]
- Facchini, F.; Silvestri, B.; Digiesi, S.; Lucchese, A. Agri-food loss and waste management: Win-win strategies for edible discarded fruits and vegetables sustainable reuse. Innov. Food Sci. Emerg. Technol. 2023, 83, 103235. [Google Scholar] [CrossRef]
- Scarano, P.; Sciarrillo, R.; Tartaglia, M.; Zuzolo, D.; Guarino, C. Circular economy and secondary raw materials from fruits as sustainable source for recovery and reuse: A Review. Trends Food Sci. Technol. 2022, 122, 157–170. [Google Scholar] [CrossRef]
- Monforte, M.T.; Fimiani, V.; Lanuzza, F.; Naccari, C.; Restuccia, S.; Galati, E.M. Feijoa sellowiana Berg fruit juice: Anti-inflammatory effect and activity on superoxide anion generation. J. Med. Food. 2014, 17, 455–461. [Google Scholar] [CrossRef]
- Mosbah, H.; Louati, H.; Boujbiha, M.A.; Chahdoura, H.; Snoussi, M.; Flamini, G.; Ascrizzi, R.; Bouslema, A.; Achour, L.; Selmi, B. Phytochemical characterization, antioxidant, antimicrobial and pharmacological activities of Feijoa sellowiana leaves growing in Tunisia. Ind. Crops Prod. 2018, 112, 521–531. [Google Scholar] [CrossRef]
- Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antimicrobial Activities of Ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef] [PubMed]
- Minich, A.; Levarski, Z.; Mikulášová, M.; Straka, M.; Liptáková, A.; Stuchlík, S. Complex Analysis of Vanillin and Syringic Acid as Natural Antimicrobial Agents against Staphylococcus epidermidis Biofilms. Int. J. Mol. Sci. 2022, 23, 1816. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, P.; Mita, L.; Miceli, M.; Doto, A.; Nebbioso, A.; De Bellis, F.; Conte, M.; Minichiello, A.; Manzo, F.; Carafa, V.; et al. Feijoa sellowiana derived natural Flavone exerts anti-cancer action displaying HDAC inhibitory activities. Int. J. Biochem. Cell Biol. 2007, 39, 1902–1914, Erratum in Elife. Int. J. Biochem. Cell Biol. 2024, 173, 106619. [Google Scholar] [CrossRef]
- Peng, Y.; Bishop, K.S.; Ferguson, L.R.; Quek, S.Y. Phenolic-rich feijoa extracts from flesh, peel and whole fruit activate apoptosis pathways in the LNCaP cell line. Food Chem. 2022, 383, 132285. [Google Scholar] [CrossRef] [PubMed]
- Nasef, N.A.; Mehta, S.; Murray, P.; Marlow, G.; Ferguson, L.R. Anti-inflammatory activity of fruit fractions in vitro, mediated through toll-like receptor 4 and 2 in the context of inflammatory bowel disease. Nutrients 2014, 6, 5265–5279. [Google Scholar] [CrossRef]
- Nasef, N.A.; Mehta, S.; Powell, P.; Marlow, G.; Wileman, T.; Ferguson, L.R. Extracts of Feijoa Inhibit Toll-Like Receptor 2 Signaling and Activate Autophagy Implicating a Role in Dietary Control of IBD. PLoS ONE 2015, 10, e0130910. [Google Scholar] [CrossRef]
- Aoyama, H.; Sakagami, H.; Hatano, T. Three new flavonoids, proanthocyanidin, and accompanying phenolic constituents from Feijoa sellowiana. Biosci. Biotechnol. Biochem. 2018, 82, 31–41. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Chaliha, M.; Sultanbawa, Y.; Netzel, M.E. Nutritional Characteristics and Antimicrobial Activity of Australian Grown Feijoa (Acca sellowiana). Foods 2019, 8, 376. [Google Scholar] [CrossRef]
- Mosbah, H.; Chahdoura, H.; Adouni, K.; Kamoun, J.; Boujbiha, M.A.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Ciudad-Mulero, M.; Morales, P.; Fernández-Ruiz, V.; et al. Nutritional properties, identification of phenolic compounds, and enzyme inhibitory activities of Feijoa sellowiana leaves. J. Food Biochem. 2019, 43, e13012. [Google Scholar] [CrossRef]
- Bini, L.; Gori, M.; Novello, M.A.; Biricolti, S.; Giordani, E.; Lara, M.V.; Niella, F.; Nunziata, A.; Rocha, P.; Filippi, J.M.; et al. Assessing the Genetic Diversity of Wild and Commercial Feijoa sellowiana Accessions Using AFLPs. Horticulturae 2024, 10, 366. [Google Scholar] [CrossRef]
- Cheli, F.; Baldi, A. Nutrition-based health: Cell-based bioassays for food antioxidant activity evaluation. J. Food Sci. 2011, 76, R197–R205. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 113–124. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 107–115. ISBN 9781119135388. [Google Scholar]
- Tuncel, N.B.; Yılmaz, N. Optimizing the extraction of phenolics and antioxidants from feijoa (Feijoa sellowiana, Myrtaceae). J. Food Sci. Technol. 2015, 52, 141–150. [Google Scholar] [CrossRef]
- de Oliveira Schmidt, H.; Rockett, F.C.; Klen, A.V.B.; Schmidt, L.; Rodrigues, E.; Tischer, B.; Augusti, P.R.; de Oliveira, V.R.; da Silva, V.L.; Flôres, S.H.; et al. New insights into the phenolic compounds and antioxidant capacity of feijoa and cherry fruits cultivated in Brazil. Food Res. Int. 2020, 136, 109564. [Google Scholar] [CrossRef] [PubMed]
- Castelucci, A.C.L.; de Toledo, N.M.V.; Juliano, F.F.; da Silva, P.P.M.; Spoto, M.H.F. Influence of processing on the phenolic compounds of feijoa pulp (Feijoa sellowiana). JFB 2020, 11, 66–74. [Google Scholar] [CrossRef]
- de Oliveira Schmidt, H.; Camboim Rockett, F.; Veloso Sartori, G.; Rezzadori, K.; Tischer, B.; Rodrigues, E.; de Oliveira Rios, A.; Vitor Manfroi, V. Influence of processing conditions on the composition of feijoa (Acca sellowiana) juices during storage. J. Food Compos. Anal. 2022, 114, 104769. [Google Scholar] [CrossRef]
- Weston, R.J. Bioactive products from fruit of the feijoa (Feijoa sellowiana, Myrtaceae): A review. Food Chem. 2010, 121, 923–926. [Google Scholar] [CrossRef]
- Bell, T.J.; Draper, S.L.; Centanni, M.; Carnachan, S.M.; Tannock, G.W.; Sims, I.M. Characterization of polysaccharides from feijoa fruits (Acca sellowiana Berg.) and their utilization as growth substrates by gut commensal bacteroides species. J. Agric. Food Chem. 2018, 66, 13277–13284. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Cuevas-Cianca, S.I.; Romero-Castillo, C.; Gálvez-Romero, J.L.; Juárez, Z.N.; Hernández, L.R. Antioxidant and Anti-Inflammatory Compounds from Edible Plants with Anti-Cancer Activity and Their Potential Use as Drugs. Molecules 2023, 28, 1488. [Google Scholar] [CrossRef]
- Allegra, M. Antioxidant and Anti-Inflammatory Properties of Plants Extract. Antioxidants 2019, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.; Mira, L.; Nogueira, J.M.; da Silva, A.P.; Helena Florêncio, M. Plant extracts with anti-inflammatory properties—a new approach for characterization of their bioactive compounds and establishment of structure-antioxidant activity relationships. Bioorg. Med. Chem. 2009, 17, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Zafrilla, P.; Ferreres, F.; Tomás-Barberán, F.A. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J. Agric. Food Chem. 2001, 49, 3651–36555. [Google Scholar] [CrossRef] [PubMed]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kaushik, P.; Incerpi, S.; Pedersen, J.Z.; Goel, S.; Prasad, A.K.; Rohil, V.; Parmar, V.S.; Saso, L.; Len, C. Evaluation of the Free Radical Scavenging Activities of Ellagic Acid and Ellagic Acid Peracetate by EPR Spectrometry. Molecules 2021, 26, 4800. [Google Scholar] [CrossRef]
- Khalid, M.; Saeed-ur-Rahman; Bilal, M.; Huang, D.-F. Role of flavonoids in plant interactions with the environment and against human pathogens—A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Faraoni, P.; Bellumori, M.; Cecchi, L.; Zonfrillo, B.; Innocenti, M.; Gnerucci, A.; Mulinacci, N.; Ranaldi, F. AGS Gastric Cells: Antioxidant Activity and Metabolic Effects of Phenolic Extracts from Different Monocultivar Virgin Olive Oils. Antioxidants 2023, 12, 1347. [Google Scholar] [CrossRef]
- Faraoni, P.; Cecchi, L.; Bellumori, M.; Gnerucci, A.; Ranaldi, F.; Mulinacci, N. Virgin Olive Oil By-Products: Biological Activity of Phenolic Extract of Pâté on AGS Gastric Cells. Int. J. Mol. Sci. 2023, 24, 7959. [Google Scholar] [CrossRef]
- Liemburg-Apers, D.C.; Willems, P.H.; Koopman, W.J.; Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015, 89, 1209–1226. [Google Scholar] [CrossRef]
- Mullarky, E.; Cantley, L.C. Diverting Glycolysis to Combat Oxidative Stress. In Innovative Medicine: Basic Research and Development; Nakao, K., Minato, N., Uemoto, S., Eds.; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Nehar, D.; Mauduit, C.; Boussouar, F.; Benahmed, M. Tumor necrosis factor-alpha-stimulated lactate production is linked to lactate dehydrogenase A expression and activity increase in porcine cultured Sertoli cells. Endocrinology 1997, 138, 1964–1971. [Google Scholar] [CrossRef]
- Lu, N.; Li, X.; Li, J.; Xu, W.; Li, H.; Gao, Z. Nitrative and oxidative modifications of enolase are associated with iron in iron-overload rats and in vitro. J. Biol. Inorg. Chem. 2011, 16, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Jiang, L.; Chen, G.; Feng, Y.; Lv, Q.; Zhang, C.; Qu, S.; Zhu, H.; Zhou, B.; Xiao, X. Constitutive heat shock protein 70 interacts with α-enolase and protects cardiomyocytes against oxidative stress. Free Radic. Res. 2011, 45, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-T.; Lin, C.-S.; Liu, C.-Y.; Chen, P.; Tsai, C.-Y.; Wei, Y.-H. Mitochondrial Plasticity and Glucose Metabolic Alterations in Human Cancer under Oxidative Stress—From Viewpoints of Chronic Inflammation and Neutrophil Extracellular Traps (NETs). Int. J. Mol. Sci. 2024, 25, 9458. [Google Scholar] [CrossRef]
- Shi, D.Y.; Xie, F.Z.; Zhai, C.; Stern, J.S.; Liu, Y.; Liu, S.L. The role of cellular oxidative stress in regulating glycolysis energy metabolism in hepatoma cells. Mol. Cancer 2009, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef]
- Spinelli, S.; Marino, A.; Morabito, R.; Remigante, A. Interplay Between Metabolic Pathways and Increased Oxidative Stress in Human Red Blood Cells. Cells 2024, 13, 2026. [Google Scholar] [CrossRef]
- Fuentes-Lemus, E.; Usgame, K.; Fierro, A.; López-Alarcón, C. Enzymes of glycolysis and the pentose phosphate pathway as targets of oxidants: Role of redox reactions on the carbohydrate catabolism. Redox Biochem. Chem. 2025, 11, 100049. [Google Scholar] [CrossRef]
- Ainsworth, E.; Gillespie, K. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Fogh, J.; Fogh, J.M.; Orfeo, T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 1977, 59, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, T.; Hagman, S.; Ristola, M.; Sukki, L.; Veijula, K.; Kreutzer, J.; Kallio, P.; Narkilahti, S. Co-stimulation with IL-1β and TNF-α induces an inflammatory reactive astrocyte phenotype with neurosupportive characteristics in a human pluripotent stem cell model system. Sci. Rep. 2019, 9, 16944. [Google Scholar] [CrossRef]
- Genah, S.; Cialdai, F.; Ciccone, V.; Sereni, E.; Morbidelli, L.; Monici, M. Effect of NIR Laser Therapy by MLS-MiS Source on Fibroblast Activation by Inflammatory Cytokines in Relation to Wound Healing. Biomedicines 2021, 9, 307. [Google Scholar] [CrossRef]
- Vindeløv, L.L.; Christensen, I.J. A review of techniques and results obtained in one laboratory by an integrated system of methods designed for routine clinical flow cytometric DNA analysis. Cytometry 1990, 11, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Gnerucci, A.; Faraoni, P.; Sereni, E.; Ranaldi, F. Scratch assay microscopy: A reaction-diffusion equation approach for common instruments and data. Math. Biosci. 2020, 330, 108482. [Google Scholar] [CrossRef] [PubMed]
- Bass, D.A.; Parce, J.W.; Dechatelet, L.R.; Szejda, P.; Seeds, M.C.; Thomas, M. Flow cytometric studies of oxidative product formation by neutrophils: A graded response to membrane stimulation. J. Immunol. 1983, 130, 1910–1917. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods of Enzymatic Analysis, 2nd ed.; Verlag Chemie: Weinheim, Germany; Academic Press: New York, NY, USA, 1974; Volume 1, pp. 449, 458–459, 481–482, 509–511. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Pearson’s Correlation Coefficient. In Encyclopedia of Public Health; Kirch, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1090–1091. [Google Scholar] [CrossRef]
- Student. Probable error of a correlation coefficient. Biometrika 1908, 6, 302–310. [Google Scholar] [CrossRef]
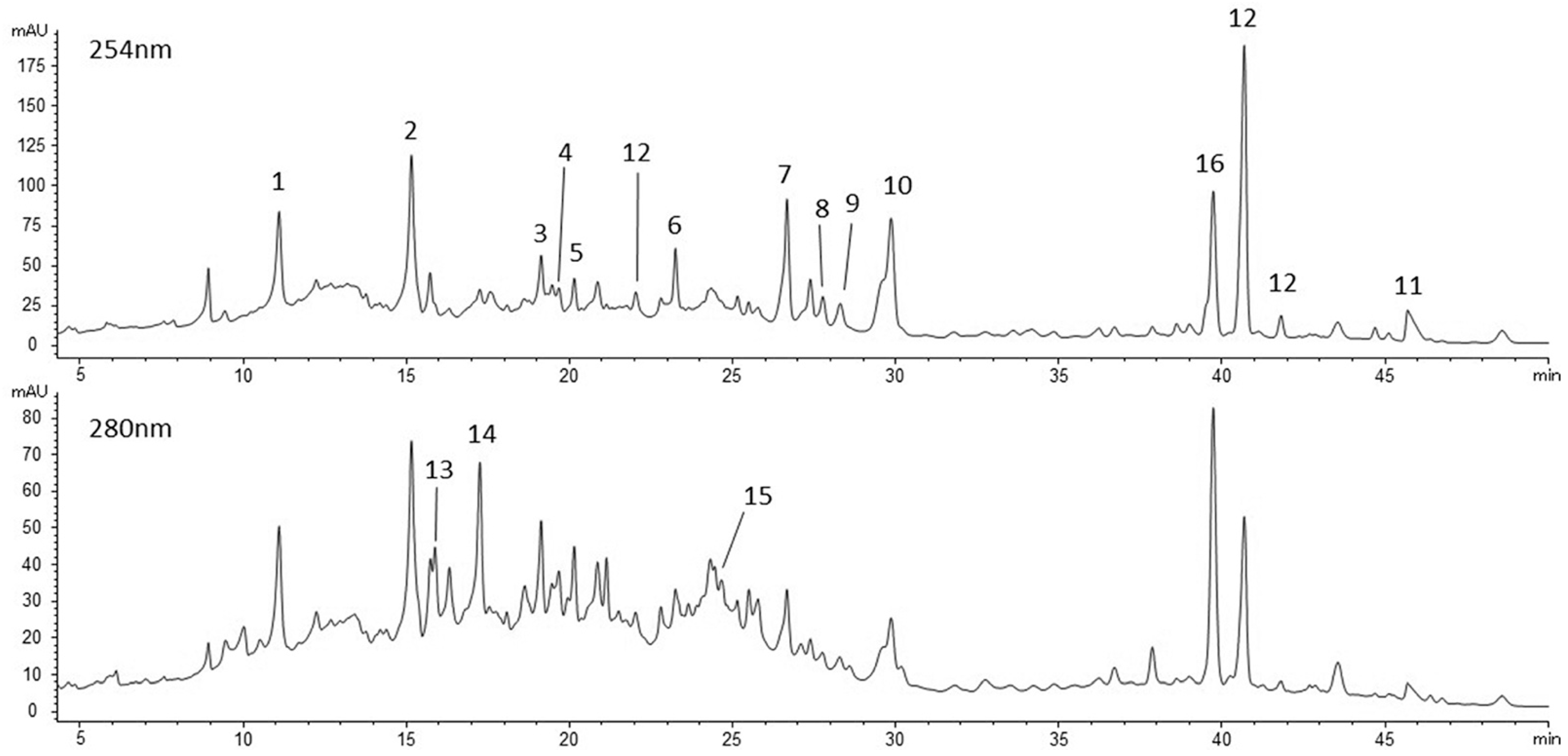


| mg/g FDP | mg/g FP | ||
|---|---|---|---|
| 1 | Pedunculagin isomer I | 0.157 | 0.027 |
| 2 | Pedunculagin isomer II | 0.245 | 0.042 |
| 3 | HHDP-glucose | 0.023 | 0.004 |
| 4 | Flavonoid derivative | traces | traces |
| 5 | Casuarictin/potentillin | 0.028 | 0.005 |
| 6 | Ellagic acid hexoside I | 0.020 | 0.003 |
| 7 | Ellagic acid arabinoside I | 0.059 | 0.010 |
| 8 | Ellagic acid arabinoside II | 0.012 | 0.002 |
| 9 | Ellagic acid hexoside II | 0.017 | 0.003 |
| 10 | Ellagic acid derivative m/z 491 | 0.120 | 0.020 |
| 11 | Ellagic acid derivative m/z 423 | 0.024 | 0.004 |
| 12 | Ellagic acid derivatives calibrated as ellagic acid | 0.109 | 0.019 |
| 13 | Procyanidin dimer I | 0.147 | 0.025 |
| 14 | Procyanidin dimer II | 0.337 | 0.057 |
| 15 | Procyanidin dimer III | 0.079 | 0.013 |
| 16 | Unknown compound calibrated as ellagic acid | 0.054 | 0.009 |
| Total polyphenols | 1.431 | 0.243 | |
| Feijoa Extract Concentration (µg/mL GAE) | Total Antioxidant Capacity | Trolox Concentration with Equivalent Antioxidant Capacity (mM) |
|---|---|---|
| 5 µg/mL | 0.899 ± 0.014 | 0.201 ± 0.004 |
| 10 µg/mL | 1.396 ± 0.014 | 0.343 ± 0.004 |
| 20 µg/mL | 2.18 ± 0.06 | 0.568 ± 0.017 |
| 40 µg/mL | 3.295 ± 0.09 | 0.887 ± 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraoni, P.; Campo, M.; Gnerucci, A.; Vignolini, P.; Ranaldi, F.; Iantomasi, T.; Bini, L.; Gori, M.; Giordani, E.; Natale, R.; et al. Polyphenolic Profile and Biological Activities in HT29 Intestinal Epithelial Cells of Feijoa sellowiana Fruit Extract. Int. J. Mol. Sci. 2025, 26, 7851. https://doi.org/10.3390/ijms26167851
Faraoni P, Campo M, Gnerucci A, Vignolini P, Ranaldi F, Iantomasi T, Bini L, Gori M, Giordani E, Natale R, et al. Polyphenolic Profile and Biological Activities in HT29 Intestinal Epithelial Cells of Feijoa sellowiana Fruit Extract. International Journal of Molecular Sciences. 2025; 26(16):7851. https://doi.org/10.3390/ijms26167851
Chicago/Turabian StyleFaraoni, Paola, Margherita Campo, Alessio Gnerucci, Pamela Vignolini, Francesco Ranaldi, Teresa Iantomasi, Lorenzo Bini, Massimo Gori, Edgardo Giordani, Roberto Natale, and et al. 2025. "Polyphenolic Profile and Biological Activities in HT29 Intestinal Epithelial Cells of Feijoa sellowiana Fruit Extract" International Journal of Molecular Sciences 26, no. 16: 7851. https://doi.org/10.3390/ijms26167851
APA StyleFaraoni, P., Campo, M., Gnerucci, A., Vignolini, P., Ranaldi, F., Iantomasi, T., Bini, L., Gori, M., Giordani, E., Natale, R., Nin, S., Carossino, R., & Biricolti, S. (2025). Polyphenolic Profile and Biological Activities in HT29 Intestinal Epithelial Cells of Feijoa sellowiana Fruit Extract. International Journal of Molecular Sciences, 26(16), 7851. https://doi.org/10.3390/ijms26167851








