Genome-Wide Identification of ATP-Binding Cassette (ABC) Transporter Gene Family and Their Expression Analysis in Response to Anthocyanin Transportation in the Fruit Peel of Eggplant (Solanum melongena L.)
Abstract
1. Introduction
2. Results
2.1. Identification and Physicochemical Analysis of the SmABC Gene Family
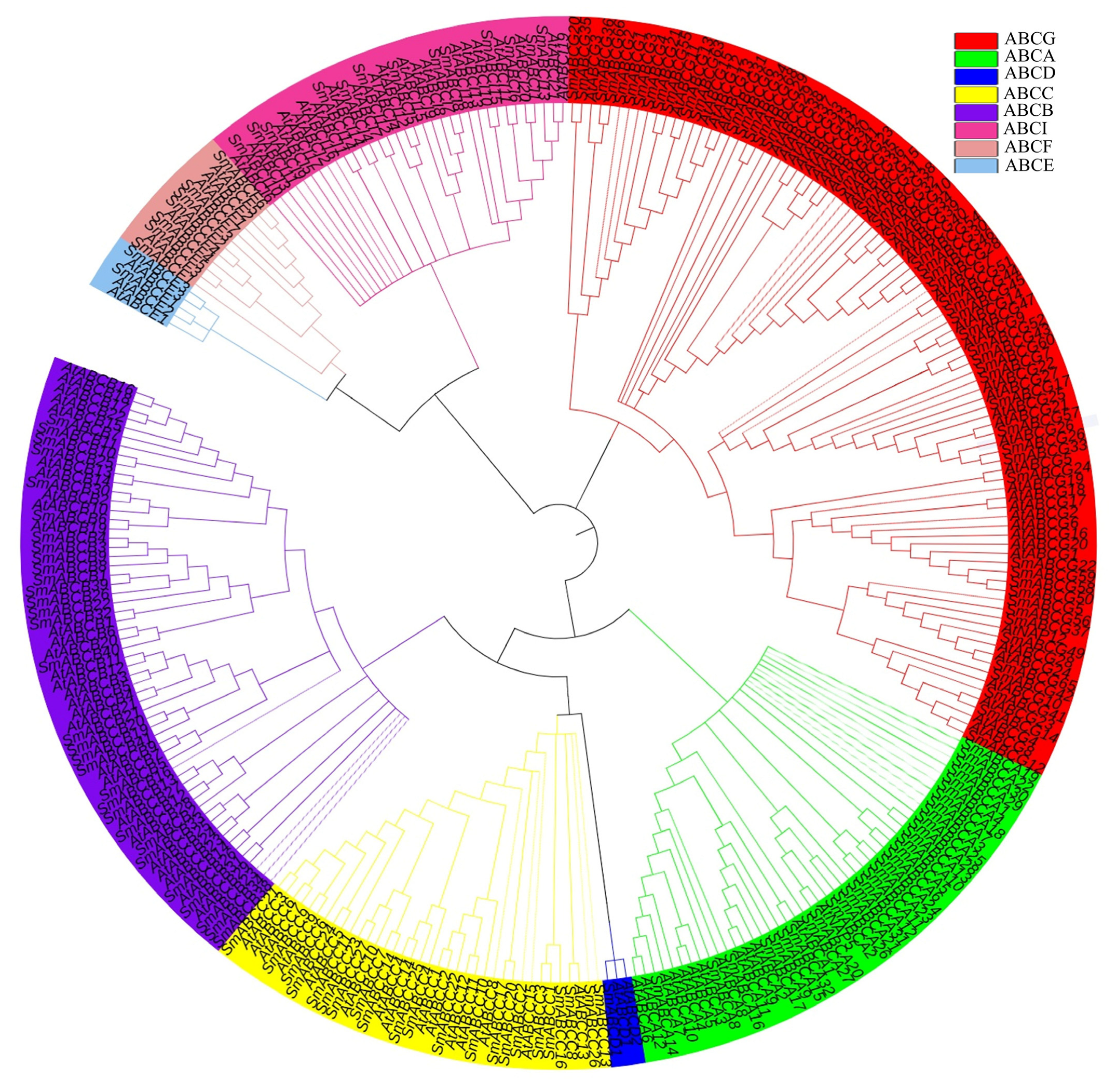
2.2. Phylogenetic Analysis of the SmABC Gene Family
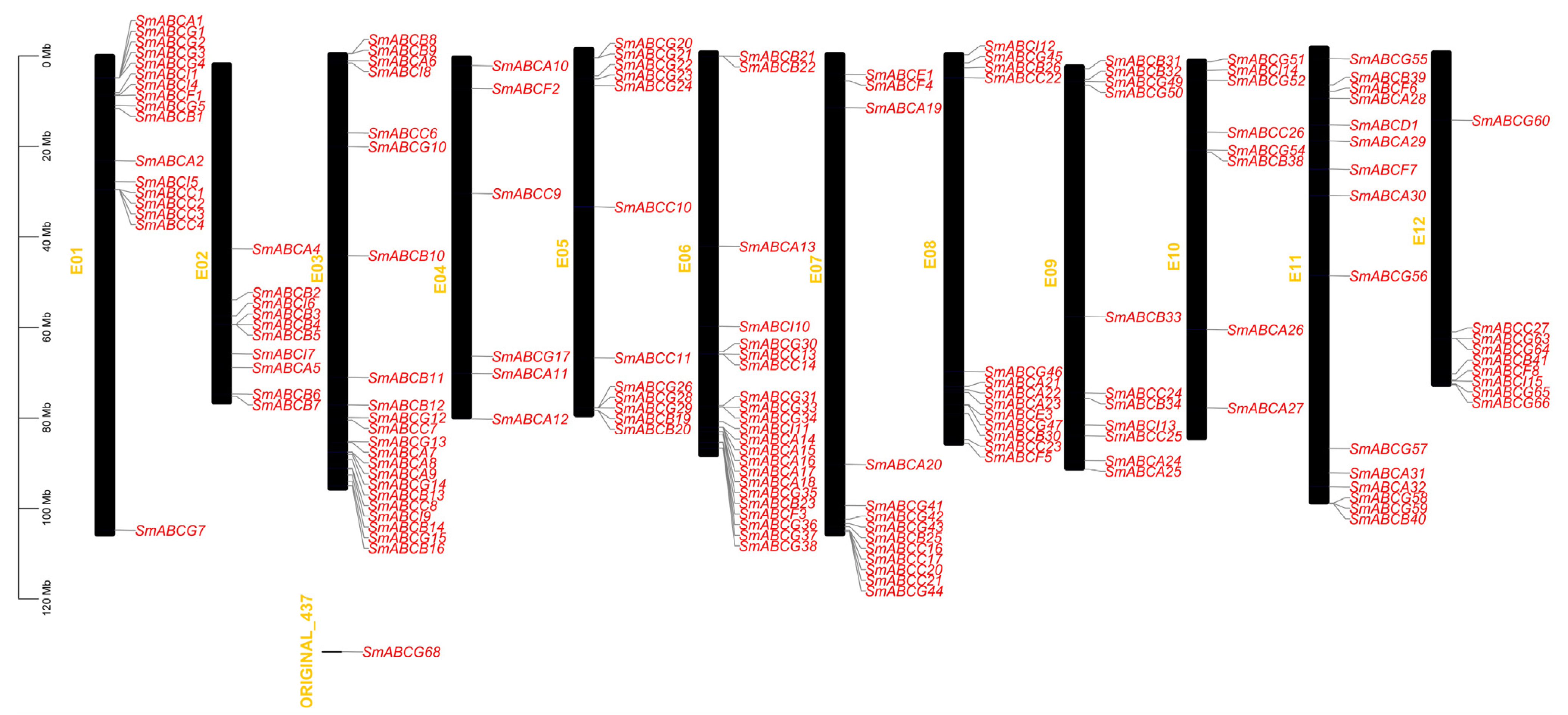
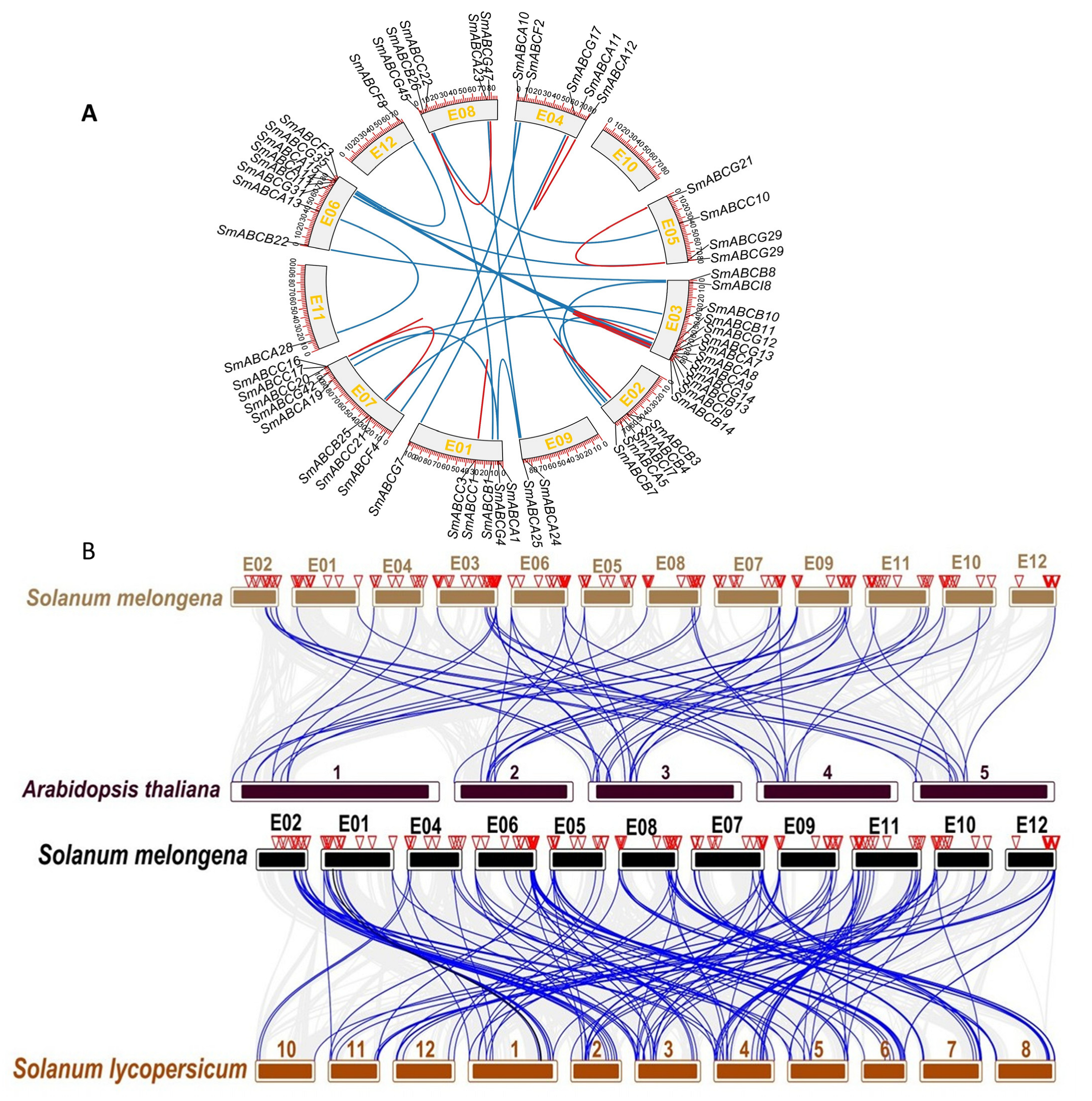
2.3. Chromosomal Distribution, Collinearity, and Gene Duplication Analysis of SmABC in Eggplant
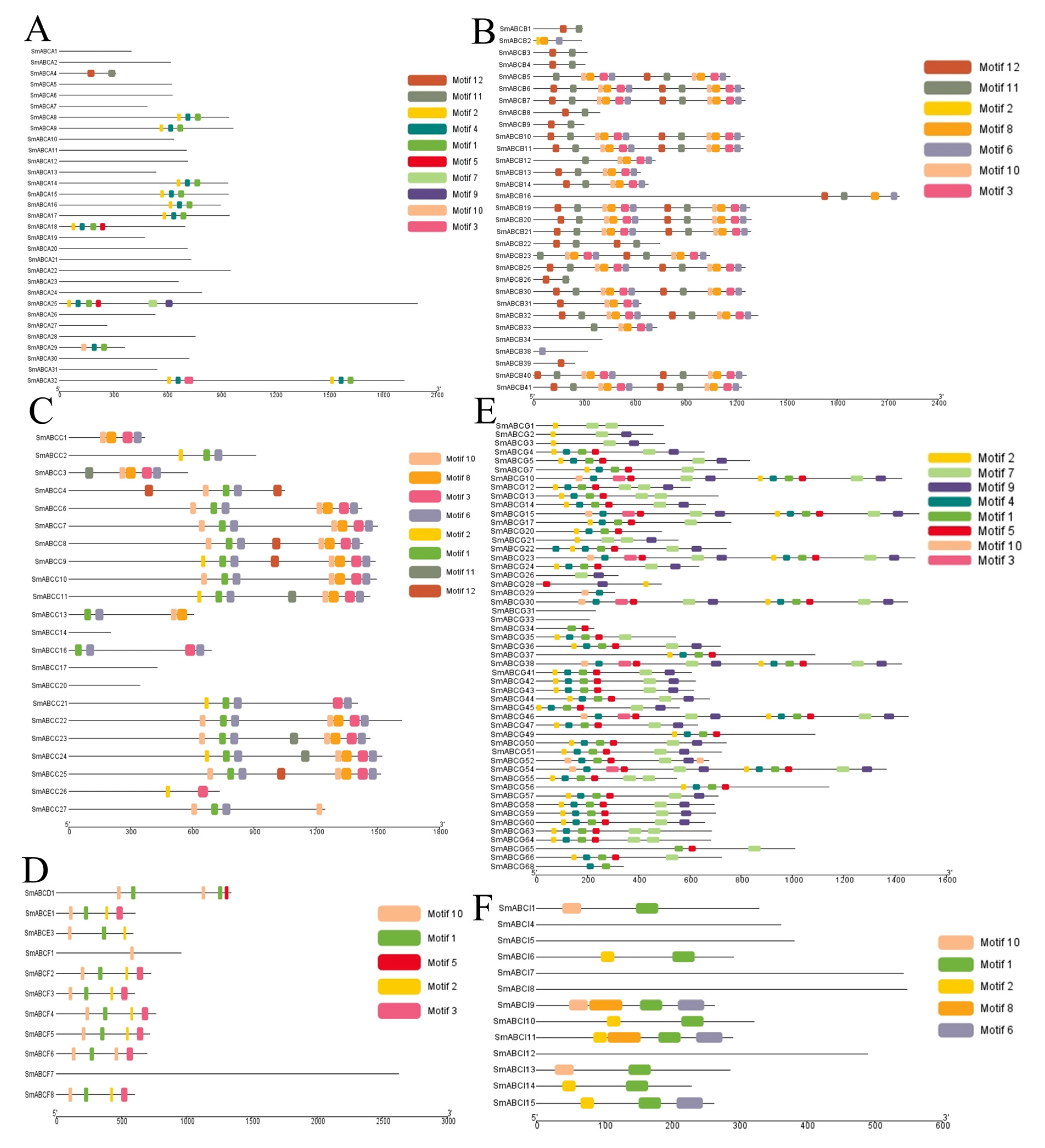
2.4. Gene Structure and Conserved Motif Analysis of SmABC in Eggplant
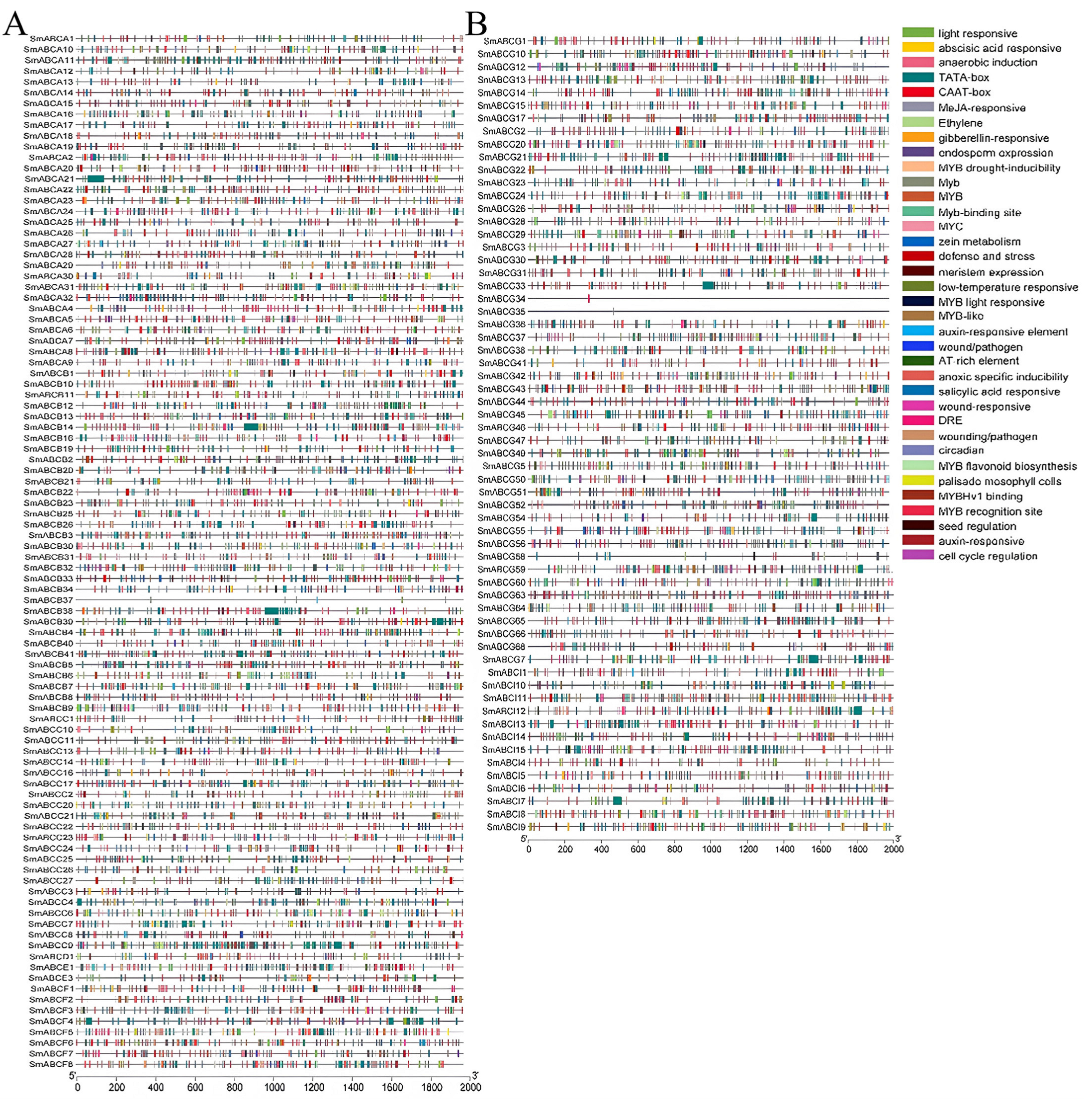
2.5. Analysis of Cis-Acting Elements in the SmABC Gene Family
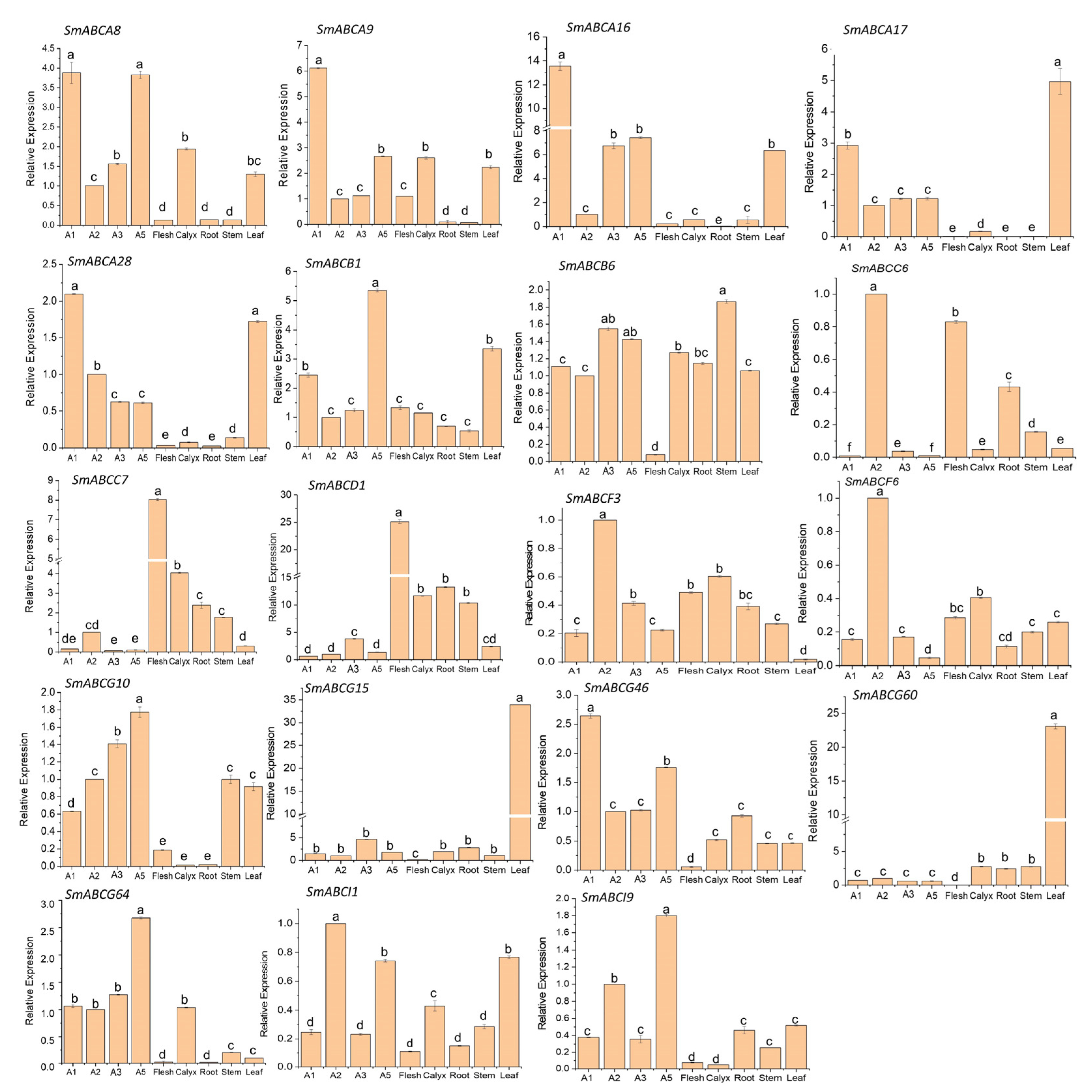
2.6. Expression of SmABC Genes on Eggplant Fruits with Different Peel Colors
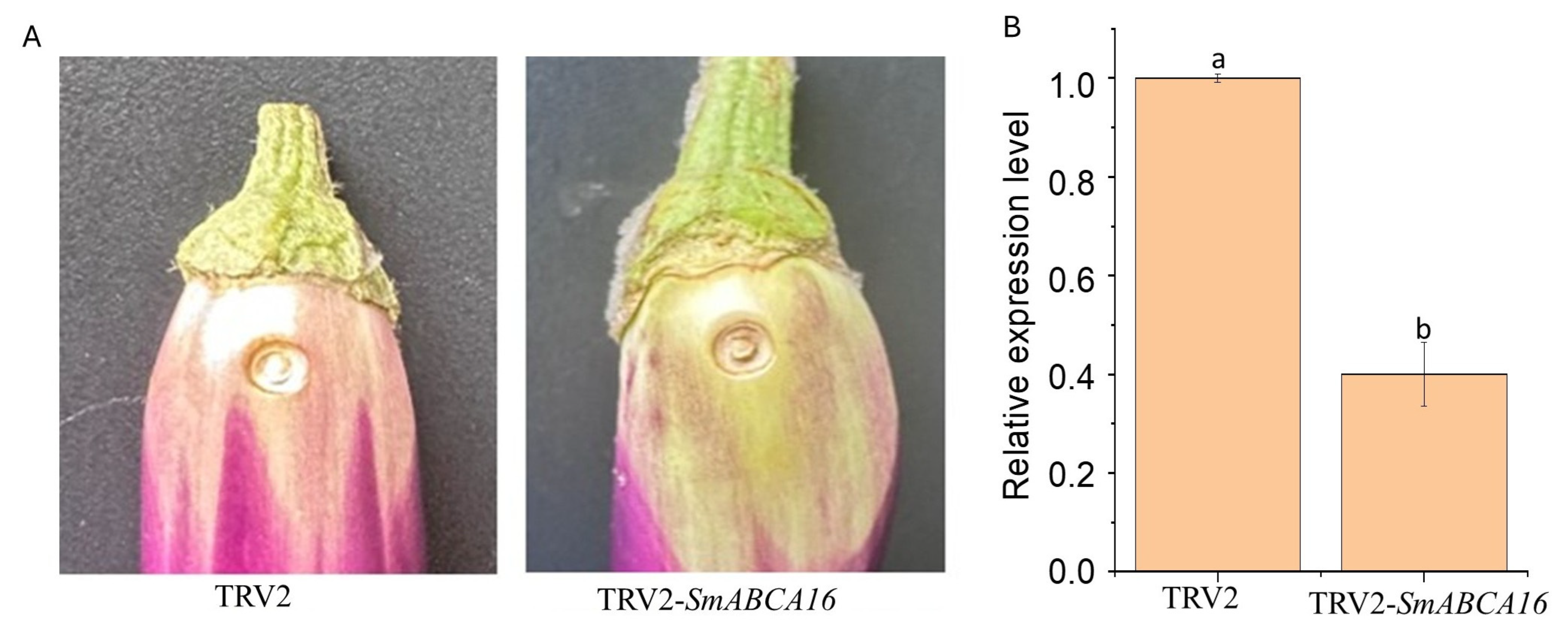
2.7. Analysis of the Effects of Silencing SmABCA16 on Fruit Color and the Expression Pattern
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification and Protein Characterization Analysis of ABC in Eggplant
4.2. Phylogenetic Analysis of ABC Genes in Eggplant
4.3. Gene Structure Conserved Motif Analysis of ABC in Eggplant
4.4. Chromosome Localization, Collinearity, and Gene Duplication Analysis of ABC in Eggplant
4.5. Identification of Cis-Acting Elements of ABC in Eggplant
4.6. Expression Analysis Using Transcriptomic Data
4.7. Quantitative Real-Time PCR Analysis of SmABC Genes
4.8. Vector Construction and Transformation of Eggplant
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banasiak, J.; Jasiński, M. ATP-binding cassette transporters in non-model plants. New Phytol. 2022, 233, 1597–1612. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T. Structural dynamics of ABC transporters: Molecular simulation studies. Biochem. Soc. Trans. 2021, 49, 405–414. [Google Scholar] [CrossRef]
- Nogia, P.; Pati, P.K. Plant secondary metabolite transporters: Diversity, functionality, and their modulation. Front. Plant Sci. 2021, 12, 758202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yu, Z.; Zeng, B.; Liu, X. Genome-wide analysis of the ABC gene family in almond and functional predictions during flower development, freezing stress, and salt stress. BMC Plant Biol. 2024, 24, 12. [Google Scholar] [CrossRef]
- Stratford, F.L.; Ramjeesingh, M.; Cheung, J.C.; Huan, L.J.; Bear, C.E. The Walker B motif of the second nucleotide-binding domain (NBD2) of CFTR plays a key role in ATPase activity by the NBD1–NBD2 heterodimer. Biochem. J. 2007, 401, 581–586. [Google Scholar] [CrossRef]
- Zolnerciks, J.K.; Andress, E.J.; Nicolaou, M.; Linton, K.J. Structure of ABC transporters. Essays Biochem. 2011, 50, 43–61. [Google Scholar] [CrossRef]
- Andolfo, G.; Ruocco, M.; Di Donato, A.; Frusciante, L.; Lorito, M.; Scala, F.; Ercolano, M.R. Genetic variability and evolutionary diversification of membrane ABC transporters in plants. BMC Plant Biol. 2015, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Lane, T.S.; Rempe, C.S.; Davitt, J.; Staton, M.E.; Peng, Y.; Soltis, D.E.; Melkonian, M.; Deyholos, M.; Leebens-Mack, J.H.; Chase, M.; et al. Diversity of ABC transporter genes across the plant kingdom and their potential utility in biotechnology. BMC Biotechnol. 2016, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, J.; Chen, H.; Luo, H. Genome-wide analysis of ATP-binding cassette transporter provides insight to genes related to bioactive metabolite transportation in Salvia miltiorrhiza. BMC Genom. 2021, 22, 315. [Google Scholar] [CrossRef]
- Kang, J.; Park, J.; Choi, H.; Burla, B.; Kretzschmar, T.; Lee, Y.; Martinoia, E. Plant ABC transporters. Arabidopsis Book 2011, 9, e0153. [Google Scholar] [CrossRef]
- Thomas, C.; Aller, S.G.; Beis, K.; Carpenter, E.P.; Chang, G.; Chen, L.; Tampé, R. Structural and functional diversity calls for a new classification of ABC transporters. FEBS Lett. 2020, 594, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Verrier, P.J.; Bird, D.; Burla, B.; Dassa, E.; Forestier, C.; Geisler, M.; Theodoulou, F.L. Plant ABC proteins–a unified nomenclature and updated inventory. Plant Sci. 2008, 13, 151–159. [Google Scholar] [CrossRef]
- Jasinski, M.; Ducos, E.; Martinoia, E.; Boutry, M. The ATP-binding cassette transporters: Structure, function, and gene family comparison between rice and Arabidopsis. Plant Physiol. 2003, 131, 1169–1177. [Google Scholar] [CrossRef]
- Çakır, B.; Kılıçkaya, O. Whole-genome survey of the putative ATP-binding cassette transporter family genes in Vitis vinifera. PLoS ONE 2013, 8, e78860. [Google Scholar] [CrossRef]
- Malik, W.A.; Afzal, M.; Chen, X.; Cui, R.; Lu, X.; Wang, S.; Ye, W. Systematic analysis and comparison of ABC proteins superfamily confer structural, functional and evolutionary insights into four cotton species. Ind. Crop. Prod 2022, 177, 114433. [Google Scholar] [CrossRef]
- Pang, K.; Li, Y.; Liu, M.; Meng, Z.; Yu, Y. Inventory and general analysis of the ATP-binding cassette (ABC) gene superfamily in maize (Zea mays L.). Gene 2013, 526, 411–428. [Google Scholar] [CrossRef]
- Cho, M.; Cho, H. The function of ABCB transporters in auxin transport. Plant Signal. Behav. 2013, 8, 642–654. [Google Scholar] [CrossRef]
- Titapiwatanakun, B.; Murphy, A.S. post-transcriptional regulation of auxin transport proteins: Cellular trafficking, protein phosphorylation, protein maturation, ubiquitination, and membrane composition. J. Exp. Bot. 2009, 60, 1093–1107. [Google Scholar] [CrossRef]
- Yang, H.; Murphy, A.S. Functional expression and characterization of Arabidopsis ABCB, AUX 1 and PIN auxin transporters in Schizosaccharomyces pombe. Plant J. 2009, 59, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, S.; Guo, H.; Wang, S.; Xu, L.; Li, C.; Jiang, D.A. Os ABCB 14 functions in auxin transport and iron homeostasis in rice (Oryza sativa L.). Plant J. 2014, 79, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Çakır, B.; Jalili, H.; Turgay, G. Genome-wide analysis of the ABCB gene family in Vitis vinifera: Its expression patterns in berries and its responses to iron and heavy metal stresses. J. Hortic. Sci. Biotechnol. 2023, 98, 591–607. [Google Scholar] [CrossRef]
- Naaz, S.; Ahmad, N.; Jameel, M.R.; Al-Huqail, A.A.; Khan, F.; Qureshi, M.I. Impact of Some Toxic Metals on Important ABC Transporters in Soybean (Glycine max L.). ACS Omega 2023, 8, 27597–27611. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Xie, Y.F.; Wu, Y.; Guo, N.; Li, D.H.; Gao, J.S. Genome-wide identification of ABCC gene family and their expression analysis in pigment deposition of fiber in brown cotton (Gossypium hirsutum). PLoS ONE 2021, 16, e0246649. [Google Scholar] [CrossRef]
- Yazaki, K. ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett. 2006, 580, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.M.; Regalado, A.; Ageorges, A.; Burla, B.J.; Bassin, B.; Eisenach, C.; Zarrouk, O.; Vialet, S.; Marlin, T.; Chaves, M.M.; et al. ABCC1, an ATP-binding cassette protein from grape berry, transports anthocyanidin 3-O-glucosides. Plant Cell 2013, 25, 1840–1854. [Google Scholar] [CrossRef]
- Behrens, C.E.; Smith, K.E.; Iancu, C.V.; Choe, J.Y.; Dean, J.V. Transport of anthocyanins and other flavonoids by the Arabidopsis ATP-binding cassette transporter AtABCC2. Sci. Rep. 2019, 9, 437. [Google Scholar] [CrossRef]
- Qian, T.; Wang, X.; Liu, J.; Shi, M.; Zhao, J.; Sun, P.; Xie, X. ATP-binding cassette protein ABCC8 promotes anthocyanin accumulation in strawberry fruits. Plant Physiol. Biochem. 2023, 203, 108037. [Google Scholar] [CrossRef]
- Sylvia, C.; Sun, J.; Zhang, Y.; Ntini, C.; Ogutu, C.; Zhao, Y.; Han, Y. Genome-wide analysis of ATP binding cassette (ABC) transporters in peach (Prunus persica) and identification of a Gene PpABCC1 involved in anthocyanin accumulation. Int. J. Mol. Sci. 2023, 24, 1931. [Google Scholar] [CrossRef] [PubMed]
- Bhati, K.K.; Sharma, S.; Aggarwal, S.; Kaur, M.; Shukla, V.; Kaur, J.; Mantri, S.; Pandey, A.K. Genome-wide identification and expression characterization of ABCC-MRP transporters in hexaploid wheat. Front. Plant Sci. 2015, 6, 488. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.D.; Casati, P.; Walbot, V. A multidrug resistance–associated protein involved in anthocyanin transport in Zea mays. Plant Cell 2004, 16, 1812–1826. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xie, X.R.; Zhang, J.; Xiang, G.; Li, Y.; Wu, H.B. In silico analysis of a MRP transporter gene reveals its possible role in anthocyanins or flavonoids transport in Oryza sativa. Am. J. Plant Sci. 2013, 4, 555–560. [Google Scholar] [CrossRef]
- Dean, J.V.; Willis, M.; Shaban, L. Transport of acylated anthocyanins by the Arabidopsis ATP-binding cassette transporters AtABCC1, AtABCC2, and AtABCC14. Physiol. Plant. 2022, 174, e13780. [Google Scholar] [CrossRef]
- Gaillard, S.; Jacquet, H.; Vavasseur, A.; Leonhardt, N.; Forestier, C. AtMRP6/AtABCC6, an ATP-binding cassette transporter gene expressed during early steps of seedling development and up-regulated by cadmium in Arabidopsis thaliana. BMC Plant Biol. 2008, 8, 22. [Google Scholar] [CrossRef]
- Raichaudhuri, A. Arabidopsis thaliana MRP1 (AtABCC1) nucleotide binding domain contributes to arsenic stress tolerance with serine triad phosphorylation. Plant Physiol. Biochem. 2016, 108, 109–120. [Google Scholar] [CrossRef]
- Rea, P.A. Plant ATP-binding cassette transporters. Annu. Rev. Plant Biol. 2007, 58, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Zolman, B.K.; Silva, I.D.; Bartel, B. The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol. 2001, 127, 1266–1278. [Google Scholar] [CrossRef]
- Dhara, A.; Raichaudhuri, A. ABCG transporter proteins with beneficial activity on plants. Phytochemistry 2021, 184, 112663. [Google Scholar] [CrossRef]
- Niu, L.; Li, H.; Song, Z.; Dong, B.; Cao, H.; Liu, T.; Fu, Y. The functional analysis of ABCG transporters in the adaptation of pigeon pea (Cajanus cajan) to abiotic stresses. PeerJ 2021, 9, e10688. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health Benefits and Bioactive Compounds of Eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Shirasawa, K.; Miyatake, K.; Nunome, T.; Negoro, S.; Ohyama, A.; Yamaguchi, H.; Sato, S.; Isobe, S.; Tabata, S.; et al. Draft genome sequence of eggplant (Solanum melongena L.): The representative solanum species indigenous to the Old World. DNA Res. 2014, 21, 649–660. [Google Scholar] [CrossRef]
- Barchi, L.; Pietrella, M.; Venturini, L.; Minio, A.; Toppino, L.; Acquadro, A.; Andolfo, G.; Aprea, G.; Avanzato, C.; Bassolino, L. A chromosome-anchored eggplant genome sequence reveals key events in Solanaceae evolution. Sci. Rep. 2021, 9, 11769. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, J.; Wang, W.; Hu, T.; Hu, H.; Bao, C. A high-quality chromosome-level genome assembly reveals genetics for important traits in eggplant. Hortic. Res. 2020, 7, 153. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, S.; Yang, Y.; Liu, J.; Zhuang, Y. Integrated metabolome and transcriptome analysis reveals a regulatory network of fruit peel pigmentation in eggplant (Solanum melongena L.). Int. J. Mol. Sci. 2022, 23, 13475. [Google Scholar] [CrossRef]
- Sánchez-Fernández, R.; Davies, T.G.; Coleman, J.O.; Rea, P.A. The Arabidopsis thaliana ABC protein superfamily, a complete inventory. J. Biol. Chem. 2001, 276, 30231–30244. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, Z.J.; Liu, J.; Nan, Z.; Yang, H. Genome-wide identification of Oryza sativa: A new insight for advanced analysis of ABC transporter genes associated with the degradation of four pesticides. Gene 2022, 834, 146613. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yuan, X.; Li, L.; Zeng, M.; Yang, J.; Tang, H.; Duan, C. Genome-wide analysis of the ATP-binding cassette (ABC) transporter family in Zea mays L. and its response to heavy metal stresses. Int. J. Mol. Sci. 2022, 23, 2109. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Choi, J.; Rabbee, M.F.; Baek, K.H. In Silico Genome-Wide Analysis of the ATP-Binding Cassette Transporter Gene Family in Soybean (Glycine max L.) and Their Expression Profiling. BioMed Res. Int. 2019, 2019, 8150523. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Li, X. Genome-wide identification and expression pattern profiling of the ATP-binding cassette gene family in tea plant (Camellia sinensis). Plant Physiol. Biochem. 2023, 202, 107930. [Google Scholar] [CrossRef]
- Zaynab, M.; Wang, Z.; Hussain, A.; Bahadar, K.; Sajid, M.; Sharif, Y.; Azam, M.; Sughra, K.; Raza, M.A.; Khan, K.A.; et al. ATP-binding cassette transporters expression profiling revealed its role in the development and regulating stress response in Solanum tuberosum. Mol. Biol. Rep. 2022, 49, 5251–5264. [Google Scholar] [CrossRef]
- Hashmi, S.S.; Bilal, S.; Jan, R.; Asif, S.; Abdelbacki, A.M.; Al-Harrasi, A.; Asaf, S. Exploring the Role of ATP-Binding Cassette (ABC) Transporters in Tomato (Solanum lycopersicum) Under Cadmium Stress Through Genome-Wide and Transcriptomic Analysis. Front. Plant Sci. 2025, 16, 1536178. [Google Scholar] [CrossRef]
- Ofori, P.A.; Mizuno, A.; Suzuki, M.; Martinoia, E.; Reuscher, S.; Aoki, K.; Shibata, D.; Otagaki, S.; Matsumoto, S.; Shiratake, K. Genome-wide analysis of ATP-binding cassette (ABC) transporters in tomato. PLoS ONE 2018, 13, e0200854. [Google Scholar] [CrossRef]
- Lopez-Ortiz, C.; Dutta, S.K.; Natarajan, P.; Peña-Garcia, Y.; Abburi, V.; Saminathan, T.; Reddy, U.K. Genome-wide identification and gene expression pattern of ABC transporter gene family in Capsicum spp. PLoS ONE 2019, 14, e0215901. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.J.; Chamberlain, S.R.; Fineberg, N. An ABC model of habit disorders: Hair-pulling, skin-picking, and other stereotypic conditions. CNS Spectr. 2006, 11, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.; Ambrosino, P.; Lanzuise, S.; Woo, S.L.; Lorito, M.; Scala, F. Four potato (Solanum tuberosum) ABCG transporters and their expression in response to abiotic factors and Phytophthora infestans infection. Plant Physiol. 2011, 168, 2225–2233. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, Q.; Zhang, G.; Wu, T.; Zhang, X.; Xu, X.; Wang, Y. Genome-wide identification and characterization of ABC transporters in nine Rosaceae species, identifying MdABCG28 as a possible cytokinin transporter linked to dwarfing. Int. J. Mol. Sci. 2019, 20, 5783. [Google Scholar] [CrossRef]
- Sidhu, D.; Gill, K.S. Distribution of genes and recombination in wheat and other eukaryotes. Plant Cell Tissue Organ Cult. 2004, 79, 257–270. [Google Scholar] [CrossRef]
- Lecharny, A.; Boudet, N.; Gy, I.; Aubourg, S.; Kreis, M. Introns in, introns out in plant gene families: A genomic approach of the dynamics of gene structure. J. Struct. Funct. Genom. 2003, 3, 111–116. [Google Scholar] [CrossRef]
- Shi, M.; Wang, S.; Zhang, Y.; Wang, S.; Zhao, J.; Feng, H.; Xie, X. Genome-wide characterization and expression analysis of ATP-binding cassette (ABC) transporters in strawberry reveal the role of FvABCC11 in cadmium tolerance. Sci. Hortic. 2020, 271, 109464. [Google Scholar] [CrossRef]
- Klein, M.; Burla, B.; Martinoia, E. The multidrug resistance-associated protein (MRP/ABCC) subfamily of ATP-binding cassette transporters in plants. FEBS Lett. 2006, 580, 1112–1122. [Google Scholar] [CrossRef]
- Panikashvili, D.; Shi, J.X.; Schreiber, L.; Aharoni, A. The Arabidopsis ABCG13 transporter is required for flower cuticle secretion and patterning of the petal epidermis. New Phytol. 2011, 190, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Siriwardana, C.L.; Risinger, J.R.; Carpenter, E.M.; Holt, B.F. Analysis of gene duplication within the Arabidopsis NUCLEAR FACTOR Y, subunit B (NF-YB) protein family reveals domains under both purifying and diversifying selection. PLoS ONE 2023, 18, e0289332. [Google Scholar] [CrossRef]
- Biłas, R.; Szafran, K.; Hnatuszko-Konka, K.; Kononowicz, A.K. Cis-regulatory elements used to control gene expression in plants. Plant Cell Tissue Organ Cult. 2016, 127, 269–287. [Google Scholar] [CrossRef]
- Zong, Y.; Zhu, X.; Liu, Z.; Xi, X.; Li, G.; Cao, D.; Wei, L.; Li, J.; Liu, B. Functional MYB transcription factor encoding gene AN2 is associated with anthocyanin biosynthesis in Lycium ruthenicum Murray. BMC Plant Biol. 2019, 19, 169. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, L.; Zhao, Y.; Zhang, Z.; Zhang, Z.; Pei, M.; Song, B. Tissue-specific expression of StMYB3 gene involves regulating potato tuber skin and vascular bundle anthocyanin biosynthesis. Potato Res. 2023, 66, 159–178. [Google Scholar] [CrossRef]
- Li, S.; He, Y.; Li, L.; Li, D.; Chen, H. New insights on the regulation of anthocyanin biosynthesis in purple Solanaceous fruit vegetables. Sci. Hortic. 2022, 297, 110917. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Salazar, J.A.; Nicolás-Almansa, M.; Razi, M.; Rubio, M.; Ruiz, D.; Martínez-Gómez, P. Molecular bases of fruit quality in Prunus species: An integrated genomic, transcriptomic, and metabolic review with a breeding perspective. Int. J. Mol. Sci. 2020, 22, 333. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, X.Y.; Zhao, Y.W.; Wang, C.K.; Sun, Q.; Hu, D.G. Role of an ATP-binding cassette (ABC) transporter MdABCI17 in the anthocyanin accumulation of apple. Sci. Hortic. 2024, 323, 112502. [Google Scholar] [CrossRef]
- Cai, G.; Wang, G.; Kim, S.C.; Li, J.; Zhou, Y.; Wang, X. Increased expression of fatty acid and ABC transporters enhances seed oil production in camelina. Biotechnol. Biofuels 2021, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Chairattanawat, C.; Yamaoka, Y.; Yang, Q.; Lee, Y.; Hwang, J.U. Early seed development requires the A-type ATP-binding cassette protein ABCA10. Plant Physiol. 2022, 189, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant. 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, w202–w208. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.A.; Haubold, B.; Mitchell-Olds, T. Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, Arabis and related genera (Brassicaceae). Mol. Biol. Evol. 2000, 17, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Ju, Y.; Zhang, C.; Li, L.; Jiang, X.; Xie, X.; Lu, Y.; Wang, K.; Li, W. Styrax japonicus functional genomics: An efficient virus induced gene silencing (VIGS) system. Hortic. Plant J. 2024, 10, 252–258. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obel, H.O.; Zhou, X.; Liu, S.; Xing, L.; Yang, Y.; Liu, J.; Zhuang, Y. Genome-Wide Identification of ATP-Binding Cassette (ABC) Transporter Gene Family and Their Expression Analysis in Response to Anthocyanin Transportation in the Fruit Peel of Eggplant (Solanum melongena L.). Int. J. Mol. Sci. 2025, 26, 7848. https://doi.org/10.3390/ijms26167848
Obel HO, Zhou X, Liu S, Xing L, Yang Y, Liu J, Zhuang Y. Genome-Wide Identification of ATP-Binding Cassette (ABC) Transporter Gene Family and Their Expression Analysis in Response to Anthocyanin Transportation in the Fruit Peel of Eggplant (Solanum melongena L.). International Journal of Molecular Sciences. 2025; 26(16):7848. https://doi.org/10.3390/ijms26167848
Chicago/Turabian StyleObel, Hesbon Ochieng, Xiaohui Zhou, Songyu Liu, Liwei Xing, Yan Yang, Jun Liu, and Yong Zhuang. 2025. "Genome-Wide Identification of ATP-Binding Cassette (ABC) Transporter Gene Family and Their Expression Analysis in Response to Anthocyanin Transportation in the Fruit Peel of Eggplant (Solanum melongena L.)" International Journal of Molecular Sciences 26, no. 16: 7848. https://doi.org/10.3390/ijms26167848
APA StyleObel, H. O., Zhou, X., Liu, S., Xing, L., Yang, Y., Liu, J., & Zhuang, Y. (2025). Genome-Wide Identification of ATP-Binding Cassette (ABC) Transporter Gene Family and Their Expression Analysis in Response to Anthocyanin Transportation in the Fruit Peel of Eggplant (Solanum melongena L.). International Journal of Molecular Sciences, 26(16), 7848. https://doi.org/10.3390/ijms26167848







