The Roles of PD-L1, Ki-67, P53, and Cyclin D1 in PitNETs: Diagnostic and Prognostic Implications in a Series of 74 Patients
Abstract
1. Introduction
- Primary objective: Assessment of the expression of PDL1, Ki-67, cyclin D1, and P53 in PitNETs depending on the transcription factor and adenoma subtype.
- Secondary objective: Assessment of the correlations between PDL1, cyclin D1, P53, and Ki-67 and tumor invasiveness, hormonal function, and parameters such as age, sex, maximum tumor size, and tumor volume.
2. Results
2.1. Patient Characteristics
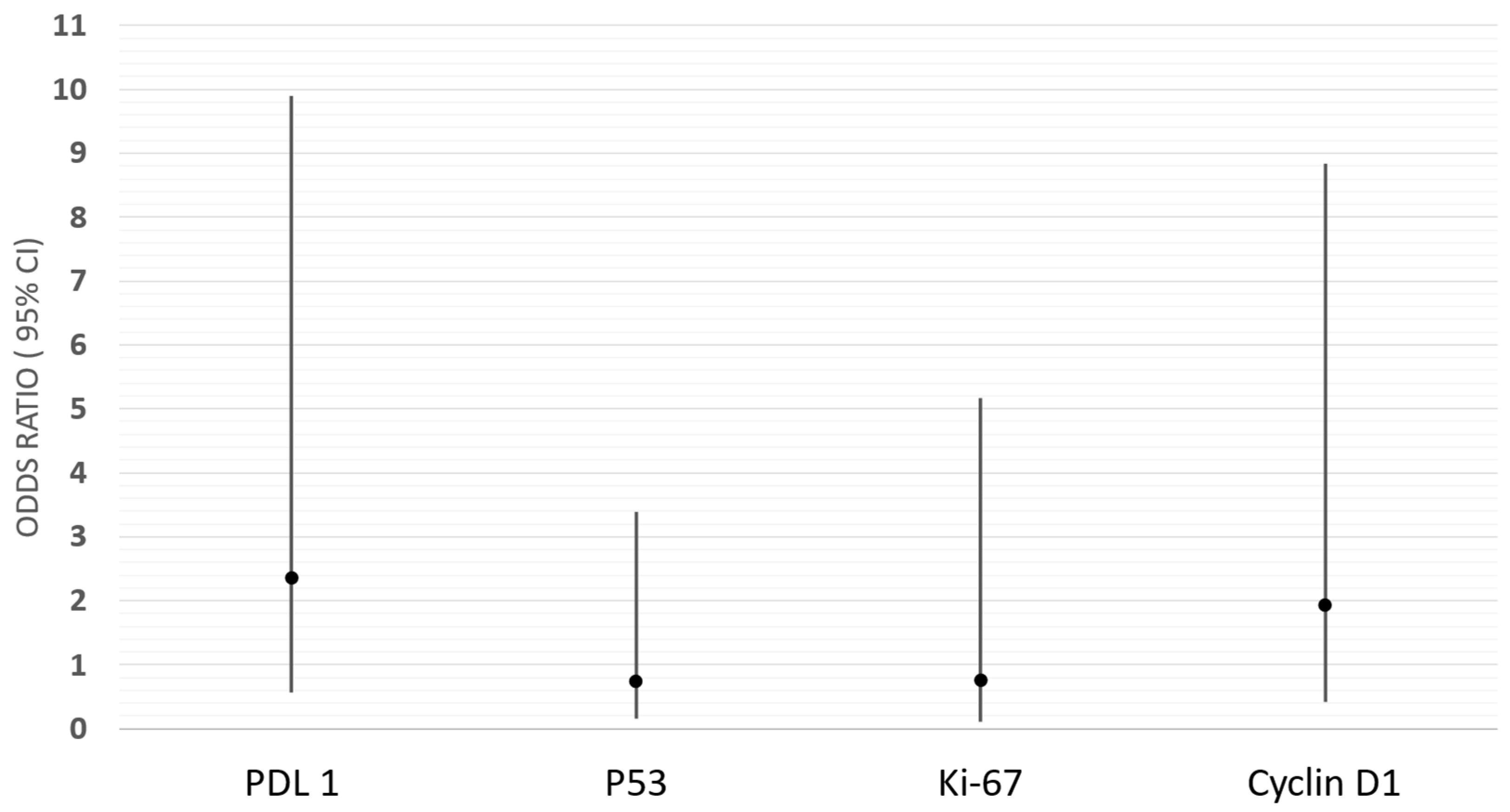
2.2. Immunoexpression of PDL-1, Ki-67, P53, and Cyclin D1 and Their Correlations with Epidemiological, Clinical, and Histopathological Features
2.3. Combined Analysis of Ki-67 and P53 Expression or Ki-67 and Cyclin D1 Expression
2.3.1. Combined Analysis of Ki-67 and P53 Factors
2.3.2. Combined Analysis of Cyclin D1 and P53 Factors
2.3.3. Analysis of the Effect of the Combination of Ki-67, P53, and Cyclin D1 on the Invasiveness of PitNETs
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Classification of Pituitary Neuroendocrine Tumors (PitNETs)
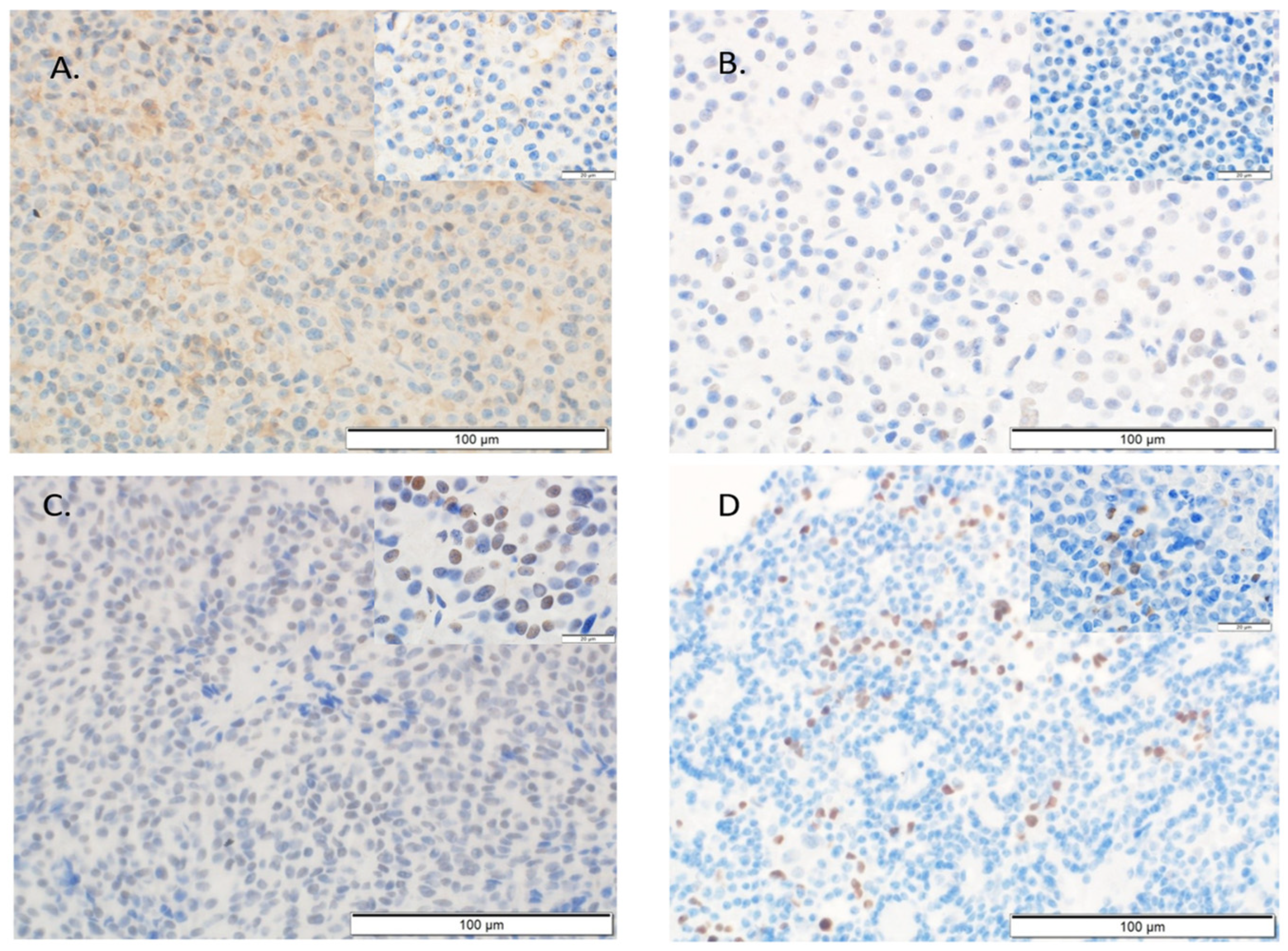
4.3. Immunohistochemical Assessment
4.3.1. Immunohistochemical Assessment of the Hormones and Transcription Factors
4.3.2. Immunohistochemistry of PD-L1, Cyclin D1, Ki-67, and P53
4.3.3. Magnetic Resonance Imaging of the Tumors
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molitch, M.E. Diagnosis and treatment of pituitary adenomas: A review. JAMA 2017, 317, 516–524. [Google Scholar] [CrossRef]
- Melmed, S. Pathogenesis of pituitary tumors. Nat. Rev. Endocrinol. 2011, 7, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Kaiser, U.B.; Lopes, M.B.; Bertherat, J.; Syro, L.V.; Raverot, G.; Reincke, M.; Johannsson, G.; Beckers, A.; Fleseriu, M.; et al. Clinical biology of the pituitary adenoma. Endocr. Rev. 2022, 43, 1003–1037. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Casar-Borota, O.; Chanson, P.; Delgrange, E.; Earls, P.; Ezzat, S.; Grossman, A.; Ikeda, H.; Inoshita, N.; Karavitaki, N.; et al. From pituitary adenoma to pituitary neuroendocrine tumor (PITNET): An International Pituitary Pathology Club proposal. Endocr. Relat. Cancer 2017, 24, C5–C8. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO classification of pituitary tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef]
- Raverot, G.; Dantony, E.; Beauvy, J.; Vasiljevic, A.; Mikolasek, S.; Borson-Chazot, F.; Jouanneau, E.; Roy, P.; Trouillas, J. Risk of recurrence in pituitary neuroendocrine tumors: A prospective study using a five-tiered classification. J. Clin. Endocrinol. Metab. 2017, 102, 3368–3374. [Google Scholar] [CrossRef]
- Raverot, G.; Burman, P.; McCormack, A.; Heaney, A.; Petersenn, S.; Popovic, V.; Trouillas, J.; Dekkers, O.M. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur. J. Endocrinol. 2018, 178, G1–G24. [Google Scholar] [CrossRef]
- Dekkers, O.M.; Karavitaki, N.; Pereira, A.M. The epidemiology of aggressive pituitary tumors (and its challenges). Rev. Endocr. Metab. Disord. 2020, 21, 209–212. [Google Scholar] [CrossRef]
- Lopes-Pinto, M.; Lacerda-Nobre, E.; Silva, A.L.; Tortosa, F.; Marques, P. The Role of Programmed Cell Death Ligand 1 Expression in Pituitary Tumours: Lessons from the Current Literature. Neuroendocrinology 2024, 114, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Gejman, R.; Swearingen, B.; Hedley-Whyte, E.T. Role of Ki-67 Proliferation Index and P53 Expression in Predicting Progression of Pituitary Adenomas. Hum. Pathol. 2008, 39, 758–766. [Google Scholar] [CrossRef]
- Suteau, V.; Collin, A.; Menei, P.; Rodien, P.; Rousselet, M.C.; Briet, C. Expression of programmed death-ligand 1 (PD-L1) in human pituitary neuroendocrine tumor. Cancer Immunol. Immunother. 2020, 69, 2053–2061. [Google Scholar] [CrossRef]
- Trouillas, J.; Roy, P.; Sturm, N.; Dantony, E.; Cortet-Rudelli, C.; Viennet, G.; Bonneville, J.F.; Assaker, R.; Auger, C.; Brue, T.; et al. Clinically aggressive pituitary ade-nomas and carcinomas: A clinicopathologic classification and long-term follow-up study. Acta Neuropathol. 2013, 126, 419–435. [Google Scholar]
- Ben-Shlomo, A.; Cooper, O. Silent corticotroph adenomas. Pituitary 2018, 21, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rivas, L.G.; Simon, J.; Albani, A.; Tang, S.; Roeber, S.; Assié, G.; Deutschbein, T.; Fassnacht, M.; Gadelha, M.R.; Hermus, A.R.; et al. TP53 mutations in functional corticotroph tumors are linked to invasion and worse clinical outcome. Acta Neuropathol. Commun. 2022, 10, 139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cha, J.H.; Chan, L.C.; Li, C.W.; Hsu, J.L.; Hung, M.C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef]
- Mei, Y.; Bi, W.L.; Greenwald, N.F.; Du, Z.; Agar, N.Y.; Kaiser, U.B.; Woodmansee, W.W.; Reardon, D.A.; Freeman, G.J.; Fecci, P.E.; et al. Increased Expression of Programmed Death Ligand 1 (PD-L1) in Human Pituitary Tumors. Oncotarget 2016, 7, 76565–76576. [Google Scholar] [CrossRef]
- Wang, P.F.; Wang, T.J.; Yang, Y.K.; Yao, K.; Li, Z.; Li, Y.M.; Yan, C.X. The Expression Profile of PD-L1 and CD8+ Lymphocyte in Pituitary Adenomas Indicating for Immunotherapy. J. Neurooncol. 2018, 139, 89–95. [Google Scholar] [CrossRef]
- Harel, E.; Hewer, E.; La Rosa, S.; Brouland, J.P.; Pitteloud, N.; Santoni, F.; Brunner, M.; Daniel, R.T.; Messerer, M.; Cossu, G. PD-L1 Expression in PITNETs: Correlations with the 2022 WHO Classification. Brain Spine 2024, 5, 104171. [Google Scholar] [CrossRef]
- Cossu, G.; La Rosa, S.; Brouland, J.P.; Pitteloud, N.; Harel, E.; Santoni, F.; Brunner, M.; Daniel, R.T.; Messerer, M. PD-L1 Expression in Pituitary Neuroendocrine Tumors/Pituitary Adenomas. Cancers 2023, 15, 4471. [Google Scholar] [CrossRef]
- Luo, M.; Tang, R.; Wang, H. Tumor Immune Microenvironment in Pituitary Neuroendocrine Tumors (PITNETs): Increased M2 Macrophage Infiltration and PD-L1 Expression in PIT1-Lineage Subset. J. Neurooncol. 2023, 163, 663–674. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Y.; Qian, Z.; Chang, M.; Zhao, Y.; Ma, W.; Wang, Y.; Xing, B. Immune Landscape and Progress in Immunotherapy for Pituitary Neuroendocrine Tumors. Cancer Lett. 2024, 592, 216908. [Google Scholar] [CrossRef]
- Di Nunno, V.; Franceschi, E.; Tosoni, A.; Gatto, L.; Maggio, I.; Lodi, R.; Bartolini, S.; Brandes, A.A. Immune-checkpoint inhibitors in pituitary malignancies. Anticancer. Drugs 2022, 33, e28–e35. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Ilie, M.D. Immunotherapy in Pituitary Carcinomas and Aggressive Pituitary Tumors. Best. Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101712. [Google Scholar] [CrossRef]
- Lopes-Pinto, M.; Lacerda-Nobre, E.; Silva, A.L.; Marques, P. Therapeutical Usefulness of PD-1/PD-L1 Inhibitors in Aggressive or Metastatic Pituitary Tumours. Cancers 2024, 16, 3033. [Google Scholar] [CrossRef] [PubMed]
- Gruppetta, M.; Formosa, R.; Falzon, S.; Ariff Scicluna, S.; Falzon, E.; Degeatano, J.; Vassallo, J. Expression of Cell Cycle Regulators and Biomarkers of Proliferation and Regrowth in Human Pituitary Adenomas. Pituitary 2017, 20, 358–371. [Google Scholar] [CrossRef]
- Yuhan, L.; Zhiqun, W.; Jihui, T.; Renlong, P. Ki-67 labeling index and Knosp classification of pituitary adenomas. Br. J. Neurosurg. 2024, 38, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Agur, A.; Scheithauer, B.W.; Kovacs, K.; Lloyd, R.V.; Cusimano, M. Ki-67 in Pituitary Neoplasms: A Review—Part I. Neurosurgery 2009, 65, 429–437. [Google Scholar] [CrossRef]
- Del Basso De Caro, M.; Solari, D.; Pagliuca, F.; Villa, A.; Guadagno, E.; Cavallo, L.M.; Colao, A.; Pettinato, G.; Cappabianca, P. Atypical Pituitary Adenomas: Clinical Characteristics and Role of Ki-67 and P53 in Prognostic and Therapeutic Evaluation. A Series of 50 Patients. Neurosurg. Rev. 2017, 40, 105–114. [Google Scholar] [CrossRef]
- Honegger, J.; Prettin, C.; Feuerhake, F.; Petrick, M.; Schulte-Mönting, J.; Reincke, M. Expression of Ki-67 Antigen in Nonfunctioning Pituitary Adenomas: Correlation with Growth Velocity and Invasiveness. J. Neurosurg. 2003, 99, 674–679. [Google Scholar] [CrossRef]
- Hasanov, R.; Aydoğan, B.İ.; Kiremitçi, S.; Erden, E.; Güllü, S. The Prognostic Roles of the Ki-67 Proliferation Index, P53 Expression, Mitotic Index, and Radiological Tumor Invasion in Pituitary Adenomas. Endocr. Pathol. 2019, 30, 49–55. [Google Scholar] [CrossRef]
- Petry, C.; Poli, J.H.Z.; de Azevedo Dossin, I.; Rech, C.G.S.L.; Pereira Lima, J.F.S.; Ferreira, N.P.; da Costa Oliveira, M. Evaluation of the Potential of the Ki67 Index to Predict Tumor Evolution in Patients with Pituitary Adenoma. Int. J. Clin. Exp. Pathol. 2019, 12, 320–326. [Google Scholar]
- Matoušek, P.; Buzrla, P.; Reguli, Š.; Krajča, J.; Dvořáčková, J.; Lipina, R. Factors That Predict the Growth of Residual Nonfunctional Pituitary Adenomas: Correlations Between Relapse and Cell Cycle Markers. Biomed. Res. Int. 2018, 2018, 1876290. [Google Scholar] [CrossRef]
- Mete, O.; Cintosun, A.; Pressman, I.; Asa, S.L. Epidemiology and Biomarker Profile of Pituitary Adenohypophysial Tumors. Mod. Pathol. 2018, 31, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Rak, B.; Maksymowicz, M.; Pękul, M.; Zieliński, G. Clinical, Biological, Radiological, Pathological and Immediate Post-Operative Remission of Sparsely and Densely Granulated Corticotroph Pituitary Tumors: A Retrospective Study of a Cohort of 277 Patients with Cushing’s Disease. Front. Endocrinol. 2021, 12, 672178. [Google Scholar] [CrossRef] [PubMed]
- George, D.H.; Scheithauer, B.W.; Kovacs, K.; Horvath, E.; Young, W.F., Jr.; Lloyd, R.V.; Meyer, F.B. Crooke’s Cell Adenoma of the Pituitary: An Aggressive Variant of Corticotroph Adenoma. Am. J. Surg. Pathol. 2003, 27, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Osamura, R.Y.; Inomoto, C.; Tahara, S.; Oyama, K.I.; Matsuno, A.; Teramoto, A. Pathology of Crooke Cells in the Human Pituitaries: A Timely Review. Appl. Immunohistochem. Mol. Morphol. 2023, 31, 485–489. [Google Scholar] [CrossRef]
- Raymond, P.; Raverot, G.; Ilie, M.D. Outcome and Prognostic Factors for Pituitary Carcinomas: Lessons from a Systematic Review. Endocr. Relat. Cancer 2023, 30, e220338. [Google Scholar] [CrossRef]
- Jordan, S.; Lidhar, K.; Korbonits, M.; Lowe, D.G.; Grossman, A.B. Cyclin D1 and Cyclin E Expression in Normal and Adenomatous Pituitary. Eur. J. Endocrinol. 2000, 143, R1–R6. [Google Scholar] [CrossRef]
- Tani, Y.; Inoshita, N.; Sugiyama, T.; Kato, M.; Yamada, S.; Shichiri, M.; Hirata, Y. Upregulation of CDKN2A and Suppression of Cyclin D1 Gene Expressions in ACTH-Secreting Pituitary Adenomas. Eur. J. Endocrinol. 2010, 163, 523–529. [Google Scholar] [CrossRef]
- Turner, H.E.; Nagy, Z.; Sullivan, N.; Esiri, M.M.; Wass, J.A. Expression Analysis of Cyclins in Pituitary Adenomas and the Normal Pituitary Gland. Clin. Endocrinol. 2000, 53, 337–344. [Google Scholar] [CrossRef]
- Fedele, M.; Fusco, A. Role of the High Mobility Group A Proteins in the Regulation of Pituitary Cell Cycle. J. Mol. Endocrinol. 2010, 44, 309–318. [Google Scholar] [CrossRef]
- Stefanidis, P.; Kyriakopoulos, G.; Seretis, A.M.; Korfias, S.; Theocharis, S.; Angelousi, A. Prognostic Factors for Invasiveness and Recurrence of Pituitary Adenomas: A Series of 94 Patients. Diagnostics 2022, 12, 2413. [Google Scholar] [CrossRef]
- Hibberts, N.A.; Simpson, D.J.; Bicknell, J.E.; Broome, J.C.; Hoban, P.R.; Clayton, R.N.; Farrell, W.E. Analysis of Cyclin D1 (CCND1) Allelic Imbalance and Overexpression in Sporadic Human Pituitary Tumors. Clin. Cancer Res. 1999, 5, 2133–2139. [Google Scholar] [PubMed]
- Hewedi, I.H.; Osman, W.M.; El Mahdy, M.M. Differential Expression of Cyclin D1 in Human Pituitary Tumors: Relation to MIB-1 and p27/Kip1 Labeling Indices. J. Egypt. Natl. Canc. Inst. 2011, 23, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Yagnik, G.; Jahangiri, A.; Chen, R.; Wagner, J.R.; Aghi, M.K. Role of a P53 Polymorphism in the Development of Nonfunctional Pituitary Adenomas. Mol. Cell. Endocrinol. 2017, 446, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Thapar, K.; Scheithauer, B.W.; Kovacs, K.; Pernicone, P.J.; Laws, E.R., Jr. P53 Expression in Pituitary Adenomas and Carcinomas: Correlation with Invasiveness and Tumor Growth Fractions. Neurosurgery 1996, 38, 765–770. [Google Scholar] [CrossRef]
- Saeger, W.; Mawrin, C.; Meinhardt, M.; Wefers, A.K.; Jacobsen, F. Two Pituitary Neuroendocrine Tumors (PITNETs) with Very High Proliferation and TP53 Mutation—High-Grade PITNET or PitNEC? Endocr. Pathol. 2022, 33, 257–262. [Google Scholar] [CrossRef]
- Trouillas, J.; Roy, P.; Sturm, N.; Dantony, E.; Cortet-Rudelli, C.; Viennet, G.; Bonneville, J.F.; Assaker, R.; Auger, C.; Brue, T.; et al. A new prognostic clinicopathological classification of pituitary adenomas: A multicentric case–control study of 410 patients with 8 years follow-up. Eur. J. Endocrinol. 2013, 168, 457–468. [Google Scholar] [CrossRef]
- Mete, O.; Lopes, M.B. Overview of the 2017 WHO classification of Pituitary Tumors. Endocr. Pathol. 2017, 28, 228–232. [Google Scholar] [CrossRef]
- Vela-Patiño, S.; Salazar, M.I.; Taniguchi-Ponciano, K.; Vadillo, E.; Gomez-Apo, E.; Escobar-España, A.; Perez-Koldenkova, V.; Bonifaz, L.; Aguilar-Flores, C.; Marrero-Rodríguez, D.; et al. The Immune Microenvironment Landscape of Pituitary Neuroendocrine Tumors, a Transcriptomic Approach. Genes 2024, 15, 531. [Google Scholar] [CrossRef]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 Expression in the Tumor Microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Gao, L.; Deng, K.; Lian, W.; Bao, X.; Feng, M.; Duan, L.; Zhu, H.; Xing, B. The immune profile of pituitary adenomas and a novel immune classification for predicting immunotherapy responsiveness. J. Clin. Endocrinol. Metab. 2020, 105, e3207–e3223. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, C.; Zhang, D.; Peng, J.; Ma, S.; Wang, X.; Guan, X.; Li, P.; Li, D.; Jia, G.; et al. Comprehensive analysis of the immunological landscape of pituitary adenomas: Implications of immunotherapy for pituitary adenomas. J. Neurooncol. 2020, 149, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Tapoi, D.A.; Popa, M.L.; Tanase, C.; Derewicz, D.; Gheorghișan-Gălățeanu, A.A. Role of Tumor Microenvironment in Pituitary Neuroendocrine Tumors: New Approaches in Classification, Diagnosis and Therapy. Cancers 2023, 15, 5301. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Q.; Adam, B.; Jack, A.S.; Lam, A.; Broad, R.W.; Chik, C.L. Immune cell infiltrates in pituitary adenomas: More macrophages in larger adenomas and more T cells in growth hormone adenomas. Endocr. Pathol. 2015, 26, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Awad, S.; Dorward, N.; Grieve, J.; Mendoza, N.; Muquit, S.; et al. Chemokines modulate the tumour microenvironment in pituitary neuroendocrine tumours. Acta Neuropathol. Commun. 2019, 7, 172. [Google Scholar] [CrossRef]
- Almeida, J.P.; Stephens, C.C.; Eschbacher, J.M.; Felicella, M.M.; Yuen, K.C.J.; White, W.L.; Mooney, M.A.; Bernat, A.L.; Mete, O.; Zadeh, G.; et al. Clinical, pathologic, and imaging characteristics of pituitary null cell adenomas as defined according to the 2017 World Health Organization criteria: A case series from two pituitary centers. Pituitary 2019, 22, 514–519. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Oh, T.; Pereira, M.P.; Joshi, R.S.; Pereira, K.M.; Osorio, R.C.; Donohue, K.C.; Peeran, Z.; Sudhir, S.; et al. Clinical characteristics and outcomes of null-cell versus silent gonadotroph adenomas in a series of 1166 pituitary adenomas from a single institution. Neurosurg. Focus. 2020, 48, E13. [Google Scholar] [CrossRef]
- Mayson, S.E.; Snyder, P.J. Silent (clinically nonfunctioning) pituitary adenomas. J. Neurooncol. 2014, 117, 429–436. [Google Scholar] [CrossRef]
- Xi, Z.; Jones, P.S.; Mikamoto, M.; Jiang, X.; Faje, A.T.; Nie, C.; Labelle, K.E.; Zhou, Y.; Miller, K.K.; Soberman, R.J.; et al. The Upregulation of Molecules Related to Tumor Immune Escape in Human Pituitary Adenomas. Front. Endocrinol. 2021, 12, 726448. [Google Scholar] [CrossRef]
- Mei, Y.; Bi, W.L.; Agolia, J.; Hu, C.; Giantini Larsen, A.M.; Meredith, D.M.; Al Abdulmohsen, S.; Bale, T.; Dunn, G.P.; Abedalthagafi, M.; et al. Immune Profiling of Pituitary Tumors Reveals Variations in Immune Infiltration and Checkpoint Molecule Expression. Pituitary 2021, 24, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Uraki, S.; Ariyasu, H.; Doi, A.; Takeshima, K.; Morita, S.; Inaba, H.; Furuta, H.; Fukuhara, N.; Inoshita, N.; Nishioka, H.; et al. MSH6/2 and PD-L1 Expressions Are Associated with Tumor Growth and Invasiveness in Silent Pituitary Adenoma Subtypes. Int. J. Mol. Sci. 2020, 21, 2831. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Jiang, X.; Wang, F.; Ling, S.; Niu, C. Prediction of Higher Ki-67 Index in Pituitary Adenomas by Pre- and Intra-Operative Clinical Characteristics. Brain Sci. 2022, 12, 1002. [Google Scholar] [CrossRef]
- Grimm, F.; Maurus, R.; Beschorner, R.; Naros, G.; Stanojevic, M.; Gugel, I.; Giese, S.; Bier, G.; Bender, B.; Honegger, J. Ki-67 labeling index and expression of p53 are non-predictive for invasiveness and tumor size in functional and nonfunctional pituitary adenomas. Acta Neurochir. 2019, 161, 1149–1156. [Google Scholar] [CrossRef]
- Mastronardi, L.; Guiducci, A.; Puzzilli, F. Lack of correlation between Ki-67 labelling index and tumor size of anterior pituitary adenomas. BMC Cancer 2001, 1, 12. [Google Scholar] [CrossRef]
- Thapar, K.; Kovacs, K.; Scheithauer, B.W.; Stefaneanu, L.; Lloyd, R.V.; Laws, E.R. Proliferative activity and invasiveness in pituitary adenomas. Neurosurgery 1996, 38, 99–107. [Google Scholar] [CrossRef]
- Flores, L.; Sleightholm, R.; Neilsen, B.; Baine, M.; Drincic, A.; Thorell, W.; Shonka, N.; Oupicky, D.; Zhang, C. Highly Aggressive and Radiation-Resistant, “Atypical” and Silent Pituitary Corticotrophic Carcinoma: A Case Report and Review of the Literature. Case Rep. Oncol. 2019, 12, 139–146. [Google Scholar] [CrossRef]
- Lasolle, H.; Vasiljevic, A.; Jouanneau, E.; Ilie, M.D.; Raverot, G. Aggressive Corticotroph Tumors and Carcinomas. J. Neuroendocrinol. 2022, 34, e13169. [Google Scholar] [CrossRef]
- Kovacs, K.; Diep, C.C.; Horvath, E.; Cusimano, M.; Smyth, H.; Lombardero, C.C.; Scheithauer, B.W.; Lloyd, R.V. Prognostic Indicators in an Aggressive Pituitary Crooke’s Cell Adenoma. Can. J. Neurol. Sci. 2005, 32, 540–545. [Google Scholar] [CrossRef]
- Liu, X. Classification Accuracy and Cut Point Selection. Stat. Med. 2012, 31, 2676–2686. [Google Scholar] [CrossRef]
| Characteristic | N (%) or Mean (SD); Median (Q1–Q3) |
|---|---|
| Age (years) | 57.4 (14.0); 60.5 (47.0–69.0) |
| Gender | Female: 29 (39.2%); Male: 45 (60.8%) |
| Tumor size | Micro: 1 (1.4%); Macro: 72 (97.3%) |
| Missing | 1 (1.4%) |
| Tumor volume (cm3) | 8.6 (8.9); 5.0 (3.1–10.2) |
| Max tumor size (mm) | 28.6 (10.2); 25.0 (21.5–34.0) |
| Transcription factors | |
| PIT-1 | 9 (12.2%) |
| SF1 | 43 (58.1%) |
| TPIT | 8 (10.8%) |
| Null cell adenoma | 3 (4.1%) |
| Multiple PitNETs | 11 (14.9%) |
| Hormonal activity | |
| Non-active/Active | 59 (79.7%)/15 (20.3%) |
| Type of PitNET | |
| Gonadotroph | 42 (56.8%) |
| Gonadotroph/lactotroph | 2 (2.7%) |
| Corticotroph | 8 (10.8%) |
| Lactotroph | 4 (5.4%) |
| Somatotroph | 1 (1.4%) |
| Thyrotroph | 1 (1.4%) |
| Null cell adenoma | 3 (4.1%) |
| Multiple synchronous PitNET | 4 (5.4%) |
| Immature PIT-lineage tumor | 6 (8.1%) |
| Mature PIT-1-lineage tumor | 3 (4.1%) |
| PD-L1 (TPS) | |
| 0%/≥1% | 31 (41.9%)/35 (47.3%) |
| Missing | 8 (10.8%) |
| Proliferative factors | |
| P53 < 10%/P53 ≥ 10% | 34 (45.9%)/33 (44.6%) |
| Missing | 7 (9.5%) |
| Ki-67 < 3%/Ki-67 ≥ 3% | 65 (87.8%)/9 (12.2%) |
| Cyclin D1 < 10%/Cyclin D1 ≥ 10% | 41 (55.4%)/29 (39.2%) |
| Missing | 4 (5.4%) |
| Knosp scale | |
| Invasive/Non-invasive | 38 (51.4%)/34 (45.9%) |
| Missing | 2 (2.7%) |
| Hardy scale | |
| Invasive/Non-invasive | 57 (77.0%)/15 (20.3%) |
| Missing | 2 (2.7%) |
| Overall invasiveness | |
| Yes/No | 58 (78.4%)/14 (18.9%) |
| Missing | 2 (2.7%) |
| PD-L1 | Ki-67 | P53 | Cyclin D1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feature | <1% (n = 31) N (%)/ Median (Q1–Q3) | ≥1% (n = 35) N (%)/ Median (Q1–Q3) | p-Value | <3% (n = 65) N (%)/ Median (Q1–Q3) | ≥3% (n = 9) N (%)/ Median (Q1–Q3) | p-Value | <10% (n = 34) N (%)/ Median (Q1–Q3) | ≥10% (n = 33) N (%)/ Median (Q1–Q3) | p-Value | <10% (n = 41) N (%)/ Median (Q1–Q3) | ≥10% (n = 29) N (%)/ Median (Q1–Q3) | p-Value |
| Age (years) | 59.0 (43.0–70.0) | 60.0 (52.0–68.0) | 0.59 ᵠ | 62.0 (50.0–69.0) | 41.0 (36.0–57.0) | 0.03 ᵠ | 62.0 (53.0–70.0) | 61.0 (45.0–67.0) | 0.57 ᵠ | 57.0 (49.0–68.0) | 62.0 (47.0–68.0) | 0.38 ᵠ |
| Gender (F/M) | 12 (46.2%)/ 19 (47.5%) | 14 (53.8%)/ 21 (52.5%) | 0.91 ᵡ | 28 (96.5%)/ 37 (82.2%) | 1 (3.5%)/ 8 (17.8%) | 0.08 ᵟ | 13 (48.1%)/ 21 (52.5%) | 14 (51.8%)/19 (47.5%) | 0.73 ᵡ | 17 (60.7%)/ 24 (57.1%) | 11 (39.3%)/ 18 (42.9%) | 0.77 ᵡ |
| Tumor volume (cm3) | 4.6 (2.1–10.0) | 6.2 (3.2–10.0) | 0.55 ᵠ | 4.8 (3.1–10.2) | 7.3 (2.8–11.0) | 0.96 ᵠ | 5.2 (2.8–10.0) | 7.3 (3.8–11.5) | 0.41 ᵠ | 4.3 (2.8–9.0) | 8.1 (3.1–12.3) | 0.31 ᵠ |
| Max tumor size (mm) | 23.0 (22.5–34.0) | 30.0 (22.0–34.0) | 0.59 ᵠ | 25.0 (22.0–33.0) | 29.0 (20.0–36.0) | 0.94 ᵠ | 28.5 (22.5–34.0) | 25.5 (20.8–33.0) | 0.59 ᵠ | 25.0 (21.0–34.0) | 26.8 (22.3–33.0) | 0.86 ᵠ |
| PIT-1 (No/Yes) | 25 (42.4%)/ 6 (85.7%) | 34 (57.6%)/ 1 (14.3%) | <0.05 ᵟ | 57 (87.7%)/ 8 (88.9%) | 8 (12.3%)/ 1 (11.1%) | 1.00 ᵟ | 30 (50.0%)/ 4 (57.1%) | 30 (50.0%)/ 3 (42.9%) | 1.00 ᵟ | 35 (57.4%)/ 6 (66.7%) | 26 (42.6%)/ 3 (33.3%) | 0.73 ᵟ |
| SF1 (No/Yes) | 16 (53.3%)/15 (41.7%) | 14 (46.7%)/21 (58.3%) | 0.34 ᵡ | 26 (83.9%)/ 39 (90.7%) | 5 (16.1%)/ 4 (9.3%) | 0.48 ᵟ | 15 (57.7%)/ 19 (46.3%) | 11 (42.3%)/22 (53.7%) | 0.36 ᵡ | 21 (67.7%)/ 20 (50.0%) | 9 (29.0%)/ 20 (50.0%) | 0.09 ᵡ |
| TPIT (No/Yes) | 28 (48.3%)/3 (37.5%) | 30 (51.7%)/ 5 (62.5%) | 0.71 ᵟ | 59 (89.4%)/ 6 (75.0%) | 7 (10.6%)/ 2 (25.0%) | 0.25 ᵟ | 30 (50.8%)/ 4 (50.0%) | 29 (49.2%)/ 4 (50.0%) | 1.00 ᵟ | 35 (56.5%)/ 6 (75.0%) | 27 (43.6%)/ 2 (25.0%) | 0.54 ᵡ |
| Null cell adenoma (No/Yes) | 30 (49.2%)/ 1 (20.0%) | 31 (50.8%)/ 4 (80.0%) | 0.24 ᵟ | 62 (87.4%)/ 3 (100.0%) | 9 (12.6%)/ 0 (0.0%) | 1.00 ᵟ | 32 (49.2%)/ 2 (100.0%) | 33 (50.8%)/ 0 (0.0%) | 0.49 ᵟ | 39 (57.4%)/ 2 (100.0%) | 29 (42.6%)/ 0 (0.0%) | 0.51 ᵟ |
| Multiple PitNETs (No/Yes) | 25 (44.6%)/ 6 (60.0%) | 31 (55.4%)/ 4 (40.0%) | 0.72 ᵟ | 56 (86.2%)/ 9 (81.8%) | 7 (13.9%)/ 2 (18.2%) | 0.62 ᵟ | 29 (50.0%)/ 5 (55.6%) | 29 (50.0%)/ 4 (44.4%) | 0.24 ᵟ | 34 (57.6%)/ 7 (63.6%) | 25 (42.4%)/ 4 (36.4%) | 1.00 ᵟ |
| Type of PitNET | - | - | - | - | ||||||||
| Gonadotroph | 17 (44.7%) | 21 (55.3%) | 39 (92.8%) | 3 (7.1%) | 19 (47.5%) | 21 (52.5%) | 20 (51.3%) | 19 (48.7%) | ||||
| Gonadotroph/Lactotroph | 0 (0.0%) | 2 (100.0%) | 2 (100.0%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 2 (100.0%) | 0 (0.0%) | ||||
| Corticotroph | 3 (37.5%) | 5 (62.5%) | 6 (75.0%) | 2 (25.0%) | 4 (50.0%) | 4 (50.0%) | 6 (75.0%) | 2 (25.0%) | ||||
| Lactotroph | 3 (100.0%) | 0 (0.0%) | 3 (75.0%) | 1 (25.0%) | 2 (50.0%) | 2 (50.0%) | 3 (75.0%) | 1 (25.0%) | ||||
| Somatroph | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (100.0%) | ||||
| Thyrotroph | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | ||||
| Null cell adenoma | 0 (0.0%) | 3 (100.0%) | 3 (100.0%) | 0 (0.0%) | 2 (100.0%) | 0 (0.0%) | 2 (100.0%) | 0 (0.0%) | ||||
| Multiple synchronous PitNET | 1 (33.3%) | 2 (66.7%) | 3 (75.0%) | 1 (25.0%) | 2 (50.0%) | 2 (50.0%) | 2 (50.0%) | 2 (50.0%) | ||||
| Immature PIT-lineage tumor | 4 (80.0%) | 1 (20.0%) | 5 (83.3%) | 1 (16.6%) | 2 (40.0%) | 3 (60.0%) | 4 (66.7%) | 2 (33.3%) | ||||
| Mature PIT-lineage tumor | 1 (50.0%) | 1 (50.0%) | 2 (66.6%) | 1 (33.3%) | 1 (50.0%) | 1 (50.0%) | 1 (33.3%) | 2 (66.7%) | ||||
| Hormonal activity Non-active Active | 23 (43.4%) 8 (61.5%) | 30 (56.6%) 5 (38.5%) | 0.24 ᵡ | 53 (89.8%) 12 (80.0%) | 6 (10.2%) 3 (20.0%) | 0.38 ᵟ | 25 (46.3%) 9 (69.2%) | 29 (53.7%) 4 (30.8%) | 0.14 ᵡ | 30 (54.5%) 11 (73.2%) | 25 (45.5%) 4 (26.6%) | 0.19 ᵡ |
| Knosp scale Invasive Non-invasive | 18 (51.4%) 16 (53.3%) | 15 (45.5%) 18 (58.1%) | 0.31 ᵡ | 34 (89.5%) 29 (85.3%) | 4 (10.5%) 5 (14.7%) | 0.73 ᵟ | 18 (51.4%) 16 (53.3%) | 17 (48.6%) 14 (46.7%) | 0.88 ᵡ | 22 (59.46%) 18 (58.1%) | 15 (40.5%) 13 (41.9%) | 0.91 ᵡ |
| Hardy scale Invasive Non-invasive | 23 (45.1%) 8 (61.5%) | 28 (54.9%) 5 (38.5%) | 0.29 ᵡ | 51 (89.5%) 12 (80.0%) | 6 (10.5%) 3 (20.0%) | 0.38 ᵟ | 28 (53.8%) 6 (46.2%) | 24 (46.2%) 7 (53.8%) | 0.62 ᵡ | 31 (58.5%) 9 (60.0%) | 22 (41.5%) 6 (40.0%) | 0.92 ᵡ |
| Invasiveness (Knosp or Hardy scale) Non-invasive Invasive | 6 (50.0%) 28 (52.8%) | 4 (33.3%) 29 (55.8%) | 0.16 ᵡ | 11 (78.6%) 52 (89.7%) | 3 (21.4%) 6 (10.3%) | 0.36 ᵟ | 6 (50.0%) 28 (52.8%) | 6 (50.0%) 25 (47.2%) | 0.86 ᵡ | 9 (64.3%) 31 (57.45) | 5 (35.7%) 23 (42.65) | 0.64 ᵡ |
| Parameter | PD-L1 TPS 0% n (%) | PD-L1 TPS ≥ 1% n (%) | p-Value |
|---|---|---|---|
| Ki-67 expression ᵟ | 0.71 | ||
| <3% | 28 (48.3%) | 30 (51.7%) | |
| ≥3% | 3 (37.5%) | 5 (62.5%) | |
| Missing | – | – | |
| P53 expression ᵡ | 0.10 | ||
| <10% | 19 (57.6%) | 14 (42.4%) | |
| ≥10% | 11 (36.7%) | 19 (63.3%) | |
| Missing | 1 (33.3%) | 2 (66.7%) | |
| Cyclin D1 expression ᵡ | 0.27 | ||
| <10% | 14 (41.2%) | 20 (58.8%) | |
| ≥10% | 16 (55.2%) | 13 (44.8%) | |
| Missing | 1 (33.3%) | 2 (66.7%) |
| Outcome | Optimal TPS Cut-Off | Sensitivity | Specificity | AUC |
|---|---|---|---|---|
| Transcription Factors | ||||
| PIT-1 | 0.00 | 0.86 | 0.58 | 0.68 |
| SF1 | ≥1.3% | 0.56 | 0.52 | 0.57 |
| TPIT | ≥5% | 0.63 | 0.59 | 0.50 |
| Null Cell Adenoma | ≥5% | 1.00 | 0.59 | 0.75 |
| Multiple PitNETs | ≤3% | 0.78 | 0.49 | 0.59 |
| Type of PitNET | ||||
| Gonadotroph | ≥1.3% | 0.55 | 0.50 | 0.54 |
| Corticotroph | ≥5% | 0.63 | 0.59 | 0.50 |
| Lactotroph | 0.00 | 1.00 | 0.56 | 0.78 |
| Immature PIT-1-lineage tumor | 0.00 | 0.80 | 0.56 | 0.67 |
| Mature PIT-1-lineage tumor | ≥85% | 0.50 | 0.98 | 0.61 |
| Type of PitNET | Ki ≥ 3 & P53 ≥ 10 (N = 6) | Ki ≥ 3 & P53 < 10 (N = 2) | Ki < 3 & P53 ≥ 10 (N = 27) | Ki < 3 & P53 < 10 (N = 32) |
|---|---|---|---|---|
| Gonadotroph | 7.7 | 0 | 46.2 | 46.2 |
| Gonadotroph/Lactotroph | 0 | 0 | 0 | 100 |
| Corticotroph | 25 | 0 | 25 | 50 |
| Lactotroph | 0 | 25 | 50 | 25 |
| Somatotroph | 0 | 0 | 0 | 0 |
| Thyrotroph | 0 | 0 | 0 | 100 |
| Null cell adenoma | 0 | 0 | 0 | 100 |
| Multiple synchronous PitNETs | 25 | 0 | 25 | 50 |
| Immature PIT-1-lineage tumor | 0 | 25 | 75 | 0 |
| Mature PIT-1-lineage tumor | 0 | 0 | 50 | 50 |
| Types of PitNET | Cyclin ≥ 10 & P53 ≥ 10 (N = 18) | Cyclin ≥ 10 & P53 < 10 (N = 10) | Cyclin < 10 & P53 ≥ 10 (N = 14) | Cyclin < 10 & P53 < 10 (N = 22) |
|---|---|---|---|---|
| Gonadotroph | 26.3 | 23.7 | 26.3 | 23.7 |
| Gonadotroph/Lactotroph | 0 | 0 | 0 | 100 |
| Corticotroph | 25 | 0 | 25 | 50 |
| Lactotroph | 25 | 0 | 25 | 50 |
| Somatroph | 0 | 0 | 0 | 0 |
| Thyrotroph | 0 | 0 | 0 | 100 |
| Null cell adenoma | 0 | 0 | 0 | 100 |
| Multiple synchronous PitNETs | 50 | 0 | 0 | 50 |
| Immature PIT-lineage tumor | 40 | 0 | 20 | 40 |
| Mature PIT-1-lineage tumor | 50 | 50 | 0 | 0 |
| Features | Knosp Scale | Hardy Scale | ||
|---|---|---|---|---|
| Non-Invasive n (%) | Invasive n (%) | Non-Invasive n (%) | Invasive n (%) | |
| Combined analysis of Ki-67 and P53 | ||||
| Ki-67 ≥ 3% & P53 ≥ 10% | 3 (50.0%) | 3 (50.0%) | 1 (16.7%) | 5 (83.3%) |
| Ki-67 ≥ 3% & P53 < 10% | 1 (50.0%) | 1 (50.0%) | 1 (50.0%) | 1 (50.05) |
| Ki-67 < 3% & P53 ≥ 10% | 11 (44.0%) | 14 (56.0%) | 6 (24.0%) | 19 (76.0%) |
| Ki-67 < 3% & P53 < 10% | 15 (46.9%) | 17 (53.1%) | 5 (15.6%) | 27 (84.4%) |
| p value ᵡ | 0.992 | 0.615 | ||
| Combined analysis of cyclin D1 and P53 | ||||
| Cyclin D1 ≥ 10% & P53 ≥ 10% | 8 (47.1%) | 9 (52.9%) | 5 (29.4%) | 12 (70.6%) |
| Cyclin D1 ≥ 10% & P53 < 10% | 4 (40.0%) | 6 (60.0%) | 0 (0.0%) | 10 (100.0%) |
| Cyclin D1 < 10% & P53 ≥ 10% | 5 (38.5%) | 8 (61.5%) | 2 (15.4%) | 11 (84.6%) |
| Cyclin D1 < 10% & P53 < 10% | 11 (50.0%) | 11 (50.0%) | 6 (27.3%) | 16 (72.7%) |
| p value ᵡ | 0.902 | 0.245 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzentowska, A.; Biesaga, B.; Czepko, R.; Merklinger-Gruchała, A.; Adamek, D.; Jasińska, M.; Pluta, B.; Michalska, W.; Wróblewska, K.; Janczy, F.; et al. The Roles of PD-L1, Ki-67, P53, and Cyclin D1 in PitNETs: Diagnostic and Prognostic Implications in a Series of 74 Patients. Int. J. Mol. Sci. 2025, 26, 7830. https://doi.org/10.3390/ijms26167830
Krzentowska A, Biesaga B, Czepko R, Merklinger-Gruchała A, Adamek D, Jasińska M, Pluta B, Michalska W, Wróblewska K, Janczy F, et al. The Roles of PD-L1, Ki-67, P53, and Cyclin D1 in PitNETs: Diagnostic and Prognostic Implications in a Series of 74 Patients. International Journal of Molecular Sciences. 2025; 26(16):7830. https://doi.org/10.3390/ijms26167830
Chicago/Turabian StyleKrzentowska, Anna, Beata Biesaga, Ryszard Czepko, Anna Merklinger-Gruchała, Dariusz Adamek, Małgorzata Jasińska, Barbara Pluta, Wiktoria Michalska, Katarzyna Wróblewska, Filip Janczy, and et al. 2025. "The Roles of PD-L1, Ki-67, P53, and Cyclin D1 in PitNETs: Diagnostic and Prognostic Implications in a Series of 74 Patients" International Journal of Molecular Sciences 26, no. 16: 7830. https://doi.org/10.3390/ijms26167830
APA StyleKrzentowska, A., Biesaga, B., Czepko, R., Merklinger-Gruchała, A., Adamek, D., Jasińska, M., Pluta, B., Michalska, W., Wróblewska, K., Janczy, F., & Gołkowski, F. (2025). The Roles of PD-L1, Ki-67, P53, and Cyclin D1 in PitNETs: Diagnostic and Prognostic Implications in a Series of 74 Patients. International Journal of Molecular Sciences, 26(16), 7830. https://doi.org/10.3390/ijms26167830







