TRP Channels in Skin Cancer: Focus on Malignant Melanoma
Abstract
1. Introduction
2. Characteristics of TRP Channels
3. Physiological Role and Expression of TRP Channels in Melanocytes
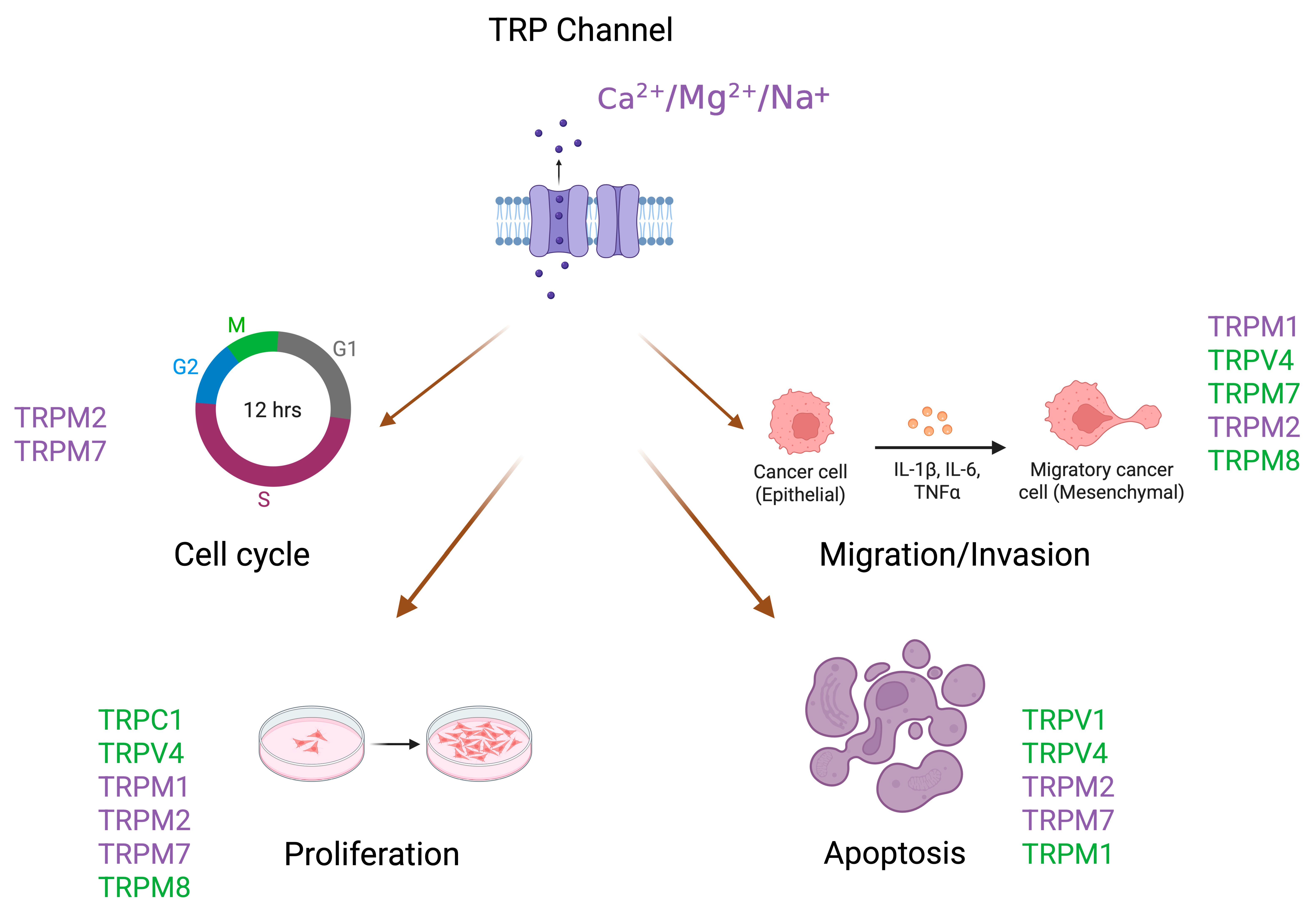
4. TRP Channels and Melanoma Development and Progression
5. TRP Channels as Potential Therapeutic Targets
6. Current Limitations and Future Directions in TRP Channel-Targeted Melanoma Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TRP | Transient Receptor Potential |
| TRPM | Transient Receptor Potential Melastatin |
| TRPV | Transient Receptor Potential Vanilloid |
| TRPC | Transient Receptor Potential Canonical |
| TRPA | Transient Receptor Potential Ankyrin |
| TRPP | Transient Receptor Potential Polycystin |
| TRPML | Transient Receptor Potential Mucolipin |
| ROS | Reactive Oxygen Species |
| EMT | Epithelial–Mesenchymal Transition |
| MITF | Microphthalmia-Associated Transcription Factor |
| miR-211 | microRNA-211 |
| KCNMA1 | Potassium Calcium-Activated Channel Subfamily M Alpha 1 |
References
- Heistein, J.B.; Acharya, U.; Mukkamalla, S.K.R. Malignant Melanoma; StatPearls: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK470409/ (accessed on 1 July 2025).
- Naik, P.P. Cutaneous Malignant Melanoma: A Review of Early Diagnosis and Management. World J. Oncol. 2021, 12, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Qadir, M.I.; Ghafoor, S. Malignant Melanoma: Skin Cancer-Diagnosis, Prevention, and Treatment. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, Y.; Liu, Q.; Zhang, H.; Jia, L.; Chai, Y.; Jiang, H.; Wu, M.; Li, Y. Global trends in melanoma burden: A comprehensive analysis from the Global Burden of Disease Study, 1990–2021. J. Am. Acad. Dermatol. 2025, 92, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pethő, Z.; Najder, K.; Bulk, E.; Schwab, A. Mechanosensitive ion channels push cancer progression. Cell Calcium 2019, 8, 79–90. [Google Scholar] [CrossRef]
- Marini, M.; Titiz, M.; Souza Monteiro De Araújo, D.; Geppetti, P.; Nassini, R.; De Logu, F. TRP Channels in Cancer: Signaling Mechanisms and Translational Approaches. Biomolecules 2023, 13, 1557. [Google Scholar] [CrossRef]
- Ho, J.-C.; Lee, C.-H. TRP channels in skin: From physiological implications to clinical significances. Biomolecules 2015, 11, 17–24. [Google Scholar] [CrossRef]
- Dhennin-Duthille, I.; Gautier, M.; Faouzi, M.; Guilbert, A.; Brevet, M.; Vaudry, D.; Ahidouch, A.; Sevestre, H.; Ouadid-Ahidouch, H. High Expression of Transient Receptor Potential Channels in Human Breast Cancer Epithelial Cells and Tissues: Correlation with Pathological Parameters. Cell Physiol. Biochem. 2011, 28, 813–822. [Google Scholar] [CrossRef]
- Santoni, G.; Farfariello, V. TRP Channels and Cancer: New Targets for Diagnosis and Chemotherapy. Endocr. Metab. Immune Disord.—Drug Targets 2011, 11, 54–67. [Google Scholar] [CrossRef]
- Bai, S.; Wei, Y.; Liu, R.; Chen, Y.; Ma, W.; Wang, M.; Chen, L.; Luo, Y.; Du, J. The role of transient receptor potential channels in metastasis. Biomed. Pharmacother. 2023, 158, 114074. [Google Scholar] [CrossRef]
- Chen, J.; Luan, Y.; Yu, R.; Zhang, Z.; Zhang, J.; Wang, W. Transient receptor potential (TRP) channels, promising potential diagnostic and therapeutic tools for cancer. Biosci. Trends 2014, 8, 1–10. [Google Scholar] [CrossRef]
- Bödding, M. TRP proteins and cancer. Cell. Signal. 2007, 19, 617–624. [Google Scholar] [CrossRef]
- Shapovalov, G.; Ritaine, A.; Skryma, R.; Prevarskaya, N. Role of TRP ion channels in cancer and tumorigenesis. Semin. Immunopathol. 2016, 38, 357–369. [Google Scholar] [CrossRef]
- Vrenken, K.S.; Jalink, K.; Van Leeuwen, F.N.; Middelbeek, J. Beyond ion-conduction: Channel-dependent and -independent roles of TRP channels during development and tissue homeostasis. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1436–1446. [Google Scholar] [CrossRef]
- Jia, Q.; Tian, W.; Li, B.; Chen, W.; Zhang, W.; Xie, Y.; Cheng, N.; Chen, Q.; Xiao, J.; Zhang, Y.; et al. Transient Receptor Potential channels, TRPV1 and TRPA1 in melanocytes synergize UV-dependent and UV-independent melanogenesis. Br. J. Pharmacol. 2021, 178, 4646–4662. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.F.; Bayet, E.; Leverrier-Penna, S.; Le Devedec, D.; Mallavialle, A.; Marionneau-Lambot, S.; Rambow, F.; Perret, R.; Joussaume, A.; Viel, R.; et al. The mechanosensitive TRPV2 calcium channel promotes human melanoma invasiveness and metastatic potential. EMBO Rep. 2023, 24, e55069. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Gu, M.; Hu, M.; Pinchi, P.; Chen, W.; Ryan, M.; Nold, T.; Bannaga, A.; Xu, H. Lysosomal Zn2+ release triggers rapid, mitochondria-mediated, non-apoptotic cell death in metastatic melanoma. Cell Rep. 2021, 37, 109848. [Google Scholar] [CrossRef]
- Huang, J.; Korsunsky, A.; Yazdani, M.; Chen, J. Targeting TRP channels: Recent advances in structure, ligand binding, and molecular mechanisms. Front. Mol. Neurosci. 2024, 16, 1334370. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP channels in cancer. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2007, 1772, 937–946. [Google Scholar] [CrossRef]
- Wong, K.K.; Banham, A.H.; Yaacob, N.S.; Nur Husna, S.M. The oncogenic roles of TRPM ion channels in cancer. J. Cell. Physiol. 2019, 234, 14556–14573. [Google Scholar] [CrossRef]
- Huang, Y.; Anderle, P.; Bussey, K.J.; Barbacioru, C.; Shankavaram, U.; Dai, Z.; Reinhold, W.C.; Papp, A.; Weinstein, J.N.; Sade, W. Membrane transporters and channels: Role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res. 2004, 64, 4294–4301. [Google Scholar] [CrossRef]
- Yang, P.; Feng, J.; Luo, J.; Madison, M.; Hu, H. A Critical Role for TRP Channels in the Skin. In Neurobiology of TRP Channels, 1st ed.; Emir, T.L.R., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 95–111. [Google Scholar]
- Holzer, P.; Izzo, A.A. The pharmacology of TRP channels. Br. J. Pharmacol. 2014, 171, 2469–2473. [Google Scholar] [CrossRef]
- Guo, H.; Carlson, J.A.; Slominski, A. Role of TRPM in melanocytes and melanoma. Exp. Dermatol. 2012, 21, 650–654. [Google Scholar] [CrossRef]
- Hu, Q.; Yi, W.; Su, M.; Jiang, S.; Xu, S.; Lei, T. Induction of retinal-dependent calcium influx in human melanocytes by UVA or UVB radiation contributes to the stimulation of melanosome transfer. Cell Prolif. 2017, 50, e12372. [Google Scholar] [CrossRef]
- Devi, S.; Markandeya, Y.; Maddodi, N.; Dhingra, A.; Vardi, N.; Balijepalli, R.C.; Setaluri, V. Metabotropic glutamate receptor 6 signaling enhances TRPM1 calcium channel function and increases melanin content in human melanocytes. Pigment. Cell Melanoma Res. 2013, 26, 348–356. [Google Scholar] [CrossRef]
- Oancea, E.; Vriens, J.; Brauchi, S.; Jun, J.; Splawski, I.; Clapham, D.E. TRPM1 Forms Ion Channels Associated with Melanin Content in Melanocytes. Sci. Signal. 2009, 2, ra21. [Google Scholar] [CrossRef] [PubMed]
- Oancea, E.; Wicks, N.L. TRPM1: New Trends for an Old, T.R.P. Adv. Exp. Med. Biol. 2011, 704, 135–145. [Google Scholar]
- Yee, N. Role of TRPM7 in Cancer: Potential as Molecular Biomarker and Therapeutic Target. Pharmaceuticals 2017, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.S.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 Provides an Ion Channel Mechanism for Cellular Entry of Trace Metal Ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Yogi, A.; Callera, G.E.; Antunes, T.T.; Tostes, R.C.; Touyz, R.M. Transient Receptor Potential Melastatin 7 (TRPM7) Cation Channels, Magnesium and the Vascular System in Hypertension. Circ. J. 2011, 75, 237–245. [Google Scholar] [CrossRef]
- Hayes, P.; Meadows, H.J.; Gunthorpe, M.J.; Harries, M.H.; Duckworth, M.D.; Cairns, W.; Harrison, D.C.; Clarke, C.E.; Ellington, K.; Prinjha, R.K.; et al. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain 2000, 88, 205–215. [Google Scholar] [CrossRef]
- Yamamura, H.; Ugawa, S.; Ueda, T.; Morita, A.; Shimada, S. TRPM8 activation suppresses cellular viability in human melanoma. Am. J. Physiol.-Cell Physiol. 2008, 295, C296–C301. [Google Scholar] [CrossRef]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.-L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Julius, D. TRP Channels and Pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Nam, D.Y.; Kim, H.J.; Hong, P.T.L.; Kim, W.K.; Nam, J.H. Nootkatol prevents ultraviolet radiation-induced photoaging via ORAI1 and TRPV1 inhibition in melanocytes and keratinocytes. Korean J. Physiol. Pharmacol. 2021, 25, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Park, S.-Y.; Jo, J.Y.; Kang, G.; Park, J.B.; Kim, J.-G.; Hong, S.-G.; Kim, C.-D.; Lee, J.-H.; Yoon, T.-J. Endogenous expression of TRPV1 channel in cultured human melanocytes. J. Dermatol. Sci. 2009, 56, 128–130. [Google Scholar] [CrossRef]
- Boudaka, A.; Al-Yazeedi, M.; Al-Lawati, I. Role of Transient Receptor Potential Vanilloid 4 Channel in Skin Physiology and Pathology. Sultan Qaboos Univ. Med. J. 2020, 20, e138–e146. [Google Scholar] [CrossRef]
- Fusi, C.; Materazzi, S.; Minocci, D.; Maio, V.; Oranges, T.; Massi, D.; Nassini, R. Transient Receptor Potential Vanilloid 4 (TRPV4) Is Downregulated in Keratinocytes in Human Non-Melanoma Skin Cancer. J. Investig. Dermatol. 2014, 134, 2408–2417. [Google Scholar] [CrossRef]
- Moore, C. The role of TRPV4 channels in cutaneous epithelia. Curr. Top. Membr. 2022, 89, 139–154. [Google Scholar]
- Olivan-Viguera, A.; Garcia-Otin, A.L.; Lozano-Gerona, J.; Abarca-Lachen, E.; Garcia-Malinis, A.J.; Hamilton, K.L.; Gilaberte, Y.; Pueyo, E.; Köhler, R. Pharmacological activation of TRPV4 produces immediate cell damage and induction of apoptosis in human melanoma cells and HaCaT keratinocytes. PLoS ONE 2018, 13, e0190307. [Google Scholar] [CrossRef]
- Oda, K.; Umemura, M.; Nakakaji, R.; Tanaka, R.; Sato, I.; Nagasako, A.; Oyamada, C.; Baljinnyam, E.; Katsumata, M.; Xie, L.-H.; et al. Transient receptor potential cation 3 channel regulates melanoma proliferation and migration. J. Physiol. Sci. 2017, 67, 497–505. [Google Scholar] [CrossRef]
- Margue, C.; Philippidou, D.; Reinsbach, S.E.; Schmitt, M.; Behrmann, I.; Kreis, S. New Target Genes of MITF-Induced microRNA-211 Contribute to Melanoma Cell Invasion. PLoS ONE 2013, 8, e73473. [Google Scholar] [CrossRef]
- Boyle, G.M.; Woods, S.L.; Bonazzi, V.F.; Stark, M.S.; Hacker, E.; Aoude, L.G.; Dutton-Regester, K.; Cook, A.L.; Sturm, R.A.; Hayward, N.K. Melanoma cell invasiveness is regulated by miR-211 suppression of the BRN2 transcription factor. Pigment Cell Melanoma Res. 2011, 24, 525–537. [Google Scholar] [CrossRef]
- Mazar, J.; DeYoung, K.; Khaitan, D.; Meister, E.; Almodovar, A.; Goydos, J.; Ray, A.; Perera, R.J. The Regulation of miRNA-211 Expression and Its Role in Melanoma Cell Invasiveness. PLoS ONE 2010, 5, e13779. [Google Scholar] [CrossRef]
- Hantute-Ghesquier, A.; Haustrate, A.; Prevarskaya, N.; Lehen’kyi, V. TRPM Family Channels in Cancer. Pharmaceuticals 2018, 11, 58. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Rodboon, N.; Samart, P.; Vinayanuwattikun, C.; Klamkhlai, S.; Chanvorachote, P.; Rojanasakul, Y.; Issaragrisil, S. A novel TRPM7/O-GlcNAc axis mediates tumour cell motility and metastasis by stabilising c-Myc and caveolin-1 in lung carcinoma. Br. J. Cancer 2020, 123, 1289–1301. [Google Scholar] [CrossRef]
- Egawa, M.; Schmücker, E.; Grimm, C.; Gudermann, T.; Chubanov, V. Expression Profiling Identified TRPM7 and HER2 as Potential Targets for the Combined Treatment of Cancer Cells. Cells 2024, 13, 1801. [Google Scholar] [CrossRef]
- Xing, Y.; Wei, X.; Wang, M.; Liu, Y.; Sui, Z.; Wang, X.; Zhang, Y.; Fei, Y.; Jiang, Y.; Lu, C.; et al. Stimulating TRPM7 suppresses cancer cell proliferation and metastasis by inhibiting autophagy. Cancer Lett. 2022, 52, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Kijpornyongpan, T.; Sereemaspun, A.; Chanchao, C. Dose-Dependent Cytotoxic Effects of Menthol on Human Malignant Melanoma A-375 Cells: Correlation with TRPM8 Transcript Expression. Asian Pac. J. Cancer Prev. 2014, 15, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, H.; Wei, Z.; Wang, X.; Shen, P.; Wang, S.; Chen, W.; Lu, Y. TRPM8: A potential target for cancer treatment. J. Cancer Res. Clin. Oncol. 2016, 142, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Hemida, A.S.; Hammam, M.A.; Heriz, N.A.E.M.; Shehata, W.A. Expression of Transient Receptor Potential Channel of Melastatin number 8 (TRPM8) in Non- Melanoma Skin Cancer: A Clinical and Immunohistochemical study. J. Immunoass. Immunochem. 2021, 42, 620–632. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, W.; Ma, J.; Xu, P.; Zhang, W.; Guo, S.; Liu, L.; Ma, J.; Shi, Q.; Jian, Z.; et al. Downregulated TRPV1 Expression Contributes to Melanoma Growth via the Calcineurin-ATF3-p53 Pathway. J. Investig. Dermatol. 2018, 138, 2205–2215. [Google Scholar] [CrossRef]
- Li, M.; Zheng, J.; Wu, T.; He, Y.; Guo, J.; Xu, J.; Gao, C.; Qu, S.; Zhang, Q.; Zhao, J.; et al. Activation of TRPV4 Induces Exocytosis and Ferroptosis in Human Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 4146. [Google Scholar] [CrossRef]
- Elzamzamy, O.M.; Penner, R.; Hazlehurst, L.A. The Role of TRPC1 in Modulating Cancer Progression. Cells 2020, 9, 388. [Google Scholar] [CrossRef]
- Foster, H.M.; Carle, M.N.; Jira, L.R.; Koh, D.W. TRPM2 Channels: A Potential Therapeutic Target in Melanoma? Int. J. Mol. Sci. 2023, 24, 10437. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.A. TRPM2 in Cancer. Cell Calcium 2019, 8, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Syed Mortadza, S.A.; Wang, L.; Li, D.; Jiang, L.-H. TRPM2 Channel-Mediated ROS-Sensitive Ca2+ Signaling Mechanisms in Immune Cells. Front. Immunol. 2015, 6, 407. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shimizu, S. Targeting TRPM2 in ROS-Coupled Diseases. Pharmaceuticals 2016, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Maliougina, M.; El Hiani, Y. TRPM2: Bridging calcium and ROS signaling pathways—Implications for human diseases. Front. Physiol. 2023, 14, 1217828. [Google Scholar] [CrossRef]
- Hopmann, L.; Heider, J.; Niebel, D.; Evert, K.; Zeman, F.; Hammers, C.M.; Ettl, T.; Brochhausen, C.; Schreml, S. pH-Sensitive TRPC5 Is Differentially Expressed in Various Common Skin Tumors. Biology 2025, 14, 823. [Google Scholar] [CrossRef]
- Kurz, B.; Michael, H.P.; Förch, A.; Wallner, S.; Zeman, F.; Decking, S.-M.; Ugele, I.; Hintschich, C.; Haubner, F.; Ettl, T.; et al. Expression of pH-Sensitive TRPC4 in Common Skin Tumors. Int. J. Mol. Sci. 2023, 24, 1037. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Zhang, A.; Gupte, A.A.; Hamilton, D.J. The Role of Calcium Signaling in Melanoma. Int. J. Mol. Sci. 2022, 23, 1010. [Google Scholar] [CrossRef]
- Lei, J.; Tominaga, M. Unlocking the therapeutic potential of TRPV3: Insights into thermosensation, channel modulation, and skin homeostasis involving TRPV3. BioEssays 2024, 46, 2400047. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, H.; Li, Z.; Wang, T.; Yu, D.; Jin, H.; Cao, Y. TRPV3 promotes the angiogenesis through HIF-1α-VEGF signaling pathway in A549 cells. Acta Histochem. 2022, 124, 151955. [Google Scholar] [CrossRef] [PubMed]
- cBioPortal for Cancer Genomics: TRPM1, TRPM2 and 4 other genes in Skin Cutaneous Melanoma (TCGA, PanCancer Atlas). Available online: https://www.cbioportal.org/results/download?cancer_study_list=skcm_tcga_pan_can_atlas_2018&tab_index=tab_visualize&case_set_id=skcm_tcga_pan_can_atlas_2018_all&Action=Submit&gene_list=TRPM1%2520TRPM2%2520TRPM8%2520TRPM7%2520TRPV1%2520TRPV4&Z_SCORE_THRESHOLD=2.0&RPPA_SCORE_THRESHOLD=2.0&profileFilter=mutations%2Cstructural_variants%2Cgistic&geneset_list=%20&plots_horz_selection=%7B%22selectedGeneOption%22%3A7226%2C%22dataType%22%3A%22COPY_NUMBER_ALTERATION%22%2C%22selectedDataSourceOption%22%3A%22gistic%22%7D&plots_vert_selection=%7B%22selectedGeneOption%22%3A-20000%2C%22dataType%22%3A%22MRNA_EXPRESSION%22%2C%22selectedDataSourceOption%22%3A%22rna_seq_v2_mrna%22%2C%22logScale%22%3A%22true%22%7D&plots_coloring_selection=%7B%7D&comparison_selectedGroups=%5B%22Unaltered%20group%22%2C%22Altered%20group%22%5D (accessed on 4 August 2025).
- Liu, Q.; Hu, M.; Li, S.; Zhang, X.; Zhang, R.; Lyu, H.; Xiao, S.; Guo, D.; Chen, X.-Z.; Tang, J.; et al. TRPM channels in human cancers: Regulatory mechanism and therapeutic prospects. Biomark Res. 2024, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Zhang, W.; Guo, H.; Pan, X.; Chen, X.; He, Q.; Yang, B.; Ding, L. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm. Sin. B 2022, 12, 1761–1780. [Google Scholar] [CrossRef]
- Köles, L.; Ribiczey, P.; Szebeni, A.; Kádár, K.; Zelles, T.; Zsembery, Á. The Role of TRPM7 in Oncogenesis. Int. J. Mol. Sci. 2024, 25, 719. [Google Scholar] [CrossRef]
- Zierler, S.; Yao, G.; Zhang, Z.; Kuo, W.C.; Pörzgen, P.; Penner, R.; Horgen, F.D.; Fleig, A. Waixenicin A Inhibits Cell Proliferation through Magnesium-dependent Block of Transient Receptor Potential Melastatin 7 (TRPM7) Channels. J. Biol. Chem. 2011, 286, 39328–39335. [Google Scholar] [CrossRef]
- Erin, N.; Szallasi, A. Carcinogenesis and Metastasis: Focus on TRPV1-Positive Neurons and Immune Cells. Biomolecules 2023, 13, 983. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Chiang, C.; Xiao, T.; Chen, Y.; Zhao, Y.; Zheng, D. The Impact of TRPV1 on Cancer Pathogenesis and Therapy: A Systematic Review. Int. J. Biol. Sci. 2021, 17, 2034–2049. [Google Scholar] [CrossRef]
- Liu, Y.; Mikrani, R.; He, Y.; Faran Ashraf Baig, M.M.; Abbas, M.; Naveed, M.; Tang, M.; Zhang, Q.; Li, C.; Zhou, X. TRPM8 channels: A review of distribution and clinical role. Eur. J. Pharmacol. 2020, 88, 173312. [Google Scholar] [CrossRef]
- Cai, X.; Yu, X.; Yang, J.; Lu, L.; Hua, N.; Duan, X.; Ye, P.; Ni, L.; Jiang, L.; Yang, W.; et al. TRPM2 regulates cell cycle through the Ca2+-CaM-CaMKII signaling pathway to promote HCC. Hepatol. Commun. 2023, 7, e0101. [Google Scholar] [CrossRef]
- Ciaglia, T.; Vestuto, V.; Bertamino, A.; González-Muñiz, R.; Gómez-Monterrey, I. On the modulation of TRPM channels: Current perspectives and anticancer therapeutic implications. Front. Oncol. 2023, 1, 1065935. [Google Scholar] [CrossRef]
| Gene Symbol | Percent Samples Altered (%) |
|---|---|
| TRPM2 | 14 |
| TRPM8 | 9 |
| TRPM1 | 8 |
| TRPM7 | 8 |
| TRPV1 | 5 |
| TRPV4 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Twardak, D.; Havryliuk, V.; Gagat, M. TRP Channels in Skin Cancer: Focus on Malignant Melanoma. Int. J. Mol. Sci. 2025, 26, 7829. https://doi.org/10.3390/ijms26167829
Twardak D, Havryliuk V, Gagat M. TRP Channels in Skin Cancer: Focus on Malignant Melanoma. International Journal of Molecular Sciences. 2025; 26(16):7829. https://doi.org/10.3390/ijms26167829
Chicago/Turabian StyleTwardak, Damian, Vita Havryliuk, and Maciej Gagat. 2025. "TRP Channels in Skin Cancer: Focus on Malignant Melanoma" International Journal of Molecular Sciences 26, no. 16: 7829. https://doi.org/10.3390/ijms26167829
APA StyleTwardak, D., Havryliuk, V., & Gagat, M. (2025). TRP Channels in Skin Cancer: Focus on Malignant Melanoma. International Journal of Molecular Sciences, 26(16), 7829. https://doi.org/10.3390/ijms26167829





