The Role of Receptor Tyrosine Kinase-like Orphan Receptor 1 (ROR1) in Cancer Stem Cell Signaling
Abstract
1. Introduction
2. ROR1 Structure and Function in Development and Cancer
3. Cancer Stem Cells
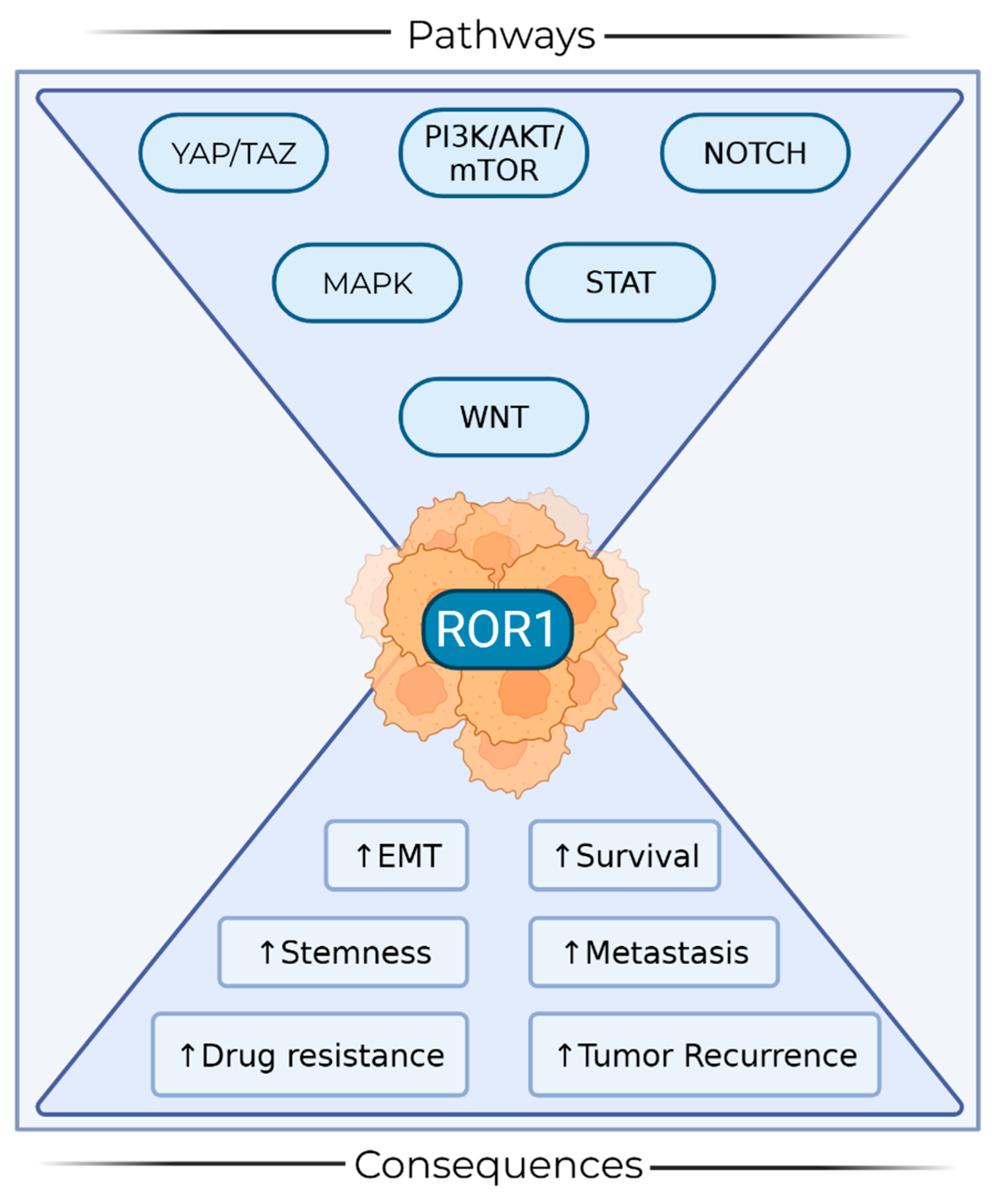
4. ROR1 in Cancer Stem Cells
4.1. Gynecological Cancers
4.2. Breast Cancer
4.3. Glioblastoma
4.4. Pancreatic Ductal Adenocarcinoma
4.5. Osteosarcoma
4.6. Hematologic Malignancies
5. ROR1 in Targeted Therapeutics and Clinical Trials
6. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3′UTR | 3′ untranslated region |
| ADCs | Antibody–drug conjugate |
| ALDH1 | Aldehyde dehydrogenase 1 |
| AURKB | Aurora kinase B |
| B-ALL | B-cell acute lymphoblastic leukemia |
| BC | Breast cancer |
| CLL | Chronic lymphocytic leukemia |
| CRD | Cysteine-rich domain |
| CSC | Cancer stem cell |
| DLBCL | Diffuse large B-cell lymphoma |
| EC | Endometrial cancer |
| EMT | Epithelial to mesenchymal transition |
| FZD | Frizzled |
| GSC | Glioblastoma stem cells |
| HIF1α | Hypoxia-inducible factor 1 alpha |
| Ig | Immunoglobulin |
| IGFBP5 | Insulin-like growth factor-binding protein 5 |
| KD | Kringle domain |
| LBCL | Large B-cell lymphoma |
| LC | Lung cancer |
| mAb | Monoclonal antibody |
| MCL | Mantle cell lymphoma |
| MSC | Mesenchymal stem cells |
| NICD | Notch intracellular domain |
| NSCLC | Non-small cell lung cancer |
| OC | Ovarian cancer |
| PC | Prostate cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDX | Patient-derived xenograft |
| PRD | Proline-rich domain |
| ROR1 | Receptor tyrosine kinase-like orphan receptor 1 |
| RTK | Receptor tyrosine kinase |
| T-DM1 | Trastuzumab |
| TKD | Tyrosine kinase domain |
| TNBC | Triple-negative breast cancer |
References
- Ebben, J.D.; Treisman, D.M.; Zorniak, M.; Kutty, R.G.; Clark, P.A.; Kuo, J.S. The cancer stem cell paradigm: A new understanding of tumor development and treatment. Expert Opin. Ther. Targets 2010, 14, 621–632. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.; Park, J.; Park, S.; Yoo, G.; Yum, S.; Kang, W.; Lee, J.-M.; Youn, H.; Youn, B. Cancer stem cells: Landscape, challenges and emerging therapeutic innovations. Signal Transduct. Target. Ther. 2025, 10, 248. [Google Scholar] [CrossRef]
- Bisht, S.; Nigam, M.; Kunjwal, S.S.; Sergey, P.; Mishra, A.P.; Sharifi-Rad, J. Cancer Stem Cells: From an Insight into the Basics to Recent Advances and Therapeutic Targeting. Stem Cells Int. 2022, 2022, 9653244. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.; Pirzada, R.H.; Ul Ain, Q.; Choi, S. Wnt signaling in the regulation of immune cell and cancer therapeutics. Cells 2019, 8, 1380. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Katoh, M. Molecular genetics and targeted therapy of WNT-related human diseases (Review). Int. J. Mol. Med. 2017, 40, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Widhopf, G.F.; Ghia, E.M.; Kidwell, R.L.; Hasan, M.K.; Yu, J.; Rassenti, L.Z.; Chen, L.; Chen, Y.; Pittman, E.; et al. Phase I Trial: Cirmtuzumab Inhibits ROR1 Signaling and Stemness Signatures in Patients with Chronic Lymphocytic Leukemia. Cell Stem Cell 2018, 22, 951–959.e3. [Google Scholar] [CrossRef]
- Borcherding, N.; Kusner, D.; Liu, G.-H.; Zhang, W. ROR1, an embryonic protein with an emerging role in cancer biology. Protein Cell 2014, 5, 496–502. [Google Scholar] [CrossRef]
- Menck, K.; Heinrichs, S.; Baden, C.; Bleckmann, A. The WNT/ROR pathway in cancer: From signaling to therapeutic intervention. Cells 2021, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Jiang, V.C.; Liu, Y.; Jordan, A.; McIntosh, J.; Li, Y.; Che, Y.; Jessen, K.A.; Lannutti, B.J.; Wang, M. The antibody drug conjugate VLS-101 targeting ROR1 is effective in CAR T-resistant mantle cell lymphoma. J. Hematol. Oncol. 2021, 14, 132. [Google Scholar] [CrossRef]
- Caras, I.W. Two cancer stem cell-targeted therapies in clinical trials as viewed from the standpoint of the cancer stem cell model. Stem Cells Transl. Med. 2020, 9, 821–826. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, L.; Liu, D.; Ma, X.; Chen, Z.; Fan, H.; Li, R.; Zhang, Y.; Mi, H.; Li, J.; et al. ROR1 facilitates glioblastoma growth via stabilizing GRB2 to promote c-Fos expression in glioma stem cells. Neuro-Oncol. 2025, 27, 695–710. [Google Scholar] [CrossRef]
- Illendula, A.; Fultang, N.; Peethambaran, B. Retinoic acid induces differentiation in neuroblastoma via ROR1 by modulating retinoic acid response elements. Oncol. Rep. 2020, 44, 1013–1024. [Google Scholar] [CrossRef]
- Perkins, R.S.; Murray, G.; Suthon, S.; Davis, L.; Perkins, N.B.; Fletcher, L.; Bozzi, A.; Schreiber, S.L.; Lin, J.; Laxton, S.; et al. WNT5B drives osteosarcoma stemness, chemoresistance and metastasis. Clin. Transl. Med. 2024, 14, e1670. [Google Scholar] [CrossRef]
- Liu, M.; Shi, Y.; Hu, Q.; Qin, Y.; Ji, S.; Liu, W.; Zhuo, Q.; Fan, G.; Ye, Z.; Song, C.; et al. SETD8 induces stemness and epithelial-mesenchymal transition of pancreatic cancer cells by regulating ROR1 expression. Acta Biochim. Biophys. Sin. 2021, 53, 1614–1624. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishita, M.; Hoshi, K.; Honda, T.; Kakeji, Y.; Minami, Y. Mesenchymal stem cell-derived CXCL16 promotes progression of gastric cancer cells by STAT3-mediated expression of Ror1. Cancer Sci. 2020, 111, 1254–1265. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chu, T.Y.; Ding, D.C. Human fallopian tube epithelial cells exhibit stemness features, self-renewal capacity, and Wnt-related organoid formation. J. Biomed. Sci. 2020, 27, 32. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, J.; Ye, C.; Yang, D.; Gao, L.; Su, Y.; Tang, X.; Xu, N.; Zhang, D.; Xiong, L.; et al. ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Sci. Rep. 2014, 4, 5811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, H.; Ghia, E.M.; Huang, J.; Wu, L.; Zhang, J.; Lam, S.; Lei, Y.; He, J.; Cui, B.; et al. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc. Natl. Acad. Sci. USA 2019, 116, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Quezada, M.J.; Lopez-Bergami, P. The signaling pathways activated by ROR1 in cancer. Cell Signal 2023, 104, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, D.; Guo, Y.; Lu, B.; Zhao, Z.J.; Xu, X.; Chen, Y. Tyrosine Kinase ROR1 as a Target for Anti-Cancer Therapies. Front. Oncol. 2021, 11, 680834. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, H.; Barker, H.; Kaleva, L.; Niininen, W.; Ungureanu, D. Molecular mechanisms associated with ROR1-mediated drug resistance: Crosstalk with Hippo-YAP/TAZ and BMI-1 pathways. Cells 2019, 8, 812. [Google Scholar] [CrossRef]
- Katoh, M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int. J. Oncol. 2017, 51, 1357–1369. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, H.; Luo, W.; Zhang, Q.; Liu, J.; Yao, K. 5T4-specific chimeric antigen receptor modification promotes the immune efficacy of cytokine-induced killer cells against nasopharyngeal carcinoma stem cell-like cells. Sci. Rep. 2017, 7, 4859. [Google Scholar] [CrossRef] [PubMed]
- Bengoa-Vergniory, N.; Gorroño-Etxebarria, I.; López-Sánchez, I.; Marra, M.; Di Chiaro, P.; Kypta, R. Identification of non-canonical Wnt receptors required for Wnt-3a-induced early differentiation of human neural stem cells. Mol. Neurobiol. 2017, 54, 6213–6224. [Google Scholar] [CrossRef]
- Lin, W.; Niu, R.; Park, S.M.; Zou, Y.; Kim, S.S.; Xia, X.; Xing, S.; Yang, Q.; Sun, X.; Yuan, Z.; et al. IGFBP5 is an ROR1 ligand promoting glioblastoma invasion via ROR1/HER2-CREB signaling axis. Nat. Commun. 2023, 14, 1578. [Google Scholar] [CrossRef]
- Karvonen, H.; Arjama, M.; Kaleva, L.; Niininen, W.; Barker, H.; Koivisto-Korander, R.; Tapper, J.; Pakarinen, P.; Lassus, H.; Loukovaara, M.; et al. Glucocorticoids induce differentiation and chemoresistance in ovarian cancer by promoting ROR1-mediated stemness. Cell Death Dis. 2020, 11, 790. [Google Scholar] [CrossRef]
- Yamazaki, M.; Hino, S.; Usuki, S.; Miyazaki, Y.; Oda, T.; Nakao, M.; Ito, T.; Yamagata, K. YAP/BRD4-controlled ROR1 promotes tumor-initiating cells and hyperproliferation in pancreatic cancer. EMBO J. 2023, 42, e112614. [Google Scholar] [CrossRef]
- Endo, M.; Kamizaki, K.; Minami, Y. The Ror-Family Receptors in Development, Tissue Regeneration and Age-Related Disease. Front. Cell Dev. Biol. 2022, 10, 891763. [Google Scholar] [CrossRef]
- Mao, Y.; Xu, L.; Wang, J.; Zhang, L.; Hou, N.; Xu, J.; Wang, L.; Yang, S.; Chen, Y.; Xiong, L.; et al. ROR1 associates unfavorable prognosis and promotes lymphoma growth in DLBCL by affecting PI3K/Akt/mTOR signaling pathway. BioFactors 2019, 45, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Heabah, N.A.E.-G.; Darwish, S.A.; Eid, A.M. Evaluation of the prognostic significance of receptor tyrosine kinase-like orphan receptor 1 (ROR1) in lung carcinoma and its relation to lymphangiogenesis and epithelial mesenchymal transition. Pathol. Res. Pract. 2023, 248, 154703. [Google Scholar] [CrossRef] [PubMed]
- Yikilmaz, A.Ş.; Bakanay, Ş.M.; Nurdan Avcı, D.; Akinci, S.; Falay, M.; Özet, G.; Dilek, İ. Prognostic Value of the Expression of Receptor Tyrosine Kinase-Like Orphan Receptor 1 (ROR-1) in Chronic Lymphocytic Leukemia. Int. J. Hematol. Oncol. Stem Cell Res. 2023, 17, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-K.; Zheng, Y.-Z.; Liu, X.-S.; Gou, Q.; Ma, R.; Guo, C.-L.; Croce, C.M.; Liu, L.; Peng, Y. ROR1 expression as a biomarker for predicting prognosis in patients with colorectal cancer. Oncotarget 2017, 8, 32864–32872. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-P.; Ueng, S.-H.; Chen, S.-C.; Chang, Y.-S.; Lin, Y.-C.; Lo, Y.-F.; Chang, H.-K.; Chuang, W.-Y.; Huang, Y.-T.; Cheung, Y.-C.; et al. Expression of ROR1 has prognostic significance in triple negative breast cancer. Virchows Arch. 2016, 468, 589–595. [Google Scholar] [CrossRef]
- Ishikawa, T.; Ogura, Y.; Tanaka, K.; Nagashima, H.; Sasayama, T.; Endo, M.; Minami, Y. Ror1 is expressed inducibly by Notch and hypoxia signaling and regulates stem cell-like property of glioblastoma cells. Cancer Sci. 2023, 114, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pestell, T.G.; Lisanti, M.P.; Pestell, R.G. Cancer stem cells. Int. J. Biochem. Cell Biol. 2012, 44, 2144–2151. [Google Scholar] [CrossRef]
- Kreso, A.; Dick, J.E. Evolution of the cancer stem cell model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef]
- Tu, S.-M.; Guo, C.C.; Chow, D.S.-L.; Zacharias, N.M. Stem cell theory of cancer: Implications for drug resistance and chemosensitivity in cancer care. Cancers 2022, 14, 1548. [Google Scholar] [CrossRef]
- Sugihara, E.; Saya, H. Complexity of cancer stem cells. Int. J. Cancer 2013, 132, 1249–1259. [Google Scholar] [CrossRef]
- Lee, J.; Kotliarova, S.; Kotliarov, Y.; Li, A.; Su, Q.; Donin, N.M.; Pastorino, S.; Purow, B.W.; Christopher, N.; Zhang, W.; et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006, 9, 391–403. [Google Scholar] [CrossRef]
- Ignatova, T.N.; Kukekov, V.G.; Laywell, E.D.; Suslov, O.N.; Vrionis, F.D.; Steindler, D.A. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 2002, 39, 193–206. [Google Scholar] [CrossRef]
- Lu, B.; Morrison, R.; Schleicher, S.M.; Sun, Y.; Niermann, K.J.; Kim, S.; Spratt, D.E.; Chung, C.H. Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J. Oncol. 2011, 2011, 941876. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Kim, W.-T.; Ryu, C.J. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017, 50, 285–298. [Google Scholar] [CrossRef]
- Kipps, T.J. ROR1: An orphan becomes apparent. Blood 2022, 140, 1583–1591. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, N.H.; Kim, H.S.; Kim, Y.; Lee, J.J.; Kim, J.H.; Cho, H.Y.; Jeong, S.Y.; Park, S.T. The Role of ROR1 in Chemoresistance and EMT in Endometrial Cancer Cells. Medicina 2023, 59, 994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cui, B.; Lai, H.; Liu, G.; Ghia, E.M.; Widhopf, G.F.; Zhang, Z.; Wu, C.C.N.; Chen, L.; Wu, R.; et al. Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy. Proc. Natl. Acad. Sci. USA 2014, 111, 17266–17271. [Google Scholar] [CrossRef]
- Cao, J.; Wang, X.; Dai, T.; Wu, Y.; Zhang, M.; Cao, R.; Zhang, R.; Wang, G.; Jiang, R.; Zhou, B.P.; et al. Twist promotes tumor metastasis in basal-like breast cancer by transcriptionally upregulating ROR1. Theranostics 2018, 8, 2739–2751. [Google Scholar] [CrossRef] [PubMed]
- Shatsky, R.A.; Batra-Sharma, H.; Helsten, T.; Schwab, R.B.; Pittman, E.I.; Pu, M.; Weihe, E.; Ghia, E.M.; Rassenti, L.Z.; Molinolo, A.; et al. A phase 1b study of zilovertamab in combination with paclitaxel for locally advanced/unresectable or metastatic Her2-negative breast cancer. Breast Cancer Res. 2024, 26, 32. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.S.; Al-Tweigeri, T.; Al-Harbi, L.; Ujjahan, S.; Al-Mozaini, M.; Tulbah, A.; Aboussekhra, A. Long noncoding RNA DLEU2 and ROR1 pathway induces epithelial-to-mesenchymal transition and cancer stem cells in breast cancer. Cell Death Discov. 2024, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.S.; Uddin, M.; Noman, A.S.M.; Akter, H.; Dity, N.J.; Basiruzzman, M.; Uddin, F.; Ahsan, J.; Annoor, S.; Alaiya, A.A.; et al. Antibody-drug conjugate T-DM1 treatment for HER2+ breast cancer induces ROR1 and confers resistance through activation of Hippo transcriptional coactivator YAP1. EBioMedicine 2019, 43, 211–224. [Google Scholar] [CrossRef]
- Jung, E.H.; Lee, H.N.; Han, G.Y.; Kim, M.J.; Kim, C.W. Targeting ROR1 inhibits the self-renewal and invasive ability of glioblastoma stem cells. Cell Biochem. Funct. 2016, 34, 149–157. [Google Scholar] [CrossRef]
- Dai, B.; Shen, Y.; Yan, T.; Zhang, A. Wnt5a/ROR1 activates DAAM1 and promotes the migration in osteosarcoma cells. Oncol. Rep. 2020, 43, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Ghia, E.M.; Rassenti, L.Z.; Choi, M.Y.; Quijada-Álamo, M.; Chu, E.; Widhopf, G.F.; Kipps, T.J. High expression level of ROR1 and ROR1-signaling associates with venetoclax resistance in chronic lymphocytic leukemia. Leukemia 2022, 36, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.Y.; Widhopf, G.F.; Wu, C.C.N.; Cui, B.; Lao, F.; Sadarangani, A.; Cavagnaro, J.; Prussak, C.; Carson, D.A.; Jamieson, C.; et al. Pre-clinical Specificity and Safety of UC-961, a First-In-Class Monoclonal Antibody Targeting ROR1. Clin. Lymphoma Myeloma Leuk. 2015, 15, S167–S169. [Google Scholar] [CrossRef] [PubMed]
- Widhopf, G.F.; Cui, B.; Ghia, E.M.; Chen, L.; Messer, K.; Shen, Z.; Briggs, S.P.; Croce, C.M.; Kipps, T.J. ROR1 can interact with TCL1 and enhance leukemogenesis in E|i-TCL1 transgenic mice. Proc. Natl. Acad. Sci. USA 2014, 111, 793–798. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Gutierrez, M.; Sanz-Garcia, E.; Villa, D.; Zhang, J.; Friedmann, J.; Yan, F.; Socinski, M.A.; Sarantopoulos, J.; Raez, L.E.; et al. Phase 2 Study of Zilovertamab Vedotin in Participants With Metastatic Solid Tumors. Cancer Res. Commun. 2025. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pu, W.; He, H.; Fan, X.; Zheng, Y.; Zhou, J.-K.; Ma, R.; He, J.; Zheng, Y.; Wu, K.; et al. Novel ROR1 inhibitor ARI-1 suppresses the development of non-small cell lung cancer. Cancer Lett. 2019, 458, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Daneshmanesh, A.H.; Hojjat-Farsangi, M.; Ghaderi, A.; Moshfegh, A.; Hansson, L.; Schultz, J.; Vågberg, J.; Byström, S.; Olsson, E.; Olin, T.; et al. A receptor tyrosine kinase ROR1 inhibitor (KAN0439834) induced significant apoptosis of pancreatic cells which was enhanced by erlotinib and ibrutinib. PLoS ONE 2018, 13, e0198038. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, M.; Siddiqi, T.; Barrientos, J.; Wierda, W.G.; Isufi, I.; Tuscano, J.; Lamanna, N.; Subbiah, S.; Koff, J.L.; et al. Phase 1b/2 Study of Cirmtuzumab and Ibrutinib in Mantle Cell Lymphoma (MCL) or Chronic Lymphocytic Leukemia (CLL). Blood 2021, 138, 3534. [Google Scholar] [CrossRef]
- Yam, A.; Hanson, J.; Bajjuri, K.; Zhou, S.; Moreira, D.; Smith, J.; Abrahams, C.; Li, X.; Lee, G.; Armstrong, S.; et al. 1191 STRO-003 Is a Novel ROR1-Targeted ADC for Breast and Lung Cancer. Regular and Young Investigator Award Abstracts; BMJ Publishing Group Ltd.: London, UK, 2022; p. A1235. [Google Scholar] [CrossRef]
- Wang, M.; Barrientos, J.C.; Furman, R.R.; Mei, M.; Barr, P.M.; Choi, M.Y.; de Vos, S.; Kallam, A.; Patel, K.; Rule, S.; et al. VLS-101, a ROR1-Targeting Antibody-Drug Conjugate, Demonstrates a Predictable Safety Profile and Clinical Efficacy in Patients with Heavily Pretreated Mantle Cell Lymphoma and Diffuse Large B-Cell Lymphoma. Blood 2020, 136, 13–14. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Han, G.; Xie, J.; Bai, X. A ROR1 targeted bispecific T cell engager shows high potency in the pre-clinical model of triple negative breast cancer. Breast Cancer Res. 2025, 27, 47. [Google Scholar] [CrossRef]
- Wang, M.; Johnson, P.C.; Yazji, S.; Katz, Y.; Pietrofeso, A.; Robinson, J.; Mei, M.; Frigault, M.J.; Breitmeyer, J.B.; Jacobson, C.A. A Phase 1/2 Study of a ROR1-Targeting CAR T Cell Therapy (ONCT-808) in Adult Patients with Relapsed/Refractory (R/R) Aggressive B Cell Lymphomas (BCL). Blood 2024, 144, 1743. [Google Scholar] [CrossRef]
- Chan, T.; Scott, S.P.; Du, M.; Bolinger, C.; Poortman, C.; Shepard, L.; Koenitzer, B.; Govekung, A.; Sailor, C.; Johnson, R.; et al. Preclinical Evaluation of Prgn-3007, a Non-Viral, Multigenic, Autologous ROR1 Ultracar-T® Therapy with Novel Mechanism of Intrinsic PD-1 Blockade for Treatment of Hematological and Solid Cancers. Blood 2021, 138, 1694. [Google Scholar] [CrossRef]
- Hudecek, M.; Schmitt, T.M.; Baskar, S.; Lupo-Stanghellini, M.T.; Nishida, T.; Yamamoto, T.N.; Bleakley, M.; Turtle, C.J.; Chang, W.C.; Greisman, H.A.; et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood 2010, 116, 4532–4541. [Google Scholar] [CrossRef] [PubMed]
- Jaeger-Ruckstuhl, C.A.; Specht, J.M.; Voutsinas, J.M.; MacMillan, H.R.; Wu, Q.; Muhunthan, V.; Berger, C.; Pullarkat, S.; Wright, J.H.; Yeung, C.C.S.; et al. Phase I Study of ROR1-Specific CAR-T Cells in Advanced Hematopoietic and Epithelial Malignancies. Clin. Cancer Res. 2025, 31, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2016, 109, 314–341. [Google Scholar] [CrossRef]
- Noorbakhsh, N.; Hayatmoghadam, B.; Jamali, M.; Golmohammadi, M.; Kavianpour, M. The Hippo signaling pathway in leukemia: Function, interaction, and carcinogenesis. Cancer Cell Int. 2021, 21, 705. [Google Scholar] [CrossRef]
- Paul, S.; Xie, S.; Yao, X.; Dey, A. Transcriptional Regulation of the Hippo Pathway: Current Understanding and Insights from Single-Cell Technologies. Cells 2022, 11, 2225. [Google Scholar] [CrossRef]
- Rybak, A.P.; Bristow, R.G.; Kapoor, A. Prostate cancer stem cells: Deciphering the origins and pathways involved in prostate tumorigenesis and aggression. Oncotarget 2015, 6, 1900–1919. [Google Scholar] [CrossRef] [PubMed]
- Avanes, A.; Lenz, G.; Momand, J. Darpp-32 and t-Darpp protein products of PPP1R1B: Old dogs with new tricks. Biochem. Pharmacol. 2019, 160, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer stem cells: Advances in knowledge and implications for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 170. [Google Scholar] [CrossRef]
- Ma, F.; Arai, S.; Wang, K.; Calagua, C.; Yuan, A.R.; Poluben, L.; Gu, Z.; Russo, J.W.; Einstein, D.J.; Ye, H.; et al. Autocrine Canonical Wnt Signaling Primes Noncanonical Signaling through ROR1 in Metastatic Castration-Resistant Prostate Cancer. Cancer Res. 2022, 82, 1518–1533. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Hoque, M.O. Targeting Cancer Stem Cells: A Strategy for Effective Eradication of Cancer. Cancers 2019, 11, 732. [Google Scholar] [CrossRef]

| Therapeutic Class | Specific Therapeutic | Cancers in Trial | Clinical Status | References |
|---|---|---|---|---|
| mAb | Zilovertamab (Cirmtuzumab or UC-961) | B-ALL, DLBCL, neuroblastoma and Ewing sarcoma. | Phase 1; Recruiting | ClinicalTrials.gov ID NCT06395103 |
| Zilovertamab (Cirmtuzumab or UC-961) + Paclitaxel | HER2- BC | Phase 1b; Completed | [49] | |
| Antibody–Drug Conjugate (ADC) | Zilovertamab (Cirmtuzumab or UC-961) | CLL | Preclinical, Phase 1, 2 (phase 1b-2 = cirmtuzumab + ibrutinib); Terminated (strategic directions) | [6,55,60] |
| STRO-003 | BC and LC | Preclinical | [61] | |
| VLS-101 | TNBC, BC HER2- BC, NSCLC, GC, PDAC, and OC | Phase 2; Terminated (business reasons) | ClinicalTrials.gov ID NCT04504916; [57] | |
| VLS-101 | MCL | Preclinical, Phase 1; Completed | [9,62] | |
| Bispecific Antibody | Anti-ROR1 × CD3 BiAb | TNBC | Preclinical | [63] |
| CAR T-Cell Therapy | LYL-797 | TNBC, NSCLC, OC, and EC | Phase 1; Active | ClinicalTrials.gov, NCT05274451 |
| ONCT-808 | MCL, LBCL | Phase 1/2; Terminated (strategic directions) | [64] | |
| PRGN-3007 | ROR1+ Hematologic and Solid Tumors | Preclinical, Phase 1/1b; Active | ClinicalTrials.gov, NCT05694364 [65] | |
| ROR1-CAR T | ALL, CLL, MCL, NSCLC, Osteosarcoma, PC, TNBC | Preclinical, Phase 1; Terminated (slow patient accrual) | ClinicalTrials.gov, (NCT02706392) [66,67] | |
| Small Molecule Inhibitor | ARI-1 | NSCLC | Preclinical | [58] |
| KAN0439044 | PDAC | Preclinical | [59] | |
| Strictinin | Glioblastoma | Preclinical | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, M.S.; Choi, W.-Y.; Zhang, W.; Barrera, F.N.; Perkins, R.S. The Role of Receptor Tyrosine Kinase-like Orphan Receptor 1 (ROR1) in Cancer Stem Cell Signaling. Int. J. Mol. Sci. 2025, 26, 7828. https://doi.org/10.3390/ijms26167828
Jung MS, Choi W-Y, Zhang W, Barrera FN, Perkins RS. The Role of Receptor Tyrosine Kinase-like Orphan Receptor 1 (ROR1) in Cancer Stem Cell Signaling. International Journal of Molecular Sciences. 2025; 26(16):7828. https://doi.org/10.3390/ijms26167828
Chicago/Turabian StyleJung, Matthew S., Won-Young Choi, Wenjing Zhang, Francisco N. Barrera, and Rachel S. Perkins. 2025. "The Role of Receptor Tyrosine Kinase-like Orphan Receptor 1 (ROR1) in Cancer Stem Cell Signaling" International Journal of Molecular Sciences 26, no. 16: 7828. https://doi.org/10.3390/ijms26167828
APA StyleJung, M. S., Choi, W.-Y., Zhang, W., Barrera, F. N., & Perkins, R. S. (2025). The Role of Receptor Tyrosine Kinase-like Orphan Receptor 1 (ROR1) in Cancer Stem Cell Signaling. International Journal of Molecular Sciences, 26(16), 7828. https://doi.org/10.3390/ijms26167828






