Effects of a Novel Mammalian-Derived Collagen Matrix on Human Articular Cartilage-Derived Chondrocytes from Osteoarthritis Patients
Abstract
1. Introduction
2. Results
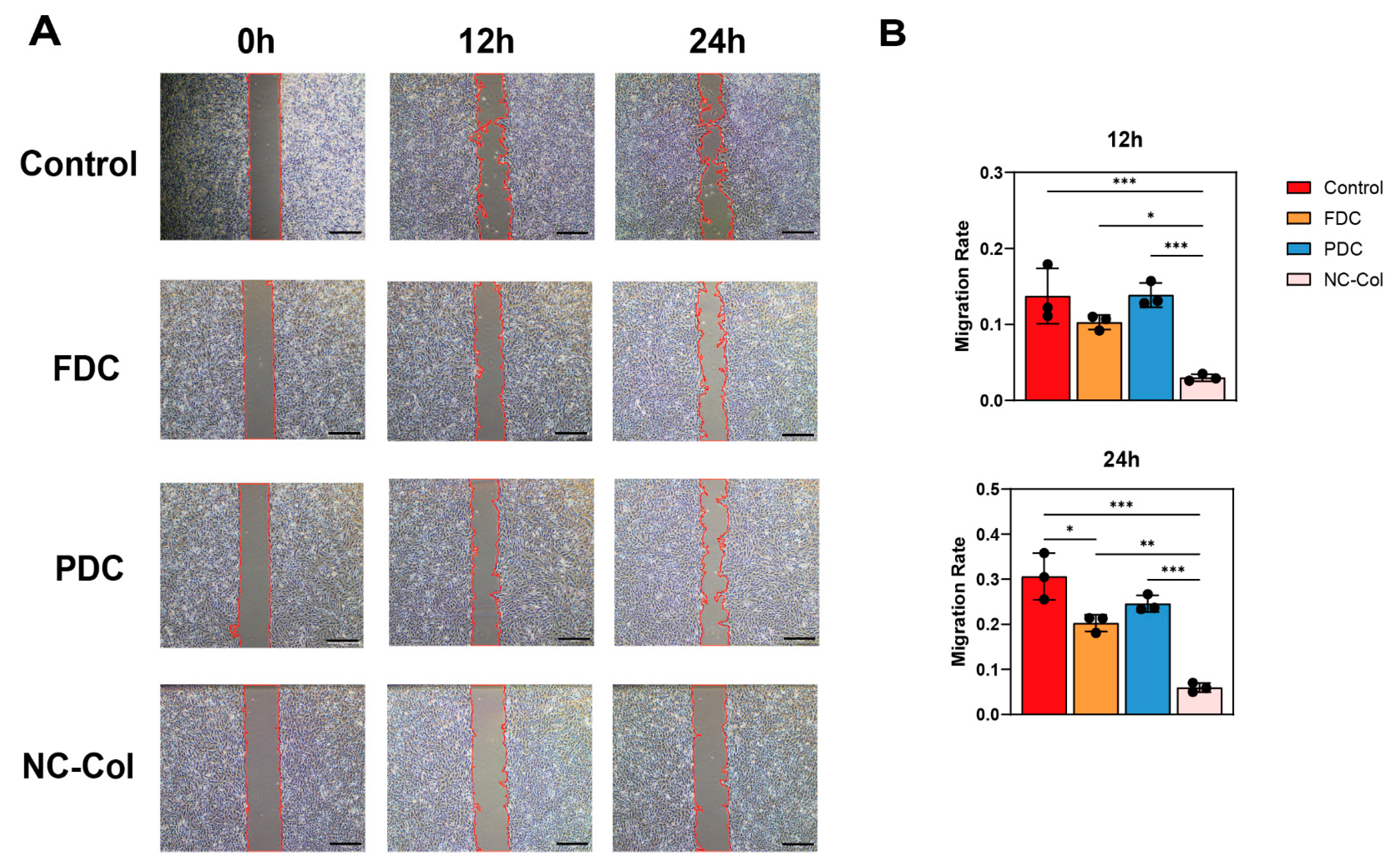
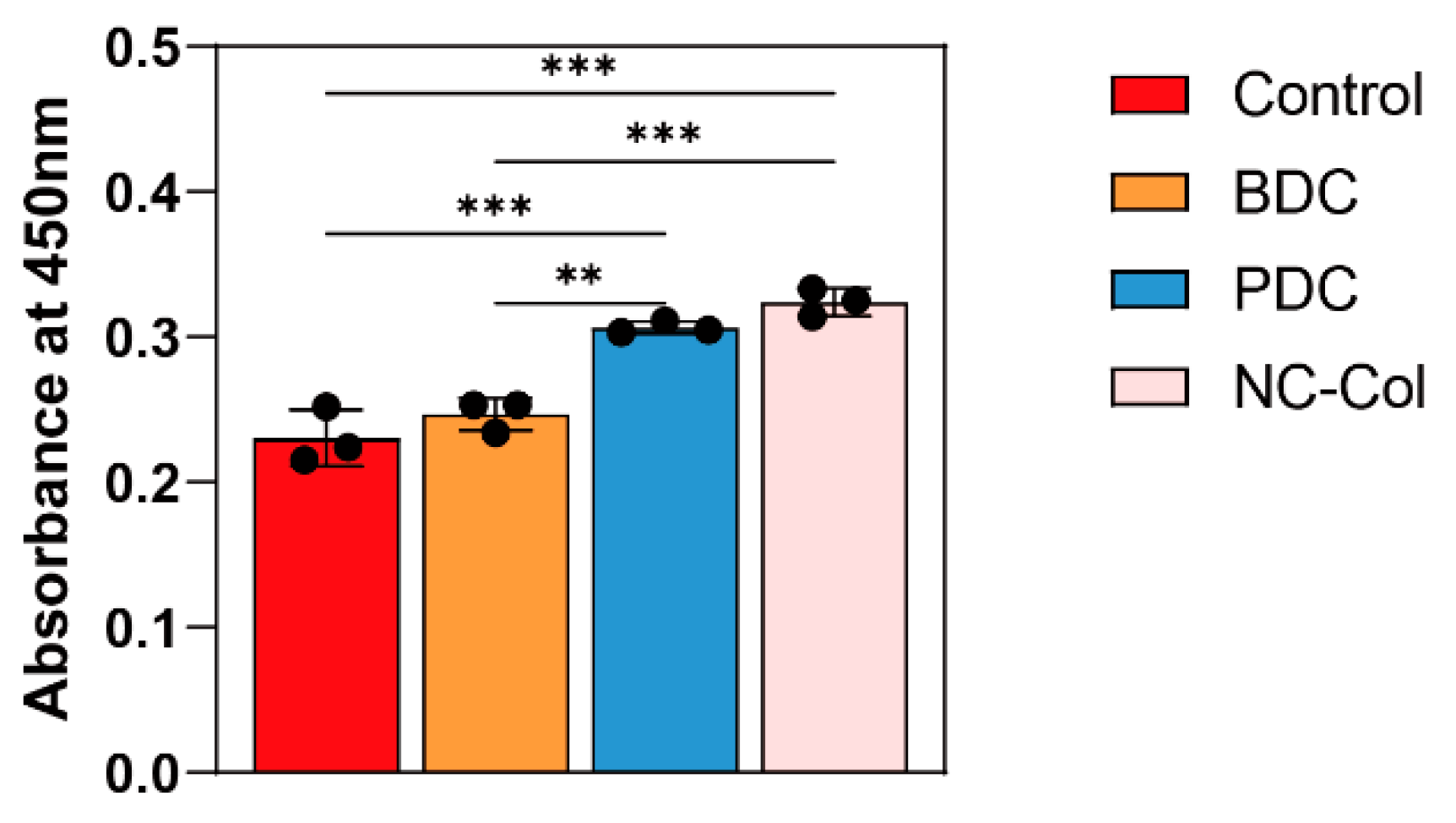
2.1. NC-Col Promoted the Proliferation, Inhibited the Migration, and Enhanced the Adhesion Ability of Human Articular Cartilage-Derived Chondrocytes
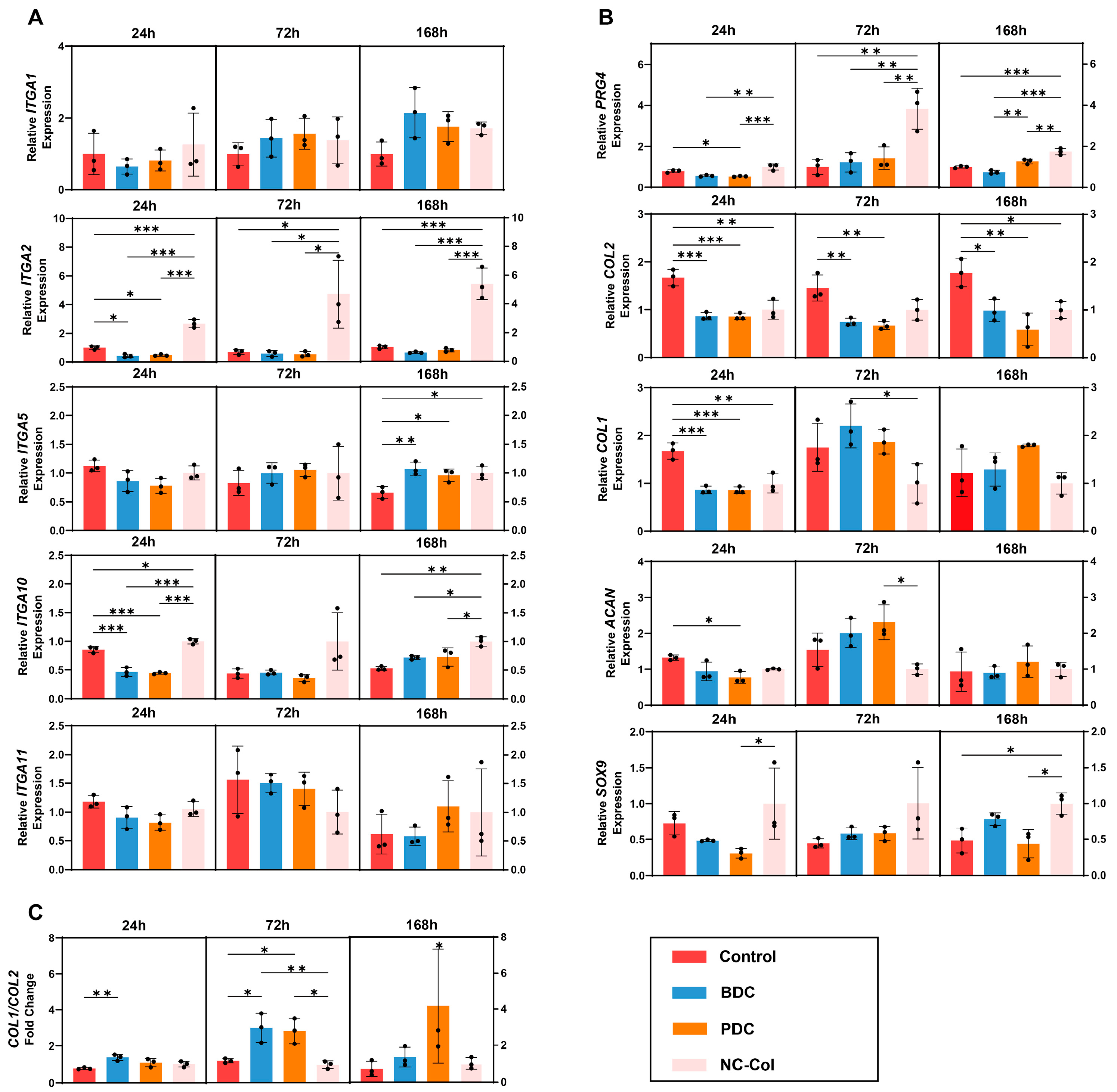
2.2. NC-Col Upregulated the Expression of ITGA2, ITGA10, and PRG4 in Chondrocytes
2.3. RNA Sequencing Revealed Significant Differences Between NC-Col and PDC Groups in the Gene Expression Patterns of Chondrocytes
2.4. NC-Col Regulated ITGA10 and PRG4 Expression and Inhibited Chondrocyte Migration via the PI3K/Akt Signalling Pathway
3. Discussion
4. Materials and Methods
4.1. Human Samples and Ethics Statement
4.2. Materials
4.3. Cell Isolation and Culture
4.4. Preparation of Collagen-Coated Plates and Dishes
4.5. Cell Proliferation Assay
4.6. Wound Healing Assay
4.7. Cell Adhesion Assay
4.8. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.9. Construction and Sequencing of RNA-Seq Libraries
4.10. WB
4.11. Quantification and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| ACI | Autologous chondrocyte implantation |
| MACI | Matrix-induced autologous chondrocyte implantation |
| PRG4 | Proteoglycan 4 |
| PDC | Porcine-derived collagen matrix |
| FDC | Fish-derived collagen matrix |
| BDC | Bovine-derived collagen matrix |
| ACAN | Aggrecan |
| SOX9 | SRY-Box transcription factor 9 |
| qRT-PCR | Quantitative real-time PCR |
| RNA-Seq | RNA sequencing |
| WB | Western blotting |
| ECM | Extracellular matrix |
| PBS | Phosphate-buffered saline |
| OD | Optical density |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| DEGs | Differentially expressed genes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Ecke, A.; Lutter, A.H.; Scholka, J.; Hansch, A.; Becker, R.; Anderer, U. Tissue Specific Differentiation of Human Chondrocytes Depends on Cell Microenvironment and Serum Selection. Cells 2019, 8, 934. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; King, L.K. The Burden of Osteoarthritis in Older Adults. Clin. Geriatr. Med. 2022, 38, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, C.; Oo, W.M.; Fu, K.; Risberg, M.A.; Bierma-Zeinstra, S.M.; Neogi, T.; Atukorala, I.; Malfait, A.M.; Ding, C.; et al. Osteoarthritis. Nat. Rev. Dis. Primers 2025, 11, 10. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Sun, K.; Guo, J.; Guo, Z.; Hou, L.; Liu, H.; Hou, Y.; He, J.; Guo, F.; Ye, Y. The roles of the Hippo-YAP signalling pathway in Cartilage and Osteoarthritis. Ageing Res. Rev. 2023, 90, 102015. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Kanamoto, T.; Hikida, M.; Sato, S.; Oyama, S.; Tachi, Y.; Kuroda, S.; Mazuka, T.; Ebina, K.; Nakai, T.; Nakata, K. Integrin alpha2beta1 plays an important role in the interaction between human articular cartilage-derived chondrocytes and atelocollagen gel. Sci. Rep. 2021, 11, 1757. [Google Scholar] [CrossRef]
- Miao, Z.; Lu, Z.; Wu, H.; Liu, H.; Li, M.; Lei, D.; Zheng, L.; Zhao, J. Collagen, agarose, alginate, and Matrigel hydrogels as cell substrates for culture of chondrocytes in vitro: A comparative study. J. Cell. Biochem. 2018, 119, 7924–7933. [Google Scholar] [CrossRef]
- Oesser, S.; Seifert, J. Stimulation of type II collagen biosynthesis and secretion in bovine chondrocytes cultured with degraded collagen. Cell Tissue Res. 2003, 311, 393–399. [Google Scholar] [CrossRef]
- Tohyama, H.; Yasuda, K.; Minami, A.; Majima, T.; Iwasaki, N.; Muneta, T.; Sekiya, I.; Yagishita, K.; Takahashi, S.; Kurokouchi, K.; et al. Atelocollagen-associated autologous chondrocyte implantation for the repair of chondral defects of the knee: A prospective multicenter clinical trial in Japan. J. Orthop. Sci. 2009, 14, 579–588. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.L.; Kuiper, N.J. Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy. Bioengineering 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Ebert, J.R.; Edwards, P.K.; Klinken, S.; Wood, D.J.; Janes, G.C. Prior procedures, graft location, preoperative physical health, postoperative strength and graft integrity are associated with 10-year clinical outcome after matrix-induced autologous chondrocyte implantation. Knee Surg. Sports Traumatol. Arthrosc. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nixon, A.J.; Rickey, E.; Butler, T.J.; Scimeca, M.S.; Moran, N.; Matthews, G.L. A chondrocyte infiltrated collagen type I/III membrane (MACI(R) implant) improves cartilage healing in the equine patellofemoral joint model. Osteoarthr. Cartil. 2015, 23, 648–660. [Google Scholar] [CrossRef]
- Hinckel, B.B.; Gomoll, A.H. Autologous Chondrocytes and Next-Generation Matrix-Based Autologous Chondrocyte Implantation. Clin. Sports Med. 2017, 36, 525–548. [Google Scholar] [CrossRef]
- Behrendt, P.; Feldheim, M.; Preusse-Prange, A.; Weitkamp, J.T.; Haake, M.; Eglin, D.; Rolauffs, B.; Fay, J.; Seekamp, A.; Grodzinsky, A.J.; et al. Chondrogenic potential of IL-10 in mechanically injured cartilage and cellularized collagen ACI grafts. Osteoarthr. Cartil. 2018, 26, 264–275. [Google Scholar] [CrossRef]
- Colombini, A.; Libonati, F.; Lopa, S.; Peretti, G.M.; Moretti, M.; de Girolamo, L. Autologous chondrocyte implantation provides good long-term clinical results in the treatment of knee osteoarthritis: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2338–2348. [Google Scholar] [CrossRef]
- Lyu, J.; Geng, H.; Zhu, W.; Li, D.; Chen, K.; Ye, H.; Xia, J. Correlation between the quality of cartilage repair tissue and patellofemoral osteoarthritis after matrix-induced autologous chondrocyte implantation at three-year follow-up: A cross-sectional study. Int. Orthop. 2023, 47, 2383–2390. [Google Scholar] [CrossRef]
- Willershausen, I.; Barbeck, M.; Boehm, N.; Sader, R.; Willershausen, B.; Kirkpatrick, C.J.; Ghanaati, S. Non-cross-linked collagen type I/III materials enhance cell proliferation: In vitro and in vivo evidence. J. Appl. Oral. Sci. 2014, 22, 29–37. [Google Scholar] [CrossRef]
- Gonzalez-Molina, J.; Hahn, P.; Falcao, R.M.; Gultekin, O.; Kokaraki, G.; Zanfagnin, V.; Braz Petta, T.; Lehti, K.; Carlson, J.W. MMP14 expression and collagen remodelling support uterine leiomyosarcoma aggressiveness. Mol. Oncol. 2024, 18, 850–865. [Google Scholar] [CrossRef]
- Vuoriluoto, K.; Jokinen, J.; Kallio, K.; Salmivirta, M.; Heino, J.; Ivaska, J. Syndecan-1 supports integrin alpha2beta1-mediated adhesion to collagen. Exp. Cell Res. 2008, 314, 3369–3381. [Google Scholar] [CrossRef]
- White, D.J.; Puranen, S.; Johnson, M.S.; Heino, J. The collagen receptor subfamily of the integrins. Int. J. Biochem. Cell Biol. 2004, 36, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Lee, K.; Woo, J.H.; Choi, J.H. Macrophages Promote Ovarian Cancer-Mesothelial Cell Adhesion by Upregulation of ITGA2 and VEGFC in Mesothelial Cells. Cells 2023, 12, 384. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liu, X.; Liu, C.; Xu, Y.; Geng, W.; Li, J. Integrin alpha10 regulates adhesion, migration, and osteogenic differentiation of alveolar bone marrow mesenchymal stem cells in type 2 diabetic patients who underwent dental implant surgery. Bioengineered 2022, 13, 13252–13268. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol. 2014, 39, 11–16. [Google Scholar] [CrossRef]
- Fan, Y.; Dong, Z.; Shi, Y.; Sun, S.; Wei, B.; Zhan, L. NLRC5 promotes cell migration and invasion by activating the PI3K/AKT signaling pathway in endometrial cancer. J. Int. Med. Res. 2020, 48, 300060520925352. [Google Scholar] [CrossRef]
- Yu, X.; Qi, Y.; Zhao, T.; Fang, J.; Liu, X.; Xu, T.; Yang, Q.; Dai, X. NGF increases FGF2 expression and promotes endothelial cell migration and tube formation through PI3K/Akt and ERK/MAPK pathways in human chondrocytes. Osteoarthr. Cartil. 2019, 27, 526–534. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Collins, C.; Nelson, W.J. Running with neighbors: Coordinating cell migration and cell-cell adhesion. Curr. Opin. Cell Biol. 2015, 36, 62–70. [Google Scholar] [CrossRef]
- Lechuga, S.; Amin, P.H.; Wolen, A.R.; Ivanov, A.I. Adducins inhibit lung cancer cell migration through mechanisms involving regulation of cell-matrix adhesion and cadherin-11 expression. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 395–408. [Google Scholar] [CrossRef]
- Du, J.; Zhang, S.; Zhao, J.; Li, S.; Chen, W.; Cui, H.; Su, Y. Draxin inhibits chick trunk neural crest delamination and migration by increasing cell adhesion. Dev. Growth Differ. 2021, 63, 501–515. [Google Scholar] [CrossRef]
- Xie, H.; Rutz, J.; Maxeiner, S.; Grein, T.; Thomas, A.; Juengel, E.; Chun, F.K.; Cinatl, J.; Haferkamp, A.; Tsaur, I.; et al. Sulforaphane Inhibits Adhesion and Migration of Cisplatin- and Gemcitabine-Resistant Bladder Cancer Cells In Vitro. Nutrients 2024, 16, 623. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-C.; Hua, W.-J.; Yeh, H.; Lin, Z.-H.; Huang, L.-C.; Ciou, Y.-R.; Ruan, R.; Lin, K.-F.; Tseng, A.-J.; Wu, A.T.H.; et al. GMI, a Ganoderma microsporum protein, abolishes focal adhesion network to reduce cell migration and metastasis of lung cancer. Life Sci. 2023, 335, 122255. [Google Scholar] [CrossRef]
- Karlsen, T.A.; Shahdadfar, A.; Brinchmann, J.E. Human primary articular chondrocytes, chondroblasts-like cells and dedifferentiated chondrocytes: Differences in gene, microRNA and protein expression and phenotype. Tissue Eng. Part C Methods 2011, 17, 219–227. [Google Scholar] [CrossRef]
- Marlovits, S.; Hombauer, M.; Truppe, M.; Vècsei, V.; Schlegel, W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J. Bone Jt. Surg. Br. 2004, 86, 286–295. [Google Scholar] [CrossRef]
- Rhee, D.K.; Marcelino, J.; Baker, M.; Gong, Y.; Smits, P.; Lefebvre, V.; Jay, G.D.; Stewart, M.; Wang, H.; Warman, M.L.; et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Investig. 2005, 115, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Takahata, Y.; Hagino, H.; Kimura, A.; Urushizaki, M.; Yamamoto, S.; Wakamori, K.; Murakami, T.; Hata, K.; Nishimura, R. Regulatory Mechanisms of Prg4 and Gdf5 Expression in Articular Cartilage and Functions in Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 4672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Yu, Z.L.; Wu, B.B.; Sun, F.R. PENK inhibits osteosarcoma cell migration by activating the PI3K/Akt signaling pathway. J. Orthop. Surg. Res. 2020, 15, 162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Liu, Q.; Su, W.; Wang, J.; Sun, Y.; Zhang, J.; Shang, K.; Chen, Z.; Cheng, S.; Wu, H. Genome-wide analysis of differentially expressed genes and the modulation of PEDV infection in Vero E6 cells. Microb. Pathog. 2018, 117, 247–254. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbelaez, P.; Cruz, J.C.; Munoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Humphries, M.J. Cell adhesion assays. Methods Mol. Biol. 2009, 522, 203–210. [Google Scholar] [CrossRef]
- Nakao, M.; Shimizu, I.; Katsuumi, G.; Yoshida, Y.; Suda, M.; Hayashi, Y.; Ikegami, R.; Hsiao, Y.T.; Okuda, S.; Soga, T.; et al. Empagliflozin maintains capillarization and improves cardiac function in a murine model of left ventricular pressure overload. Sci. Rep. 2021, 11, 18384. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]



| No. | Age (Years) | Gender | Affected Knee | ICRS Grade Score of Cartilage Lesion * |
|---|---|---|---|---|
| 1 | 78 | Female | Left | 4 |
| 2 | 75 | Female | Right | 3 |
| 3 | 80 | Female | Left | 3 |
| 4 | 82 | Male | Left | 4 |
| Primers | Forward (5′—3′) | Reverse (5′—3′) |
|---|---|---|
| GAPDH | TCTCTGCTCCTCCTGTTCGAC | GTTGACTCCGACCTTCACCTTC |
| ITGA1 | CAGCCCCACATTTCAAGTCGT | ACCTGTGTCTGTTTAGGACCA |
| ITGA2 | GCAACTGGTTACTGGTTGGTT | GAGGCTCATGTTGGTTTTCATCT |
| ITGA5 | GCCTGTGGAGTACAAGTCCTT | AATTCGGGTGAAGTTATCTGTGG |
| ITGA10 | CTTCAGTTCTGGGATATGTGCC | CCAGTCTTCGTAGGAAGGTCT |
| ITGA11 | TCACGGACACCTTCAACATGG | CCAGCCACTTATTGCCACTGA |
| SOX9 | AGGCAAGCAAAGGAGATGAA | TGGTGTTCTGAGAGGCACAG |
| ACAN | ACAGCTGGGGACATTAGTGG | GTGGAATGCAGAGGTGGTTT |
| PRG4 | TCCATTCAGTCCACCATCTCC | TGTCCAGTTATCCTCCAAATCCT |
| COL1A1 | GACCAGCAGACTGGCAACCT | GCTGAGGTGAAGCGGCTGT |
| COL2A1 | TGGACGCCATGAAGGTTTTCT | TGGGAGCCAGATTGTCATCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Iwahashi, T.; Kasuya, T.; Konishi, M.; Konishi, K.; Kawanaka, M.; Kanamoto, T.; Tanaka, H.; Nakata, K. Effects of a Novel Mammalian-Derived Collagen Matrix on Human Articular Cartilage-Derived Chondrocytes from Osteoarthritis Patients. Int. J. Mol. Sci. 2025, 26, 7826. https://doi.org/10.3390/ijms26167826
Wang M, Iwahashi T, Kasuya T, Konishi M, Konishi K, Kawanaka M, Kanamoto T, Tanaka H, Nakata K. Effects of a Novel Mammalian-Derived Collagen Matrix on Human Articular Cartilage-Derived Chondrocytes from Osteoarthritis Patients. International Journal of Molecular Sciences. 2025; 26(16):7826. https://doi.org/10.3390/ijms26167826
Chicago/Turabian StyleWang, Mingyuan, Toru Iwahashi, Taisuke Kasuya, Mai Konishi, Katsuyuki Konishi, Miki Kawanaka, Takashi Kanamoto, Hiroyuki Tanaka, and Ken Nakata. 2025. "Effects of a Novel Mammalian-Derived Collagen Matrix on Human Articular Cartilage-Derived Chondrocytes from Osteoarthritis Patients" International Journal of Molecular Sciences 26, no. 16: 7826. https://doi.org/10.3390/ijms26167826
APA StyleWang, M., Iwahashi, T., Kasuya, T., Konishi, M., Konishi, K., Kawanaka, M., Kanamoto, T., Tanaka, H., & Nakata, K. (2025). Effects of a Novel Mammalian-Derived Collagen Matrix on Human Articular Cartilage-Derived Chondrocytes from Osteoarthritis Patients. International Journal of Molecular Sciences, 26(16), 7826. https://doi.org/10.3390/ijms26167826






