Recent Advances and Future Directions in Alzheimer’s Disease Genetic Research
Abstract
1. Introduction
2. Hypothesis of Alzheimer’s Disease Etiology
2.1. The Cholinergic Hypothesis
2.2. The Amyloid Hypothesis
2.3. Genetic Etiology
2.3.1. Genetics of Early-Onset Alzheimer’s Disease
2.3.2. Genetics of Late-Onset Alzheimer’s Disease
2.3.3. Somatic Mutations in Alzheimer’s Disease
2.4. Microbiome
2.5. Metabolomics
3. Stages and Symptoms
4. Diagnostics
4.1. Neurobehavioral and Socio-Cognitive Assessment
4.2. Laboratory Markers and Imaging Tools
4.3. Current and Future Digital Approaches
4.4. Molecular Diagnostic Tools
5. Therapy
5.1. Current and Potential Treatment
5.2. Future Treatment Directions
5.2.1. Gene Therapy
5.2.2. Gene-Editing Technology
5.2.3. mRNA Vaccine
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cipriani, G.; Danti, S.; Picchi, L.; Nuti, A.; Di Fiorino, M. Daily Functioning and Dementia. Dement. E Neuropsychol. 2020, 14, 93–102. [Google Scholar] [CrossRef]
- Cloak, N.; Al Khalili, Y. Behavioral and Psychological Symptoms in Dementia: Continuing Education Activity. In Behavioral and Psychological Symptoms in Dementia; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA J. Am. Med. Assoc. 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Lewis, F.; Schaffer, S.K.; Sussex, J.; O’Neill, P.; Cockcroft, L. The Trajectory of Dementia in the UK–Making a Difference. Rep. Alzheimer’s Res. UK OHE Consult. 2014, 2013, 1–55. [Google Scholar]
- Muzambi, R.; Bhaskaran, K.; Brayne, C.; Davidson, J.A.; Smeeth, L.; Warren-Gash, C. Common Bacterial Infections and Risk of Dementia or Cognitive Decline: A Systematic Review. J. Alzheimer’s Dis. 2020, 76, 1609–1626. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Fox, C.; Maidment, I.; Steel, N.; Loke, Y.K.; Arthur, A.; Myint, P.K.; Grossi, C.M.; Mattishent, K.; Bennett, K.; et al. Anticholinergic Drugs and Risk of Dementia: Case-Control Study. BMJ 2018, 361, k1315. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.H.; Nelson, L.D.; Sharon, J.D.; McCrea, M.A.; Dikmen, S.S.; Markowitz, A.J.; Manley, G.T.; Temkin, N.R. Association between TBI-Related Hearing Impairment and Cognition: A TRACK-TBI Study. J. Head Trauma Rehabil. 2022, 37, E327–E335. [Google Scholar] [CrossRef]
- Emmady, P.D.; Schoo, C.; Tadi, P. Major Neurocognitive Disorder (Dementia); StatPearls: Petersburg, FL, USA, 2022. [Google Scholar]
- Karantzoulis, S.; Galvin, J.E. Distinguishing Alzheimer’s Disease from Other Major Forms of Dementia. Expert Rev. Neurother. 2011, 11, 1579–1591. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Mieling, M. Structural Degeneration of the Nucleus Basalis of Meynert in Mild Cognitive Impairment and Alzheimer’s Disease—Evidence from an MRI-Based Meta-Analysis. Neurosci. Biobehav. Rev. 2023, 154, 105393. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Martinez, J. Basal Forebrain Cholinergic Neurons: Linking Down Syndrome and Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 703876. [Google Scholar] [CrossRef]
- Ananth, M.R.; Rajebhosale, P.; Kim, R.; Talmage, D.A.; Role, L.W. Basal Forebrain Cholinergic Signalling: Development, Connectivity and Roles in Cognition. Nat. Rev. Neurosci. 2023, 24, 233–251. [Google Scholar] [CrossRef]
- Tryphena Kamatham, P. Pathogenesis, Diagnostics, and Therapeutics for Alzheimer’s Disease: Breaking the Memory Barrier. Ageing Res. Rev. 2024, 101, 102481. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Common Genetic Signatures of Alzheimer’s Disease in Down Syndrome. F1000Research 2021, 9, 1299. [Google Scholar] [CrossRef]
- Fortea, J. Down Syndrome-Associated Alzheimer’s Disease: A Genetic Form of Dementia. Lancet Neurol. 2021, 20, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B. National Institute on Aging–Alzheimer’s Association Guidelines for the Neuropathologic Assessment of Alzheimer’s Disease. Alzheimer’s Dement. 2012, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, G. Protein Misfolding and Aggregation in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS Neurol. Disord. Drug Targets 2016, 13, 1280–1293. [Google Scholar] [CrossRef]
- Tackenberg, C.; Kulic, L.; Nitsch, R.M. Familial Alzheimer’s Disease Mutations at Position 22 of the Amyloid β-Peptide Sequence Differentially Affect Synaptic Loss, Tau Phosphorylation and Neuronal Cell Death in an Ex Vivo System. PLoS ONE 2020, 15, e0239584. [Google Scholar] [CrossRef] [PubMed]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fà, M.; Amato, A.; Palmeri, A.; D’Adamio, L.; Grassi, C.; Devanand, D.P.; Honig, L.S.; et al. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimer’s Dis. 2018, 64, S611–S631. [Google Scholar] [CrossRef]
- Medeiros, R.; Baglietto-Vargas, D.; Laferla, F.M. The Role of Tau in Alzheimer’s Disease and Related Disorders. CNS Neurosci. Ther. 2011, 17, 514–524. [Google Scholar] [CrossRef]
- Tenchov, R. Alzheimer’s Disease: Exploring the Landscape of Cognitive Decline. ACS Chem. Neurosci. 2024, 15, 3800–3827. [Google Scholar] [CrossRef]
- Buchholz, S. The Six Brain-specific TAU Isoforms and Their Role in Alzheimer’s Disease and Related Neurodegenerative Dementia Syndromes. Alzheimer’s Dement. 2024, 20, 3606–3628. [Google Scholar] [CrossRef] [PubMed]
- Stoothoff, W. Differential Effect of Three-Repeat and Four-Repeat Tau on Mitochondrial Axonal Transport. J. Neurochem. 2009, 111, 417–427. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The Significance of the Cholinergic System in the Brain during Aging and in Alzheimer’s Disease. J. Neural Transm. 2006, 113, 1625–1644. [Google Scholar] [CrossRef]
- Vilchez, D.; Saez, I.; Dillin, A. The Role of Protein Clearance Mechanisms in Organismal Ageing and Age-Related Diseases. Nat. Commun. 2014, 5, 5659. [Google Scholar] [CrossRef]
- Jansen, I.E.; Savage, J.E.; Watanabe, K.; Bryois, J.; Williams, D.M.; Steinberg, S.; Sealock, J.; Karlsson, I.K.; Hägg, S.; Athanasiu, L.; et al. Genome-Wide Meta-Analysis Identifies New Loci and Functional Pathways Influencing Alzheimer’s Disease Risk. Nat. Genet. 2019, 51, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Price, B. Reactive Astrocytes: The Nexus of Pathological and Clinical Hallmarks of Alzheimer’s Disease. Ageing Res. Rev. 2021, 68, 101335. [Google Scholar] [CrossRef]
- Maccioni, R.B.; González, A.; Andrade, V.; Cortés, N.; Tapia, J.P.; Guzmán-Martínez, L. Alzheimer’s Disease in the Perspective of Neuroimmunology. Open Neurol. J. 2018, 12, 50–56. [Google Scholar] [CrossRef]
- Sochocka, M.; Zwolińska, K.; Leszek, J. The Infectious Etiology of Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Tellechea, P.; Pujol, N.; Esteve-Belloch, P.; Echeveste, B.; García-Eulate, M.R.; Arbizu, J.; Riverol, M. Early- and Late-Onset Alzheimer Disease: Are They the Same Entity? Neurologia 2018, 33, 244–253. [Google Scholar] [CrossRef]
- Durmaz, A.; Kumral, E.; Durmaz, B.; Onay, H.; Aslan, G.I.; Ozkinay, F.; Pehlivan, S.; Orman, M.; Cogulu, O. Genetic Factors Associated with the Predisposition to Late Onset Alzheimer’s Disease. Gene 2019, 707, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Piaceri, I.; Nacmias, B.; Sorbi, S. Genetics of Familial and Sporadic Alzheimer’s Disease. Front. Biosci. Elite 2013, E5, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F. Early-Onset Alzheimer Disease and Its Variants. Contin. Lifelong Learn. Neurol. 2019, 25, 34–51. [Google Scholar] [CrossRef]
- Kamboh, M.I. Genomics and Functional Genomics of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 152–172. [Google Scholar] [CrossRef]
- Cacace, R.; Sleegers, K.; Van Broeckhoven, C. Molecular Genetics of Early-Onset Alzheimer’s Disease Revisited. Alzheimer’s Dement. 2016, 12, 733–748. [Google Scholar] [CrossRef]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of Aβ-Deposition in the Human Brain and Its Relevance for the Development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- TCW, J.; Goate, A. Genetics of β-Amyloid Precursor Protein in Alzheimer’s Disease. Cold Spring Harb. Perspect. Med. 2017, 26, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Nalivaeva, N.N.; Turner, A.J. The Amyloid Precursor Protein: A Biochemical Enigma in Brain Development, Function and Disease. FEBS Lett. 2013, 587, 2046–2054. [Google Scholar] [CrossRef]
- Nystuen, K.L.; McNamee, S.M.; Akula, M.; Holton, K.M.; DeAngelis, M.M.; Haider, N.B. Alzheimer’s Disease: Models and Molecular Mechanisms Informing Disease and Treatments. Bioengineering 2024, 11, 45. [Google Scholar] [CrossRef]
- Belyaev, N.D.; Kellett, K.A.B.; Beckett, C.; Makova, N.Z.; Revett, T.J.; Nalivaeva, N.N.; Hooper, N.M.; Turner, A.J. The Transcriptionally Active Amyloid Precursor Protein (APP) Intracellular Domain Is Preferentially Produced from the 695 Isoform of APP in a β-Secretase-Dependent Pathway. J. Biol. Chem. 2010, 285, 41443–41454. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.M.; Prokopenko, D.; Liang, Y.; Zhen, S.Y.; Weigle, I.Q.; Han, W.; Aryal, M.; Tanzi, R.E.; Sisodia, S.S. An APP Ectodomain Mutation Outside of the Aβ Domain Promotes Aβ Production in Vitro and Deposition in Vivo. J. Exp. Med. 2021, 218, e20210313. [Google Scholar] [CrossRef]
- Lanoiselée, H.M.; Nicolas, G.; Wallon, D.; Rovelet-Lecrux, A.; Lacour, M.; Rousseau, S.; Richard, A.C.; Pasquier, F.; Rollin-Sillaire, A.; Martinaud, O.; et al. APP, PSEN1, and PSEN2 Mutations in Early-Onset Alzheimer Disease: A Genetic Screening Study of Familial and Sporadic Cases. PLoS Med. 2017, 14, e1002270. [Google Scholar] [CrossRef]
- Dai, M.H.; Zheng, H.; Zeng, L.D.; Zhang, Y. The Genes Associated with Early-Onset Alzheimer’s Disease. Oncotarget 2018, 9, 15132–15143. [Google Scholar] [CrossRef]
- Kasuga, K.; Shimohata, T.; Nishimura, A.; Shiga, A.; Mizuguchi, T.; Tokunaga, J.; Ohno, T.; Miyashita, A.; Kuwano, R.; Matsumoto, N.; et al. Identification of Independent APP Locus Duplication in Japanese Patients with Early-Onset Alzheimer Disease. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Jeong, M.S.; Jang, S.B. Molecular Characteristics of Amyloid Precursor Protein (App) and Its Effects in Cancer. Int. J. Mol. Sci. 2021, 22, 4999. [Google Scholar] [CrossRef]
- Hur, J.Y. γ-Secretase in Alzheimer’s Disease. Exp. Mol. Med. 2022, 54, 433–446. [Google Scholar] [CrossRef]
- Ryan, N.S.; Nicholas, J.M.; Weston, P.S.J.; Liang, Y.; Lashley, T.; Guerreiro, R.; Adamson, G.; Kenny, J.; Beck, J.; Chavez-Gutierrez, L.; et al. Clinical Phenotype and Genetic Associations in Autosomal Dominant Familial Alzheimer’s Disease: A Case Series. Lancet Neurol. 2016, 15, 1326–1335. [Google Scholar] [CrossRef]
- Giau, V.V.; Bagyinszky, E.; Yang, Y.S.; Youn, Y.C.; An, S.S.A.; Kim, S.Y. Genetic Analyses of Early-Onset Alzheimer’s Disease Using next Generation Sequencing. Sci. Rep. 2019, 9, 8368. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; An, S.S.A.; Kim, S. Mutations in Presenilin 2 and Its Implications in Alzheimer’s Disease and Other Dementia-Associated Disorders. Clin. Interv. Aging 2015, 10, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Valdes, P.; Caldwell, A.B.; Liu, Q.; Fitzgerald, M.Q.; Ramachandran, S.; Karch, C.M.; Adams, S.; Allegri, R.; Araki, A.; Barthelemy, N.; et al. Integrative multiomics reveals common endotypes across PSEN1, PSEN2, and APP mutations in familial Alzheimer’s disease. Alzheimer’s Res. Ther. 2025, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, W.G.; Cochran, J.N.; Taylor, J.W.; Broce, I.J.; Geier, E.G.; Bonham, L.W.; Anderson, A.G.; Sirkis, D.W.; Joie, R.L.; Iaccarino, L.; et al. Early-Onset Alzheimer’s Disease Explained by Polygenic Risk of Late-Onset Disease? Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12482. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New Insights into the Genetic Etiology of Alzheimer’s Disease and Related Dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.C.; Bu, G. Apolipoprotein E and Alzheimer Disease: Pathobiology and Targeting Strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef]
- Suri, S.; Heise, V.; Trachtenberg, A.J.; Mackay, C.E. The Forgotten APOE Allele: A Review of the Evidence and Suggested Mechanisms for the Protective Effect of APOE E2. Neurosci. Biobehav. Rev. 2013, 37, 2878–2886. [Google Scholar] [CrossRef]
- Linard, M.; Letenneur, L.; Garrigue, I.; Doize, A.; Dartigues, J.F.; Helmer, C. Interaction between APOE4 and Herpes Simplex Virus Type 1 in Alzheimer’s Disease. Alzheimer’s Dement. 2020, 16, 200–208. [Google Scholar] [CrossRef]
- Sepulveda-Falla, D.; Sanchez, J.S.; Almeida, M.C.; Boassa, D.; Acosta-Uribe, J.; Vila-Castelar, C.; Ramirez-Gomez, L.; Baena, A.; Aguillon, D.; Villalba-Moreno, N.D.; et al. Distinct Tau Neuropathology and Cellular Profiles of an APOE3 Christchurch Homozygote Protected against Autosomal Dominant Alzheimer’s Dementia. Acta Neuropathol. 2022, 144, 589–601. [Google Scholar] [CrossRef]
- Strang, K.H.; Golde, T.E.; Giasson, B.I. MAPT Mutations, Tauopathy, and Mechanisms of Neurodegeneration. Lab. Investig. 2019, 99, 912–928. [Google Scholar] [CrossRef] [PubMed]
- Schoonhoven, D.N.; Coomans, E.M.; Millán, A.P.; Van Nifterick, A.M.; Visser, D.; Ossenkoppele, R.; Tuncel, H.; Van Der Flier, W.M.; Golla, S.S.V.; Scheltens, P.; et al. Tau Protein Spreads through Functionally Connected Neurons in Alzheimer’s Disease: A Combined MEG/PET Study. Brain 2023, 146, 4040–4054. [Google Scholar] [CrossRef]
- Karch, C.M.; Goate, A.M. Alzheimer’s Disease Risk Genes and Mechanisms of Disease Pathogenesis. Biol. Psychiatry 2015, 77, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Perdigao, C.; Barata, M. Alzheimer’s Disease BIN1 Coding Variants Increase Intracellular Aβ Levels by Interfering with BACE1 Recycling. J. Biol. Chem. 2021, 26, 1553–1562. [Google Scholar] [CrossRef]
- Jones, R.E.; Andrews, R.; Holmans, P.; Hill, M.; Taylor, P.R. Modest Changes in Spi1 Dosage Reveal the Potential for Altered Microglial Function as Seen in Alzheimer’s Disease. Sci. Rep. 2021, 11, 14935. [Google Scholar] [CrossRef]
- Griciuc, A.; Federico, A.N.; Natasan, J.; Forte, A.M.; McGinty, D.; Nguyen, H.; Volak, A.; Leroy, S.; Gandhi, S.; Lerner, E.P.; et al. Gene Therapy for Alzheimer’s Disease Targeting CD33 Reduces Amyloid Beta Accumulation and Neuroinflammation. Hum. Mol. Genet. 2020, 29, 2920–2935. [Google Scholar] [CrossRef]
- Zhu, X.C.; Yu, J.T.; Jiang, T.; Wang, P.; Cao, L.; Tan, L. CR1 in Alzheimer’s Disease. Mol. Neurobiol. 2015, 51, 753–765. [Google Scholar] [CrossRef]
- Luo, J.; Li, S.; Qin, X.; Song, L.; Peng, Q.; Chen, S.; Xie, Y.; Xie, L.; Li, T.; He, Y.; et al. Meta-Analysis of the Association between CR1 Polymorphisms and Risk of Late-Onset Alzheimer’s Disease. Neurosci. Lett. 2014, 578, 165–170. [Google Scholar] [CrossRef]
- Foster, E.M.; Dangla-Valls, A.; Lovestone, S.; Ribe, E.M.; Buckley, N.J. Clusterin in Alzheimer’s Disease: Mechanisms, Genetics, and Lessons from Other Pathologies. Front. Neurosci. 2019, 13, 164. [Google Scholar] [CrossRef]
- Harper, A.R.; Nayee, S.; Topol, E.J. Protective Alleles and Modifier Variants in Human Health and Disease. Nat. Rev. Genet. 2015, 16, 689–701. [Google Scholar] [CrossRef]
- Parikh, I.; Fardo, D.W.; Estus, S. Genetics of PICALM Expression and Alzheimer’s Disease. PLoS ONE 2014, 9, e91242. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Nagaraj, S.; Küçükali, F.; de Fisenne, M.A.; Kosa, A.C.; Doeraene, E.; Lopez Gutierrez, L.; Brion, J.P.; Leroy, K. PICALM and Alzheimer’s Disease: An Update and Perspectives. Cells 2022, 11, 3994. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic Meta-Analysis of Diagnosed Alzheimer’s Disease Identifies New Risk Loci and Implicates Aβ, Tau, Immunity and Lipid Processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.; Joseph, S.A.; Tayler, H.; Abraham, R.; Owen, M.J.; Williams, J.; Kehoe, P.G.; Love, S. Distribution and Expression of Picalm in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2010, 69, 1071–1077. [Google Scholar] [CrossRef]
- Li, H.; Karl, T.; Garner, B. Understanding the Function of ABCA7 in Alzheimer’s Disease. Biochem. Soc. Trans. 2015, 43, 920–923. [Google Scholar] [CrossRef]
- Albrecht, C.; Viturro, E. The ABCA Subfamily-Gene and Protein Structures, Functions and Associated Hereditary Diseases. Pflug. Arch. 2007, 453, 581–589. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Aikawa, T.; Holm, M.L.; Kanekiyo, T. ABCA7 and Pathogenic Pathways of Alzheimer’s Disease. Brain Sci. 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, C.; Boada, M.; González-Pérez, A.; Gayán, J.; Ramírez-Lorca, R.; Marín, J.; Hernández, I.; Moreno-Rey, C.; Morón, F.J.; López-Arrieta, J.; et al. The Membrane-Spanning 4-Domains, Subfamily A (MS4A) Gene Cluster Contains a Common Variant Associated with Alzheimer’s Disease. Genome Med. 2011, 3, 33. [Google Scholar] [CrossRef]
- Silva-Gomes, R.; Mapelli, S.N.; Boutet, M.A.; Mattiola, I.; Sironi, M.; Grizzi, F.; Colombo, F.; Supino, D.; Carnevale, S.; Pasqualini, F.; et al. Differential Expression and Regulation of MS4A Family Members in Myeloid Cells in Physiological and Pathological Conditions. J. Leukoc. Biol. 2022, 111, 817–836. [Google Scholar] [CrossRef]
- Dodson, S.E.; Gearing, M.; Lippa, C.F.; Montine, T.J.; Levey, A.I.; Lah, J.J. LR11/SorLA Expression Is Reduced in Sporadic Alzheimer Disease but Not in Familial Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2006, 65, 866–872. [Google Scholar] [CrossRef]
- Small, S.A.; Kent, K.; Pierce, A.; Leung, C.; Kang, M.S.; Okada, H.; Honig, L.; Vonsattel, J.P.; Kim, T.W. Model-Guided Microarray Implicates the Retromer Complex in Alzheimer’s Disease. Ann. Neurol. 2005, 58, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Simoes, S.; Guo, J.; Buitrago, L.; Qureshi, Y.H.; Feng, X.; Kothiya, M.; Cortes, E.; Patel, V.; Kannan, S.; Kim, Y.H.; et al. Alzheimer’s Vulnerable Brain Region Relies on a Distinct Retromer Core Dedicated to Endosomal Recycling. Cell Rep. 2021, 37, 110182. [Google Scholar] [CrossRef]
- Andersen, O.M.; Reiche, J.; Schmidt, V.; Gotthardt, M.; Spoelgen, R.; Behlke, J.; Von Arnim, C.A.F.; Breiderhoff, T.; Jansen, P.; Wu, X.; et al. Neuronal Sorting Protein-Related Receptor sorLA/LR11 Regulates Processing of the Amyloid Precursor Protein. Proc. Natl. Acad. Sci. USA 2005, 102, 13461–13466. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Zhang, F.; Zhang, M.; Wang, Q.; Wang, J. The Common Genes Involved in the Pathogenesis of Alzheimer’s Disease and Type 2 Diabetes and Their Implication for Drug Repositioning. Neuropharmacology 2023, 223, 109327. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tan, M.S.; Wang, H.F.; Zhang, W.; Wang, Z.X.; Jiang, T.; Yu, J.T.; Tan, L. ZCWPW1 Is Associated with Late-Onset Alzheimer’s Disease in Han Chinese: A Replication Study and Meta-Analyses. Oncotarget 2016, 7, 20305–20311. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, N.; Liu, J.; Huang, J.; Shi, J.; Jin, F. AMPK: A Bridge between Diabetes Mellitus and Alzheimer’s Disease. Behav. Brain Res. 2021, 400, 113043. [Google Scholar] [CrossRef]
- Pathak, G.A.; Silzer, T.K.; Sun, J.; Zhou, Z.; Daniel, A.A.; Johnson, L.; O’Bryant, S.; Phillips, N.R.; Barber, R.C. Genome-Wide Methylation of Mild Cognitive Impairment in Mexican Americans Highlights Genes Involved in Synaptic Transport, Alzheimer’s Disease-Precursor Phenotypes, and Metabolic Morbidities. J. Alzheimer’s Dis. 2019, 72, 733–749. [Google Scholar] [CrossRef]
- Reagan, A.M.; Christensen, K.E.; Graham, L.C.; Bedwell, A.A.; Eldridge, K.; Speedy, R.; Figueiredo, L.L.; Persohn, S.C.; Bottiglieri, T.; Nho, K.; et al. The 677C > T Variant in Methylenetetrahydrofolate Reductase Causes Morphological and Functional Cerebrovascular Deficits in Mice. J. Cereb. Blood Flow Metab. 2022, 42, 2333–2350. [Google Scholar] [CrossRef] [PubMed]
- Cruchaga, C.; Kauwe, J.S.K.; Harari, O.; Jin, S.C.; Cai, Y.; Karch, C.M.; Benitez, B.A.; Jeng, A.T.; Skorupa, T.; Carrell, D.; et al. GWAS of Cerebrospinal Fluid Tau Levels Identifies Risk Variants for Alzheimer’s Disease. Neuron 2013, 78, 256–268. [Google Scholar] [CrossRef]
- Manzine, P.R.; Ettcheto, M.; Cano, A.; Busquets, O.; Marcello, E.; Pelucchi, S.; Di Luca, M.; Endres, K.; Olloquequi, J.; Camins, A.; et al. ADAM10 in Alzheimer’s Disease: Pharmacological Modulation by Natural Compounds and Its Role as a Peripheral Marker. Biomed. Pharmacother. 2019, 113, 108661. [Google Scholar] [CrossRef]
- Migliore, L. Gene–Environment Interactions in Alzheimer Disease: The Emerging Role of Epigenetics. Nat. Rev. Neurol. 2022, 18, 643–660. [Google Scholar] [CrossRef]
- Basavarajappa, B. Unlocking the Epigenetic Symphony: Histone Acetylation’s Impact on Neurobehavioral Change in Neurodegenerative Disorders. Epigenomics 2024, 26, 1553–1562. [Google Scholar] [CrossRef]
- Quiroz, Y. APOE3 Christchurch Heterozygosity and Autosomal Dominant Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, M. The First Genome-Wide Association Study in the Argentinian and Chilean Populations Identifies Shared Genetics with Europeans in Alzheimer’s Disease. Alzheimer’s Dement. 2024, 20, 1298–1308. [Google Scholar] [CrossRef]
- Miyashita, A. Genetics of Alzheimer’s Disease: An East Asian Perspective. J. Hum. Genet. 2023, 68, 115–124. [Google Scholar] [CrossRef]
- Mazure, C. Sex Differences in Alzheimer’s Disease and Other Dementias. Lancet Neurol. 2016, 15, 451–452. [Google Scholar] [CrossRef]
- Vila-Castelar, C.; Tariot, P.N.; Sink, K.M.; Clayton, D.; Langbaum, J.B.; Thomas, R.G.; Chen, Y.; Su, Y.; Chen, K.; Hu, N.; et al. Sex Differences in Cognitive Resilience in Preclinical Autosomal-Dominant Alzheimer’s Disease Carriers and Non-Carriers: Baseline Findings from the API ADAD Colombia Trial. Alzheimer’s Dement. 2022, 18, 2272–2282. [Google Scholar] [CrossRef]
- Li, R. Sex Differences in Cognitive Impairment and Alzheimer’s Disease. Front. Neuroendocrinol. 2014, 35, 385–403. [Google Scholar] [CrossRef]
- Altmann, A. Sex Modifies the APOE-Related Risk of Developing Alzheimer Disease. Ann. Neurol. 2014, 75, 563–573. [Google Scholar] [CrossRef]
- Nazarian, A.; Yashin, A.I.; Kulminski, A.M. Genome-Wide Analysis of Genetic Predisposition to Alzheimer’s Disease and Related Sex Disparities. Alzheimer’s Res. Ther. 2019, 11, 5. [Google Scholar] [CrossRef]
- Pollard, C.; Aston, K.; Emery, B.R.; Hill, J.; Jenkins, T. Detection of Neuron-Derived cfDNA in Blood Plasma: A New Diagnostic Approach for Neurodegenerative Conditions. Front. Neurol. 2023, 14, 1272960. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, B.A.; Luquin, I.B.; Corroza, J.; Miguel, M.S.; Roldan, M.; Zueco, S.; Beiras, I.E.; Cabello, C.; Agudo, M.Á.G.; Jericó, I.; et al. Liquid Biopsy Shows Differences in cfDNA Fragmentation Pattern between AD Patients and Controls. Alzheimer’s Dement. 2020, 16, e039748. [Google Scholar] [CrossRef]
- Li, C. Messengers From the Gut: Gut Microbiota-Derived Metabolites on Host Regulation. Front. Microbiol. 2022, 13, 863407. [Google Scholar] [CrossRef]
- Kim, S. Gut Microbiota-Derived Metabolites Tune Host Homeostasis Fate. Semin. Immunopathol. 2024, 26, 1553–1562. [Google Scholar] [CrossRef]
- Basiji, K. The Critical Role of Gut-Brain Axis Microbiome in Mental Disorders. Metab. Brain Dis. 2023, 38, 2547–2561. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G. From Gut Dysbiosis to Altered Brain Function and Mental Illness: Mechanisms and Pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef]
- Bercik, P. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mic. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef]
- Tarawneh, R. The Gut Microbiome and Alzheimer’s Disease: Complex and Bidirectional Interactions. Neurosci. Biobehav. Rev. 2022, 141, 104814. [Google Scholar] [CrossRef] [PubMed]
- Brown, G. The Endotoxin Hypothesis of Alzheimer’s Disease. Mol. Neurodegener. 2024, 19, 30. [Google Scholar] [CrossRef]
- Zhan, X. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Front. Aging Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef]
- Intili, G. From Dysbiosis to Neurodegenerative Diseases through Different Communication Pathways: An Overview. Biology 2023, 12, 195. [Google Scholar] [CrossRef]
- Sarmiento-Ordonez, J.M. Association Between Porphyromonas Gingivalis and Alzheimer’s Disease: A Comprehensive Review. Infect. Drug Resist. 2025, 18, 2119–2136. [Google Scholar] [CrossRef]
- Ain Adil, N. The Oral–Gut Microbiome–Brain Axis in Cognition. Microorganisms 2025, 13, 814. [Google Scholar] [CrossRef]
- Berezhnoy, G. Metabolomic Profiling of CSF and Blood Serum Elucidates General and Sex-Specific Patterns for Mild Cognitive Impairment and Alzheimer’s Disease Patients. Front. Aging Neurosci. 2023, 15, 1219718. [Google Scholar] [CrossRef]
- Jutten, R. Identifying Sensitive Measures of Cognitive Decline at Different Clinical Stages of Alzheimer’s Disease. J. Int. Neuropsychol. Soc. 2021, 27, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Wattmo, C.; Minthon, L.; Wallin, Å.K. Mild versus Moderate Stages of Alzheimer’s Disease: Three-year Outcomes in a Routine Clinical Setting of Cholinesterase Inhibitor Therapy. Alzheimer’s Res. Ther. 2016, 8, 7. [Google Scholar]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease: A Primer. Nat. Rev. Dis. Primer 2015, 1, 15056. [Google Scholar] [CrossRef]
- Jessen, F. Subjective and Objective Cognitive Decline at the Pre-Dementia Stage of Alzheimer’s Disease. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 3–7. [Google Scholar] [CrossRef]
- Steinberg, M.; Shao, H.; Zandi, P.; Lyketsos, C.G.; Welsh-Bohmer, K.A.; Norton, M.C.; Breitner, J.; Steffens, D.C.; Tschanz, J.A.T.; Hayden, K.; et al. Point and 5-Year Period Prevalence of Neuropsychiatric Symptoms in Dementia: The Cache County Study. Int. J. Geriatr. Psychiatry 2008, 23, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Advances in Therapeutics to Alleviate Cognitive Decline and Neuropsychiatric Symptoms of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5169. [Google Scholar] [CrossRef]
- Nlemolisa, O.R. Groundwater Contamination and Health Risks near Waste Dumps and Mechanic Workshops: A Seasonal Perspective. Clean. Water 2024, 4, 100090. [Google Scholar] [CrossRef]
- Aupperle, P. Management of Aggression, Agitation, and Psychosis in Dementia: Focus on Atypical Antipsychotics. Am. J. Alzheimer’s Dis. Other Demen. 2006, 21, 101–108. [Google Scholar] [CrossRef]
- Fong, T.G.; Inouye, S.K. The Inter-Relationship between Delirium and Dementia: The Importance of Delirium Prevention. Nat. Rev. Neurol. 2022, 18, 579–596. [Google Scholar] [CrossRef]
- Kumar Patra, A. MRI Patterns of the Hippocampus and Amygdala for Predicting Stages of Alzheimer’s Progression: A Minimal Feature Machine Learning Framework. arXiv 2025. [Google Scholar] [CrossRef]
- Cummings, J. Alzheimer’s Disease Diagnostic Criteria: Practical Applications. Alzheimer’s Res. Ther. 2012, 4, 35–36. [Google Scholar] [CrossRef]
- Ercole Jaqua, E. Alzheimer Disease: Treatment of Cognitive and Functional Symptoms. Am. Fam. Physician 2024, 110, 281–293. [Google Scholar]
- Bomasang-Layno, E.; Bronsther, R. Diagnosis and Treatment of Alzheimer’s Disease: An Update. Del. J. Public Health 2021, 7, 74–85. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Fowler, N. Feasibility and Acceptability of Implementing a Digital Cognitive Assessment for Alzheimer Disease and Related Dementias in Primary Care. Ann. Fam. Med. 2025, 23, 191–198. [Google Scholar] [CrossRef]
- Maeshima, S. Neuropsychological Tests Used for Dementia Assessment in Japan: Current Status. Geriatr. Gerontol. Int. 2023, 24, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Smith, S. Measurement of Health-Related Quality of Life for People with Dementia: Development of a New Instrument (DEMQOL) and an Evaluation of Current Methodology. Health Technol. Assess. 2005, 9, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, R. Quality of Life in Alzheimer’s Disease: Patient and Caregiver Reports. J. Ment. Health 1999, 5, 21–32. [Google Scholar]
- Brod, M. Conceptualization and Measurement of Quality of Life in Dementia: The Dementia Quality of Life Instrument (DQoL). Gerontologist 1999, 39, 25–36. [Google Scholar] [CrossRef]
- Kale, M. AI-Driven Innovations in Alzheimer’s Disease: Integrating Early Diagnosis, Personalized Treatment, and Prognostic Modelling. Ageing Res. Rev. 2024, 101, 102497. [Google Scholar] [CrossRef]
- Ren, F. Artificial Intelligence-Driven Multi-Omics Approaches in Alzheimer’s Disease: Progress, Challenges, and Future Directions. Acta Pharm. Sin. B, 2025; in press. [Google Scholar] [CrossRef]
- Mohamed, A.Z. Response to the Letter Concerning the Publication: Amyloid Pathology Fingerprint Differentiates Post-Traumatic Stress Disorder and Traumatic Brain Injury. NeuroImage Clin. 2019, 19, 716–726. [Google Scholar] [CrossRef]
- Yoshida, S. New Insights into the Roles for DYRK Family in Mammalian Development and Congenital Diseases. Genes. Dis. 2023, 10, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Gozt, A. Emerging Applications for Quantitative Susceptibility Mapping in the Detection of Traumatic Brain Injury Pathology. Neurosci. Forefr. Rev. 2021, 467, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Dimitsu Assfaw, A. Advances in Blood Biomarkers for Alzheimer Disease (AD): A Review. Kaohsiung J. Med. Sci. 2024, 40, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R.; Scheltens, P.; Thompson, P.M. The Clinical Use of Structural MRI in Alzheimer Disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef]
- Sudlow, C. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Muhammed Niyas, K.P.; Thiyagarajan, P. A Systematic Review on Early Prediction of Mild Cognitive Impairment to Alzheimers Using Machine Learning Algorithms. Int. J. Intell. Netw. 2023, 4, 74–88. [Google Scholar] [CrossRef]
- Coorey, G. The Health Digital Twin: Advancing Precision Cardiovascular Medicine. Nat. Rev. Cardiol. 2021, 18, 803–804. [Google Scholar] [CrossRef]
- Wen, X. The Applications of CircRNA in the Diagnosis and Treatment of Alzheimer’s Disease. Mol. Neurobiol. 2024, 61, 6501–6510. [Google Scholar] [CrossRef]
- Adadi-Pooya, A. Late-Onset Idiopathic (Genetic) Generalized Epilepsies: Clinical and EEG Findings. J. Clin. Neurosci. 2020, 76, 58–60. [Google Scholar] [CrossRef]
- Leidinger, P. A Blood Based 12-nniRNA Signature of Alzheimer Disease Patients. Genome Biol. 2013, 14, R78. [Google Scholar] [CrossRef]
- Fletcher, K. Pilot Testing in the Wild: Feasibility, Acceptability, Usage Patterns, and Efficacy of an Integrated Web and Smartphone Platform for Bipolar II Disorder. JMIR Form. Res. 2023, 6, e32740. [Google Scholar] [CrossRef]
- Deardorff, W.J.; Feen, E.; Grossberg, G.T. The Use of Cholinesterase Inhibitors Across All Stages of Alzheimer’s Disease. Drugs Aging 2015, 32, 537–547. [Google Scholar] [CrossRef]
- Tanvir Kabir, M.; Sahab Uddin, M.; Al Mamun, A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Md Ashraf, G.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D. Research Progress and Future Development Direction of Alzheimer’s Therapy. Theor. Nat. Sci. 2024, 33, 236–241. [Google Scholar] [CrossRef]
- Keller, L.-A. Intranasal Drug Delivery: Opportunities and Toxicologic Challenges during Drug Development. Drug Deliv. Transl. Res. 2021, 2, 735–757. [Google Scholar] [CrossRef] [PubMed]
- Doty, K. The Role of the Immune System in Neurodegenerative Disorders: Adaptive or Maladaptive? Brain Res. 2015, 1617, 155–173. [Google Scholar] [CrossRef]
- Strzelec, M. Immunomodulation—A General Review of the Current State-of-the-Art and New Therapeutic Strategies for Targeting the Immune System. Front. Immunol. 2023, 14, 1127704. [Google Scholar] [CrossRef]
- Burmeister, A. The Interleukin-10 Family of Cytokines and Their Role in the CNS. Front. Cell. Neurosci. 2018, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Study of the Safety & Efficacy of Leukine® in the Treatment of Alzheimer’s Disease. Available online: https://clinicaltrials.gov/study/NCT01409915 (accessed on 13 July 2025).
- Hill, J. Fenamates as Potential Therapeutics for Neurodegenerative Disorders. Cells 2021, 10, 702. [Google Scholar] [CrossRef]
- Song, C. Immunotherapy for Alzheimer’s Disease: Targeting β-Amyloid and Beyond. Transl. Neurodegener. 2022, 11, 18. [Google Scholar] [CrossRef]
- Abushouk, A.I. Bapineuzumab for Mild to Moderate Alzheimer’s Disease: A Meta-Analysis of Randomized Controlled Trials. BMC Neurol. 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F. Applications of Nanobodies in Brain Diseases. Front. Immunol. 2022, 13, 978513. [Google Scholar] [CrossRef] [PubMed]
- Huang, W. Advances in immunoPET/SPECT Imaging: The Role of Fab and F(Ab′)2 Fragments in Theranostics. Acta Pharm. Sin. B, 2025; in press. [Google Scholar] [CrossRef]
- Steeland, S. Nanobodies as Therapeutics: Big Opportunities for Small Antibodies. Drug Discov. Today 2016, 21, 1076–1113. [Google Scholar] [CrossRef]
- Kim, B. A Probabilistic Strategy for Understanding Action Selection. J. Neurosci. 2010, 30, 2340–2355. [Google Scholar] [CrossRef]
- Zhao, Z. Current Anti-Amyloid-β Therapy for Alzheimer’s Disease Treatment: From Clinical Research to Nanomedicine. Int. J. Nanomed. 2023, 18, 7825–7845. [Google Scholar] [CrossRef]
- Budni, J. The Involvement of BDNF, NGF and GDNF in Aging and Alzheimer’s Disease. Aging Dis. 2015, 6, 331–341. [Google Scholar] [CrossRef]
- Herman, F. Principles of Inflammasome Priming and Inhibition: Implications for Psychiatric Disorders. Brain Behav. Immun. 2018, 73, 66–84. [Google Scholar] [CrossRef]
- Zhang, Y. Ganoderic Acid A To Alleviate Neuroinflammation of Alzheimer’s Disease in Mice by Regulating the Imbalance of the Th17/Tregs Axis. J. Agric. Food Chem. 2021, 69, 14204–14214. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia Prevention, Intervention, and Care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Hickman, R.A.; Faustin, A.; Wisniewski, T. Alzheimer Disease and Its Growing Epidemic: Risk Factors, Biomarkers, and the Urgent Need for Therapeutics. Neurol. Clin. 2016, 34, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N. Physical Activity, Diet, and Risk of Alzheimer Disease. JAMA 2009, 302, 627–637. [Google Scholar] [CrossRef]
- Clare Morris, M. MIND Diet Slows Cognitive Decline with Aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Butterfield, A. Perspectives on Oxidative Stress in Alzheimer’s Disease and Predictions of Future Research Emphases. J. Alzheimer’s Dis. 2018, 64, S469–S479. [Google Scholar] [CrossRef] [PubMed]
- Browne, D. Vitamin E and Alzheimer’s Disease: What Do We Know so Far? Clin. Interv. Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef]
- Sevigny, J. The Antibody Aducanumab Reduces Aβ Plaques in Alzheimer’s Disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J. Conformational Stability, Vibrational Spectra, HOMO-LUMO and NBO Analysis of 1,3,4-Thiadiazolidine-2,5-Dithione with Experimental (FT-IR and FT-Raman) Techniques and Scaled Quantum Mechanical Calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 113, 171–181. [Google Scholar] [CrossRef]
- Lam, D. Distribution and Neurochemical Characterization of Neurons within the Nucleus of the Solitary Tract Responsive to Serotonin Agonist-Induced Hypophagia. Behav. Brain Res. 2009, 196, 139–143. [Google Scholar] [CrossRef]
- Neelakantan, P. Histologic Assessment of Debridement of the Root Canal Isthmus of Mandibular Molars by Irrigant Activation Techniques Ex Vivo. J. Endod. 2016, 42, 1268–1272. [Google Scholar] [CrossRef]
- Jack, A. SARS-CoV-2 Nucleocapsid Protein Forms Condensates with Viral Genomic RNA. PLoS Biol. 2021, 19, e3001425. [Google Scholar] [CrossRef]
- Congdon, E. Tau-Targeting Therapies for Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Mummery, C. Tau-Targeting Antisense Oligonucleotide MAPTRx in Mild Alzheimer’s Disease: A Phase 1b, Randomized, Placebo-Controlled Trial. Nat. Med. Vol. 2023, 29, 1437–1447. [Google Scholar] [CrossRef]
- Wei, J. Genome-Wide CRISPR Screens Reveal Host Factors Critical for SARS-CoV-2 Infection. Cell 2021, 184, 76–91.e13. [Google Scholar] [CrossRef] [PubMed]
- Katel, S. AAV-Mediated Peripheral Single Chain Variable Fragments’ Administration to Reduce Cerebral Tau in Adult P301S Transgenic Mice: Mono- vs Combination Therapy. bioRxiv 2025. [Google Scholar] [CrossRef]
- Ising, C. AAV-Mediated Expression of Anti-Tau scFvs Decreases Tau Accumulation in a Mouse Model of Tauopathy. J. Exp. Med. 2017, 214, 1227–1238. [Google Scholar] [CrossRef]
- Mecozzi, V.J.; Berman, D.E.; Simoes, S.; Vetanovetz, C.; Awal, M.R.; Patel, V.M.; Schneider, R.T.; Petsko, G.A.; Ringe, D.; Small, S.A. Pharmacological Chaperones Stabilize Retromer to Limit APP Processing. Nat. Chem. Biol. 2014, 10, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Hernandez, P.; Liow, K.; Damiano, E.; Zetterberg, H.; Blennow, K.; Feng, D.; Chen, M.; Maccecchini, M. Buntanetap, a Novel Translational Inhibitor of Multiple Neurotoxic Proteins, Proves to Be Safe and Promising in Both Alzheimer’s and Parkinson’s Patients. J. Prev. Alzheimer’s Dis. 2023, 10, 25–33. [Google Scholar] [CrossRef]
- Doudna, J.; Charpentier, E. The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 364, 1258096. [Google Scholar] [CrossRef]
- Komor, A. Programmable Editing of a Target Base in Genomic DNA without Double-Stranded DNA Cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Tan, D.C.; Yao, S.; Ittner, A.; Bertz, J.; Ke, Y.D.; Ittner, L.M.; Delerue, F. Generation of a New Tau Knockout (tauΔex1) Line Using CRISPR/Cas9 Genome Editing in Mice. J. Alzheimer’s Dis. 2018, 62, 571–578. [Google Scholar] [CrossRef]
- Weiner, D. Polygenic Transmission Disequilibrium Confirms That Common and Rare Variation Act Additively to Create Risk for Autism Spectrum Disorders. Nat. Genet. 2017, 49, 978–985. [Google Scholar] [CrossRef]
- Terrinoni, A. The Circulating miRNAs as Diagnostic and Prognostic Markers. Clin. Chem. Lab. Med. 2019, 57, 932–953. [Google Scholar] [CrossRef]
- Shawkatova, I. Alzheimer’s Disease: Recent Developments in Pathogenesis, Diagnosis, and Therapy. Life 2025, 15, 549. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; Dekosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing Research Diagnostic Criteria for Alzheimer’s Disease: The IWG-2 Criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.D.; Chen-Plotkin, A.S. The Post-GWAS Era: From Association to Function. Am. J. Hum. Genet. 2018, 102, 717–730. [Google Scholar] [CrossRef] [PubMed]
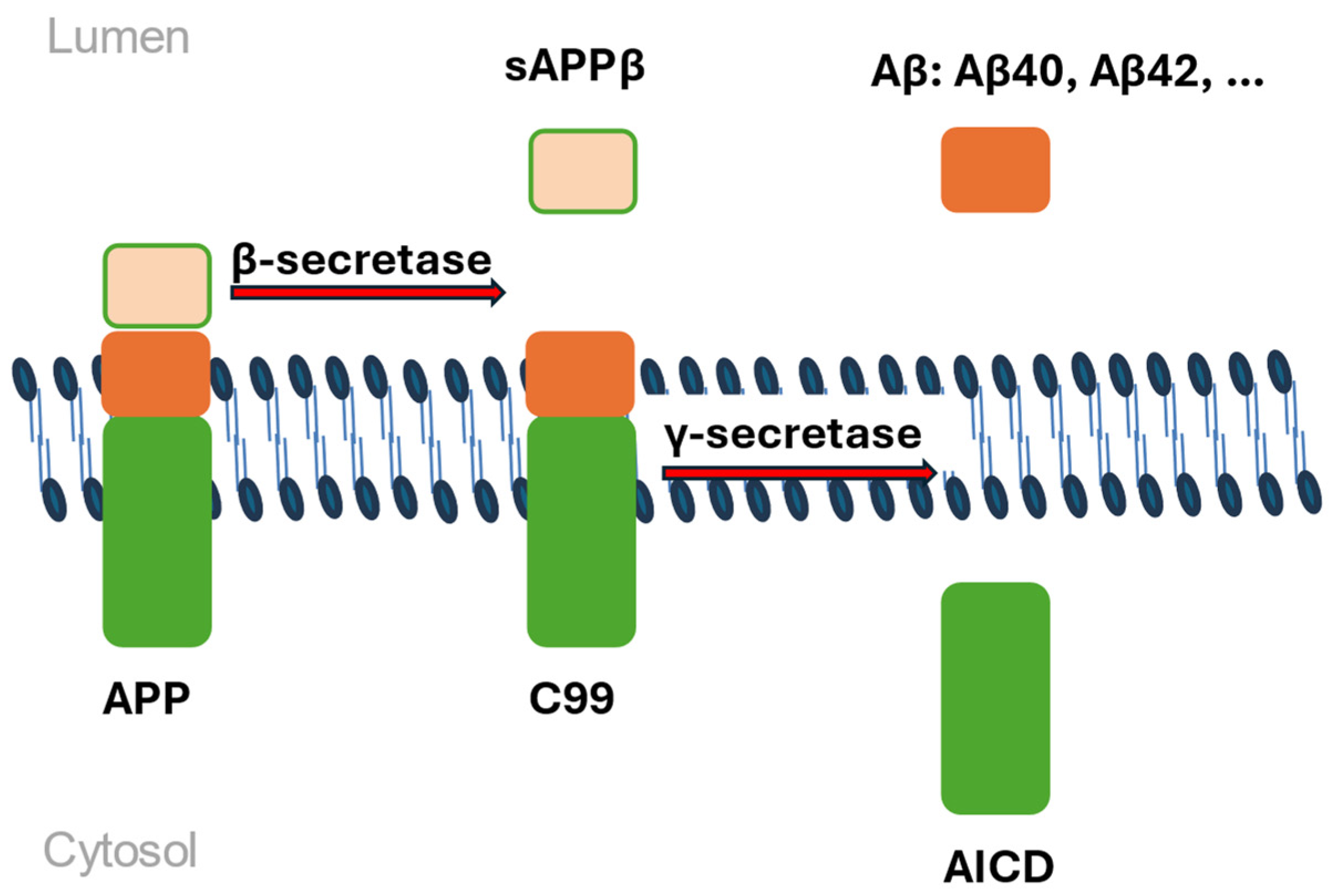
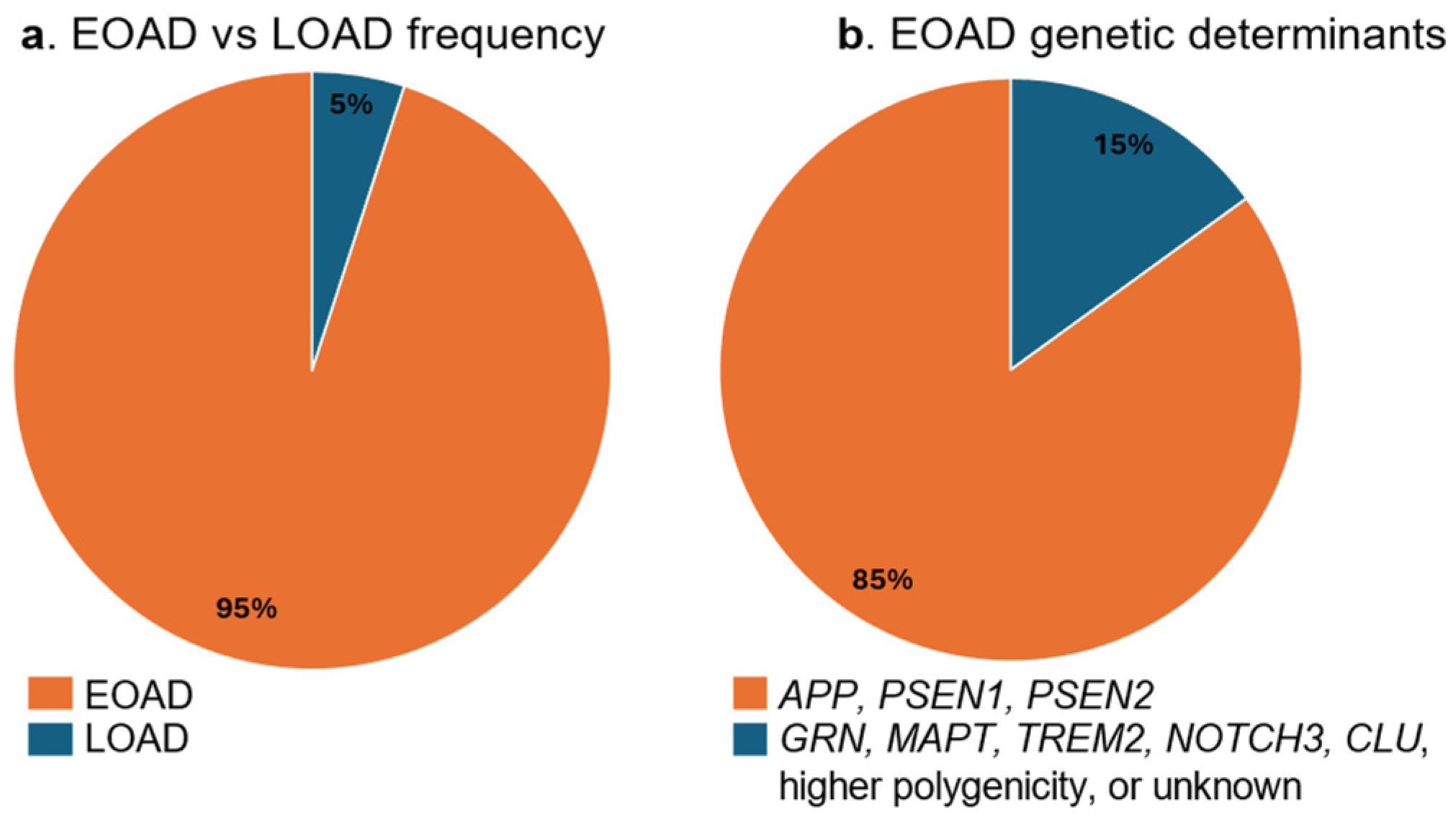
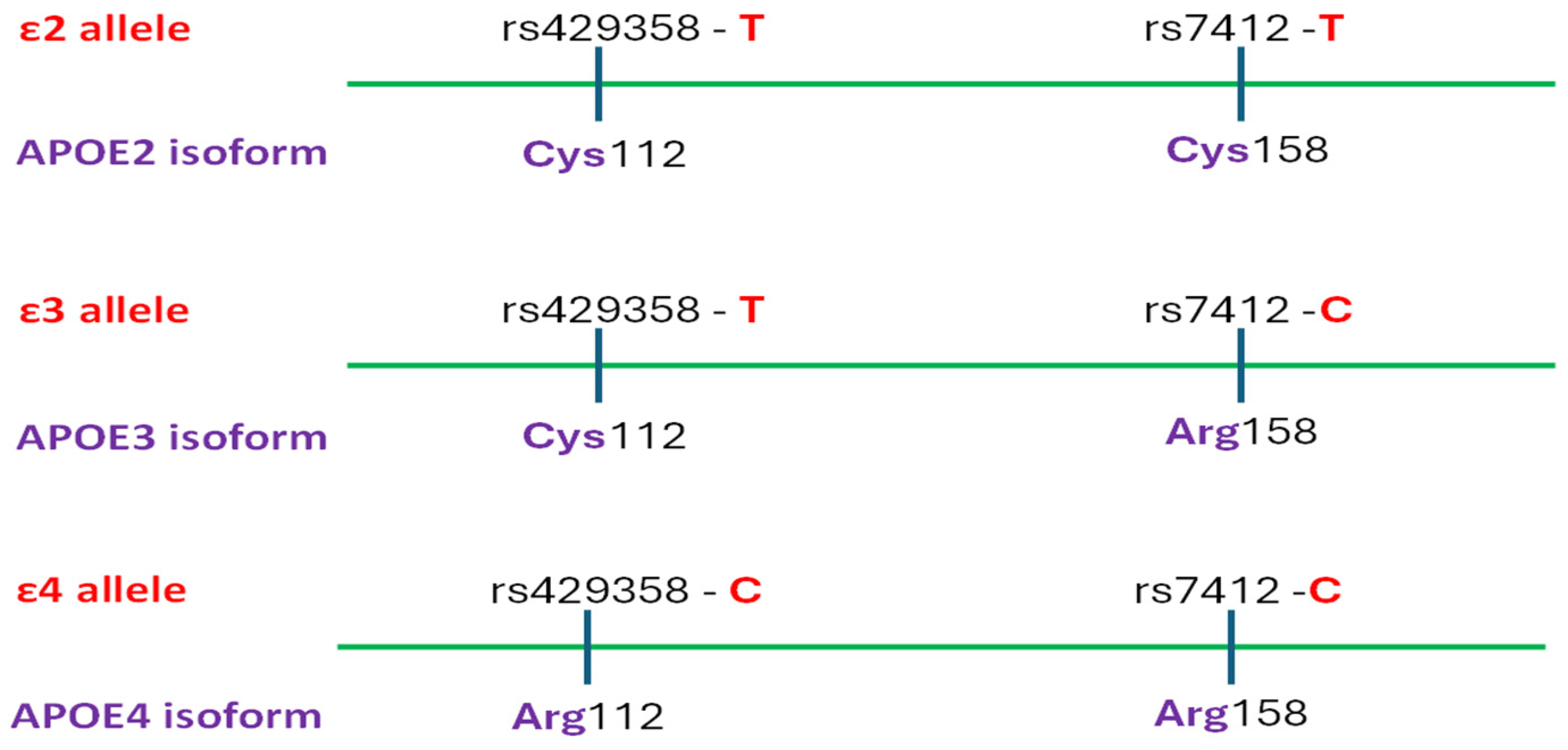
| Gene Name | Biological Function | Prevailing Mutation Type | Mechanism Relevant to AD Pathogenesis | Reference |
|---|---|---|---|---|
| Amyloid precursor protein (APP) | Involved in neurite growth, neuronal adhesion | Mainly missense mutations and gross insertions/duplications | Increased total Aβ or Aβ42 levels or Aβ fibrillogenesis | [37] |
| Presenilin 1 (PSEN1) | Catalytic subunit of the gamma-secretase complex | Missense mutations, small insertions, deletions, and genomic deletions, complete penetrance | Cause of the most severe forms of EOAD (as early as 24 years) | [37] |
| Presenilin 2 (PSEN2) | Probable catalytic subunit of the gamma-secretase complex | Missense/nonsense; incomplete penetrance | Found in dementia-associated disorders; EOAD, but later than PSEN1 | [37] |
| Triggering receptor expressed on myeloid cells-2 (TREM2) | Receptor for Aβ42; mediates multiple pro-inflammatory cytokine processes | Missense/nonsense | Mutated tau accelerates the neurodegenerative process | [54] |
| Apolipoprotein E (APOE) | Binds lipids to form lipoproteins, central role in CNS lipid transport | ε4 allele | Increased intra-neuronal accumulation of Aβ and plaque deposition | [56] |
| Microtubule associated protein tau (MAPT) | Assembly and stabilization of microtubules | Missense | Accelerates the neurodegenerative process in AD | [54] |
| Bridging integrator 1 (BIN1) | Membrane tubulation, endocytosis and intracellular endosome trafficking | Missense/nonsense | Intracellular beta-amyloid accumulation and early endosome enlargement | [62] |
| Siglec-3 (sialic acid binding Ig-like lectin 3) (CD33) | Negative regulation of cytokine production | Missense/nonsense | Decreased Aβ42 uptake and increased expression of full-length CD33 and TREM2 in monocytes | [65] |
| Complement receptor 1 (CR1) | Missense/nonsense, gross insertions or duplications | Aβ accumulation in brain | [66] | |
| Phosphatidylinositol-binding clathrin-assembly protein (PICALM) | Clathrin-mediated endocytosis, membrane repair of synaptic vesicles | Missense/nonsense, regulatory | Aβ production and clearance, tau-mediated neurodegeneration | [70] |
| ATP-Binding Cassette Transporter 7 (ABCA7) | Lipid transport and immune responses | Missense/nonsense | Impaired clearance of amyloid-beta | [74] |
| Membrane-spanning 4-Domains A4A, A4E, and A6E, respectively (MS4A4A, MS4A4E, and MS4A6E) | Proteins with four or more transmembrane domains | Missense/nonsense | Disrupted clearance process, amyloid plaque buildup, and increased neuroinflammation | [78] |
| Sortilin-related Receptor 1 (sorLA) | Type 1 transmembrane protein involved in regulating APP intracellular trafficking and processing | Missense/nonsense | Truncating mutations are shown to be highly pathogenic | [80] |
| Zinc finger CW-type PWWP domain protein 1 (ZCWPW1) | Modulates epigenetic regulation | Point mutations | Protective and risk effect depending on population background | [85] |
| Disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) | α-secretase that cleaves APP in the non-amyloidogenic pathway | Missense/nonsense | Can lead to age-related downregulation of α-secretase | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stancheva, M.; Toncheva, D.; Karachanak-Yankova, S. Recent Advances and Future Directions in Alzheimer’s Disease Genetic Research. Int. J. Mol. Sci. 2025, 26, 7819. https://doi.org/10.3390/ijms26167819
Stancheva M, Toncheva D, Karachanak-Yankova S. Recent Advances and Future Directions in Alzheimer’s Disease Genetic Research. International Journal of Molecular Sciences. 2025; 26(16):7819. https://doi.org/10.3390/ijms26167819
Chicago/Turabian StyleStancheva, Mikaela, Draga Toncheva, and Sena Karachanak-Yankova. 2025. "Recent Advances and Future Directions in Alzheimer’s Disease Genetic Research" International Journal of Molecular Sciences 26, no. 16: 7819. https://doi.org/10.3390/ijms26167819
APA StyleStancheva, M., Toncheva, D., & Karachanak-Yankova, S. (2025). Recent Advances and Future Directions in Alzheimer’s Disease Genetic Research. International Journal of Molecular Sciences, 26(16), 7819. https://doi.org/10.3390/ijms26167819







