Identification of Key Differentially Expressed Genes in Arabidopsis thaliana Under Short- and Long-Term High Light Stress
Abstract
1. Introduction
- The identification of differentially expressed genes (DEGs) is inherently probabilistic and may yield false positives, introducing uncertainty into the interpretation of results.
- The authors used diverse bioinformatics pipelines, software tools, and statistical thresholds for RNA-seq data processing, which complicates direct comparisons between studies.
- The individual studies employed different protocols for plant cultivation and stress exposure, and the biological materials analyzed (e.g., plant age, tissue type) also varied.
2. Results and Discussion
2.1. The Initial Step of the AraLightMeta Analysis
2.1.1. Classification of Experimental Conditions
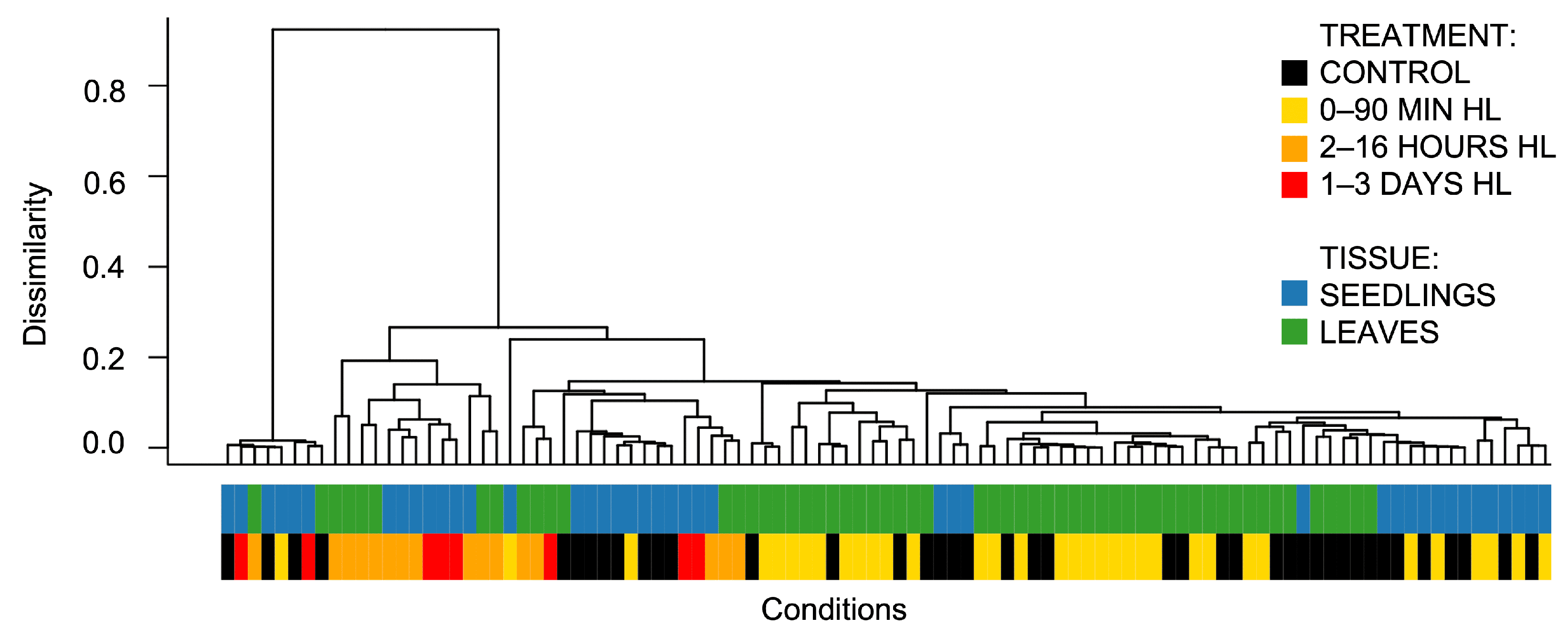
- Focusing on the main analysis of leaf tissue. This dataset comprises twice as many experiments as those involving seedlings and is more balanced in terms of average plant age. In particular, 75% of the included experiments used plants in a mature developmental stage (21–30 days after germination, Figure 2B). This significantly reduces the batch effect associated with early developmental transitions.
- To perform the primary grouping of experimental conditions based on high light treatment duration, two groups were defined: short-term (up to 90 min) and long-term (2 h to 3 days). This classification reflects the observed transcriptomic patterns: responses to medium- and long-term HL (≥2 h) show high consistency during clustering, while short-term responses (≤90 min) exhibit heterogeneity and limited separation from control samples.
- Identification of consistent DEGs-specific regulations. Rather than relying on fold changes in individual experiments, our meta-analysis revealed DEGs with frequent upregulation and downregulation across multiple independent experiments.
- Exclusion of outlier datasets. The experiments PRJNA699408 and GSE251796 showed pronounced batch effects (Figure 1) and were removed from further analysis.
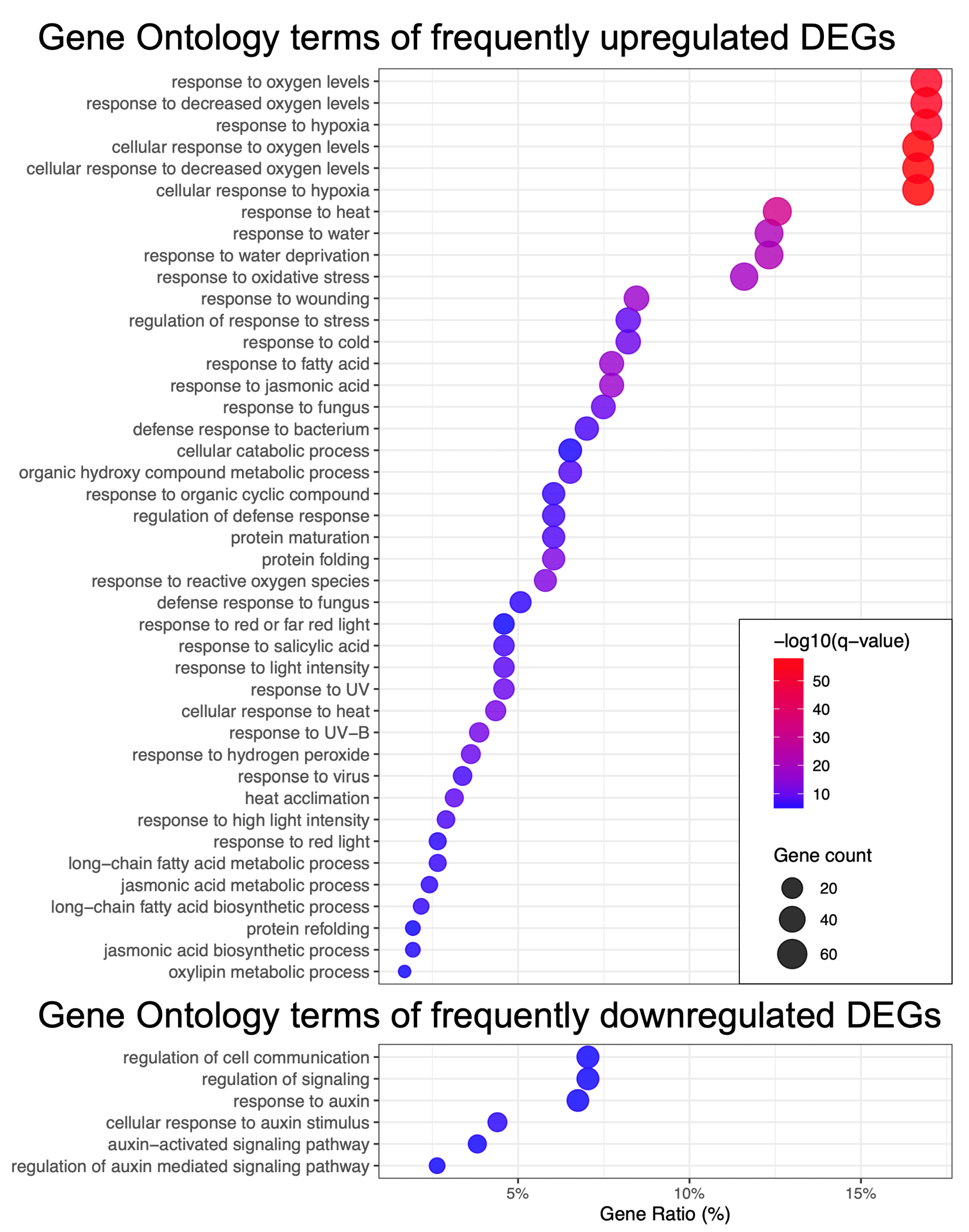
2.1.2. Functional Analysis of Most Frequent DEGs
2.2. The Second Step of the AraLightMeta Analysis
2.2.1. Identification of Short-Term and Long-Term Specific DEGs
2.2.2. Regulation of Key Pathways Related to Long-Term High Light Response
2.3. The Third Step of the AraLightMeta Analysis
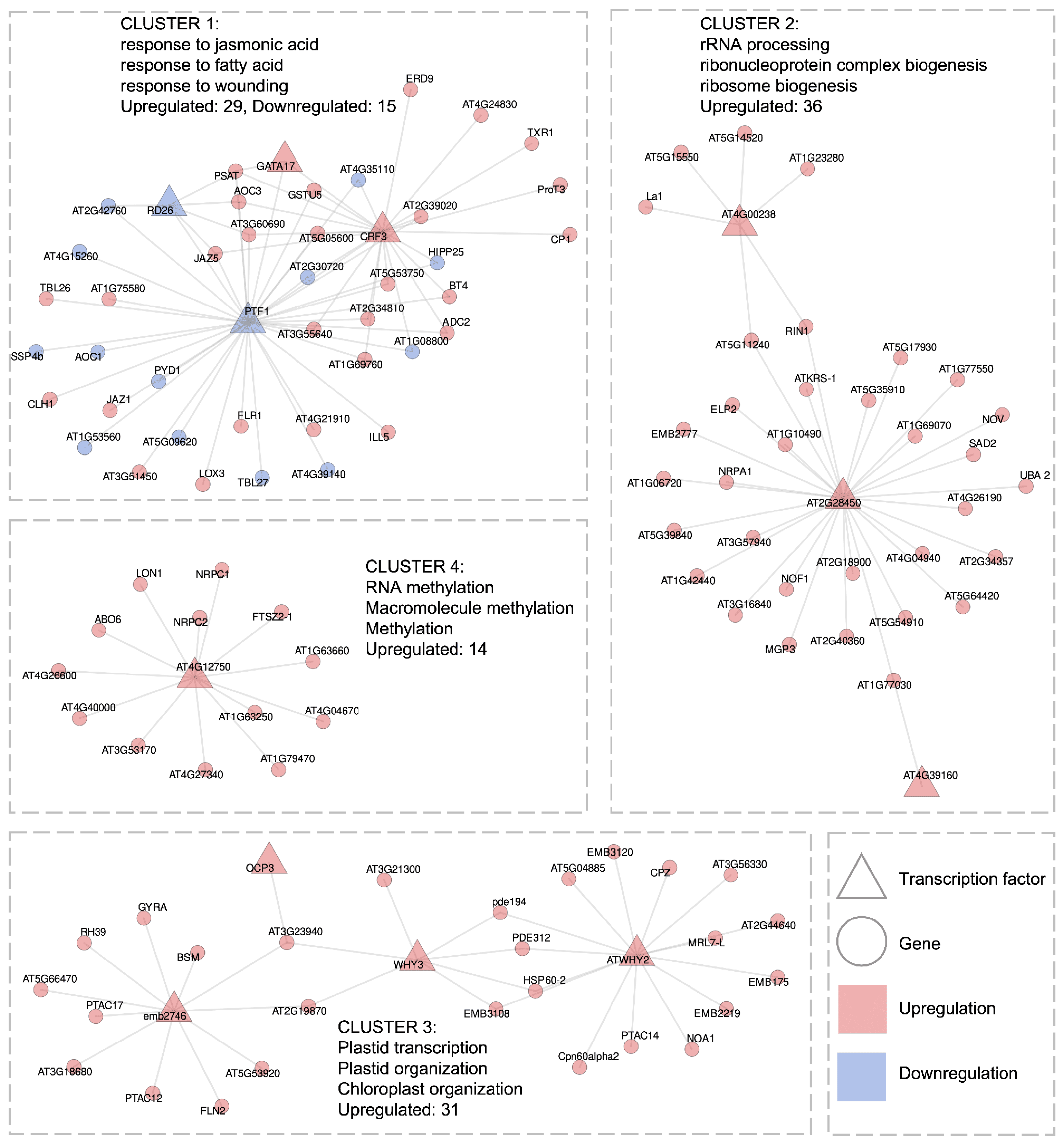
2.3.1. Gene Regulatory Network of Long-Term High Light Response
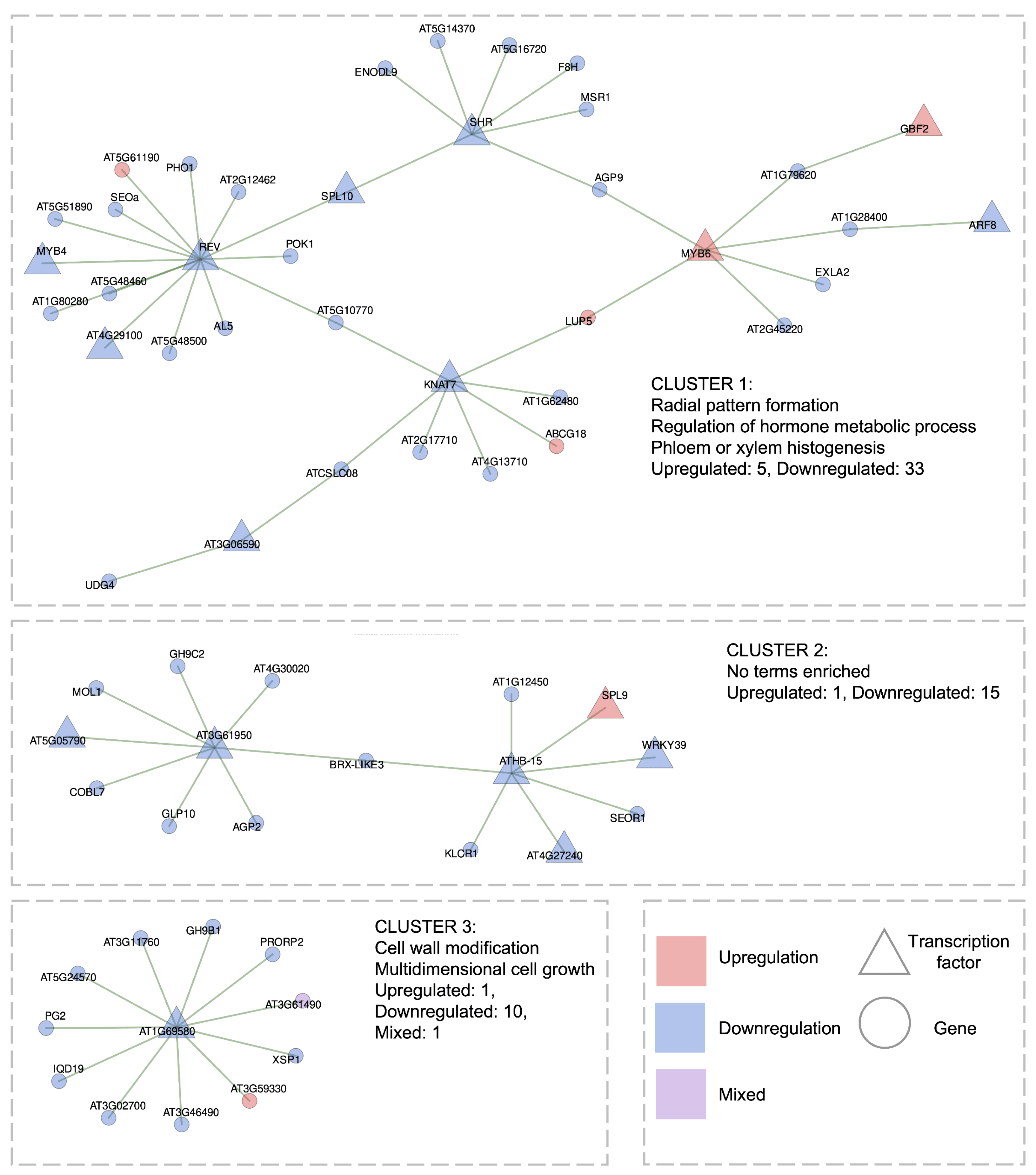
2.3.2. Gene Regulatory Network of Short-Term High Light Response
2.4. Conceptual Scheme of the Transcriptional Response of A. thaliana Leaves to Long-Term High Light
- Shared regulation of glutathione S-transferases and jasmonic acid pathway genes: We predict, for the first time, that two GSTs (GSTU5, GSTU17) are co-regulated with seven JA-response genes (JAZ1, JAZ5, LOX3, and others) via the upregulated TF CRF3 and the downregulated PTF1, which could act antagonistically (GRN Cluster 1, Figure 6). This raises Q1: whether other upregulated GSTs indirectly associate with the JA pathway.
- Ribosome biogenesis regulation: TF AT2G28450 is predicted to regulate ribosome biogenesis genes, which are consistently upregulated during long-term HL stress (GRN Cluster 2, Figure 6).
- Chloroplast–mitochondrial interplay: We revealed HL-induced upregulation of organelle-specific genes, mediated by TFs ATWHY2, WHY3, and emb2746, with the latter being shown for the first time to have this functional role (GRN Cluster 3, Figure 6).
- Methyltransferase upregulation: AT4G04670, AT4G27340, AT4G40000 are consistently upregulated, and potentially controlled by TF AT4G12750 (GRN Cluster 4, Figure 6).
2.5. Main Findings of Transcriptomic Response of Seedlings to High Light
3. Materials and Methods
3.1. Search and Selection of Relevant Transcriptomic Experiments for Analysis
- The organism studied was A. thaliana, genotype Columbia (Col-0);
- The experiments analyzed transcriptomes of photosynthetic tissues;
- The stress condition involved exposure to excess light, with a minimum light intensity threshold of 500 µmol· up to 2000 µmol· and up to five days of exposure duration;
- Each dataset included control groups of plants grown under normal light conditions, defined as light intensities ranging from 50 to 150 µmol .
3.2. Preliminary Data Analysis and Quality Control
3.3. Identification of Differentially Expressed Genes in Individual Experimental Conditions and Classification of Transcriptomic Datasets
- Read count matrices (from Section 3.2) were loaded from CSV files, including the first column (gene identifiers) and the first row (sample names). An experimental design vector was created, specifying the treatment conditions: control libraries (photosynthetic tissues under non-stress conditions) and stress-induced libraries (photosynthetic tissues exposed to excess light; light intensity ≥ 500 µmol·.
- A DGEList object was created, containing the count matrix and the experimental design vector for downstream analysis.
- Genes with low expression were filtered out using a threshold of in more than half of the samples.
- Normalization was performed using the TMM (trimmed mean of M-values) method via the calcNormFactors function.
- Differential expression analysis was conducted using the exactTest function, comparing control and excess light conditions. False discovery rate (FDR) correction was applied using the Benjamini–Hochberg method. The following thresholds were used to identify DEGs:
- Upregulated: FDR ≤ 0.05 and log2FC ≥ 0.5;
- Downregulated: FDR ≤ 0.05 and log2FC ≤ –0.5.
Two comparison strategies were applied depending on the availability of time-matched controls:- I
- For six experiments with time-matched controls (GSE111062, GSE117298, GSE137650, GSE138196, GSE251796, PRJNA817005), statistical comparisons were made between paired time points (e.g., HL stress at 6 h vs. control at 6 h; HL stress at 12 h vs. control at 12 h, etc.).
- II
- For the remaining 15 experiments with a single control group, all stress-exposed samples were compared against the common control (e.g., HL stress at 6 h vs. control; HL stress at 12 h vs. control, etc.), i.e., all time points of light stress were analyzed relative to the same baseline.
3.4. Construction of the AraLightDEGs Knowledge Base for High Light-Responsive DEGs
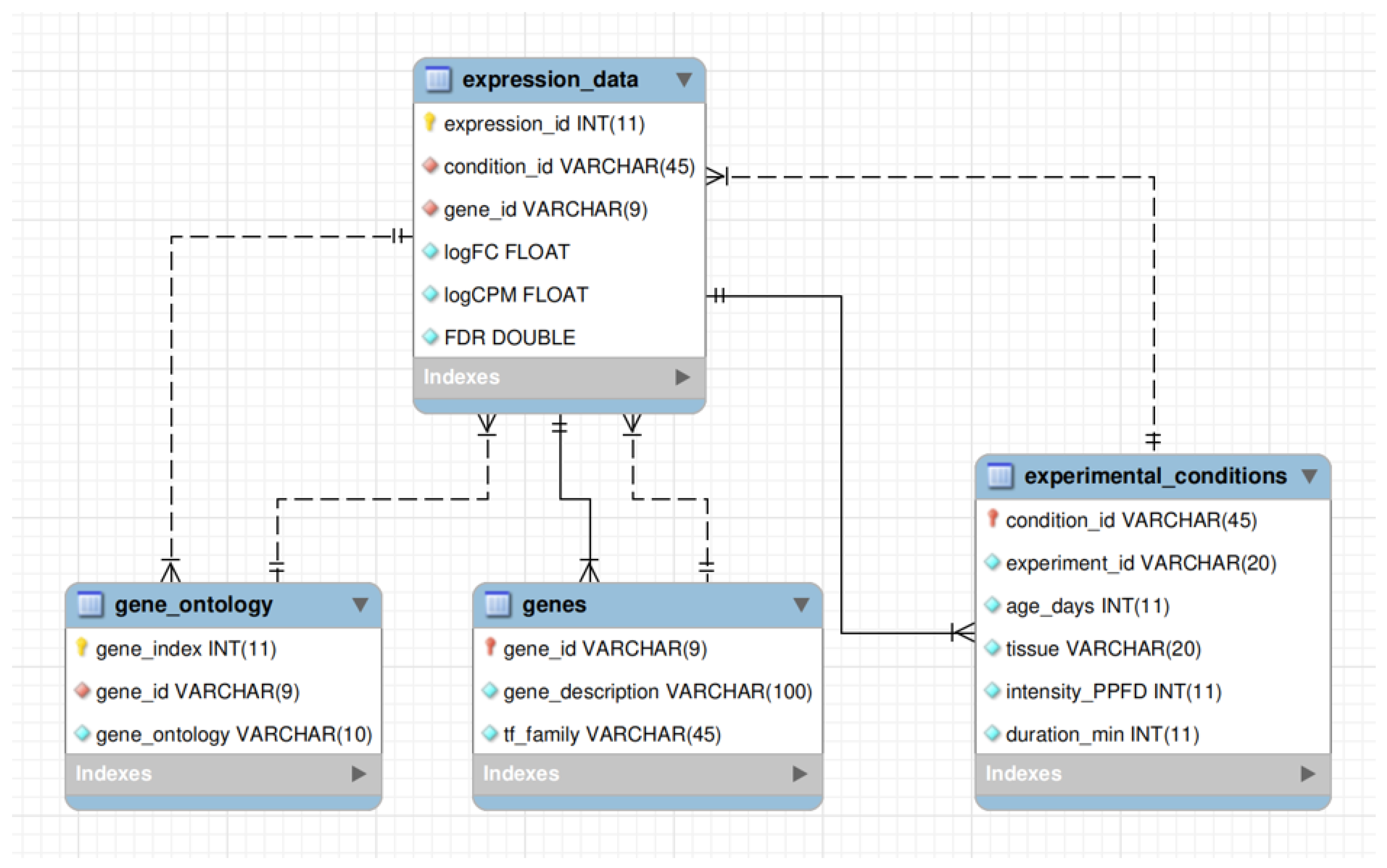
3.4.1. Structure of the Database
- expression_id is the primary key; a sequential identifier of each record.
- condition_id is the identifier of the excess light condition, representing a unique combination of key experimental details (e.g., GSE132626_LEAF_15_500_1_hour).
- gene_id is the gene identifier in TAIR10 format (e.g., AT1G01010).
- logFC is the fold change in gene expression under excess light conditions relative to the control.
- logCPM is the average gene expression in counts-per-million units across the experiment to which the condition belongs.
- FDR is the false discovery rate-adjusted p-value, calculated using the Benjamini–Hochberg procedure, indicating the statistical significance of the DEG.
- gene_index is the primary key; a sequential identifier of the record.
- gene_id is the gene identifier in TAIR10 format (e.g., AT1G01010).
- gene_ontology is the GO term associated with the corresponding gene.
- condition_id is the primary key, identical to the field of the same name in the expression_data table; it represents a meaningful combination of the remaining five fields.
- experiment_id is the identifier of the corresponding experiment (in GEO or BioProject NCBI format).
- age_days is plant age in days at the time of sampling.
- tissue is the tissue type analyzed (either leaf or seedling).
- intensity_PPFD is the intensity of excess light in µmol .
- duration_min is the duration of excess light exposure in minutes.
3.4.2. Implementation of the Server-Side and User Interface Components
- Filters by experimental conditions (light intensity and exposure duration, tissue type, and plant age).
- Filters by DEG identification frequency and expression levels.
- Filters by TAIR10 gene identifiers, GO terms, or selection of TFs only.
- Query predicted protein–protein interactions via the STRING database v12.0 [103] (https://string-db.org/, accessed on 19 January 2025), implemented via the STRING API (https://string-db.org/help/api/, accessed on 19 January 2025).
- Download a line-by-line list of DEG identifiers for downstream analysis outside the knowledge base.
3.5. Development of Transcriptomic Meta-Analysis Pipeline AraLightMeta
- Clustering and classification of experimental conditions. This includes GO analysis of the most frequently identified DEGs in the AraLightDEGs database.
- Selection and classification of the most relevant DEGs associated with short- and long-term high light responses, distinguishing between upregulated and downregulated genes.
- Coexpression and machine learning analysis aimed at reconstructing GRNs associated with specific stress conditions.
3.5.1. Needed Packages and Input Data Structure
- aralightdegs_counts_metadata.csv: This file contains raw count matrices across all experimental conditions (280 in total), along with metadata such as tissue type, light intensity, duration of exposure, and experimental ID. It is utilized for data clustering and classification (Step 1) and for GRN reconstruction (Step 3).
- aralightdegs_all_degs.csv: A precomputed table of DEGs derived from the AraLightDEGs database, containing gene IDs, descriptions, TF family annotations, up/downregulation statistics, fold changes, and lists of experiments in which up- or downregulation was detected. The table was generated from the AraLightDEGs database (https://www.sysbio.ru/aralightdegs/search, accessed on 16 July 2025) using the following parameters:
- Remove outlier stress conditions from analysis.
- Minimum light intensity: 500 µmol·.
- Maximum light intensity: 2000 µmol·.
- Tissue types: Leaves and seedlings.
- Minimum plant age: 5 days.
- Maximum plant age: 49 days.
- Minimum duration of high light treatment: 0 min.
- Maximum duration of high light treatment: 7200 min.
- Minimum frequency threshold (total number of DEG identifications across selected experimental conditions): 1.
- Minimum average expression (in units): 2.
Essentially, these parameters correspond to the following query to the database: Retrieve all available DEGs except those from the two outlier experiments. The result table can be regenerated with alternative parameters if required.The downloadable result table includes the following:- Gene descriptions.
- TF family annotations.
- Lists of experimental conditions showing upregulation.
- Lists of experimental conditions showing downregulation.
- Lists of values for conditions with upregulation.
- Lists of values for conditions with downregulation.
This dataset is used in all stages of the analysis: for GO enrichment of the most frequent DEGs (Step 1), for classifying DEGs into short- and long-term responses in both seedlings and leaves (Step 2), and for adding metadata to GRNs (Step 3). - ATH_GO_GOSLIM.txt: A file containing A. thaliana GO terms describing biological processes, used for parsing the most relevant DEGs and generating heatmaps in Step 2.
- Four RDS files containing permutation-tested edges generated by the test_edges function of the DIANE package: edges_test_(long/short)_(leaves/seedlings).rds. These files are derived from GENIE3 weighted matrices computed separately for seedlings and leaves under short- and long-term HL-specific conditions. Inclusion of these precomputed files helps to bypass the computationally intensive step, which otherwise requires at least 128 GB of RAM. However, users can opt to recompute this step within the main pipeline if desired.
3.5.2. First Step of AraLightMeta: Experiments Classification and Frequent Differentially Expressed Genes Analysis
3.5.3. Second Step of AraLightMeta: Differentially Expressed Genes Classification, Functional Annotation, and Visualization
3.5.4. Third Step of AraLightMeta: Gene Regulatory Networks Reconstruction
- For long-term HL response in leaves, the correlation threshold is set to 0.3 (weak coexpression);
- For short-term HL leaves and long-term HL seedlings, the threshold is set to 0.5 (strong coexpression);
- For short-term HL seedlings, the threshold is set to 0.6 (strong coexpression).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPM | Counts-per-million |
| DEG(s) | Differentially expressed gene(s) |
| FC | Fold change |
| FDR | False discovery rate |
| GO | Gene Ontology |
| GST(s) | Glutathione S-transferase(s) |
| GRN(s) | Gene regulatory network(s) |
| HL | High light |
| JA | Jasmonic acid |
| ROS | Reactive oxygen species |
| TF(s) | Transcription factor(s) |
| WGCNA | Weighted gene coexpression network analysis |
References
- Didaran, F.; Kordrostami, M.; Ghasemi-Soloklui, A.A.; Pashkovskiy, P.; Kreslavski, V.; Kuznetsov, V.; Allakhverdiev, S.I. The mechanisms of photoinhibition and repair in plants under high light conditions and interplay with abiotic stressors. J. Photochem. Photobiol. Biol. 2024, 259, 113004. [Google Scholar] [CrossRef]
- Li, M.; Kim, C. Chloroplast ROS and stress signaling. Plant Commun. 2022, 3, 100264. [Google Scholar] [CrossRef]
- Brunetti, C.; Guidi, L.; Sebastiani, F.; Tattini, M. Isoprenoids and phenylpropanoids are key components of the antioxidant defense system of plants facing severe excess light stress. Environ. Exp. Bot. 2015, 119, 54–62. [Google Scholar] [CrossRef]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Gotoh, E.; Suetsugu, N.; Yamori, W.; Ishishita, K.; Kiyabu, R.; Fukuda, M.; Higa, T.; Shirouchi, B.; Wada, M. Chloroplast accumulation response enhances leaf photosynthesis and plant biomass production. Plant Physiol. 2018, 178, 1358–1369. [Google Scholar] [CrossRef]
- Kulheim, C.; Ågren, J.; Jansson, S. Rapid regulation of light harvesting and plant fitness in the field. Science 2002, 297, 91–93. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, X.; Chory, J. The Arabidopsis transcriptome responds specifically and dynamically to high light stress. Cell Rep. 2019, 29, 4186–4199. [Google Scholar] [CrossRef] [PubMed]
- Schröder, P.; Hsu, B.Y.; Gutsche, N.; Winkler, J.B.; Hedtke, B.; Grimm, B.; Schwechheimer, C. B-GATA factors are required to repress high-light stress responses in Marchantia polymorpha and Arabidopsis thaliana. Plant Cell Environ. 2023, 46, 2376–2390. [Google Scholar] [CrossRef] [PubMed]
- Arend, M.; Yuan, Y.; Ruiz-Sola, M.Á.; Omranian, N.; Nikoloski, Z.; Petroutsos, D. Widening the landscape of transcriptional regulation of green algal photoprotection. Nat. Commun. 2023, 14, 2687. [Google Scholar] [CrossRef]
- Balfagón, D.; Zandalinas, S.I.; dos Reis de Oliveira, T.; Santa-Catarina, C.; Gómez-Cadenas, A. Reduction of heat stress pressure and activation of photosystem II repairing system are crucial for citrus tolerance to multiple abiotic stress combination. Physiol. Plant. 2022, 174, e13809. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Sengupta, S.; Burks, D.; Azad, R.K.; Mittler, R. Identification and characterization of a core set of ROS wave-associated transcripts involved in the systemic acquired acclimation response of Arabidopsis to excess light. Plant J. 2019, 98, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic signaling during abiotic stress combination in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13810–13820. [Google Scholar] [CrossRef]
- Fichman, Y.; Zandalinas, S.I.; Sengupta, S.; Burks, D.; Myers Jr, R.J.; Azad, R.K.; Mittler, R. MYB30 orchestrates systemic reactive oxygen signaling and plant acclimation. Plant Physiol. 2020, 184, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Sengupta, S.; Fritschi, F.B.; Azad, R.K.; Nechushtai, R.; Mittler, R. The impact of multifactorial stress combination on plant growth and survival. New Phytol. 2021, 230, 1034–1048. [Google Scholar] [CrossRef]
- Balfagón, D.; Sengupta, S.; Gómez-Cadenas, A.; Fritschi, F.B.; Azad, R.K.; Mittler, R.; Zandalinas, S.I. Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol. 2019, 181, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, K.; Veluchamy, A.; Fatima, A.; García-Ramírez, G.X.; Reichheld, J.P.; Artyukh, O.; Fröhlich, K.; Polussa, A.; Parween, S.; Nagarajan, A.P.; et al. Microbe-induced coordination of plant iron–sulfur metabolism enhances high-light-stress tolerance of Arabidopsis. Plant Commun. 2024, 5, 101012. [Google Scholar] [CrossRef]
- Araguirang, G.E.; Venn, B.; Kelber, N.M.; Feil, R.; Lunn, J.; Kleine, T.; Leister, D.; Mühlhaus, T.; Richter, A.S. Spliceosomal complex components are critical for adjusting the C: N balance during high-light acclimation. Plant J. 2024, 119, 153–175. [Google Scholar] [CrossRef]
- Balfagón, D.; Pascual, L.S.; Sengupta, S.; Halliday, K.J.; Gómez-Cadenas, A.; Peláez-Vico, M.Á.; Sinha, R.; Mittler, R.; Zandalinas, S.I. WRKY48 negatively regulates plant acclimation to a combination of high light and heat stress. Plant J. 2024, 117, 1642–1655. [Google Scholar] [CrossRef]
- Bobrovskikh, A.V.; Zubairova, U.S.; Bondar, E.I.; Lavrekha, V.V.; Doroshkov, A.V. Transcriptomic data meta-analysis sheds light on high light response in Arabidopsis thaliana L. Int. J. Mol. Sci. 2022, 23, 4455. [Google Scholar] [CrossRef]
- Oliwa, J.; Skoczowski, A. Different response of photosynthetic apparatus to high-light stress in sporotrophophyll and nest leaves of Platycerium bifurcatum. Photosynthetica 2019, 57, 147–159. [Google Scholar] [CrossRef]
- Lin, C.W.; Huang, L.Y.; Huang, C.L.; Wang, Y.C.; Lai, P.H.; Wang, H.V.; Chang, W.C.; Chiang, T.Y.; Huang, H.J. Common stress transcriptome analysis reveals functional and genomic architecture differences between early and delayed response genes. Plant Cell Physiol. 2017, 58, 546–559. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Meng, K.; Thomas, H.R.; Yang, Y.; Williams, B.; Kang, H.; Zhou, Y. Reframing agriculture by light: The role of light-mediated jasmonates/salicylic acid regulation in plant defense, development and beyond. Veg. Res. 2024, 4, e027. [Google Scholar] [CrossRef]
- Liu, J.X.; Howell, S.H. Managing the protein folding demands in the endoplasmic reticulum of plants. New Phytol. 2016, 211, 418–428. [Google Scholar] [CrossRef]
- Tivendale, N.D.; Millar, A.H. How is auxin linked with cellular energy pathways to promote growth? New Phytol. 2022, 233, 2397–2404. [Google Scholar] [CrossRef]
- Kasahara, M.; Kagawa, T.; Oikawa, K.; Suetsugu, N.; Miyao, M.; Wada, M. Chloroplast avoidance movement reduces photodamage in plants. Nature 2002, 420, 829–832. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef]
- Sharma, N.; Nagar, S.; Thakur, M.; Suriyakumar, P.; Kataria, S.; Shanker, A.; Landi, M.; Anand, A. Photosystems under high light stress: Throwing light on mechanism and adaptation. Photosynthetica 2023, 61, 250. [Google Scholar] [CrossRef]
- Kirchhoff, H. Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Philos. Trans. R. Soc. Biol. Sci. 2014, 369, 20130225. [Google Scholar] [CrossRef]
- Garcia-Molina, A.; Kleine, T.; Schneider, K.; Mühlhaus, T.; Lehmann, M.; Leister, D. Translational components contribute to acclimation responses to high light, heat, and cold in Arabidopsis. iScience 2020, 23, 101331. [Google Scholar] [CrossRef]
- Zhang, M.; Zeng, Y.; Peng, R.; Dong, J.; Lan, Y.; Duan, S.; Chang, Z.; Ren, J.; Luo, G.; Liu, B.; et al. N6-methyladenosine RNA modification regulates photosynthesis during photodamage in plants. Nat. Commun. 2022, 13, 7441. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, L.; Chen, D.; Gan, T.; Cao, M.; Luo, J. Alterations of amino acid concentrations and photosynthetic indices in light irradiated Arabidopsis thaliana during phytoextraction. Sustainability 2021, 13, 7720. [Google Scholar] [CrossRef]
- Griffin, J.H.; Toledo-Ortiz, G. Plant photoreceptors and their signalling components in chloroplastic anterograde and retrograde communication. J. Exp. Bot. 2022, 73, 7126–7138. [Google Scholar] [CrossRef]
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N. ROS-dependent signalling pathways in plants and algae exposed to high light: Comparisons with other eukaryotes. Free Radic. Biol. Med. 2018, 122, 52–64. [Google Scholar] [CrossRef]
- Huq, E.; Lin, C.; Quail, P.H. Light signaling in plants—A selective history. Plant Physiol. 2024, 195, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Bobrovskikh, A.; Zubairova, U.; Kolodkin, A.; Doroshkov, A. Subcellular compartmentalization of the plant antioxidant system: An integrated overview. PeerJ 2020, 8, e9451. [Google Scholar] [CrossRef]
- Noshi, M.; Yamada, H.; Hatanaka, R.; Tanabe, N.; Tamoi, M.; Shigeoka, S. Arabidopsis dehydroascorbate reductase 1 and 2 modulate redox states of ascorbate-glutathione cycle in the cytosol in response to photooxidative stress. Biosci. Biotechnol. Biochem. 2017, 81, 523–533. [Google Scholar] [CrossRef]
- Attia, H.; Karray, N.; Lachaâl, M. Light interacts with salt stress in regulating superoxide dismutase gene expression in Arabidopsis. Plant Sci. 2009, 177, 161–167. [Google Scholar] [CrossRef]
- Maruta, T.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2010, 51, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Gallé, Á.; Czékus, Z.; Bela, K.; Horváth, E.; Ördög, A.; Csiszár, J.; Poór, P. Plant glutathione transferases and light. Front. Plant Sci. 2019, 9, 1944. [Google Scholar] [CrossRef]
- Nikkanen, L.; Rintamäki, E. Thioredoxin-dependent regulatory networks in chloroplasts under fluctuating light conditions. Philos. Trans. R. Soc. Biol. Sci. 2014, 369, 20130224. [Google Scholar] [CrossRef]
- Laporte, D.; Olate, E.; Salinas, P.; Salazar, M.; Jordana, X.; Holuigue, L. Glutaredoxin GRXS13 plays a key role in protection against photooxidative stress in Arabidopsis. J. Exp. Bot. 2012, 63, 503–515. [Google Scholar] [CrossRef]
- Rodriguez-Heredia, M.; Saccon, F.; Wilson, S.; Finazzi, G.; Ruban, A.V.; Hanke, G.T. Protection of photosystem I during sudden light stress depends on ferredoxin: NADP (H) reductase abundance and interactions. Plant Physiol. 2022, 188, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Doroshkov, A.; Bobrovskikh, A. Using the methods of systems biology for predicting perspective target genes to select C3 and C4 cereals for oxidative stress resistance. Vavilov J. Genet. Breed. 2018, 22, 122–131. [Google Scholar] [CrossRef]
- Floris, M.; Bassi, R.; Robaglia, C.; Alboresi, A.; Lanet, E. Post-transcriptional control of light-harvesting genes expression under light stress. Plant Mol. Biol. 2013, 82, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, L.; Last, R.L.; Hua, W.; Liu, J. Psb28 protein is indispensable for stable accumulation of PSII core complexes in Arabidopsis. Plant J. 2024, 119, 1226–1238. [Google Scholar] [CrossRef]
- Song, Z.; Bian, Y.; Liu, J.; Sun, Y.; Xu, D. B-box proteins: Pivotal players in light-mediated development in plants. J. Integr. Plant Biol. 2020, 62, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Song, Z.; Liu, C.; Song, Z.; Dong, J.; Xu, D. The BBX7/8-CCA1/LHY transcription factor cascade promotes shade avoidance by activating PIF4. New Phytol. 2025, 245, 637–652. [Google Scholar] [CrossRef]
- Rahul, P.V.; Yadukrishnan, P.; Sasidharan, A.; Datta, S. The B-box protein BBX13/COL15 suppresses photoperiodic flowering by attenuating the action of CONSTANS in Arabidopsis. Plant Cell Environ. 2024, 47, 5358–5371. [Google Scholar] [CrossRef]
- Yadav, A.; Ravindran, N.; Singh, D.; Rahul, P.V.; Datta, S. Role of Arabidopsis BBX proteins in light signaling. J. Plant Biochem. Biotechnol. 2020, 29, 623–635. [Google Scholar] [CrossRef]
- Song, Z.; Yan, T.; Liu, J.; Bian, Y.; Heng, Y.; Lin, F.; Jiang, Y.; Wang Deng, X.; Xu, D. BBX28/BBX29, HY5 and BBX30/31 form a feedback loop to fine-tune photomorphogenic development. Plant J. 2020, 104, 377–390. [Google Scholar] [CrossRef]
- Alvarez-Fernandez, R.; Penfold, C.A.; Galvez-Valdivieso, G.; Exposito-Rodriguez, M.; Stallard, E.J.; Bowden, L.; Moore, J.D.; Mead, A.; Davey, P.A.; Matthews, J.S.; et al. Time-series transcriptomics reveals a BBX32-directed control of acclimation to high light in mature Arabidopsis leaves. Plant J. 2021, 107, 1363–1386. [Google Scholar] [CrossRef]
- Atanasov, V.; Schumacher, J.; Muino, J.M.; Larasati, C.; Wang, L.; Kaufmann, K.; Leister, D.; Kleine, T. Arabidopsis BBX14 is involved in high light acclimation and seedling development. Plant J. 2024, 118, 141–158. [Google Scholar] [CrossRef]
- Zhao, X.; Heng, Y.; Wang, X.; Deng, X.W.; Xu, D. A positive feedback loop of BBX11–BBX21–HY5 promotes photomorphogenic development in Arabidopsis. Plant Commun. 2020, 1, 100045. [Google Scholar] [CrossRef] [PubMed]
- Balcerowicz, M.; Mahjoub, M.; Nguyen, D.; Lan, H.; Stoeckle, D.; Conde, S.; Jaeger, K.E.; Wigge, P.A.; Ezer, D. An early-morning gene network controlled by phytochromes and cryptochromes regulates photomorphogenesis pathways in Arabidopsis. Mol. Plant 2021, 14, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Won, J.H.; Park, J.; Lee, H.G.; Shim, S.; Lee, H.; Oh, E.; Seo, P.J. The PRR–EC complex and SWR1 chromatin remodeling complex function cooperatively to repress nighttime hypocotyl elongation by modulating PIF4 expression in Arabidopsis. Plant Commun. 2024, 5, 100981. [Google Scholar] [CrossRef]
- Sharma, A.; Pridgeon, A.J.; Liu, W.; Segers, F.; Sharma, B.; Jenkins, G.I.; Franklin, K.A. ELONGATED HYPOCOTYL5 (HY5) and HY5 HOMOLOGUE (HYH) maintain shade avoidance suppression in UV-B. Plant J. 2023, 115, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Y.; Huai, J.; Jing, Y.; Lin, R. The RNA helicase UAP56 and the E3 ubiquitin ligase COP1 coordinately regulate alternative splicing to repress photomorphogenesis in Arabidopsis. Plant Cell 2022, 34, 4191–4212. [Google Scholar] [CrossRef]
- Qin, N.; Xu, D.; Li, J.; Deng, X.W. COP9 signalosome: Discovery, conservation, activity, and function. J. Integr. Plant Biol. 2020, 62, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Enderle, B.; Sheerin, D.J.; Paik, I.; Kathare, P.K.; Schwenk, P.; Klose, C.; Ulbrich, M.H.; Huq, E.; Hiltbrunner, A. PCH1 and PCHL promote photomorphogenesis in plants by controlling phytochrome B dark reversion. Nat. Commun. 2017, 8, 2221. [Google Scholar] [CrossRef]
- Cho, J.N.; Ryu, J.Y.; Jeong, Y.M.; Park, J.; Song, J.J.; Amasino, R.M.; Noh, B.; Noh, Y.S. Control of seed germination by light-induced histone arginine demethylation activity. Dev. Cell 2012, 22, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Johansson, H.; Hettiarachchi, C.; Irigoyen, M.L.; Desai, M.; Rubio, V.; Holm, M. LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant Cell 2008, 20, 2324–2338. [Google Scholar] [CrossRef]
- Peng, H.; Phung, J.; Zhai, Y.; Neff, M.M. Self-transcriptional repression of the Arabidopsis NAC transcription factor ATAF2 and its genetic interaction with phytochrome A in modulating seedling photomorphogenesis. Planta 2020, 252, 48. [Google Scholar] [CrossRef]
- Favero, D.S. Leaf Position Makes a Difference: The ABCB19 Auxin Transporter Affects Light Perception. Plant Physiol. 2020, 184, 1219–1220. [Google Scholar] [CrossRef]
- López-García, C.M.; Ruíz-Herrera, L.F.; López-Bucio, J.S.; Huerta-Venegas, P.I.; Peña-Uribe, C.A.; de la Cruz, H.R.; López-Bucio, J. ALTERED MERISTEM PROGRAM 1 promotes growth and biomass accumulation influencing guard cell aperture and photosynthetic efficiency in Arabidopsis. Protoplasma 2020, 257, 573–582. [Google Scholar] [CrossRef]
- Yan, J.; Kim, Y.J.; Somers, D.E. Post-translational mechanisms of plant circadian regulation. Genes 2021, 12, 325. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, Y.; Hu, Y.; Yang, Y.; Cai, J.; Liu, H.; Zhang, C.; Liu, X.; Hou, X. Arabidopsis NF-YCs play dual roles in repressing brassinosteroid biosynthesis and signaling during light-regulated hypocotyl elongation. Plant Cell 2021, 33, 2360–2374, Correction in Plant Cell 2021, 33, 3745. [Google Scholar] [CrossRef]
- Guo, Q.; Jing, Y.; Gao, Y.; Liu, Y.; Fang, X.; Lin, R. The PIF1/PIF3-MED25-HDA19 transcriptional repression complex regulates phytochrome signaling in Arabidopsis. New Phytol. 2023, 240, 1097–1115. [Google Scholar] [CrossRef] [PubMed]
- Patitaki, E.; Schivre, G.; Zioutopoulou, A.; Perrella, G.; Bourbousse, C.; Barneche, F.; Kaiserli, E. Light, chromatin, action: Nuclear events regulating light signaling in Arabidopsis. New Phytol. 2022, 236, 333–349. [Google Scholar] [CrossRef] [PubMed]
- de Silva, K.; Coelho, C.; Gao, J.; Brooks, M.D. Shining light on Arabidopsis regulatory networks integrating nitrogen use and photosynthesis. Plant J. 2025, 122, e70211. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Y.Y.; Liu, X.; Yang, S.; Lu, Q.; Cui, Y.; Wu, K. HD2C interacts with HDA6 and is involved in ABA and salt stress response in Arabidopsis. J. Exp. Bot. 2012, 63, 3297–3306. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Fan, K.; Li, Z.; Jia, Q.; Lin, W.; Zhang, Y. PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) negatively regulates anthocyanin accumulation by inhibiting PAP1 transcription in Arabidopsis seedlings. Plant Sci. 2021, 303, 110788. [Google Scholar] [CrossRef]
- Chao, L.M.; Liu, Y.Q.; Chen, D.Y.; Xue, X.Y.; Mao, Y.B.; Chen, X.Y. Arabidopsis transcription factors SPL1 and SPL12 confer plant thermotolerance at reproductive stage. Mol. Plant 2017, 10, 735–748. [Google Scholar] [CrossRef]
- Huang, D.; Lin, W.; Deng, B.; Ren, Y.; Miao, Y. Dual-located WHIRLY1 interacting with LHCA1 alters photochemical activities of photosystem I and is involved in light adaptation in Arabidopsis. Int. J. Mol. Sci. 2017, 18, 2352. [Google Scholar] [CrossRef] [PubMed]
- Leister, D.; Kleine, T. Definition of a core module for the nuclear retrograde response to altered organellar gene expression identifies GLK overexpressors as gun mutants. Physiol. Plant. 2016, 157, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Lee, J.; Akter, S.; Rogers, M.; Grene, R.; Li, S. Prediction of condition-specific regulatory genes using machine learning. Nucleic Acids Res. 2020, 48, e62. [Google Scholar] [CrossRef] [PubMed]
- Bobrovskikh, A.V.; Zubairova, U.S.; Naumenko, L.G.; Doroshkov, A.V. Catching the Big Fish in Big Data: A Meta-Analysis of Zebrafish Kidney scRNA-Seq Datasets Highlights Conserved Molecular Profiles of Macrophages and Neutrophils in Vertebrates. Biology 2024, 13, 773. [Google Scholar] [CrossRef]
- Chen, H.; Zou, W.; Zhao, J. Ribonuclease J is required for chloroplast and embryo development in Arabidopsis. J. Exp. Bot. 2015, 66, 2079–2091. [Google Scholar] [CrossRef]
- Shim, S.; Lee, H.G.; Seo, P.J. MET1-dependent DNA methylation represses light signaling and influences plant regeneration in Arabidopsis. Mol. Cells 2021, 44, 746–757. [Google Scholar] [CrossRef]
- Dannfald, A.; Favory, J.J.; Deragon, J.M. Variations in transfer and ribosomal RNA epitranscriptomic status can adapt eukaryote translation to changing physiological and environmental conditions. RNA Biol. 2021, 18, 4–18. [Google Scholar] [CrossRef]
- Yi, R.; Yan, J.; Xie, D. Light promotes jasmonate biosynthesis to regulate photomorphogenesis in Arabidopsis. Sci. China Life Sci. 2020, 63, 943–952. [Google Scholar] [CrossRef]
- Jiang, H.W.; Liu, M.J.; Chen, I.C.; Huang, C.H.; Chao, L.Y.; Hsieh, H.L. A glutathione S-transferase regulated by light and hormones participates in the modulation of Arabidopsis seedling development. Plant Physiol. 2010, 154, 1646–1658. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Bashir, T.; Hashem, A.; Abd_Allah, E.F.; Khan, A.L.; Al-Harrasi, A.S. Early events in plant abiotic stress signaling: Interplay between calcium, reactive oxygen species and phytohormones. J. Plant Growth Regul. 2018, 37, 1033–1049. [Google Scholar] [CrossRef]
- Trinh, M.D.L.; Masuda, S. Chloroplast pH homeostasis for the regulation of photosynthesis. Front. Plant Sci. 2022, 13, 919896. [Google Scholar] [CrossRef]
- Giese, J.; Eirich, J.; Walther, D.; Zhang, Y.; Lassowskat, I.; Fernie, A.R.; Elsässer, M.; Maurino, V.G.; Schwarzländer, M.; Finkemeier, I. The interplay of post-translational protein modifications in Arabidopsis leaves during photosynthesis induction. Plant J. 2023, 116, 1172–1193. [Google Scholar] [CrossRef]
- Minai, L.; Wostrikoff, K.; Wollman, F.A.; Choquet, Y. Chloroplast biogenesis of photosystem II cores involves a series of assembly-controlled steps that regulate translation. Plant Cell 2006, 18, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Bobrovskikh, A.; Doroshkov, A.; Mazzoleni, S.; Cartenì, F.; Giannino, F.; Zubairova, U. A sight on single-cell transcriptomics in plants through the prism of cell-based computational modeling approaches: Benefits and challenges for data analysis. Front. Genet. 2021, 12, 652974. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G. Plant responses to light. In Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–166. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [PubMed]
- Küpers, J.J.; van Gelderen, K.; Pierik, R. Location matters: Canopy light responses over spatial scales. Trends Plant Sci. 2018, 23, 865–873. [Google Scholar] [CrossRef]
- Suzuki, N.; Devireddy, A.R.; Inupakutika, M.A.; Baxter, A.; Miller, G.; Song, L.; Shulaev, E.; Azad, R.K.; Shulaev, V.; Mittler, R. Ultra-fast alterations in mRNA levels uncover multiple players in light stress acclimation in plants. Plant J. 2015, 84, 760–772. [Google Scholar] [CrossRef]
- Li, L.; Duncan, O.; Ganguly, D.R.; Lee, C.P.; Crisp, P.A.; Wijerathna-Yapa, A.; Salih, K.; Trösch, J.; Pogson, B.J.; Millar, A.H. Enzymes degraded under high light maintain proteostasis by transcriptional regulation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2022, 119, e2121362119. [Google Scholar] [CrossRef] [PubMed]
- Weise, S.E.; Liu, T.; Childs, K.L.; Preiser, A.L.; Katulski, H.M.; Perrin-Porzondek, C.; Sharkey, T.D. Transcriptional regulation of the glucose-6-phosphate/phosphate translocator 2 is related to carbon exchange across the chloroplast envelope. Front. Plant Sci. 2019, 10, 460290. [Google Scholar] [CrossRef]
- Smith, A.B.; Ganguly, D.R.; Moore, M.; Bowerman, A.F.; Janapala, Y.; Shirokikh, N.E.; Pogson, B.J.; Crisp, P.A. Dynamics of mRNA fate during light stress and recovery: From transcription to stability and translation. Plant J. 2024, 117, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Scherer, V.; Bellin, L.; Schwenkert, S.; Lehmann, M.; Rinne, J.; Witte, C.P.; Jahnke, K.; Richter, A.; Pruss, T.; Lau, A.; et al. Uracil phosphoribosyltransferase is required to establish a functional cytochrome b 6f complex. Plant J. 2024, 120, 1064–1078. [Google Scholar] [CrossRef]
- Kılıç, M.; Gollan, P.J.; Aro, E.M.; Rintamäki, E. Jasmonic acid signaling and glutathione coordinate plant recovery from high light stress. Plant Physiol. 2025, 197, kiaf143. [Google Scholar] [CrossRef]
- Barczak-Brzyżek, A.; Brzyżek, G.; Koter, M.; Siedlecka, E.; Gawroński, P.; Filipecki, M. Plastid retrograde regulation of miRNA expression in response to light stress. BMC Plant Biol. 2022, 22, 150. [Google Scholar] [CrossRef]
- Gawroński, P.; Enroth, C.; Kindgren, P.; Marquardt, S.; Karpiński, S.; Leister, D.; Jensen, P.E.; Vinther, J.; Scharff, L.B. Light-dependent translation change of Arabidopsis psbA correlates with RNA structure alterations at the translation initiation region. Cells 2021, 10, 322. [Google Scholar] [CrossRef]
- Balcke, G.U.; Vahabi, K.; Giese, J.; Finkemeier, I.; Tissier, A. Coordinated metabolic adaptation of Arabidopsis thaliana to high light. Plant J. 2024, 120, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Nastou, K.; Koutrouli, M.; Kirsch, R.; Mehryary, F.; Hachilif, R.; Hu, D.; Peluso, M.E.; Huang, Q.; Fang, T.; et al. The STRING database in 2025: Protein networks with directionality of regulation. Nucleic Acids Res. 2025, 53, D730–D737. [Google Scholar] [CrossRef]
- Smedley, D.; Haider, S.; Ballester, B.; Holland, R.; London, D.; Thorisson, G.; Kasprzyk, A. BioMart–biological queries made easy. BMC Genom. 2009, 10, 22. [Google Scholar] [CrossRef]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. “Circlize” implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Galili, T. dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Wickham, H. stringr: Modern, consistent string processing. R J. 2010, 2, 38–40. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Huynh-Thu, V.A.; Irrthum, A.; Wehenkel, L.; Geurts, P. Inferring regulatory networks from expression data using tree-based methods. PLoS ONE 2010, 5, e12776. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobrovskikh, A.V.; Zubairova, U.S.; Doroshkov, A.V. Identification of Key Differentially Expressed Genes in Arabidopsis thaliana Under Short- and Long-Term High Light Stress. Int. J. Mol. Sci. 2025, 26, 7790. https://doi.org/10.3390/ijms26167790
Bobrovskikh AV, Zubairova US, Doroshkov AV. Identification of Key Differentially Expressed Genes in Arabidopsis thaliana Under Short- and Long-Term High Light Stress. International Journal of Molecular Sciences. 2025; 26(16):7790. https://doi.org/10.3390/ijms26167790
Chicago/Turabian StyleBobrovskikh, Aleksandr V., Ulyana S. Zubairova, and Alexey V. Doroshkov. 2025. "Identification of Key Differentially Expressed Genes in Arabidopsis thaliana Under Short- and Long-Term High Light Stress" International Journal of Molecular Sciences 26, no. 16: 7790. https://doi.org/10.3390/ijms26167790
APA StyleBobrovskikh, A. V., Zubairova, U. S., & Doroshkov, A. V. (2025). Identification of Key Differentially Expressed Genes in Arabidopsis thaliana Under Short- and Long-Term High Light Stress. International Journal of Molecular Sciences, 26(16), 7790. https://doi.org/10.3390/ijms26167790







