Therapeutic Effects of Sulforaphane on Helicobacter pylori-Infected Mice: Insights from High-Coverage Metabolomics and Lipidomics Analyses of Serum and Liver
Abstract
1. Introduction
2. Results
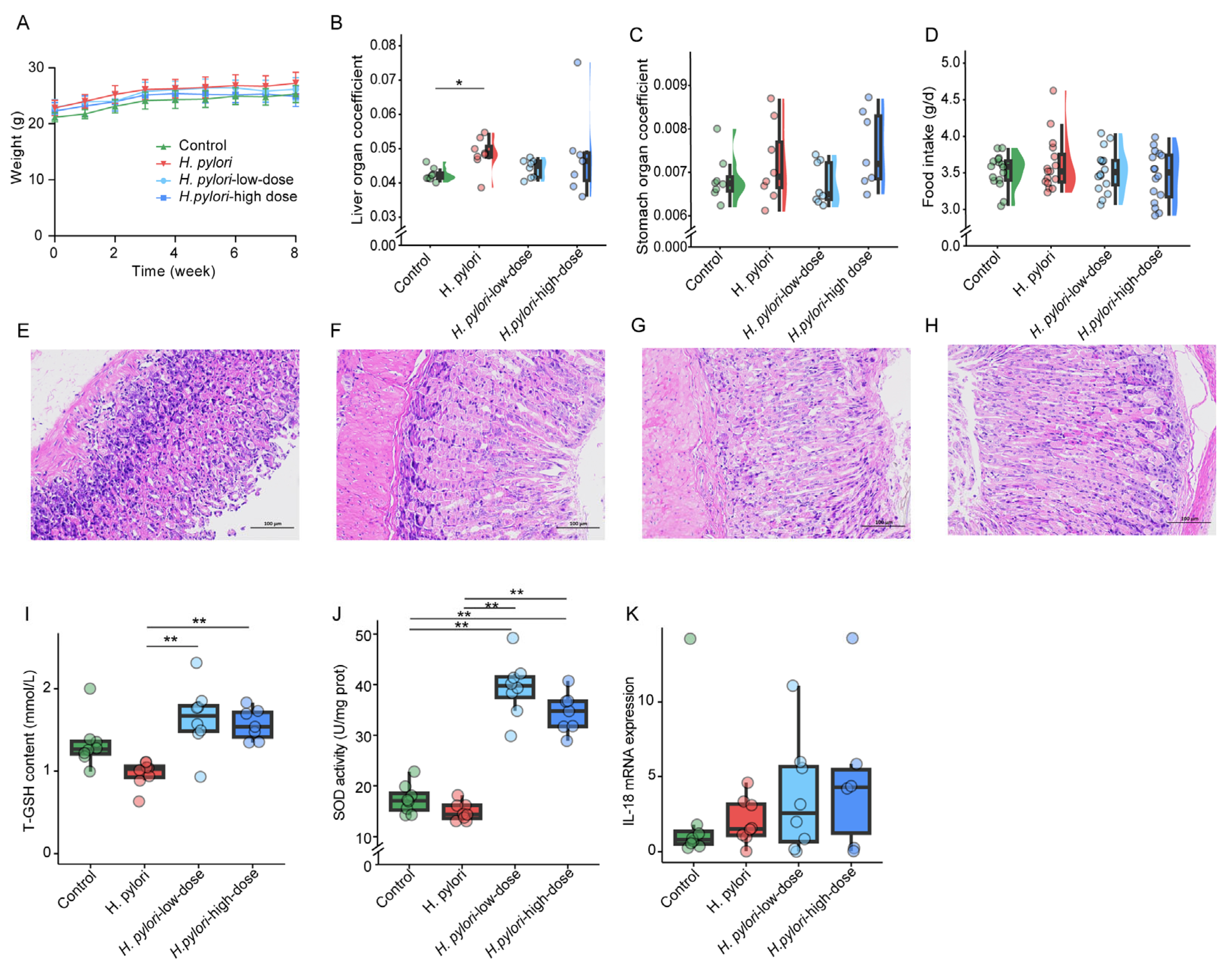
2.1. Detection of H. pylori Colonization and Biological Characteristics of Mice
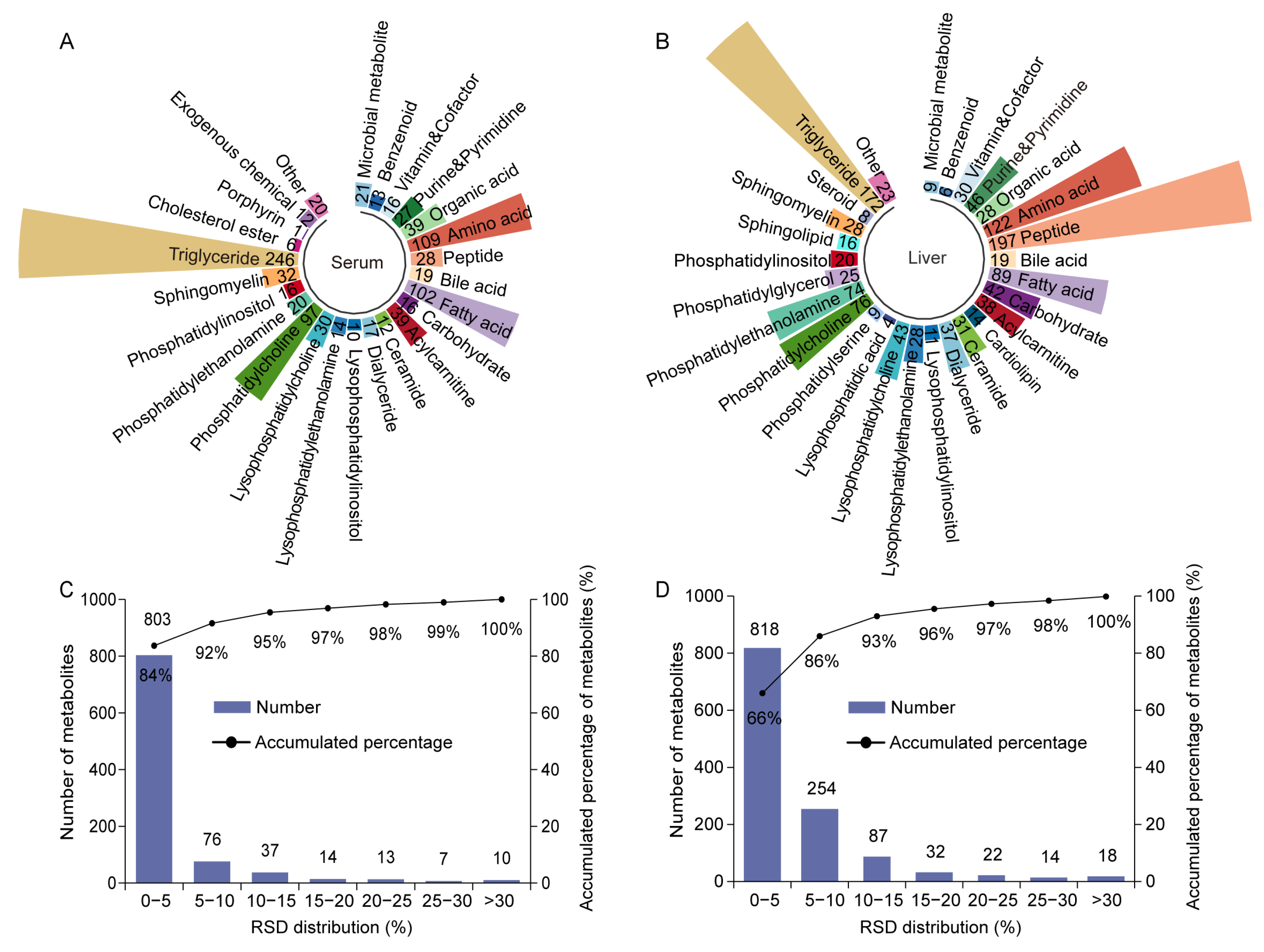
2.2. Data Quality Assessment of High-Coverage Metabolomics
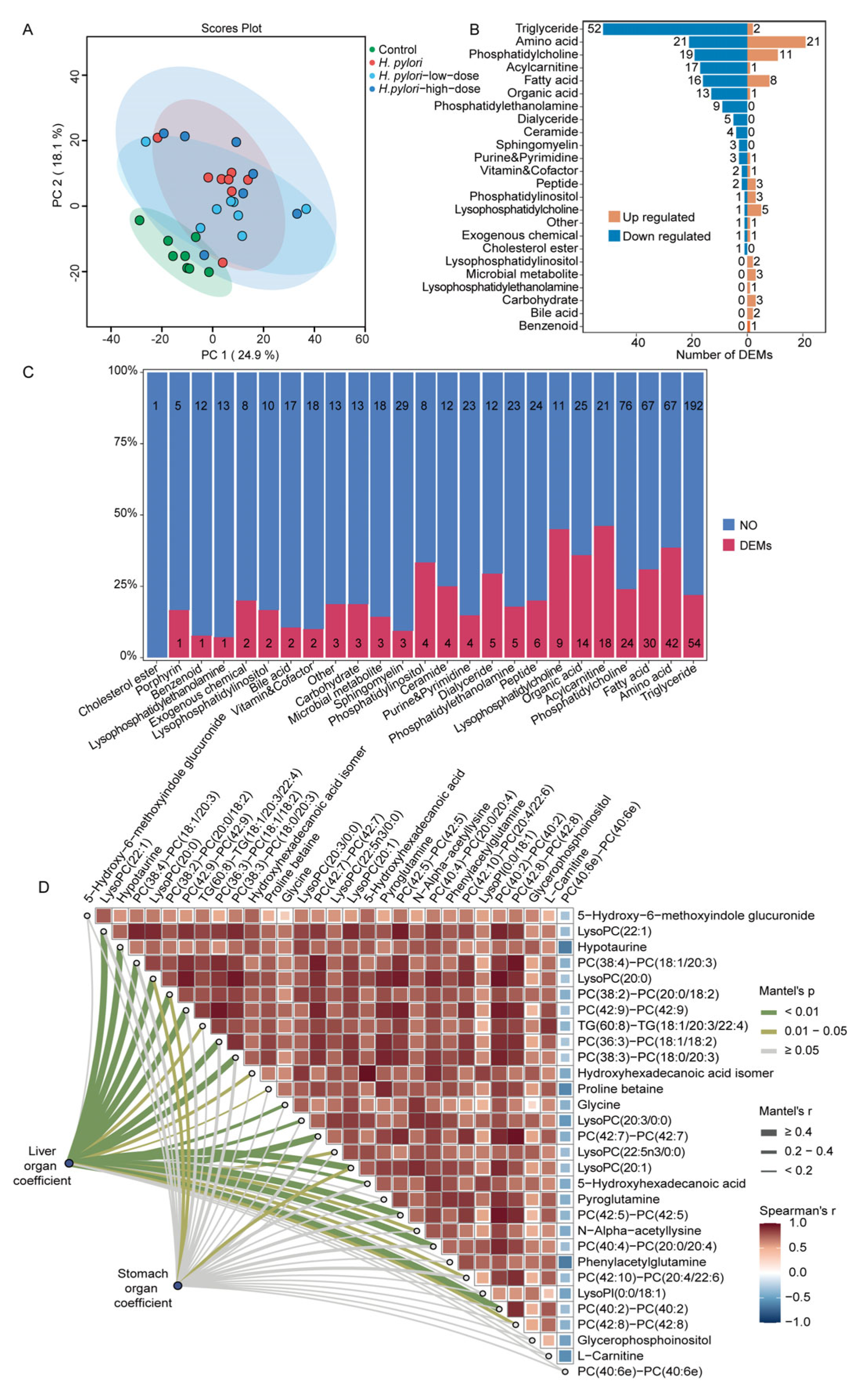
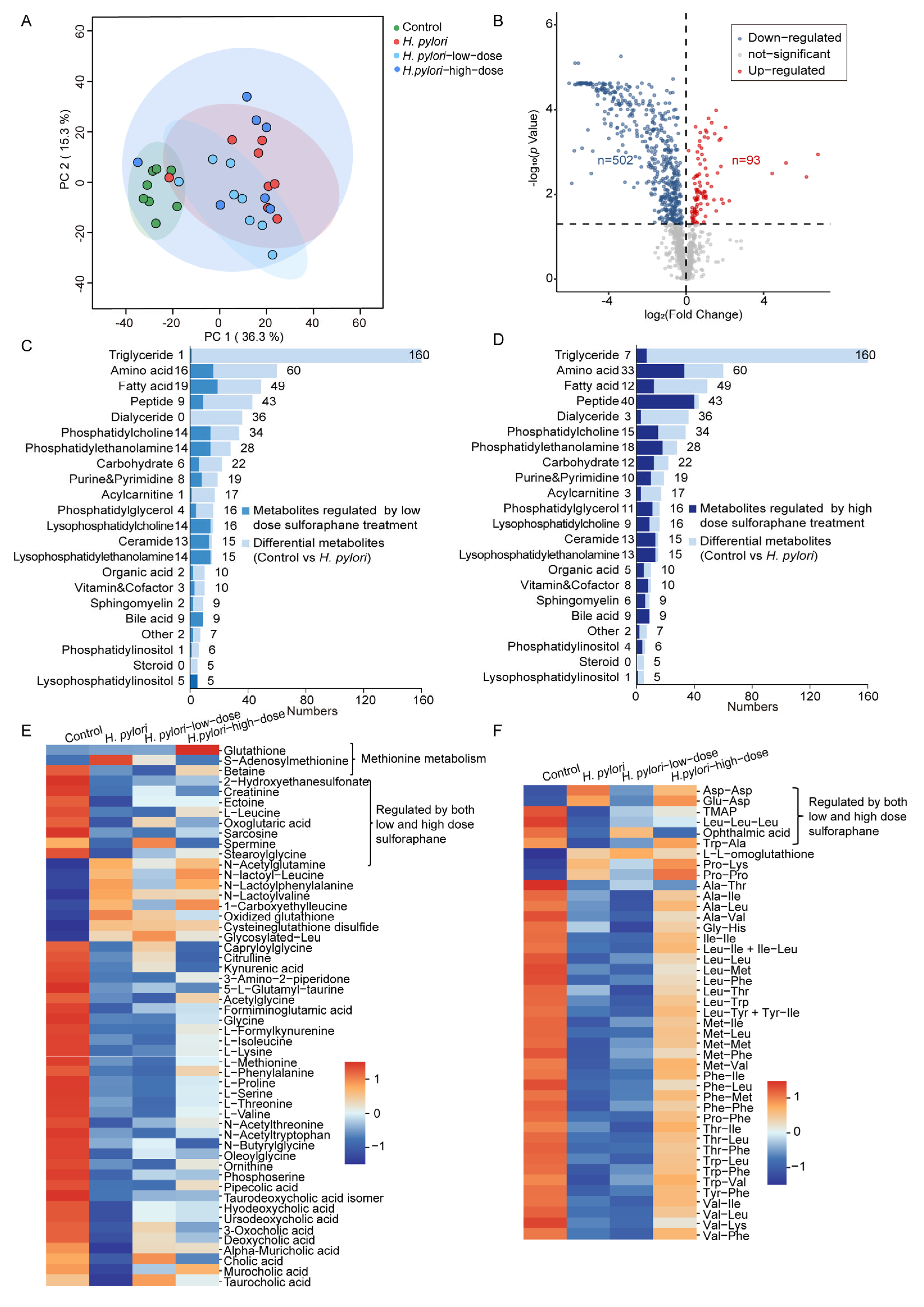
2.3. Integrated Metabolomics and Lipidomics Analysis of Mouse Serum
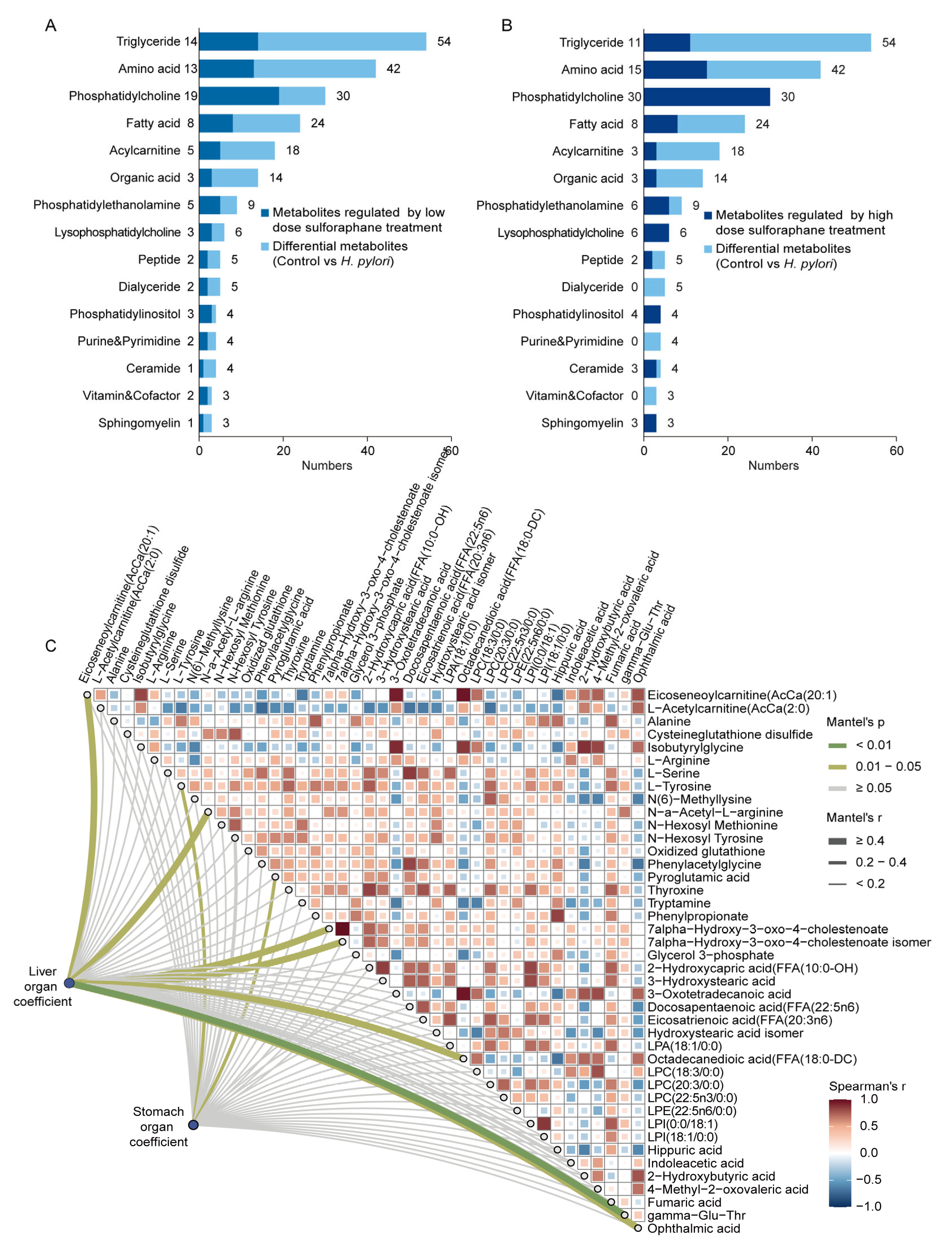
2.4. Mouse Liver Metabolomics and Lipidomics Analysis
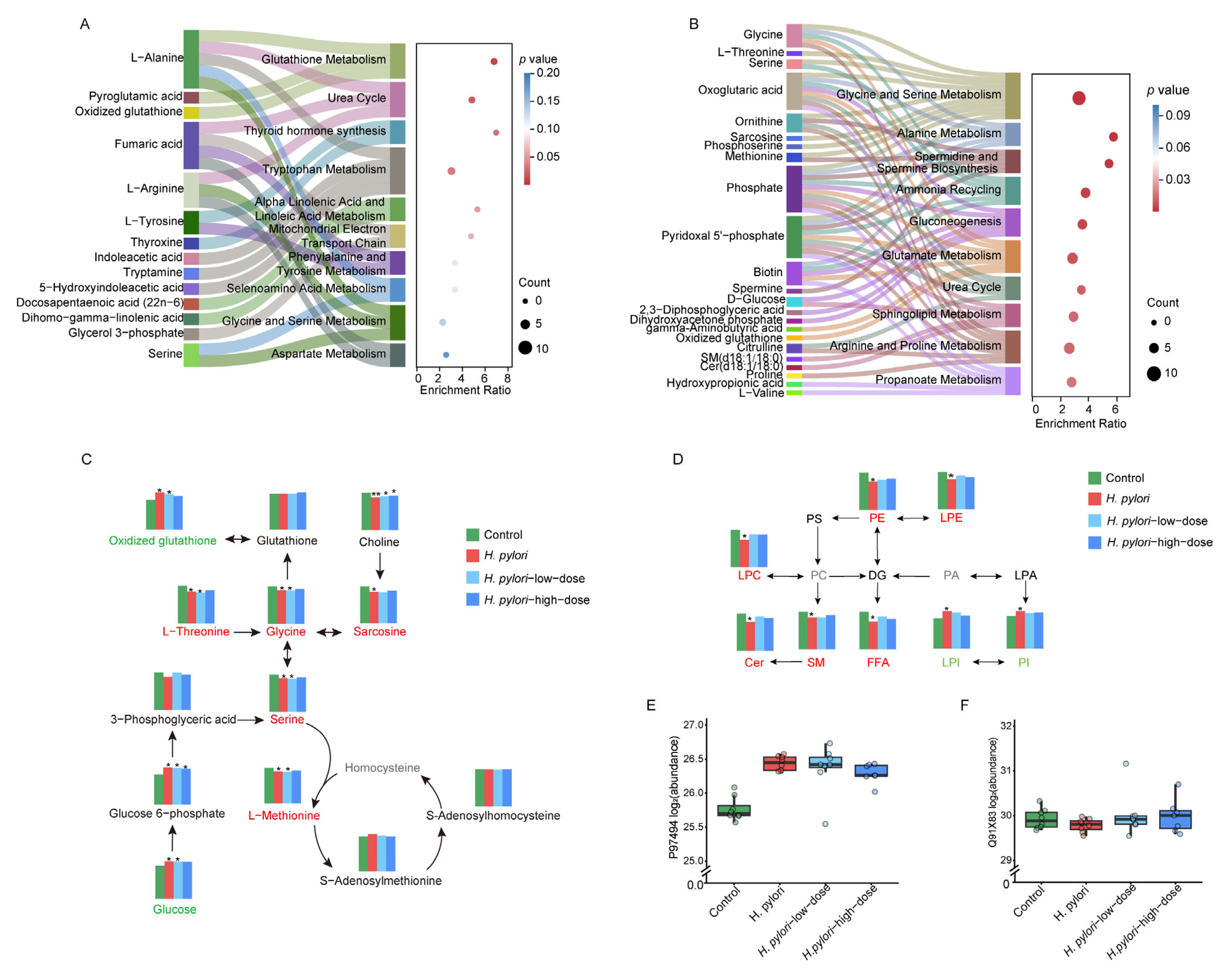
2.5. Metabolic Pathway Analysis and Proteomic Analysis
3. Discussion
3.1. Therapeutic Effects of Sulforaphane on Stomach and Liver
3.2. Sulforaphane Restores Amino Acid and Glutathione Metabolism Disrupted by H. pylori
3.3. Bile-Acid-Related Metabolism
3.4. Lipid-Related Metabolism
3.5. Energy Metabolism
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Animal Experiment
4.3. Assessment of H. pylori Colonization
4.4. Histological Evaluation
4.5. Determination of Oxidative Stress Indexes
4.6. RNA Extraction and Real-Time Quantitative RT-PCR
4.7. Sample Pre-Treatment
4.8. Non-Targeted Metabolomics and Lipidomics Analysis
4.9. Proteomic Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansari, S.; Yamaoka, Y. Helicobacter pylori virulence factors exploiting gastric colonization and its pathogenicity. Toxins 2019, 11, 677. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed]
- de Brito, B.B.; da Silva, F.A.F.; Soares, A.S.; Pereira, V.A.; Santos, M.L.C.; Sampaio, M.M.; Neves, P.H.M.; de Melo, F.F. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 2019, 25, 5578–5589. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, A. Role of sulforaphane in protection of gastrointestinal tract against H. pylori and NSAID-induced oxidative stress. Curr. Pharm. Des. 2017, 23, 4066–4075. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chun, F.K.-H.; Rutz, J.; Blaheta, R.A. Sulforaphane impact on reactive oxygen species (ROS) in bladder carcinoma. Int. J. Mol. Sci. 2021, 22, 5938. [Google Scholar] [CrossRef]
- Janczewski, Ł. Sulforaphane and its bifunctional analogs: Synthesis and biological activity. Molecules 2022, 27, 1750. [Google Scholar] [CrossRef]
- Chang, Y.W.; Jang, J.Y.; Kim, Y.H.; Kim, J.-W.; Shim, J.-J. The effects of broccoli sprout extract containing sulforaphane on lipid peroxidation and Helicobacter pylori infection in the gastric mucosa. Gut Liver 2015, 9, 486–493. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef]
- Fahey, J.W.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem. Biophys. Res. Commun. 2013, 435, 1–7. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, P.; Sha, W.; Sang, S. Urinary biomarkers of whole grain wheat intake identified by non-targeted and targeted metabolomics approaches. Sci. Rep. 2016, 6, 36278. [Google Scholar] [CrossRef]
- Wang, R.; Li, B.; Lam, S.M.; Shui, G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genom. 2020, 47, 69–83. [Google Scholar] [CrossRef]
- Butcher, L.D.; Hartog, G.D.; Ernst, P.B.; Crowe, S.E. Oxidative stress resulting from Helicobacter pylori infection contributes to gastric carcinogenesis. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 316–322. [Google Scholar] [CrossRef]
- Tran, L.S.; Ying, L.; D’Costa, K.; Wray-McCann, G.; Kerr, G.; Le, L.; Allison, C.C.; Ferrand, J.; Chaudhry, H.; Emery, J.; et al. NOD1 mediates interleukin-18 processing in epithelial cells responding to Helicobacter pylori infection in mice. Nat. Commun. 2023, 14, 3804. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tian, X.-F.; Fan, X.-G.; Fu, C.-Y.; Zhu, C. The pathological effect of Helicobacter pylori infection on liver tissues in mice. Clin. Microbiol. Infect. 2009, 15, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S.; Essa, M.M. Multifarious beneficial effect of nonessential amino acid, glycine: A review. Oxidative Med. Cell. Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.-X.; Guo, C.; Chen, Z.-G.; Yang, T.-C.; Zhang, J.-Y.; Wang, J.; Zhu, J.-X.; Li, D.; Zhang, T.-T.; Li, H.; et al. Glycine, serine and threonine metabolism confounds efficacy of complement-mediated killing. Nat. Commun. 2019, 10, 3325. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Han, S.C.; Huang, R.P.; Jiang, M.-Y.; Yan, C.-Y.; Li, X.-Y.; Zhan, Y.-J.; Li, X.-M.; Li, Y.-F.; Kurihara, H.; et al. Cyclo(-Phe-Phe) alleviates chick embryo liver injury via activating the Nrf2 pathway. Food Funct. 2022, 13, 6962–6974. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Wawrzyniak-Gramacka, E.; Książek, A.; Zagrodna, A.; Kopeć, W.; Słowińska-Lisowska, M. Dipeptide extract modulates the oxi-antioxidant response to intense physical exercise. Nutrients 2022, 14, 2402. [Google Scholar] [CrossRef]
- Winston, J.A.; Theriot, C.M. Diversification of host bile acids by members of the gut microbiota. Gut Microbes 2020, 11, 158–171. [Google Scholar] [CrossRef]
- Yang, M.; Gu, Y.; Li, L.; Liu, T.; Song, X.; Sun, Y.; Cao, X.; Wang, B.; Jiang, K.; Cao, H. Bile acid-gut microbiota axis in inflammatory bowel disease: From bench to Bedside. Nutrients 2021, 13, 3143. [Google Scholar] [CrossRef]
- Kuang, J.; Wang, J.; Li, Y.; Li, M.; Zhao, M.; Ge, K.; Zheng, D.; Cheung, K.C.; Liao, B.; Wang, S.; et al. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 2023, 35, 1752–1766.e8. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Q.; Chen, P.; Zhou, C.; Zhang, X.; Chen, L. Ursodeoxycholic acid treatment restores gut microbiota and alleviates liver inflammation in non-alcoholic steatohepatitic mouse model. Front. Pharmacol. 2021, 12, 788558. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Chen, M.; Jiang, X.; Ren, J.; Ganapathiraju, S.V.; Lei, P.; Yang, H.; Pannu, P.R.; Zhao, Y.; Zhang, X. Sulforaphane improves liver metabolism and gut microbiota in circadian rhythm disorder mice models fed with high-fat diets. Mol. Nutr. Food Res. 2024, 68, e2400535. [Google Scholar] [CrossRef]
- Liu, T.; Li, R.; Cui, Y.; Yu, Z.; Zhao, Y. Metabonomic analysis of plasma biochemical changes in pyrexia rats after treatment with Gegenqinlian decoction, aspirin and itraconazole by UHPLC-FT-ICR-MS. J. Pharm. Anal. 2020, 10, 581–587. [Google Scholar] [CrossRef]
- Balonov, I.; Mattis, M.; Jarmusch, S.; Koletzko, B.; Heinrich, K.; Neumann, J.; Werner, J.; Angele, M.K.; Heiliger, C.; Jacob, S. Metabolomic profiling of upper GI malignancies in blood and tissue: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2024, 150, 331. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Wei, T.; Ma, X.; Zhu, X.-L.; Ren, L.-F.; Zhang, L.; Ding, F.-H.; Li, X.; Wang, H.-P.; Bai, Z.-T.; et al. Effect of Helicobacter pylori on plasma metabolic phenotype in patients with gastric cancer. Cancer Control 2021, 28, 10732748211041881. [Google Scholar] [CrossRef]
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Zhao, Z.; Dong, Y.; Wang, X.; Niu, J. No evidence for a causal link between Helicobacter pylori infection and nonalcoholic fatty liver disease: A bidirectional mendelian randomization study. Front. Microbiol. 2022, 13, 1018322. [Google Scholar] [CrossRef]
- Haeri, M.; Parham, M.; Habibi, N.; Vafaeimanesh, J. Effect of Helicobacter pylori infection on serum lipid profile. J. Lipids 2018, 2018, 6734809. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, J.; Zeng, R.; Xie, Y. Association between Helicobacter pylori infection and triglyceride levels: A nested cross-sectional study. Front. Endocrinol. 2023, 14, 1220347. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, R.M.; Bott, S.J.; Harding, M.; Coward, W.A.; Bluck, L.J.; Prentice, A.M. De novo lipogenesis during controlled overfeeding with sucrose or glucose in lean and obese women. Am. J. Clin. Nutr. 2001, 74, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Pérez-Felpete, N.; Fernández-Fernández, C.; Donapetry-García, C.; Pazos-García, C. Liver glucose metabolism in humans. Biosci. Rep. 2016, 36, e00416. [Google Scholar] [CrossRef]
- Yanaka, A.; Zhang, S.; Tauchi, M.; Suzuki, H.; Shibahara, T.; Matsui, H.; Nakahara, A.; Tanaka, N. Daily intake of sulforaphane-rich broccoli sprouts prevents progression of high salt diet-induced gastric atrophy in H. pylori-infected C57/BL6 mice in vivo. Cancer Res. 2005, 65, 1220. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Williams, D.E.; Dashwood, R.H.; Stevens, J.F.; Yamamoto, M.; Ho, E. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm. Res. 2011, 28, 3171–3179. [Google Scholar] [CrossRef]
- Holloway, P.M.; Gillespie, S.; Becker, F.; Vital, S.A.; Nguyen, V.; Alexander, J.S.; Evans, P.C.; Gavins, F.N. Sulforaphane induces neurovascular protection against a systemic inflammatory challenge via both Nrf2-dependent and independent pathways. Vasc. Pharmacol. 2016, 85, 29–38. [Google Scholar] [CrossRef]
- Socała, K.; Nieoczym, D.; Kowalczuk-Vasilev, E.; Wyska, E.; Wlaź, P. Increased seizure susceptibility and other toxicity symptoms following acute sulforaphane treatment in mice. Toxicol. Appl. Pharmacol. 2017, 326, 43–53. [Google Scholar] [CrossRef]
- Du, B.; Ding, D.; Ma, C.; Guo, W.; Kang, L. Locust density shapes energy metabolism and oxidative stress resulting in divergence of flight traits. Proc. Natl. Acad. Sci. USA 2021, 119, e2115753118. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Li, J.; Miao, B.; Wang, S.; Dong, W.; Xu, H.; Si, C.; Wang, W.; Duan, S.; Lou, J.; Bao, Z.; et al. Hiplot: A comprehensive and easy-to-use web service for boosting publication-ready biomedical data visualization. Brief. Bioinform. 2022, 23, bbac261. [Google Scholar] [CrossRef]
- Lyu, F.; Han, F.; Ge, C.; Mao, W.; Chen, L.; Hu, H.; Chen, G.; Lang, Q.; Fang, C. OmicStudio: A composable bioinformatics cloud platform with real-time feedback that can generate high-quality graphs for publication. iMeta 2023, 2, e85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Sun, L.; Chen, J.; Li, Y.; Pan, Y.; Su, A.; Mao, Q.; Hu, J.; Feng, D.; Ouyang, Y. Therapeutic Effects of Sulforaphane on Helicobacter pylori-Infected Mice: Insights from High-Coverage Metabolomics and Lipidomics Analyses of Serum and Liver. Int. J. Mol. Sci. 2025, 26, 7791. https://doi.org/10.3390/ijms26167791
He S, Sun L, Chen J, Li Y, Pan Y, Su A, Mao Q, Hu J, Feng D, Ouyang Y. Therapeutic Effects of Sulforaphane on Helicobacter pylori-Infected Mice: Insights from High-Coverage Metabolomics and Lipidomics Analyses of Serum and Liver. International Journal of Molecular Sciences. 2025; 26(16):7791. https://doi.org/10.3390/ijms26167791
Chicago/Turabian StyleHe, Shuling, Lvyun Sun, Jiali Chen, Yixin Li, Ying Pan, Amei Su, Qiuyao Mao, Jiaqian Hu, Disheng Feng, and Yang Ouyang. 2025. "Therapeutic Effects of Sulforaphane on Helicobacter pylori-Infected Mice: Insights from High-Coverage Metabolomics and Lipidomics Analyses of Serum and Liver" International Journal of Molecular Sciences 26, no. 16: 7791. https://doi.org/10.3390/ijms26167791
APA StyleHe, S., Sun, L., Chen, J., Li, Y., Pan, Y., Su, A., Mao, Q., Hu, J., Feng, D., & Ouyang, Y. (2025). Therapeutic Effects of Sulforaphane on Helicobacter pylori-Infected Mice: Insights from High-Coverage Metabolomics and Lipidomics Analyses of Serum and Liver. International Journal of Molecular Sciences, 26(16), 7791. https://doi.org/10.3390/ijms26167791




