Mechanism of H2S in Inhibiting the Senescence and Browning of Fresh-Cut Potatoes
Abstract
1. Introduction
2. Results
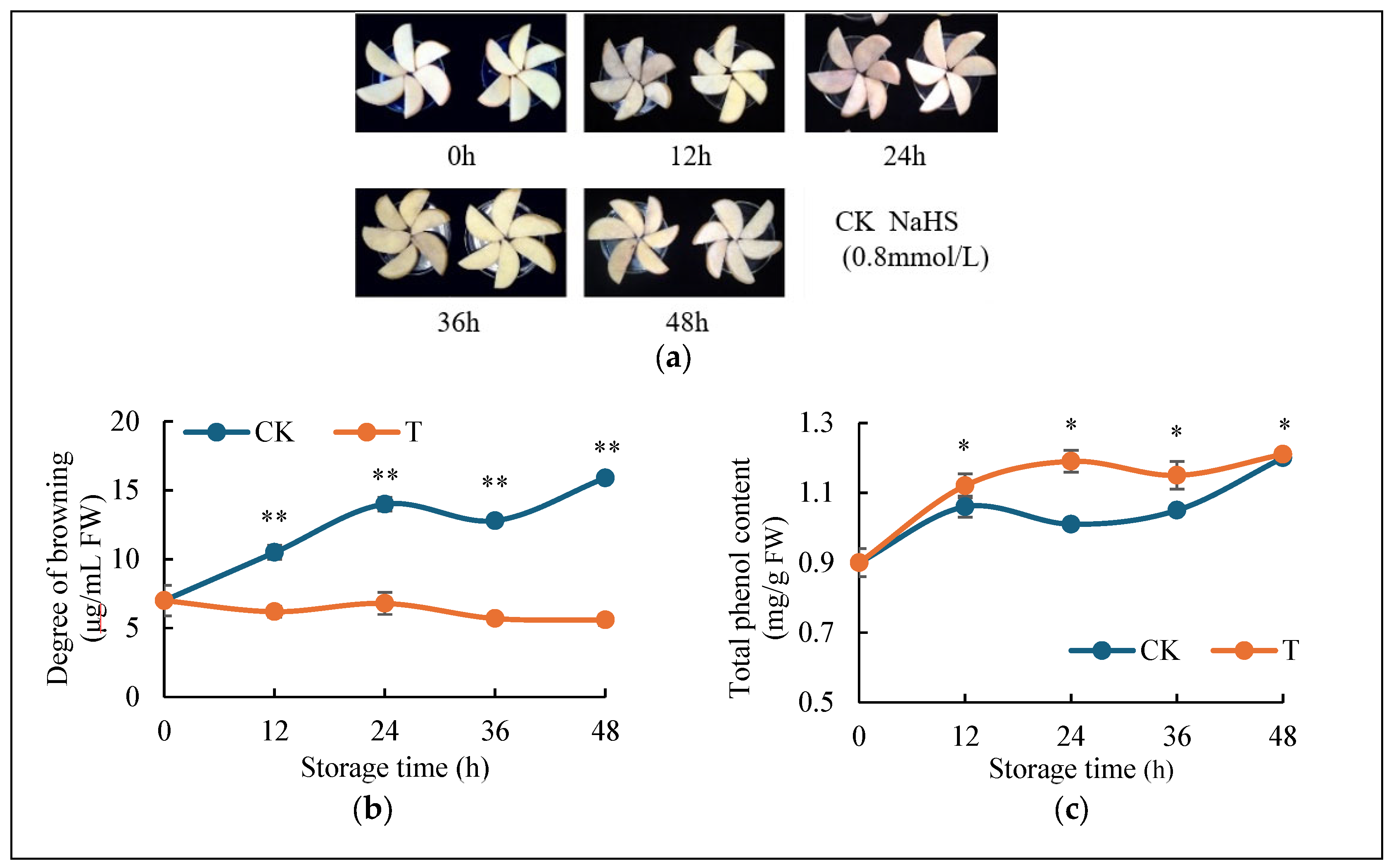
2.1. H2S Reduces Browning of Fresh-Cut Potatoes
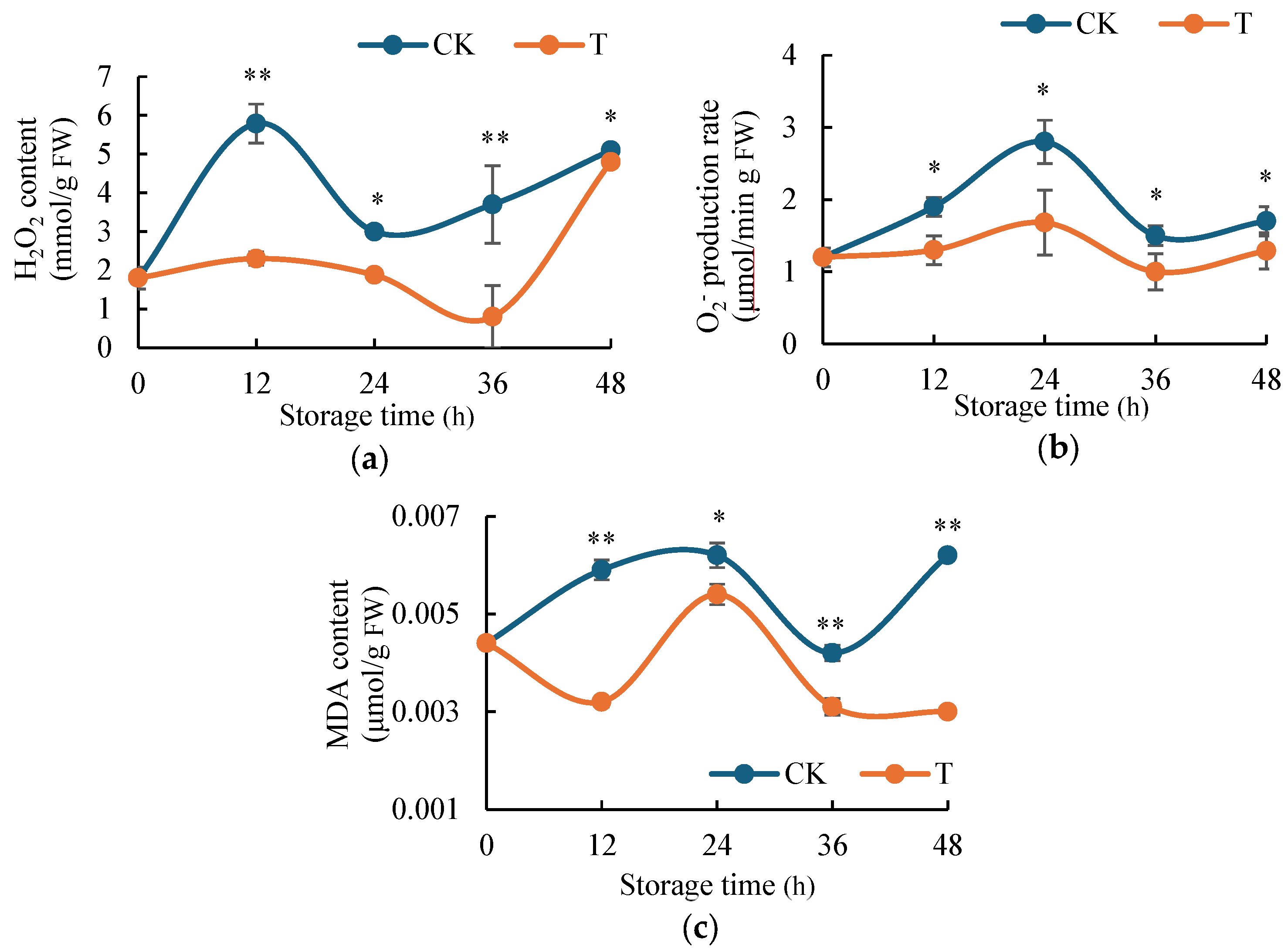
2.2. H2S Inhibits the Accumulation of ROS Content in Potato
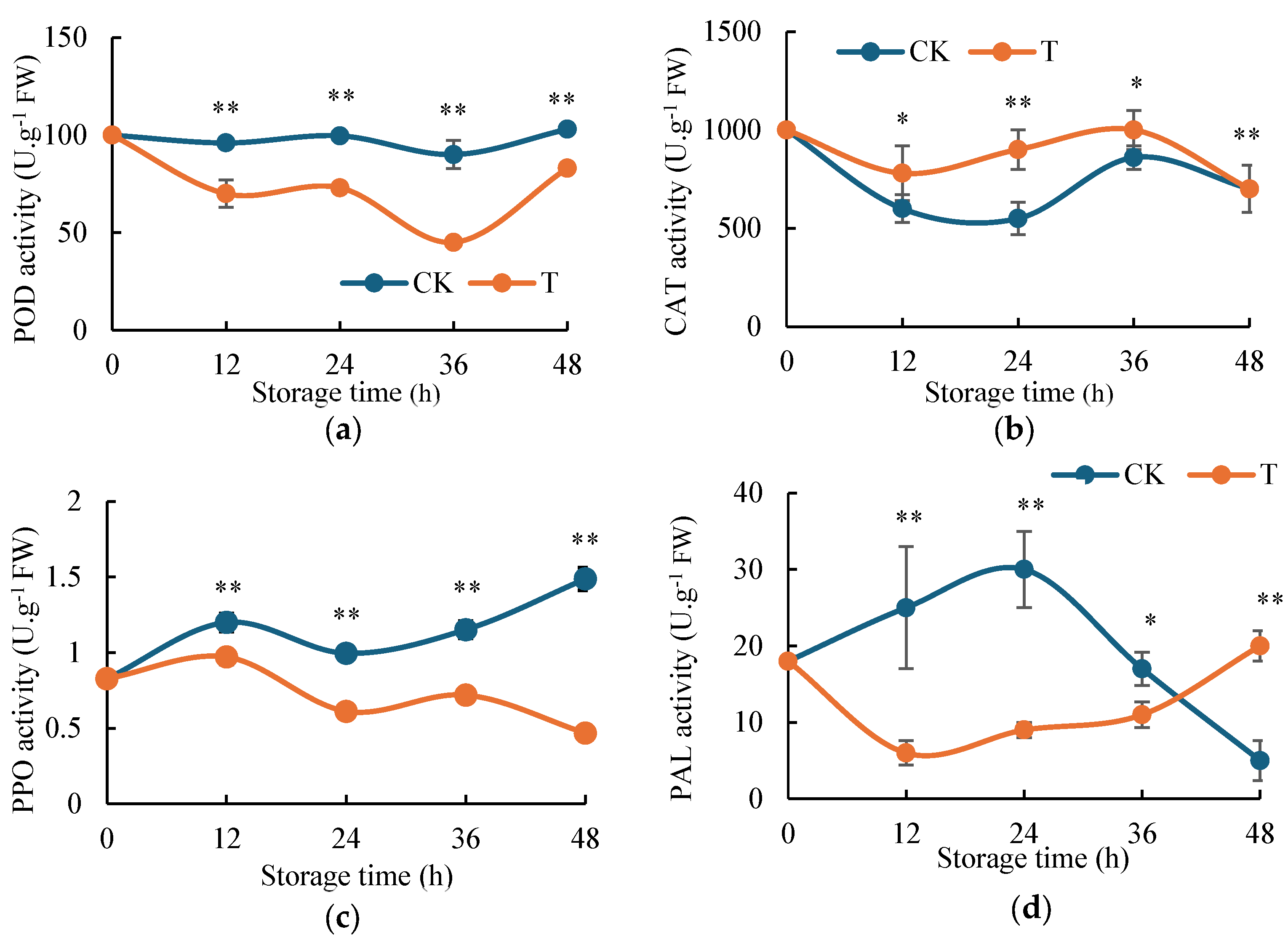
2.3. Effect of H2S on Enzyme Activities of Potato
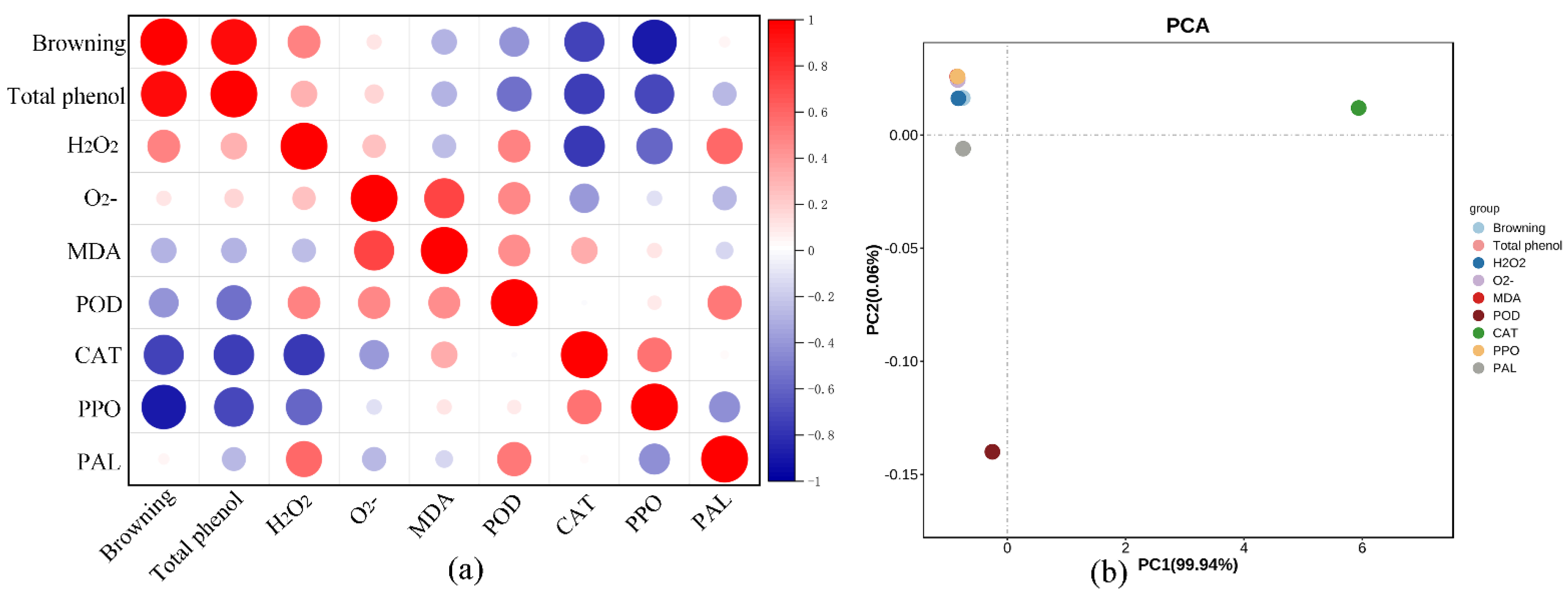
2.4. Correlation Analysis and Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Material
4.2. Experimental Methods
4.2.1. Sample Treatment
4.2.2. Determination of Browning Degree and Total Phenol Content
4.2.3. Determination of ROS Content
4.2.4. Determination of Enzyme Activity
4.2.5. Statistical Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, A.Z. Development status and countermeasures of potato industry. Agric. Dev. Equip. 2021, 27, 42–43. [Google Scholar]
- Visse-Mansiaux, M.; Tallant, M.; Brostaux, Y.; Delaplace, P.; Vanderschuren, H.; Dupuis, B. Assessment of pre-and post-harvest anti-sprouting treatments to replace CIPC for potato storage. Postharvest Biol. Technol. 2021, 178, 111540. [Google Scholar] [CrossRef]
- Rashid, M.H.; Khan, M.R.; Roobab, U.; Rajoka, M.S.R.; Inam-Ur-Raheem, M.; Anwar, R.; Ahmed, W.; Jahan, M.; Ijaz, M.R.A.; Asghar, M.M.; et al. Enhancing the shelf stability of fresh-cut potatoes via chemical and nonthermal treatments. J. Food Process. Preserv. 2021, 45, e15582. [Google Scholar] [CrossRef]
- Furrer, A.N.; Chegeni, M.; Ferruzzi, M.G. Impact of potato processing on nutrients, phytochemicals, and human health. Crit. Rev. Food Sci. Nutr. 2018, 58, 146–168. [Google Scholar] [CrossRef]
- Cheng, L.J.; Wang, S.M.; Li, Z.; Cheng, J.P.; Liu, J.J.; Yu, P.L. Study on browning inhibition of fresh-cut potatoes in southwest cold highlan. Storage Process. 2021, 21, 8–14, 22. [Google Scholar]
- Hu, P.P. Effects of different pre-coating methods on storage quality of fresh-cut potatoes. Food Res. Dev. 2021, 42, 135–140. [Google Scholar]
- Ru, X.; Tao, N.; Feng, Y.; Li, Q.; Wang, Q. A novel anti-browning agent 3-mercapto-2-butanol for inhibition of fresh-cut potato browning. Postharvest Biol. Technol. 2020, 170, 111324. [Google Scholar] [CrossRef]
- Hu, L.S.; Wang, Y.Y.; Jiang, Y.Y.; Yu, R.Q.; Gu, J.; Zhang, R. Effect of vacuum packaging at different temperatures on storage quality of fresh-cut potatoes. Contemp. Hortic. 2018, 21, 24–26. [Google Scholar]
- Tian, X.J.; Liu, Y.L.; Long, M.; Chen, S.E.; Gao, D.D.; Li, M.S.; Ma, Z.R.; Nazariyah, Y. Comparison of color-preservation method for fresh cut potato slices and process optimization. Sci. Technol. Food Ind. 2018, 39, 137–141. [Google Scholar]
- Wang, X.Y.; Yang, L.Z.; Wang, T.; Wang, R.R.; Liu, J.; San, Y.; Zhang, Q.; Ding, S.H. Recent progress toward understanding the physiological function, purification, and enzymatic browning control of plant polyphenol oxidases. Food Sci. 2020, 41, 222–237. [Google Scholar]
- Ma, Y.; Wang, H.; Yan, H.; Malik, A.U.; Dong, T.T.; Wang, Q.G. Pre-cut NaCl solution treatment effectively inhibited the browning of fresh-cut potato by influencing polyphenol oxidase activity and several free amino acids contents. Postharvest Biol. Technol. 2021, 178, 111543. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, C.; Lai, D.; Sun, Y.; Samma, M.K.; Zhang, J.; Shen, W. Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J. Plant Physiol. 2014, 171, 53–62. [Google Scholar] [CrossRef]
- Hou, L.; Zhu, D.; Ma, Q.; Zhang, D.; Liu, X. H2S synthetase AtD-CDes involves in ethylene and drought regulated stomatal movement. Sci. Bull. 2016, 61, 1171–1175. [Google Scholar] [CrossRef]
- Mathur, P.; Roy, S.; Nasir Khan, M.; Mukherjee, S. Hydrogen sulphide (H2S) in the hidden half: Role in root growth, stress signalling and rhizospheric interactions. Plant Biol. 2022, 24, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Singh, P.; Tripathi, D.K.; Corpas, F.J.; Singh, V.P. Nitric oxide and hydrogen sulfide: An indispensable combination for plant functioning. Trends Plant Sci. 2021, 26, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Chang, L.; Pan, C.; Qi, Y.; Zhao, J.; Pang, Y.; Du, J.; Tang, C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem. Biophys. Res. Commun. 2004, 318, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Manganaris, G.A.; Papadopoulos, I.; Fotopoulos, V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013, 64, 1953–1966. [Google Scholar] [CrossRef]
- De Bont, L.; Mu, X.; Wei, B.; Han, Y. Abiotic stress-triggered oxidative challenges: Where does H2S act? J. Genet. Genom. 2022, 49, 748–755. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.; Chen, L.; Wang, C. The role of hydrogen sulfide in plant roots during development and in response to abiotic stress. Int. J. Mol. Sci. 2022, 23, 1024. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant. 2020, 168, 289–300. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, L.; Hu, S.; Hou, Y.; Wang, J.; Zheng, Y.; Jin, P. Hydrogen sulfide-induced chilling resistance in peach fruit is performed via sustaining the homeostasis of ROS and RNS. Food Chem. 2023, 398, 133940. [Google Scholar] [CrossRef]
- Li, S.P.; Hu, K.D.; Hu, L.Y.; Li, Y.H.; Jiang, A.M.; Xiao, F.; Han, Y.; Liu, Y.S.; Zhang, H. Hydrogen sulfide alleviates postharvest senescence of broccoli by modulating antioxidant defense and senescence-related gene expression. J. Agric. Food. Chem. 2014, 62, 1119–1129. [Google Scholar] [CrossRef]
- Cui, W.Y.; Li, X.; Xu, X.Y. Research progress in the role of H2S as signal molecule and its regulation of physiological metabolism in postharvest fruits and vegetables. Storage Process. 2020, 20, 226–229. [Google Scholar]
- Liu, D.; Pei, Y. The secret of H2S to keep plants young and fresh and its products. Plant Biol. 2022, 24, 587–593. [Google Scholar] [CrossRef]
- Li, X.J.; Li, M.X.; Nie, Y.H.; Zhou, X.W.; Zhang, B.; Li, M. Effect of exogenous H2S on antioxidant capacity of strawberry fruit during shelf and low temperature storage. Sci. Technol. Food Ind. 2019, 40, 291–296. [Google Scholar]
- Wang, S.S. Physiological Mechanisms of H2S and D/L-CD Regulating Ripening and Aging of Tomato Fruits. MA/PhD Thesis, Hefei University of Technology, Hefei, China, 2018. [Google Scholar]
- Hu, K.D.; Zhang, X.Y.; Yao, G.F.; Rong, Y.L.; Ding, C.; Tang, J.; Yang, F.; Huang, Z.Q.; Xu, Z.M.; Chen, X.Y.; et al. A nuclear-localized cysteine desulfhydrase plays a role in fruit ripening in tomato. Hortic. Res. 2020, 7, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.Y.; Yao, G.F.; Wang, S.S.; Li, T.T.; Sun, K.K.; Tang, J.; Huang, Z.Q.; Yang, F.; Li, Y.H.; Chen, X.Y.; et al. Hydrogen sulfide maintains good nutrition and delays postharvest senescence in postharvest tomato fruits by regulating antioxidative metabolism. J. Plant Growth Regul. 2021, 40, 2548–2559. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, Y.Q.; Wang, Q.; Zhuo, J.H.; Yuan, S.Z.; Liu, J.; Yan, R.H.; Feng, B.H. Shelf quality changes of fresh-cut potatoes during storage. Food Ferment. Ind. 2022, 48, 208–215. [Google Scholar]
- Liu, D.M.; Li, J.; Li, Z.W.; Pei, Y.X. Hydrogen sulfide inhibits ethylene-induced petiole abscission in tomato (Solanum lycopersicum L.). Hortic Res. 2020, 7, 14. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Deshi, V.; Homa, F.; Aftab, M.; Aftab, T. Inhibitory effects of hydrogen sulfide on oxidative damage and pericarp browning in harvested litchi. J. Plant Growth Regul. 2021, 40, 2560–2569. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, A.; Liu, C.; Wagstaff, C.; Zhao, Q.; Zhang, Y.; Hu, W. Hydrogen sulfide inhibits the browning of fresh-cut apple by regulating the antioxidant, energy and lipid metabolism. Postharvest Biol. Technol. 2021, 175, 111487. [Google Scholar] [CrossRef]
- Jin, Z.P.; Sun, L.M.; Yang, G.D.; Pei, Y.X. Hydrogen Sulfide Regulates Energy Production to Delay Leaf Senescence Induced by Drought Stress in Arabidopsis. Front. Plant Sci. 2018, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lin, Y.Z.; Fan, Z.Q. Research progress on the relationship between the occurrence of postharvest diseases and reactive oxygen species and membrane lipid metabolism in fruits and vegetables. Subtrop. Agric. Res. 2020, 16, 132–137. [Google Scholar]
- Qiong, T.; Zheng, X.D.; Jun, G.; Ting, Y. Tomato SlPti5 plays a regulative role in the plant immune response against Botrytis cinerea through modulation of ROS system and hormone pathways. J. Integr. Agric. 2022, 21, 697–709. [Google Scholar] [CrossRef]
- Wang, J.; Sun, G.Y.; Ji, Q.Q.; Liu, Z.H.; He, J.; Wei, Y. The role and control of reactive oxygen species in the postharvest aging process of fruits and vegetables. Pack. Food Mach. 2015, 33, 51–54, 58. [Google Scholar]
- Zheng, J.L.; Hu, L.Y.; Hu, K.D.; Wu, J.; Yang, F.; Zhang, H. Hydrogen sulfide alleviates senescence of fresh-cut apple by regulating antioxidant defense system and senescence-related gene expression. Hortscience 2016, 51, 152–158. [Google Scholar] [CrossRef]
- Liu, C.B.; Li, S.Y.; Ye, N.; Tang, Y.; Cao, Y.X.; Ma, H.L. Browning inhibition and decolourisation methods and shelf-life preservation effects of fresh walnuts. Food Sci. 2022, 43, 232–241. [Google Scholar]
- Lin, Q.; Zhou, C.; Li, X.J.; Li, L.; Li, X.H.; Li, J.X. Effect of Amaranthus extract on browning of fresh-cut apples. J. Food Sci. Technol. 2021, 39, 108–114. [Google Scholar]
- Switala, J.; Loewen, P.C. Diversity of properties among catalases. Arch. Biochem. Biophys. 2002, 401, 145–154. [Google Scholar] [CrossRef]
- Jiang, D.; Hu, W.Z.; Chen, C.; Jiang, A.L. Regulation of benzopropane metabolism in fresh-cut pumpkin by salicylic acid and hydrogen sulfide. Food Technol. 2016, 41, 42–46. [Google Scholar]
- Li, Z.; Zhang, Y.; Ge, H. The membrane may be an important factor in browning of fresh-cut pear. Food Chem. 2017, 230, 265–270. [Google Scholar] [CrossRef]
- Zhao, K.; Xiao, Z.; Zeng, J.; Xie, H. Effects of different storage conditions on the browning degree, PPO activity, and content of chemical components in fresh Lilium bulbs (Liliumbrownii FE Brown var. viridulum Baker.). Agriculture 2021, 11, 184. [Google Scholar] [CrossRef]
- Cheng, D.; Ma, Q.; Zhang, J.; Jiang, K.; Cai, S.; Wang, W.; Wang, J.; Sun, J. Cactus polysaccharides enhance preservative effects of ultrasound treatment on fresh-cut potatoes. Ultrason. Sonochem. 2022, 90, 106205. [Google Scholar] [CrossRef]
- Ge, Y.; Hu, K.D.; Wang, S.S.; Hu, L.Y.; Chen, X.Y.; Li, Y.H.; Yang, Y.; Yang, F.; Zhang, H. Hydrogen sulfide alleviates postharvest ripening and senescence of banana by antagonizing the effect of ethylene. PLoS ONE 2017, 12, e0180113. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Peng, X.; Yao, G.; Zhou, Z.; Yang, F.; Li, W.; Zhao, Y.; Li, Y.; Han, Z.; Chen, X.; et al. Roles of a cysteine desulfhydrase LCD1 in regulating leaf senescence in tomato. Int. J. Mol. Sci. 2021, 22, 13078. [Google Scholar] [CrossRef]
- Yang, T.; Peng, Q.; Lin, H.; Xi, D. Alpha-momorcharin preserves catalase activity to inhibit viral infection by disrupting the 2b–CAT interaction in Solanum lycopersicum. Mol. Plant Pathol. 2023, 24, 107–122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Liu, N.; Li, W.; Guan, L.; Yao, G.; Zhang, H.; Hu, K. Mechanism of H2S in Inhibiting the Senescence and Browning of Fresh-Cut Potatoes. Int. J. Mol. Sci. 2025, 26, 7785. https://doi.org/10.3390/ijms26167785
Lu Z, Liu N, Li W, Guan L, Yao G, Zhang H, Hu K. Mechanism of H2S in Inhibiting the Senescence and Browning of Fresh-Cut Potatoes. International Journal of Molecular Sciences. 2025; 26(16):7785. https://doi.org/10.3390/ijms26167785
Chicago/Turabian StyleLu, Zixu, Nannan Liu, Wanjie Li, Lisheng Guan, Gaifang Yao, Hua Zhang, and Kangdi Hu. 2025. "Mechanism of H2S in Inhibiting the Senescence and Browning of Fresh-Cut Potatoes" International Journal of Molecular Sciences 26, no. 16: 7785. https://doi.org/10.3390/ijms26167785
APA StyleLu, Z., Liu, N., Li, W., Guan, L., Yao, G., Zhang, H., & Hu, K. (2025). Mechanism of H2S in Inhibiting the Senescence and Browning of Fresh-Cut Potatoes. International Journal of Molecular Sciences, 26(16), 7785. https://doi.org/10.3390/ijms26167785







