Sex-Dependent Regulation of Liver Fibrosis in Primary Sclerosing Cholangitis: The Role of miR-125b, Androgen Receptors, TGF-β, and Apelin Signalling
Abstract
1. Introduction
2. Results
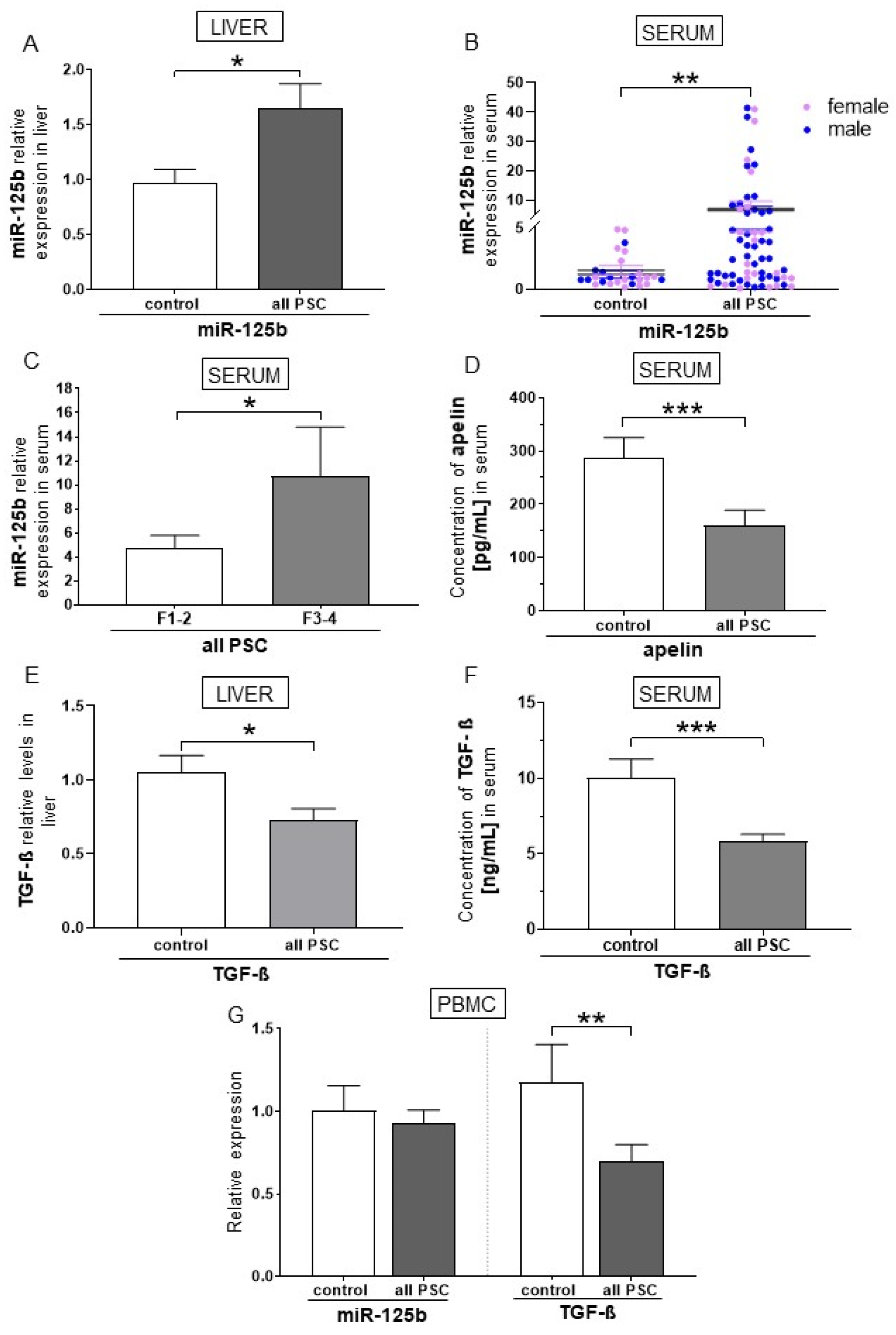
2.1. miR-125b Is a Negative Regulator of TGF-β and Apelin in Patients with PSC
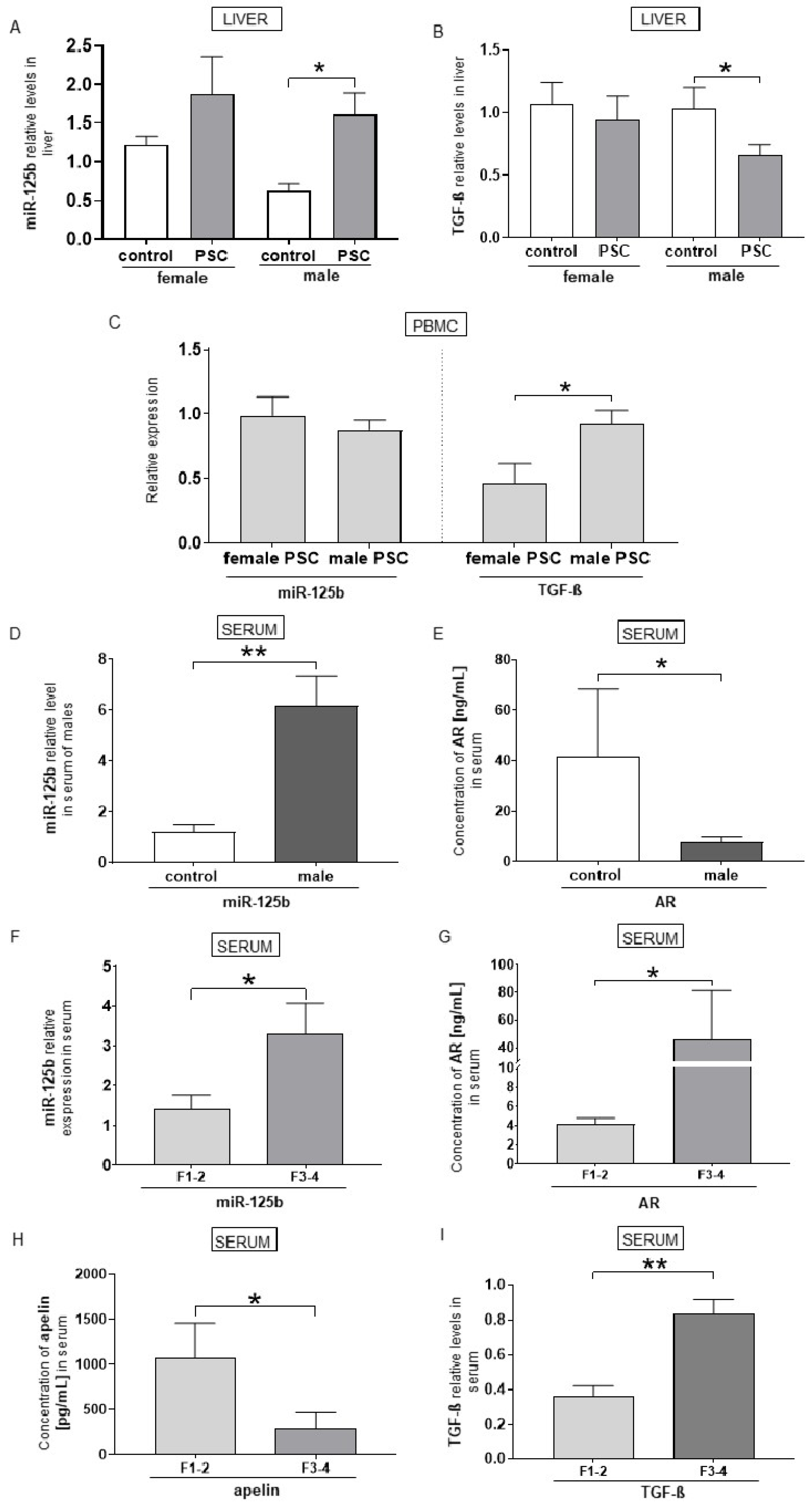
2.2. Relationship Between miR-125b and TGF-β, ARs, and Apelin Expression in Male PSC Patients with Liver Fibrosis
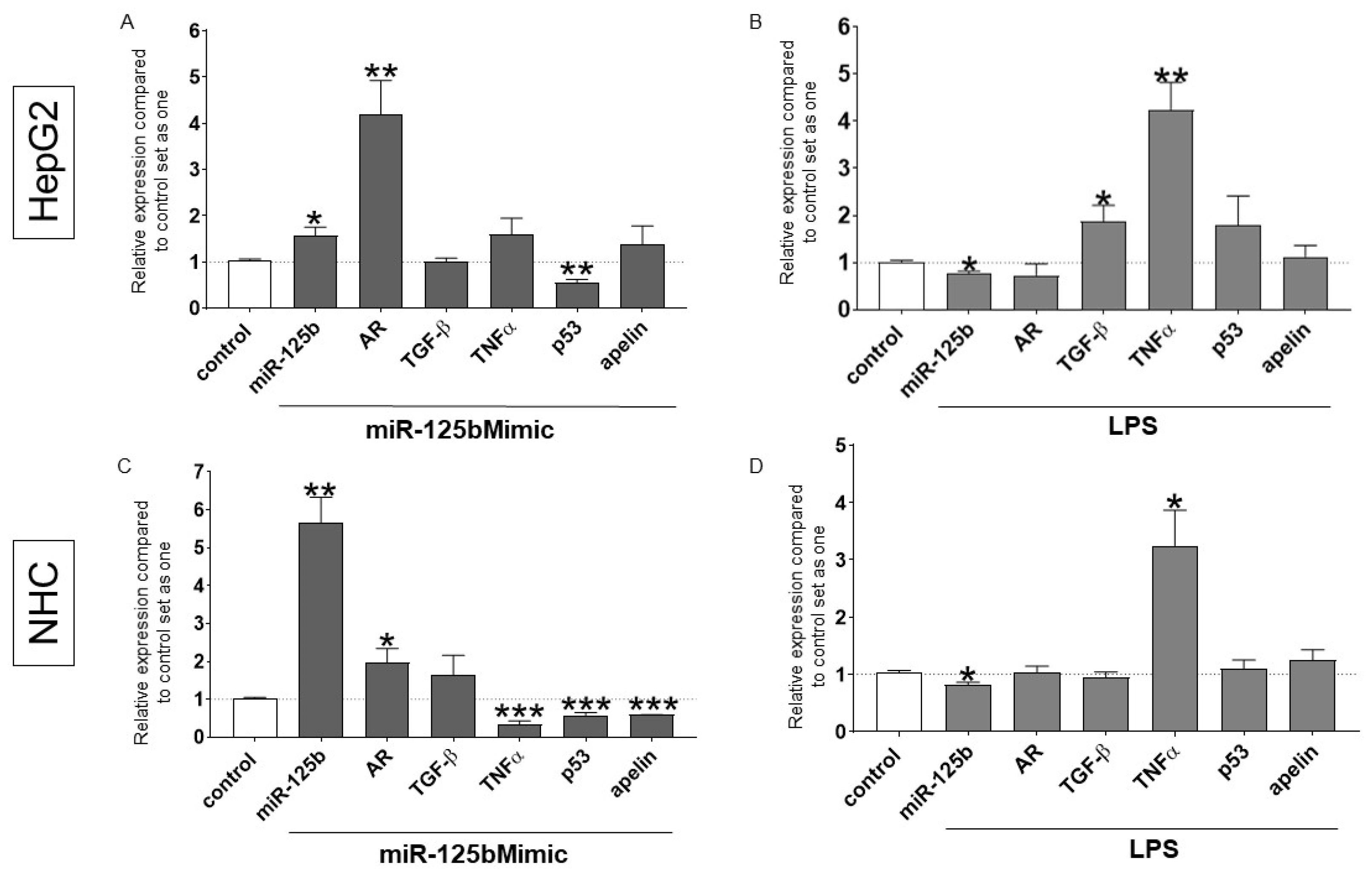
2.3. The Effect of Experimentally Induced miR-125b Overexpression and LPS-Stimulation in Hepatocytes and Cholangiocyte Cells
2.4. UDCA Treatment Reduces the Expression of miR-125b
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Cell Culture
4.3. Cell Transfection and Treatments
4.4. RNA and miRNA Expression Analysis
4.5. ELISA Analyses
4.6. Statistical Analysis
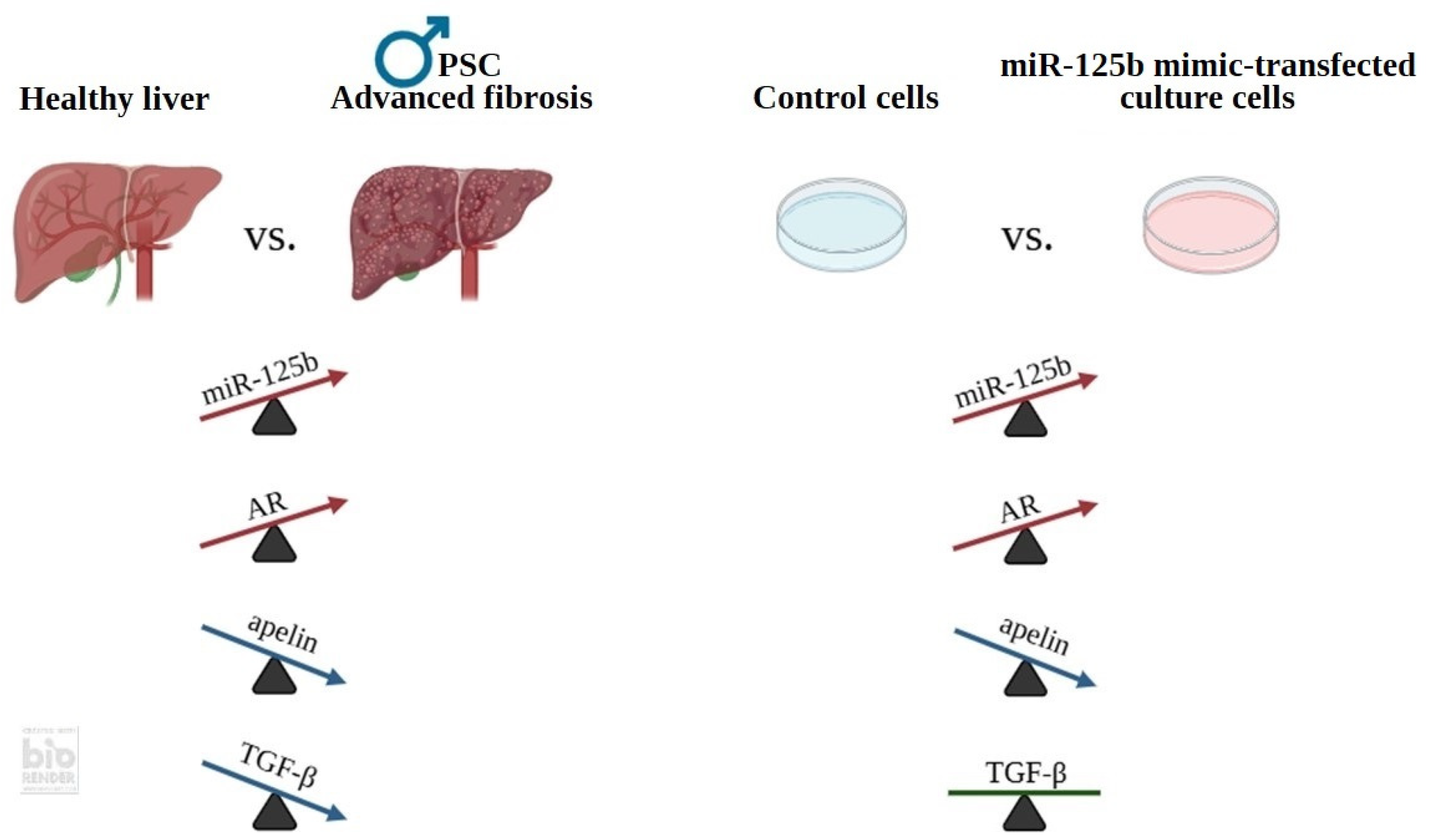
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirschfield, G.M.; Heathcote, E.J.; Gershwin, M.E. Pathogenesis of Cholestatic Liver Disease and Therapeutic Approaches. Gastroenterology 2010, 139, 1481–1496. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Wang, B.E.; Shen, F.J.; Wang, A.M.; Jia, J.D.; Ma, H. Dynamic Evolution of Mmp-13, Timp-1, Type I and Iii Collagen and Their Interaction in Experimental Liver Fibrosis. Chin. J. Hepatol. 2004, 12, 612–615. [Google Scholar]
- Tacke, F.; Weiskirchen, R. Update on Hepatic Stellate Cells: Pathogenic Role in Liver Fibrosis and Novel Isolation Techniques. Expert Rev. Gastroenterol. Hepatol. 2012, 6, 67–80. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R. Key Discoveries in Bile Acid Chemistry and Biology and Their Clinical Applications: History of the Last Eight Decades. J. Lipid Res. 2014, 55, 1553–1595. [Google Scholar] [CrossRef]
- Worthington, J.J.; Kelly, A.; Smedley, C.; Bauche, D.; Campbell, S.; Marie, J.C.; Travis, M.A. Integrin Alphavbeta8-Mediated TGF-Beta Activation by Effector Regulatory T Cells Is Essential for Suppression of T-Cell-Mediated Inflammation. Immunity 2015, 42, 903–915. [Google Scholar] [CrossRef]
- Pchejetski, D.; Foussal, C.; Alfarano, C.; Lairez, O.; Calise, D.; Guilbeau-Frugier, C.; Schaak, S.; Seguelas, M.H.; Wanecq, E.; Valet, P.; et al. Apelin Prevents Cardiac Fibroblast Activation and Collagen Production Through Inhibition of Sphingosine Kinase 1. Eur. Heart J. 2012, 33, 2360–2369. [Google Scholar] [CrossRef]
- Sutton, T.A.; Hato, T.; Mai, E.; Yoshimoto, M.; Kuehl, S.; Anderson, M.; Mang, H.; Plotkin, Z.; Chan, R.J.; Dagher, P.C. p53 Is Renoprotective After Ischemic Kidney Injury by Reducing Inflammation. J. Am. Soc. Nephrol. 2013, 24, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Kleinz, M.J.; Davenport, A.P. Emerging Roles of Apelin in Biology and Medicine. Pharmacol. Ther. 2005, 107, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Owen, N.E.; Nyimanu, D.; Kuc, R.E.; Upton, P.D.; Morrell, N.W.; Alexander, G.J.; Maguire, J.J.; Davenport, A.P. Plasma Levels of Apelin Are Reduced in Patients with Liver Fibrosis and Cirrhosis but Are Not Correlated with Circulating Levels of Bone Morphogenetic Protein 9 and 10. Peptides 2021, 136, 170440. [Google Scholar] [CrossRef]
- Ismail, A.; Kennedy, L.; Francis, H. Sex-Dependent Differences in Cholestasis: Why Estrogen Signaling May Be a Key Pathophysiological Driver. Am. J. Pathol. 2023, 193, 1355–1362. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Zhou, J.; Fang, H.; Wang, J. Overexpression of Estrogen Receptor β Inhibits Cellular Functions of Human Hepatic Stellate Cells and Promotes the Anti-Fibrosis Effect of Calycosin via Inhibiting Stat3 Phosphorylation. BMC Pharmacol. Toxicol. 2022, 23, 77. [Google Scholar] [CrossRef]
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and Menopause Impact Severity of Fibrosis Among Patients with Nonalcoholic Steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef]
- Poynard, T.; Mathurin, P.; Lai, C.L.; Guyader, D.; Poupon, R.; Tainturier, M.H.; Myers, R.P.; Muntenau, M.; Ratziu, V.; Manns, M.; et al. A Comparison of Fibrosis Progression in Chronic Liver Diseases. J. Hepatol. 2003, 38, 257–265. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, W.L.; Ma, W.L.; Chang, C.; Ou, J.H. Enhancement of Gene Transactivation Activity of Androgen Receptor by Hepatitis B Virus X Protein. Virology 2007, 363, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Steele, R.; Ray, R.; Ray, R.B. Hepatitis C Virus Core Protein Augments Androgen Receptor-Mediated Signaling. J. Virol. 2008, 82, 11066–11072. [Google Scholar] [CrossRef] [PubMed]
- Panella, M.; Carotenuto, P.; Braconi, C. MicroRNAs Link Inflammation and Primary Biliary Cholangitis. Non Coding RNA Investig. 2018, 2, 48. [Google Scholar] [CrossRef]
- Nagpal, V.; Rai, R.; Place, A.T.; Murphy, S.B.; Verma, S.K.; Ghosh, A.K.; Vaughan, D.E. MiR-125b Is Critical for Fibroblast-to-Myofibroblast Transition and Cardiac Fibrosis. Circulation 2016, 133, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Onori, P.; Hargrove, L.; Han, Y.; Kennedy, L.; Graf, A.; Hodges, K.; Ueno, Y.; Francis, T.; Gaudio, E.; et al. Regulation of the Histamine/VEGF Axis by miR-125b During Cholestatic Liver Injury in Mice. Am. J. Pathol. 2014, 184, 662–673. [Google Scholar] [CrossRef]
- Tan, G.; Niu, J.; Shi, Y.; Ouyang, H.; Wu, Z.H. NF-κB-Dependent microRNA-125b up-Regulation Promotes Cell Survival by Targeting P38α Upon Ultraviolet Radiation. J. Biol. Chem. 2012, 287, 33036–33047. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Zang, W.; Wang, H.; Chu, H.; Li, P.; Li, M.; Zhang, G.; Zhao, G. Downregulation of microRNA-182 Inhibits Cell Growth and Invasion by Targeting Programmed Cell Death 4 in Human Lung Adenocarcinoma Cells. Tumor Biol. 2014, 35, 39–46. [Google Scholar] [CrossRef]
- Kwekel, J.C.; Vijay, V.; Han, T.; Moland, C.L.; Desai, V.G.; Fuscoe, J.C. Sex and Age Differences in the Expression of Liver microRNAs During the Life Span of F344 Rats. Biol. Sex Differ. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bemis, L.; Su, L.J.; Gao, D.; Flaig, T.W. miR-125b Regulation of Androgen Receptor Signaling Via Modulation of the Receptor Complex Co-Repressor NCOR2. BioRes. Open Access 2012, 1, 55–62. [Google Scholar] [CrossRef]
- Ma, W.L.; Lai, H.C.; Yeh, S.; Cai, X.J.; Chang, C.S. Androgen Receptor Roles in Hepatocellular Carcinoma, Fatty Liver, Cirrhosis and Hepatitis. Endocr. Relat. Cancer 2014, 21, R165–R182. [Google Scholar] [CrossRef]
- Ye, H.L.; Zhang, J.W.; Chen, X.Z.; Wu, P.B.; Chen, L.; Zhang, G. Ursodeoxycholic Acid Alleviates Experimental Liver Fibrosis Involving Inhibition of Autophagy. Life Sci. 2020, 242, 117175. [Google Scholar] [CrossRef]
- Corpechot, C.; Carrat, F.; Bonnand, A.M.; Poupon, R.E.; Poupon, R. The Effect of Ursodeoxycholic Acid Therapy on Liver Fibrosis Progression in Primary Biliary Cirrhosis. Hepatology 2000, 32, 1196–1199. [Google Scholar] [CrossRef]
- Isayama, H.; Tazuma, S.; Kokudo, N.; Tanaka, A.; Tsuyuguchi, T.; Nakazawa, T.; Notohara, K.; Mizuno, S.; Akamatsu, N.; Serikawa, M.; et al. Clinical Guidelines for Primary Sclerosing Cholangitis 2017. J. Gastroenterol. 2018, 53, 1006–1034. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Dyson, J.K.; Alexander, G.J.M.; Chapman, M.H.; Collier, J.; Hubscher, S.; Patanwala, I.; Pereira, S.P.; Thain, C.; Thorburn, D.; et al. The British Society of Gastroenterology/UK-PBC Primary Biliary Cholangitis Treatment and Management Guidelines. Gut 2018, 67, 1568–1594. [Google Scholar] [CrossRef]
- Dyson, J.K.; Beuers, U.; Jones, D.E.J.; Lohse, A.W.; Hudson, M. Primary Sclerosing Cholangitis. Lancet 2018, 391, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Karlsen, T.H.; Lindor, K.D.; Adams, D.H. Primary Sclerosing Cholangitis. Lancet 2013, 382, 1587–1599. [Google Scholar] [CrossRef]
- Yang, D.K.; Yuan, Q.G.; Balakrishnan, A.; Bantel, H.; Klusmann, J.H.; Manns, M.P.; Ott, M.; Cantz, T.; Sharma, A.D. MicroRNA-125b-5p Mimic Inhibits Acute Liver Failure. Nat. Commun. 2016, 7, 11916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Liu, Y.; Xiao, L.L.; Li, S.F.; Jiang, J.H.; Zhao, Y.; Qian, S.W.; Tang, Q.Q.; Li, X. Upregulation of miR-125b by Estrogen Protects Against Non-Alcoholic Fatty Liver in Female Mice. J. Hepatol. 2015, 63, 1466–1475. [Google Scholar] [CrossRef]
- Liang, L.; Wong, C.M.; Ying, Q.; Fan, D.N.; Huang, S.; Ding, J.; Yao, J.; Yan, M.; Li, J.; Yao, M.; et al. MicroRNA-125b Suppressesed Human Liver Cancer Cell Proliferation and Metastasis by Directly Targeting Oncogene LIN28B2. Hepatology 2010, 52, 1731–1740. [Google Scholar] [CrossRef]
- Kalafateli, M.; Triantos, C.; Tsochatzis, E.; Michalaki, M.; Koutroumpakis, E.; Thomopoulos, K.; Kyriazopoulou, V.; Jelastopulu, E.; Burroughs, A.; Lambropoulou-Karatza, C.; et al. Adipokines Levels are Associated with the Severity of Liver Disease in Patients with Alcoholic Cirrhosis. World J. Gastroenterol. 2015, 21, 3020–3029. [Google Scholar] [CrossRef]
- Harmon, C.; Jameson, G.; Almuaili, D.; Houlihan, D.D.; Hoti, E.; Geoghegan, J.; Robinson, M.W.; O’Farrelly, C. Liver-Derived TGF-Beta Maintains the Eomes(Hi)Tbet(Lo) Phenotype of Liver Resident Natural Killer Cells. Front. Immunol. 2019, 10, 1502. [Google Scholar] [CrossRef]
- Morris, S.M.; Baek, J.Y.; Koszarek, A.; Kanngurn, S.; Knoblaugh, S.E.; Grady, W.M. Transforming Growth Factor-Beta Signaling Promotes Hepatocarcinogenesis Induced by P53 Loss. Hepatology 2012, 55, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Bhattacharyya, S.; Varga, J. The Tumor Suppressor P53 Abrogates Smad-Dependent Collagen Gene Induction in Mesenchymal Cells. J. Biol. Chem. 2004, 279, 47455–47463. [Google Scholar] [CrossRef]
- Dagher, P.C.; Mai, E.M.; Hato, T.; Lee, S.Y.; Anderson, M.D.; Karozos, S.C.; Mang, H.E.; Knipe, N.L.; Plotkin, Z.; Sutton, T.A. The p53 Inhibitor Pifithrin-α Can Stimulate Fibrosis in a Rat Model of Ischemic Acute Kidney Injury. Am. J. Physiol. Ren. Physiol. 2012, 302, F284–F291. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.N.; Teh, C.; Shyh-Chang, N.; Xie, H.M.; Zhou, B.Y.; Korzh, V.; Lodish, H.F.; Lim, B. MicroRNA-125b Is a Novel Negative Regulator of P53. Genes Dev. 2009, 23, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Shaham, L.; Binder, V.; Gefen, N.; Borkhardt, A.; Izraeli, S. MiR-125 in Normal and Malignant Hematopoiesis. Leukemia 2012, 26, 2011–2018. [Google Scholar] [CrossRef]
- Yeh, S.H.; Chen, P.J. Gender Disparity of Hepatocellular Carcinoma: The Roles of Sex Hormones. Oncology 2010, 78, 172–179. [Google Scholar] [CrossRef]
- Tanaka, K.; Sakai, H.; Hashizume, M.; Hirohata, T. Serum Testosterone: Estradiol Ratio and the Development of Hepatocellular Carcinoma Among Male Cirrhotic Patients. Cancer Res. 2000, 60, 5106–5110. [Google Scholar]
- Yu, M.W.; Cheng, S.W.; Lin, M.W.; Yang, S.Y.; Liaw, Y.F.; Chang, H.C.; Hsiao, T.J.; Lin, S.M.; Lee, S.D.; Chen, P.J.; et al. Androgen-Receptor Gene CAG Repeats, Plasma Testosterone Levels, and Risk of Hepatitis B-Related Hepatocellular Carcinoma. J. Natl. Cancer Inst. 2000, 92, 2023–2028. [Google Scholar] [CrossRef]
- Allam, S.; Elsakka, E.G.E.; Ismail, A.; Doghish, A.S.; Yehia, A.M.; Elkady, M.A.; Mokhlis, H.A.; Sayed, S.M.; Abd Elaziz, A.I.; Hashish, A.A.; et al. Androgen Receptor Blockade by Flutamide Down-Regulates Renal Fibrosis, Inflammation, and Apoptosis Pathways in Male Rats. Life Sci. 2023, 323, 121697. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.; Lu, S.; Yan, L.; Hu, F.; Wang, Z.; Cheng, B. Androgen Receptor Regulates Cardiac Fibrosis in Mice with Experimental Autoimmune Myocarditis by Increasing microRNA-125b Expression. Biochem. Biophys. Res. Commun. 2018, 506, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sheng, J.; Hu, S.; Cui, Y.; Xiao, J.; Yu, W.; Peng, J.; Han, W.; He, Q.; Fan, Y.; et al. Estrogen and G Protein-Coupled Estrogen Receptor Accelerate the Progression of Benign Prostatic Hyperplasia by Inducing Prostatic Fibrosis. Cell Death Dis. 2022, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, K.; Schonfeld, M.; Rodriguez, A.; Sharma, M.; Weinman, S.; Tikhanovich, I. Androgen Effects on Alcohol-Induced Liver Fibrosis Are Controlled by a Notch-Dependent Epigenetic Switch. Cell. Mol. Gastroenterol. Hepatol. 2025, 19, 101414. [Google Scholar] [CrossRef]
- Shi, X.B.; Xue, L.; Yang, J.; Ma, A.H.; Zhao, J.; Xu, M.; Tepper, C.G.; Evans, C.P.; Kung, H.J.; White, R.W.D. An Androgen-Regulated miRNA Suppresses Bak1 Expression and Induces Androgen-Independent Growth of Prostate Cancer Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 19983–19988. [Google Scholar] [CrossRef]
- Shi, X.B.; Xue, L.R.; Ma, A.H.; Tepper, C.G.; Kung, H.J.; White, R.W.D. miR-125b Promotes Growth of Prostate Cancer Xenograft Tumor Through Targeting Pro-Apoptotic Genes. Prostate 2011, 71, 538–549. [Google Scholar] [CrossRef]
- Calabrese, F.; Valente, M.; Giacometti, C.; Pettenazzo, E.; Benvegnu, L.; Alberti, A.; Gatta, A.; Pontisso, P. Parenchymal Transforming Growth Factor Beta-1: Its Type II Receptor and Smad Signaling Pathway Correlate with Inflammation and Fibrosis in Chronic Liver Disease of Viral Etiology. J. Gastroenterol. Hepatol. 2003, 18, 1302–1308. [Google Scholar] [CrossRef]
- Casini, A.; Ceni, E.; Salzano, R.; Biondi, P.; Parola, M.; Galli, A.; Foschi, M.; Caligiuri, A.; Pinzani, M.; Surrenti, C. Neutrophil-Derived Superoxide Anion Induces Lipid Peroxidation and Stimulates Collagen Synthesis in Human Hepatic Stellate Cells: Role of Nitric Oxide. Hepatology 1997, 25, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J.; Cimino, A.; Costinean, S.; Dumitru, C.D.; Adair, B.; Fabbri, M.; Alder, H.; Liu, C.G.; Calin, G.A.; et al. Modulation of miR-155 and miR-125b Levels Following Lipopolysaccharide/TNF-α Stimulation and Their Possible Roles in Regulating the Response to Endotoxin Shock. J. Immunol. 2007, 179, 5082–5089. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, J.T.; Sohal, A.; Handa, P.; Maliken, B.D.; Kim, T.K.; Wang, K.; Gochanour, E.; Li, Y.; Rose, J.B.; Nelson, J.E.; et al. Serum miRNA Profiles Are Altered in Patients with Primary Sclerosing Cholangitis Receiving High-Dose Ursodeoxycholic Acid. JHEP Rep. 2023, 5, 100729. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Patients with PSC | |||||
|---|---|---|---|---|---|---|
| Females n = 23 | Males n = 43 | Total n = 66 | ||||
| Rho | p-Value * | Rho | p-Value * | Rho | p-Value * | |
| miR-125 and ALP | NS | NS | 0.4 | 0.01 | ||
| miR-125 and ALT | 0.8 | 0.0001 | 0.6 | 0.001 | 0.6 | 0.0001 |
| miR-125 and AST | 0.7 | 0.002 | 0.6 | 0.0009 | 0.6 | 0.002 |
| miR-125 and GGTP. | 0.8 | 0.0001 | NS | NS | ||
| miR-125 and bilirubin | NS | 0.5 | 0.02 | 0.4 | 0.03 | |
| Parameter | Control (n = 15) | PSC (n = 14) |
|---|---|---|
| Sex (Female/Male) | 7/8 | 4/10 |
| Age (years) | N/A | 36.5 ± 3.5 |
| Bilirubin (mgL/dL, NR: 0.1–1.1) | N/A | 4.69 ± 1.25 |
| ALP (IU/L, NR: 40–120) | N/A | 611.3 ± 220.6 |
| ALT (IU/L, NR: <40) | N/A | 130.8 ± 43.7 |
| AST (IU/L, NR: 5–35) | N/A | 207.5 ± 54.0 |
| GGTP (IU/L, normal F < 66, M < 100) | N/A | 123.4 ± 27.4 |
| miR-125b | 0.91 ± 0.12 | 1.69 ± 0.23 |
| Parameter | Control (n = 27) | PSC (n = 66) |
|---|---|---|
| Sex (Female/Male) | 17/10 | 23/43 |
| Age (years) | N/A | 35.3 ± 2.8/30.4 ± 1.7 |
| Bilirubin (mgL/dL, NR: 0.1–1.1) | N/A | 2.0 ± 0.35 |
| ALP (IU/L, NR: 40–120) | N/A | 312.3 ± 30.7 |
| ALT (IU/L, NR: <40) | N/A | 102.6 ± 9.5 |
| AST (IU/L, NR: 5–35) | N/A | 73.1 ± 6.6 |
| GGTP (IU/L, normal F < 66, M < 100) | N/A | 331 ± 46.3 |
| Fibrosis from FibroScan (yes/no) | N/A | 28/38 |
| Fibrosis (yes/no) | N/A | 28/38 |
| AST/ALT ratio cirrhosis | N/A | 14/52 |
| Cirrhosis (yes/no) | N/A | 13/53 |
| miR-125b | 1.4 ± 0.3 | 6.63 ± 1.27 |
| Parameter | Before UDCA (n = 9) | After UDCA (n = 9) | |
|---|---|---|---|
| Sex (Female/Male) | 3/6 | ||
| Age (years) | 34.7 ± 2.5 | 37.7 ± 2.5 | |
| Bilirubin (mgL/dL, NR: 0.2–1.1) | 0.95 ± 0.86 | 0.70 ± 0.35 | |
| ALP (IU/L, NR: 30–120) | 303 ± 236 | 154 ± 87 | |
| GGTP (IU/L, normal F < 66, M < 100) | 511 ± 370 | 259 ± 285 | |
| AST (IU/l, NR 5–35) | 59 ± 36 | 48 ± 27 |
| Parameter | Control (n = 10) | Males with PSC (n = 36) |
|---|---|---|
| Sex (Female/Male) | 0/10 | 0/36 |
| Age (years) | N/A | 32.3 ± 2.1 |
| Bilirubin (mgL/dL, NR: 0.1–1.1) | N/A | 1.6 ± 0.3 |
| ALP (IU/L, NR: 40–120) | N/A | 293.1 ± 42 |
| ALT (IU/L, NR: <40) | N/A | 121.4 ± 13.2 |
| AST (IU/L, NR: 5–35) | N/A | 76.8 ± 9.4 |
| GGTP (IU/L, normal F < 66, M < 100) | N/A | 330.6 ± 55.5 |
| Fibrosis (yes/no) | N/A | 15/21 |
| Cirrhosis (yes/no) | N/A | 6/30 |
| miR-125b | 1.2 ± 0.3 | 5.45 ± 1.35 |
| Parameter | Control (n = 17) | PSC (n = 29) |
|---|---|---|
| Sex (Female/Male) | 10/7 | 14/15 |
| Age (years) | N/A | 35.2 ± 1.8 |
| Bilirubin (mgL/dL, NR: 0.1–1.1) | N/A | 1.8 ± 0.33 |
| ALP (IU/L, NR: 40–120) | N/A | 359.7 ± 45.7 |
| ALT (IU/L, NR: <40) | N/A | 75.5 ± 9.3 |
| AST (IU/L, NR: 5–35) | N/A | 70 ± 10.4 |
| GGTP (IU/L, normal F < 66, M < 100) | N/A | 248.7 ± 58.9 |
| Cirrhosis (yes/no) | N/A | 7/22 |
| miR-125b | 1.22 ± 0.26 | 0.93 ± 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramczyk, J.; Milkiewicz, M.; Łaba, A.; Milkiewicz, P.; Banales, J.M.; Kempinska-Podhorodecka, A. Sex-Dependent Regulation of Liver Fibrosis in Primary Sclerosing Cholangitis: The Role of miR-125b, Androgen Receptors, TGF-β, and Apelin Signalling. Int. J. Mol. Sci. 2025, 26, 7784. https://doi.org/10.3390/ijms26167784
Abramczyk J, Milkiewicz M, Łaba A, Milkiewicz P, Banales JM, Kempinska-Podhorodecka A. Sex-Dependent Regulation of Liver Fibrosis in Primary Sclerosing Cholangitis: The Role of miR-125b, Androgen Receptors, TGF-β, and Apelin Signalling. International Journal of Molecular Sciences. 2025; 26(16):7784. https://doi.org/10.3390/ijms26167784
Chicago/Turabian StyleAbramczyk, Joanna, Malgorzata Milkiewicz, Alicja Łaba, Piotr Milkiewicz, Jesus M. Banales, and Agnieszka Kempinska-Podhorodecka. 2025. "Sex-Dependent Regulation of Liver Fibrosis in Primary Sclerosing Cholangitis: The Role of miR-125b, Androgen Receptors, TGF-β, and Apelin Signalling" International Journal of Molecular Sciences 26, no. 16: 7784. https://doi.org/10.3390/ijms26167784
APA StyleAbramczyk, J., Milkiewicz, M., Łaba, A., Milkiewicz, P., Banales, J. M., & Kempinska-Podhorodecka, A. (2025). Sex-Dependent Regulation of Liver Fibrosis in Primary Sclerosing Cholangitis: The Role of miR-125b, Androgen Receptors, TGF-β, and Apelin Signalling. International Journal of Molecular Sciences, 26(16), 7784. https://doi.org/10.3390/ijms26167784






