Anticancer Activity of the Marine-Derived Compound Bryostatin 1: Preclinical and Clinical Evaluation
Abstract
1. Introduction
2. Marine Compounds
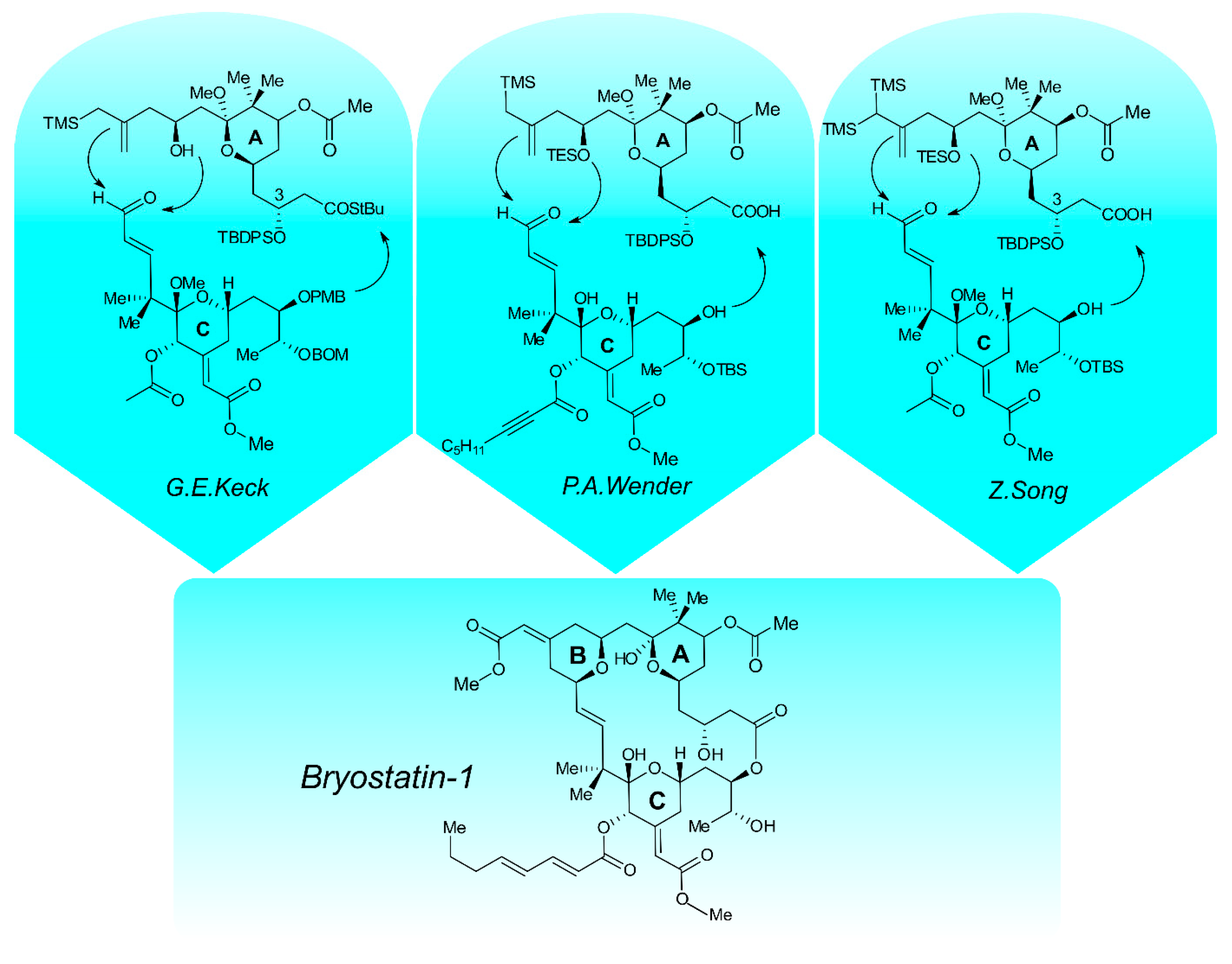
3. Chemistry and Synthesis of Bryostatin 1
4. Mechanism of Action
4.1. Modulation of Protein Kinase C (PKC)
4.2. Induction of Apoptosis in Cancer Cells Through Activation of Signaling Pathways
4.3. Pharmacokinetics of Bryostatin 1
5. Preclinical and Clinical Studies
5.1. In Vitro Studies of Bryostatin 1
5.2. In Vivo Studies on Bryostatin 1
5.3. Clinical Trials on Bryostatin 1
6. Conclusions, Future Perspectives and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO) Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 31 March 2025).
- Cancer TODAY DataViz. Available online: https://gco.iarc.fr/today/en/dataviz/pie?mode=population&group_populations=0 (accessed on 31 March 2025).
- Sharifi-Rad, J.; Ozleyen, A.; Boyunegmez Tumer, T.; Oluwaseun Adetunji, C.; El Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules 2019, 9, 679. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting Cancer Signaling Pathways by Natural Products: Exploring Promising Anti-Cancer Agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Gupta, A.P.; Pandotra, P.; Sharma, R.; Kushwaha, M.; Gupta, S. Marine Resource: A Promising Future for Anticancer Drugs. Stud. Nat. Prod. Chem. 2013, 40, 229–325. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Tamzi, N.N.; Rahman, M.M.; Das, S. Recent Advances in Marine-Derived Bioactives towards Cancer Therapy. Int. J. Transl. Med. 2024, 4, 740–781. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Montalbano, A.; Cueto, M.; Díaz Marrero, A.R.; Deniz, I.; Erdoğan, A.; Lukić Bilela, L.; Moulin, C.; Taffin-de-Givenchy, E.; et al. Marine Anticancer Agents: An Overview with a Particular Focus on Their Chemical Classes. Mar. Drugs 2020, 18, 619. [Google Scholar] [CrossRef]
- Wu, L.; Ye, K.; Jiang, S.; Zhou, G. Marine Power on Cancer: Drugs, Lead Compounds, and Mechanisms. Mar. Drugs 2021, 19, 488. [Google Scholar] [CrossRef]
- Sun, M.-K.; Alkon, D.L. Bryostatin-1: Pharmacology and Therapeutic Potential as a CNS Drug. CNS Drug Rev. 2006, 12, 1–8. [Google Scholar] [CrossRef]
- Kortmansky, J.; Schwartz, G.K. Bryostatin-1: A Novel PKC Inhibitor in Clinical Development. Cancer Investig. 2003, 21, 924–936. [Google Scholar] [CrossRef]
- Trindade-Silva, A.E.; Lim-Fong, G.E.; Sharp, K.H.; Haygood, M.G. Bryostatins: Biological Context and Biotechnological Prospects. Curr. Opin. Biotechnol. 2010, 21, 834–842. [Google Scholar] [CrossRef]
- Smithsonian Environmental Research Center Bugula Neritina. Available online: https://invasions.si.edu/nemesis/species_summary/-95 (accessed on 2 April 2025).
- Sharp, K.H.; Davidson, S.K.; Haygood, M.G. Localization of “Candidatus Endobugula Sertula” and the Bryostatins throughout the Life Cycle of the Bryozoan Bugula Neritina. ISME J. 2007, 1, 693–702. [Google Scholar] [CrossRef]
- National Library of Medicine (NIH) Bryostatin 1. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5280757 (accessed on 1 April 2025).
- Sigma Aldrich NMR Chemical Shifts of Impurities. Available online: https://www.sigmaaldrich.com/MX/en/technical-documents/technical-article/genomics/cloning-and-expression/blue-white-screening (accessed on 1 April 2025).
- Saeed, A.F.U.H.; Su, J.; Ouyang, S. Marine-Derived Drugs: Recent Advances in Cancer Therapy and Immune Signaling. Biomed. Pharmacother. 2021, 134, 111091. [Google Scholar] [CrossRef]
- Quitério, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine Health-Promoting Compounds: Recent Trends for Their Characterization and Human Applications. Foods 2021, 10, 3100. [Google Scholar] [CrossRef]
- DrugBank Trabectedin. Available online: https://go.drugbank.com/drugs/DB05109 (accessed on 1 April 2025).
- Smithsonian Environmental Research Center Ecteinascidia Turbinata. Available online: https://invasions.si.edu/nemesis/species_summary/159142 (accessed on 1 April 2025).
- Larsen, A.K.; Galmarini, C.M.; D’Incalci, M. Unique Features of Trabectedin Mechanism of Action. Cancer Chemother. Pharmacol. 2015, 77, 663–671. [Google Scholar] [CrossRef]
- Gadducci, A.; Cosio, S. Trabectedin and Lurbinectedin: Mechanisms of Action, Clinical Impact, and Future Perspectives in Uterine and Soft Tissue Sarcoma, Ovarian Carcinoma, and Endometrial Carcinoma. Front. Oncol. 2022, 12, 914342. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Kaklamani, V.; Kalinsky, K. Perspectives on the Mechanism of Action and Clinical Application of Eribulin for Metastatic Breast Cancer. Future Oncol. 2019, 15, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.; Gupta, S. Eribulin Drug Review. South Asian J. Cancer 2014, 3, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.J.; Zheng, W.; Seletsky, B.M.; Littlefield, B.A.; Kishi, Y. Case History: Discovery of Eribulin (HALAVENTM), a Halichondrin B Analogue That Prolongs Overall Survival in Patients with Metastatic Breast Cancer. Annu. Rep. Med. Chem. 2011, 46, 227–241. [Google Scholar] [CrossRef]
- Seshadri, P.; Deb, B.; Kumar, P. Multifarious Targets beyond Microtubules—Role of Eribulin in Cancer Therapy. Front. Biosci.-Sch. 2021, 13, 157–172. [Google Scholar] [CrossRef]
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.D.; Mateos, M.V.; Martin, A. Plitidepsin: Design, Development, and Potential Place in Therapy. Drug Des. Dev. Ther. 2017, 11, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Washington Invasive Species Council Tunicate. Available online: https://invasivespecies.wa.gov/priorityspecies/tunicate/ (accessed on 1 April 2025).
- Losada, A.; Muñoz-Alonso, M.J.; García, C.; Sánchez-Murcia, P.A.; Martínez-Leal, J.F.; Domínguez, J.M.; Lillo, M.P.; Gago, F.; Galmarini, C.M. Translation Elongation Factor EEF1A2 Is a Novel Anticancer Target for the Marine Natural Product Plitidepsin. Sci. Rep. 2016, 6, 35100. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Annex Scientific Conclusions and Grounds for Refusal Presented by the European Medicines Agency; European Comission: Brussel, Belgium, 2018.
- Nigam, M.; Suleria, H.A.R.; Farzaei, M.H.; Mishra, A.P. Marine Anticancer Drugs and Their Relevant Targets: A Treasure from the Ocean. DARU J. Pharm. Sci. 2019, 27, 491–515. [Google Scholar] [CrossRef] [PubMed]
- DrugBank Cytarabine. Available online: https://go.drugbank.com/drugs/DB00987 (accessed on 26 July 2025).
- Cerrano, C.; Pansini, M.; Valisano, L.; Calcinai, B.; Sarà, M.; Bavestrello, G. Lagoon sponges from carrie bow cay (Belize): Ecological benefits of selective sediment incorporation. BMIB-Boll. Dei Musei E Degli Ist. Biol. 2004, 68, 239–252. [Google Scholar]
- DrugBank Vidarabine. Available online: https://go.drugbank.com/drugs/DB00194 (accessed on 26 July 2025).
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral Lead Compounds from Marine Sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef]
- Shilpi, J.A.; Uddin, S.J. Analgesic and Antipyretic Natural Products. Annu. Rep. Med. Chem. 2020, 55, 435–458. [Google Scholar] [CrossRef]
- Montalvão, S.I.G.H.M.; Singh, V.; Haque, S. Bioassays for Bioactivity Screening. Compr. Anal. Chem. 2014, 65, 79–114. [Google Scholar] [CrossRef]
- DrugBank Ziconotide. Available online: https://go.drugbank.com/drugs/DB06283 (accessed on 26 July 2025).
- Raghuvanshi, R.; Bharate, S.B. Preclinical and Clinical Studies on Bryostatins, a Class of Marine-Derived Protein Kinase c Modulators: A Mini-Review. Curr. Top. Med. Chem. 2020, 20, 1124–1135. [Google Scholar] [CrossRef]
- Davidson, S.K.; Haygood, M.G. Identification of Sibling Species of the Bryozoan Bugula Neritina That Produce Different Anticancer Bryostatins and Harbor Distinct Strains of the Bacterial Symbiont “Candidatus Endobugula Sertula”. Biol. Bull. 1999, 196, 273–280. [Google Scholar] [CrossRef]
- Slocum, S.T.; Lowell, A.N.; Tripathi, A.; Shende, V.V.; Smith, J.L.; Sherman, D.H. Chemoenzymatic Dissection of Polyketide β-Branching in the Bryostatin Pathway. Methods Enzymol. 2018, 604, 207–236. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Nature as Source of Medicines; Novel Drugs from Nature; Screening for Antitumor Activity. Compr. Nat. Prod. II 2010, 3, 135–175. [Google Scholar] [CrossRef]
- Kedei, N.; Lewin, N.E.; Géczy, T.; Selezneva, J.; Braun, D.C.; Chen, J.; Herrmann, M.A.; Heldman, M.R.; Lim, L.; Mannan, P.; et al. Biological Profile of the Less Lipophilic and Synthetically More Accessible Bryostatin 7 Closely Resembles that of Bryostatin 1. ACS Chem. Biol. 2013, 8, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Wang, Y.; Buckl, A.K.; Huang, Z.; Nguyen, M.H.; Kuzmina, O. Total Synthesis of Bryostatin 3. Science 2020, 368, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.M.; Carter, P.H.; Carreira, E.M.; Charette, A.B.; Prunet, J.A.; Lautens, M. Total Synthesis of Bryostatin 2. J. Am. Chem. Soc. 1999, 121, 7540–7552. [Google Scholar] [CrossRef]
- DrugBank Bryostatin 1: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB11752 (accessed on 2 April 2025).
- National Library of Medicine (NIH) Antineoplastic Agents—MeSH—NCBI. Available online: https://www.ncbi.nlm.nih.gov/mesh/68000970 (accessed on 2 April 2025).
- Guo, Q.; Yang, Y.; Zhang, H.; Wang, D.; Li, B.; Jiang, D.; Tu, X.; Gao, X.; Zhang, C.; Qin, Y.; et al. Total Syntheses of Bryostatins 1, 7, 9 and 9-N3. Angew. Chem. Int. Ed. 2025, 64, e202423465. [Google Scholar] [CrossRef]
- Pettit, G.R.; Herald, C.L.; Doubek, D.L.; Herald, D.L.; Arnold, E.; Clardy, J. Isolation and Structure of Bryostatin 1. J. Am. Chem. Soc. 1982, 104, 6846–6848. [Google Scholar] [CrossRef]
- Sudek, S.; Lopanik, N.B.; Waggoner, L.E.; Hildebrand, M.; Anderson, C.; Liu, H.; Patel, A.; Sherman, D.H.; Haygood, M.G. Identification of the Putative Bryostatin Polyketide Synthase Gene Cluster from “CandidatusEndobugula Sertula”, the Uncultivated Microbial Symbiont of the Marine BryozoanBugula Neritina. J. Nat. Prod. 2007, 70, 67–74. [Google Scholar] [CrossRef]
- Miller, I.J.; Vanee, N.; Fong, S.S.; Lim-Fong, G.E.; Kwan, J.C. Lack of Overt Genome Reduction in the Bryostatin-Producing Bryozoan Symbiont “Candidatus Endobugula Sertula”. Appl. Environ. Microbiol. 2016, 82, 6573–6583. [Google Scholar] [CrossRef]
- Schaufelberger, D.E.; Koleck, M.P.; Beutler, J.A.; Vatakis, A.M.; Alvarado, A.B.; Andrews, P.; Marzo, L.V.; Muschik, G.M.; Roach, J.; Ross, J.T.; et al. The Large-Scale Isolation of Bryostatin 1 from Bugula Neritina Following Current Good Manufacturing Practices. J. Nat. Prod. 1991, 54, 1265–1270. [Google Scholar] [CrossRef]
- Castor, T.P. Method and Apparatus for Isolating Therapeutic Compositions from Source Materials 1998. U.S. Patent 5,750,709, 12 May 1998. [Google Scholar]
- Keck, G.E.; Poudel, Y.B.; Cummins, T.J.; Rudra, A.; Covel, J.A. Total Synthesis of Bryostatin 1. J. Am. Chem. Soc. 2010, 133, 744–747. [Google Scholar] [CrossRef]
- Keck, G.E.; Truong, A.P. Synthetic Studies on the Bryostatins: Preparation of a Truncated BC-Ring Intermediate by Pyran Annulation. Org. Lett. 2005, 7, 2149–2152. [Google Scholar] [CrossRef]
- Keck, G.E.; Welch, D.S.; Poudel, Y.B. Synthetic Studies toward Bryostatin 1: Preparation of a C1–C16 Fragment by Pyran Annulation. Tetrahedron Lett. 2006, 47, 8267–8270. [Google Scholar] [CrossRef]
- Wender, P.A.; Hardman, C.T.; Ho, S.; Jeffreys, M.S.; Maclaren, J.K.; Quiroz, R.V.; Ryckbosch, S.M.; Shimizu, A.J.; Sloane, J.L.; Stevens, M.C. Scalable Synthesis of Bryostatin 1 and Analogs, Adjuvant Leads against Latent HIV. Science 2017, 358, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Tamao, K.; Sumitani, K.; Kumada, M. Selective Carbon-Carbon Bond Formation by Cross-Coupling of Grignard Reagents with Organic Halides. Catal. By Nickel-Phosphine Complexes. J. Am. Chem. Soc. 1972, 94, 4374–4376. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Masse, J.P. Activation of Grignard Reagents by Transition-Metal Complexes. A New and Simple Synthesis of Trans-Stilbenes and Polyphenyls. J. Chem. Soc. Chem. Commun. 1972, 144a. [Google Scholar] [CrossRef]
- Chu, Z.; Tong, R.; Yang, Y.; Song, X.; Hu, T.B.; Fan, Y.; Zhao, C.; Gao, L.; Song, Z. Diverse Synthesis of the c Ring Fragment of Bryostatins via Zn/Cu-Promoted Conjugate Addition of α-Hydroxy Iodide with Enone. Chin. Chem. Lett. 2021, 32, 1–4. [Google Scholar] [CrossRef]
- Rosse, C.; Linch, M.; Kermorgant, S.; Cameron, A.J.M.; Boeckeler, K.; Parker, P.J. PKC and the Control of Localized Signal Dynamics. Nat. Rev. Mol. Cell Biol. 2010, 11, 103–112. [Google Scholar] [CrossRef]
- Nishizuka, Y. The Role of Protein Kinase c in Cell Surface Signal Transduction and Tumour Promotion. Nature 1984, 308, 693–698. [Google Scholar] [CrossRef]
- Li, W.; Zhu, K.; Liu, Y.; Liu, M.; Chen, Q. Recent Advances in PKC Inhibitor Development: Structural Design Strategies and Therapeutic Applications. Eur. J. Med. Chem. 2025, 287, 117290. [Google Scholar] [CrossRef]
- Geraldes, P.; King, G.L. Activation of Protein Kinase c Isoforms and Its Impact on Diabetic Complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef]
- Zhang, D.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Neuroprotective Effect of Protein Kinase Cδ Inhibitor Rottlerin in Cell Culture and Animal Models of Parkinson’s Disease. J. Pharmacol. Exp. Ther. 2007, 322, 913–922. [Google Scholar] [CrossRef]
- Isakov, N. Protein Kinase c (PKC) Isoforms in Cancer, Tumor Promotion and Tumor Suppression. Semin. Cancer Biol. 2018, 48, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; De Ciucis, C.; Ricciarelli, R.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Protein Kinase C: An Attractive Target for Cancer Therapy. Cancers 2011, 3, 531–567. [Google Scholar] [CrossRef] [PubMed]
- Konopatskaya, O.; Poole, A.W. Protein Kinase Cα: Disease Regulator and Therapeutic Target. Trends Pharmacol. Sci. 2010, 31, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hirai, H. Protein Kinase c in the Cerebellum: Its Significance and Remaining Conundrums. Cerebellum 2018, 17, 23–27. [Google Scholar] [CrossRef]
- Mochly-Rosen, D.; Das, K.; Grimes, K.V. Protein Kinase C, an Elusive Therapeutic Target? Nat. Rev. Drug Discov. 2012, 11, 937–957. [Google Scholar] [CrossRef]
- Tian, Z.; Lu, X.-T.; Jiang, X.; Tian, J. Bryostatin-1: A Promising Compound for Neurological Disorders. Front. Pharmacol. 2023, 14, 118411. [Google Scholar] [CrossRef]
- Yi, P.; Schrott, L.M.; Castor, T.; Alexander, J. Bryostatin-1 vs. TPPB: Dose-Dependent APP Processing and PKC-α, -δ, and -ε Isoform Activation in SH-SY5Y Neuronal Cells. J. Mol. Neurosci. 2012, 48, 234–244. [Google Scholar] [CrossRef]
- Nelson, T.R.; Cui, C.; Wang, F.; Alkon, D.L. Reduction of β-Amyloid Levels by Novel Protein Kinase Cϵ Activators. J. Biol. Chem. 2009, 284, 34514–34521. [Google Scholar] [CrossRef]
- Sun, M.-K.; Hongpaisan, J.; Nelson, T.; Alkon, D. Poststroke Neuronal Rescue and Synaptogenesis Mediated in Vivo by Protein Kinase c in Adult Brains. Proc. Natl. Acad. Sci. USA 2008, 105, 13620–13625. [Google Scholar] [CrossRef]
- Farlow, M.; Thompson, R.; Wei, L.-J.; Tuchman, A.; Grenier, E.; Crockford, D.; Wilke, S.; Benison, J.; Alkon, D. A Randomized, Double-Blind, Placebo-Controlled, Phase II Study Assessing Safety, Tolerability, and Efficacy of Bryostatin in the Treatment of Moderately Severe to Severe Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2019, 67, 555–570. [Google Scholar] [CrossRef]
- Thomas, A.; Pepper, C.; Hoy, T.; Bentley, P. Bryostatin Induces Protein Kinase c Modulation, Mcl-1 Up-Regulation and Phosphorylation of Bcl-2 Resulting in Cellular Differentiation and Resistance to Drug-Induced Apoptosis in B-Cell Chronic Lymphocytic Leukemia Cells. Leuk. Lymphoma 2004, 45, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ruan, H.; Demeter, M.R.; Comb, M.J. P90RSK Blocks Bad-Mediated Cell Death via a Protein Kinase C-Dependent Pathway. J. Biol. Chem. 1999, 274, 34859–34867. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P.; Deng, X.; Carr, B.K.; May, W.S. A Functional Role for Mitochondrial Protein Kinase Cα in Bcl2 Phosphorylation and Suppression of Apoptosis. J. Biol. Chem. 1998, 273, 25436–25442. [Google Scholar] [CrossRef] [PubMed]
- al-Katib, A.; Mohammad, R.M.; Dan, M.; Hussein, M.E.; Akhtar, A.; Pettit, G.R.; Sensenbrenner, L.L. Bryostatin 1-Induced Hairy Cell Features on Chronic Lymphocytic Leukemia Cells in Vitro. Exp. Hematol. 1993, 21, 61–65. [Google Scholar]
- Mohammad, R.M.; Beck, F.W.J.; Katato, K.; Hamdy, N.; Wall, N.; Al-Katib, A. Potentiation of 2-Chlorodeoxyadenosine Activity by Bryostatin 1 in the Resistant Chronic Lymphocytic Leukemia Cell Line (WSU-CLL): Association with Increased Ratios of DCK/5′-NT and Bax/Bcl-2. Biol. Chem. 1998, 379, 1253–1262. [Google Scholar] [CrossRef]
- Kitada, S.; Zapata, J.M.; Andreeff, M.; Reed, J.C. Bryostatin and CD40-Ligand Enhance Apoptosis Resistance and Induce Expression of Cell Survival Genes in B-Cell Chronic Lymphocytic Leukaemia. Br. J. Haematol. 1999, 106, 995–1004. [Google Scholar] [CrossRef]
- Asiedu, C.; Biggs, J.; Lilly, M.; Kraft, A.S. Inhibition of Leukemic Cell Growth by the Protein Kinase c Activator Bryostatin 1 Correlates with the Dephosphorylation of Cyclin-Dependent Kinase 2. Cancer Res. 1995, 55, 3716–3720. [Google Scholar]
- Vrana, J.A.; Saunders, A.M.; Chellappan, S.P.; Grant, S. Divergent Effects of Bryostatin 1 and Phorbol Myristate Acetate on Cell Cycle Arrest and Maturation in Human Myelomonocytic Leukemia Cells (U937). Differentiation 1998, 63, 33–42. [Google Scholar] [CrossRef]
- Vrana, J.A.; Kramer, L.B.; Saunders, A.M.; Zhang, X.-F.; Dent, P.; Povirk, L.F.; Grant, S. Inhibition of Protein Kinase c Activator-Mediated Induction of P21CIP1 and P27KIP1 by Deoxycytidine Analogs in Human Leukemia Cells. Biochem. Pharmacol. 1999, 58, 121–131. [Google Scholar] [CrossRef]
- Rajabi, S.; Maresca, M.; Yumashev, A.V.; Choopani, R.; Hajimehdipoor, H. The Most Competent Plant-Derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules 2021, 11, 534. [Google Scholar] [CrossRef] [PubMed]
- Dalisay, D.S.; Tenebro, C.P.; Sabido, E.M.; Faith, A.; June, M.; Reyes-Salarda, R.; Saludes, J.P. Marine-Derived Anticancer Agents Targeting Apoptotic Pathways: Exploring the Depths for Novel Cancer Therapies. Mar. Drugs 2024, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Kumar, D.; Bansal, N.; Narasimhan, B.; Marwaha, R.K.; Sharma, P.C. Understanding Mechanistic Aspects and Therapeutic Potential of Natural Substances as Anticancer Agents. Phytomed. Plus 2023, 3, 100418. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Dai, Y.; Grant, S. Bryostatin 1 Increases 1-β-d-Arabinofuranosylcytosine-Induced Cytochrome c Release and Apoptosis in Human Leukemia Cells Ectopically Expressing Bcl-XL. J. Pharmacol. Exp. Ther. 2002, 301, 568–577. [Google Scholar] [CrossRef]
- Wall, N.R.; Mohammad, R.M.; Reddy, K.B.; Al-Katib, A.M. Bryostatin 1 Induces Ubiquitination and Proteasome Degradation of Bcl-2 in the Human Acute Lymphoblastic Leukemia Cell Line, Reh. Int. J. Mol. Med. 2000, 5, 165–236. [Google Scholar] [CrossRef]
- Vrana, J.A.; Grant, S. Synergistic Induction of Apoptosis in Human Leukemia Cells (U937) Exposed to Bryostatin 1 and the Proteasome Inhibitor Lactacystin Involves Dysregulation of the PKC/MAPK Cascade. Blood 2001, 97, 2105–2114. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Wang, Z.; Boise, L.; Dent, P.; Grant, S. Bryostatin 1 Enhances Paclitaxel-Induced Mitochondrial Dysfunction and Apoptosis in Human Leukemia Cells (U937) Ectopically Expressing Bcl-XL. Leukemia 1999, 13, 1564–1573. [Google Scholar] [CrossRef]
- Von Burstin, V.A.; Xiao, L.; Kazanietz, M.G. Bryostatin 1 Inhibits Phorbol Ester-Induced Apoptosis in Prostate Cancer Cells by Differentially Modulating Protein Kinase c (PKC) δ Translocation and Preventing PKCδ-Mediated Release of Tumor Necrosis Factor-α. Mol. Pharmacol. 2010, 78, 325–332. [Google Scholar] [CrossRef]
- Stang, S.L.; Lopez-Campistrous, A.; Song, X.; Dower, N.A.; Blumberg, P.M.; Wender, P.A.; Stone, J.C. A Proapoptotic Signaling Pathway Involving RasGRP, Erk, and Bim in B Cells. Exp. Hematol. 2009, 37, 122–134.e2. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Zhao, H.; Cai, H.; Gush, K.A.; Kerr, R.G.; Pettit, G.R.; Kraft, A.S. Preclinical Pharmacology of the Natural Product Anticancer Agent Bryostatin 1, an Activator of Protein Kinase C. Cancer Res. 1996, 56, 802–808. [Google Scholar]
- Khan, P.; McGown, A.T.; Dawson, M.J.; Jayson, G.; Prendiville, J.A.; Pettit, G.R.; Crowther, D. High-Performance Liquid Chromatographic Assay for the Novel Antitumor Drug, Bryostatin-1, Incorporating a Serum Extraction Technique. J. Chromatogr. B Biomed. Sci. Appl. 1998, 709, 113–117. [Google Scholar] [CrossRef]
- Zhao, M.; Rudek, M.A.; He, P.; Smith, B.D.; Baker, S.D. Validation and Implementation of a Method for Determination of Bryostatin 1 in Human Plasma by Using Liquid Chromatography/Tandem Mass Spectrometry. Anal. Biochem. 2005, 337, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Korecka, M.; Shaw, L.M. Review of the Newest HPLC Methods with Mass Spectrometry Detection for Determination of Immunosuppressive Drugs in Clinical Practice. Ann. Transplant. 2009, 14, 61–72. [Google Scholar] [PubMed]
- McGown, A.T.; Jayson, G.; Pettit, G.R.; Haran, M.S.; Ward, T.H.; Crowther, D. Bryostatin 1-Tamoxifen Combinations Show Synergistic Effects on the Inhibition of Growth of P388 Cells in Vitro. Br. J. Cancer 1998, 77, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Biberacher, V.; Decker, T.; Oelsner, M.; Wagner, M.; Bogner, C.; Schmidt, B.; Kreitman, R.J.; Peschel, C.; Pastan, I.; Meyer, C.; et al. The Cytotoxicity of Anti-CD22 Immunotoxin Is Enhanced by Bryostatin 1 in B-Cell Lymphomas through CD22 Upregulation and PKC-βII Depletion. Haematologica 2012, 97, 771–779. [Google Scholar] [CrossRef][Green Version]
- Koutcher, J.A.; Motwani, M.; Zakian, K.L.; Li, X.K.; Matei, C.; Dyke, J.P.; Ballon, D.; Yoo, H.H.; Schwartz, G.K. The in Vivo Effect of Bryostatin-1 on Paclitaxel-Induced Tumor Growth, Mitotic Entry, and Blood Flow. Clin. Cancer Res. 2000, 6, 1498–1507. [Google Scholar][Green Version]
- Mohammad, R.M.; Varterasian, M.L.; Almatchy, V.P.; Hannoudi, G.N.; Pettit, G.; Al-Katib, A. Successful Treatment of Human Chronic Lymphocytic Leukemia Xenografts with Combination Biological Agents Auristatin PE and Bryostatin 1. Clin. Cancer Res. 1998, 4, 1337–1343. [Google Scholar][Green Version]
- Mohanty, S.; Huang, J.; Basu, A. Enhancement of Cisplatin Sensitivity of Cisplatin-Resistant Human Cervical Carcinoma Cells by Bryostatin 1. Clin. Cancer Res. 2005, 11, 6730–6737. [Google Scholar] [CrossRef]
- Szallasi, Z.; Smith, C.B.; Pettit, G.R.; Blumberg, P.M. Differential Regulation of Protein Kinase c Isozymes by Bryostatin 1 and Phorbol 12-Myristate 13-Acetate in NIH 3T3 Fibroblasts. J. Biol. Chem. 1994, 269, 2118–2124. [Google Scholar] [CrossRef]
- Yan, W.; Chen, W.C.; Liu, Z.; Huang, L. Bryostatin-I: A Dendritic Cell Stimulator for Chemokines Induction and a Promising Adjuvant for a Peptide Based Cancer Vaccine. Cytokine 2010, 52, 238–244. [Google Scholar] [CrossRef]
- Schuchter, L.M.; Esa, A.H.; May, S.; Laulis, M.K.; Pettit, G.R.; Hess, A.D. Successful Treatment of Murine Melanoma with Bryostatin 1. Cancer Res. 1991, 51, 682–687. [Google Scholar]
- Mohammad, R.M.; Wall, N.R.; Dutcher, J.A.; Al-Katib, A.M. The Addition of Bryostatin 1 to Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (CHOP) Chemotherapy Improves Response in a CHOP-Resistant Human Diffuse Large Cell Lymphoma Xenograft Model. Clin. Cancer Res. 2000, 6, 4950–4956. [Google Scholar] [PubMed]
- Erin, N.; Tavşan, E.; Akdeniz, Ö.; Isca, V.M.S.; Rijo, P. Rebound Increases in Chemokines by CXCR2 Antagonist in Breast Cancer Can Be Prevented by PKCδ and PKCε Activators. Cytokine 2021, 142, 155498. [Google Scholar] [CrossRef] [PubMed]
- Drexler, H.G.; Gignac, S.M.; Jones, R.A.; Scott, C.S.; Pettit, G.R.; Hoffbrand, A.V. Bryostatin 1 Induces Differentiation of B-Chronic Lymphocytic Leukemia Cells. Blood 1989, 74, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; Sharkis, S.J.; Miller, C.B.; Rowinsky, E.K.; Burke, P.J.; May, W.S. Bryostatin 1, a Unique Biologic Response Modifier: Anti-Leukemic Activity in Vitro. Blood 1990, 75, 1319–1323. [Google Scholar] [CrossRef]
- Scheid, C.; Prendiville, J.; Jayson, G.; Crowther, D.; Fox, B.; Pettit, G.R.; Stern, P.L. Immunomodulation in Patients Receiving Intravenous Bryostatin 1 in a Phase I Clinical Study: Comparison with Effects of Bryostatin 1 on Lymphocyte Function in Vitro. Cancer Immunol. Immunother. 1994, 39, 223–230. [Google Scholar] [CrossRef]
- Ariza, M.E.; Ramakrishnan, R.; Singh, N.P.; Chauhan, A.; Nagarkatti, P.S.; Nagarkatti, M. Bryostatin-1, a Naturally Occurring Antineoplastic Agent, Acts as a Toll-like Receptor 4 (TLR-4) Ligand and Induces Unique Cytokines and Chemokines in Dendritic Cells. J. Biol. Chem. 2010, 286, 24–34. [Google Scholar] [CrossRef]
- Spitaler, M.; Utz, I.; Hilbe, W.; Hofmann, J.; Grunicke, H.H. PKC-Independent Modulation of Multidrug Resistance in Cells with Mutant (V185) but Not Wild-Type (G185) P-Glycoprotein by Bryostatin 1. Biochem. Pharmacol. 1998, 56, 861–869. [Google Scholar] [CrossRef]
- Al-Katib, A.M.; Smith, M.R.; Kamanda, W.S.; Pettit, G.R.; Hamdan, M.; Mohamed, A.N.; Chelladurai, B.; Mohammad, R.M. Bryostatin 1 Down-Regulates Mdr1 and Potentiates Vincristine Cytotoxicity in Diffuse Large Cell Lymphoma Xenografts. Clin. Cancer Res. 1998, 4, 1305–1314. [Google Scholar]
- Wang, H.; Mohammad, R.M.; Werdell, J.; Shekhar, P.V. P53 and Protein Kinase c Independent Induction of Growth Arrest and Apoptosis by Bryostatin 1 in a Highly Metastatic Mammary Epithelial Cell Line: In Vitro versus in Vivo Activity. Int. J. Mol. Med. 1998, 1, 915–938. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Sun, Y.; Liu, D. Bryostatin-1 Inhibits Cell Proliferation of Hepatocarcinoma and Induces Cell Cycle Arrest by Activation of GSK3β. Biochem. Biophys. Res. Commun. 2019, 512, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Xu, Y.; Wu, Y.; Hongbo, T.; Wu, M. Bryostatin 1 Causes Attenuation of TPA-Mediated Tumor Promotion in Mouse Skin. Mol. Med. Rep. 2017, 17, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, A.; Lazarus, H.M.; Hartman, P.; Jacobberger, J.W.; Whitacre, C.; Gerson, S.L.; Ksenich, P.; Cooper, B.W.; Frisa, P.S.; Gottlieb, M.; et al. Phase I and Correlative Study of Combination Bryostatin 1 and Vincristine in Relapsed B-Cell Malignancies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 5929–5935. [Google Scholar]
- Roberts, J.D.; Smith, M.R.; Feldman, E.J.; Cragg, L.; Millenson, M.M.; Roboz, G.J.; Honeycutt, C.; Thune, R.; Padavic-Shaller, K.; Carter, W.H.; et al. Phase I Study of Bryostatin 1 and Fludarabine in Patients with Chronic Lymphocytic Leukemia and Indolent (Non-Hodgkin’s) Lymphoma. Clin. Cancer Res. 2006, 12, 5809–5816. [Google Scholar] [CrossRef]
- Haas, N.B.; Smith, M.; Lewis, N.; Littman, L.; Yeslow, G.; Joshi, I.D.; Murgo, A.; Bradley, J.; Gordon, R.; Wang, H.; et al. Weekly Bryostatin-1 in Metastatic Renal Cell Carcinoma: A Phase II Study. Clin. Cancer Res. 2003, 9, 109–114. [Google Scholar]
- Prendiville, J.; Crowther, D.; Thatcher, N.; Woll, P.J.; Fox, B.W.; McGown, A.; Testa, N.; Stern, P.; McDermott, R.; Potter, M.; et al. A Phase I Study of Intravenous Bryostatin 1 in Patients with Advanced Cancer. Br. J. Cancer 1993, 68, 418–424. [Google Scholar] [CrossRef]
- Philip, P.A.; Rea, D.; Thavasu, P.; Carmichael, J.; Stuart, N.S.; Rockett, H.; Talbot, D.C.; Ganesan, T.; Pettit, G.R.; Balkwill, F.; et al. Phase I Study of Bryostatin 1: Assessment of Interleukin 6 and Tumor Necrosis Factor Induction in Vivo. J. Natl. Cancer Inst. 1993, 85, 1812–1818. [Google Scholar] [CrossRef]
- Ahmad, I.; Al-Katib, A.M.; Beck, F.W.; Mohammad, R.M. Sequential Treatment of a Resistant Chronic Lymphocytic Leukemia Patient with Bryostatin 1 Followed by 2-Chlorodeoxyadenosine: Case Report. Clin. Cancer Res. 2000, 6, 1328–1332. [Google Scholar]
- Zonder, J.A.; Shields, A.F.; Zalupski, M.; Chaplen, R.; Heilbrun, L.K.; Arlauskas, P.; Philip, P.A. A Phase II Trial of Bryostatin 1 in the Treatment of Metastatic Colorectal Cancer1. Clin. Cancer Res. 2001, 7, 38–42. [Google Scholar]
- Tozer, R.G.; Burdette-Radoux, S.; Berlanger, K.; Davis, M.L.; Lohmann, R.C.; Rusthoven, J.R.; Wainman, N.; Zee, B.; Seymour, L. A Randomized Phase II Study of Two Schedules of Bryostatin-1 (NSC339555) in Patients with Advanced Malignant Melanoma—A National Cancer Institute of Canada Clinical Trials Group Study. Investig. New Drugs 2002, 20, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Varterasian, M.L.; Mohammad, R.M.; Eilender, D.S.; Hulburd, K.; Rodriguez, D.H.; Pemberton, P.A.; Pluda, J.M.; Dan, M.D.; Pettit, G.R.; Chen, B.D.; et al. Phase I Study of Bryostatin 1 in Patients with Relapsed Non-Hodgkin’s Lymphoma and Chronic Lymphocytic Leukemia. J. Clin. Oncol. 1998, 16, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Crowther, D.; Prendiville, J.A.; McGown, A.T.; Scheid, C.; Stern, P.L.; Young, R.; Brenchley, P.; Chang, J.; Owens, S.M. A Phase I Trial of Bryostatin 1 in Patients with Advanced Malignancy Using a 24 Hour Intravenous Infusion. Br. J. Cancer 1995, 72, 461–468. [Google Scholar] [CrossRef]
- Varterasian, M.L.; Mohammad, R.M.; Shurafa, M.S.; Hulburd, K.; Pemberton, P.A.; Rodriguez, D.H.; Spadoni, V.; Eilender, D.S.; Murgo, A.; Wall, N.; et al. Phase II Trial of Bryostatin 1 in Patients with Relapsed Low-Grade Non-Hodgkin’s Lymphoma and Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2000, 6, 825–828. [Google Scholar] [PubMed]
- El-Rayes, B.F.; Gadgeel, S.; Shields, A.F.; Manza, S.; Lorusso, P.; Philip, P.A. Phase I Study of Bryostatin 1 and Gemcitabine. Clin. Cancer Res. 2006, 12, 7059–7062. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Propper, D.J.; Macaulay, V.; O’Byrne, K.J.; Braybrooke, J.P.; Wilner, S.M.; Ganesan, T.S.; Talbot, D.C.; Harris, A.L. A Phase II Study of Bryostatin 1 in Metastatic Malignant Melanoma. Br. J. Cancer 1998, 78, 1337–1341. [Google Scholar] [CrossRef]
- Cragg, L.H.; Andreeff, M.; Feldman, E.; Roberts, J.; Murgo, A.; Winning, M.; Tombes, M.B.; Roboz, G.; Kramer, L.; Grant, S. Phase I Trial and Correlative Laboratory Studies of Bryostatin 1 (NSC 339555) and High-Dose 1-B-D-Arabinofuranosylcytosine in Patients with Refractory Acute Leukemia. Clin. Cancer Res. 2002, 8, 2123–2133. [Google Scholar]
- Grant, S.; Roberts, J.; Poplin, E.; Tombes, M.B.; Kyle, B.; Welch, D.; Carr, M.; Bear, H.D. Phase Ib Trial of Bryostatin 1 in Patients with Refractory Malignancies. Clin. Cancer Res. 1998, 4, 611–618. [Google Scholar]
- Morgan, R.J.; Leong, L.; Chow, W.; Gandara, D.; Frankel, P.; Garcia, A.; Lenz, H.-J.; Doroshow, J.H. Phase II Trial of Bryostatin-1 in Combination with Cisplatin in Patients with Recurrent or Persistent Epithelial Ovarian Cancer: A California Cancer Consortium Study. Investig. New Drugs 2010, 30, 723–728. [Google Scholar] [CrossRef]

| Parameter | Intravenous (i.v.) | Intraperitoneal (i.p.) |
|---|---|---|
| Pharmacokinetic model | Two-compartment | One-compartment with first-order absorption |
| T1/2 (distribution) | 1.05 h | – |
| T1/2 (elimination) | 22.97 h | 28.76 h |
| T1/2 (absorption) | – | 0.81 h |
| AUC | 376.7 ng/mL * h | 620.2 ng/mL * h |
| Cmax | 92.9 ng/mL | 13.5 ng/mL |
| Volume of distribution | 2.37 mL/kg | 2.72 mL/kg |
| Clearance | 0.11 mL/(kg * h) | 0.06 (kg * h) |
| Elimination route | Urine + feces | Urine + feces |
| Brain penetration | Rapid, short-lasting | Longer duration, lower intensity |
| Cell Line | IC50 | Activity/Mechanism/Effects | Ref. |
|---|---|---|---|
| MKN-74 gastric cancer cell line. | 1 µM | Treatment of tumor cells with bryostatin 1 before paclitaxel decreases mitotic entry of the cells. | [101] |
| WSU-CLL human chronic lymphocytic leukemia cell line. | Bryostatin 1: 200 nM Auristatin-PE: various Dolastatin 10: various | There is a synergetic effect between these agents (auristatin-PE, dolastatin 10) and bryostatin 1 as regards apoptosis and cell death. It is more apparent in the bryostatin 1 + auristatin PE combination. | [102] |
| Human cervical carcinoma HeLa cells. HeLa cisplatin-resistant variants (HeLa/CP). | 1 nmol/L | PKCy acts as a proapoptotic protein but in full length may inhibit cisplatin-induced cell death. Persistent activation/downregulation of PKCy by bryostatin 1 leads to cisplatin sensitization. Pretreatment with bryostatin 1 enhanced cisplatin-induced cell death in HeLa and HeLa cells, overexpressing PKCy gene to 30% and 19%, respectively. Bryostatin 1 decreased the IC50 of cisplatin from 6.4 to 1.7 µmol/L in HeLa cells and >30 µmol/L to and 14 µmol/L in HeLa/CP cells. | [103] |
| NIH 3T3 fibroblast cell line. | Bryostatin 1: 0.01; 0.1; 1; 10; 100; 1000 nM Combination: 1 µM of both Bryostatin 1 and PMA | Bryostatin 1 was more potent than PMA in terms of translocating and downregulating PKCα, -δ, and -ε. | [104] |
| Dendritic cells (DC) derived from bone marrow of C75BL/6 female mice. | 0.1; 0.5; 1; 5; 10; 50 nM | Bryostatin 1 induced CC chemokine release from bone marrow DC, including CCL2 and CCL3, via ERK pathway in a dose- and time-dependent way. Maximal effect observed at 5 and 10 nM. No significant induction occurred at 50 nM. Bryostatin 1 is a strong adjuvant which potentiates a peptide-based cancer vaccine. | [105] |
| B16 melanoma cells and REH human leukemia cells. | 10−10; 10−9; 10−8; 10−7; 10−6 M | Bryostatin 1 has a direct antiproliferative effect against B16 melanoma, more potent as 10−6 M, but shows no evidence that it can stimulate nonspecific cell-mediated cytotoxicity. Against REH, bryostatin 1 showed a significant antiproliferative effect over all the concentrations. | [106] |
| WSU-DLCL2 human diffuse large cell lymphoma cells. | 10 nM | Bryostatin 1 increased Bax expression but only modestly induced apoptosis. Adding bryostatin 1 to CHO (cyclophosphamide monophosphate) leads to an inhibition of the cell growth by over 100%. | [107] |
| 4TBM and 4THM cells originated from 4 T1 breast cancer cells. | 10 ng/mL | The inhibitory effects of bryostatin 1 on chemokine secretion induced by CXCR2 antagonist seems to be mediated mainly by PKCδ followed by PKCε. | [108] |
| Leukemia cells from heparinized peripheral blood of patients with B-CLL. | 10−6 to 10−10 mol/L | Bryostatin 1 can induce differentiation of B-CLL cells. | [109] |
| Human leukemia cell lines: HL-60 and K562. T lymphoblast cells: CEM. REH Human Lymphoblastic leukemia cells. | 10−11 to 10−7 mol/L | Bryostatin 1 (possibly by activation of protein kinase C) inhibits clonogenic leukemia cells at concentrations that stimulate normal hematopoietic progenitors. Bryostatin 1 may be valuable in the treatment of leukemias and MDS. | [110] |
| Peripheral Blood Mononuclear Cells (PBMCs). | 1 pM–100 nM | Bryostatin 1 increased IL-2 receptor expression on CD4+, CD8+ and CD56+ cells. Maximal IL-2 receptor induction and proliferation at 10 nM. | [111] |
| HEK293 Human Embryonic Kidney and Murine Bone Marrow-derived dendritic cells (DCs). | 10 ng/mL | Bryostatin 1-induced production of IFN-β, MIP1-α, and RANTES, regulated, at least in part, through TLR4 activation. Also induced NF-kB activation, with TLR4 involved. TLRs are involved in Bryostatin 1-mediated activation of IRF-3, specifically via TLR4 in BMDCs. | [112] |
| Bone Marrow Dendritic Cells generated from C57BL/6 mice. | 10 nM | Bryostatin 1 caused the maturation of DCs with a significant increase in the expression of CD40, CD80 and CD86. Bryostatin 1-induced activation of dendritic cells is not mediated by low levels of endotoxin contamination. | [112] |
| KB-3-1 Human papillomavirus related cervical adenocarcinoma cells. KB-C1 carcinoma cells. HeLa-MDR1-G185 multidrug-resistant HeLa cells transfected with a wild-type multidrug resistance gene 1 (MDR1). HeLa-MDR1-V185 multidrug-resistant HeLa cells transfected with a mutant MDR1 gene. | 1 µM | Bryostatin 1 is able to reverse resistance to vinblastine, colchicine and adriamycin in cells V185, but not in the parental cells expressing G185. In cells expressing MDR1-V185, bryostatin 1 can inhibit the efflux of the PGP-substrate rhodamine 123 but not in the G185-expressing cells. Bryostatin 1 specifically reverses a mutant PGP. | [113] |
| WSU-DLCL2 human diffuse large cell lymphoma cells. | 200 nM | Bryostatin 1 decreases P-glycoprotein by downregulating MDR1 expression. Exposure of culture cells to bryostatin 1 reversed the multidrug resistance phenotype within 24 h. A four-fold increase in vincristine accumulation was recorded in bryostatin l-treated cells compared with the control group. | [114] |
| LNCaP prostate cancer cells. | 0.1; 1; 10; 100 nM | Bryostatin 1 is not an efficient killing agent for LNCaP prostate cancer cells. Moreover, as it inhibits the effect of other anticancer drugs, bryo-1 possesses antiapoptotic activity in LNCaP cells. | [93] |
| 4T1 mouse breast cancer cells. | 20; 200 and 400 nM | Approximately 60% of 4T1 cells undergo apoptosis within 48 h of treatment with bryostatin 1. Bryostatin 1-mediated effects on growth and apoptosis of 4T1 cells are not related to its ability to enhance translocation or downregulation of total PKC or PKC isoforms α and δ. | [115] |
| REH human acute lymphoblastic leukemia cells. | 1 nM | Bryostatin 1 induces the ubiquitination, proteasome degradation and downregulation of the proto-oncogene Bcl-2 in human pre-B acute lymphoblastic leukemia cells. Bcl-2 acts like a multidrug resistance protein and its increased expression can protect tumor cells from the cytotoxic effects of most anticancer drugs. In contrast, decreased expression of Bcl-2 is associated with improved response to chemotherapy. | [90] |
| Organism | Dose | n | Exposure Time | Activity/Mechanism/Effects | Ref. |
|---|---|---|---|---|---|
| BALB/c nude mice | 5, 25 and 50 µg/kg/day | 15 | n/a (i.p. injection) | Bryostatin 1 treatment hindered development of liver tumors as there were remissions in tumor size and invasion. This treatment had no adverse effects on the mice. | [116] |
| Four-week-old female ICR-SCID mice | 75 µg/kg/ injection | 20 | n/a (s.c. injection) | Bryostatin is considered active in terms of tumor growth inhibition. Auristatin PE with bryostatin 1 is highly active in tumor growth inhibition and delay and kill value. In this group, all the mice treated were free of tumors for 150 days and thus were considered cured. The combination of dolastatin 10 with bryostatin 1 was considered active and only two of five mice were free of tumor. Bryostatin 1 in doses of 100 and 75 µg/kg were considered too toxic if administered alone and together with the other drugs, respectively. | [102] |
| C57BL/6 mice | Bryostatin 1: 20 ng E7 peptide: 20 µg | 4–6 | n/a (s.p. injection) | Bryostatin1 induces the CC chemokines release from BMDC, including CCL2 and CCL3. The Bryostatin 1 is a potent adjuvant which potentiates a peptide based cancer vaccine. | [105] |
| Female C57BL | 1, 10, 50, 100 µg/kg as i.p. injections in 0.5 mL, during 5 days | 6 | n/a (i.p. injection) | Short term treatment of animals causes a dramatic reduction in the number of identified pulmonary melanoma metastases. Bryostatin 1 may be easily combined with other anticancer therapies without significant toxicity. | [106] |
| SCID mice | Bryostatin 1: 75 mg/kg, Cyclophosphamide: 40 mg/kg, Doxorubicin: 3.3 mg/kg, Vincristine: 0.5 mg/kg Prednisone: 0.2 mg/kg | 39 | n/a (i.p. injection) | The combination of Bryostatin 1 with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) is highly active in tumor growth delay and inhibition, even better than Bryostatin 1 or CHOP alone. | [107] |
| Seven-week-old female BALB/c nude mice | Bryostatin 1: 5, 10, 15, 20, 25, 30 µM | 48 la | n/a (not described) | Bryostatin 1 inhibited the development and progression of skin tumors in mice through the prevention of inflammation-inducing processes (such as activation of COX-2) and the quenching of radicals (H2O2). The 30 µM dose was the most effective in reducing inflammation and tumor progression. | [117] |
| Adult (from six to eight weeks old) female C57BL/6 mice | 75 µg/kg body weight | Not described | n/a (i.v. injection) | Bryostatin 1 triggered a TLR-4-dependent T helper cell 2 (Th2) cytokine response, which can help treat disorders that are mediated by Th1inflammatory cells, and expanded a subset of myeloid dendritic cells that expressed a CD11c+ CD8+-CD11b+ CD4+ phenotype. Bryostatin 1 can act as a TLR4 ligand and activate innate immunity. | [112] |
| SCID mice | Bryostatin 1: 50–75 µg/kg Vincristine: 0.5 mg/kg Doxorubicin: 3.3 mg/kg Ara-C: 450 mg/kg | 50 | n/a (i.p. injection) | The combination of Bryostatin 1 and Vincristine is well tolerated and was associated with significant antitumor activity, regarding tumor growth delay and inhibition. Bryostatin 1 downregulates MDR1 in tumor tissue, enhancing significantly vincristine-induced tumor regression. Bryostatin 1 in combination with Doxorubicin or Ara-C was too toxic or had low antitumor activity, respectively. | [114] |
| From five- to six-week-old BALB/c mice | 75 µg/kg body weight/injection | 15 | n/a (i.p. injection) | Bryostatin 1 inhibits both primary tumor growth and lung metastasis by 50%. It is equally effective in decreasing tumor if it is administered immediately after tumor challenge or several days after the detection of palpable tumors. Bryostatin 1 can effectively inhibit growth and metastasis of mammary tumor (especially p53 mutant or p53-null), although not eradicate tumors. | [115] |
| Trial | Dose and Schedule | Disease | No. of Patients | Exposure Time | Activity/Mechanism/Effects | Ref. |
|---|---|---|---|---|---|---|
| Phase II | 35–40 µg/m2 on days 1, 8 and 15 of each four-week cycle. | Renal cell carcinoma | 32 | 1 h | Limited antitumor activity as a single agent. Common toxicities: myalgias, dyspnea, fatigue. | [120] |
| Phase I | 5; 10; 20; 35; 50; 65 µg/m2. | Ovarian carcinoma, sarcoma, colon cancer, mesothelioma | 19 | 1 h | No antitumor activity was observed. Recommended dose for phase II studies is 35–50 µg/m2 twice a week. The dose-limiting toxicity was myalgia. | [121] |
| Phase I | 25 µg/m2 weekly; 35 µg/m2 biweekly; 25 µg/m2 for three weeks in a four-week cycle. | Non-small cell lung cancer, carcinoid tumor, esophageal carcinoma, small cell lung cancer, breast carcinoma, ovarian adenocarcinoma, hypernephroma, maxillary carcinoma, malignant melanoma, colorectal carcinoma, adenocarcinoma of unknown origin and pancreatic carcinoma | 35 | 1 h | The dose-limiting toxicity was myalgia. Plasma IL-6 and TNF-α increased, and antitumor activity against malignant melanoma was observed early in the treatment. | [122] |
| Case Report | Bryostatin 1 (120 µg/m2) followed by 2-CdA (0.06 mg/kg) for five days and three cycles of chemotherapy. | Resistant chronic lymphocytic leukemia | 1 (69-year-old male patient) | 72 h | Decreased significantly peripheral blood lymphocytes (CLL cells), while simultaneously increasing their differentiation. Activated Bax/Bcl-2 apoptotic pathway, increasing its ratio. Enhanced sensitivity to 2-hCdA. | [123] |
| Phase II | Group 1—25 µg/m2 and escalated to 35 mg/m2. Group 2—25 µg/m2 to 35 µg/m2. Doses were given weekly followed by a week break, in four-week cycles. | Advanced colorectal cancer | 28 | 24 h | Bryostatin 1 showed no complete or partial responses regarding antitumor activity. A different treatment schedule might enhance its effect. | [124] |
| Phase II | Group 1—25 µg/m2 weekly; Group 2—120 µg/m2 biweekly. | Metastatic melanoma | 32 | 24 h (group 1) 72 h (group 2) | Although seven patients had stable disease, it was concluded that there were no partial or complete responses as the remaining patients had early progressive disease. The study treatment was not apparently effective. | [125] |
| Phase I | Doses were given every two weeks with escalation: 12; 20; 30; 42; 75; 120; 180 µg/m2. | Chronic lymphocytic leukemia Non-Hodgkin’s lymphoma | 29 | 72 h | The maximum tolerated and recommended dose for phase II is 120 pg/m2 (40 pg/m2/d for three days). Myalgia was the dose-limiting toxicity. | [126] |
| Phase I | 25; 35; 50 µg/m2 weekly for eight weeks | Advanced malignancy: ovarian carcinoma, renal carcinoma, melanoma, liposarcoma and low-grade non-Hodgkin’s lymphoma | 19 | 24 h | Myalgia was the dose-limiting toxicity. The maximum tolerated and recommended dose for phase II trial was 25 µg/m2 per week. Partial and minor responses were seen both in four patients, two of them with ovarian carcinoma and the other two with low grade non-Hodgkin’s lymphoma. | [127] |
| Phase II | 120 µg/m2 every two weeks. If disease progressed, patients could receive vincristine (with dose escalation) after completing bryostatin 1. | Non-Hodgkin’s lymphoma, Chronic lymphocytic leukemia | 25 | 72 h | Bryostatin 1 alone resulted in one complete remission and two partial remissions. Sequential therapy with vincristine in doses up to 2 mg is feasible and well tolerated. | [128] |
| Phase I | Gemcitabine (mg/m2), followed by bryostatin 1 (µg/m2) on days 1, 8, and 15 of a 28-day cycle with dose escalation: 600/25; 800/25; 1000/25; 1000/30; 1000/35; 1000/45, respectively. | Nonhematologic cancer refractory to conventional treatment | 36 | 24 h | Two heavily pretreated patients had a partial response, lasting 22 and eight months. Eight patients had stable disease. Three patients with non-small cell lung cancer had disease stabilization for more than four months. The combination of bryostatin 1 and gemcitabine is well-tolerated with limited grade 3 toxicity. The recommended dose of bryostatin 1 in combination with full doses of gemcitabine is 35 µg/m2. | [129] |
| Phase II | 25 µg/m2 weekly for three weeks followed by a rest week. | Malignant melanoma | 15 | 1 h | Toxicity in this study, apart from myalgia, was low. Bryostatin 1 is not effective as a single agent in this disease. Evidence suggests it may warrant further study in combination with cytotoxic or biological agents. | [130] |
| Phase I | Bryostatin 1: 12.5 escalated to 50 µg/m2 in increments of 12.5 µg/m2. HiDAC: 1.5 or 3 g/m2 dose every 12 h for two days | Refractory/relapsed acute leukemia: acute myelogenous leukemia, acute lymphoblastic leukemia, blast crisis in chronic myeloid leukemia | 30 | 24 h | The combination of bryostatin 1 with HiDAC demonstrated some activity in heavily pretreated leukemia patients. Bryostatin 1 modulated PKC, induced apoptosis and showed potential synergy with HiDAC. The maximum tolerated dose was 50 µg/m2. | [131] |
| Phase Ib | 25 µg/m2 weekly for three weeks or 12.5 µg/m2 on days 1 and 4 of each week. | Refractory malignancies: pancreatic cancer, breast cancer, melanoma, colon cancer, non-small cell lung cancer, soft tissue sarcoma, kidney cancer. | 12 | 30 min (12.5 µg/m2); 1 or 24 h (25 µg/m2) | Three patients (with sarcoma, pancreatic and breast cancer), had stable disease for at least eight weeks, while eight patients showed disease progression. Lower bryostatin 1 doses may be more appropriate when this agent is intended as an immunomodulator since the split doses showed an increase in IL-2. PKC activity reductions were observed in some patients. | [132] |
| Phase II | Bryostatin 1: 45 μg/m2 followed immediately by Cisplatin: 50 mg/m2. | Recurrent and persistent epithelial ovarian cancer | 8 | 72 h (Bryostatin 1) and 1 h (Cisplatin) | There was a modest response rate in patients with recurrent or persistent ovarian cancer treated with the combination of bryostatin and cisplatin. Its severe incidence of myalgias shown in this study limits its ability to be delivered in effective doses as patients required narcotics for pain control. | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, T.; Staszewski, M.; Markowicz-Piasecka, M.; Sikora, J.; Amaro, C.; Picot, L.; Sitarek, P. Anticancer Activity of the Marine-Derived Compound Bryostatin 1: Preclinical and Clinical Evaluation. Int. J. Mol. Sci. 2025, 26, 7765. https://doi.org/10.3390/ijms26167765
Kowalczyk T, Staszewski M, Markowicz-Piasecka M, Sikora J, Amaro C, Picot L, Sitarek P. Anticancer Activity of the Marine-Derived Compound Bryostatin 1: Preclinical and Clinical Evaluation. International Journal of Molecular Sciences. 2025; 26(16):7765. https://doi.org/10.3390/ijms26167765
Chicago/Turabian StyleKowalczyk, Tomasz, Marek Staszewski, Magdalena Markowicz-Piasecka, Joanna Sikora, Catarina Amaro, Laurent Picot, and Przemysław Sitarek. 2025. "Anticancer Activity of the Marine-Derived Compound Bryostatin 1: Preclinical and Clinical Evaluation" International Journal of Molecular Sciences 26, no. 16: 7765. https://doi.org/10.3390/ijms26167765
APA StyleKowalczyk, T., Staszewski, M., Markowicz-Piasecka, M., Sikora, J., Amaro, C., Picot, L., & Sitarek, P. (2025). Anticancer Activity of the Marine-Derived Compound Bryostatin 1: Preclinical and Clinical Evaluation. International Journal of Molecular Sciences, 26(16), 7765. https://doi.org/10.3390/ijms26167765











