Enhanced Photo-Fenton Removal of Oxytetracycline Hydrochloride via BP/Bi2MoO6 Z-Scheme Heterojunction Photocatalyst
Abstract
1. Introduction
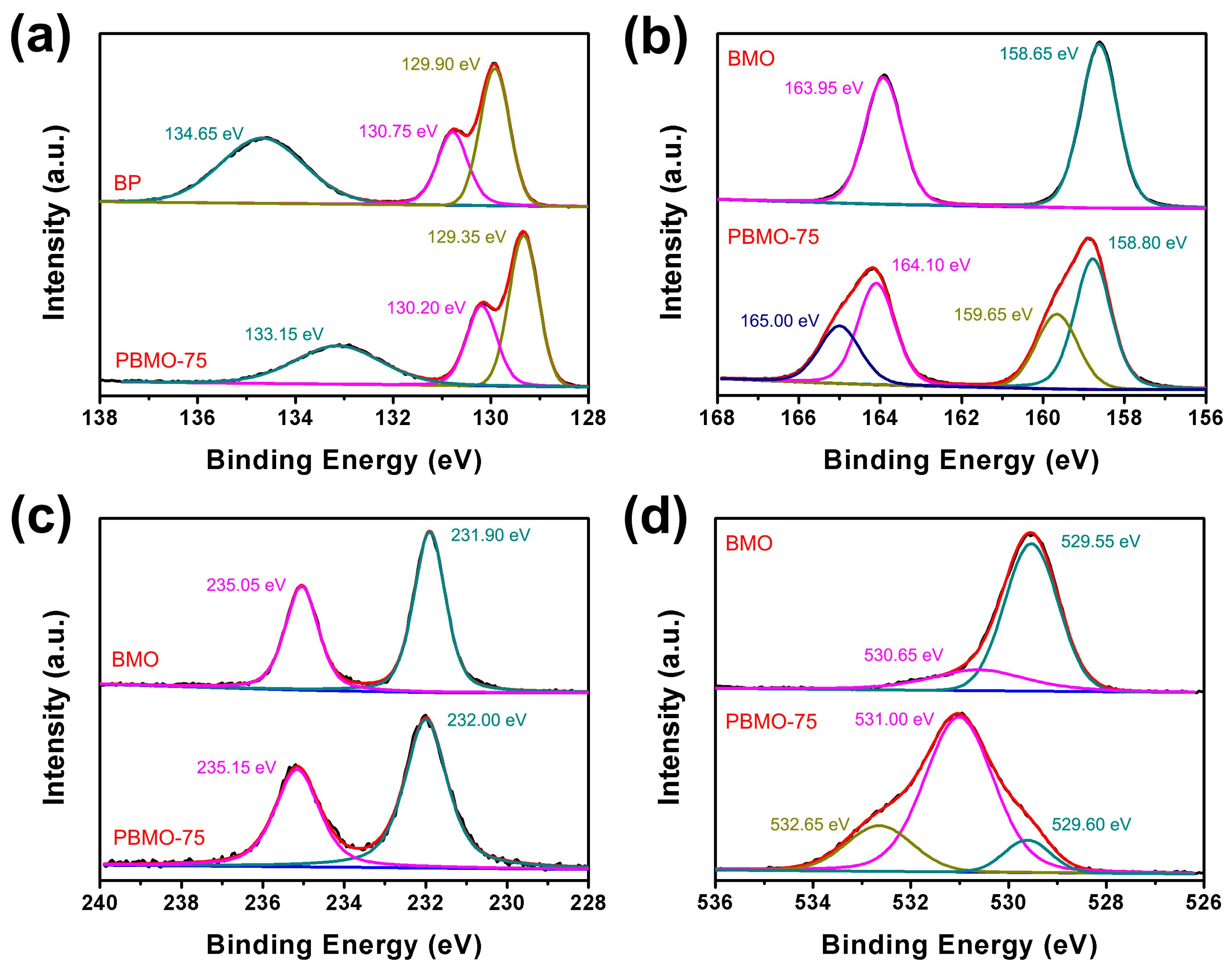
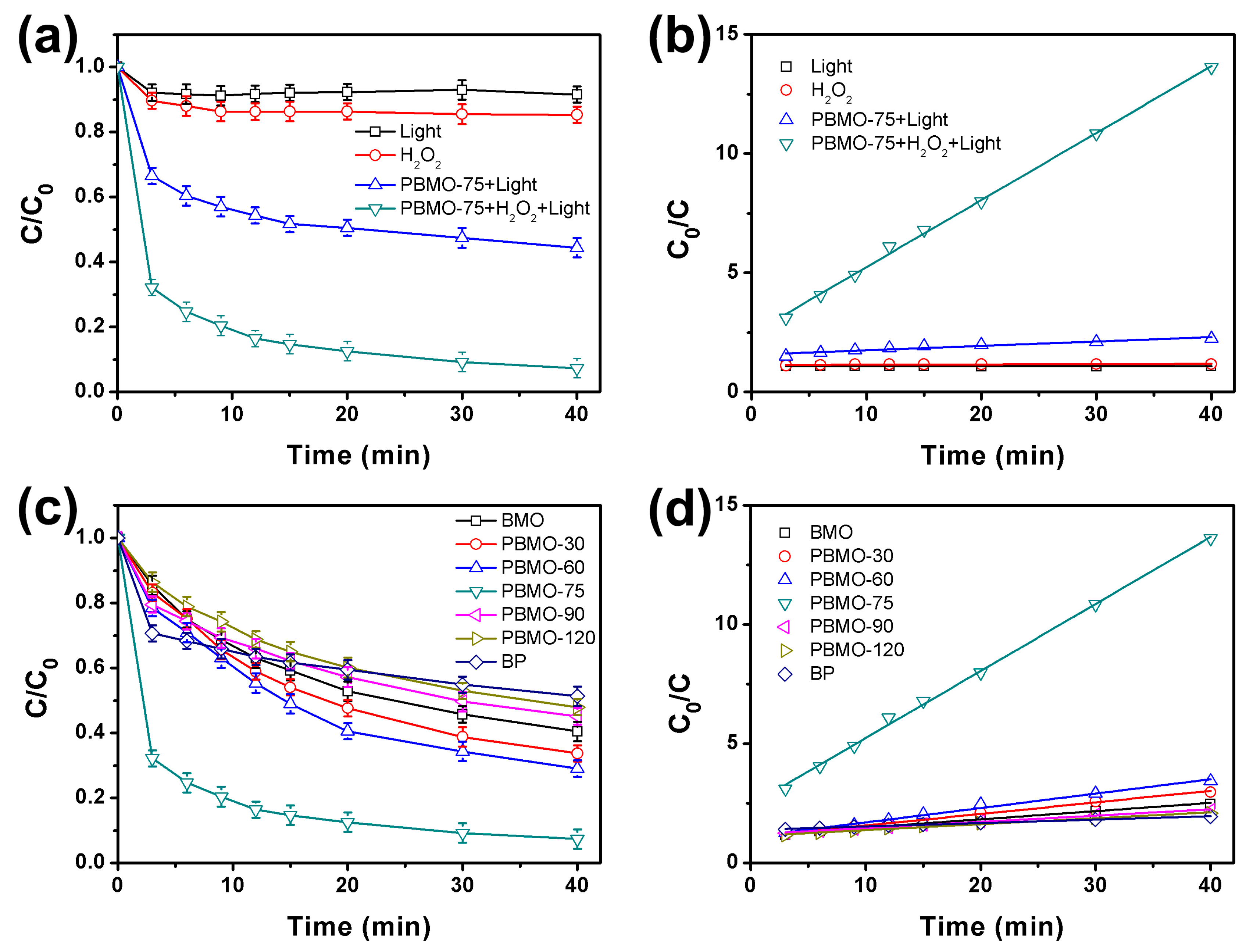
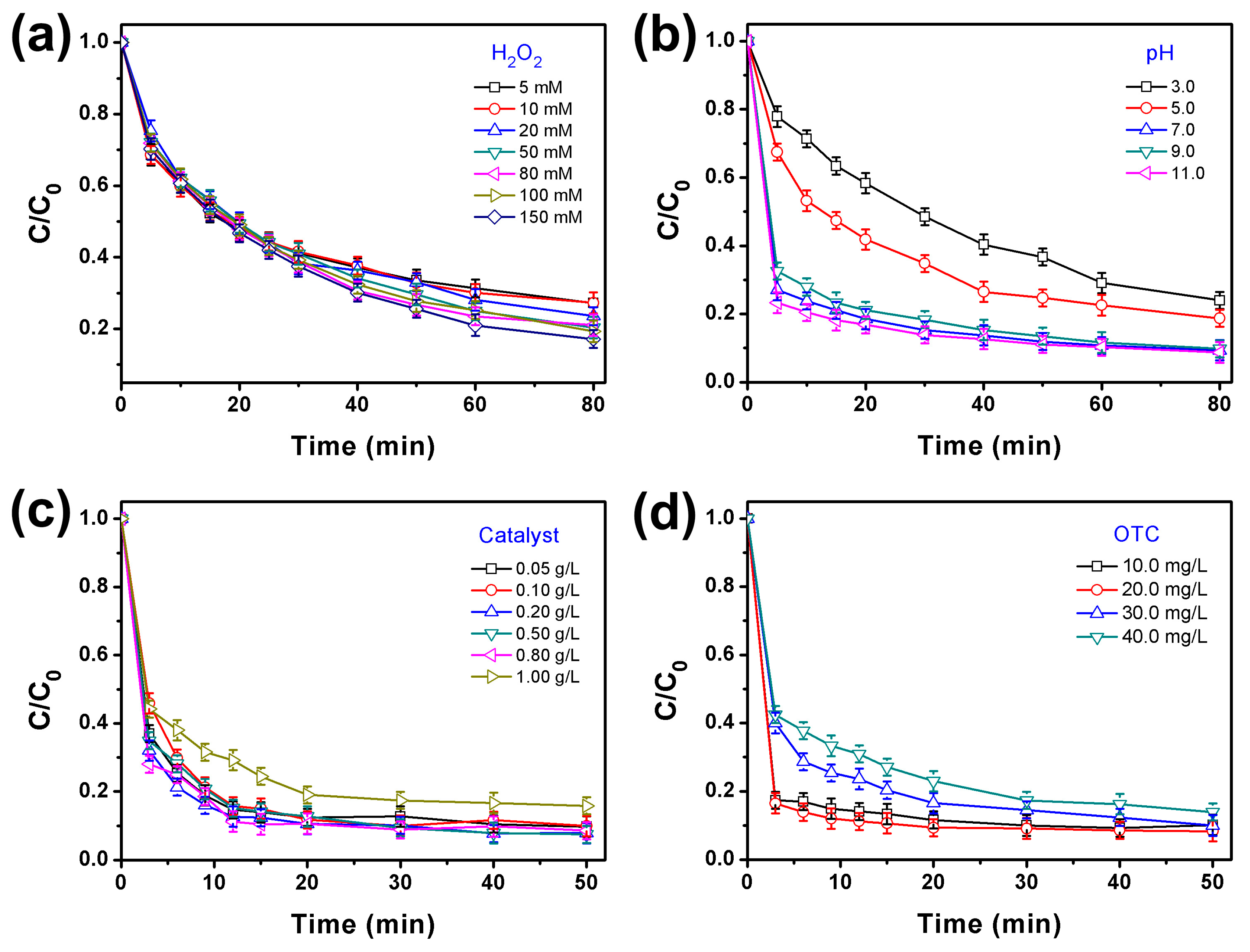
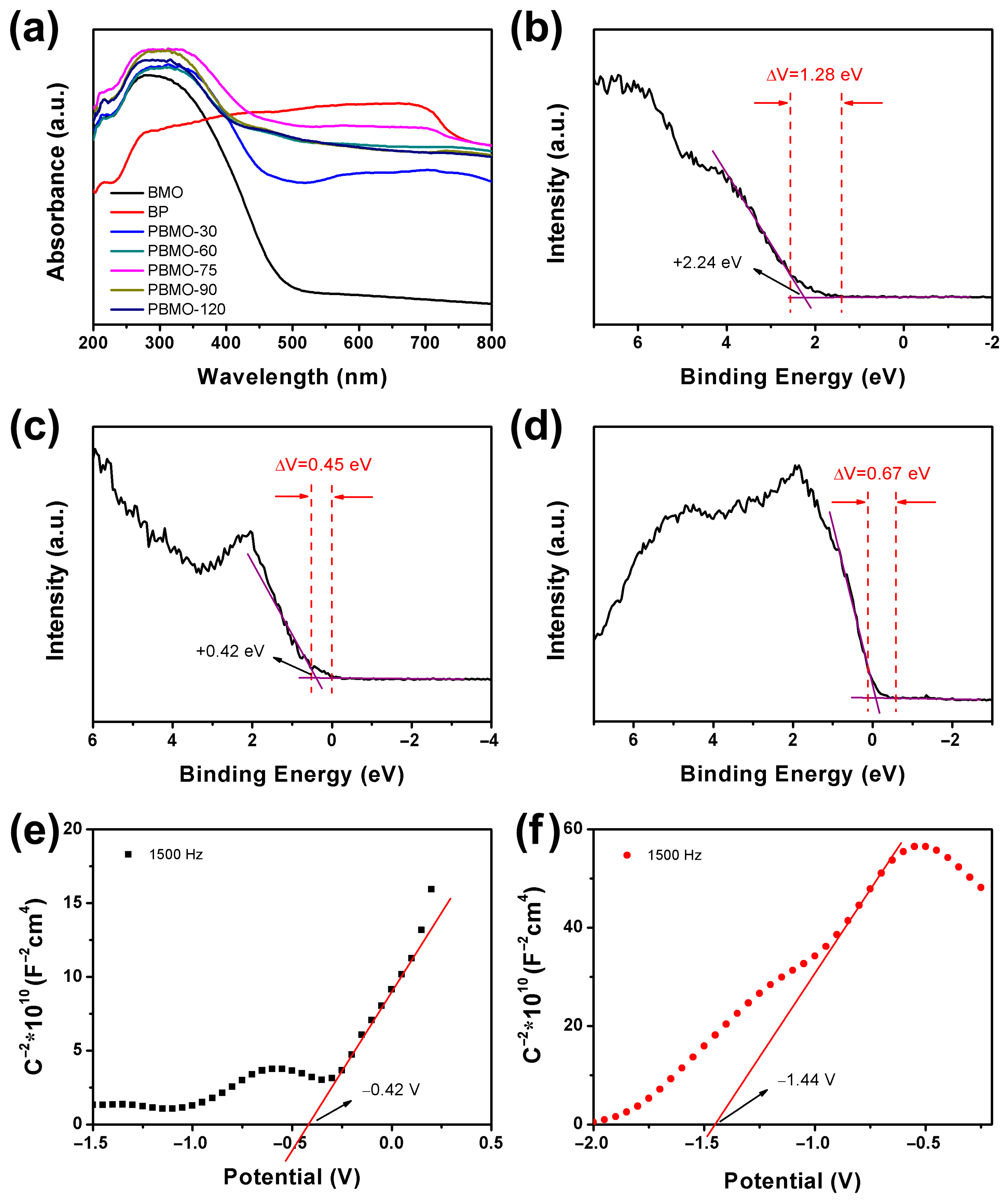
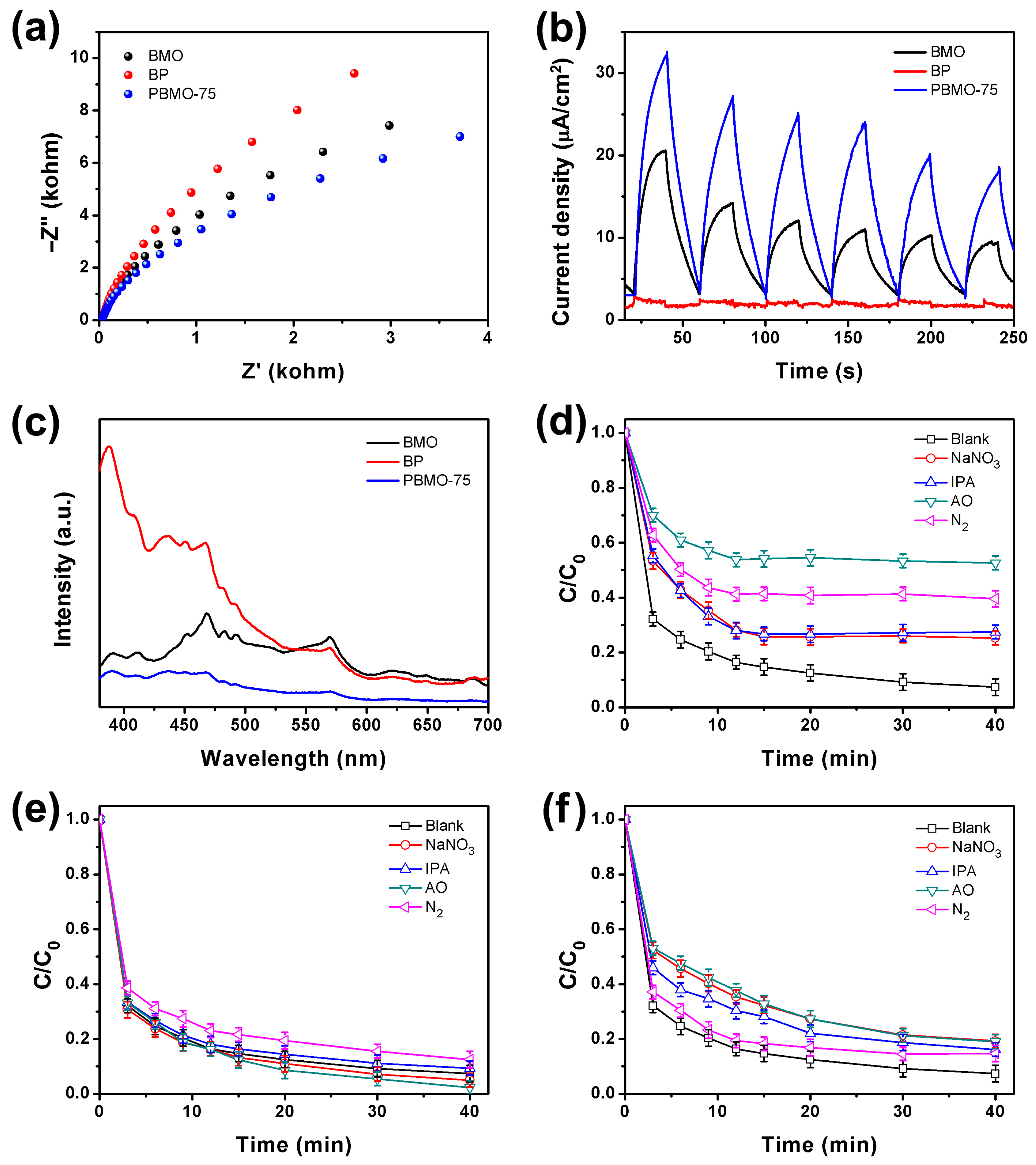
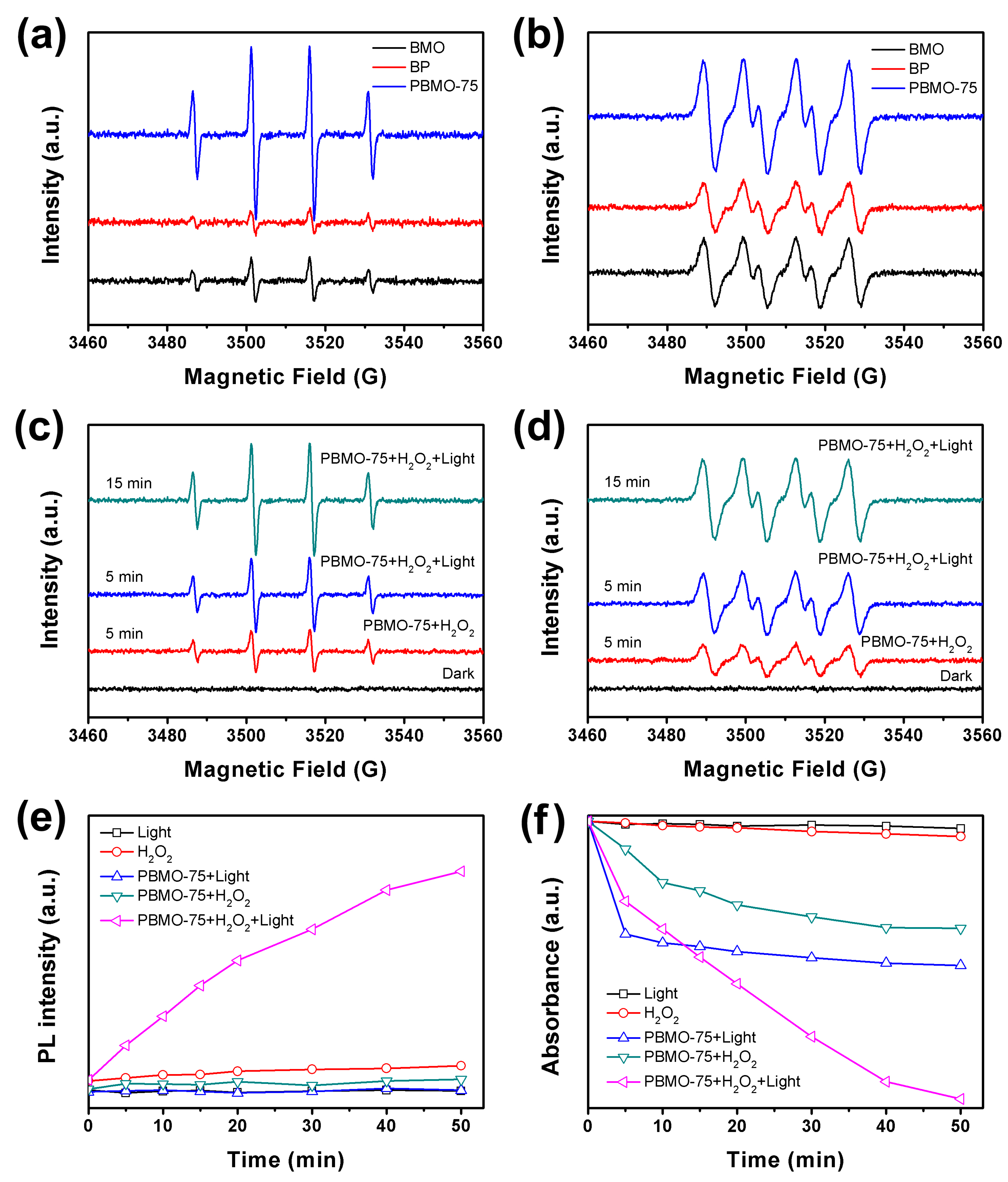
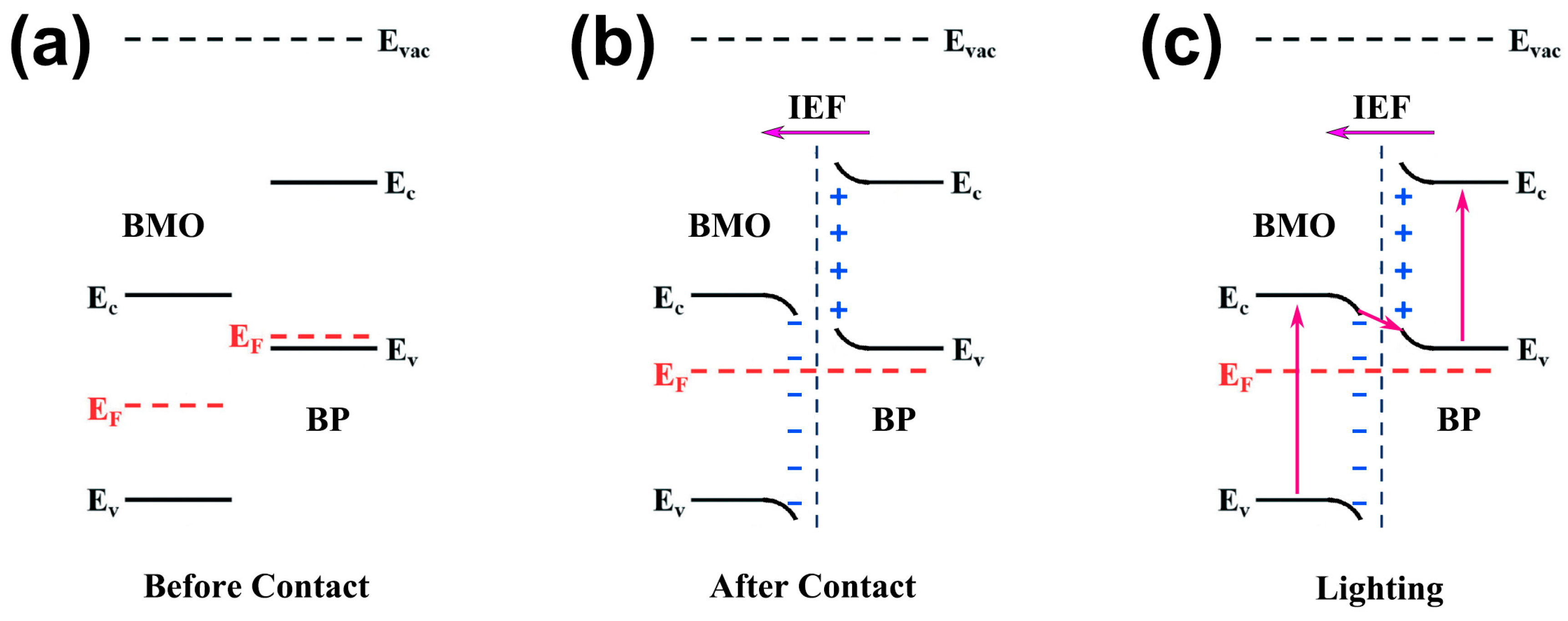
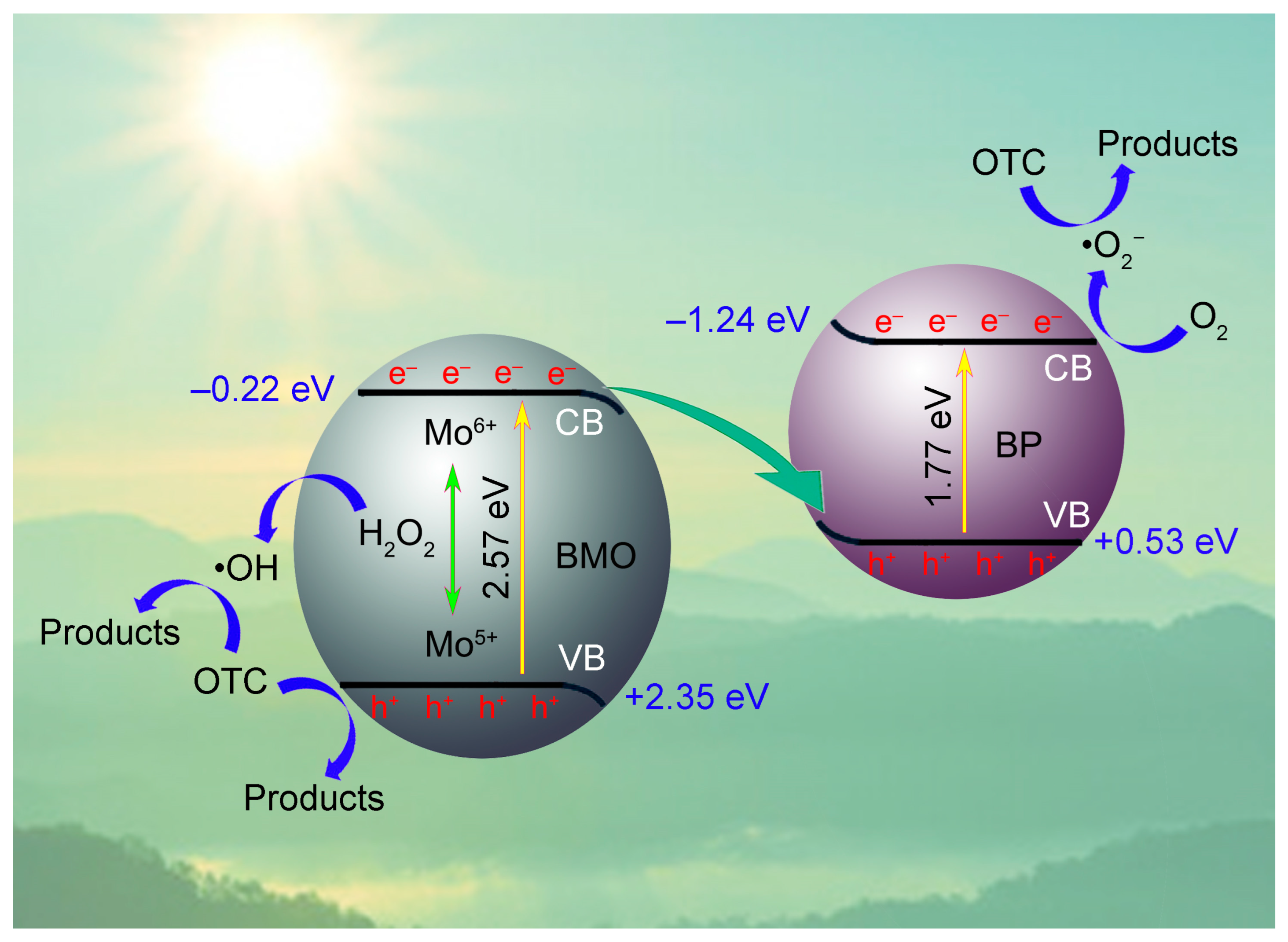
2. Results
3. Experiment
3.1. Chemicals and Materials
3.2. Preparation of PBMO Heterojunction
3.3. Characterization
3.4. Photo-Fenton Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Zhou, X.; Zheng, Y.; Yang, H.; Cao, W. How far do we still need to go with antibiotics in aquatic environments? Antibiotic occurrence, chemical-free or chemical-limited strategies, key challenges, and future perspectives. Water Res. 2025, 275, 123179. [Google Scholar] [CrossRef]
- Pham, M.N.; Nishimura, F.; Lan, J.C.W.; Khoo, K.S. Recent advancement of eliminating antibiotic resistance bacteria and antibiotic resistance genes in livestock waste: A review. Environ. Technol. Innov. 2024, 36, 103751. [Google Scholar] [CrossRef]
- Luo, Q.; Zhuang, W.; Sui, M. Combating Antibiotic Resistance in Persulfate-Based Advanced Oxidation Processes: Activation Methods and Energy Consumption. Environ. Res. 2025, 270, 120932. [Google Scholar] [CrossRef] [PubMed]
- Obi, C.C.; Abonyi, M.N.; Ohale, P.E.; Onu, C.E.; Nwabanne, J.T.; Igwegbe, C.A.; Kamuche, T.T.; Ozofor, I.H. Adsorption of antibiotics from aqueous media using nanocomposites: Insight into the current status and future perspectives. Chem. Eng. J. 2024, 497, 154767. [Google Scholar] [CrossRef]
- Ji, J.; Li, H.; Liu, S. Current Natural Degradation and Artificial Intervention Removal Techniques for Antibiotics in the Aquatic Environment: A Review. Appl. Sci. 2025, 15, 5182. [Google Scholar] [CrossRef]
- Hidayatullah, I.M.; Steven, S.; Kusmayadi, A.; Rahmatika, I.; Reski, M.; Boopathy, R.; Alfatan, M.A.; Purba, P.W.G. Advances in Antibiotic Biodegradation: Emerging Strategies and Challenges in Mitigating Environmental Contamination and Resistance. Curr. Pollut. Rep. 2025, 11, 35. [Google Scholar] [CrossRef]
- Lau, P.L.; Trzcinski, A.P. A review of modified and hybrid anaerobic baffled reactors for municipal wastewater treatment with a focus on emerging contaminants. Environ. Sci. Water Res. Technol. 2024, 10, 1335–1354. [Google Scholar] [CrossRef]
- Xiao, J.; Guo, S.; Wang, D.; An, Q. Fenton-Like Reaction: Recent Advances and New Trends. Chem. Eur. J. 2024, 30, e202304337. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, D.; Bai, B.; Ma, Z.; Zong, S. Insight into antibiotic removal by advanced oxidation processes (AOPs): Performance, mechanism, degradation pathways, and ecotoxicity assessment. Chem. Eng. J. 2024, 500, 157134. [Google Scholar] [CrossRef]
- Moradeeya, P.G.; Sharma, A.; Anil Kumar, M.; Basha, S. Titanium dioxide based nanocomposites—Current trends and emerging strategies for the photocatalytic degradation of ruinous environmental pollutants. Environ. Res. 2022, 204, 112384. [Google Scholar] [CrossRef]
- Rathod, M.; Moradeeya, P.G.; Haldar, S.; Basha, S. Nanocellulose/TiO2 composites: Preparation, characterization and application in the photocatalytic degradation of a potential endocrine disruptor, mefenamic acid, in aqueous media. Photochem. Photobiol. Sci. 2018, 17, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Moradeeya, P.G.; Kumar, M.A.; Sharma, A.; Basha, S. Conductive polymer layered semiconductor for degradation of triclopyr acid and 2,4-dichlorophenoxyacetic acid from aqueous stream using coalesce adsorption-photocatalysis technique. Chemosphere 2022, 298, 134360. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, U.; De, A.K.; Sinha, I. Neutral pH Fenton and photo-Fenton activity of Mo-doped iron-pyrite particles. Phys. Chem. Chem. Phys. 2024, 26, 22442–22453. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sudhaik, A.; Sonu; Raizada, P.; Ahamad, T.; Thakur, S.; Le, Q.V.; Selvasembian, R.; Nguyen, V.-H.; Mishra, A.K.; et al. Critical review on the tetracycline degradation using photo-Fenton assisted g-C3N4-based photocatalysts: Modification strategies, reaction parameters, and degradation pathway. J. Environ. Chem. Eng. 2024, 12, 112984. [Google Scholar] [CrossRef]
- Ran, Y.; Cui, R.; Wang, X.; Wang, H.; Zhang, L.; Xu, L.; Zhu, J.; Huang, Q.; Yuan, W. Advancements in iron-based photocatalytic degradation for antibiotics and dyes. J. Environ. Manag. 2025, 374, 123991. [Google Scholar] [CrossRef]
- Lin, J.; Tian, W.; Guan, Z.; Zhang, H.; Duan, X.; Wang, H.; Sun, H.; Fang, Y.; Huang, Y.; Wang, S. Functional Carbon Nitride Materials in Photo-Fenton-Like Catalysis for Environmental Remediation. Adv. Funct. Mater. 2022, 32, 2201743. [Google Scholar] [CrossRef]
- Mao, S.; Liu, C.; Wu, Y.; Xia, M.; Wang, F. Porous P, Fe-doped g-C3N4 nanostructure with enhanced photo-Fenton activity for removal of tetracycline hydrochloride: Mechanism insight, DFT calculation and degradation pathways. Chemosphere 2022, 291, 133039. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, H.; Wen, J.; Peng, H. In2S3-NiS co-decorated MoO3@MoS2 composites for enhancing the solar-light induced CO2 photoreduction activity. Int. J. Hydrogen Energy 2021, 46, 36848–36858. [Google Scholar] [CrossRef]
- Zhang, T.; Peng, H.; Chen, R.; Guo, Y.; Wang, J.; Chen, X.; Wei, J.; Yu, Q. Enhancing simultaneous desulfurization and denitrification efficiency of cement kiln exhaust gas via co-catalytic Fenton oxidation process. J. Clean. Prod. 2023, 426, 139105. [Google Scholar] [CrossRef]
- Wang, L.; Ran, X.; Xiao, B.; Lei, L.; Zhu, J.; Xi, X.; Feng, G.; Li, R.; Feng, J. Visible light assisted Fenton degradation of oxytetracycline over perovskite ErFeO3/porous g-C3N4 nanosheets p-n heterojunction. J. Environ. Chem. Eng. 2022, 10, 108330. [Google Scholar] [CrossRef]
- James, A.; Shivakumar; Rodney, J.D.; Joshi, S.; Dalimba, U.; Kim, B.C.; Udayashankar, N.K. Mechanistic insights and DFT analysis of bimetal doped styrofoam-like LaFeO3 perovskites with in-built dual redox couples for enhanced Photo-Fenton degradation of Tetracycline. Chem. Eng. J. 2024, 481, 148466. [Google Scholar] [CrossRef]
- Tong, Z.; Wang, H.; An, W.; Li, G.; Cui, W.; Hu, J. FeCu bimetallic metal organic frameworks photo-Fenton synergy efficiently degrades organic pollutants: Structure, properties, and mechanism insight. J. Colloid Interface Sci. 2024, 661, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.L.; Li, Y.X.; Li, W.Z.; Luan, J. Controllable synthesis of copper-organic frameworks via ligand adjustment for enhanced photo-Fenton-like catalysis. J. Colloid Interface Sci. 2023, 646, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Huang, H. Black phosphorus-based heterostructures for photocatalysis and photoelectrochemical water splitting. J. Energy Chem. 2022, 67, 745–779. [Google Scholar] [CrossRef]
- Goren, A.Y.; Gungormus, E.; Vatanpour, V.; Yoon, Y.; Khataee, A. Recent Progress on Synthesis and Properties of Black Phosphorus and Phosphorene As New-Age Nanomaterials for Water Decontamination. ACS Appl. Mater. Interfaces 2024, 16, 20055–20078. [Google Scholar] [CrossRef]
- Yan, J.; Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Recent Progress on Black Phosphorus-Based Materials for Photocatalytic Water Splitting. Small Methods 2018, 2, 1800212. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, L.; Wang, H.; Huang, B.; Yuan, X.; Huang, J.; Zhang, J.; Zeng, G. Modulation of Bi2MoO6-Based Materials for Photocatalytic Water Splitting and Environmental Application: A Critical Review. Small 2019, 15, 1901008. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, J.; Gu, H.; Huang, J.; Lin, J.; Shao, J.; Wang, D.; Li, H. A review on research progress in photocatalytic degradation of organic pollutants by Bi2MoO6. J. Environ. Chem. Eng. 2023, 11, 110911. [Google Scholar] [CrossRef]
- Shimodaira, Y.; Kato, H.; Kobayashi, H.; Kudo, A. Photophysical Properties and Photocatalytic Activities of Bismuth Molybdates under Visible Light Irradiation. J. Phys. Chem. B 2006, 110, 17790–17797. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Zhang, S.; Shen, J.; Feng, W.; Du, Y.; Wan, L.; Zhang, S. Defect state of indium-doped bismuth molybdate nanosheets for enhanced photoreduction of chromium(vi) under visible light illumination. Dalton Trans. 2018, 47, 8110–8120. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.-Q.; Liao, J.; Zhang, X.; Feng, D.; Deng, H.; Ge, C. Infrared-to-visible energy transfer photocatalysis over black phosphorus quantum dots/carbon nitride. Chem. Eng. J. 2022, 431, 133453. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, H.; Wang, B.; He, W.; Dong, H.; Sui, L.; Gan, Z.; Ma, S.; Pang, B.; Dong, L.; et al. Synthesis and photocatalytic performance of MoS2/Polycrystalline black phosphorus heterojunction composite. Int. J. Hydrogen Energy 2021, 46, 3530–3538. [Google Scholar] [CrossRef]
- Bi, Q.; Hu, K.; Chen, J.; Zhang, Y.; Riaz, M.S.; Xu, J.; Han, Y.; Huang, F. Black phosphorus coupled black titania nanocomposites with enhanced sunlight absorption properties for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2021, 295, 120211. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Zhou, G.; Jin, M.; Li, X. In-situ growth of metal phosphide-black phosphorus heterojunction for highly selective and efficient photocatalytic carbon dioxide conversion. J. Colloid Interface Sci. 2022, 616, 641–648. [Google Scholar] [CrossRef]
- Chen, C.; Hu, J.; Yang, X.; Yang, T.; Qu, J.; Guo, C.; Li, C.M. Ambient-Stable Black Phosphorus-Based 2D/2D S-Scheme Heterojunction for Efficient Photocatalytic CO2 Reduction to Syngas. ACS Appl. Mater. Interfaces 2021, 13, 20162–20173. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Shao, W.; Zhang, X.; Chen, S.; Sun, X.; Zhang, Q.; Luo, Y.; Xie, Y. Optically Switchable Photocatalysis in Ultrathin Black Phosphorus Nanosheets. J. Am. Chem. Soc. 2018, 140, 3474–3480. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, S.; Deng, J.; Gangadharan, D.T.; Yang, F.; Xu, Z.; Giorgi, G.; Palummo, M.; Chaker, M.; Ma, D. Ice-Assisted Synthesis of Black Phosphorus Nanosheets as a Metal-Free Photocatalyst: 2D/2D Heterostructure for Broadband H2 Evolution. Adv. Funct. Mater. 2019, 29, 1902486. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, R.; Hui, J.; Zhang, Z.; Wei, S. Exposure to hypoxic Bi2MoO6 nanobelts with {001} crystal faces and face-dependent visible light driven photocatalytic activity. J. Alloys Compd. 2024, 1003, 175609. [Google Scholar] [CrossRef]
- Yang, J.; Xie, T.; Mei, Y.; Chen, J.; Sun, H.; Feng, S.; Zhang, Y.; Zhao, Y.; Wang, J.; Li, X.; et al. High-efficiency V-Mediated Bi2MoO6 photocatalyst for PMS activation: Modulation of energy band structure and enhancement of surface reaction. Appl. Catal. B Environ. 2023, 339, 123149. [Google Scholar] [CrossRef]
- Hu, J.; Chen, D.; Mo, Z.; Li, N.; Xu, Q.; Li, H.; He, J.; Xu, H.; Lu, J. Z-Scheme 2D/2D Heterojunction of Black Phosphorus/Monolayer Bi2WO6 Nanosheets with Enhanced Photocatalytic Activities. Angew. Chem. Int. Ed. 2019, 58, 2073–2077. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Huang, L.; Liu, S.; Zhu, M.; Qiu, L.; Huang, J.; Fu, Y.; Huang, L. Synergistic photocatalysis for bacteria inactivation and organic pollutant removal by S-scheme heterojunction InVO4/Bi5O7I: Performance evaluation and mechanism investigation. J. Colloid Interface Sci. 2025, 677, 234–249. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, J.; Li, S.; Zhang, L.; Shao, C.; Wang, X.; Bai, H. Z-scheme heterojunction composed of Fe-doped g-C3N4 and Bi2MoO6 for photo-fenton degradation of antibiotics over a wide pH range: Activity and toxicity assessment. Environ. Res. 2024, 252, 118886. [Google Scholar] [CrossRef]
- Feng, J.; Ran, X.; Wang, Y.; Zhan, S.; Wang, L.; Xiao, B.; Zhong, Y.; Xu, D.; Feng, G.; Dai, Z. Modulation of surface oxygen vacancies on bismuth-rich Bi5O7I nanoparticles for efficiently boosting photocatalytic overall water splitting. Appl. Surf. Sci. 2024, 655, 159517. [Google Scholar] [CrossRef]
- Ren, X.; Gao, M.; Zhang, Y.; Zhang, Z.; Cao, X.; Wang, B.; Wang, X. Photocatalytic reduction of CO2 on BiOX: Effect of halogen element type and surface oxygen vacancy mediated mechanism. Appl. Catal. B Environ. 2020, 274, 119063. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Dong, F.; Deng, F.; Han, L.; Gao, X.; Xu, J.; Hou, X.; Feng, Z.; Chen, Z.; et al. BiOBr with oxygen vacancies capture 0D black phosphorus quantum dots for high efficient photocatalytic ofloxacin degradation. Appl. Surf. Sci. 2022, 593, 153422. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Y.; Zhang, Z.; Liu, J.; Yu, Y.; Zuo, S.; Li, B. A superior ternary Z-scheme photocatalyst of Bi/Black Phosphorus nanosheets/P-doped BiOCl containing interfacial P–P bond and metallic mediator for H2O2 production and RhB degradation. Chemosphere 2023, 330, 138717. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Luo, Q.; Zhen, W.; Ge, Q.; Zhou, Y.; Ma, C.; Sun, P.; Xu, J.; Chen, B. The Photocatalytic Ozonation Oxidation Synergistic Degradation on Organic Pollutants In Coal Chemical Phenol-Ammonia Wastewater by Two-Dimensional Nanosheets Bi2WO6. Water Air Soil Pollut. 2024, 235, 371. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Ran, X.; Xiao, B.; Lei, L.; Zhu, J.; Li, R.; Xi, X.; Feng, G. Adsorption and photocatalytic synergistic removal of ciprofloxacin on mesoporous ErFeO3/g-C3N4 heterojunction. Environ. Technol. Innov. 2022, 28, 102785. [Google Scholar] [CrossRef]
- Abidi, M.; Assadi, A.A.; Aouida, S.; Tahraoui, H.; Khezami, L.; Zhang, J.; Amrane, A.; Hajjaji, A. Photocatalytic Activity of Cu2O-Loaded TiO2 Heterojunction Composites for the Simultaneous Removal of Organic Pollutants and Bacteria in Indoor Air. Catalysts 2025, 15, 360. [Google Scholar] [CrossRef]
- Moradeeya, P.G.; Kumar, M.A.; Sharma, A.; Basha, S. Sequential sorption-photocatalytic method using polypyrrole@TiO2 nanocomposites for the removal of agrochemicals. Indian J. Chem. Technol. 2025, 32, 395–406. [Google Scholar]
- Huang, X.; Zhu, N.; Mao, F.; Ding, Y.; Zhang, S.; Liu, H.; Li, F.; Wu, P.; Dang, Z.; Ke, Y. Enhanced heterogeneous photo-Fenton catalytic degradation of tetracycline over yCeO2/Fh composites: Performance, degradation pathways, Fe2+ regeneration and mechanism. Chem. Eng. J. 2020, 392, 123636. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, Y.; Wang, Y.; Sun, S.-P.; Wu, W.D.; Wu, Z. Nanostructured semiconductor supported iron catalysts for heterogeneous photo-Fenton oxidation: A review. J. Mater. Chem. A 2020, 8, 15513–15546. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, K.; Zhao, H.; Peng, B.; Liang, J.; Chen, H.; Huang, R.; Wei, Y.; Chen, H. Synergetic promotional effect of dual oxygen vacancies and 2D/2D TiO2/BiOBr S-scheme heterostructure for boosted photocatalytic oxytetracycline removal. Colloid. Surf. A 2025, 715, 136636. [Google Scholar] [CrossRef]
- Deng, C.; Li, S.; Wang, J.; Li, Y.; Chen, J.; Tang, F.; Yang, X. Enhanced visible light photocatalytic degradation of oxytetracycline hydrochloride using heterojunction BiOBr/TiO2 composites. Appl. Surf. Sci. 2025, 710, 163945. [Google Scholar] [CrossRef]
- Liang, X.; Li, L.; Wu, Y.; Liu, F.; Wang, Y. Enhancement of CuS/Fe3O4@MIL-100(Fe) ternary composite by co-catalyst CuS for photo-Fenton degradation of oxytetracycline hydrochloride. Appl. Surf. Sci. 2025, 697, 163029. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, Q.; Li, Y.; Cui, S.; Zhao, C.; Yang, J. Enhanced photocatalytic degradation of oxytetracycline by Z-scheme heterostructure ZIF-8/Bi4O5Br2. Appl. Catal. A-Gen. 2024, 681, 119786. [Google Scholar] [CrossRef]
- Xin, S.; Liu, G.; Ma, X.; Gong, J.; Ma, B.; Yan, Q.; Chen, Q.; Ma, D.; Zhang, G.; Gao, M.; et al. High efficiency heterogeneous Fenton-like catalyst biochar modified CuFeO2 for the degradation of tetracycline: Economical synthesis, catalytic performance and mechanism. Appl. Catal. B Environ. 2021, 280, 119386. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.; Mou, R. Rice hull-derived biochar with photocatalytic activity for efficient degradation of oxytetracycline in water under sunlight. Process Saf. Environ. 2024, 188, 1550–1561. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, X.; Tang, Y.; Zeng, Y.; Wang, L.; Zhang, S.; Cai, T.; Liu, Y.; Luo, S.; Pei, Y.; et al. Positioning cyanamide defects in g-C3N4: Engineering energy levels and active sites for superior photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 237, 24–31. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Gao, X.; Ma, J.; Chen, Z.; Kang, B.; Liu, J.; Li, H.; Feng, Z.; Huang, J. Novel BP/BiOBr S-scheme nano-heterojunction for enhanced visible-light photocatalytic tetracycline removal and oxygen evolution activity. J. Hazard. Mater. 2020, 387, 121690. [Google Scholar] [CrossRef]
- Moradian, S.; Badiei, A.; Ziarani, G.M.; Mohajer, F.; Varma, R.S.; Iravani, S. Black Phosphorus-based Photocatalysts: Synthesis, Properties, and Applications. Environ. Res. 2023, 237, 116910. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Si, Q.; Jia, W.; Zheng, S.; Wang, H.; Zhao, Q.; Luo, H.; Jiang, J.; Ren, N. Atomically dispersed cobalt on carbon nitride for peroxymonosulfate activation: Switchable catalysis enabled by light irradiation. Chem. Eng. J. 2022, 446, 137277. [Google Scholar] [CrossRef]
- Zhao, M.; Qian, H.; Niu, X.; Wang, W.; Guan, L.; Sha, J.; Wang, Y. Growth Mechanism and Enhanced Yield of Black Phosphorus Microribbons. Cryst. Growth Des. 2016, 16, 1096–1103. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, T.; Kang, J.; Zhao, Y.; Zhang, Y.; Wang, T.; Yin, X. High-efficient degradation of oxytetracycline by visible photo-Fenton process using BP/Bi2MoO6: Performance and mechanisms. Sep. Purif. Technol. 2022, 299, 121771. [Google Scholar] [CrossRef]
- He, Y.; Lv, H.; Daili, Y.; Yang, Q.; Junior, L.B.; Liu, D.; Liu, H.; Ma, Z. Construction of a new cascade photogenerated charge transfer system for the efficient removal of bio-toxic levofloxacin and rhodamine B fromaqueous solution: Mechanism, degradation pathways and intermediates study. Environ. Res. 2020, 187, 109647. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Niu, C.; Guo, H.; Zhang, L.; Liang, C.; Zeng, G. Photocatalytic degradation of levofloxacin by ternary Ag2CO3/CeO2/AgBr photocatalyst under visible-light irradiation: Degradation pathways, mineralization ability, and an accelerated interfacial charge transfer process study. J. Catal. 2018, 358, 211. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, H.; Deng, J.; Li, M.; Tang, C.; Hu, X.; Zhu, M. Z-scheme γ-Fe2O3/g-C3N4 in Photo-Fenton reaction for oxytetracycline degradation: Mechanism study and DFT calculation. Sep. Purif. Technol. 2025, 354, 129185. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, M.; Guan, J.; Yao, Y.; Sun, C.; Liu, Q.; Chen, X. Mixed-valent Fe-MOF accelerated Fe(III)/Fe(II) cycle for highly efficient photo-Fenton-like catalytic degradation of organic pollutants: Boosting mechanism and degradation pathways. J. Environ. Chem. Eng. 2024, 12, 113577. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Li, X.; Ran, X.; Wang, L.; Xiao, B.; Li, R.; Feng, G. Enhanced Photo-Fenton Removal of Oxytetracycline Hydrochloride via BP/Bi2MoO6 Z-Scheme Heterojunction Photocatalyst. Int. J. Mol. Sci. 2025, 26, 7751. https://doi.org/10.3390/ijms26167751
Feng J, Li X, Ran X, Wang L, Xiao B, Li R, Feng G. Enhanced Photo-Fenton Removal of Oxytetracycline Hydrochloride via BP/Bi2MoO6 Z-Scheme Heterojunction Photocatalyst. International Journal of Molecular Sciences. 2025; 26(16):7751. https://doi.org/10.3390/ijms26167751
Chicago/Turabian StyleFeng, Jian, Xiaohui Li, Xia Ran, Li Wang, Bo Xiao, Rong Li, and Guangwei Feng. 2025. "Enhanced Photo-Fenton Removal of Oxytetracycline Hydrochloride via BP/Bi2MoO6 Z-Scheme Heterojunction Photocatalyst" International Journal of Molecular Sciences 26, no. 16: 7751. https://doi.org/10.3390/ijms26167751
APA StyleFeng, J., Li, X., Ran, X., Wang, L., Xiao, B., Li, R., & Feng, G. (2025). Enhanced Photo-Fenton Removal of Oxytetracycline Hydrochloride via BP/Bi2MoO6 Z-Scheme Heterojunction Photocatalyst. International Journal of Molecular Sciences, 26(16), 7751. https://doi.org/10.3390/ijms26167751






