Evaluation of CoFe2O4-L-Au (L: Citrate, Glycine) as Superparamagnetic–Plasmonic Nanocomposites for Enhanced Cytotoxic Activity Towards Oncogenic (A549) Cells
Abstract
1. Introduction
2. Results and Discussion
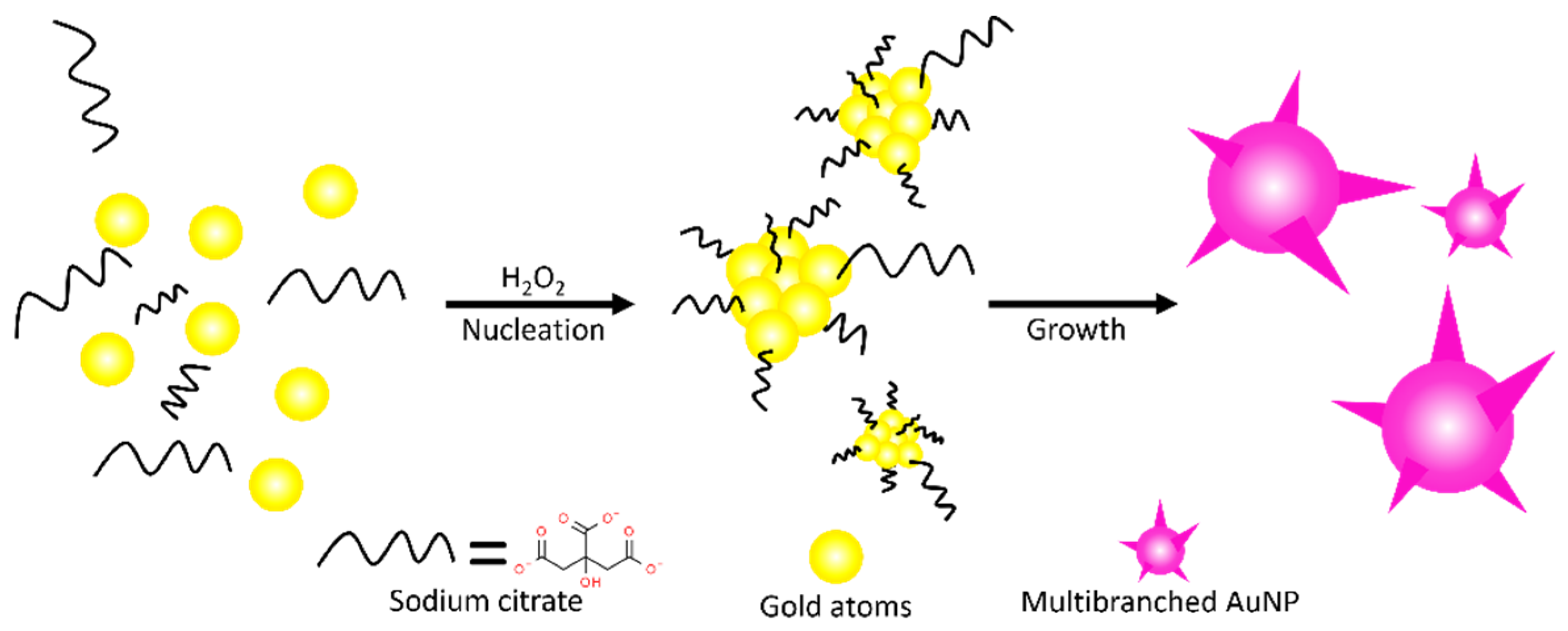
2.1. Synthesis of MCF, MCF-AuCit, and MCF-AuGly
2.2. Characterization of Materials
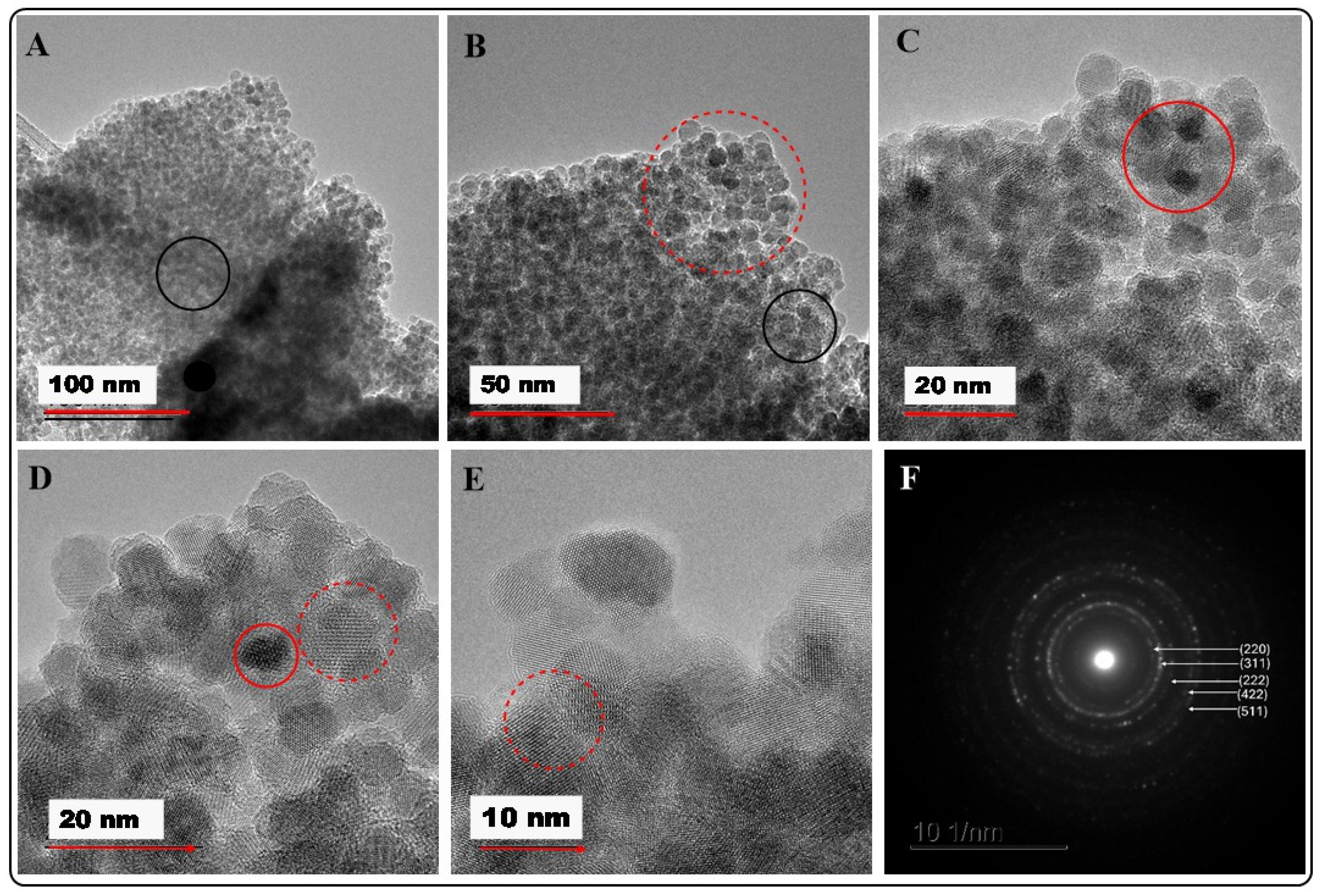
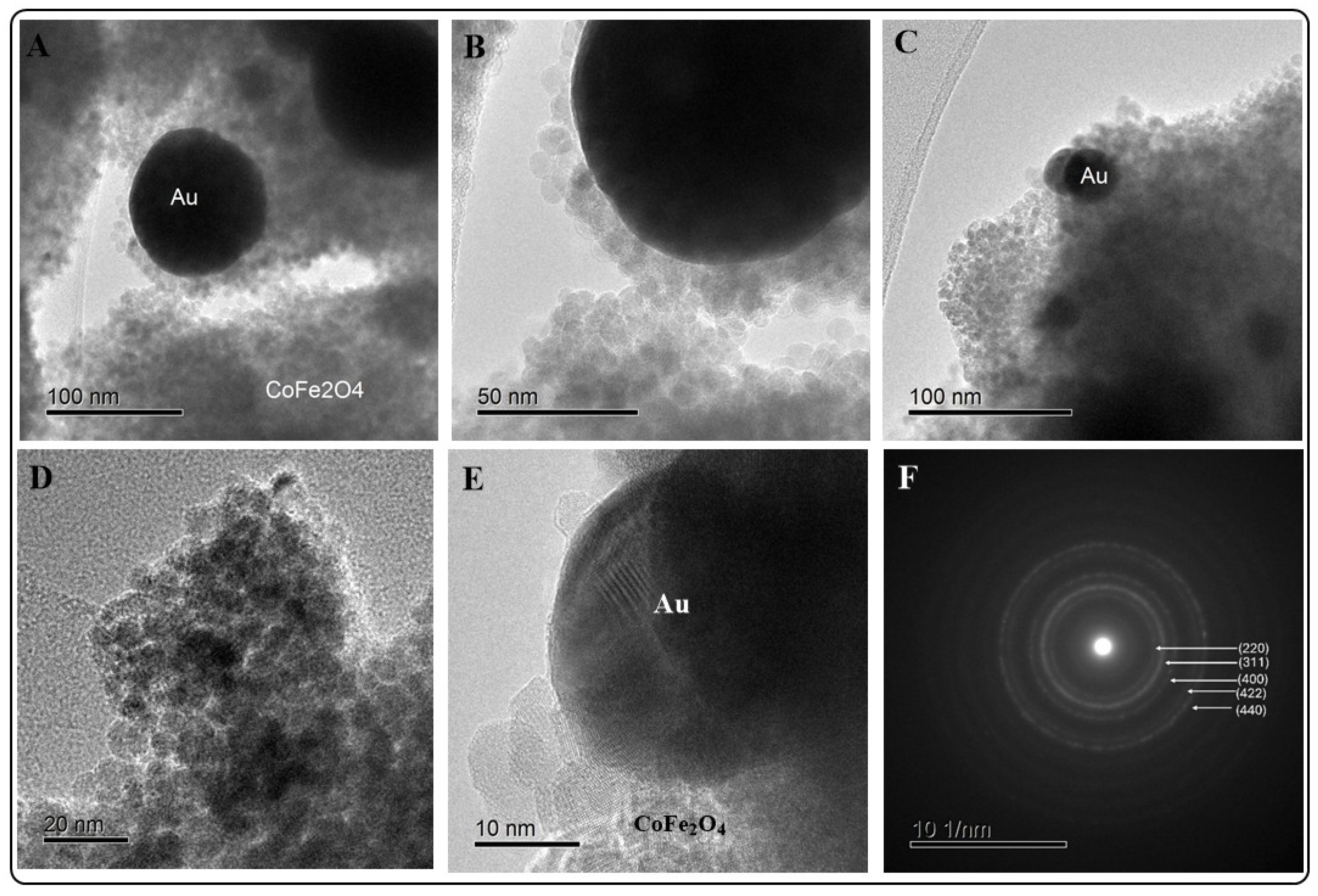
2.2.1. HRTEM/EDX Analysis
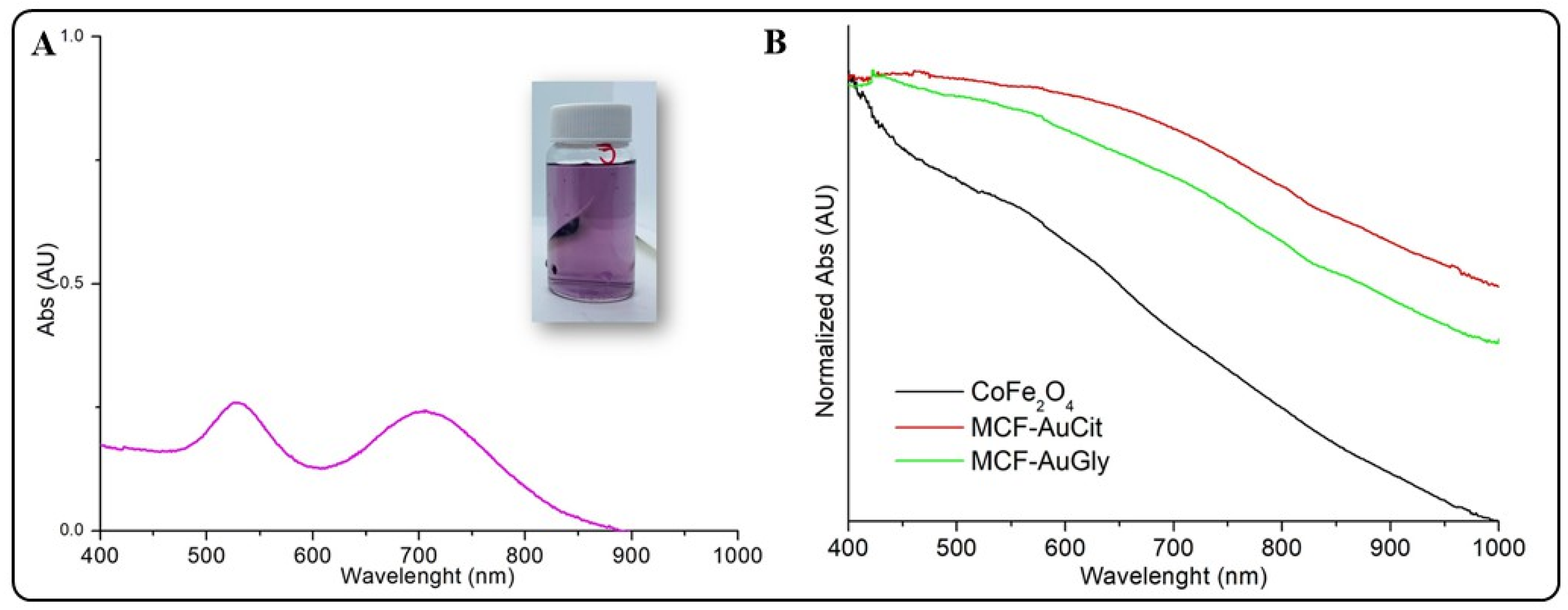
2.2.2. UV-Vis Spectrophotometry
2.2.3. XRD Analysis
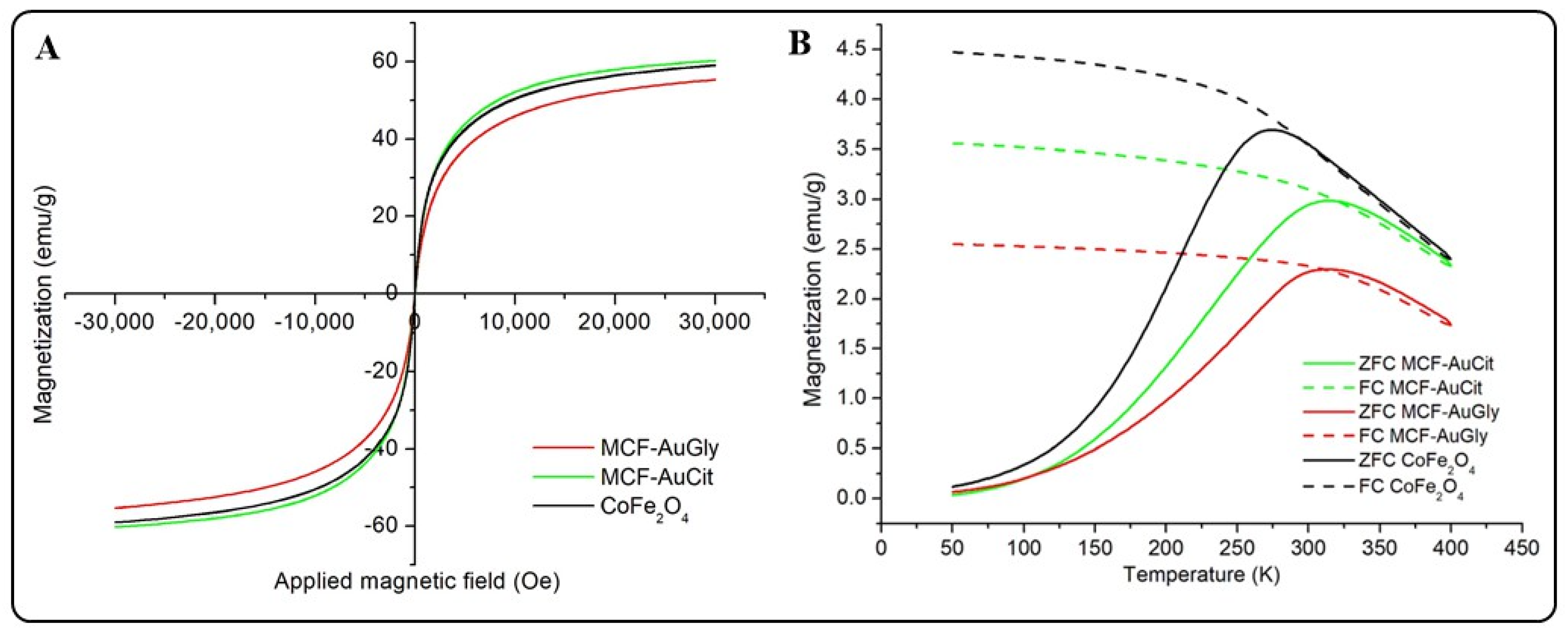
2.2.4. Analysis of Magnetic Properties
2.2.5. FTIR Spectroscopy
2.2.6. TGA Analyses
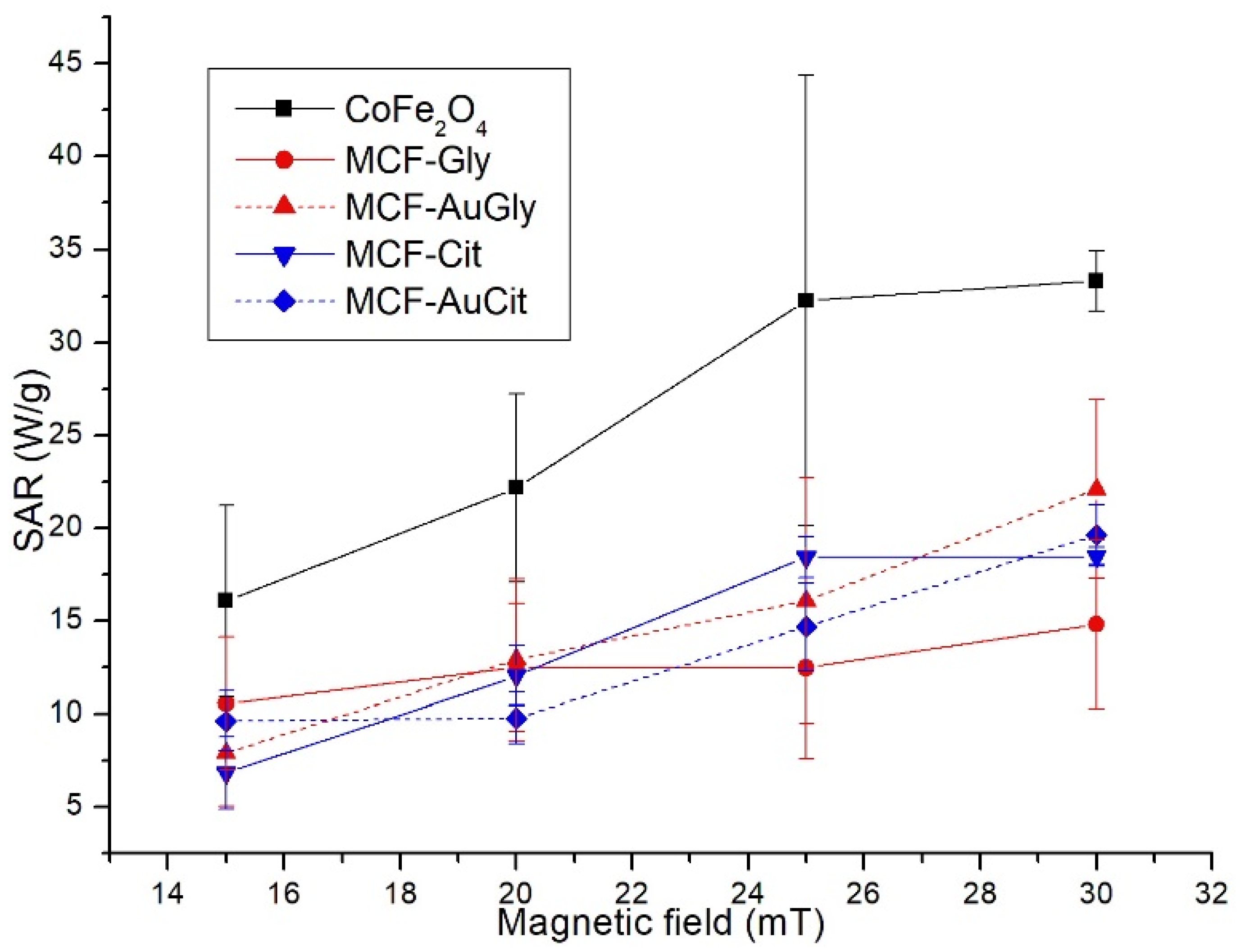
2.3. Calorimetric Tests
2.4. Biocompatibility or Cytotoxic Activity Evaluation
2.4.1. Hemolysis Tests
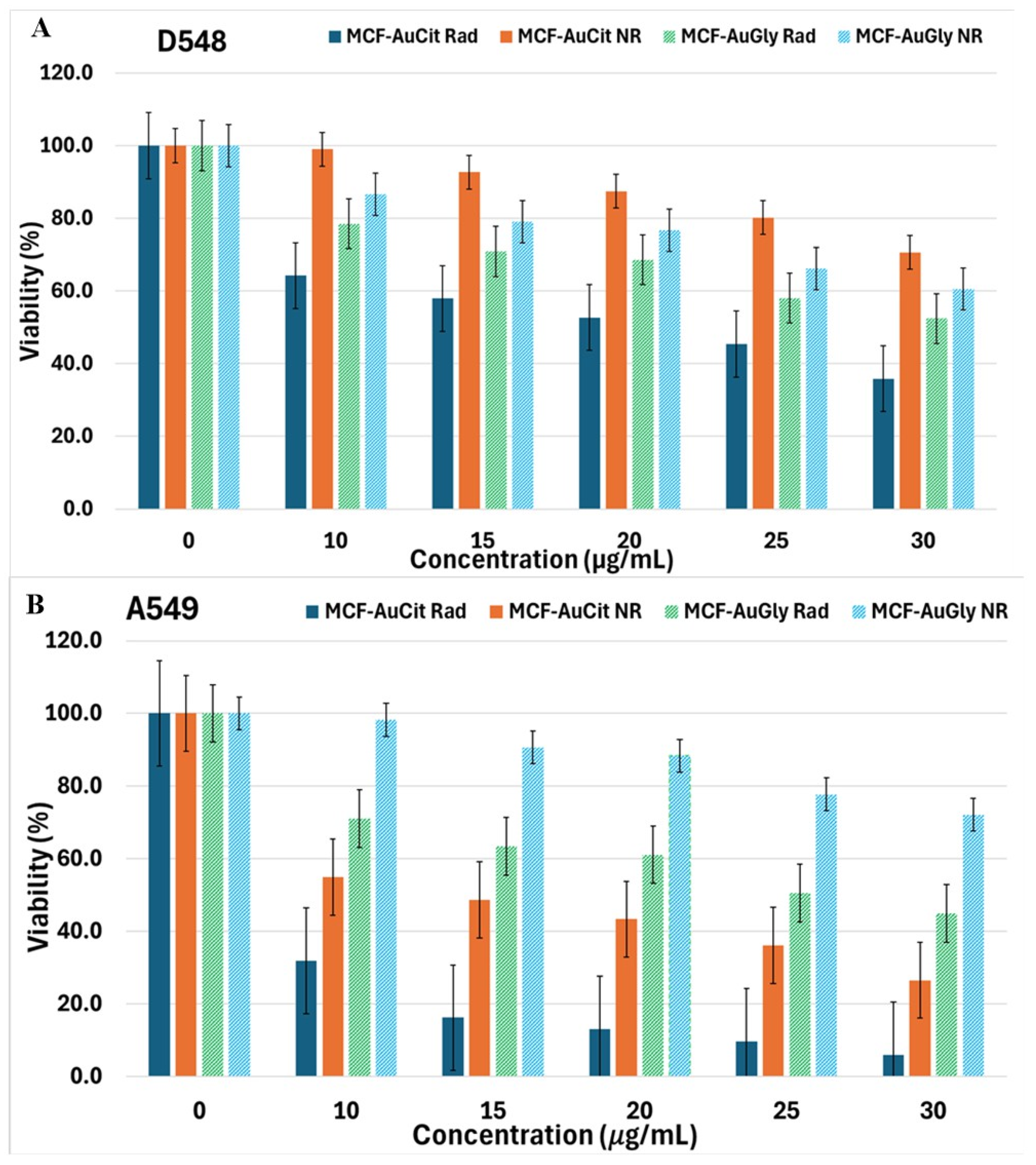
2.4.2. Cell Viability Evaluation
2.5. Evaluation of the Interactions of NMs with A549 and D548 Cells Using Advanced Microscopy Techniques
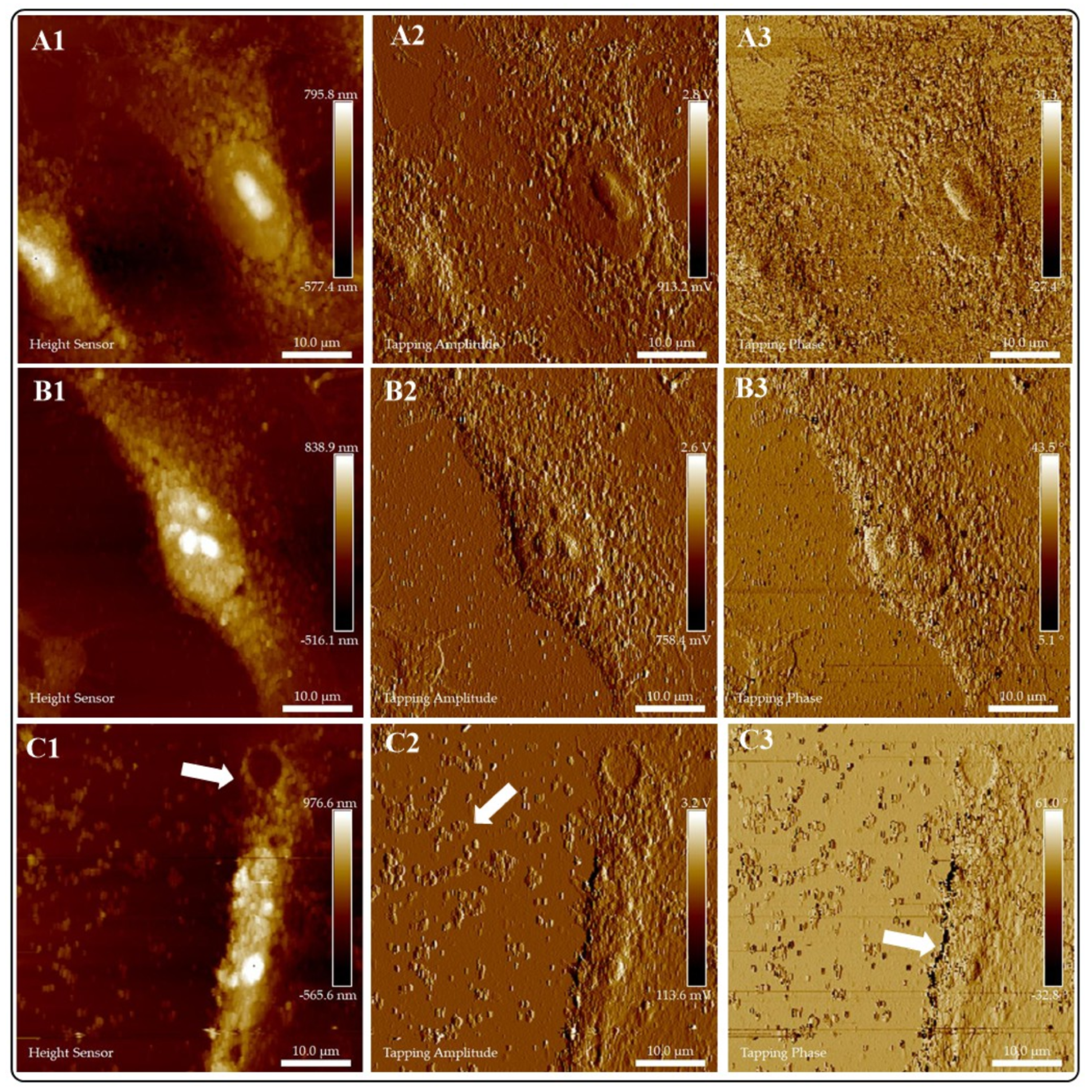
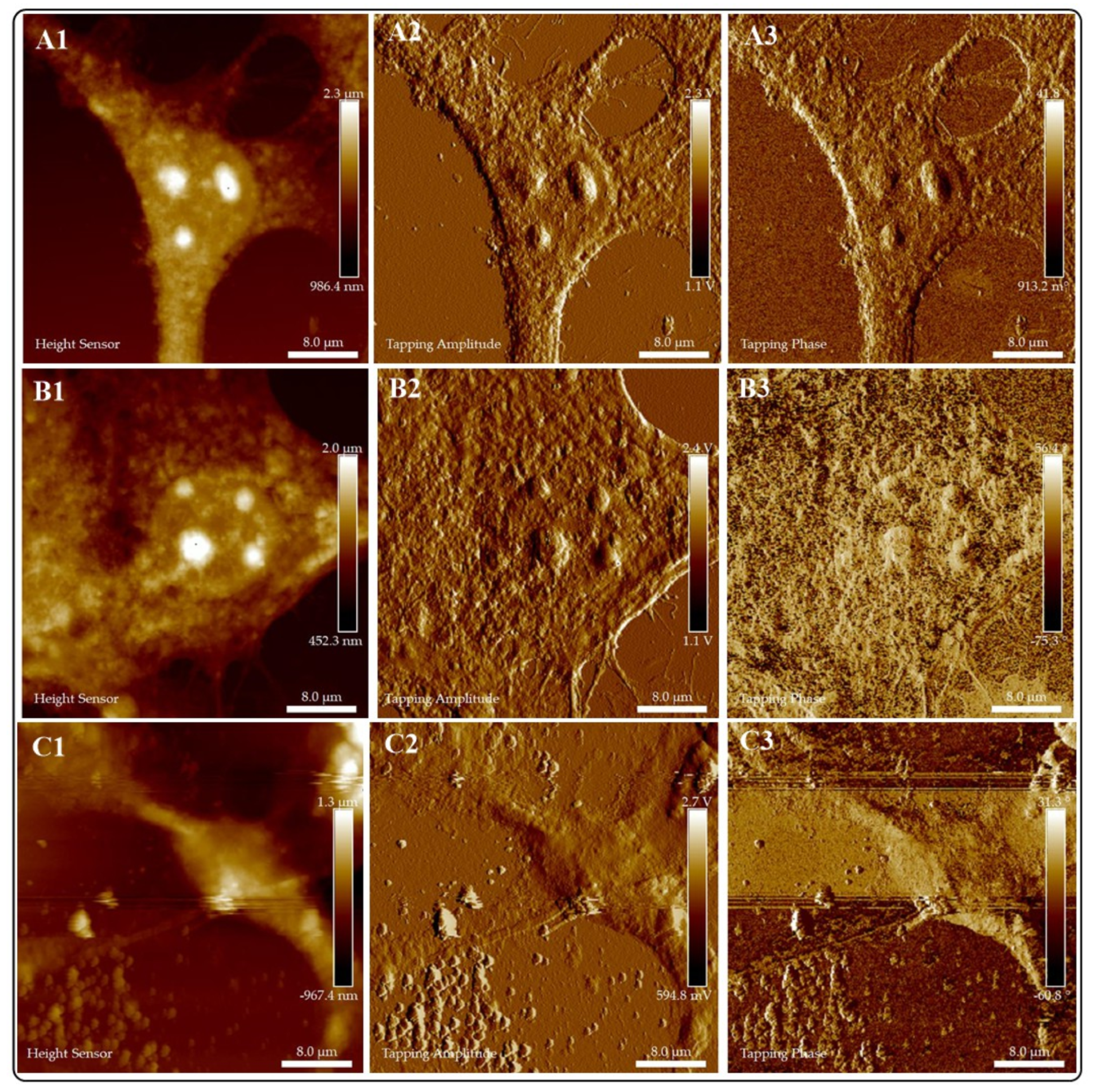
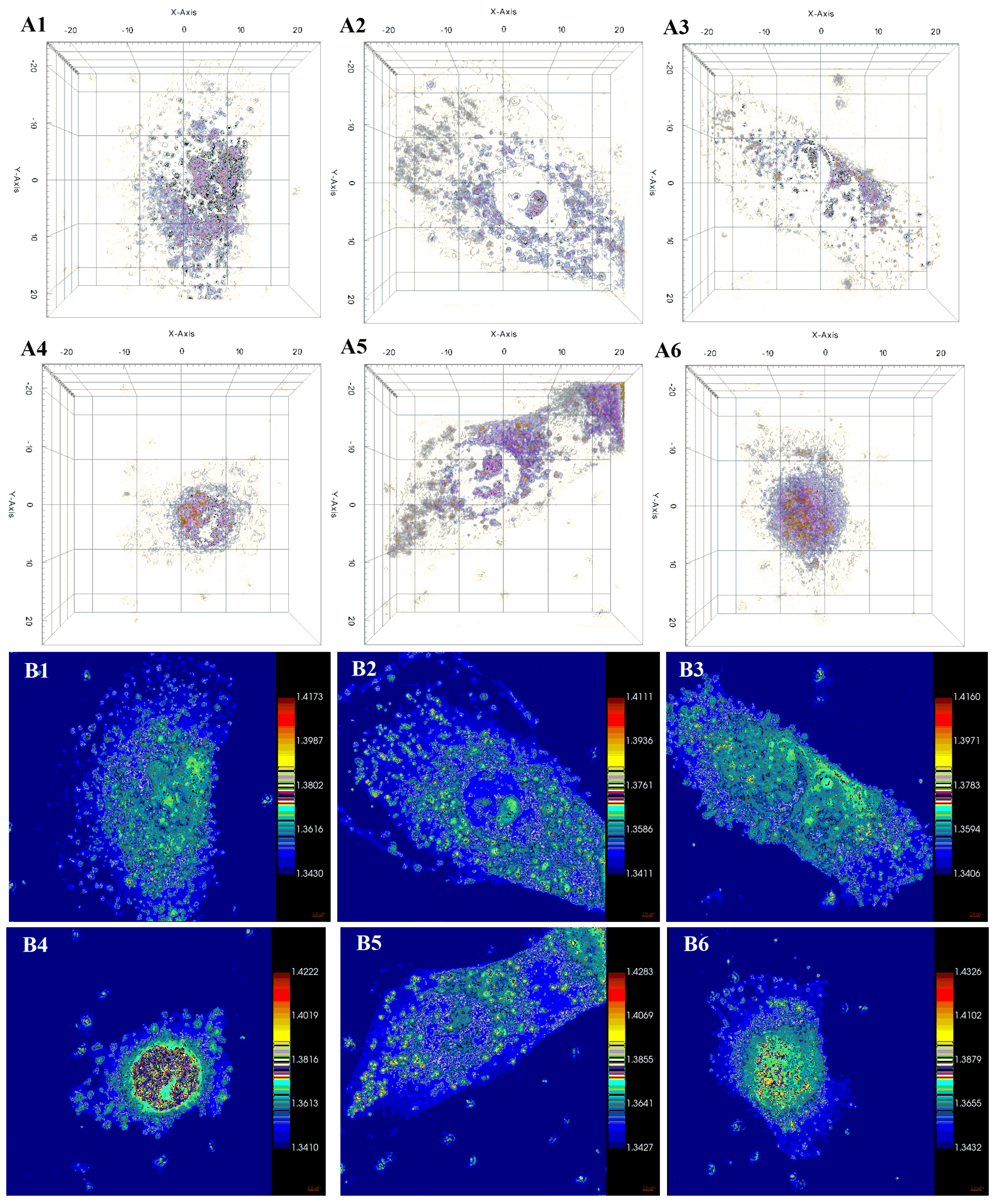
2.5.1. Atomic Force Microscopy
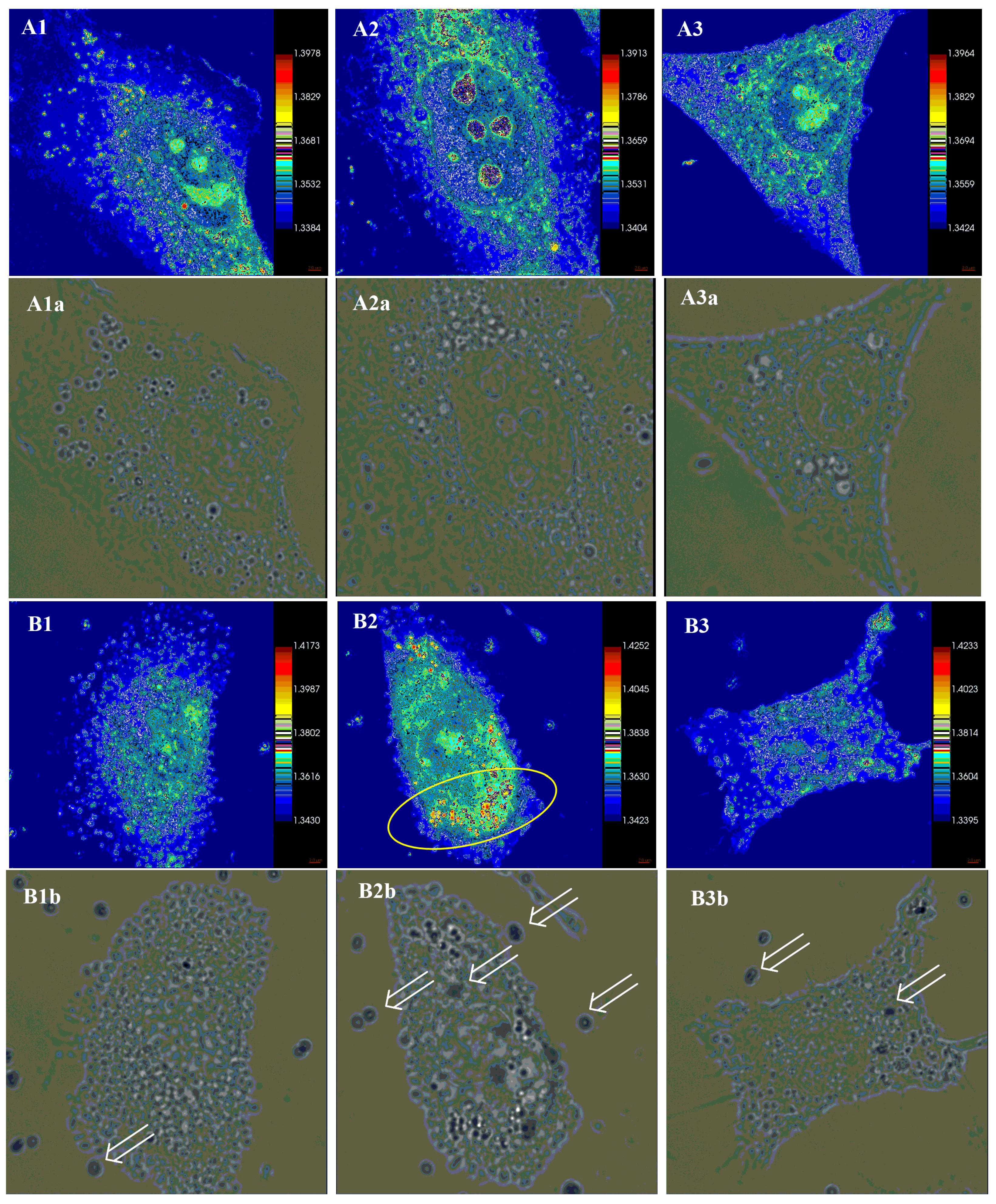
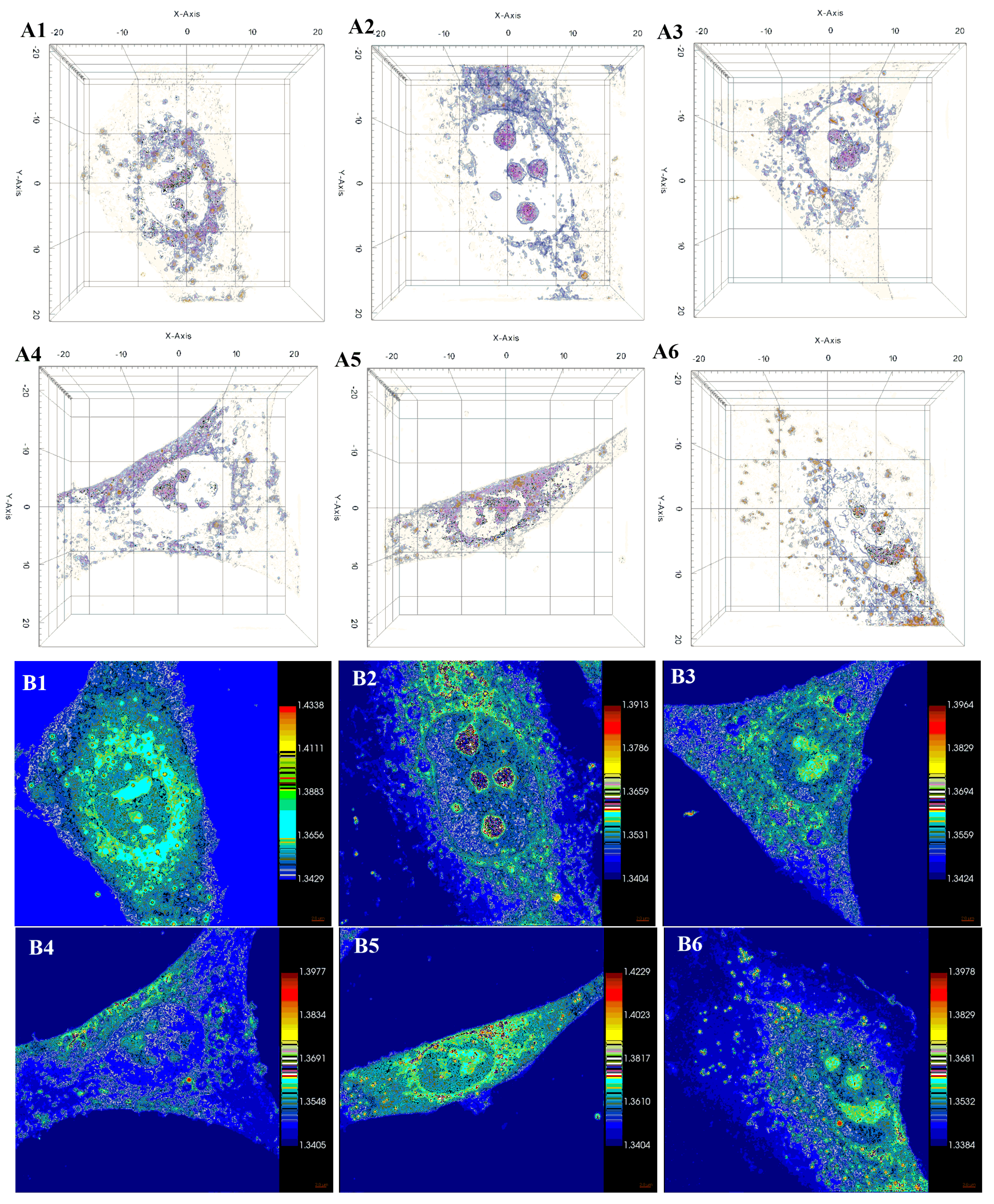
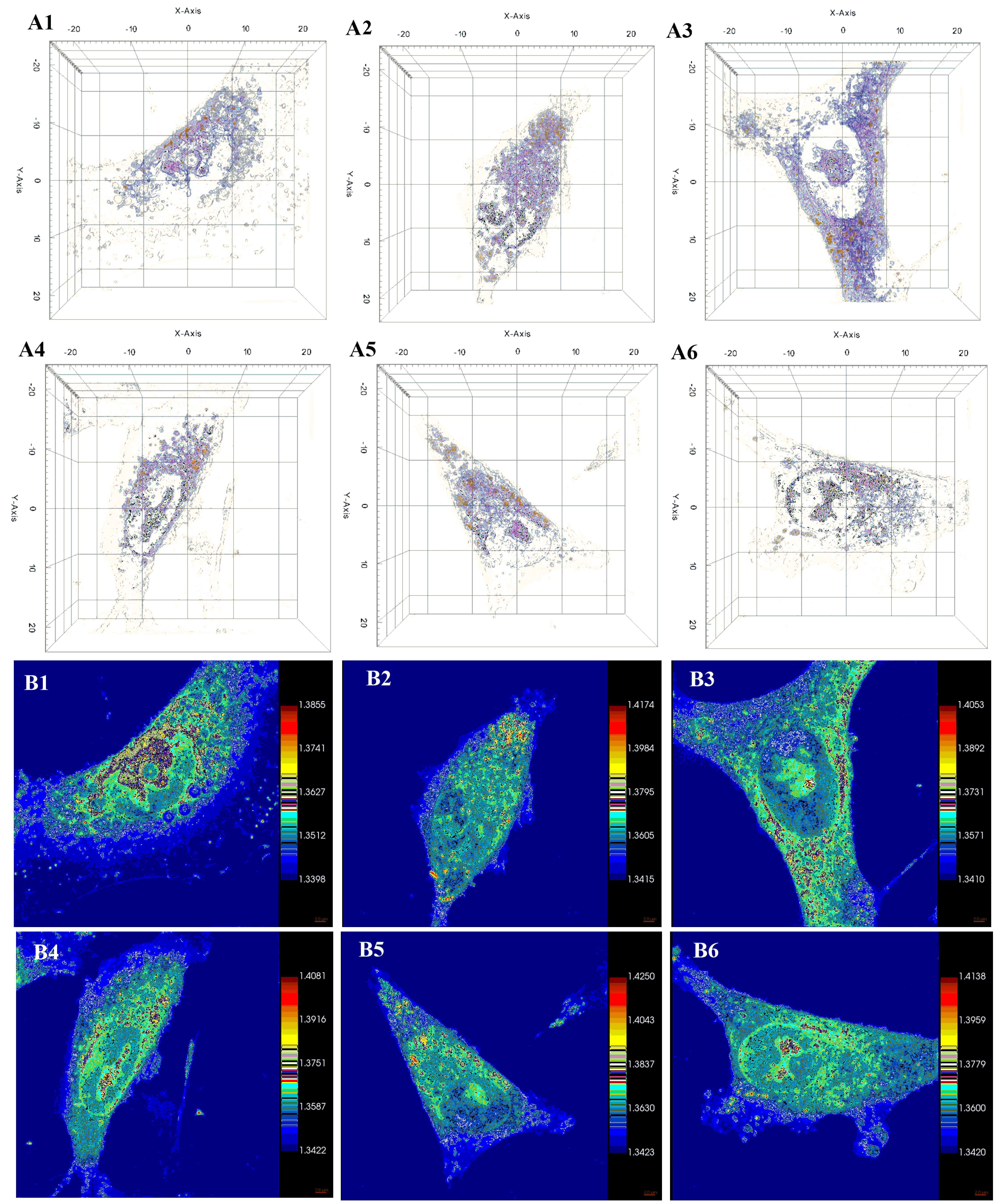
2.5.2. Holotomographic Microscopy Analysis
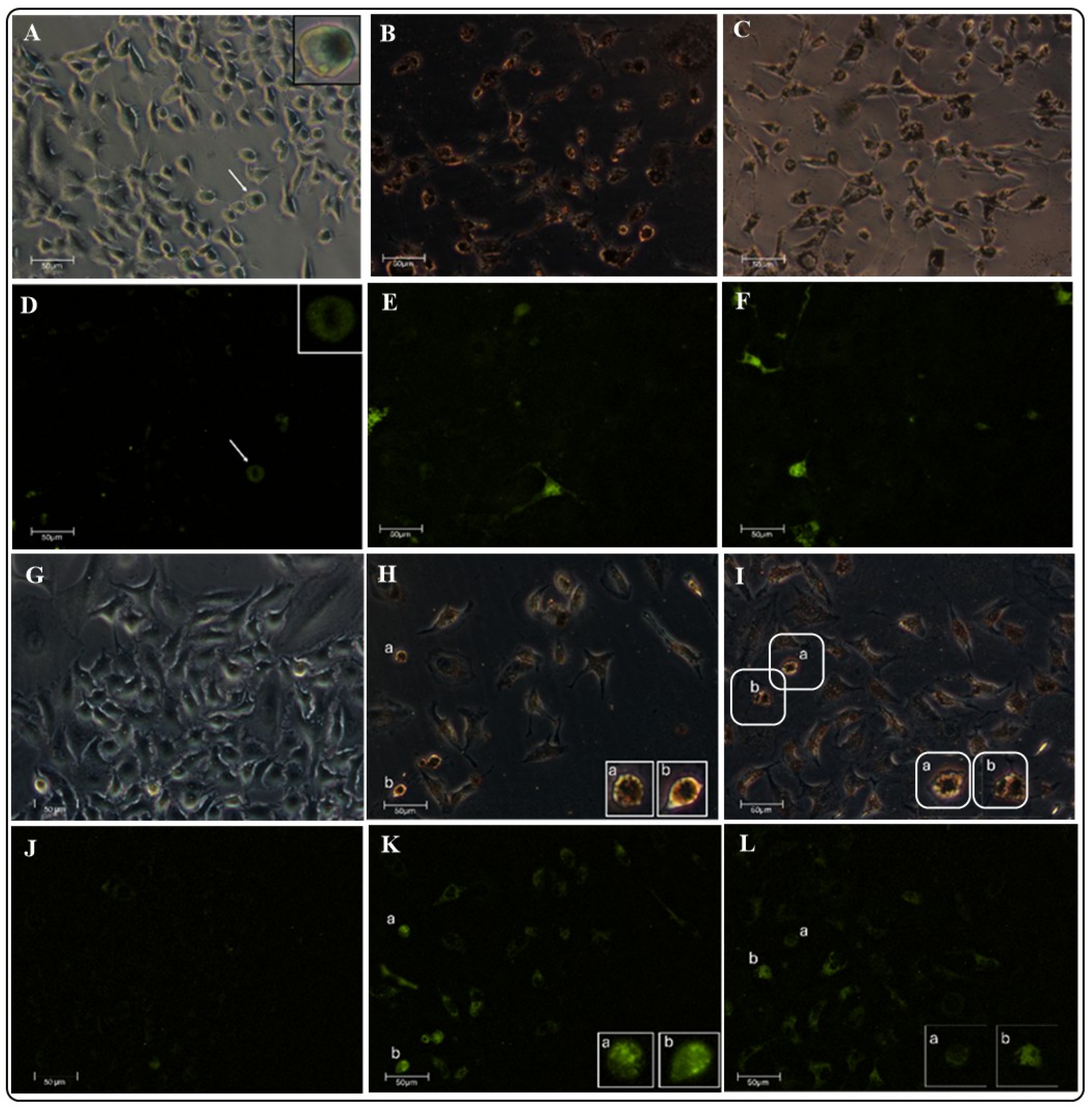
2.5.3. Evaluation of the ROS Oxidative Damage Using Fluorescence Microscopy
3. Materials and Methods
3.1. Synthesis of CoFe2O4
3.1.1. CoFe2O4 Functionalization
3.1.2. Au Nanoparticle Synthesis
3.1.3. AuNP Deposition on MCF-Cit and MCF-Gly (MCF-AuCit and MCF-AuGly)
3.2. Characterization
3.3. Calorimetric Analyses
3.4. Biocompatibility or Cytotoxic Activity Evaluation
3.4.1. Hemolysis Assay
3.4.2. CCK-8 Assay
3.5. Evaluation of the Interactions of NMs with A549 and D548 Cells Using Advanced Microscopy Techniques
3.5.1. Atomic Force Microscopy (AFM) Analysis
3.5.2. Holotomographic Microscopy (HTM) Analysis
3.5.3. Evaluation of the ROS Oxidative Damage Using Fluorescence Microscopy
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| L | Ligand (functionalization molecule) |
| Cit | Sodium citrate |
| Gly | Glycine |
| MCF | Magnetic cobalt ferrite (CoFe2O4) |
| NMs | Nanomaterials |
| MCF-Au-L | Multifunctional plasmonic nanostructured materials |
| Au NPs | Gold nanoparticles |
| PDT | Photodynamic therapy |
| LSPR | Localized surface plasmon resonance |
| PTT | Photothermal therapy |
| PCE | Photoconversion Energy |
| A549 | Lung alveolar cancer cells |
| Detroit 548 | Skin fibroblasts cells |
| HR-TEM | High-resolution transmission electron microscopy |
| EDX | Energy-dispersive X-ray spectroscopy |
| UV-Vis | Ultraviolet-visible |
| LSP | Localized surface plasmon |
| FT-IR | Fourier transformed-infrared spectroscopy |
| TGA | Thermogravimetric analysis |
| CCK-8 | Cell Counting Kit-8 |
| AFM | Atomic force microscopy |
| CFM | Confocal fluorescence microscopy |
| HTM | Holotomographic microscopy |
| MHT | Magnetic hyperthermia therapy |
| MNMs | Magnetic nanomaterials |
| DNA | Desoxyribonucleic acid |
| ROS | Reactive oxygen species |
References
- Medina-Ramírez, I.E.; Díaz de León Olmos, M.A.; Muñoz Ortega, M.H.; Zapien, J.A.; Betancourt, I.; Santoyo-Elvira, N. Development and Assessment of Nano-Technologies for Cancer Treatment: Cytotoxicity and Hyperthermia Laboratory Studies. Cancer Investig. 2020, 38, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.P.; Moreira, J.A.; Monteiro, F.J.; Laranjeira, M.S. Nanomaterials in Cancer: Reviewing the Combination of Hyperthermia and Triggered Chemotherapy. J. Control. Release 2022, 347, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Cihoric, N.; Tsikkinis, A.; van Rhoon, G.; Crezee, H.; Aebersold, D.M.; Bodis, S.; Beck, M.; Nadobny, J.; Budach, V.; Wust, P.; et al. Hyperthermia-Related Clinical Trials on Cancer Treatment Within the ClinicalTrials.Gov Registry. Int. J. Hyperth. 2015, 31, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Zhang, M. Magnetite Nanoparticles for Cancer Diagnosis, Treatment, and Treatment Monitoring: Recent Advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive Understanding of Magnetic Hyperthermia for Improving Antitumor Therapeutic Efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Szwed, M.; Marczak, A. Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge. Cancers 2024, 16, 1156. [Google Scholar] [CrossRef]
- Skitzki, J.J.; Repasky, E.A.; Evans, S.S. Hyperthermia as an Immunotherapy Strategy for Cancer. Curr. Opin. Investig. Drugs 2009, 10, 550–558. [Google Scholar]
- Wang, Z.; Zhang, F.; Shao, D.; Chang, Z.; Wang, L.; Hu, H.; Zheng, X.; Li, X.; Chen, F.; Tu, Z.; et al. Janus Nanobullets Combine Photodynamic Therapy and Magnetic Hyperthermia to Potentiate Synergetic Anti-Metastatic Immunotherapy. Adv. Sci. 2019, 6, 1901690. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Zhang, M. Recent Progress in the Synergistic Combination of Nanoparticle-Mediated Hyperthermia and Immunotherapy for Treatment of Cancer. Adv. Healthc. Mater. 2021, 10, e2001415. [Google Scholar] [CrossRef]
- Yi, G.Y.; Kim, M.J.; Kim, H.I.; Park, J.; Baek, S.H. Hyperthermia Treatment as a Promising Anti-Cancer Strategy: Therapeutic Targets, Perspective Mechanisms and Synergistic Combinations in Experimental Approaches. Antioxidants 2022, 11, 625. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-Mediated near-Infrared Thermal Therapy of Tumors under Magnetic Resonance Guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, B.; Auh, Y.; Kim, E. Conjugated Organic Photothermal Films for Spatiotemporal Thermal Engineering. Adv. Mater. 2021, 33, 2005940. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Su, S.; Xu, T.; Zhong, Y.; Zapien, J.A.; Li, J.; Fan, C.; Lee, S.T. Silicon Nanowires-Based Highly-Efficient SERS-Active Platform for Ultrasensitive DNA Detection. Nano Today 2011, 6, 122–130. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Duan, X.; Sun, H.; Wang, S. Photothermal Catalysis: From Fundamentals to Practical Applications. Mater. Today 2023, 68, 234–253. [Google Scholar] [CrossRef]
- Koç, M.M.; Paksu, U.; Kurnaz Yetim, N.; Coşkun, B.; Hasanoğlu Özkan, E.; Erkovan, M. Nanoparticles in Photothermal Therapy-Based Medical and Theranostic Applications: An Extensive Review. Eur. Phys. J. Plus 2025, 140, 514. [Google Scholar] [CrossRef]
- Paramasivam, G.; Kayambu, N.; Rabel, A.M.; Sundramoorthy, A.K.; Sundaramurthy, A. Anisotropic Noble Metal Nanoparticles: Synthesis, Surface Functionalization and Applications in Biosensing, Bioimaging, Drug Delivery and Theranostics. Acta Biomater. 2017, 49, 45–65. [Google Scholar] [CrossRef]
- Curcio, A.; Silva, A.K.A.; Cabana, S.; Espinosa, A.; Baptiste, B.; Menguy, N.; Wilhelm, C.; Abou-Hassan, A. Iron Oxide Nanoflowers @ Cus Hybrids for Cancer Tri-Therapy: Interplay of Photothermal Therapy, Magnetic Hyperthermia and Photodynamic Therapy. Theranostics 2019, 9, 1288–1302. [Google Scholar] [CrossRef]
- Mai, B.T.; Fernandez-Cabada, T.; Conteh, J.S.; Nucci, G.E.P.; Fiorito, S.; Gavilán, H.; Debellis, D.; Gjurgjaj, L.; Pellegrino, T. Nanoplatforms for Magnetic-Photo-Heating of Thermo-Resistant Tumor Cells: Singular Synergic Therapeutic Effects at Mild Temperature. Small 2024, 20, e2310522. [Google Scholar] [CrossRef]
- Debnath, M.; Debnath, S.K.; Talpade, M.V.; Bhatt, S.; Gupta, P.P.; Srivastava, R. Surface Engineered Nanohybrids in Plasmonic Photothermal Therapy for Cancer: Regulatory and Translational Challenges. Nanotheranostics 2024, 8, 202–218. [Google Scholar] [CrossRef]
- Medina-Ramírez, I.E.; Díaz de León-Macias, C.E.; Pedroza-Herrera, G.; Gonzáles-Segovia, R.; Zapien, J.A.; Rodríguez-López, J.L. Evaluation of the Biocompatibility and Growth Inhibition of Bacterial Biofilms by ZnO, Fe3O4 and ZnO@Fe3O4 Photocatalytic Magnetic Materials. Ceram. Int. 2020, 46, 8979–8994. [Google Scholar] [CrossRef]
- Medina-Ramírez, I.E.; Marroquin-Zamudio, A.; Martínez-Montelongo, J.H.; Romo-Lozano, Y.; Zapien, J.A.; Perez-Larios, A. Enhanced Photocatalytic and Antifungal Activity of ZnO–Cu2+ and Ag@ZnO–Cu2+ Materials. Ceram. Int. 2022, 48, 12660–12674. [Google Scholar] [CrossRef]
- Mokhosi, S.R.; Mdlalose, W.; Nhlapo, A.; Singh, M. Advances in the Synthesis and Application of Magnetic Ferrite Nanoparticles for Cancer Therapy. Pharmaceutics 2022, 14, 937. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of Functionalization: Strategies to Explore Potential Nano-Bio Applications of Magnetic Nanoparticles. RSC Adv. 2016, 6, 43989–44012. [Google Scholar] [CrossRef]
- Martínez-Montelongo, J.H.; Hernandez-Rangel, R.; Campos-Avelar, I.; Betancourt, I.; Quintero, L.H.; Cano, M.E.; Zapien, J.A.; Medina-Ramirez, I.E. Antimicrobial Activity of CuFe2O4 and CuFe2O4/ZnO: Squaring Colorimetric and Traditional Microbiology Assays with Atomic Force- and Holotomography-Microscopies. Ceram. Int. 2025, 51, 2452–2466. [Google Scholar] [CrossRef]
- Sampaio, W.S.; Dalmaschio, C.J. Functionalization of Nanostructured Surfaces: From the Impact on Morphological Control during Synthesis to the Effect on Colloidal Dispersion. J. Mater. Res. 2024, 39, 2379–2396. [Google Scholar] [CrossRef]
- Guan, H.; Harris, C.; Sun, S. Metal-Ligand Interactions and Their Roles in Controlling Nanoparticle Formation and Functions. Acc. Chem. Res. 2023, 56, 1591–1601. [Google Scholar] [CrossRef]
- He, M.; Cao, B.; Gao, X.; Liu, B.; Yang, J. Synthesis of Multi-Branched Gold Nanostructures and Their Surface-Enhanced Raman Scattering Properties of 4-Aminothiophenol. J. Mater. Res. 2019, 34, 2928–2934. [Google Scholar] [CrossRef]
- Gao, Y.; Torrente-Murciano, L. Mechanistic Insights of the Reduction of Gold Salts in the Turkevich Protocol. Nanoscale 2020, 12, 2740–2751. [Google Scholar] [CrossRef]
- Panda, B.R.; Chattopadhyay, A. Synthesis of Au Nanoparticles at “All” PH by H2O2 Reduction of HAuCl4. J. Nanosci. Nanotechnol. 2007, 7, 1911–1915. [Google Scholar] [CrossRef]
- Cheng, L.C.; Huang, J.H.; Chen, H.M.; Lai, T.C.; Yang, K.Y.; Liu, R.S.; Hsiao, M.; Chen, C.H.; Her, L.J.; Tsai, D.P. Seedless, Silver-Induced Synthesis of Star-Shaped Gold/Silver Bimetallic Nanoparticles as High Efficiency Photothermal Therapy Reagent. J. Mater. Chem. 2012, 22, 2244–2253. [Google Scholar] [CrossRef]
- Amiri, M.; Salavati-Niasari, M.; Akbari, A. Magnetic Nanocarriers: Evolution of Spinel Ferrites for Medical Applications. Adv. Colloid Interface Sci. 2019, 265, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Yockell-Lelièvre, H.; Lussier, F.; Masson, J.-F. Influence of the Particle Shape and Density of Self-Assembled Gold Nanoparticle Sensors on LSPR and SERS. J. Phys. Chem. C 2015, 119, 28577–28585. [Google Scholar] [CrossRef]
- Yoo, S.; Nam, D.H.; Singh, T.I.; Leem, G.; Lee, S. Effect of Reducing Agents on the Synthesis of Anisotropic Gold Nanoparticles. Nano Converg. 2022, 9, 5. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Saikova, S.; Pavlikov, A.; Trofimova, T.; Mikhlin, Y.; Karpov, D.; Asanova, A.; Grigoriev, Y.; Volochaev, M.; Samoilo, A.; Zharkov, S.; et al. Hybrid Nanoparticles Based on Cobalt Ferrite and Gold: Preparation and Characterization. Metals 2021, 11, 705. [Google Scholar] [CrossRef]
- Gerina, M.; Sanna Angotzi, M.; Mameli, V.; Gajdošová, V.; Rainer, D.N.; Dopita, M.; Steinke, N.J.; Aurélio, D.; Vejpravová, J.; Zákutná, D. Size Dependence of the Surface Spin Disorder and Surface Anisotropy Constant in Ferrite Nanoparticles. Nanoscale Adv. 2023, 5, 4563–4570. [Google Scholar] [CrossRef]
- Kumar, L.; Kumar, P.; Kar, M. Cation Distribution by Rietveld Technique and Magnetocrystalline Anisotropy of Zn Substituted Nanocrystalline Cobalt Ferrite. J. Alloys Compd. 2013, 551, 72–81. [Google Scholar] [CrossRef]
- Pimentel, B.; Caraballo-Vivas, R.J.; Checca, N.R.; Zverev, V.I.; Salakhova, R.T.; Makarova, L.A.; Pyatakov, A.P.; Perov, N.S.; Tishin, A.M.; Shtil, A.A.; et al. Threshold Heating Temperature for Magnetic Hyperthermia: Controlling the Heat Exchange with the Blocking Temperature of Magnetic Nanoparticles. J. Solid State Chem. 2018, 260, 34–38. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley and Sons: Middlesex, UK, 2001; ISBN 0-471-85298-8. [Google Scholar]
- Rana, S.; Philip, J.; Raj, B. Micelle Based Synthesis of Cobalt Ferrite Nanoparticles and Its Characterization Using Fourier Transform Infrared Transmission Spectrometry and Thermogravimetry. Mater. Chem. Phys. 2010, 124, 264–269. [Google Scholar] [CrossRef]
- Habibi, M.H.; Parhizkar, H.J. FTIR and UV–Vis Diffuse Reflectance Spectroscopy Studies of the Wet Chemical (WC) Route Synthesized Nano-Structure CoFe2O4 from CoCl2 and FeCl3. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 127, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Feitoza, N.C.; Gonçalves, T.D.; Mesquita, J.J.; Menegucci, J.S.; Santos, M.-K.M.S.; Chaker, J.A.; Cunha, R.B.; Medeiros, A.M.M.; Rubim, J.C.; Sousa, M.H. Fabrication of Glycine-Functionalized Maghemite Nanoparticles for Magnetic Removal of Copper from Wastewater. J. Hazard. Mater. 2014, 264, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.J.; Correa, J.R.; Peláez-Abellán, E.; Urones-Garrote, E. A Novel Method for the Functionalization of Aminoacids L-Glycine, l-Glutamic Acid and l-Arginine on Maghemite/Magnetite Nanoparticles. J. Magn. Magn. Mater. 2018, 456, 87–91. [Google Scholar] [CrossRef]
- Cholico, F.A.; Sámano, A.H.; Casta˜neda-Priego, R.; Paz, J.A.; Quintero, L.H.; Cano, M.E. Analysis of the Magnetic Properties of Core-Shell Iron Oxide Nanoparticles. Rev. Mex. Fis. 2022, 68, 041004. [Google Scholar] [CrossRef]
- Pilati, V.; Gomide, G.; Gomes, R.C.; Goya, G.F.; Depeyrot, J. Colloidal Stability and Concentration Effects on Nanoparticle Heat Delivery for Magnetic Fluid Hyperthermia. Langmuir 2021, 37, 1129–1140. [Google Scholar] [CrossRef]
- Sabale, S.; Jadhav, V.; Mane-Gavade, S.; Yu, X.-Y. Superparamagnetic CoFe2O4@Au with High Specific Absorption Rate and Intrinsic Loss Power for Magnetic Fluid Hyperthermia Applications. Acta Metall. Sin. (Engl. Lett.) 2019, 32, 719–725. [Google Scholar] [CrossRef]
- Yedgar, S.; Barshtein, G.; Gural, A. Hemolytic Activity of Nanoparticles as a Marker of Their Hemocompatibility. Micromachines 2022, 13, 2091. [Google Scholar] [CrossRef]
- Medina-Ramirez, I.E.; Macias-Diaz, J.E.; Masuoka-Ito, D.; Zapien, J.A. Holotomography and Atomic Force Microscopy: A Powerful Combination to Enhance Cancer, Microbiology and Nanotoxicology Research. Discov. Nano 2024, 19, 64. [Google Scholar] [CrossRef]
- Dai, J.; Song, J.; Qiu, Y.; Wei, J.; Hong, Z.; Li, L.; Yang, H. Gold Nanoparticles Decorated G-C3N4 Nanosheets for Controlled of Reactive Oxygen Species upon 670 Nm Illumination. ACS Appl. Mater. Interfaces 2019, 11, 10589–10596. [Google Scholar] [CrossRef]
- Soriano, J.; Villanueva, A.; Stockert, J.C.; Cañete, M. Regulated Necrosis in HeLa Cells Induced by ZnPc Photodynamic Treatment: A New Nuclear Morphology. Int. J. Mol. Sci. 2014, 15, 22772–22785. [Google Scholar] [CrossRef]
- Tabero, A.; Planas, O.; Gallavardin, T.; Nieves, I.; Nonell, S.; Villanueva, A. Smart Dual-Functionalized Gold Nanoclusters for Spatio-Temporally Controlled Delivery of Combined Chemo-and Photodynamic Therapy. Nanomaterials 2020, 10, 2474. [Google Scholar] [CrossRef]
- Miller, M.A.; Arlauckas, S.; Weissleder, R. Prediction of Anti-Cancer Nanotherapy Efficacy by Imaging. Nanotheranostics 2017, 1, 296–312. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of Gold Nanoparticles (AuNPs): A Review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Fard, J.K.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N.M.; Tirelli, N. Gateways for the Intracellular Access of Nanocarriers: A Review of Receptor-Mediated Endocytosis Mechanisms and of Strategies in Receptor Targeting. Expert Opin. Drug Deliv. 2010, 7, 895–913. [Google Scholar] [CrossRef] [PubMed]
- Zadoorian, A.; Du, X.; Yang, H. Lipid Droplet Biogenesis and Functions in Health and Disease. Nat. Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef]
- Jimenez-Chavez, A.; Pedroza-Herrera, G.; Betancourt-Reyes, I.; De Vizcaya Ruiz, A.; Masuoka-Ito, D.; Zapien, J.A.; Medina-Ramirez, I.E. Aluminum Enhances the Oxidative Damage of ZnO NMs in the Human Neuroblastoma SH-SY5Y Cell Line. Discov. Nano 2024, 19, 36. [Google Scholar] [CrossRef]
- Chang, H.Y.; Shih, M.H.; Huang, H.C.; Tsai, S.R.; Juan, H.F.; Lee, S.C. Middle Infrared Radiation Induces G2/M Cell Cycle Arrest in A549 Lung Cancer Cells. PLoS ONE 2013, 8, e54117. [Google Scholar] [CrossRef]
- Rello, S.; Stockert, J.C.; Moreno, V.; Amez, A.G.; Pacheco, M.; Juarranz, A.; Cã, M.; Villanueva, A. Morphological Criteria to Distinguish Cell Death Induced by Apoptotic and Necrotic Treatments; Springer Science + Business Media, Inc.: Berlin/Heidelberg, Germany, 2005; Volume 10. [Google Scholar]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Balvan, J.; Krizova, A.; Gumulec, J.; Raudenska, M.; Sladek, Z.; Sedlackova, M.; Babula, P.; Sztalmachova, M.; Kizek, R.; Chmelik, R.; et al. Multimodal Holographic Microscopy: Distinction between Apoptosis and Oncosis. PLoS ONE 2015, 10, e0121674. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell Death: A Review of the Major Forms of Apoptosis, Necrosis and Autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Pecchillo Cimmino, T.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOX Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086. [Google Scholar] [CrossRef] [PubMed]
- Yeshchenko, O.; Khort, P.; Fedotov, O.; Chumachenko, V.; Virych, P.; Warren, H.S.; Booth, B.W.; Bliznyuk, V.; Kutsevol, N. Third-Generation Anticancer Photodynamic Therapy Systems Based on Star-like Anionic Polyacrylamide Polymer, Gold Nanoparticles, and Temoporfin Photosensitizer. Molecules 2024, 29, 2224. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Yang, M.; Liu, S.B. Research Progress on Morphology and Mechanism of Programmed Cell Death. Cell Death Dis. 2024, 15, 327. [Google Scholar] [CrossRef]
- Chota, A.; George, B.P.; Abrahamse, H. In Vitro Cell Death Mechanisms Induced by Dicoma Anomala Root Extract in Combination with ZnPcS4 Mediated-Photodynamic Therapy in A549 Lung Cancer Cells. Cells 2022, 11, 3288. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, R.; Kim, E.; Lee, S.; Park, Y. Il Near-Infrared Light-Triggered Photodynamic Therapy and Apoptosis Using Upconversion Nanoparticles with Dual Photosensitizers. Front. Bioeng. Biotechnol. 2020, 8, 275. [Google Scholar] [CrossRef]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which Cell Death Modality Wins the Contest for Photodynamic Therapy of Cancer? Cell Death Dis. 2022, 13, 455. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The Role of Apoptosis in Response to Photodynamic Therapy: What, Where, Why, and How. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [CrossRef]
- Lieven, C.J.; Thurber, K.A.; Levin, E.J.; Levin, L.A. Ordering of Neuronal Apoptosis Signaling: A Superoxide Burst Precedes Mitochondrial Cytochrome c Release in a Growth Factor Deprivation Model. Apoptosis 2012, 17, 591–599. [Google Scholar] [CrossRef][Green Version]
- Soriano, J.; Mora-Espí, I.; Alea-Reyes, M.E.; Pérez-García, L.; Barrios, L.; Ibáñez, E.; Nogués, C. Cell Death Mechanisms in Tumoral and Non-Tumoral Human Cell Lines Triggered by Photodynamic Treatments: Apoptosis, Necrosis and Parthanatos. Sci. Rep. 2017, 7, srep41340. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Hessler, J.A.; Budor, A.; Putchakayala, K.; Mecke, A.; Rieger, D.; Holl, M.M.B.; Orr, B.G.; Bielinska, A.; Beals, J.; Baker, J. Atomic Force Microscopy Study of Early Morphological Changes during Apoptosis. Langmuir 2005, 21, 9280–9286. [Google Scholar] [CrossRef]
- Park, J.; Baek, S.H. Combination Therapy with Cinnamaldehyde and Hyperthermia Induces Apoptosis of A549 Non-Small Cell Lung Carcinoma Cells via Regulation of Reactive Oxygen Species and Mitogen-Activated Protein Kinase Family. Int. J. Mol. Sci. 2020, 21, 6229. [Google Scholar] [CrossRef] [PubMed]
- Al-Majmaie, R.; Kennedy, E.; Al-Rubeai, M.; Rice, J.H.; Zerulla, D. AFM-Based Bivariate Morphological Discrimination of Apoptosis Induced by Photodynamic Therapy Using Photosensitizer-Functionalized Gold Nanoparticles. RSC Adv. 2015, 5, 82983–82991. [Google Scholar] [CrossRef]
- Naessens, F.; Demuynck, R.; Vershinina, O.; Efimova, I.; Saviuk, M.; De Smet, G.; Mishchenko, T.A.; Vedunova, M.V.; Krysko, O.; Catanzaro, E.; et al. CX3CL1 Release during Immunogenic Apoptosis Is Associated with Enhanced Anti-Tumour Immunity. Front. Immunol. 2024, 15, 1396349. [Google Scholar] [CrossRef] [PubMed]
- Rosales, S.; Hernández-Gutiérrez, R.; Oaxaca, A.; López, Z.; Casillas, N.; Knauth, P.; Quintero, L.H.; Paz, J.A.; Cholico, F.; Velásquez, C.; et al. The Fluorescent Cell Line SW620-GFP Is a Valuable Model to Monitor Magnetic Hyperthermia. Bioengineering 2024, 11, 638. [Google Scholar] [CrossRef]
| Material | 10 µg/mL | 15 µg/mL | 20 µg/mL | 25 µg/mL | 30 µg/mL |
|---|---|---|---|---|---|
| MCF NR | 0 | 0 | 0 | 0 | 0 |
| MCF R | 0 | 0 | 0 | 0 | 0 |
| MCF-AuCit NR | 0 | 0 | 0 | 0 | 0 |
| MCF-AuCit R | 0 | 0 | 1.7 | 2.0 | 2.0 |
| MCF-AuGly NR | 0 | 0 | 0 | 0 | 0 |
| MCF-AuGly R | 0 | 0 | 3.7 | 1.2 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-López, A.; Cano-González, M.E.; Ventura-Juárez, J.; Muñoz-Ortega, M.H.; Betancourt, I.; Zapien, J.A.; Medina-Ramirez, I.E. Evaluation of CoFe2O4-L-Au (L: Citrate, Glycine) as Superparamagnetic–Plasmonic Nanocomposites for Enhanced Cytotoxic Activity Towards Oncogenic (A549) Cells. Int. J. Mol. Sci. 2025, 26, 7732. https://doi.org/10.3390/ijms26167732
Lozano-López A, Cano-González ME, Ventura-Juárez J, Muñoz-Ortega MH, Betancourt I, Zapien JA, Medina-Ramirez IE. Evaluation of CoFe2O4-L-Au (L: Citrate, Glycine) as Superparamagnetic–Plasmonic Nanocomposites for Enhanced Cytotoxic Activity Towards Oncogenic (A549) Cells. International Journal of Molecular Sciences. 2025; 26(16):7732. https://doi.org/10.3390/ijms26167732
Chicago/Turabian StyleLozano-López, Alberto, Mario E. Cano-González, J. Ventura-Juárez, Martín H. Muñoz-Ortega, Israel Betancourt, Juan Antonio Zapien, and Iliana E. Medina-Ramirez. 2025. "Evaluation of CoFe2O4-L-Au (L: Citrate, Glycine) as Superparamagnetic–Plasmonic Nanocomposites for Enhanced Cytotoxic Activity Towards Oncogenic (A549) Cells" International Journal of Molecular Sciences 26, no. 16: 7732. https://doi.org/10.3390/ijms26167732
APA StyleLozano-López, A., Cano-González, M. E., Ventura-Juárez, J., Muñoz-Ortega, M. H., Betancourt, I., Zapien, J. A., & Medina-Ramirez, I. E. (2025). Evaluation of CoFe2O4-L-Au (L: Citrate, Glycine) as Superparamagnetic–Plasmonic Nanocomposites for Enhanced Cytotoxic Activity Towards Oncogenic (A549) Cells. International Journal of Molecular Sciences, 26(16), 7732. https://doi.org/10.3390/ijms26167732








