Low Antibody Dosing in Cancer Therapy: Targeted Cytotoxicity Combined with Anti-Tumour Immunostimulation
Abstract
1. Introduction
2. Tumour-Associated Antigens and Challenges of Targeted Immunotherapy
3. Therapeutic Monoclonal Antibodies in Cancer Treatment: Mechanisms and Classification
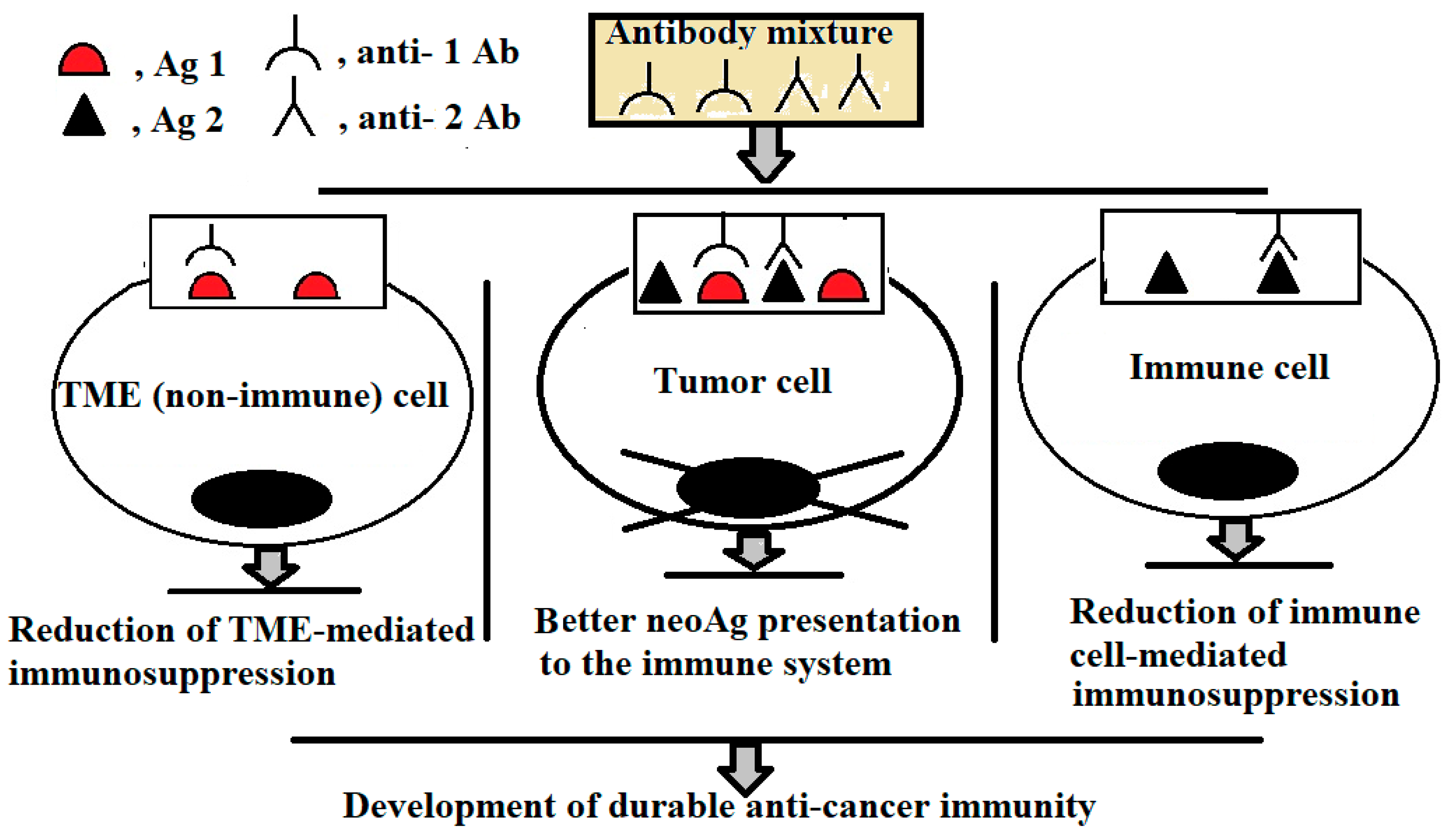
4. Designing Low-Dose, Multi-Target mAb Therapies for Treatment of Solid Tumours
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pulappadi, V.P.; Rangarajan, K.; Pramanik, R.; Yadav, M.K.; Thulkar, S. Toxicity of anti-cancer drugs: The imaging findings to watch out for in common cancers. Br. J. Radiol. 2025, 98, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Noren, H.; Jove, R.; Beljanski, V.; Grinnemo, K.H. Differences and similarities between cancer and somatic stem cells: Therapeutic implications. Stem Cell Res. Ther. 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thommen, D.S.; Schumacher, T.N. T cell dysfunction in cancer. Cancer Cell 2018, 33, 547–562. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seledtsov, V.I.; Darinskas, A.; Von Delwig, A.; Seledtsova, G.V. Inflammation control and immunotherapeutic strategies in comprehensive cancer treatment. Metabolites 2023, 13, 123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Xiao, L.; Li, H.; Cui, W. Global research trends of immunosenescence and immunotherapy: A bibliometric study. Hum. Vaccin. Immunother. 2025, 21, 2469403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajagopal, D.; MacLeod, E.; Corogeanu, D.; Vessillier, S. Immune-related adverse events of antibody-based biological medicines in cancer therapy. J. Cell. Mol. Med. 2024, 28, e18470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coulie, P.G.; Van den Eynde, B.J.; van der Bruggen, P.; Boon, T. Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Seledtsov, V.I.; Goncharov, A.G.; Seledtsova, G.V. Multiple-purpose immunotherapy for cancer. Biomed. Pharmacother. 2015, 76, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.; Pan, X.; Wang, Y.; Zhang, Y. Targeting neoantigens for cancer immunotherapy. Biomark. Res. 2021, 9, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Q.; Jia, Q.; Zhang, J.; Zhu, B. Neoantigens in precision cancer immunotherapy: From identification to clinical applications. Chin. Med. J. 2022, 135, 1285–1298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thelen, M.; Keller, D.; Lehmann, J.; Wennhold, K.; Weitz, H.; Bauer, E.; Gathof, B.; Brüggemann, M.; Kotrova, M.; Quaas, A.; et al. Immune responses against shared antigens are common in esophago-gastric cancer and can be enhanced using CD40-activated B cells. J. Immunother. Cancer 2022, 10, e005200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsu, J.L.; Hung, M.C. The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016, 35, 575–588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akbay, E.A.; Koyama, S.; Carretero, J.; Altabef, A.; Tchaicha, J.H.; Christensen, C.L.; Mikse, O.R.; Cherniack, A.D.; Beauchamp, E.M.; Pugh, T.J.; et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013, 3, 1355–1363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toledo-Stuardo, K.; Ribeiro, C.H.; González-Herrera, F.; Matthies, D.J.; Le Roy, M.S.; Dietz-Vargas, C.; Latorre, Y.; Campos, I.; Guerra, Y.; Tello, S.; et al. Therapeutic antibodies in oncology: An immunopharmacological overview. Cancer Immunol. Immunother. 2024, 73, 242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, S.; Sun, Y.; Liang, X.; Gu, X.; Ning, J.; Xu, Y.; Chen, S.; Pan, L. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, T.H.; Patz, E.F., Jr.; Ackerman, M.E. Coming together at the hinges: Therapeutic prospects of IgG3. MAbs 2021, 13, 1882028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, J.; Song, Y.; Tian, W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J. Hematol. Oncol. 2020, 13, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferris, R.L.; Jaffee, E.M.; Ferrone, S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: Clinical response, cellular immunity, and immunoescape. J. Clin. Oncol. 2010, 28, 4390–4399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noris, M.; Remuzzi, G. Overview of complement activation and regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, M.; Linstra, R.; van Vugt, M.A.T.M. Genomic instability, inflammatory signaling and response to cancer immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188661. [Google Scholar] [CrossRef] [PubMed]
- Seledtsov, V.I.; Seledtsova, G.V. Attaining threshold antibody cytotoxicity for selective tumor cell destruction: An opinion article. Oncotarget 2018, 9, 35790–35794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Cheresh, D.A.; Honsik, C.J.; Staffileno, L.K.; Jung, G.; Reisfeld, R.A. Disialoganglioside GD3 on human melanoma serves as a relevant target antigen for monoclonal antibody-mediated tumor cytolysis. Proc. Natl. Acad. Sci. USA 1985, 82, 5155–5159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greiner, J.W.; Guadagni, F.; Hand, P.H.; Pestka, S.; Noguchi, P.; Fisher, P.B.; Schlom, J. Augmentation of tumor antigen expression by recombinant human interferons: Enhanced targeting of monoclonal antibodies to carcinomas. Cancer Treat. Res. 1990, 51, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Seledtsov, V.I.; Seledtsova, G.V. Total threshold cytotoxicity of therapeutic antibodies for selective destruction of pathogenic memory T cells: Implications for immunotherapy of autoimmune and allergenic disorders. Expert Rev. Clin. Immunol. 2019, 15, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Singh, V.J.; Chawla, P.A. Epidermal growth factor receptor inhibitors as potential anticancer agents: An update of recent progress. Bioorg. Chem. 2021, 116, 105393. [Google Scholar] [CrossRef] [PubMed]
- Stanowicka-Grada, M.; Senkus, E. Anti-HER2 drugs for the treatment of advanced HER2 positive breast cancer. Curr. Treat. Options Oncol. 2023, 24, 1633–1650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadek, I.; Keaton, M.; Maihle, N.J.; Tang, S.C. Anti-HER-2 therapy following severe trastuzumab-induced cardiac toxicity. Genes Dis. 2017, 4, 159–162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016, 9, 5023–5039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Javed, S.A.; Najmi, A.; Ahsan, W.; Zoghebi, K. Targeting PD-1/PD-L-1 immune checkpoint inhibition for cancer immunotherapy: Success and challenges. Front. Immunol. 2024, 15, 1383456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.L.; Cui, Y.; Liu, X.; Liu, G.; Dong, X.; Tang, L.; Hung, Y.; Wang, C.; Feng, M.Q. A bispecific antibody targeting HER2 and PD-L1 inhibits tumor growth with superior efficacy. J. Biol. Chem. 2021, 297, 101420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krasniqi, E.; Barchiesi, G.; Pizzuti, L.; Mazzotta, M.; Venuti, A.; Maugeri-Saccà, M.; Sanguineti, G.; Massimiani, G.; Sergi, D.; Carpano, S.; et al. Immunotherapy in HER2-positive breast cancer: State of the art and future perspectives. J. Hematol. Oncol. 2019, 12, 111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, X. A Comprehensive review of HER2 in cancer biology and therapeutics. Genes 2024, 15, 903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonomo, P.; Desideri, I.; Mangoni, M.; Saieva, C.; Loi, M.; Becherini, C.; Cerbai, C.; Ganovelli, M.; Salvestrini, V.; Stocchi, G.; et al. Durvalumab with cetuximab and radiotherapy for locally advanced squamous cell carcinoma of the head and neck: A phase 1/2 trial. Radiother. Oncol. 2022, 169, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Abe, M.; Kitahara, S.; Sakuma, N.; Ohno, I.; Takahashi, K.; Imai, C.; Saeki, H.; Suzuki, T.; Uzawa, K.; et al. Sequential administration of PD-1 inhibitor and cetuximab causes pneumonia. Oncol. Lett. 2023, 26, 288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inoue, M.; Mimura, K.; Izawa, S.; Shiraishi, K.; Inoue, A.; Shiba, S.; Watanabe, M.; Maruyama, T.; Kawaguchi, Y.; Inoue, S.; et al. Expression of MHC Class I on breast cancer cells correlates inversely with HER2 expression. Oncoimmunology 2012, 1, 1104–1110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Wang, L.; Zhang, M.; Jin, M.; Bai, C.; Wang, X. Potential mechanism of interleukin-8 production from lung cancer cells: An involvement of EGF-EGFR-PI3K-Akt-Erk pathway. J. Cell. Physiol. 2012, 227, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.; Ujvari, B.; Ramana, V.; Donald, J. Cross-talk between EGFR and IL-6 drives oncogenic signaling and offers therapeutic opportunities in cancer. Cytokine Growth Factor Rev. 2018, 41, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, C. Anti-VEGF/VEGFR2 monoclonal antibodies and their combinations with PD-1/PD-L1 inhibitors in clinic. Curr. Cancer Drug Targets 2020, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, M.; Palle, J.; Galy-Fauroux, I.; Terme, M. Direct and indirect modulation of T cells by VEGF-A counteracted by anti-angiogenic treatment. Front. Immunol. 2021, 12, 616837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lynce, F.; Wang, H.; Petricoin, E.F.; Pohlmann, P.R.; Smaglo, B.; Hwang, J.; He, A.R.; Subramaniam, D.S.; Deeken, J.; Marshall, J.; et al. A phase I study of HER1, HER2 dual kinase inhibitor lapatinib plus the proteasome inhibitor bortezomib in patients with advanced malignancies. Cancer Chemother. Pharmacol. 2019, 84, 1145–1151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Zhang, K.; Wang, W.; Liu, Y.; Huang, J.; Zheng, M.; Li, L.; Zhang, X.; Xu, M.; Chen, G.; et al. Combined inhibition of HER2 and VEGFR synergistically improves therapeutic efficacy via PI3K-AKT pathway in advanced ovarian cancer. J. Exp. Clin. Cancer Res. 2024, 43, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bleeker, W.K.; Munk, M.E.; Mackus, W.J.; Brakel, J.H.; Pluyter, M.; Glennie, M.J.; Winkel, J.G.; Parren, P.W. Estimation of dose requirements for sustained in vivo activity of a therapeutic human anti CD20 antibody. Br. J. Haematol. 2008, 140, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Murungi, L.M.; Kamuyu, G.; Lowe, B.; Bejon, P.; Theisen, M.; Kinyanjui, S.M.; Marsh, K.; Osier, F.H. A threshold concentration of anti-merozoite antibodies is required for protection from clinical episodes of malaria. Vaccine 2013, 31, 3936–3942. [Google Scholar] [CrossRef]
- Webb, N.E.; Montefiori, D.C.; Lee, B. Dose-response curve slope helps predict therapeutic potency and breadth of HIV broadly neutralizing antibodies. Nat. Commun. 2015, 6, 8443. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Cao, Y. Which factors matter the most? Revisiting and dissecting antibody therapeutic doses. Drug Discov. Today 2021, 26, 1980–1990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajasekaran, N.; Chester, C.; Yonezawa, A.; Zhao, X.; Kohrt, H.E. Enhancement of antibody-dependent cell mediated cytotoxicity: A new era in cancer treatment. Immunotargets Ther. 2015, 4, 91–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, L.; Wang, R.; Xie, K.; Zhang, J.; Tao, F.; Pi, C.; Feng, Y.; Gu, H.; Fang, J. A HER2 target antibody drug conjugate combined with anti-PD-(L)1 treatment eliminates hHER2+ tumors in hPD-1 transgenic mouse model and contributes immune memory formation. Breast Cancer Res. Treat. 2022, 191, 51–61. [Google Scholar] [CrossRef] [PubMed]
| Name, IgG Subclass | Antigen | Indications (Tumour Types) |
|---|---|---|
| Cetuximab, chimeric IgG1 | Epidermal growth factor receptor (HER1) | Colorectal cancer (2004); head and neck squamous cell carcinoma (2006) |
| Necitumumab, human IgG1 | HER1 | Non-small-cell lung cancer (2015) |
| Trastuzumab, humanized IgG1 | Epidermal growth factor receptor 2 (HER2) | Breast cancer (1998) |
| Pertuzumab, humanized IgG1 | HER2 | Breast cancer (2012) |
| Ramucirumab, human IgG1 | Vascular endothelial growth factor receptor 2 (VEGFR2) | Gastric cancer (2014) |
| Atezolizumab, humanized IgG1 | Programmed death-ligand 1 (PD-L1) | Bladder cancer, non-small-cell lung cancer (2016); triple-negative breast cancer (2019) |
| Avelumab, human IgG1 | PD-L1 | Urothelial carcinoma, Merkel cell carcinoma (2017) |
| Durvalumab, human IgG1 | PD-L1 | Bladder cancer (2017) |
| Ag1 | Ag2 | Target Tumours |
|---|---|---|
| Epidermal growth factor receptor (ERBB1, HER1) | PD-L1 | Bladder cancer; breast cancer; colorectal cancer; gastroesophageal cancer; glioblastoma; head and neck squamous cell carcinoma (HNSCC); non-small-cell lung cancer (NSCLC); ovarian cancer |
| Human epidermal growth factor receptor 2 (ERBB2, HER2) | PD-L1 | Bladder cancer; breast cancer; colorectal cancer; endometrial cancer; gastroesophageal cancer, NSCLC; ovarian cancer; pancreatic cancer |
| Vascular endothelial growth factor receptor-2 (VEGFR-2) | PD-L1 | Breast cancer; colorectal cancer; gastroesophageal cancer; glioblastoma; hepatocellular carcinoma; NSCLC; ovarian cancer; renal cell carcinoma; soft tissue sarcoma; thyroid cancer |
| ERBB1 (HER1) | VEGFR-2 | Breast cancer; colorectal cancer; gastroesophageal cancer; glioblastoma; NSCLC; ovarian cancer |
| ERBB2 (HER2) | VEGFR-2 | Breast cancer; colorectal cancer; gastroesophageal cancer; NSCLC |
| ERBB1 (HER1) | ERBB2 (HER2) | Bladder cancer; breast cancer; colorectal cancer; endometrial cancer; gastroesophageal cancer; NSCLC; ovarian cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seledtsov, V.I.; Seledtsova, G.V.; Darinskas, A.; von Delwig, A. Low Antibody Dosing in Cancer Therapy: Targeted Cytotoxicity Combined with Anti-Tumour Immunostimulation. Int. J. Mol. Sci. 2025, 26, 7724. https://doi.org/10.3390/ijms26167724
Seledtsov VI, Seledtsova GV, Darinskas A, von Delwig A. Low Antibody Dosing in Cancer Therapy: Targeted Cytotoxicity Combined with Anti-Tumour Immunostimulation. International Journal of Molecular Sciences. 2025; 26(16):7724. https://doi.org/10.3390/ijms26167724
Chicago/Turabian StyleSeledtsov, Victor I., Galina V. Seledtsova, Adas Darinskas, and Alexei von Delwig. 2025. "Low Antibody Dosing in Cancer Therapy: Targeted Cytotoxicity Combined with Anti-Tumour Immunostimulation" International Journal of Molecular Sciences 26, no. 16: 7724. https://doi.org/10.3390/ijms26167724
APA StyleSeledtsov, V. I., Seledtsova, G. V., Darinskas, A., & von Delwig, A. (2025). Low Antibody Dosing in Cancer Therapy: Targeted Cytotoxicity Combined with Anti-Tumour Immunostimulation. International Journal of Molecular Sciences, 26(16), 7724. https://doi.org/10.3390/ijms26167724







