Not-So-Rare Defects of RBC Lipidic Composition: Four New Cases of Flippase Deficiency Due to ATP11C Mutations
Abstract
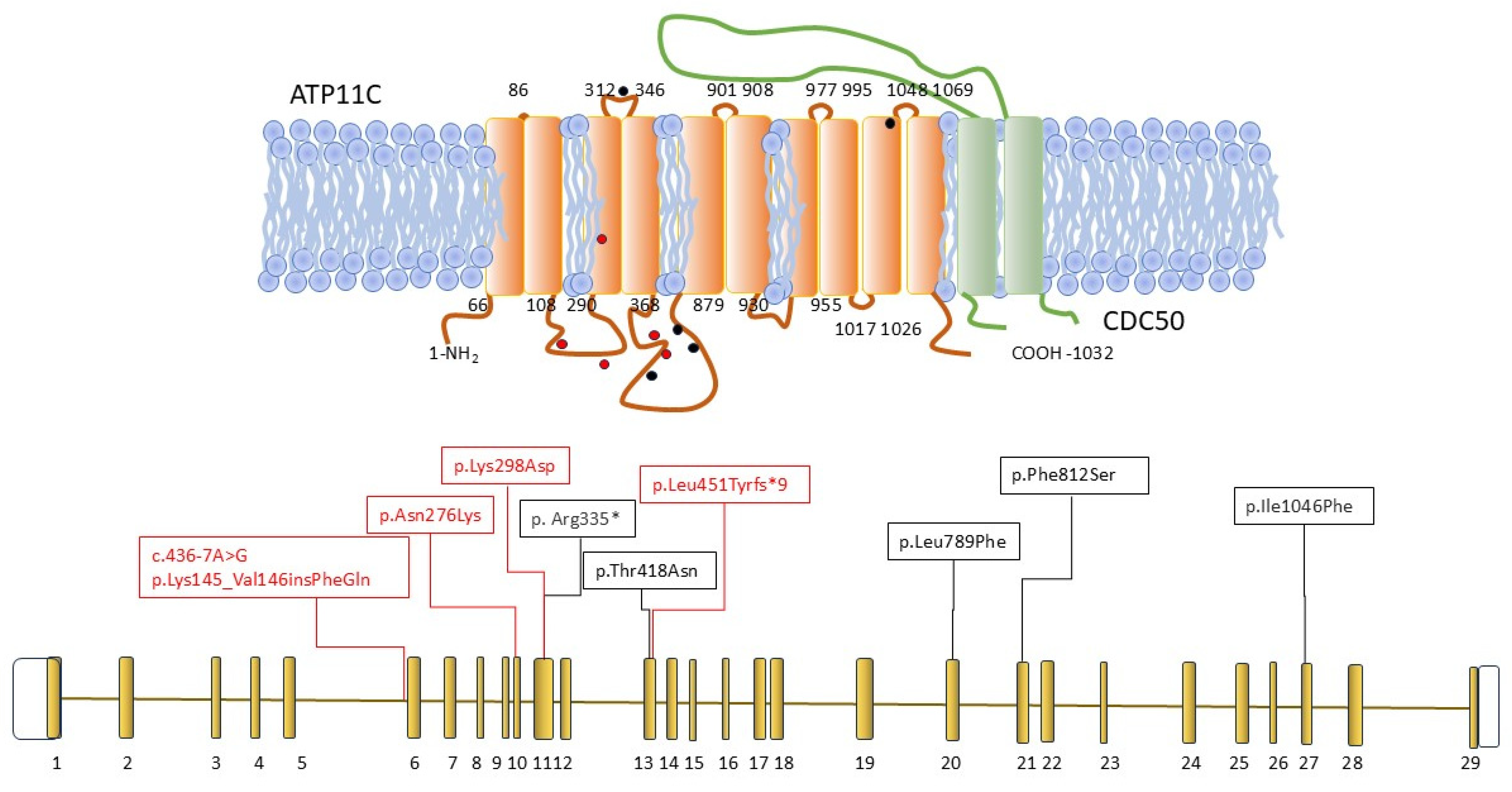
1. Introduction
2. Results
2.1. Case Reports
2.2. Laboratory Investigations
2.3. Flippase Activity Assay
2.4. X-Chromosomal Inactivation
3. Discussion
4. Materials and Methods
4.1. Hematologic Studies
4.2. Molecular Studies
4.3. Measurement of Flippase Activities in Human RBCs
4.4. X-Chromosomal Inactivation
4.5. Materials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Risinger, M.; Emberesh, M.; Kalfa, T.A. Rare Hereditary Hemolytic Anemias: Diagnostic Approach and Considerations in Management. Hematol. Oncol. Clin. N. Am. 2019, 33, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; van Beers, E.J. Mitapivat, a novel pyruvate kinase activator, for the treatment of hereditary hemolytic anemias. Ther. Adv. Hematol. 2021, 12, 20406207211066070. [Google Scholar] [CrossRef]
- Agarwal, A.M.; Rets, A.V. Molecular diagnosis of hereditary hemolytic anemias: Recent updates. Int. J. Lab. Hematol. 2023, 45, 79–86. [Google Scholar] [CrossRef]
- Manno, S.; Takakuwa, Y.; Mohandas, N. Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl. Acad. Sci. USA 2002, 99, 1943–1948. [Google Scholar] [CrossRef]
- Zwaal, R.F.A.; Comfurius, P.; Bevers, E.M. Surface exposure of phosphatidylserine in pathological cells. Cell. Mol. Life Sci. 2005, 62, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.P.; Vestergaard, A.L.; Mikkelsen, S.A.; Mogensen, L.S.; Chalat, M.; Molday, R.S. P4-ATPases as phospholipid flippases—Structure, function, and enigmas. Front. Physiol. 2016, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Arashiki, N.; Takakuwa, Y. Maintenance and regulation of asymmetric phospholipid distribution in human erythrocyte membranes: Implications for erythrocyte functions. Curr. Opin. Hematol. 2017, 24, 167–172. [Google Scholar] [CrossRef]
- Connor, J.; Pak, C.C.; Schroit, A.J. Exposure of phosphatidylserine in the outer leaflet of human red blood cells. Relationship to cell density, cell age, and clearance by mononuclear cells. J. Biol. Chem. 1994, 269, 2399–2404. [Google Scholar] [CrossRef]
- Boas, F.E.; Forman, L.; Beutler, E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc. Natl. Acad. Sci. USA 1998, 95, 3077–3081. [Google Scholar] [CrossRef]
- Seki, M.; Arashiki, N.; Takakuwa, Y.; Nitta, K.; Nakamura, F. Reduction in flippase activity contributes to surface presentation of phosphatidylserine in human senescent erythrocytes. J. Cell. Mol. Med. 2020, 24, 13991–14000. [Google Scholar] [CrossRef]
- Tkachenko, A.; Alfhili, M.A.; Alsughayyir, J.; Attanzio, A.; Al Mamun Bhuyan, A.; Bukowska, B.; Cilla, A.; Quintanar-Escorza, M.A.; Föller, M.; Havranek, O.; et al. Current understanding of eryptosis: Mechanisms, physiological functions, role in disease, pharmacological applications, and nomenclature recommendations. Cell Death Dis. 2025, 16, 467. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A. Apoptosis and eryptosis: Similarities and differences. Apoptosis 2024, 29, 482–502. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.L.; Gibson, D.F.; Tait, J.F. Increased erythrocyte phosphatidylserine exposure in sickle cell disease: Flow-cytometric measurement and clinical associations. Blood 1996, 88, 1873–1880. [Google Scholar] [CrossRef]
- Kuypers, F.A.; Yuan, J.; Lewis, R.A.; Snyder, L.M.; Kiefer, C.R.; Bunyaratvej, A.; Fucharoen, S.; Ma, L.; Styles, L.; de Jong, K.; et al. Membrane phospholipid asymmetry in human thalassemia. Blood 1998, 91, 3044–3051. [Google Scholar] [CrossRef]
- Arashiki, N.; Takakuwa, Y.; Mohandas, N.; Hale, J.; Yoshida, K.; Ogura, H.; Utsugisawa, T.; Ohga, S.; Miyano, S.; Ogawa, S.; et al. ATP11C is a major flippase in human erythrocytes and its defect causes congenital hemolytic anemia. Haematologica 2016, 101, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Mansour-Hendili, L.; Aissat, A.; Badaoui, B.; Sakka, M.; Gameiro, C.; Ortonne, V.; Wagner-Ballon, O.; Pissard, S.; Picard, V.; Ghazal, K.; et al. Exome sequencing for diagnosis of congenital hemolytic anemia. Orphanet J. Rare Dis. 2020, 15, 180. [Google Scholar] [CrossRef]
- Zhang, H.M.; Yang, L.; Dong, W.; Liu, Q.C. Congenital hemolytic anemia caused by a new mutation of ATP11C gene: A case report. Zhonghua Xue Ye Xue Za Zhi 2022, 43, 528. [Google Scholar]
- van Dijk, M.J.; van Oirschot, B.A.; Harrison, A.N.; Recktenwald, S.M.; Qiao, M.; Stommen, A.; Cloos, A.S.; Vanderroost, J.; Terrasi, R.; Dey, K.; et al. A novel missense variant in ATP11C is associated with reduced red blood cell phosphatidylserine flippase activity and mild hereditary hemolytic anemia. Am. J. Hematol. 2023, 98, 1877–1887. [Google Scholar] [CrossRef]
- Xu, W.; Ma, M.; Zhao, S.; Yuan, Y.; Tian, Z. Case of Congenital Hemolytic Anemia with ATP11C and ANK1 Variants. Children 2023, 10, 1600. [Google Scholar] [CrossRef]
- Sai, K.V.; Lee JY., E. Crossing the membrane – what does it take to flip a phospholipid? Structural and biochemical advances on P4-ATPase flippases. J. Biol. Chem. 2024, 300, 107738. [Google Scholar] [CrossRef]
- Hankins, H.M.; Baldridge, R.D.; Xu, P.; Graham, T.R. Role of flippases, scramblases and transfer proteins in phosphatidylserine subcellular distribution. Traffic 2015, 16, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Čopič, A.; Dieudonné, T.; Lenoir, G. Phosphatidylserine transport in cell life and death. Curr. Opin. Cell Biol. 2023, 83, 102192. [Google Scholar] [CrossRef]
- Föller, M.; Lang, F. Ion Transport in Eryptosis, the Suicidal Death of Erythrocytes. Front. Cell Dev. Biol. 2020, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Romana, M.; Guillot, N.; Fort, R.; Stauffer, E.; Lemonne, N.; Garnier, Y.; Chambers Skinner, S.; Etienne-Julan, M.; Robert, M.; et al. Association Between Nitric Oxide, Oxidative Stress, Eryptosis, Red Blood Cell Microparticles, and Vascular Function in Sickle Cell Anemia. Front. Immunol. 2020, 4, 551441. [Google Scholar] [CrossRef]
- Bouguerra, G.; Talbi, K.; Trabelsi, N.; Chaouachi, D.; Boudriga, I.; Abbès, S.; Menif, S. Enhanced Eryptosis in Glucose-6-Phosphate Dehydrogenase Deficiency. Cell. Physiol. Biochem. 2021, 55, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, Y.; Hayashi, H.; Kusuhara, H. Impaired Hepatic Uptake by Organic Anion-Transporting Polypeptides Is Associated with Hyperbilirubinemia and Hypercholanemia in Atp11c Mutant Mice. Mol. Pharmacol. 2015, 88, 1085–1092. [Google Scholar] [CrossRef]
- Yabas, M.; Coupland, L.A.; Cromer, D.; Winterberg, M.; Teoh, N.C.; D’Rozario, J.; Kirk, K.; Bröer, S.; Parish, C.R.; Enders, A. Mice deficient in the putative phospholipid flippase Atp11c exhibit altered erythrocyte shape, anemia, and reduced erythrocyte life span. J. Biol. Chem. 2014, 289, 19531–19537. [Google Scholar] [CrossRef]
- Arashiki, N.; Niitsuma, K.; Seki, M.; Takakuwa, Y.; Nakamura, F. ATP11C T418N, a gene mutation causing congenital hemolytic anemia, reduces flippase activity due to improper membrane trafficking. Biochem. Biophys. Res. Commun. 2019, 516, 705–712. [Google Scholar] [CrossRef]
- Hanayo Nakanishi, H.; Irie, K.; Segawa, H.; Hasegawa, K.; Fujiyoshi, Y.; Nagata, S.; Abe, K. Crystal structure of a human plasma membrane phospholipid flippase. J. Biol. Chem. 2020, 295, 10180–10194. [Google Scholar] [CrossRef]
- Vercellati, C.; Zaninoni, A.; Marcello, A.P.; Fermo, E.; Fattizzo, B.; Giannotta, J.A.; Bianchi, P.; Zanella, A.; Barcellini, W. Changing trends of splenectomy in hereditary spherocytosis: The experience of a reference Centre in the last 40 years. Br. J. Haematol. 2022, 198, 912–915. [Google Scholar] [CrossRef]
- Bianchi, P.; Fermo, E.; Vercellati, C.; Marcello, A.P.; Porretti, L.; Cortelezzi, A.; Barcellini, W.; Zanella, A. Diagnostic power of laboratory tests for hereditary spherocytosis: A comparison study in 150 patients grouped according to molecular and clinical characteristics. Haematologica 2012, 97, 516–523. [Google Scholar] [CrossRef]
- Zaninoni, A.; Fermo, E.; Vercellati, C.; Consonni, D.; Marcello, A.P.; Zanella, A.; Cortelezzi, A.; Barcellini, W.; Bianchi, P. Use of laser assisted optical rotational cell analyzer (LoRRca MaxSis) in the diagnosis of RBC membrane disorders, enzyme defects, and congenital dyserythropoietic anemias: A monocentric study on 202 patients. Front. Physiol. 2018, 9, 451. [Google Scholar] [CrossRef]
- Mariani, M.; Barcellini, W.; Vercellati, C.; Marcello, A.P.; Fermo, E.; Pedotti, P.; Boschetti, C.; Zanella, A. Clinical and hematologic features of 300 patients affected by hereditary spherocytosis grouped according to the type of the membrane protein defect. Haematologica. 2008, 93, 1310–1317. [Google Scholar] [CrossRef]
- Fermo, E.; Vercellati, C.; Marcello, A.P.; Keskin, E.Y.; Perrotta, S.; Zaninoni, A.; Brancaleoni, V.; Zanella, A.; Giannotta, J.A.; Barcellini, W.; et al. Targeted next generation sequencing and diagnosis of congenital hemolytic anemias: A three years experience monocentric study. Front. Physiol. 2021, 21, 684569. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, V.; Brancaleoni, V.; Granata, F.; Graziadei, G.; Di Pierro, E. The assessment of noncoding variant of PPOX gene in variegate porphyria reveals post-transcriptional role of the 5’ untranslated exon 1. Blood Cells Mol. Dis. 2016, 61, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Brancaleoni, V.; Balwani, M.; Granata, F.; Graziadei, G.; Missineo, P.; Fiorentino, V.; Fustinoni, S.; Cappellini, M.D.; Naik, H.; Desnick, R.J.; et al. X-chromosomal inactivation directly influences the phenotypic manifestation of X-linked protoporphyria. Clin. Genet. 2016, 89, 20–26. [Google Scholar] [CrossRef] [PubMed]
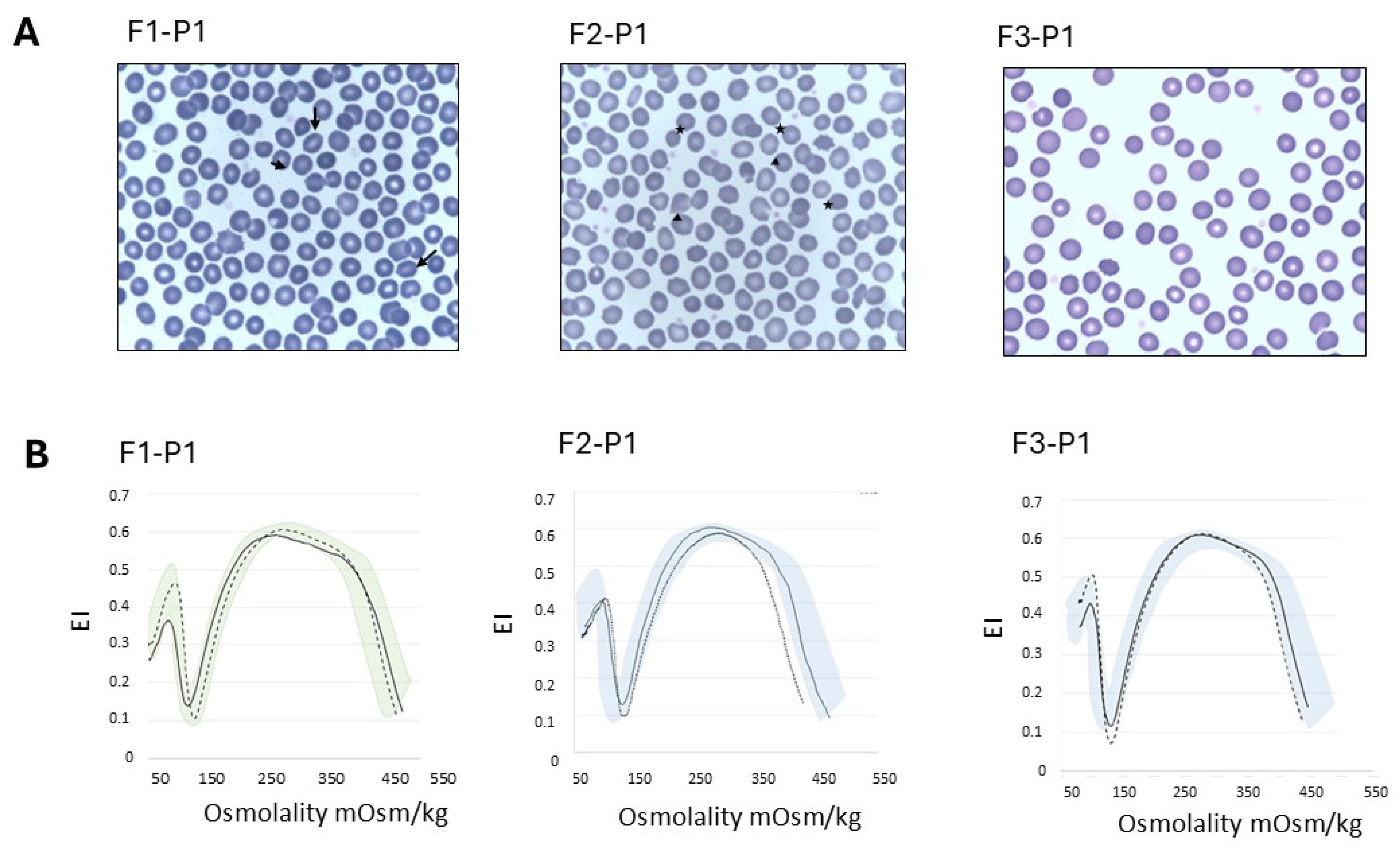
| F1-P1 | F1-P2 | F1-Mo | F1-Fa | F2-P1 | F2-Mo | F2-Fa | F3-P1 | F3-Mo | F3-Fa | Ref. Values | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | 16 | 9 | 41 | 54 | 9 | 45 | 47 | 19 | 56 | 57 | |
| Neonatal jaundice | yes | no | no | no | no | no | no | yes | no | no | |
| Splenomegaly | yes (13cm) | no | no | no | yes | no | no | nd | no | no | |
| Transfusions | no | no | no | no | yes (2 units) # | no | no | no | No | no | |
| Hb (g/dL) | 15.6 | 13.3 | 11.2 | 14.9 | 12.2 | 14.4 | 14.7 | 14.4 | 10.7 | 15.3 | 12.1–16.7 |
| MCV (fL) | 90.7 | 84.5 | 90.3 | 82.6 | 92.6 | 86.6 | 88 | 94.1 | 65.6 ° | 83.7 | 78–99 |
| RDW (%) | 13 | 13.1 | 15.8 | 13 | 14.7 | 13 | 11.9 | 12.7 | 15.5 | 13.2 | 11.6–16.5 |
| Reticulocytes (109/L) | 164 | 202 | 95 | 78 | 361 | 73 | 55 | 188 | 109 | 78 | 24–84 |
| RBC morphology | rare spherocytes rare stomatocytes | n.a. | n.a. | n.a. | 3% ovalocytes, rare spherocytes, rare mushroom cells | n.a. | n.a. | 5% echinocytes 2% spherocytes rare ovalocytes | n.a. | n.a. | n.a. |
| Unconjugated bilirubin (mg/dL) | 1.81 | n.a. | n.a. | n.a. | 2.56 | n.a. | n.a. | 5 | 0.88 | n.a. | <1.00 |
| Haptoglobin (mg/dL) | 139 | n.a. | n.a. | n.a. | <7 | n.a. | n.a. | 58 | 115 | 145 | 30–200 |
| LDH (IU/L) | 234 | n.a. | n.a. | n.a. | 464 | n.a. | n.a. | 108 | 158 | 170 | 125–220 |
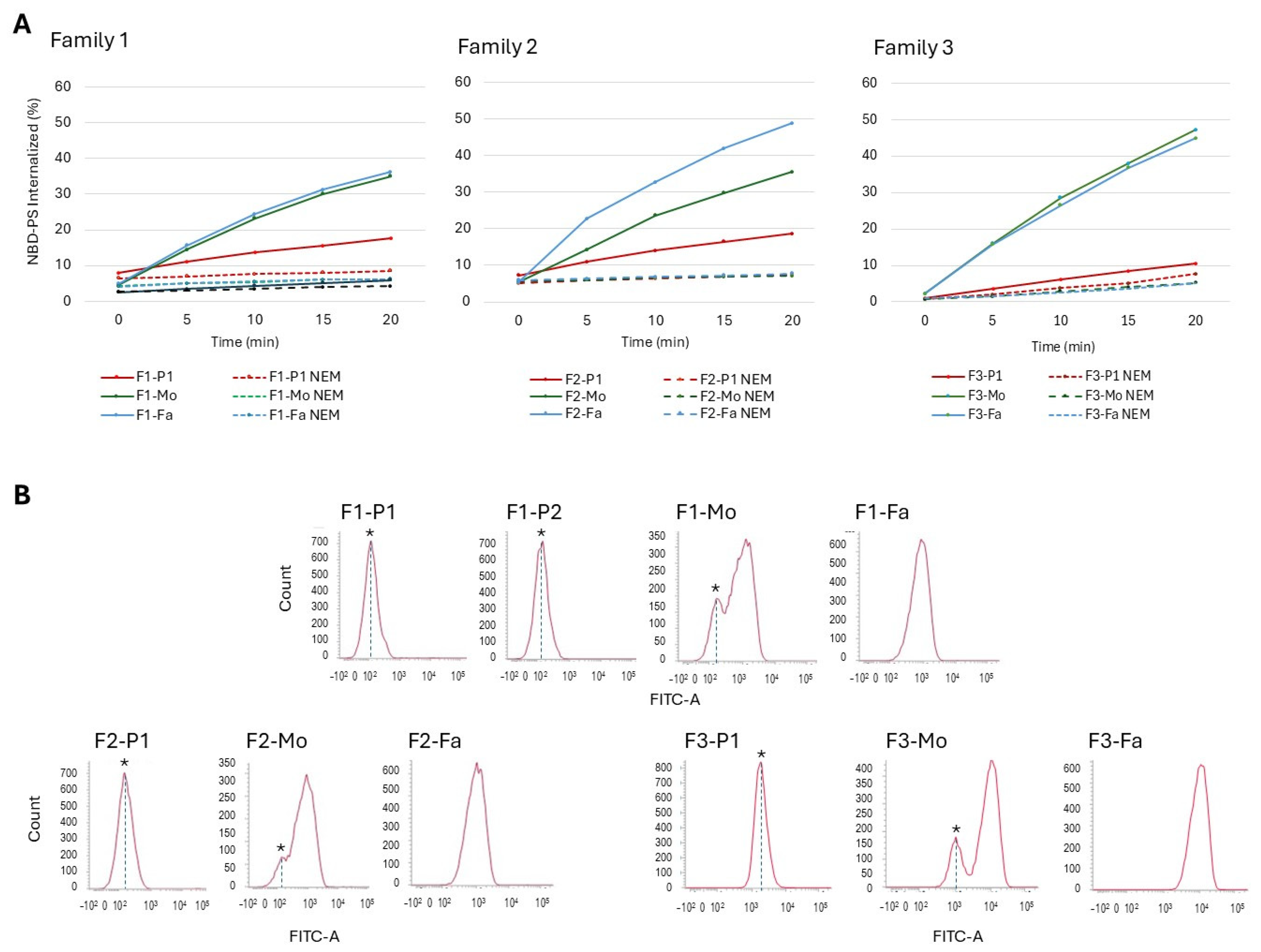
| Residual flippase activity (%) | 17.6 | 5.8 | 35 | 36 | 18.6 | 35.5 | 48.9 | 10.6 | 47.23 | 44.9 | 43–62 |
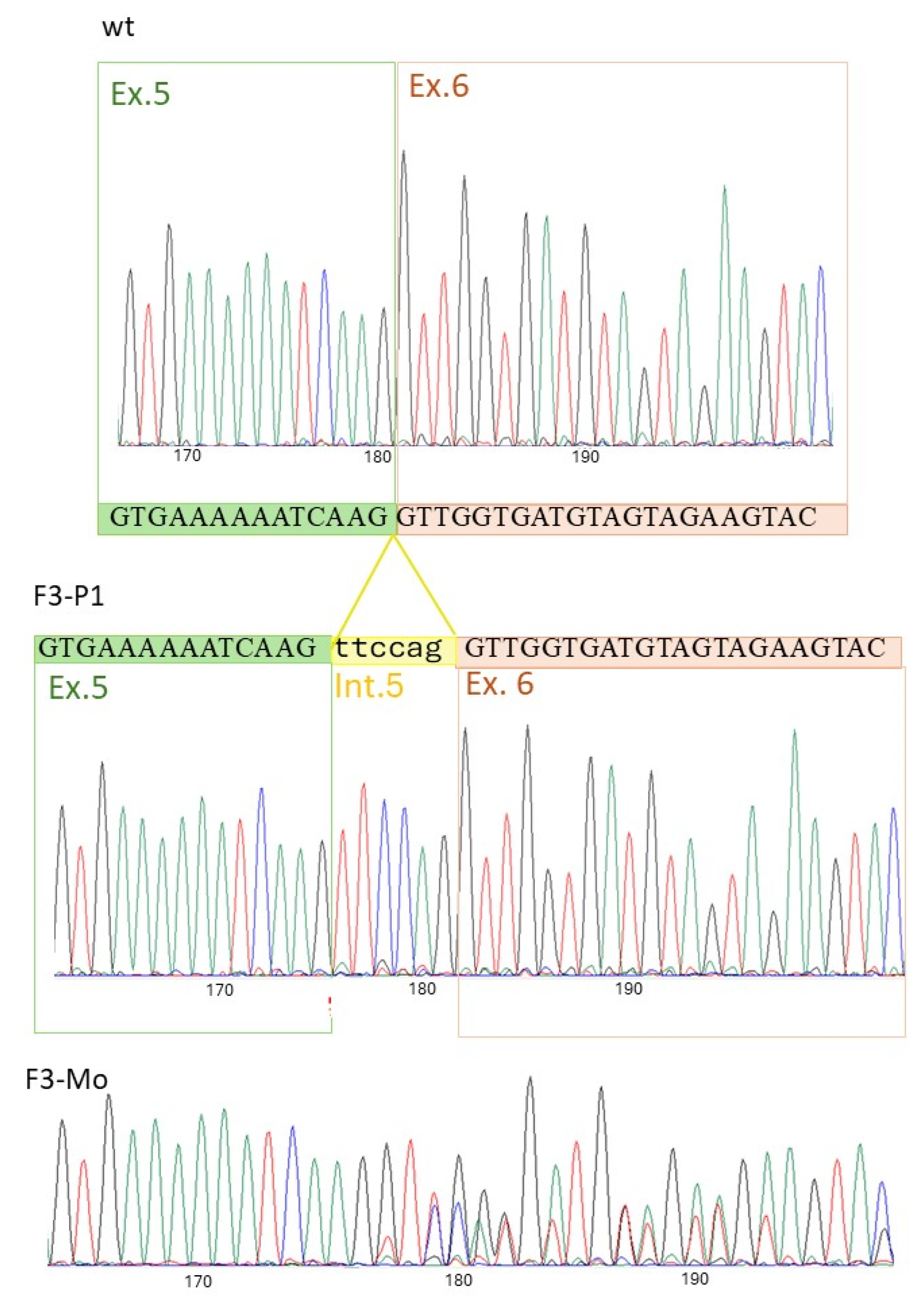
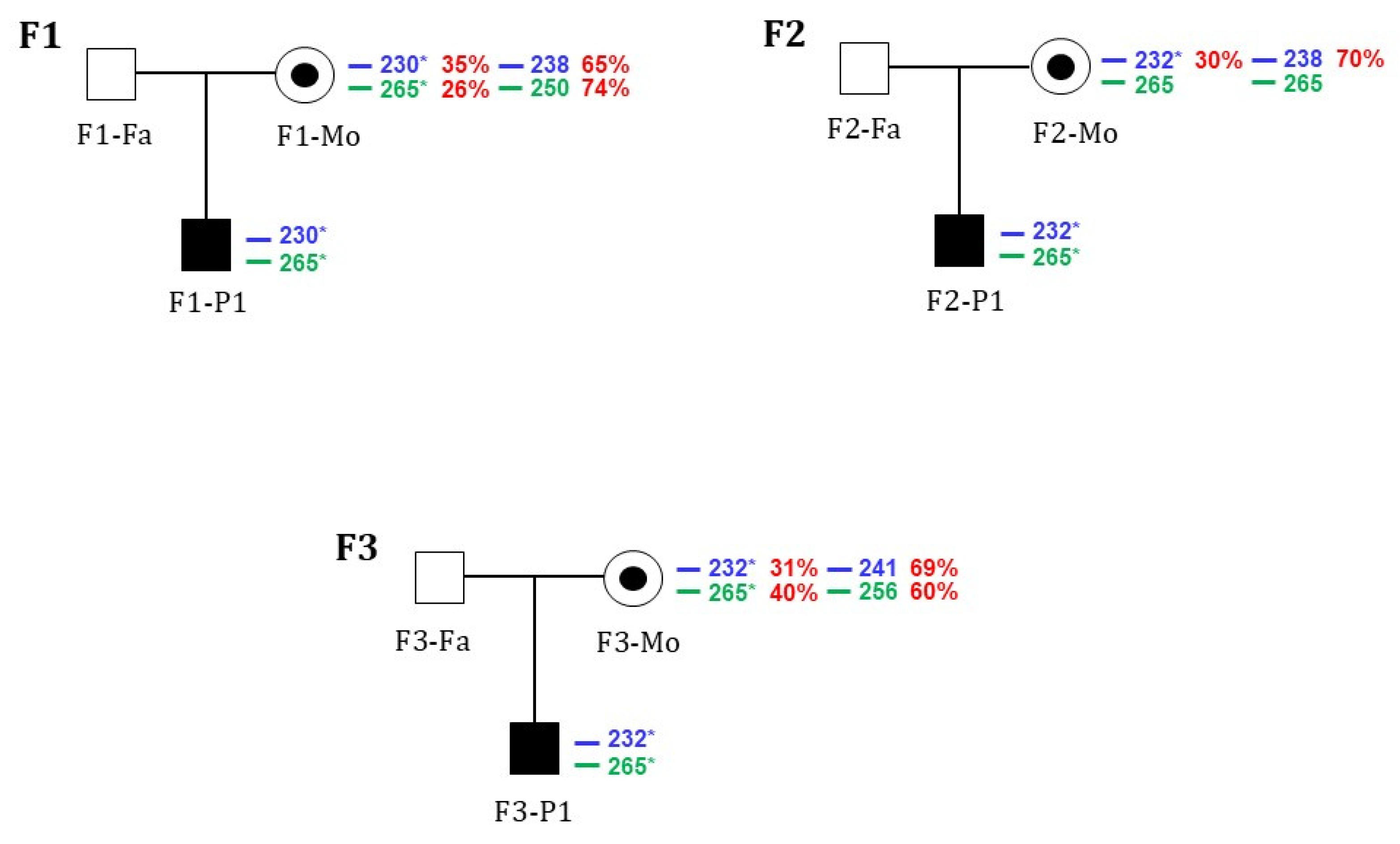
| ATP11C variant | c.828C>G; (p. Asn276Lys) c.892T>G; (p. Tyr298Asp) | wt | §c.1349_1350insATATTTTGACATA; (p.Leu451Tyrfs*9) | wt | c.436-7A>G; (Lys145_Val146 ins PheGln) | wt | |||||
| Case | Age at Diagnosis | Clinical Presentation | Hb (g/dL) | Reticulocytes (109/L) | Unconj. Bilirubin (mg/dL) | ATP11C Gene Mutation | Residual Flippase Activity (%) |
|---|---|---|---|---|---|---|---|
| [15] | 14 | pigmented urine (4yrs) mild anemia (13yrs) | 11.8 | 53 | 1 | c.1253C>A (p.Thr418Asn) | 3% |
| [17] | 15 | jaundice, pigmented urine | 11.9 | 208 | 4 | c.1003C>T (p.Arg335*) | n.a |
| [18] | 37 | mild splenomegaly, fatigue, reduced exercise capacity | 13.2 | 194 | 1.8 # | c.2365C> T (p. Leu789Phe) | 10.8% |
| [16] ° | n.a | splenomegaly, compensated hemolysis | 14.7 | 107 | 1.5 | c.2434C>T (p.Phe812Ser) | n.a |
| [19] § | birth | anemia, neonatal jaundice | 6 | 92-233 | 12.2 | c.3136A>T (p.Ile1046Phe) | n.a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fermo, E.; Trombetta, E.; Marcello, A.P.; Vercellati, C.; Ferrari, G.M.; Zaninoni, A.; Brancaleoni, V.; Di Pierro, E.; Beneventi, S.; Tornese, M.; et al. Not-So-Rare Defects of RBC Lipidic Composition: Four New Cases of Flippase Deficiency Due to ATP11C Mutations. Int. J. Mol. Sci. 2025, 26, 7722. https://doi.org/10.3390/ijms26167722
Fermo E, Trombetta E, Marcello AP, Vercellati C, Ferrari GM, Zaninoni A, Brancaleoni V, Di Pierro E, Beneventi S, Tornese M, et al. Not-So-Rare Defects of RBC Lipidic Composition: Four New Cases of Flippase Deficiency Due to ATP11C Mutations. International Journal of Molecular Sciences. 2025; 26(16):7722. https://doi.org/10.3390/ijms26167722
Chicago/Turabian StyleFermo, Elisa, Elena Trombetta, Anna Paola Marcello, Cristina Vercellati, Giulia Maria Ferrari, Anna Zaninoni, Valentina Brancaleoni, Elena Di Pierro, Sara Beneventi, Marta Tornese, and et al. 2025. "Not-So-Rare Defects of RBC Lipidic Composition: Four New Cases of Flippase Deficiency Due to ATP11C Mutations" International Journal of Molecular Sciences 26, no. 16: 7722. https://doi.org/10.3390/ijms26167722
APA StyleFermo, E., Trombetta, E., Marcello, A. P., Vercellati, C., Ferrari, G. M., Zaninoni, A., Brancaleoni, V., Di Pierro, E., Beneventi, S., Tornese, M., Fattizzo, B., Casini, T., Corti, P., & Bianchi, P. (2025). Not-So-Rare Defects of RBC Lipidic Composition: Four New Cases of Flippase Deficiency Due to ATP11C Mutations. International Journal of Molecular Sciences, 26(16), 7722. https://doi.org/10.3390/ijms26167722







