1. Introduction
Cancer is a multifaceted and dynamic disease, shaped by the complex interplay among genomic alterations, signaling redundancy, clonal evolution, and the surrounding tumor microenvironment. In recent years, the paradigm of precision oncology—focused on targeting specific oncogenic drivers—has led to significant therapeutic progress. However, growing evidence underscores the limitations of single-target strategies in the context of tumor heterogeneity, resistance mechanisms, and pathway plasticity.
In response to this evolving complexity, the concept of “continuously responsive oncology” is particularly compelling. This paradigm envisions cancer treatment as a dynamic and iterative process, guided by real-time molecular data, adaptive therapeutic combinations, and predictive modeling, capable of responding to the evolving biology of each patient’s tumor. Instead of relying on fixed, one-time treatment decisions, this approach seeks to continuously incorporate new layers of information to refine and optimize therapeutic strategies over time.
This scoping review aims to map emerging conceptual and technological trajectories, including the integration of artificial intelligence (AI), that have the potential to redefine future cancer treatment paradigms. While not intended as a comprehensive overview, it offers a forward-looking perspective on how the limitations of monotherapy are being addressed through systems-level, multi-targeted, and computationally guided strategies.
The review is structured around several key dimensions of cancer complexity. First, it delineates the mechanistic limitations of single-target inhibition. It then examines the rationale for and outcomes of combination therapies, emphasizing their potential to counteract pathway compensation and clonal selection. The subsequent sections explore how liquid biopsy and next-generation sequencing (NGS) technologies enable real-time tracking of tumor evolution, and how machine learning models applied to these datasets can predict resistance trajectories and inform treatment selection. A dedicated section addresses the critical yet often underappreciated role of the tumor microenvironment (TME), highlighting advanced spatial and single-cell technologies that facilitate high-resolution profiling of TME dynamics. The final sections delve into synthetic lethality and drug repurposing, uncovering novel pharmacological opportunities that may be further accelerated through AI-driven discovery pipelines. By synthesizing key axes of cancer biology—including tumor-intrinsic complexity, evolutionary dynamics, immune contexture, and computational modeling—this review proposes a forward-facing framework for future oncology. It encourages readers to move beyond the linear “target-and-block” approach and embrace a more integrated, adaptive, and AI-enhanced vision for cancer care.
2. Limitations of the Single-Target Approach in the Precision Oncology Paradigm
In contemporary oncology, the paradigm of precision medicine has ushered in transformative therapeutic strategies, particularly through the identification and targeting of dominant oncogenic drivers. This approach has yielded significant clinical benefits in malignancies such as chronic myeloid leukemia (CML) with
BCR-
ABL1 fusion and
NTRK fusion-positive tumors, where targeted monotherapies have demonstrated remarkable efficacy [
1,
2]. Nonetheless, resistance inevitably emerges in a substantial subset of patients. In CML, secondary kinase domain mutations—most notably the p.T315I gatekeeper mutation—confer high-level resistance to first- and second-generation
BCR-
ABL1 inhibitors, necessitating the development of third-generation agents such as ponatinib [
3]. Similarly, in tumors harboring
NTRK gene fusions, acquired resistance often arises through solvent front mutations (e.g.,
NTRK1 p.G595R,
NTRK3 p.G623R) or xDFG motif substitutions that impair drug binding, prompting the design of next-generation inhibitors with improved conformational tolerance [
4]. These examples illustrate how tumor plasticity and selective pressure drive the emergence of resistance, highlighting the importance of anticipatory therapeutic strategies to prolong clinical benefit. Even if not exhaustive, additional examples can be provided to illustrate the efficacy of biologically targeted monotherapies in tumors harboring defined molecular lesions. In non-small cell lung cancer (NSCLC), activating mutations in the epidermal growth factor receptor (
EGFR) gene have been effectively targeted by tyrosine kinase inhibitors (TKIs) such as erlotinib, resulting in improved progression-free survival. However, resistance mechanisms, including secondary mutations, such as p.T790M and the activation of alternative signaling pathways, often emerge, necessitating the development of next-generation inhibitors [
5]. Similarly, rearrangements involving the anaplastic lymphoma kinase (
ALK) gene in NSCLC have been successfully targeted by crizotinib. Despite initial responses, resistance frequently develops through secondary
ALK mutations, gene amplification, or activation of bypass signaling pathways, leading to the exploration of second-generation
ALK inhibitors to overcome resistance [
6]. In melanoma, the
BRAF p.V600E mutation has been a critical target, with inhibitors such as vemurafenib demonstrating substantial clinical activity. Nevertheless, resistance mechanisms such as
NRAS mutations,
BRAF splice variants, and the activation of parallel pathways such as PI3K/AKT have been identified, prompting combination therapies to enhance efficacy [
7].
HER2 amplification in breast cancer has been effectively targeted by trastuzumab, improving survival rates. Yet, resistance can occur through mechanisms such as PI3K pathway activation, expression of truncated HER2 forms (p95HER2), and cross-talk with other receptors, leading to the development of additional HER2-targeted agents and combination therapies to address resistance [
8].
In other words, cancer is not a static entity, but rather a dynamic, evolving ecosystem shaped by intratumor heterogeneity, genomic instability, and the selective pressures exerted by the TME [
9]. In many solid tumors, the presence of multiple co-occurring mutations reflects a non-linear and multifactorial oncogenic trajectory. In this context, targeting a single signaling node is often insufficient to achieve sustained clinical benefit. Resistance—whether intrinsic or acquired—frequently arises through pathway redundancy, compensatory signaling mechanisms, or the clonal selection of resistant subpopulations [
10].
Figure 1 shows a schematic representation highlighting the complexity of intracellular signaling pathways and how this intricacy impacts their therapeutic inhibition.
3. Rationale for Combination Therapies Involving Biologic Agents
A concrete example illustrating the limitations of single-target approaches and the therapeutic potential of multi-target inhibition is the combination of sotorasib (a
KRAS p.G12C inhibitor) and panitumumab (an anti-EGFR monoclonal antibody) in advanced and chemotherapy-refractory colorectal cancer. Dual blockade of both KRAS-dependent and EGFR-mediated signaling pathways led to significantly improved progression-free survival and objective response rates compared to standard therapies [
11]. This underscores the clinical benefit of simultaneously targeting multiple oncogenic drivers.
Beyond this recent example, where the objective response rate (ORR) increased from 0% with sotorasib monotherapy to 26.4% when combined with panitumumab, there are additional compelling instances across oncology in which the strategic combination of targeted agents has resulted in a marked improvement in tumor response. Without claiming to be exhaustive, in HER2-positive breast cancer, the combination of trastuzumab and lapatinib significantly outperformed trastuzumab alone, achieving a pathologic complete response (pCR) rate of 51.3% versus 29.5% in the neoadjuvant setting [
12]. Similarly, in the hormone receptor-positive/HER2-positive metastatic breast cancer population, the triplet regimen of trastuzumab, lapatinib, and an aromatase inhibitor yielded an ORR of 31.7%, clearly superior to the 13.7% observed with the dual combination of trastuzumab and endocrine therapy [
13]. In
BRAF V600-mutant melanoma, the addition of the MEK inhibitor cobimetinib to the BRAF inhibitor vemurafenib increased the ORR from 45% to 68% [
14]. These studies converge on a unifying principle: cancer results from multiple dysregulated biological pathways, and rational combinations of targeted agents can produce therapeutic synergy and enhanced tumor regression (
Table 1). In other words, simultaneous inhibition of several altered nodes—whether convergent within the same pathway or divergent across distinct pathways—is a rational strategy that may enhance therapeutic efficacy by comprehensively targeting tumor complexity and signaling redundancy.
4. Mapping Tumor Evolution Through Liquid Biopsy: A Road to Precision Oncology Empowered by Artificial Intelligence
The development of acquired resistance to therapy, which accounts for approximately 90% of cancer-related deaths, remains one of the foremost challenges in contemporary oncology. At the core of this issue lies the emergence of drug-tolerant persister (DTP) cells that survive initial therapeutic assaults and serve as reservoirs for clonal evolution, facilitating the development of genetically distinct subpopulations and fueling intratumoral heterogeneity (ITH) [
15,
16]. ITH, a hallmark of malignancy, is not a static phenomenon but a dynamic and adaptive process characterized by spatial and temporal variability in tumor molecular architecture. Traditionally, our understanding of ITH and resistance mechanisms has relied on serial tissue biopsies, which, although informative, present several critical limitations—including procedural invasiveness, sampling bias, and the inability to capture the full extent of tumor heterogeneity over time, particularly in metastatic or anatomically inaccessible lesions. In contrast, liquid biopsy, particularly the genomic analysis of circulating tumor DNA (ctDNA) and other circulating biomarkers, has emerged as the most advanced and clinically viable tool for capturing tumor dynamics in a minimally invasive and temporally resolved manner [
17]. Liquid biopsy enables real-time monitoring of tumor evolution by facilitating the detection of emerging resistance-associated mutations, subclonal dynamics, and longitudinal assessment of molecular alterations in response to therapy. By providing a comprehensive snapshot of the genetic landscape across multiple tumor sites, it offers a holistic view of spatial heterogeneity and therapeutic adaptation. Importantly, liquid biopsy can detect low-frequency variants and clonal expansions that precede radiological progression, enabling earlier therapeutic interventions and adaptive treatment strategies [
18,
19].
Moreover, integration of NGS technologies with liquid biopsy has significantly expanded its utility. High-depth targeted panels, whole-exome sequencing (WES), and even emerging whole-genome approaches applied to ctDNA allow for the reconstruction of tumor phylogenies, identification of driver mutations, and mapping of resistance trajectories over time [
20]. The convergence of liquid biopsy with computational oncology and machine learning algorithms further enables prediction of resistance pathways, inference of subclonal architecture, and development of patient-specific models of tumor progression [
21]. However, widespread adoption of liquid biopsy and comprehensive genetic assessment in routine oncology practice is hindered by several barriers. Technologically, improvements in ctDNA detection sensitivity and specificity remain necessary—especially in early-stage disease or low-shedding tumors—as do standardization of pre-analytical variables, sequencing protocols, and bioinformatic pipelines to ensure reproducibility and clinical reliability [
22]. Furthermore, economically, high-throughput sequencing remains costly, particularly in resource-limited healthcare systems [
23]. Cost reduction strategies include the development of targeted panels focused on clinically actionable mutations, streamlined analytical workflows, and the incorporation of liquid biopsy into broader diagnostic and treatment algorithms to establish clear cost-effectiveness. Additionally, regulatory and reimbursement frameworks must evolve to support clinical utility beyond niche applications. Ethical and logistical challenges also accompany dynamic genomic surveillance: sampling frequency, interpretation of low-level variants, management of incidental findings, and psychological impact on patients require careful policy and clinical guidelines. Nonetheless, the trajectory of precision oncology is moving toward more agile, minimally invasive, and data-rich approaches, with liquid biopsy and real-time genetic profiling at the forefront.
As the clinical use of liquid biopsy expands, its role is evolving from a passive monitoring tool to an active predictor of therapeutic response and tumor evolution. During the course of treatment, serial genetic reassessments through ctDNA analysis can reveal the emergence of resistance-conferring mutations before clinical progression becomes evident [
24].
This early molecular insight allows for timely therapeutic adjustments and may also reveal co-occurring alterations that can be co-targeted in a synergistic manner—offering a strategy to overcome the limited efficacy often associated with single-agent therapies. However, capturing and interpreting the full spectrum of tumor heterogeneity through serial liquid biopsies presents a formidable analytical challenge. The volume of data generated by these longitudinal analyses increasingly exceeds our capacity for interpretation, creating a critical bottleneck in the translation of molecular complexity into actionable therapeutic strategies. At the intersection of oncology and AI, however, a transformative opportunity is emerging [
25]. In fact, bioinformatics and AI are indispensable in this context, allowing for the extraction of actionable molecular signatures from complex and large multi-omic datasets—including genomic, transcriptomic, epigenomic, proteomic, and metabolomic layers. These integrative models will not only inform dynamic treatment selection but also support the rational design of multi-targeted therapeutic strategies aimed at circumventing resistance mechanisms. In such models, genomic data are continuously integrated into AI algorithms to determine whether therapy should be maintained, intensified, or modified over time (
Figure 2).
Ultimately, the transformation of liquid biopsy from a descriptive to a predictive—and eventually prescriptive—clinical tool hinges on interdisciplinary collaboration among oncologists, molecular scientists, bioinformaticians, and data scientists. Longitudinal patient cohorts and adaptive clinical trials incorporating serial liquid biopsies will be essential for validating biomarkers of therapeutic efficacy and resistance [
26], paving the way for a truly personalized oncology paradigm.
5. Not Only the Tumor, but Also Its Microenvironment: A Critical Determinant of Therapy Response
In this context, a key contributor to cancer complexity and therapeutic resistance is the TME, which plays a central role in promoting immune evasion, metabolic adaptation, and the formation of immunosuppressive niches [
27]. The TME consists of a complex ecosystem of immune cells, fibroblasts, endothelial cells, extracellular matrix components, and soluble mediators that collectively shape tumor behavior and influence response to therapy.
Unlike tumor cells, the components of the TME are predominantly composed of genetically stable, non-malignant cells with more static biological features [
28]. A limitation of liquid biopsy is its inability to directly characterize the TME [
29]. While associations between tumor genomic alterations and specific TME profiles are plausible, they remain poorly defined and far from being systematically validated. Therefore, within a holistic view of cancer biology, a comprehensive analysis of tumor tissue remains indispensable.
Notably, the complexity of the TME is increasingly recognized as a key determinant of therapeutic outcome, reinforcing the value of integrated spatial and temporal profiling. Technologies such as multiplex immunohistochemistry, spatial transcriptomics, and single-cell RNA sequencing offer high-resolution characterization of the cellular and molecular interactions occurring within the tumor and its microenvironment [
30,
31]. These approaches complement liquid biopsy by providing spatial context and cellular specificity, which are essential for understanding the dynamic interplay between tumor cells and their surrounding niche.
Indeed, recent studies have shown that clonal diversity and cooperation among tumor subclones can modulate the immune landscape, promoting tumor progression and metastatic dissemination [
32]. Spatial and temporal heterogeneity within the TME adds yet another layer of complexity, directly impacting the efficacy of immunotherapies and other targeted strategies [
33]. The interaction between tumor cells and the TME is bidirectional: while the microenvironment influences tumor evolution, tumor-derived signals actively remodel the TME to support survival, proliferation, and immune escape.
The integration of AI into digital pathology and precision oncology is transforming modern cancer care, particularly by offering novel insights into the TME. In this context, AI enables the integration and interrogation of large-scale, multi-dimensional biological datasets, uncovering complex spatial and molecular patterns within the TME that would be difficult to discern through conventional analysis [
34]. Deep learning models trained on multi-omic inputs can now predict protein structures, model molecular interactions, and anticipate tumor evolution under therapeutic pressure [
35]. This predictive capability is already informing rational drug design, virtual screening, and the real-time generation of patient-specific therapeutic hypotheses [
36,
37].
AI-driven approaches applied to histopathology are advancing rapidly. Novel architectures, such as dual-path neural networks [
38] and multi-instance learning models coupled with foundation models [
39], enable the integration of morphological data from whole slide images (WSI) with spatially resolved transcriptomic and proteomic data. These models have been shown to accurately predict molecular subtypes of complex tumors, such as gliomas or virus-associated cancers [
38,
40,
41]. Furthermore, computational techniques such as Histology-Inferred Protein Information (HIPI) now allow the inference of multiplexed protein expression directly from H&E-stained slides, enhancing both spatial and molecular resolution [
42]. These innovations are redefining diagnostic paradigms, enabling granular classification of tumors based on morphology, genomics, and immune features. Technologies for in situ mutation detection and spatial genomics have improved the ability to identify subclonal niches, providing essential information for personalized therapeutic strategies [
43,
44,
45].
In the previous paragraph, we discussed liquid biopsy as a valuable tool for monitoring tumor dynamics. However, a recognized limitation of this approach is its inability to directly characterize the TME. Emerging research is beginning to address this gap through novel analytical strategies. For instance, methylation profiling of ctDNA can reflect the cell-of-origin and tissue-specific epigenetic states, indirectly informing on TME composition, including stromal and immune cell interactions [
46]. Similarly, fragmentomic analyses, examining the size distribution, fragmentation patterns, and end motifs of ctDNA, are being explored as surrogates of chromatin structure and cellular processes within the TME [
47,
48]. Additionally, circulating extracellular vesicles and exosomal proteins are gaining attention as promising biomarkers that may capture immune-related features of the TME, such as T-cell suppression [
49,
50]. These approaches collectively hold potential to expand the interpretive power of liquid biopsies beyond tumor-intrinsic genomic alterations.
6. Synthetic Lethality: Mechanistic Foundations, Computational Discovery, and Therapeutic Opportunities
Synthetic lethality (SL) describes a genetic interaction in which the simultaneous perturbation of two genes—neither of which is lethal on its own—results in cell death. In oncology, one of these perturbations is typically a tumor-specific lesion (germline or somatic), whereas the second is an intentionally induced inhibition of a seemingly unrelated pathway [
51]. The therapeutic appeal is straightforward: if a cancer cell harbors mutation A, then pharmacologically inhibiting partner B will selectively kill the malignant clone while sparing normal tissue that retains wild-type A. This concept, first observed in Drosophila and then formalized in yeast genetics, has matured into a pillar of precision oncology, most notably exemplified by the clinical success of PARP inhibitors in
BRCA1/
2-defective malignancies [
52,
53]. Yet, BRCA–PARP is merely the visible tip of a far broader network of lethal genetic dependencies that remain to be fully charted.
Tumor cells accumulate mutations that rewire signaling, metabolic, and DNA-repair circuits. Although these alterations confer growth advantages, they also create hidden liabilities because the cell’s homeostatic buffers—redundant or compensatory pathways—must take up the slack created by the primary lesion. When a compensatory node is disabled, the fragile new equilibrium collapses and the cell undergoes mitotic catastrophe, ferroptosis, or other fatal outcomes. For instance,
BRCA1/2 loss cripples homologous recombination repair; PARP inhibition further blocks base-excision repair, producing unrepaired single-strand breaks that convert into lethal double-strand lesions during replication [
54]. Other well-studied pairs include
PTEN loss with PI3Kβ blockade [
55],
IDH1/2 mutations with glutaminase inhibition [
56],
ARID1A deficiency with EZH2 or ATR inhibitors [
57], and
SMARCA4 inactivation with BRD9 degraders [
58]. Across these examples, the synthetic-lethal partner is rarely the most obvious downstream effector of the driver lesion; instead, it is often a parallel pathway that has silently assumed essential housekeeping duties.
Early SL discovery relied on intuition-driven, low-throughput experiments. Today, genome-wide CRISPR and shRNA dropout screens in isogenic cell lines or patient-derived organoids systematically map lethal pairs across thousands of mutational contexts. These datasets, combined with transcriptional, proteomic, phospho-signaling, and metabolomic layers, have revealed that SL interactions are highly context-specific: a dependency observed in
KRAS-mutant pancreatic cells may not hold in
KRAS-mutant colorectal cells because of lineage-restricted circuitry [
59]. Therefore, comprehensive biological insight—spanning DNA-repair, cell-cycle checkpoints, chromatin architecture, epigenetic state, metabolic flux, and immune crosstalk—is indispensable for prioritizing actionable vulnerabilities.
The combinatorial space of potential gene–drug or gene–gene interactions is astronomically large, outstripping human cognitive capacity. AI is uniquely suited to navigate this space by integrating heterogeneous omics, chemical, and clinical outcome datasets. Graph convolutional networks can embed genes, proteins, metabolites, and drugs into a unified knowledge graph, enabling the algorithm to infer probable SL edges based on topological proximity, shared ontologies, and learned patterns of co-essentiality [
60]. Variational autoencoders trained on CRISPR screen matrices can generate latent representations that highlight conditional lethalities under specific mutational or microenvironmental constraints. Moreover, transformer-based language models pre-trained on biomedical literature (e.g., PubMedGPT variants) extract mechanistic cues from millions of abstracts, suggesting testable SL pairs that have never been experimentally probed [
61,
62].
Coupling these AI pipelines with in silico virtual screening closes the loop between target discovery and drug identification. For a predicted partner gene lacking bespoke inhibitors, algorithms can interrogate >20,000 clinically approved or investigational molecules for binding motifs, off-target polypharmacology, and ADMET profiles, rapidly nominating repurposing candidates [
63]. The iterative cycle proceeds as follows: (i) AI predicts an SL interaction; (ii) docking and molecular-dynamics simulations identify druggable pockets; (iii) repurposed compounds or de novo-designed ligands are prioritized; (iv) in vitro viability assays and single-cell transcriptomics validate selective lethality; (v) feedback refines the model. Such closed-loop learning has already accelerated the path from computational hypothesis to clinically actionable insight in less than a year for certain targets [
64].
SL is evolving into a dynamic, AI-driven concept. Beyond static gene pairs such as BRCA–PARP, new approaches identify context-specific and stress-induced vulnerabilities using knowledge graphs and single-cell data. AI can forecast when tumor clones will develop dependencies that can be targeted before resistance emerges. Moreover, linking SL discovery to in silico drug screening allows rapid identification of repurposable compounds. These strategies can personalize SL targeting across tumor types and timepoints, turning cancer’s adaptability into an opportunity for proactive, precision-based interventions.
7. Drug Repurposing and Uncovering Hidden Pharmacological Opportunities
Repurposed drugs—such as statins, metformin, bisphosphonates, non-selective β-blockers, and certain antifungals—frequently exhibit anticancer effects that were initially unexplained. Emerging evidence now indicates that many of these effects arise from context-dependent synthetic lethality (SL). For example, statins inhibit HMG-CoA reductase, reducing mevalonate-pathway flux; tumors bearing p53 gain-of-function mutations or alterations in the mevalonate—YAP/TAZ axis become exquisitely sensitive because they rely on prenylation-dependent small GTPases for survival [
65]. Metformin, long known to activate AMPK and impair mitochondrial respiration, can be synthetically lethal in LKB1-deficient or mitochondrial-DNA-mutant contexts, where energetic buffering is already compromised [
66]. Crucially, the pre-existing clinical safety data for such agents lowers the translational barrier, facilitating rapid initiation of biomarker-driven trials.
However, as with targeted therapies, repurposed drugs should not be administered indiscriminately to unselected patient populations. Instead, their clinical use ought to be guided by specific molecular or metabolic features that predict therapeutic vulnerability. Administering these agents without biological stratification risks diluting their apparent clinical efficacy, as only a subset of patients may harbor the molecular dependencies required for response. This principle underscores the necessity of integrating biomarker-based patient selection even in the context of drug repurposing strategies. Beyond these examples,
Table 2 summarizes selected repurposed drugs, their approved indications, reported anti-cancer activity, mechanisms of action, and possible rational selection of patients based on tumor biology.
SL extends beyond coding mutations. Epigenetic silencing of tumor suppressors (e.g.,
MLH1 promoter methylation) creates dependencies exploitable by DNMT or HDAC inhibitors in combinatorial regimens [
67]. Similarly, non-coding RNAs that fine-tune pathway redundancy can create Achilles’ heels: an oncogenic lncRNA that buffers DNA-damage tolerance might render the cell dependent on alternative repair routes, which can be blocked pharmacologically [
68,
69]. AI models trained on chromatin-accessibility and RNA-interactome maps are beginning to reveal these subtler layers of SL, which were invisible to gene-centric screens.
Despite its promise, clinical translation of SL faces hurdles. First, tumor heterogeneity means the biomarker lesion may not be ubiquitous across all metastatic sites, risking outgrowth of resistant clones. Second, many synthetic-lethal inhibitors target ubiquitous cellular processes (e.g., replication stress), raising toxicity concerns. AI can mitigate these issues by modelling clone-level heterogeneity from liquid-biopsy sequencing, predicting the likelihood of pre-existing escape variants, and simulating therapeutic windows across normal tissue transcriptomes. Integration of differential gene-essentiality data from CRISPR screens in normal organoids further informs safety profiles [
70].
The future lies in the dynamic exploitation of SL. Serial ctDNA sampling combined with AI-driven evolutionary forecasting will detect emergent subclones and suggest the next-line SL partner before clinical progression. Master-protocol trials, embedding Bayesian adaptive randomization, can test multiple SL-guided combinations in parallel, continuously updating enrolment criteria based on accumulating molecular response data [
71,
72]. In silico twin models of each enrolled patient—fed by longitudinal multi-omics—will simulate various intervention sequences, nominating the regimen with the highest probability of durable response.
SL crystallizes the essence of precision oncology: turning cancer’s own wiring errors into selective liabilities. To fully unlock this therapeutic space, a comprehensive understanding of cellular biology under stress—including compensatory signaling, metabolic flux, and chromatin remodeling—is required. AI, with its capacity to integrate vast biological knowledge and uncover latent patterns, is the indispensable ally in this endeavor. By marrying AI-driven discovery with pragmatic drug repurposing and adaptive clinical designs, the field stands poised to translate the theoretical elegance of synthetic lethality into tangible, patient-centered benefit.
Thus, these recent advances reposition drug repurposing as a precision-guided strategy rather than “opportunistic” and empiric reuse. AI models now integrate tumor genomics, drug-target networks, and treatment evolution to predict when a repurposed drug (such as metformin or statins) can trigger SL in specific molecular contexts. Reinforcement learning can simulate effective combinations and dosing [
73], while adaptive trial designs enable dynamic patient reassignment based on biomarkers. Public pharmacogenomic datasets combined with AI tools help uncover unexpected therapeutic matches, transforming repurposed agents into tailored treatments for biomarker-defined cancer subtypes.
8. Conceptual Framework Narrative: AI-Driven Oncology Model
In the post-genomic oncology paradigm, AI serves as a central integrative engine that unifies patient-specific molecular inputs and dynamic profiling to drive predictive modeling, therapeutic repurposing, combination strategy optimization, patient stratification, and novel clinical trial design. At its core, this framework begins with multi-omic data integration, where AI synthesizes genomics, epigenomics, transcriptomics, proteomics, and metabolomics data to delineate tumor heterogeneity and uncover latent biomarkers [
74]. Complementing this is dynamic molecular profiling, leveraging longitudinal samples such as ctDNA or serial biopsies, enabling deep learning models to capture temporal tumor evolution and emerging resistance mechanisms. Next, predictive modeling uses these fused datasets to forecast treatment response and prognosis at the individual level, accommodating inter-patient variability. In parallel, AI-driven drug repurposing applies graph-based, transformer, or network-mining methods to identify novel therapeutic uses for existing agents, expediting clinical translation [
75]. The framework further advances with multi-targeted strategy optimization, where reinforcement learning and combination-design algorithms propose synergistic regimens and dose schedules tailored to multi-pathway dependencies. By integrating this information, AI can classify subpopulations with distinct phenotypic and therapeutic response profiles. Finally, AI-guided clinical trial innovation offers adaptive designs and synthetic control arms that enhance trial efficiency, which is especially beneficial for rare molecular subtypes. This conceptual model provides an organizational structure, linking each AI application domain to specific aspects of cancer complexity. It positions AI not merely as a prognostic or predictive tool but as a therapeutic enabler, fostering precision interventions informed by layered data inputs (
Figure 3).
9. Barriers and Limitations in the Clinical Implementation of AI in Oncology
While the integration of AI into oncology holds transformative potential, a balanced and realistic perspective must account for the substantial practical challenges and systemic limitations that hinder clinical implementation. First, economic constraints represent a major barrier. The costs associated with serial NGS, multi-omics profiling, and high-resolution imaging (necessary for feeding AI models with robust input) remain prohibitive for many healthcare systems. This raises concerns regarding equitable access to AI-guided precision oncology, particularly in under-resourced or publicly funded contexts where reimbursement pathways for such technologies are unclear or absent. Second, regulatory hurdles remain significant. The complexity, adaptiveness, and opacity of many AI algorithms (particularly those employing deep learning) pose difficulties for standard validation, auditing, and approval by regulatory agencies such as the EMA or FDA. Current frameworks are better suited to static diagnostics or single-gene tests than to continuously learning, multifactorial models that evolve as new data are incorporated. Third, data infrastructure and standardization issues further complicate deployment. AI requires large, harmonized datasets that integrate molecular, clinical, and radiological data; yet, in practice, such datasets are fragmented across institutions, lack standardized formats, and often suffer from batch effects or inconsistent bioinformatics pipelines. Without uniform wet-lab protocols and interoperable data architectures, reproducibility and model generalizability remain compromised.
In addition to these operational challenges, it is crucial to acknowledge the intrinsic limitations and risks of AI itself. Despite its impressive analytical capabilities, AI is not a panacea. Many deep learning systems operate as “black boxes,” offering high accuracy without interpretability, an issue that undermines clinician trust and poses ethical and legal dilemmas in medical decision-making. Furthermore, AI models trained on retrospective, often Western-centric datasets risk perpetuating or amplifying biases, particularly in patient populations that are underrepresented in training cohorts. This can lead to suboptimal or even harmful recommendations when deployed broadly. Finally, the translation of AI-derived therapeutic hypotheses into clinical benefit requires prospective validation in controlled clinical trials, a step that remains underdeveloped in current literature and practice. Without rigorous validation, there is a danger of overfitting to past data rather than truly predicting future outcomes. Thus, while the promise of AI in oncology is considerable, realizing its full potential requires careful navigation of these technical, logistical, and ethical challenges through interdisciplinary collaboration, regulatory innovation, and continued investment in infrastructure and clinical validation.
10. Conclusions: Towards Continuously Responsive Oncology
The reductionist model of single-target, single-agent therapies must give way to rational and dynamic multi-targeted strategies that account for the adaptive and evolving nature of cancer. Beyond serving as computational accelerants, AI platforms may evolve into true hypothesis-generating engines, capable of identifying emergent vulnerabilities and designing adaptive, multi-target therapeutic approaches. This is especially relevant not only for identifying novel actionable direct targets but also in complex contexts such as synthetic lethality, where targeting compensatory pathways can induce tumor collapse.
To realize this potential, the integration of real-time liquid biopsy with serial genomic profiling and AI-supported decision-making may enable “continuously responsive oncology,” wherein treatment regimens are dynamically adapted based on longitudinal tumor evolution. Predictive models may soon forecast not only resistance mutations but also their timing and likelihood, allowing preemptive therapeutic reprogramming.
Future clinical trials may increasingly adopt adaptive designs, comparing conventional line-based therapies with AI-guided approaches in real time. Within such frameworks, therapeutic decisions are continuously optimized.
Ultimately, a systems biology approach enhanced by AI may enable a paradigm shift from reactive treatment to anticipatory control of cancer, improving long-term outcomes and transforming therapeutic intent from disease management to durable remission or cure.
Author Contributions
Conceptualization, A.D.M., G.S., A.O. and M.B.; software, A.O. and M.S.; validation, A.D.M., G.F., G.S. and A.O.; writing—original draft preparation, A.D.M., A.O. and C.P.; writing—review and editing, M.S. and G.S.; supervision, A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated or analyzed in this study.
Acknowledgments
Alessandro Ottaiano, the supervisor of this work, would like to express his deepest gratitude to his parents: his mother, Anna Borrelli, and his father, Bruno, for their unwavering, unconditional, and constant support throughout his personal and professional journey. He owes everything to them. He sincerely thanks them for having continuously inspired and encouraged him. This multidisciplinary and visionary work on the future of oncology is dedicated to them.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADMET | Absorption, Distribution, Metabolism, Excretion, and Toxicity |
| AKT | AKT Serine/Threonine Kinase |
| ALK | Anaplastic Lymphoma Kinase |
| AMPK | AMP-Activated Protein Kinase |
| ARID1A | AT-Rich Interaction Domain 1A |
| ATR | Ataxia Telangiectasia and Rad3-Related Protein |
| BCR-ABL1 | Breakpoint Cluster Region–Abelson Murine Leukemia Viral Oncogene Homolog 1 |
| BRAF | B-Raf Proto-Oncogene, Serine/Threonine Kinase |
| BRCA1/2 | Breast Cancer 1/2 Genes |
| CML | Chronic Myeloid Leukemia |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| ctDNA | Circulating Tumor DNA |
| DNMT | DNA Methyltransferase |
| DTP | Drug-Tolerant Persister |
| EGFR | Epidermal Growth Factor Receptor |
| EZH2 | Enhancer of Zeste Homolog 2 |
| GTPases | Guanosine Triphosphatases |
| HDAC | Histone Deacetylase |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HIPI | Histology-Inferred Protein Information |
| HMG-CoA | 3-Hydroxy-3-Methylglutaryl-Coenzyme A |
| HR | Hormone Receptor |
| IDH1/2 | Isocitrate Dehydrogenase 1/2 |
| ITH | Intratumoral Heterogeneity |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene Homolog |
| lncRNA | Long Non-Coding RNA |
| LKB1 | Liver Kinase B1 |
| mAb | Monoclonal Antibody |
| MEK | Mitogen-Activated Protein Kinase Kinase (MAP2K) |
| MLH1 | MutL Homolog 1 |
| NGS | Next-Generation Sequencing |
| NRAS | Neuroblastoma RAS Viral Oncogene Homolog |
| NSCLC | Non-Small Cell Lung Cancer |
| NTRK | Neurotrophic Tyrosine Receptor Kinase |
| ORR | Objective Response Rate |
| PARP | Poly (ADP-ribose) Polymerase |
| pCR | Pathologic Complete Response |
| PD | Progressive Disease |
| PI3K | Phosphoinositide 3-Kinase |
| PTEN | Phosphatase and Tensin Homolog |
| shRNA | Short Hairpin RNA |
| SL | Synthetic Lethality |
| SMARCA4 | SWI/SNF Related, Matrix Associated, Actin Dependent Regulator of Chromatin Subfamily A Member 4 |
| TME | Tumor Microenvironment |
| TKI | Tyrosine Kinase Inhibitor |
| WES | Whole-Exome Sequencing |
| YAP/TAZ axis | Yes-Associated Protein/Transcriptional Coactivator with PDZ-Binding Motif Axis |
References
- O’Brien, S.G.; Guilhot, F.; Larson, R.A.; Gathmann, I.; Baccarani, M.; Cervantes, F.; Cornelissen, J.J.; Fischer, T.; Hochhaus, A.; Hughes, T.; et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 2003, 348, 994–1004. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kim, D.-W.; Pinilla-Ibarz, J.; Le Coutre, P.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. Ponatinib in refractory Philadelphia chromosome–positive leukemias. N. Engl. J. Med. 2013, 369, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Nagasubramanian, R.; Blake, J.F.; Ku, N.; Tuch, B.B.; Ebata, K.; Smith, S.; Lauriault, V.; Kolakowski, G.R.; Brandhuber, B.J.; et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. 2017, 7, 963–972. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-C.; Liang, S.-B.; Luo, M.; Wang, X.-P. Intratumoral heterogeneity and drug resistance in cancer. Cancer Cell Int. 2025, 25, 103. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.-J.; Jang, H. A New View of Pathway-Driven Drug Resistance in Tumor Proliferation. Trends Pharmacol. Sci. 2017, 38, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Santorsola, M. Revolutionizing KRAS p.G12C therapy in metastatic colorectal cancer: The triumph of dual inhibition. Med 2023, 4, 857–859. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Bradbury, I.; Eidtmann, H.; Di Cosimo, S.; de Azambuja, E.; Aura, C.; Gómez, H.; Dinh, P.; Fauria, K.; Van Dooren, V.; et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet 2012, 379, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.D.; Hegg, R.; Im, S.-A.; Park, I.H.; Burdaeva, O.; Kurteva, G.; Press, M.F.; Tjulandin, S.; Iwata, H.; Simon, S.D.; et al. Phase III, Randomized Study of Dual Human Epidermal Growth Factor Receptor 2 (HER2) Blockade With Lapatinib Plus Trastuzumab in Combination With an Aromatase Inhibitor in Postmenopausal Women With HER2-Positive, Hormone Receptor–Positive Metastatic Breast Cancer: Updated Results of ALTERNATIVE. J. Clin. Oncol. 2021, 39, 79–89. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Sharma, S.V.; Lee, D.Y.; Li, B.; Quinlan, M.P.; Takahashi, F.; Maheswaran, S.; McDermott, U.; Azizian, N.; Zou, L.; Fischbach, M.A.; et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 2010, 141, 69–80. [Google Scholar] [CrossRef]
- Dhanyamraju, P.K.; Schell, T.D.; Amin, S.; Robertson, G.P. Drug-Tolerant Persister Cells in Cancer Therapy Resistance. Cancer Res. 2022, 82, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Ianniello, M.; Santorsola, M.; Ruggiero, R.; Sirica, R.; Sabbatino, F.; Perri, F.; Cascella, M.; Di Marzo, M.; Berretta, M.; et al. From Chaos to Opportunity: Decoding Cancer Heterogeneity for Enhanced Treatment Strategies. Biology 2023, 12, 1183. [Google Scholar] [CrossRef]
- Abbosh, C.; Birkbak, N.J.; Swanton, C. Early stage NSCLC—Challenges to implementing ctDNA-based screening and MRD detection. Nat. Rev. Clin. Oncol. 2018, 15, 577–586. [Google Scholar] [CrossRef]
- Mauri, G.; Vitiello, P.P.; Sogari, A.; Crisafulli, G.; Sartore-Bianchi, A.; Marsoni, S.; Siena, S.; Bardelli, A. Liquid biopsies to monitor and direct cancer treatment in colorectal cancer. Br. J. Cancer 2022, 127, 394–407. [Google Scholar] [CrossRef]
- De Luca, G.; Dono, M. The Opportunities and Challenges of Molecular Tagging Next-Generation Sequencing in Liquid Biopsy. Mol. Diagn. Ther. 2021, 25, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Ginghina, O.; Hudita, A.; Zamfir, M.; Spanu, A.; Mardare, M.; Bondoc, I.; Buburuzan, L.; Georgescu, S.E.; Costache, M.; Negrei, C.; et al. Liquid Biopsy and Artificial Intelligence as Tools to Detect Signatures of Colorectal Malignancies: A Modern Approach in Patient’s Stratification. Front. Oncol. 2022, 12, 856575. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Fagery, M.; Khorshidi, H.A.; Wong, S.Q.; Vu, M.; Ijzerman, M. Health Economic Evidence and Modeling Challenges for Liquid Biopsy Assays in Cancer Management: A Systematic Literature Review. Pharmacoeconomics 2023, 41, 1229–1248. [Google Scholar] [CrossRef]
- Siravegna, G.; Mussolin, B.; Venesio, T.; Marsoni, S.; Seoane, J.; Dive, C.; Papadopoulos, N.; Kopetz, S.; Corcoran, R.; Siu, L.; et al. How liquid biopsies can change clinical practice in oncology. Ann. Oncol. 2019, 30, 1580–1590. [Google Scholar] [CrossRef]
- Liu, X.; Peng, T.; Xu, M.; Lin, S.; Hu, B.; Chu, T.; Liu, B.; Xu, Y.; Ding, W.; Li, L.; et al. Spatial multi-omics: Deciphering technological landscape of integration of multi-omics and its applications. J. Hematol. Oncol. 2024, 17, 72. [Google Scholar] [CrossRef]
- Gouda, M.A.; Huang, H.J.; Piha-Paul, S.A.; Call, S.G.; Karp, D.D.; Fu, S.; Naing, A.; Subbiah, V.; Pant, S.; Dustin, D.J.; et al. Longitudinal Monitoring of Circulating Tumor DNA to Predict Treatment Outcomes in Advanced Cancers. JCO Precis. Oncol. 2022, 6, e2100512. [Google Scholar] [CrossRef]
- Wu, P.; Gao, W.; Su, M.; Nice, E.C.; Zhang, W.; Lin, J.; Xie, N. Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 641469. [Google Scholar] [CrossRef] [PubMed]
- Langsten, K.L.; Kim, J.H.; Sarver, A.L.; Dewhirst, M.; Modiano, J.F. Comparative Approach to the Temporo-Spatial Organization of the Tumor Microenvironment. Front. Oncol. 2019, 9, 1185. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, I.; Ačkar, L.; Mossahebi Mohammadi, P.; Pantel, K. Liquid biopsies: Potential and challenges. Int. J. Cancer 2021, 148, 528–545. [Google Scholar] [CrossRef]
- Walsh, L.A.; Quail, D.F. Decoding the tumor microenvironment with spatial technologies. Nat. Immunol. 2023, 24, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.E.; Forder, A.; Marshall, E.A.; Vucic, E.A.; Stewart, G.L.; Noureddine, K.; Lockwood, W.W.; MacAulay, C.E.; Guillaud, M.; Lam, W.L. Delineating spatial cell-cell interactions in the solid tumour microenvironment through the lens of highly multiplexed imaging. Front. Immunol. 2023, 14, 1275890. [Google Scholar] [CrossRef] [PubMed]
- Roerden, M.; Spranger, S. Cancer immune evasion, immunoediting and intratumour heterogeneity. Nat. Rev. Immunol. 2025, 25, 353–369. [Google Scholar] [CrossRef]
- Chen, J.; Larsson, L.; Swarbrick, A.; Lundeberg, J. Spatial landscapes of cancers: Insights and opportunities. Nat. Rev. Clin. Oncol. 2024, 21, 660–674. [Google Scholar] [CrossRef]
- Gao, S.; Fang, A.; Huang, Y.; Giunchiglia, V.; Noori, A.; Schwarz, J.R.; Ektefaie, Y.; Kondic, J.; Zitnik, M. Empowering biomedical discovery with AI agents. Cell 2024, 187, 6125–6151. [Google Scholar] [CrossRef]
- Torrisi, M.; Pollastri, G.; Le, Q. Deep learning methods in protein structure prediction. Comput. Struct. Biotechnol. J. 2020, 18, 1301–1310. [Google Scholar] [CrossRef]
- Zhou, G.; Rusnac, D.-V.; Park, H.; Canzani, D.; Nguyen, H.M.; Stewart, L.; Bush, M.F.; Nguyen, P.T.; Wulff, H.; Yarov-Yarovoy, V.; et al. An artificial intelligence accelerated virtual screening platform for drug discovery. Nat. Commun. 2024, 15, 7761. [Google Scholar] [CrossRef]
- Rubio-Perez, C.; Tamborero, D.; Schroeder, M.P.; Antolín, A.A.; Deu-Pons, J.; Perez-Llamas, C.; Mestres, J.; Gonzalez-Perez, A.; Lopez-Bigas, N. In silico prescription of anticancer drugs to cohorts of 28 tumor types reveals targeting opportunities. Cancer Cell 2015, 27, 382–396. [Google Scholar] [CrossRef]
- Ning, Z.; Yang, B.; Wang, Y.; Shi, Z.; Yu, J.; Wu, G. Dual-path neural network extracts tumor microenvironment information from whole slide images to predict molecular typing and prognosis of Glioma. Comput. Methods Programs Biomed. 2025, 261, 108580. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, M.; Shi, D.; Qin, H.; Zhang, Y.; Liu, Z.; Madabhushi, A.; Gao, P.; Cong, F.; Lu, C. When multiple instance learning meets foundation models: Advancing histological whole slide image analysis. Med. Image Anal. 2025, 101, 103456. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Daylan, A.E.C.; Shevkoplias, A.; Postovalova, E.; Wang, M.; Tyshevich, A.; Lee, M.; Narvel, H.; Zornikova, K.; Shin, N.; et al. Transcriptomic Profiling and Tumor Microenvironment Classification Reveal Unique and Dynamic Immune Biology in HIV-Associated Kaposi Sarcoma. Cells 2025, 14, 134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeira, R.; Anavy, L.; Yakhini, Z.; Rivlin, E.; Freedman, D.; Naxerova, K. HIPI: Spatially resolved multiplexed protein expression inferred from H&E WSIs. PLoS Comput. Biol. 2024, 20, e1012501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grundberg, I.; Kiflemariam, S.; Mignardi, M.; Imgenberg-Kreuz, J.; Edlund, K.; Micke, P.; Sundström, M.; Sjöblom, T.; Botling, J.; Nilsson, M. In situ mutation detection and visualization of intratumor heterogeneity for cancer research and diagnostics. Oncotarget 2013, 4, 2407–2418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Casasent, A.K.; Schalck, A.; Gao, R.; Sei, E.; Long, A.; Pangburn, W.; Casasent, T.; Meric-Bernstam, F.; Edgerton, M.E.; Navin, N.E. Multiclonal Invasion in Breast Tumors Identified by Topographic Single Cell Sequencing. Cell 2018, 172, 205–217.e12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Chiang, Z.D.; Morriss, J.W.; LaFave, L.M.; Murray, E.M.; Del Priore, I.; Meli, K.; Lareau, C.A.; Nadaf, N.M.; Li, J.; et al. Spatial genomics enables multi-modal study of clonal heterogeneity in tissues. Nature 2022, 601, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Barefoot, M.E.; Loyfer, N.; Kiliti, A.J.; McDeed, A.P., 4th; Kaplan, T.; Wellstein, A. Detection of Cell Types Contributing to Cancer From Circulating, Cell-Free Methylated DNA. Front. Genet. 2021, 12, 671057. [Google Scholar] [CrossRef]
- Helzer, K.T.; Sharifi, M.N.; Sperger, J.M.; Shi, Y.; Annala, M.; Bootsma, M.L.; Reese, S.R.; Taylor, A.; Kaufmann, K.R.; Krause, H.K.; et al. Fragmentomic analysis of circulating tumor DNA-targeted cancer panels. Ann. Oncol. 2023, 34, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hong, S.-P.; Lee, S.; Lee, W.; Lee, D.; Kim, R.; Park, Y.J.; Moon, S.; Park, K.; Cha, B.; et al. Multidimensional fragmentomic profiling of cell-free DNA released from patient-derived organoids. Hum. Genom. 2023, 17, 96. [Google Scholar] [CrossRef]
- Xie, F.; Zhou, X.; Li, H.; Su, P.; Liu, S.; Li, R.; Zou, J.; Wei, X.; Pan, C.; Zhang, Z.; et al. USP8 promotes cancer progression and extracellular vesicle-mediated CD8+ T cell exhaustion by deubiquitinating the TGF-β receptor TβRII. EMBO J. 2022, 41, e108791. [Google Scholar] [CrossRef]
- Xie, F.; Zhou, X.; Su, P.; Li, H.; Tu, Y.; Du, J.; Pan, C.; Wei, X.; Zheng, M.; Jin, K.; et al. Breast cancer cell-derived extracellular vesicles promote CD8+ T cell exhaustion via TGF-β type II receptor signaling. Nat. Commun. 2022, 13, 4461. [Google Scholar] [CrossRef]
- O’NEil, N.J.; Bailey, M.L.; Hieter, P. Synthetic lethality and cancer. Nat. Rev. Genet. 2017, 18, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Lucchesi, J.C. Synthetic lethality and semi-lethality among functionally related mutants of Drosophila melanfgaster. Genetics 1968, 59, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T. The underlying mechanism for the PARP and BRCA synthetic lethality: Clearing up the misunderstandings. Mol. Oncol. 2011, 5, 387–393. [Google Scholar] [CrossRef]
- Cong, K.; Peng, M.; Kousholt, A.N.; Lee, W.T.C.; Lee, S.; Nayak, S.; Krais, J.; VanderVere-Carozza, P.S.; Pawelczak, K.S.; Calvo, J.; et al. Replication gaps are a key determinant of PARP inhibitor synthetic lethality with BRCA deficiency. Mol. Cell. 2021, 81, 3128–3144.e7. [Google Scholar] [CrossRef]
- Juric, D.; Castel, P.; Griffith, M.; Griffith, O.L.; Won, H.H.; Ellis, H.; Ebbesen, S.H.; Ainscough, B.J.; Ramu, A.; Iyer, G.; et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 2015, 518, 240–244. [Google Scholar] [CrossRef]
- Emadi, A.; Jun, S.A.; Tsukamoto, T.; Fathi, A.T.; Minden, M.D.; Dang, C.V. Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations. Exp. Hematol. 2014, 42, 247–251. [Google Scholar] [CrossRef]
- Bitler, B.G.; Aird, K.M.; Garipov, A.; Li, H.; Amatangelo, M.; Kossenkov, A.V.; Schultz, D.C.; Liu, Q.; Shih, I.-M.; Conejo-Garcia, J.; et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat. Med. 2015, 21, 231–238. [Google Scholar] [CrossRef]
- Dreier, M.R.; Walia, J.; de la Serna, I.L. Targeting SWI/SNF Complexes in Cancer: Pharmacological Approaches and Implications. Epigenomes 2024, 8, 7. [Google Scholar] [CrossRef]
- Ravichandran, M.; Maddalo, D. Applications of CRISPR-Cas9 for advancing precision medicine in oncology: From target discovery to disease modeling. Front. Genet. 2023, 14, 1273994. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Tang, S.; Gökbağ, B.; Cheng, L.; Li, L. Multi-view graph convolutional network for cancer cell-specific synthetic lethality prediction. Front. Genet. 2023, 13, 1103092. [Google Scholar] [CrossRef]
- Ma, J.; Yu, M.K.; Fong, S.; Ono, K.; Sage, E.; Demchak, B.; Sharan, R.; Ideker, T. Using deep learning to model the hierarchical structure and function of a cell. Nat. Methods 2018, 15, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Nerella, S.; Bandyopadhyay, S.; Zhang, J.; Contreras, M.; Siegel, S.; Bumin, A.; Silva, B.; Sena, J.; Shickel, B.; Bihorac, A.; et al. Transformers and large language models in healthcare: A review. Artif. Intell. Med. 2024, 154, 102900. [Google Scholar] [CrossRef] [PubMed]
- Issa, N.T.; Stathias, V.; Schürer, S.; Dakshanamurthy, S. Machine and deep learning approaches for cancer drug repurposing. Semin. Cancer Biol. 2021, 68, 132–142. [Google Scholar] [CrossRef]
- Tanoli, Z.; Vähä-Koskela, M.; Aittokallio, T. Artificial intelligence, machine learning, and drug repurposing in cancer. Expert Opin. Drug Discov. 2021, 16, 977–989. [Google Scholar] [CrossRef]
- Parrales, A.; Thoenen, E.; Iwakuma, T. The interplay between mutant p53 and the mevalonate pathway. Cell Death Differ. 2018, 25, 460–470. [Google Scholar] [CrossRef]
- Wang, Y.; An, H.; Liu, T.; Qin, C.; Sesaki, H.; Guo, S.; Radovick, S.; Hussain, M.; Maheshwari, A.; Wondisford, F.E.; et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019, 29, 1511–1523.e5. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Kasa, P.; Dariya, B.; Surepalli, N.; Peela, S.; Ahmad, S. Epigenetics and therapeutic targets in gastrointestinal malignancies. Drug Discov. Today 2021, 26, 2303–2314. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Y.-H.; Xu, H.; Yuan, C.-S.; Zhou, H.-H.; Huang, W.-H.; Wang, H.; Zhang, W. LncRNA linc00312 suppresses radiotherapy resistance by targeting DNA-PKcs and impairing DNA damage repair in nasopharyngeal carcinoma. Cell Death Dis. 2021, 12, 69. [Google Scholar] [CrossRef]
- Yu, R.; Hu, Y.; Zhang, S.; Li, X.; Tang, M.; Yang, M.; Wu, X.; Li, Z.; Liao, X.; Xu, Y.; et al. LncRNA CTBP1-DT-encoded microprotein DDUP sustains DNA damage response signalling to trigger dual DNA repair mechanisms. Nucleic Acids Res. 2022, 50, 8060–8079. [Google Scholar] [CrossRef]
- Pun, F.W.; Ozerov, I.V.; Zhavoronkov, A. AI-powered therapeutic target discovery. Trends Pharmacol. Sci. 2023, 44, 561–572. [Google Scholar] [CrossRef]
- Fountzilas, E.; Tsimberidou, A.M.; Vo, H.H.; Kurzrock, R. Clinical trial design in the era of precision medicine. Genome Med. 2022, 14, 101. [Google Scholar] [CrossRef]
- Pallmann, P.; Bedding, A.W.; Choodari-Oskooei, B.; Dimairo, M.; Flight, L.; Hampson, L.V.; Holmes, J.; Mander, A.P.; Odondi, L.; Sydes, M.R.; et al. Adaptive designs in clinical trials: Why use them, and how to run and report them. BMC Med. 2018, 16, 29. [Google Scholar] [CrossRef]
- Eckardt, J.-N.; Wendt, K.; Bornhäuser, M.; Middeke, J.M. Reinforcement Learning for Precision Oncology. Cancers 2021, 13, 4624. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, X.; Zuo, F.; Shi, H.; Jing, J. Artificial intelligence-based multi-omics analysis fuels cancer precision medicine. Semin. Cancer Biol. 2023, 88, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Sun, X.; Li, Y.; Chu, T.; Hao, X.; Cao, Y.; Zhang, P. Applications of Artificial Intelligence in Drug Repurposing. Adv. Sci. 2025, 12, e2411325. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Cancer represents the phenotypic outcome of a multitude of mutations that disrupt intracellular signaling pathways. These molecular pathways are inherently redundant and highly interconnected. Several intracellular nodes (depicted as black spheres) function as hubs for signal communication, integration, regulation, amplification, or attenuation. The flow of these signals, ultimately transmitted to the nucleus, is represented by black lines. The mutated and hyperactivated signaling cascades are highlighted in red lines and depicted with increased line thickness. The stops in red represent potentially druggable pathways. It is within the nucleus that the cell modulates gene expression programs encoding proteins responsible for key cellular behaviors such as proliferation, migration, and invasion (three hallmark capabilities that are hyperactivated and deregulated in malignant cells). Panel (A) illustrates a simplified view of a signaling pathway downstream of three surface receptors. Due to the genetic and evolutionary plasticity of neoplastic cells, inhibition of a single pathway often results in the scenario depicted in Panel (B), where not only alternative signaling routes become activated, but also a selective advantage is conferred to subclones already capable of exploiting such compensatory mechanisms. As a consequence, the tumor mass evolves towards a composition enriched in cells that sustain proliferation and metastatic dissemination through these alternative pathways. However, the scenario illustrated in Panel (C) is more realistic: the malignant phenotype arises from multiple coexisting alterations that require comprehensive identification and simultaneous inhibition. In all cases, the volume and complexity of biological information to be processed is substantial (including signaling cascades, regulatory nodes, feedback mechanisms, the dynamic evolution of molecular alterations over time, etc.). For this reason, the integration of artificial intelligence with multi-targeted pathway inhibition represents a promising and necessary therapeutic frontier.
Figure 1.
Cancer represents the phenotypic outcome of a multitude of mutations that disrupt intracellular signaling pathways. These molecular pathways are inherently redundant and highly interconnected. Several intracellular nodes (depicted as black spheres) function as hubs for signal communication, integration, regulation, amplification, or attenuation. The flow of these signals, ultimately transmitted to the nucleus, is represented by black lines. The mutated and hyperactivated signaling cascades are highlighted in red lines and depicted with increased line thickness. The stops in red represent potentially druggable pathways. It is within the nucleus that the cell modulates gene expression programs encoding proteins responsible for key cellular behaviors such as proliferation, migration, and invasion (three hallmark capabilities that are hyperactivated and deregulated in malignant cells). Panel (A) illustrates a simplified view of a signaling pathway downstream of three surface receptors. Due to the genetic and evolutionary plasticity of neoplastic cells, inhibition of a single pathway often results in the scenario depicted in Panel (B), where not only alternative signaling routes become activated, but also a selective advantage is conferred to subclones already capable of exploiting such compensatory mechanisms. As a consequence, the tumor mass evolves towards a composition enriched in cells that sustain proliferation and metastatic dissemination through these alternative pathways. However, the scenario illustrated in Panel (C) is more realistic: the malignant phenotype arises from multiple coexisting alterations that require comprehensive identification and simultaneous inhibition. In all cases, the volume and complexity of biological information to be processed is substantial (including signaling cascades, regulatory nodes, feedback mechanisms, the dynamic evolution of molecular alterations over time, etc.). For this reason, the integration of artificial intelligence with multi-targeted pathway inhibition represents a promising and necessary therapeutic frontier.
![Ijms 26 07723 g001 Ijms 26 07723 g001]()
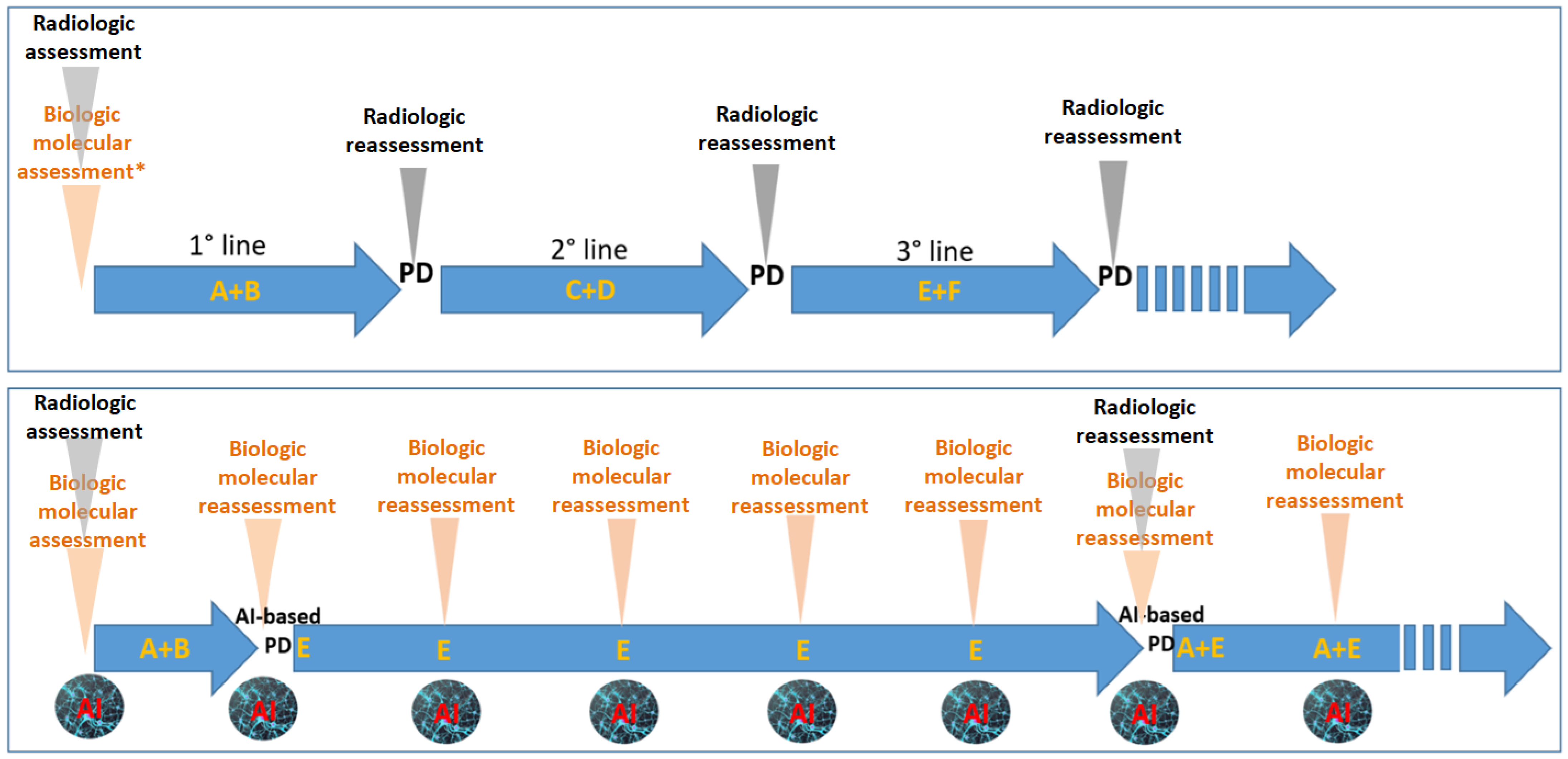
Figure 2.
The figure highlights the differences between a standard oncologic treatment strategy (top panel) and a potential AI-driven innovative approach (bottom panel). In the conventional model, therapies are administered sequentially in predefined lines (e.g., first-line, second-line, etc.) based on non-cross-resistant combinations. These decisions are primarily guided by periodic radiological evaluations. Genomic profiling—typically performed on the primary tumor—plays a pivotal role in initial treatment planning (e.g., assessment of RAS/BRAF or MSI status in colorectal cancer), but subsequent treatment choices are predominantly informed by imaging. The bottom panel illustrates a theoretical AI-integrated strategy. Here, periodic genomic reassessments capture the dynamic evolution of the tumor—whether spontaneous or therapy-induced. In this model, AI-based interpretation of serial molecular data could detect the emergence of resistance to drug combination A+B, prompting the early initiation of drug E, even before radiological progression is apparent. Further AI-guided evaluations suggest continuation of E until a subsequent reassessment supports rechallenge with A in combination with E. This conceptual framework envisions a reduced reliance on imaging, favoring biologic/molecular assessments (including circulating tumor DNA [ctDNA] genomic data, digital pathology features, and proteomic profiles). Such an approach may better accommodate the inherent complexity and heterogeneity of cancer biology. A, B, C, D, E, F: different anticancer agents; AI: artificial intelligence; PD: progressive disease.
Figure 2.
The figure highlights the differences between a standard oncologic treatment strategy (top panel) and a potential AI-driven innovative approach (bottom panel). In the conventional model, therapies are administered sequentially in predefined lines (e.g., first-line, second-line, etc.) based on non-cross-resistant combinations. These decisions are primarily guided by periodic radiological evaluations. Genomic profiling—typically performed on the primary tumor—plays a pivotal role in initial treatment planning (e.g., assessment of RAS/BRAF or MSI status in colorectal cancer), but subsequent treatment choices are predominantly informed by imaging. The bottom panel illustrates a theoretical AI-integrated strategy. Here, periodic genomic reassessments capture the dynamic evolution of the tumor—whether spontaneous or therapy-induced. In this model, AI-based interpretation of serial molecular data could detect the emergence of resistance to drug combination A+B, prompting the early initiation of drug E, even before radiological progression is apparent. Further AI-guided evaluations suggest continuation of E until a subsequent reassessment supports rechallenge with A in combination with E. This conceptual framework envisions a reduced reliance on imaging, favoring biologic/molecular assessments (including circulating tumor DNA [ctDNA] genomic data, digital pathology features, and proteomic profiles). Such an approach may better accommodate the inherent complexity and heterogeneity of cancer biology. A, B, C, D, E, F: different anticancer agents; AI: artificial intelligence; PD: progressive disease.
![Ijms 26 07723 g002 Ijms 26 07723 g002]()
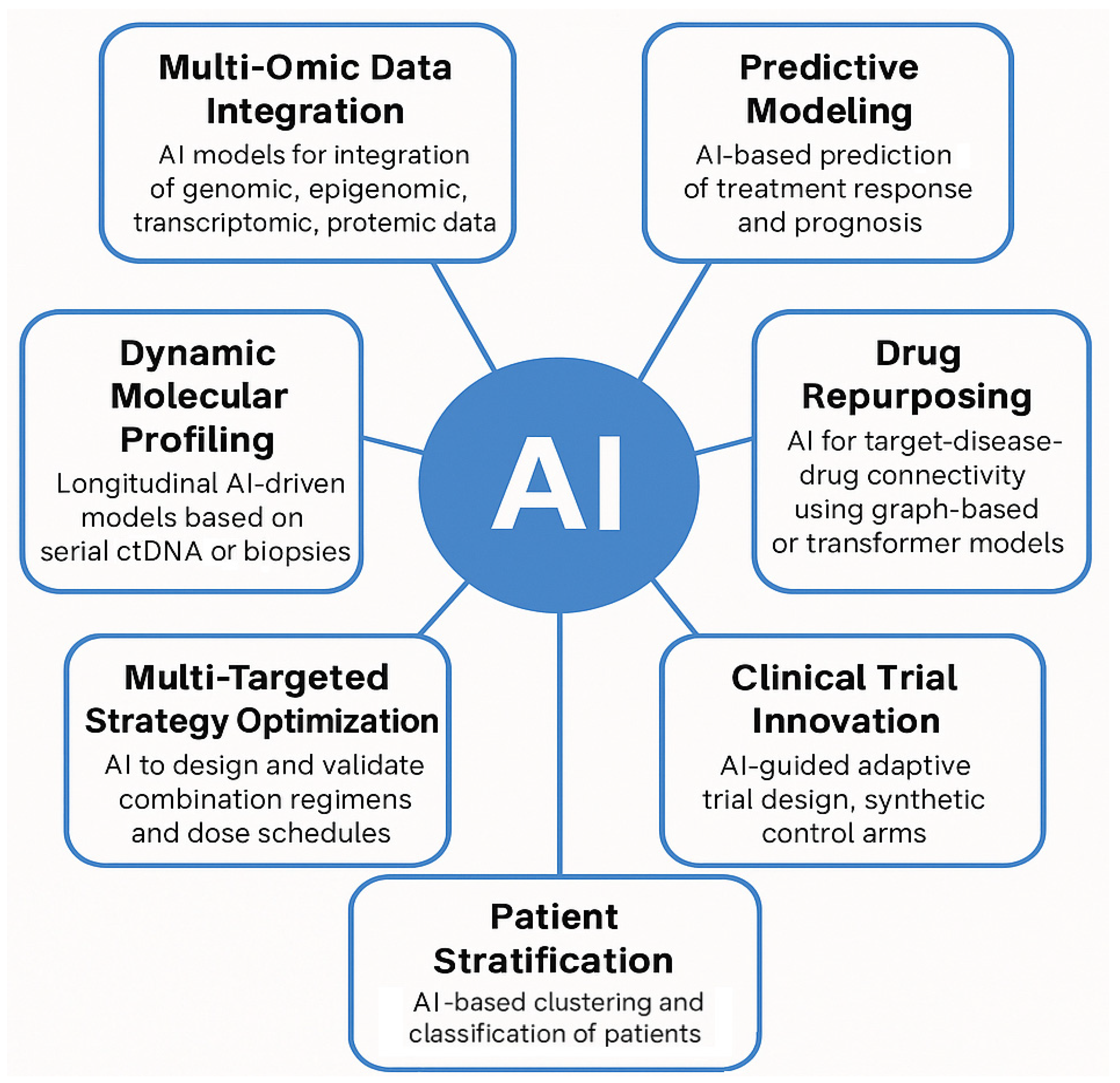
Figure 3.
This figure proposes an integrated conceptual framework for how artificial intelligence (AI) can be deployed to confront the multidimensional complexity of cancer in the post-genomic era. The framework is organized into seven functional domains, each addressing a specific layer of oncological complexity (from molecular integration to therapeutic optimization and clinical innovation). Importantly, Multi-Omic Data Integration and Dynamic Molecular Profiling represent critical input layers of the model, serving as individualized and longitudinal data sources that feed the AI-driven architecture. These inputs enable downstream modules to perform predictive modeling, therapeutic reasoning (e.g., drug repurposing and multi-targeted combination design), patient stratification, and adaptive trial structuring. The distinction between input domains and actionable output domains reflects a conceptual workflow, where AI not only processes vast datasets but actively generates clinically actionable insights, reinforcing its role as a therapeutic enabler rather than a passive analytical tool.
Figure 3.
This figure proposes an integrated conceptual framework for how artificial intelligence (AI) can be deployed to confront the multidimensional complexity of cancer in the post-genomic era. The framework is organized into seven functional domains, each addressing a specific layer of oncological complexity (from molecular integration to therapeutic optimization and clinical innovation). Importantly, Multi-Omic Data Integration and Dynamic Molecular Profiling represent critical input layers of the model, serving as individualized and longitudinal data sources that feed the AI-driven architecture. These inputs enable downstream modules to perform predictive modeling, therapeutic reasoning (e.g., drug repurposing and multi-targeted combination design), patient stratification, and adaptive trial structuring. The distinction between input domains and actionable output domains reflects a conceptual workflow, where AI not only processes vast datasets but actively generates clinically actionable insights, reinforcing its role as a therapeutic enabler rather than a passive analytical tool.
![Ijms 26 07723 g003 Ijms 26 07723 g003]()
Table 1.
Improved clinical responses with combination targeted therapies across selected solid tumors.
Table 1.
Improved clinical responses with combination targeted therapies across selected solid tumors.
| Cancer Type | Monotherapy | Outcome with
Monotherapy | Combination Therapy | Outcome with
Combination Therapy |
|---|
Metastatic colorectal
cancer (KRAS G12C+) | Sotorasib (KRAS G12C
inhibitor) | ORR: 0% | Sotorasib + Panitumumab (anti-EGFR mAb) | ORR: 26.4% |
HER2-positive breast
cancer (neoadjuvant) | Trastuzumab (anti-HER2 mAb) | pCR: 29.5% | Trastuzumab + Lapatinib (HER2 TKI) | pCR: 51.3% |
| HR+/HER2+ metastatic breast cancer | Trastuzumab + endocrine therapy | ORR: 13.7% | Trastuzumab + Lapatinib + Aromatase inhibitor | ORR: 31.7% |
BRAF V600-mutant
metastatic melanoma | Vemurafenib (BRAF
inhibitor) | ORR: 45% | Vemurafenib + Cobimetinib
(MEK inhibitor) | ORR: 68% |
Table 2.
Selected non-oncologic drugs with potential for repurposing in cancer therapy.
Table 2.
Selected non-oncologic drugs with potential for repurposing in cancer therapy.
| Repurposed Drug | Approved
Indication | Cancer Types
Showing Response | Known Anti-Tumor Mechanism | Rational Selection of
Cancer Patients |
|---|
| Metformin | Type II Diabetes | Breast, prostate, pancreatic, NSCLC | AMPK activation; inhibition of mitochondrial complex I; metabolic stress | Tumors with LKB1 deficiency or mtDNA mutations |
| Disulfiram | Alcohol dependence (aversion therapy) | Breast, glioblastoma, prostate, melanoma | Inhibition of ALDH; ROS accumulation; proteasome inhibition via Cu2+-complex | Tumors with high ALDH expression or low antioxidant
defense |
| Propranolol | Hypertension,
arrhythmias, migraine prophylaxis | Angiosarcoma, breast cancer, melanoma | Non-selective β-blockade; anti-angiogenic;
suppression of β-adrenergic signaling | Tumors expressing β-adrenergic receptors; highly vascular tumors |
| Itraconazole | Fungal infections | Basal cell carcinoma, prostate, NSCLC | Hedgehog pathway inhibition; anti-angiogenic; mTOR and P-gp inhibition | Tumors with Hedgehog
pathway activation; high
angiogenic profile |
Chloroquine/
Hydroxychloroquine | Malaria, autoimmune diseases | Glioblastoma,
pancreatic, breast | Autophagy inhibition;
lysosomal destabilization; immune modulation | RAS-mutant, hypoxic tumors, or autophagy-addicted cancers |
Statins (e.g.,
Simvastatin) | Hypercholesterolemia | Breast, ovarian,
colorectal, prostate | Inhibition of HMG-CoA
reductase; disruption of prenylation (e.g., RAS); apoptosis induction | Tumors with RAS activation, mevalonate pathway
dependency |
| Valproic Acid (VPA) | Epilepsy, bipolar
disorder | Glioblastoma, breast, prostate, myeloid
malignancies | HDAC inhibition;
chromatin remodeling;
re-expression of silenced
tumor suppressor genes | Tumors with epigenetic
silencing (e.g., low histone acetylation, hypermethylation); tumors with low expression of immune-regulatory genes |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).