Nanoparticles as an Encouraging Therapeutic Approach to Alzheimer’s Disease
Abstract
1. Introduction
2. Alzheimer’s Disease—Basic Facts
3. Nanoparticles
3.1. The Basics of NPs and Nanotechnology
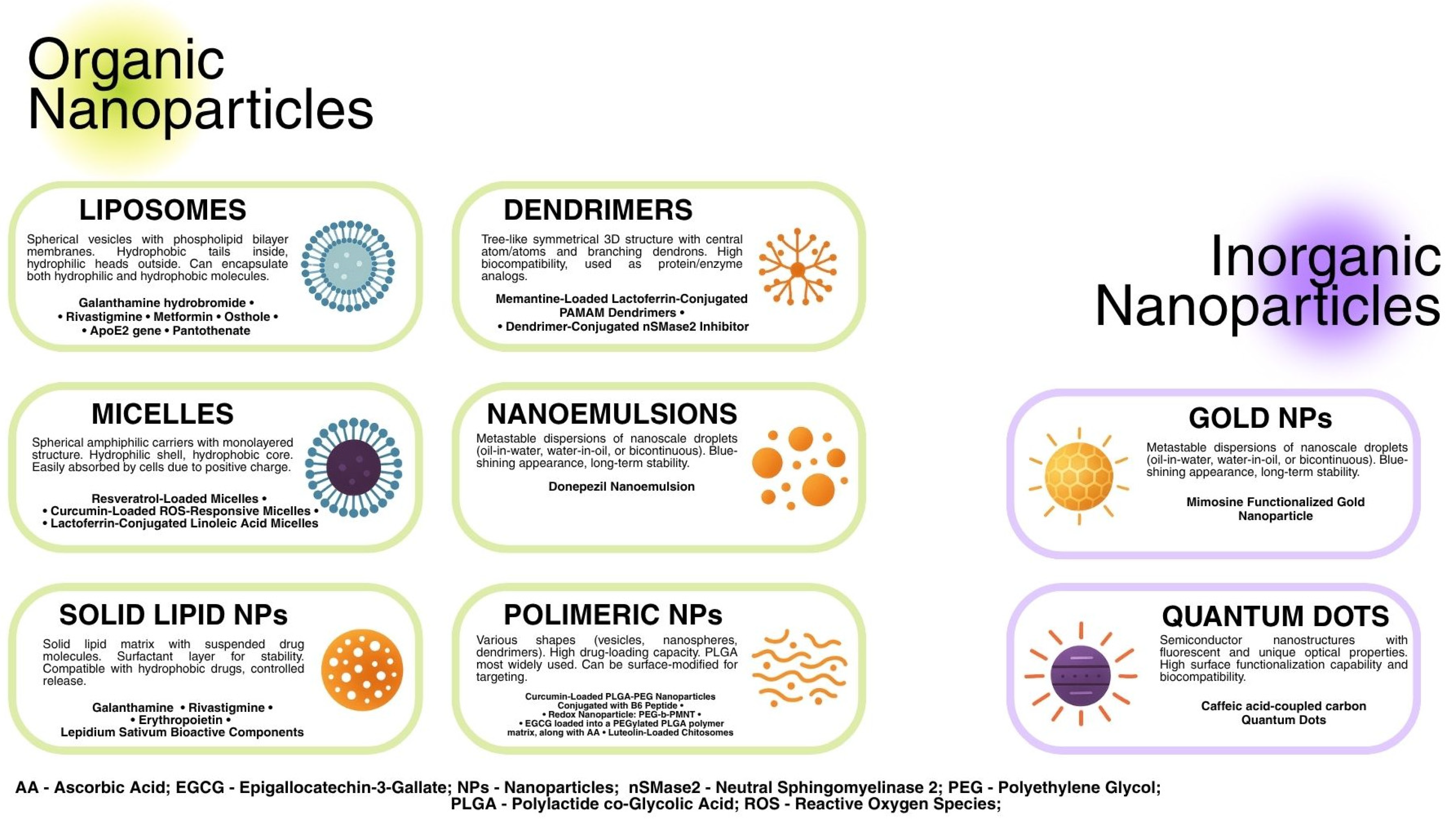
3.2. The Classification of NPs
3.2.1. Liposomes
3.2.2. Micelles
3.2.3. Solid Lipid NPs
3.2.4. Polymeric NPs
3.2.5. Dendrimers
3.2.6. Nanoemulsions
3.2.7. Inorganic NPs
3.3. Importance and Possibilities in AD Treatment
4. Nanotechnology-Based Drug Delivery Systems Through BBB in AD
5. Possible Use of NPs in Vascular Pathology
5.1. Structural Changes in Vessels in AD
5.2. Monoclonal Antibody IgG4.1 Conjugated to Iron Oxide Nanoparticles Monodisperse γ-Fe2O3 Nanoparticles (MIONs) as Potential Treatment for CAA
5.3. Common Risk Factors for AD and Cardiovascular Disorders
5.4. Role of Endothelin-1 and Angiotensin System as the Potential Therapeutic Targets
5.5. Natural Substances Use in AD Therapy
6. Neuroprotective and Anti-Inflammatory Effects of Nanoparticles
6.1. Liposomes
6.1.1. Currently Approved AD Therapeutics Encapsulated in Liposomes
6.1.2. Metformin Encapsulated in Phosphatidylserine-Based Liposomes
6.1.3. Transferrin-Modified Osthole PEGylated Liposomes
6.1.4. Surface-Modified Liposomes for ApoE2 Gene Delivery
6.1.5. Pantothenate-Encapsulated Liposomes
6.2. Micelles
6.2.1. Resveratrol-Loaded Neuronal Mitochondria-Targeted Micelles
6.2.2. Curcumin-Loaded ROS-Responsive Micelles
6.2.3. Lactoferrin-Conjugated Linoleic Acid Micelles
6.3. Solid Lipid Nanoparticles (SLN)
6.3.1. Currently Approved AD Therapeutics Encapsulated in SLNs
6.3.2. Erythropoietin-Loaded SLNs
6.3.3. SLNs Loaded with Lepidium sativum Seed Bioactive Components
6.4. Polymeric NPs (PNPs)
6.4.1. Curcumin-Loaded PLGA-PEG Nanoparticles Conjugated with B6 Peptide
6.4.2. Redox Nanoparticles
6.4.3. Epigallocatechin-3-Gallate (EGCG) Loaded into a PEGylated PLGA Polymer Matrix, Along with Ascorbic Acid (AA)
6.4.4. Luteolin-Loaded Chitosomes
6.5. Dendrimers
6.5.1. Memantine-Loaded Lactoferrin-Conjugated PAMAM Dendrimers
6.5.2. Dendrimer-Conjugated nSMase2 Inhibitor
6.6. Nanoemulsions
Donepezil Nanoemulsion
6.7. Inorganic NPs
6.7.1. Mimosine Functionalized Gold Nanoparticles
6.7.2. Quantum Dots—Caffeic Acid-Coupled Carbon Quantum Dots
7. NPs as Diagnostic Agents: Magnetic Nanoparticles for MRI-Guided Diagnosis in Alzheimer’s Disease
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Poudel, P.; Park, S. Recent Advances in the Treatment of Alzheimer’s Disease Using Nanoparticle-Based Drug Delivery Systems. Pharmaceutics 2022, 14, 835. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, D.; Le Droumaguet, B.; Nicolas, J.; Hashemi, S.H.; Wu, L.-P.; Moghimi, S.M.; Couvreur, P.; Andrieux, K. Nanotechnologies for Alzheimer’s Disease: Diagnosis, Therapy, and Safety Issues. Nanomedicine 2011, 7, 521–540. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s Disease Drug Development Pipeline: 2021. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12179. [Google Scholar] [CrossRef]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef]
- López-Espinosa, J.; Park, P.; Holcomb, M.; Godin, B.; Villapol, S. Nanotechnology-Driven Therapies for Neurodegenerative Diseases: A Comprehensive Review. Ther. Deliv. 2024, 15, 997–1024. [Google Scholar] [CrossRef]
- Shimizu, F.; Nakamori, M. Blood–Brain Barrier Disruption in Neuroimmunological Disease. Int. J. Mol. Sci. 2024, 25, 10625. [Google Scholar] [CrossRef]
- Alzheimer’s Association Report. 2024 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef]
- Kapasi, A.; Schneider, J.A. Vascular Contributions to Cognitive Impairment, Clinical Alzheimer’s Disease, and Dementia in Older Persons. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular Pathways to Neurodegeneration in Alzheimer’s Disease and Other Disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Castellani, R.; Hirai, K.; Aliev, G.; Drew, K.L.; Nunomura, A.; Takeda, A.; Cash, A.D.; Obrenovich, M.E.; Perry, G.; Smith, M.A. Role of Mitochondrial Dysfunction in Alzheimer’s Disease. J. Neurosci. Res. 2002, 70, 357–360. [Google Scholar] [CrossRef]
- Pszczołowska, M.; Walczak, K.; Miśków, W.; Antosz, K.; Batko, J.; Kurpas, D.; Leszek, J. Chronic Traumatic Encephalopathy as the Course of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 4639. [Google Scholar] [CrossRef]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum. Med. Clin. N. Am. 2019, 103, 263–293. [Google Scholar] [CrossRef]
- Harrison, J.E.; Weber, S.; Jakob, R.; Chute, C.G. ICD-11: An International Classification of Diseases for the Twenty-First Century. BMC Med. Inform. Decis. Mak. 2021, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Apostolova, L.G. Alzheimer Disease. CONTINUUM Lifelong Learn. Neurol. 2016, 22, 419–434. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Verma, M.L.; Prakash Patil, M.; Ramkumar Vijayan, S.; Altammar, K.A.; Batin, A.; Al Batin, H.; Arabia, S. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update Post COVID-19 Vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef]
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Catucci, L. Liposomes Containing Nanoparticles: Preparation and Applications. Colloids Surf. B Biointerfaces 2022, 218, 112737. [Google Scholar] [CrossRef]
- Vargas, K.M.; Shon, Y.S. Hybrid Lipid-Nanoparticle Complexes for Biomedical Applications. J. Mater. Chem. B 2019, 7, 695–708. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Basu, B.; Prajapati, B.; Dutta, A.; Paliwal, H.; Patel, S.S.K. Medical Application of Liposomes. J. Explor. Res. Pharmacol. 2024, 9, 13–22. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B.; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef] [PubMed]
- Maboudi, A.H.; Lotfipour, M.H.; Rasouli, M.; Azhdari, M.H.; MacLoughlin, R.; Bekeschus, S.; Doroudian, M. Micelle-Based Nanoparticles with Stimuli-Responsive Properties for Drug Delivery. Nanotechnol. Rev. 2024, 13, 20230218. [Google Scholar] [CrossRef]
- Zhao, J.; Stenzel, M.H. Entry of Nanoparticles into Cells: The Importance of Nanoparticle Properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric Micelles in Drug Delivery: An Insight of the Techniques for Their Characterization and Assessment in Biorelevant Conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Zhou, T.; Zhang, J.; Xia, H.; Li, H.; He, J.; He, S.; Wang, L. Positively Charged Micelles Based on a Triblock Copolymer Demonstrate Enhanced Corneal Penetration. Int. J. Nanomed. 2015, 10, 6027–6037. [Google Scholar] [CrossRef]
- Arias, J.L.; Barretta, R.; Nguyen, T.-T.-L.; Duong, V.-A. Solid Lipid Nanoparticles. Encyclopedia 2022, 2, 952–973. [Google Scholar] [CrossRef]
- Nguyen, T.T.L.; Duong, V.A.; Maeng, H.J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef]
- Tan, J.S.L.; Roberts, C.; Billa, N. Pharmacokinetics and Tissue Distribution of an Orally Administered Mucoadhesive Chitosan-Coated Amphotericin B-Loaded Nanostructured Lipid Carrier (NLC) in Rats. J. Biomater. Sci. Polym. Ed. 2020, 31, 141–154. [Google Scholar] [CrossRef]
- Misra, S.; Chopra, K.; Sinha, V.R.; Medhi, B. Galantamine-Loaded Solid-Lipid Nanoparticles for Enhanced Brain Delivery: Preparation, Characterization, in Vitro and in Vivo Evaluations. Drug Deliv. 2016, 23, 1434–1443. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Jangde, R.K. Current Updated Review on Preparation of Polymeric Nanoparticles for Drug Delivery and Biomedical Applications. Next Nanotechnol. 2023, 2, 100013. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Allahyari, M.; Mohit, E. Peptide/Protein Vaccine Delivery System Based on PLGA Particles. Hum. Vaccines Immunother. 2016, 12, 806–828. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-Based Drug Delivery Systems: History, Challenges, and Latest Developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.S.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A New Race of Pharmaceutical Nanocarriers. Biomed. Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Li, B.; Lu, D.; Di, W.; Ren, L.; Tian, L.; Zhang, P.; He, J.; Sun, D. Preparation of Polyoxypropylene Surfactant-Based Nanoemulsions Using Phase Inversion Composition Method and Their Application in Oil Recovery. J. Mol. Liq. 2021, 342, 117469. [Google Scholar] [CrossRef]
- Rigano, L.; Lionetti, N. Nanobiomaterials in Galenic Formulations and Cosmetics. In Nanobiomaterials in Galenic Formulations and Cosmetics: Applications of Nanobiomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 121–148. [Google Scholar] [CrossRef]
- Klinkova, A.; Thérien-Aubin, H. Inorganic Nanoparticles. In Nanochemistry; Elsevier: Amsterdam, The Netherlands, 2024; pp. 49–110. [Google Scholar]
- Paul, W.; Sharma, C.P. Inorganic Nanoparticles for Targeted Drug Delivery. In Biointegration of Medical Implant Materials; Elsevier: Amsterdam, The Netherlands, 2010; pp. 204–235. [Google Scholar]
- Martano, S.; De Matteis, V.; Cascione, M.; Rinaldi, R. Inorganic Nanomaterials versus Polymer-Based Nanoparticles for Overcoming Neurodegeneration. Nanomaterials 2022, 12, 2337. [Google Scholar] [CrossRef] [PubMed]
- Asad, S.; Jacobsen, A.C.; Teleki, A. Inorganic Nanoparticles for Oral Drug Delivery: Opportunities, Barriers, and Future Perspectives. Curr. Opin. Chem. Eng. 2022, 38, 100869. [Google Scholar] [CrossRef]
- Bhatti, R.; Shakeel, H.; Malik, K.; Qasim, M.; Khan, M.A.; Ahmed, N.; Jabeen, S. Inorganic Nanoparticles: Toxic Effects, Mechanisms of Cytotoxicity and Phytochemical Interactions. Adv. Pharm. Bull. 2022, 12, 757–762. [Google Scholar] [CrossRef]
- Modi, G.; Pillay, V.; Choonara, Y.E.; Ndesendo, V.M.K.; du Toit, L.C.; Naidoo, D. Nanotechnological Applications for the Treatment of Neurodegenerative Disorders. Prog. Neurobiol. 2009, 88, 272–285. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Y. Applications of Nanoparticles in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 96, 459–471. [Google Scholar] [CrossRef]
- Roney, C.; Kulkarni, P.; Arora, V.; Antich, P.; Bonte, F.; Wu, A.; Mallikarjuana, N.N.; Manohar, S.; Liang, H.-F.; Kulkarni, A.R.; et al. Targeted Nanoparticles for Drug Delivery through the Blood–Brain Barrier for Alzheimer’s Disease. J. Control. Release 2005, 108, 193–214. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Riaz, M.K.; Xie, Y.; Zhang, X.; Liu, Q.; Chen, H.; Bian, Z.; Chen, X.; Lu, A.; Yang, Z. Review of Current Strategies for Delivering Alzheimer’s Disease Drugs across the Blood-Brain Barrier. Int. J. Mol. Sci. 2019, 20, 381. [Google Scholar] [CrossRef]
- Zhong, X.; Na, Y.; Yin, S.; Yan, C.; Gu, J.; Zhang, N.; Geng, F. Cell Membrane Biomimetic Nanoparticles with Potential in Treatment of Alzheimer’s Disease. Molecules 2023, 28, 2336. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Lu, C.-T.; Zhao, Y.-Z.; Wong, H.L.; Cai, J.; Peng, L.; Tian, X.-Q. Current Approaches to Enhance CNS Delivery of Drugs across the Brain Barriers. Int. J. Nanomed. 2014, 9, 2241–2257. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Roghani, A.K.; Garcia, R.I.; Roghani, A.; Reddy, A.; Khemka, S.; Reddy, R.P.; Pattoor, V.; Jacob, M.; Reddy, P.H.; Sehar, U. Treating Alzheimer’s Disease Using Nanoparticle-Mediated Drug Delivery Strategies/Systems. Ageing Res. Rev. 2024, 97, 102291. [Google Scholar] [CrossRef]
- Bakrim, S.; Aboulaghras, S.; El Menyiy, N.; El Omari, N.; Assaggaf, H.; Lee, L.-H.; Montesano, D.; Gallo, M.; Zengin, G.; AlDhaheri, Y.; et al. Phytochemical Compounds and Nanoparticles as Phytochemical Delivery Systems for Alzheimer’s Disease Management. Molecules 2022, 27, 9043. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Yang, X.; Fu, M.; Zhai, G. Recent Progress of Drug Nanoformulations Targeting to Brain. J. Control. Release 2018, 291, 37–64. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured Lipid Carriers for Site-Specific Drug Delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C.; Mussivand, T. Can Disturbed Brain Microcirculation Cause Alzheimer’s Disease? Neurol. Res. 1993, 15, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, Y.; Isobe, Y.; Fukushima, K.; Kimura, M. Disassembly of Amyloid β-Protein Fibril by Basement Membrane Components. Life Sci. 2002, 70, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The Vascular Basement Membrane in the Healthy and Pathological Brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Zarow, C.; Barron, E.; Chui, H.C.; Perlmutter, L.S. Vascular Basement Membrane Pathology and Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 1997, 826, 147–160. [Google Scholar] [CrossRef]
- Fisher, R.A.; Miners, J.S.; Love, S. Pathological Changes within the Cerebral Vasculature in Alzheimer’s Disease: New Perspectives. Brain Pathol. 2022, 32, e13061. [Google Scholar] [CrossRef]
- Allen, N.; Robinson, A.C.; Snowden, J.; Davidson, Y.S.; Mann, D.M.A. Patterns of Cerebral Amyloid Angiopathy Define Histopathological Phenotypes in Alzheimer’s Disease. Neuropathol. Appl. Neurobiol. 2014, 40, 136–148. [Google Scholar] [CrossRef]
- Love, S.; Scott Miners, J. Small Vessel Disease, Neurovascular Regulation and Cognitive Impairment: Post-Mortem Studies Reveal a Complex Relationship, Still Poorly Understood. Clin. Sci. 2017, 131, 1579–1589. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Capuano, A.W.; Leurgans, S.E.; Bennett, D.A.; Schneider, J.A. Relation of Cerebral Vessel Disease to Alzheimer’s Disease Dementia and Cognitive Function in Elderly People: A Cross-Sectional Study. Lancet Neurol. 2016, 15, 934–943. [Google Scholar] [CrossRef]
- Poduslo, J.F.; Ramakrishnan, M.; Holasek, S.S.; Ramirez-Alvarado, M.; Kandimalla, K.K.; Gilles, E.J.; Curran, G.L.; Wengenack, T.M. In Vivo Targeting of Antibody Fragments to the Nervous System for Alzheimer’s Disease Immunotherapy and Molecular Imaging of Amyloid Plaques. J. Neurochem. 2007, 102, 1055–1065. [Google Scholar] [CrossRef]
- Ott, A.; Stolk, R.P.; Hofman, A.; Van Harskamp, F.; Grobbee, D.E.; Breteler, M.M.B. Association of Diabetes Mellitus and Dementia: The Rotterdam Study. Diabetologia 1996, 39, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, J.Q.; Tolstrup, J.S.; Benn, M.; Frikke-Schmidt, R. Type-2 Diabetes and Risk of Dementia: Observational and Mendelian Randomisation Studies in 1 Million Individuals. Epidemiol. Psychiatr. Sci. 2020, 29, e118. [Google Scholar] [CrossRef]
- Anstey, K.J.; Cherbuin, N.; Budge, M.; Young, J. Body Mass Index in Midlife and Late-Life as a Risk Factor for Dementia: A Meta-Analysis of Prospective Studies. Obes. Rev. 2011, 12, e426–e437. [Google Scholar] [CrossRef]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related Disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Tolppanen, A.M.; Ngandu, T.; Kåreholt, I.; Laatikainen, T.; Rusanen, M.; Soininen, H.; Kivipelto, M. Midlife and Late-Life Body Mass Index and Late-Life Dementia: Results from a Prospective Population-Based Cohort. J. Alzheimer’s Dis. 2014, 38, 201–209. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, R.F.A.G.; Ikram, M.A. Cardiovascular Risk Factors and Future Risk of Alzheimer’s Disease. BMC Med. 2014, 12, 130. [Google Scholar] [CrossRef]
- Silva, M.V.F.; Loures, C.D.M.G.; Alves, L.C.V.; De Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s Disease: Risk Factors and Potentially Protective Measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef]
- Nordestgaard, L.T.; Christoffersen, M.; Frikke-Schmidt, R. Shared Risk Factors between Dementia and Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 9777. [Google Scholar] [CrossRef]
- Korte, N.; Nortley, R.; Attwell, D. Cerebral Blood Flow Decrease as an Early Pathological Mechanism in Alzheimer’s Disease. Acta Neuropathol. 2020, 140, 793–810. [Google Scholar] [CrossRef]
- Ruitenberg, A.; Den Heijer, T.; Bakker, S.L.M.; Van Swieten, J.C.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M.B. Cerebral Hypoperfusion and Clinical Onset of Dementia: The Rotterdam Study. Ann. Neurol. 2005, 57, 789–794. [Google Scholar] [CrossRef]
- Wolters, F.J.; Zonneveld, H.I.; Hofman, A.; Van Der Lugt, A.; Koudstaal, P.J.; Vernooij, M.W.; Ikram, M.A. Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation 2017, 136, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Love, S.; Miners, J.S. Cerebrovascular Disease in Ageing and Alzheimer’s Disease. Acta Neuropathol. 2016, 131, 645–658. [Google Scholar] [CrossRef]
- Barker, R.; Ashby, E.L.; Wellington, D.; Barrow, V.M.; Palmer, J.C.; Kehoe, P.G.; Esiri, M.M.; Love, S. Pathophysiology of White Matter Perfusion in Alzheimer’s Disease and Vascular Dementia. Brain 2014, 137, 1524–1532. [Google Scholar] [CrossRef]
- Singh, M.; Prakash, A. Possible Role of Endothelin Receptor against Hyperhomocysteinemia and β-Amyloid Induced AD Type of Vascular Dementia in Rats. Brain Res. Bull. 2017, 133, 31–41. [Google Scholar] [CrossRef]
- Henrion, D.; Bonnin, P.; Vessieres, E.; Guihlot, A.L.; Iglarz, M.; Lévy, B.I. Endothelin Receptor Blockade Improves Cerebral Blood Flow-Mediated Dilation in a Mouse Model of Alzheimer’s Disease. J. Vasc. Res. 2023, 60, 1–12. [Google Scholar] [CrossRef]
- Krishnasailaja, A. Preparation & Characterization of Bosentan Loaded Ethyl Cellulose Nanoparticles by Solvent Evaporation Technique. Nanomed. Nanotechnol. Open Access 2018, 3, 141. [Google Scholar] [CrossRef]
- Giménez, V.M.; Sperandeo, N.; Faudone, S.; Noriega, S.; Manucha, W.; Kassuha, D. Preparation and Characterization of Bosentan Monohydrate/ε-Polycaprolactone Nanoparticles Obtained by Electrospraying. Biotechnol. Prog. 2019, 35, e2748. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, E.; Motaghian, P.; Vatanara, A. D-Optimal Design for Preparation and Optimization of Fast Dissolving Bosentan Nanosuspension. Adv. Pharm. Bull. 2016, 6, 211–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kehoe, P.G.; Passmore, P.A. The Renin-Angiotensin System and Antihypertensive Drugs in Alzheimer’s Disease: Current Standing of the Angiotensin Hypothesis? J. Alzheimer’s Dis. 2012, 30, S251–S268. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.d.A.; Giacobe, L.J.; Grassi, V.; Palmeira, A.L.R. The Use of Angiotensin Receptor Blockers in Dementia Prevention. Dement. Neuropsychol. 2023, 17, e20220052. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, H.F.; Wang, X.; Li, J.; Xing, C.M. The Association of Renin-Angiotensin System Blockade Use with the Risks of Cognitive Impairment of Aging and Alzheimer’s Disease: A Meta-Analysis. J. Clin. Neurosci. 2016, 33, 32–38. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Kwon, Y.M.; Omidi, Y.; Speth, R.C. Nanoparticle Approaches for the Renin-Angiotensin System. Heliyon 2023, 9, e17745. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Yang, W. Role of Berberine in Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Crosstalk between Obesity, Diabetes, and Alzheimer’s Disease: Introducing Quercetin as an Effective Triple Herbal Medicine. Ageing Res. Rev. 2020, 62, 101095. [Google Scholar] [CrossRef] [PubMed]
- Dan, L.; Hao, Y.; Li, J.; Wang, T.; Zhao, W.; Wang, H.; Qiao, L.; Xie, P. Neuroprotective Effects and Possible Mechanisms of Berberine in Animal Models of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2023, 14, 1204034. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Kang, C.; Che, S.; Su, J.; Sun, Q.; Ge, T.; Guo, Y.; Lv, J.; Sun, Z.; Yang, W.; et al. Berberine: A Promising Treatment for Neurodegenerative Diseases. Front. Pharmacol. 2022, 13, 845591. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, S.S.; Rathore, A.S.; Singh, S.P.; Mishra, G.; Awasthi, R.; Mishra, S.K.; Gautam, V.; Singh, S.K. Lipid-Coated MCM-41 Mesoporous Silica Nanoparticles Loaded with Berberine Improved Inhibition of Acetylcholine Esterase and Amyloid Formation. ACS Biomater. Sci. Eng. 2021, 7, 3737–3753. [Google Scholar] [CrossRef]
- Abo El-Enin, H.A.; Elkomy, M.H.; Naguib, I.A.; Ahmed, M.F.; Alsaidan, O.A.; Alsalahat, I.; Ghoneim, M.M.; Eid, H.M. Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route. Pharmaceuticals 2022, 15, 281. [Google Scholar] [CrossRef]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of A Beta-Deposition in the Human Brain and Its Relevance for the Development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Mota, I.F.L.; de Lima, L.S.; Santana, B.d.M.; Gobbo, G.d.A.M.; Bicca, J.V.M.L.; Azevedo, J.R.M.; Veras, L.G.; Taveira, R.d.A.A.; Pinheiro, G.B.; Mortari, M.R. Alzheimer’s Disease: Innovative Therapeutic Approaches Based on Peptides and Nanoparticles. Neuroscientist 2023, 29, 78–96. [Google Scholar] [CrossRef]
- Chonpathompikunlert, P.; Yoshitomi, T.; Han, J.; Isoda, H.; Nagasaki, Y. The Use of Nitroxide Radical-Containing Nanoparticles Coupled with Piperine to Protect Neuroblastoma SH-SY5Y Cells from Aβ-Induced Oxidative Stress. Biomaterials 2011, 32, 8605–8612. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Y.; Zhao, N.; Hao, B.; Wang, X.; Kong, P. Pharmacokinetic Behavior and Efficiency of Acetylcholinesterase Inhibition in Rat Brain after Intranasal Administration of Galanthamine Hydrobromide Loaded Flexible Liposomes. Environ. Toxicol. Pharmacol. 2012, 34, 272–279. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Zhang, Y.Q.; Wang, Z.Z.; Wu, K.; Lou, J.N.; Qi, X.R. Enhanced Brain Distribution and Pharmacodynamics of Rivastigmine by Liposomes Following Intranasal Administration. Int. J. Pharm. 2013, 452, 344–354. [Google Scholar] [CrossRef]
- Saffari, P.M.; Alijanpour, S.; Takzaree, N.; Sahebgharani, M.; Etemad-Moghadam, S.; Noorbakhsh, F.; Partoazar, A. Metformin Loaded Phosphatidylserine Nanoliposomes Improve Memory Deficit and Reduce Neuroinflammation in Streptozotocin-Induced Alzheimer’s Disease Model. Life Sci. 2020, 255, 117861. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Li, X.; Ni, Y.; Xiao, H.; Yao, Y.; Wang, Y.; Ju, R.; Li, H.; Liu, J.; Fu, M.; et al. Transferrin-Modified Osthole PEGylated Liposomes Travel the Blood-Brain Barrier and Mitigate Alzheimer’s Disease-Related Pathology in APP/PS-1 Mice. Int. J. Nanomed. 2020, 15, 2841–2858. [Google Scholar] [CrossRef] [PubMed]
- Conejero-Goldberg, C.; Gomar, J.J.; Bobes-Bascaran, T.; Hyde, T.M.; Kleinman, J.E.; Herman, M.M.; Chen, S.; Davies, P.; Goldberg, T.E. APOE2 Enhances Neuroprotection against Alzheimer’s Disease through Multiple Molecular Mechanisms. Mol. Psychiatry 2014, 19, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Layek, B.; Singh, J. Design and Validation of Liposomal ApoE2 Gene Delivery System to Evade Blood-Brain Barrier for Effective Treatment of Alzheimer’s Disease. Mol. Pharm. 2021, 18, 714–725. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Luo, Z.; Han, D.; Luo, W.; Wan, R.; Li, Y.; Ge, Y.; Lin, W.W.; Xie, Y.; et al. Pantothenate-Encapsulated Liposomes Combined with Exercise for Effective Inhibition of CRM1-Mediated PKM2 Translocation in Alzheimer’s Therapy. J. Control. Release 2024, 373, 336–357. [Google Scholar] [CrossRef]
- Xu, J.; Patassini, S.; Begley, P.; Church, S.; Waldvogel, H.J.; Faull, R.L.M.; Unwin, R.D.; Cooper, G.J.S. Cerebral Deficiency of Vitamin B5 (d-Pantothenic Acid; Pantothenate) as a Potentially-Reversible Cause of Neurodegeneration and Dementia in Spo-radic Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2020, 527, 676–681. [Google Scholar] [CrossRef]
- Yang, P.; Sheng, D.; Guo, Q.; Wang, P.; Xu, S.; Qian, K.; Li, Y.; Cheng, Y.; Wang, L.; Lu, W.; et al. Neuronal Mitochondria-Targeted Micelles Relieving Oxidative Stress for Delayed Progression of Alzheimer’s Disease. Biomaterials 2020, 238, 119844. [Google Scholar] [CrossRef]
- Kaur, A.; Nigam, K.; Bhatnagar, I.; Sukhpal, H.; Awasthy, S.; Shankar, S.; Tyagi, A.; Dang, S. Treatment of Alzheimer’s Dis-eases Using Donepezil Nanoemulsion: An Intranasal Approach. Drug Deliv. Transl. Res. 2020, 10, 1862–1875. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, Z.; Zhang, Y.; Li, C.; Zhang, Y.; Guo, Q.; Chen, Q.; Chen, X.; He, X.; Liu, L.; et al. Microenvironment Remodeling Micelles for Alzheimer’s Disease Therapy by Early Modulation of Activated Microglia. Adv. Sci. 2018, 6, 1801586. [Google Scholar] [CrossRef]
- Candela, P.; Gosselet, F.; Saint-Pol, J.; Sevin, E.; Boucau, M.C.; Boulanger, E.; Cecchelli, R.; Fenart, L. Apical-to-Basolateral Transport of Amyloid-β Peptides through Blood-Brain Barrier Cells Is Mediated by the Receptor for Advanced Glycation End-Products and Is Restricted by P-Glycoprotein. J. Alzheimer’s Dis. 2010, 22, 849–859. [Google Scholar] [CrossRef]
- Mehanny, M.; Hathout, R.M.; Geneidi, A.S.; Mansour, S. Exploring the Use of Nanocarrier Systems to Deliver the Magical Molecule; Curcumin and Its Derivatives. J. Control. Release 2016, 225, 1–30. [Google Scholar] [CrossRef]
- Agwa, M.M.; Abdelmonsif, D.A.; Khattab, S.N.; Sabra, S. Self-Assembled Lactoferrin-Conjugated Linoleic Acid Micelles as an Orally Active Targeted Nanoplatform for Alzheimer’s Disease. Int. J. Biol. Macromol. 2020, 162, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming Blood Brain Barrier with a Dual Purpose Te-mozolomide Loaded Lactoferrin Nanoparticles for Combating Glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef]
- Hunt, W.T.; Kamboj, A.; Anderson, H.D.; Anderson, C.M. Protection of Cortical Neurons from Excitotoxicity by Conjugated Linoleic Acid. J. Neurochem. 2010, 115, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Dara, T.; Vatanara, A.; Sharifzadeh, M.; Khani, S.; Vakilinezhad, M.A.; Vakhshiteh, F.; Nabi Meybodi, M.; Sadegh Malvajerd, S.; Hassani, S.; Mosaddegh, M.H. Improvement of Memory Deficits in the Rat Model of Alzheimer’s Disease by Erythropoietin-Loaded Solid Lipid Nanoparticles. Neurobiol. Learn. Mem. 2019, 166, 107082. [Google Scholar] [CrossRef] [PubMed]
- Al-Saran, N.; Subash-Babu, P.; Al-Harbi, L.N.; Alrfaei, B.M.; Alshatwi, A.A. Neuroprotective Effect of Solid Lipid Nanoparticles Loaded with Lepidium Sativum (L.) Seed Bioactive Components Enhance Bioavailability and Wnt/β-Catenin/Camk-II Signaling Cascade in SH-SY5Y Neuroblastoma Cells. Nanomaterials 2024, 14, 199. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-Loaded PLGA-PEG Nanoparticles Conjugated with B6 Peptide for Potential Use in Alzheimer’s Disease. Drug Deliv. 2018, 25, 1044–1055. [Google Scholar] [CrossRef]
- Boonruamkaew, P.; Chonpathompikunlert, P.; Vong, L.B.; Sakaue, S.; Tomidokoro, Y.; Ishii, K.; Tamaoka, A.; Nagasaki, Y. Chronic Treatment with a Smart Antioxidative Nanoparticle for Inhibition of Amyloid Plaque Propagation in Tg2576 Mouse Model of Alzheimer’s Disease. Sci. Rep. 2017, 7, 3785. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Redox Nanoparticles: Synthesis, Properties and Perspectives of Use for Treatment of Neu-rodegenerative Diseases. J. Nanobiotechnol. 2018, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-Drug Loaded Nanoparticles of Epigallocatechin-3-Gallate (EGCG)/Ascorbic Acid Enhance Therapeutic Efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s Disease Mice Model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.; Singh, S.; Pandey, V.P.; Dwivedi, U.N. Alzheimer’s Disease: An Overview of Amyloid Beta Dependent Pathogen-esis and Its Therapeutic Implications along with in Silico Approaches Emphasizing the Role of Natural Products. J. Neurol. Sci. 2016, 361, 256–271. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Dong, H.J.; Yin, J.J.; Zhang, H.; Wu, H.A.; Song, L.N.; Chong, Y.; Li, Z.X.; Gu, N.; et al. Sparks Fly between Ascorbic Acid and Iron-Based Nanozymes: A Study on Prussian Blue Nanoparticles. Colloids Surf. B Biointerfaces 2018, 163, 379–384. [Google Scholar] [CrossRef]
- Abbas, H.; El Sayed, N.S.; Youssef, N.A.H.A.; Gaafar, P.M.E.; Mousa, M.R.; Fayez, A.M.; Elsheikh, M.A. Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceutics 2022, 14, 1003. [Google Scholar] [CrossRef]
- Kwon, Y. Luteolin as a Potential Preventive and Therapeutic Candidate for Alzheimer’s Disease. Exp. Gerontol. 2017, 95, 39–43. [Google Scholar] [CrossRef]
- Salem, L.H.; El-Feky, G.S.; Fahmy, R.H.; El Gazayerly, O.N.; Abdelbary, A. Coated Lipidic Nanoparticles as a New Strategy for Enhancing Nose-to-Brain Delivery of a Hydrophilic Drug Molecule. J. Pharm. Sci. 2020, 109, 2237–2251. [Google Scholar] [CrossRef]
- Gothwal, A.; Kumar, H.; Nakhate, K.T.; Ajazuddin; Dutta, A.; Borah, A.; Gupta, U. Lactoferrin Coupled Lower Generation PAMAM Dendrimers for Brain Targeted Delivery of Memantine in Aluminum-Chloride-Induced Alzheimer’s Disease in Mice. Bioconjug. Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.G.; Danysz, W.; Quack, G. Memantine Is a Clinically Well Tolerated N-Methyl-D-Aspartate (NMDA) Receptor Antagonist—A Review of Preclinical Data. Neuropharmacology 1999, 38, 735–767. [Google Scholar] [CrossRef]
- Tallon, C.; Bell, B.J.; Sharma, A.; Pal, A.; Malvankar, M.M.; Thomas, A.G.; Yoo, S.W.; Hollinger, K.R.; Coleman, K.; Wilkinson, E.L.; et al. Dendrimer-Conjugated NSMase2 Inhibitor Reduces Tau Propagation in Mice. Pharmaceutics 2022, 14, 2066. [Google Scholar] [CrossRef]
- Christodoulou, C.; Melville, P.; Scherl, W.F.; MacAllister, W.S.; Elkins, L.E.; Krupp, L.B. Effects of Donepezil on Memory and Cognition in Multiple Sclerosis. J. Neurol. Sci. 2006, 245, 127–136. [Google Scholar] [CrossRef]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Qu, X. Gold-Nanoparticle-Based Multifunctional Amyloid-β Inhibitor against Alzheimer’s Disease. Chemistry 2015, 21, 829–835. [Google Scholar] [CrossRef]
- Hu, Y.; Cui, J.; Sun, J.; Liu, X.; Gao, S.; Mei, X.; Wu, C.; Tian, H. A Novel Biomimetic Nanovesicle Containing Caffeic Ac-id-Coupled Carbon Quantum Dots for the Treatment of Alzheimer’s Disease via Nasal Administration. J. Nanobiotechnol. 2024, 22, 642. [Google Scholar] [CrossRef]
- Akama, K.T.; Van Eldik, L.J. β-Amyloid Stimulation of Inducible Nitric-Oxide Synthase in Astrocytes Is Interleukin-1β- and Tumor Necrosis Factor-α (TNFα)-Dependent, and Involves a TNFα Receptor-Associated Factor- and NFκB-Inducing Kinase-Dependent Signaling Mechanism. J. Biol. Chem. 2000, 275, 7918–7924. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Kong, L.; Yao, Y.; Jiao, Y.; Song, J.; Tao, Z.; You, Z.; Yang, J. Osthole Confers Neuroprotection against Cortical Stab Wound Injury and Attenuates Secondary Brain Injury. J. Neuroinflamm. 2015, 12, 155. [Google Scholar] [CrossRef]
- Drygalski, K.; Fereniec, E.; Koryciński, K.; Chomentowski, A.; Kiełczewska, A.; Odrzygóźdź, C.; Modzelewska, B. Resveratrol and Alzheimer’s Disease. From Molecular Pathophysiology to Clinical Trials. Exp. Gerontol. 2018, 113, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.K.; Sharma, P.K. Optimization and Characterization of Rivastigmine Nanolipid Carrier Loaded Transdermal Patches for the Treatment of Dementia. Chem. Phys. Lipids 2019, 224, 104794. [Google Scholar] [CrossRef]
- Hernández, C.C.; Burgos, C.F.; Gajardo, A.H.; Silva-Grecchi, T.; Gavilan, J.; Toledo, J.R.; Fuentealba, J. Neuroprotective Effects of Erythropoietin on Neurodegenerative and Ischemic Brain Diseases: The Role of Erythropoietin Receptor. Neural Regen. Res. 2017, 12, 1381–1389. [Google Scholar] [CrossRef]
- Hallak, M.; Vazana, L.; Shpilberg, O.; Levy, I.; Mazar, J.; Nathan, I. A Molecular Mechanism for Mimosine-Induced Apoptosis Involving Oxidative Stress and Mitochondrial Activation. Apoptosis 2008, 13, 147–155. [Google Scholar] [CrossRef]
- Anand, B.G.; Wu, Q.; Karthivashan, G.; Shejale, K.P.; Amidian, S.; Wille, H.; Kar, S. Mimosine Functionalized Gold Nanoparti-cles (Mimo-AuNPs) Suppress β-Amyloid Aggregation and Neuronal Toxicity. Bioact. Mater. 2021, 6, 4491–4505. [Google Scholar] [CrossRef]
- Singh, I.; Arora, R.; Dhiman, H.; Pahwa, R. Carbon Quantum Dots: Synthesis, Characterization and Biomedical Applications. Turk. J. Pharm. Sci. 2018, 15, 219–230. [Google Scholar] [CrossRef]
- Andrade, S.; Pereira, M.C.; Loureiro, J.A. Caffeic Acid Loaded into Engineered Lipid Nanoparticles for Alzheimer’s Disease Therapy. Colloids Surf. B Biointerfaces 2023, 225, 113270. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Pervushin, K. Magnetic Nanoparticles as In Vivo Tracers for Alzheimer’s Disease. Magnetochemistry 2020, 6, 13. [Google Scholar] [CrossRef]
- Amiri, H.; Saeidi, K.; Borhani, P.; Manafirad, A.; Ghavami, M.; Zerbi, V. Alzheimer’s Disease: Pathophysiology and Applica-tions of Magnetic Nanoparticles as MRI Theranostic Agents. ACS Chem. Neurosci. 2013, 4, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
| NP System | General Characteristic | Possibilities of Appliance |
|---|---|---|
| Liposomes |
| |
| Micelles |
| |
| Solid Lipid NPs |
|
|
| .Polymeric NPs |
|
|
| Dendrimers |
|
|
| Nanoemulsions |
| |
| Inorganic NPs (e.g., gold, quantum dots) |
|
|
| Parameter | Conventional Gadolinium-Based Agents | IONPs |
|---|---|---|
| BBB Penetration | Limited | Enhanced |
| Targeting | Non-Selective | Selective AD Biomarker Targeting |
| Safety Profile | Toxic risks | Superior Safety Profile |
| Functionality | Diagnostic Only | Diagnostic and therapeutic (drug delivery) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koga-Batko, J.; Antosz-Popiołek, K.; Nowakowska, H.; Błażejewska, M.; Kowalik, E.M.; Beszłej, J.A.; Leszek, J. Nanoparticles as an Encouraging Therapeutic Approach to Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 7725. https://doi.org/10.3390/ijms26167725
Koga-Batko J, Antosz-Popiołek K, Nowakowska H, Błażejewska M, Kowalik EM, Beszłej JA, Leszek J. Nanoparticles as an Encouraging Therapeutic Approach to Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(16):7725. https://doi.org/10.3390/ijms26167725
Chicago/Turabian StyleKoga-Batko, Joanna, Katarzyna Antosz-Popiołek, Hanna Nowakowska, Marta Błażejewska, Eunika Milena Kowalik, Jan Aleksander Beszłej, and Jerzy Leszek. 2025. "Nanoparticles as an Encouraging Therapeutic Approach to Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 16: 7725. https://doi.org/10.3390/ijms26167725
APA StyleKoga-Batko, J., Antosz-Popiołek, K., Nowakowska, H., Błażejewska, M., Kowalik, E. M., Beszłej, J. A., & Leszek, J. (2025). Nanoparticles as an Encouraging Therapeutic Approach to Alzheimer’s Disease. International Journal of Molecular Sciences, 26(16), 7725. https://doi.org/10.3390/ijms26167725








