Repurposing the Antibiotic D-Cycloserine for the Treatment of Hyperpigmentation: Therapeutic Potential and Mechanistic Insights
Abstract
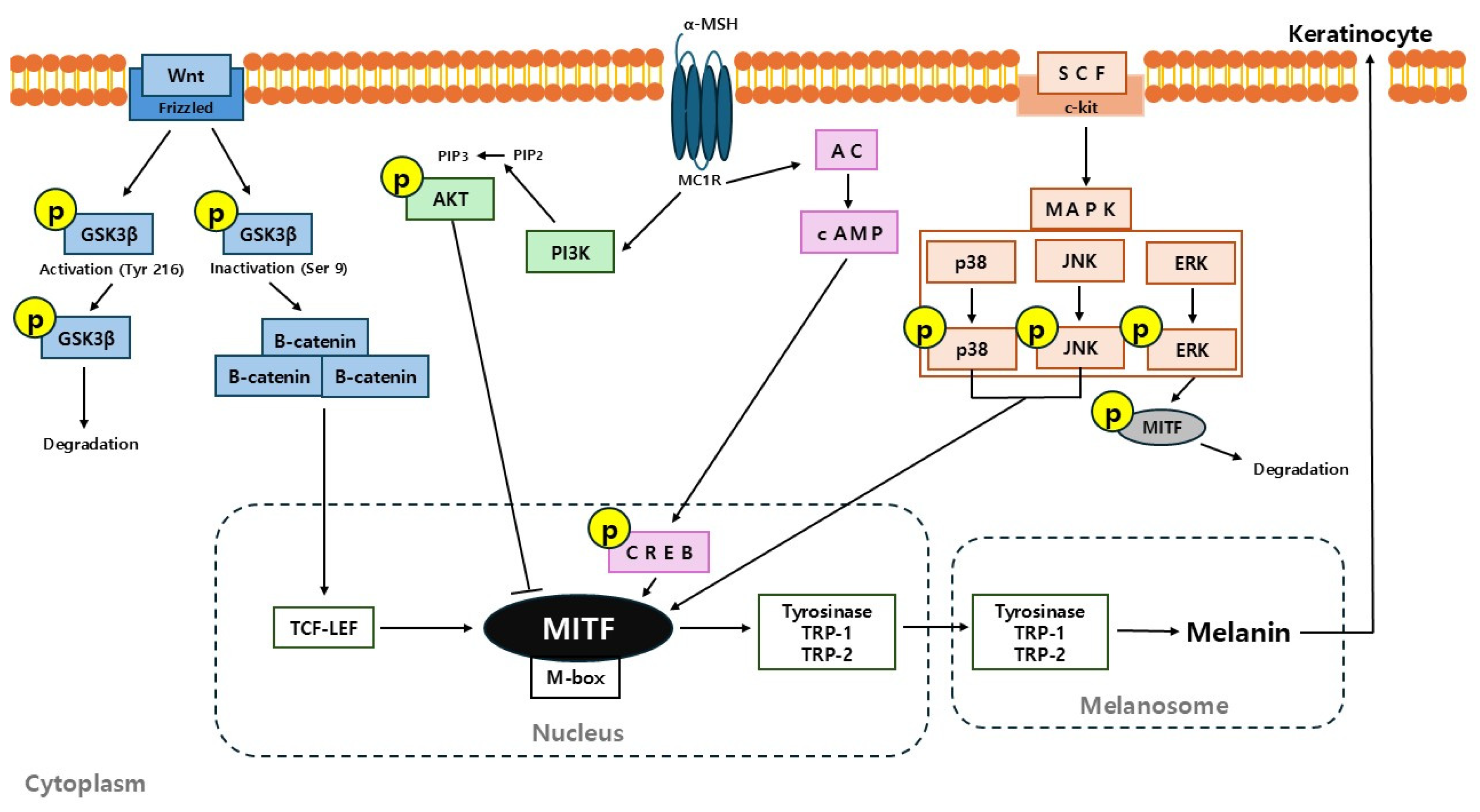
1. Introduction
2. Results and Discussion
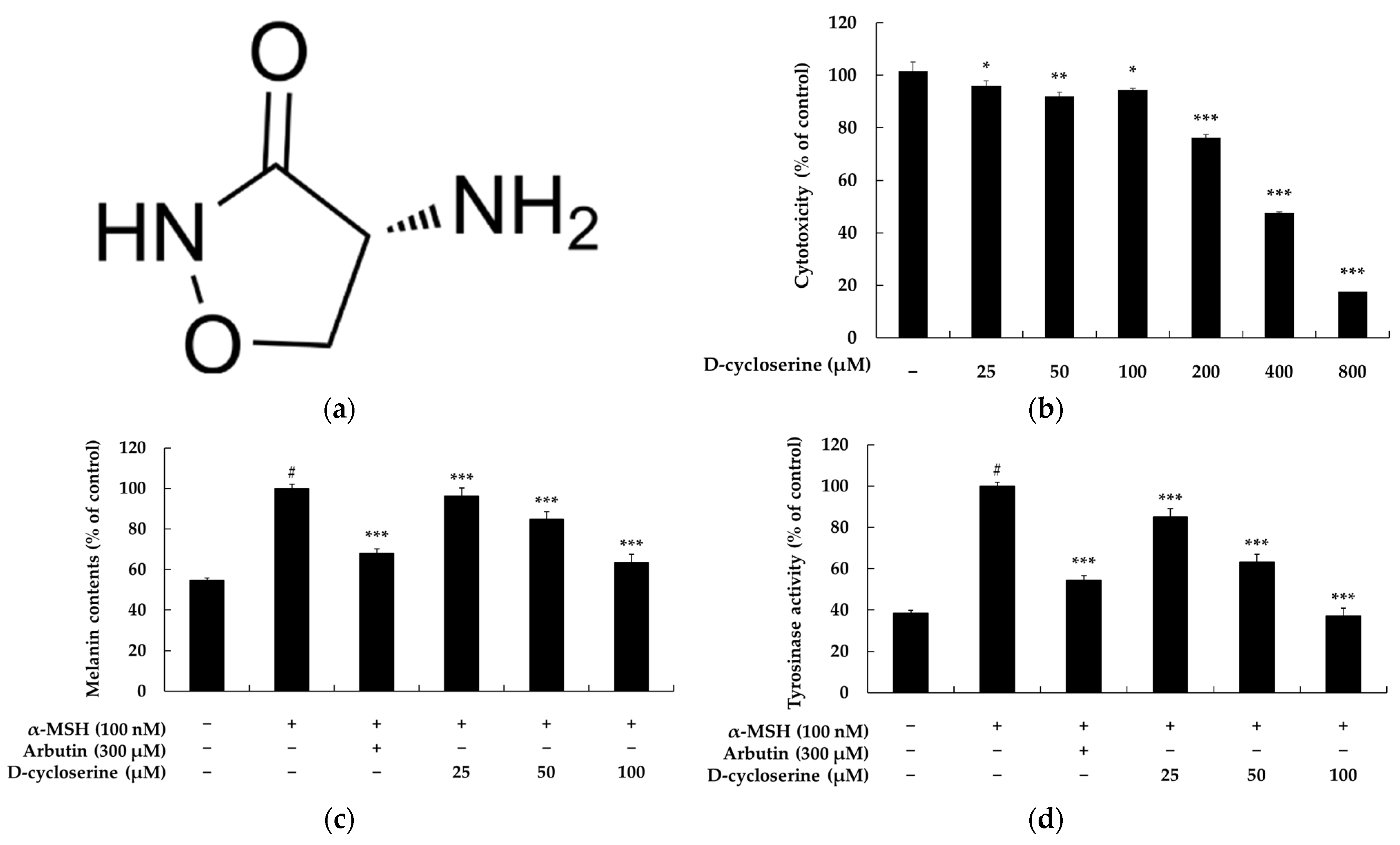
2.1. DCS Inhibits Melanin Content and Tyrosinase Activity in B16F10 Cells
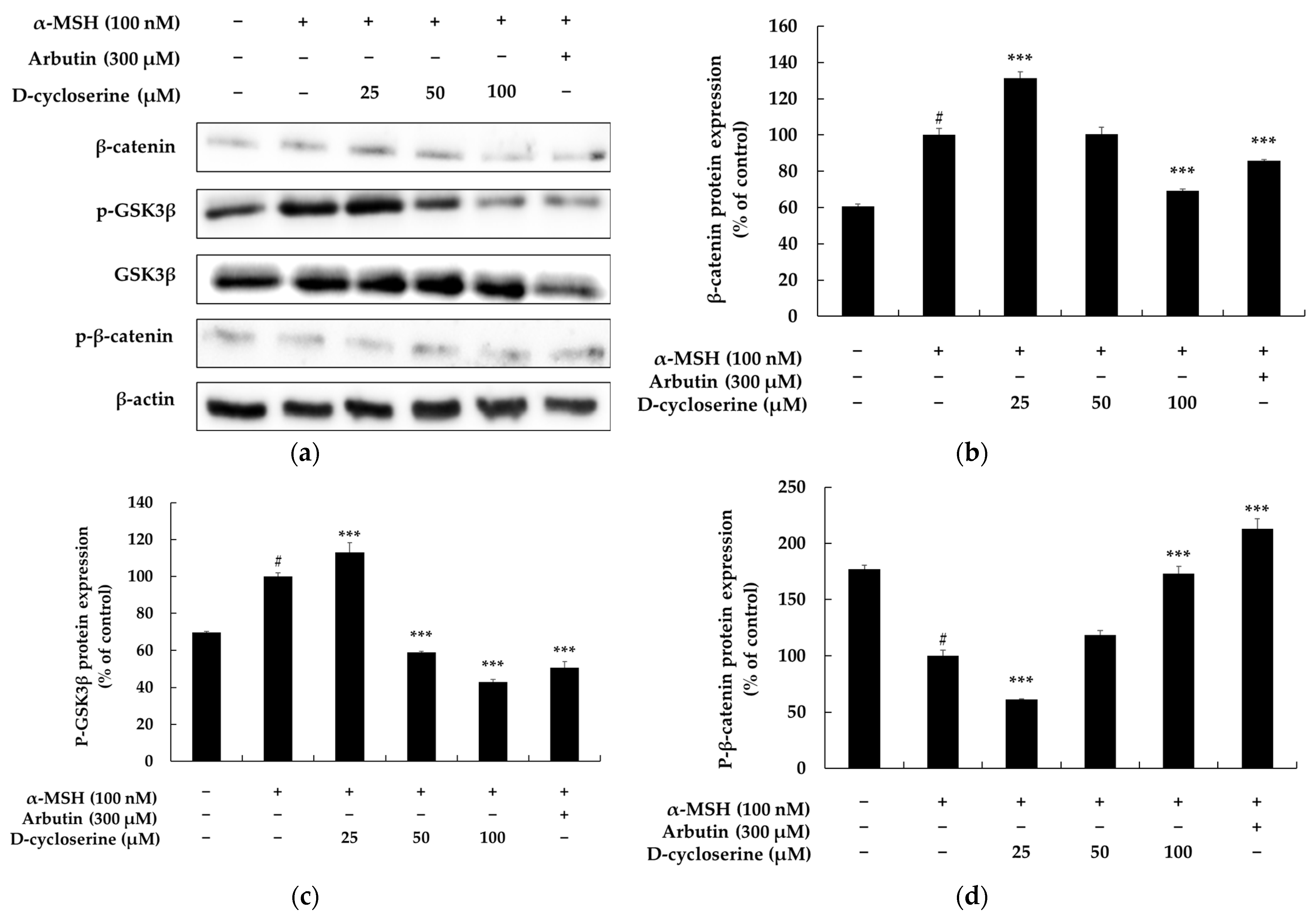
2.2. DCS Regulates the Expression of Melanogenesis-Related Proteins in B16F10 Cells
2.3. DCS Inhibits Melanogenesis Through the GSK–3β/β-Catenin Pathway in B16F10 Cells
2.4. DCS Inhibits Melanogenesis Independently of the PI3K/Akt Pathway in B16F10 Cells
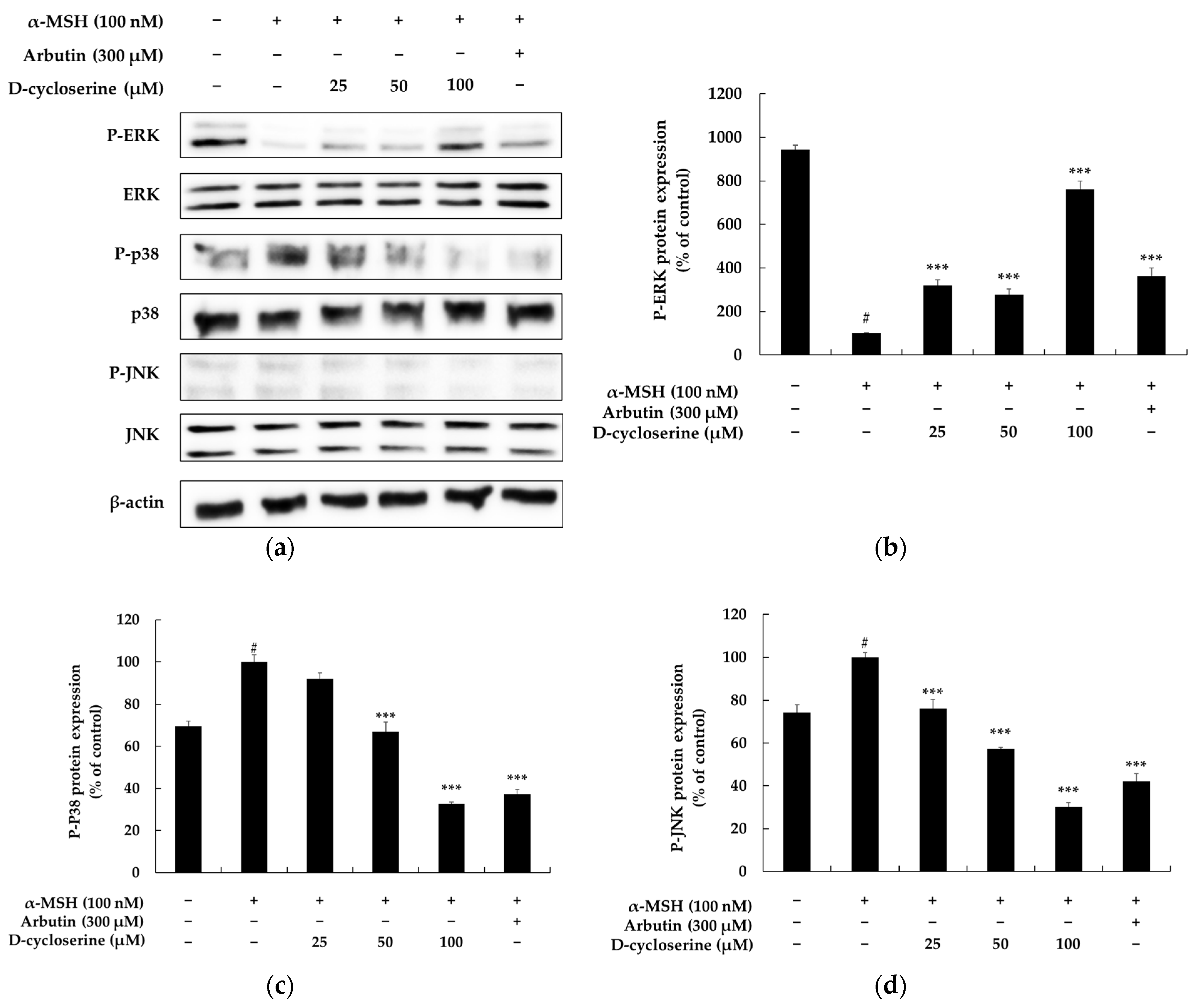
2.5. DCS Inhibits Melanogenesis Through the MAPK Pathway in B16F10 Cells
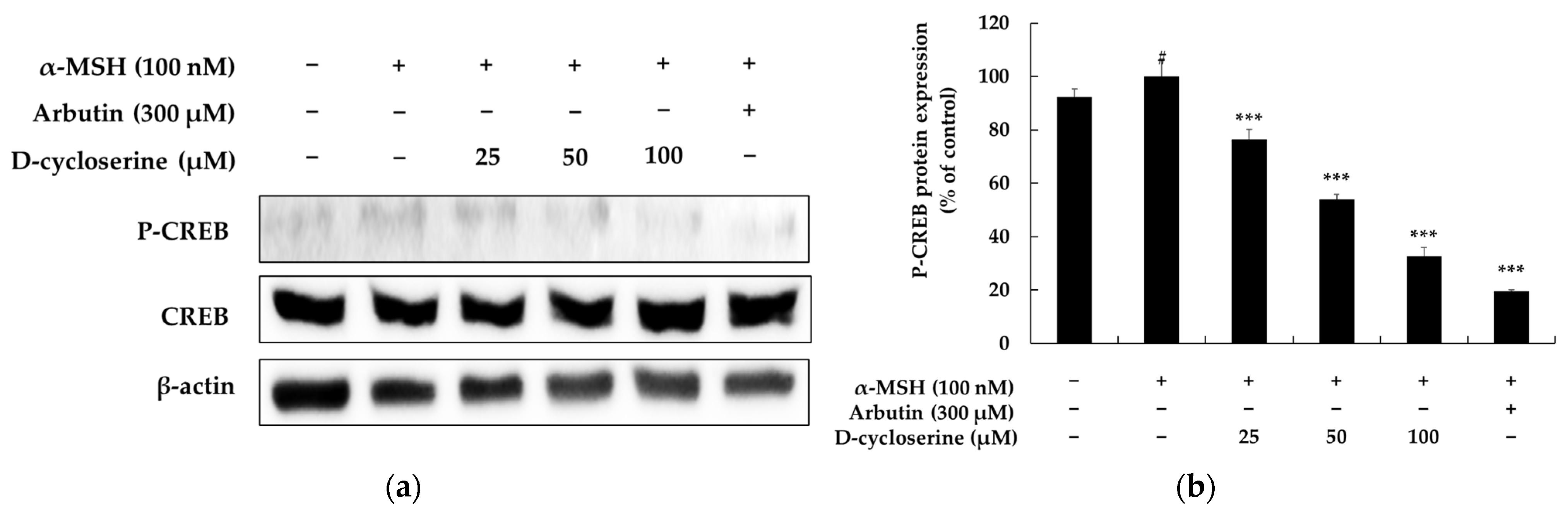
2.6. DCS Inhibits α-MSH-Induced CREB Phosphorylation in Melanocytes
2.7. Cutaneous Safety Evaluation of DCS
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell Culture
3.3. Cell-Viability (MTT) Assay
3.4. Melanin Content Assay
3.5. Intracellular Tyrosinase Activity Assay
3.6. Western Blot Analysis
3.7. Protocol for Assessing Primary Skin Irritation in Humans
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akl, J.; Lee, S.; Ju, H.J.; Parisi, R.; Kim, J.Y.; Jeon, J.J.; Heo, Y.W.; Eleftheriadou, V.; Hamzavi, I.; Griffiths, C.E.M.; et al. Global Vitiligo Atlas. Estimating the burden of vitiligo: A systematic review and modelling study. Lancet Public Health 2024, 9, e386–e396. [Google Scholar] [CrossRef]
- Ismail, I.B.; Bhat, Y.J.; Ul Islam, M.S. Treatment Advances in Vitiligo: An Updated Review. Dermatol. Pract. Concept. 2025, 15, 4600. [Google Scholar] [CrossRef]
- Dabas, G.; Vinay, K.; Parsad, D.; Kumar, A.; Kumaran, M.S. Psychological disturbances in patients with pigmentary disorders: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, L.; Bisi, N.; Rastelli, G. How drug repurposing can advance drug discovery: Challenges and opportunities. Front. Drug Discov. 2024, 4, 1460100. [Google Scholar] [CrossRef]
- Beninger, P. Thalidomide: Following Tragedy, a Repurposed Molecule With Continuing Opportunities for Clinical Benefit. Clin. Ther. 2025, 47, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Ala, M.; Mohammad Jafari, R.; Dehpour, A.R. Sildenafil beyond erectile dysfunction and pulmonary arterial hypertension: Thinking about new indications. Fundam. Clin. Pharmacol. 2021, 35, 235–259. [Google Scholar] [CrossRef]
- Banerjee, S.; Banerjee, D.; Singh, A.; Kumar, S.; Pooja, D.; Ram, V.; Kulhari, H.; Saharan, V.A. A Clinical Insight on New Discovered Molecules and Repurposed Drugs for the Treatment of COVID-19. Vaccines 2023, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Kumagai, T.; Mori, K.; Sugiyama, M. Molecular cloning of a D-cycloserine resistance gene from D-cycloserine-producing Streptomyces garyphalus. J. Antibiot. 2003, 56, 762–767. [Google Scholar] [CrossRef][Green Version]
- Kumagai, T.; Koyama, Y.; Oda, K.; Noda, M.; Matoba, Y.; Sugiyama, M. Molecular cloning and heterologous expression of a biosynthetic gene cluster for the antitubercular agent D-cycloserine produced by Streptomyces lavendulae. Antimicrob. Agents Chemother. 2010, 54, 1132–1139. [Google Scholar] [CrossRef]
- Pendela, M.; Dragovic, S.; Bockx, L.; Hoogmartens, J.; Van Schepdael, A.; Adams, E. Development of a liquid chromatographic method for the determination of related substances and assay of d-cycloserine. J. Pharm. Biomed. Anal. 2008, 47, 807–811. [Google Scholar] [CrossRef]
- Zhuang, J.; Yang, W.; Cheng, G.J. Mechanistic Insights into the Reversible Inhibition of d-Cycloserine in Alanine Racemase from Mycobacterium tuberculosis. J. Chem. Inf. Model. 2025, 65, 2610–2622. [Google Scholar] [CrossRef]
- Otto, M.W.; Kredlow, M.A.; Smits, J.A.J.; Hofmann, S.G.; Tolin, D.F.; de Kleine, R.A.; van Minnen, A.; Evins, A.E.; Pollack, M.H. Enhancement of Psychosocial Treatment With D-Cycloserine: Models, Moderators, and Future Directions. Biol. Psychiatry 2016, 80, 274–283. [Google Scholar] [CrossRef]
- Ni, J.; Wang, N.; Gao, L.; Li, L.; Zheng, S.; Liu, Y.; Ozukum, M.; Nikiforova, A.; Zhao, G.; Song, Z. The effect of the NMDA receptor-dependent signaling pathway on cell morphology and melanosome transfer in melanocytes. J. Dermatol. Sci. 2016, 84, 296–304. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Zhang, A.; Gupte, A.A.; Hamilton, D.J. The Role of Calcium Signaling in Melanoma. Int. J. Mol. Sci. 2022, 23, 1010. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Y.; Kou, Z. Bibliometric analysis of NMDA receptors: 2015–2024. Front. Pharmacol. 2025, 16, 1614831. [Google Scholar] [CrossRef] [PubMed]
- Berns, H.M.; Watkins-Chow, D.E.; Lu, S.; Louphrasitthiphol, P.; Zhang, T.; Brown, K.M.; Moura-Alves, P.; Goding, C.R.; Pavan, W.J. Single-cell profiling of MC1R-inhibited melanocytes. Pigment Cell Melanoma Res. 2024, 37, 291–308. [Google Scholar] [CrossRef]
- Kang, H.K.; Hyun, C.G. Anti-inflammatory effect of d-(+)-cycloserine through inhibition of NF-κB and MAPK signaling pathways in LPS-induced RAW 264.7 macrophages. Nat. Prod. Commun. 2020, 15, 4. [Google Scholar] [CrossRef]
- King, S.; Campbell, J.; Rowe, R.; Daly, M.L.; Moncrieff, G.; Maybury, C. A systematic review to evaluate the efficacy of azelaic acid in the management of acne, rosacea, melasma and skin aging. J. Cosmet. Dermatol. 2023, 22, 2650–2662. [Google Scholar] [CrossRef]
- Sauer, N.; Oślizło, M.; Brzostek, M.; Wolska, J.; Lubaszka, K.; Karłowicz-Bodalska, K. The multiple uses of azelaic acid in dermatology: Mechanism of action, preparations, and potential therapeutic applications. Postepy Dermatol. Alergol. 2023, 40, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Konisky, H.; Balazic, E.; Jaller, J.A.; Khanna, U.; Kobets, K. Tranexamic acid in melasma: A focused review on drug administration routes. J. Cosmet. Dermatol. 2023, 22, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gu, D.; Yan, Y.; Pan, R.; Zhong, H.; Zhang, C.; Xu, Y. Potential Role of Tranexamic Acid in Rosacea Treatment: Conquering Flushing Beyond Melasma. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1405–1412. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, H.O.; Woo, S.M.; Lee, D.H. Use of Dexpanthenol for Atopic Dermatitis-Benefits and Recommendations Based on Current Evidence. J. Clin. Med. 2022, 11, 3943. [Google Scholar] [CrossRef]
- Gorski, J.; Proksch, E.; Baron, J.M.; Schmid, D.; Zhang, L. Dexpanthenol in Wound Healing after Medical and Cosmetic Interventions (Postprocedure Wound Healing). Pharmaceuticals 2020, 13, 138. [Google Scholar] [CrossRef]
- Han, H.J.; Hyun, C.G. Acenocoumarol exerts anti-inflammatory activity via the suppression of NF-κB and MAPK pathways in RAW 264.7 cells. Molecules 2023, 28, 2075. [Google Scholar] [CrossRef]
- Kim, H.M.; Hyun, C.G. Miglitol, an oral antidiabetic drug, downregulates melanogenesis in B16F10 melanoma cells through the PKA, MAPK, and GSK3β/β-Catenin signaling pathways. Molecules 2022, 28, 115. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Hyun, C.G. Imperatorin positively regulates melanogenesis through signaling pathways involving PKA/CREB, ERK, AKT, and GSK3β/β-catenin. Molecules 2022, 27, 6512. [Google Scholar] [CrossRef]
- Moon, S.H.; Chung, Y.C.; Hyun, C.G. Tobramycin promotes melanogenesis by upregulating p38 MAPK protein phosphorylation in B16F10 melanoma cells. Antibiotics 2019, 8, 140. [Google Scholar] [CrossRef]
- Wang, F.; Ma, W.; Fan, D.; Hu, J.; An, X.; Wang, Z. The biochemistry of melanogenesis: An insight into the function and mechanism of melanogenesis-related proteins. Front. Mol. Biosci. 2024, 11, 1440187. [Google Scholar] [CrossRef]
- Yoon, J.H.; Youn, K.; Jun, M. Discovery of Pinostrobin as a Melanogenic Agent in cAMP/PKA and p38 MAPK Signaling Pathway. Nutrients 2022, 14, 3713. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Yen, H.; Lu, J.Y.; Chang, T.M.; Hii, C.H. Theophylline enhances melanogenesis in B16F10 murine melanoma cells through the activation of the MEK 1/2, and Wnt/β-catenin signaling pathways. Food Chem. Toxicol. 2020, 137, 111165. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Bejaoui, M.; Tominaga, K.; Isoda, H. Comparative Analysis of Olive-Derived Phenolic Compounds’ Pro-Melanogenesis Effects on B16F10 Cells and Epidermal Human Melanocytes. Int. J. Mol. Sci. 2024, 25, 4479. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Hyun, C.G. Mechanistic Insights into the Stimulatory Effect of Melanogenesis of 4-Methylcoumarin Derivatives in B16F10 Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 12421. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Shahzadi, S.; Kloczkowski, A. Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review. Molecules 2023, 28, 378. [Google Scholar] [CrossRef]
- Hirobe, T. Role of Dermal Factors Involved in Regulating the Melanin and Melanogenesis of Mammalian Melanocytes in Normal and Abnormal Skin. Int. J. Mol. Sci. 2024, 25, 4560. [Google Scholar] [CrossRef]
- Zolghadri, S.; Beygi, M.; Mohammad, T.F.; Alijanianzadeh, M.; Pillaiyar, T.; Garcia-Molina, P.; Garcia-Canovas, F.; Munoz-Munoz, J.; Saboury, A.A. Targeting tyrosinase in hyperpigmentation: Current status, limitations and future promises. Biochem. Pharmacol. 2023, 212, 115574. [Google Scholar] [CrossRef]
- Bin, B.H.; Kim, S.T.; Bhin, J.; Lee, T.R.; Cho, E.G. The Development of Sugar-Based Anti-Melanogenic Agents. Int. J. Mol. Sci. 2016, 17, 583. [Google Scholar] [CrossRef]
- Tanemura, A.; Yang, L.; Yang, F.; Nagata, Y.; Wataya-Kaneda, M.; Fukai, K.; Tsuruta, D.; Ohe, R.; Yamakawa, M.; Suzuki, T.; et al. An immune pathological and ultrastructural skin analysis for rhododenol-induced leukoderma patients. J. Dermatol. Sci. 2015, 77, 185–188. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Inhibitors of intracellular signaling pathways that lead to stimulated epidermal pigmentation: Perspective of anti-pigmenting agents. Int. J. Mol. Sci. 2014, 15, 8293–8315. [Google Scholar] [CrossRef]
- Lin, X.; Meng, X.; Lin, J. The possible role of Wnt/β-catenin signalling in vitiligo treatment. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2208–2221. [Google Scholar] [CrossRef]
- Bai, R.; Guo, Y.; Liu, W.; Song, Y.; Yu, Z.; Ma, X. The Roles of WNT Signaling Pathways in Skin Development and Mechanical-Stretch-Induced Skin Regeneration. Biomolecules 2023, 13, 1702. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Yang, A. Hydrolyzed conchiolin protein inhibits melanogenesis through PKA/CREB and MEK/ERK signalling pathways. Int. J. Cosmet. Sci. 2025, 47, 31–44. [Google Scholar] [CrossRef]
- Liu, C.; Nueraihemaiti, M.; Zang, D.; Edirs, S.; Zou, G.; Aisa, H.A. Quercetin 3-O-(6″-O-E-caffeoyl)-β-D-glucopyranoside, a Flavonoid Compound, Promotes Melanogenesis through the Upregulation of MAPKs and Akt/GSK3β/β-Catenin Signaling Pathways. Int, J, Mol, Sci. 2023, 24, 4780. [Google Scholar] [CrossRef]
- Liu, B.; Xie, Y.; Wu, Z. Astragaloside IV Enhances Melanogenesis via the AhR-Dependent AKT/GSK-3β/β-Catenin Pathway in Normal Human Epidermal Melanocytes. Evid.-Based Complement. Altern. Med. 2020, 2020, 8838656. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Hu, N.; Wang, H. Petanin Potentiated JNK Phosphorylation to Negatively Regulate the ERK/CREB/MITF Signaling Pathway for Anti-Melanogenesis in Zebrafish. Int. J. Mol. Sci. 2024, 25, 5939. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, X.; Zhou, J.; Sun, G.; Li, Z.; Zhai, L.; Wang, J.; Ma, R.; Zhao, D.; Jiang, R.; et al. Phenolic acids in Panax ginseng inhibit melanin production through bidirectional regulation of melanin synthase transcription via different signaling pathways. J. Ginseng Res. 2023, 47, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zou, W.; Li, M.; Yan, Y.; Yu, Y.; Li, X.; Ma, Y.L. Aloe Vera flowers extracts inhibit melanogenesis via activating PI3K/Akt signaling pathway: Network pharmacology and experimental validation. Fitoterapia 2025, 183, 106530. [Google Scholar] [CrossRef]
- Zhou, S.; Zeng, H.; Huang, J.; Lei, L.; Tong, X.; Li, S.; Zhou, Y.; Guo, H.; Khan, M.; Luo, L.; et al. Epigenetic regulation of melanogenesis. Ageing Res. Rev. 2021, 69, 101349. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, H.L.; Wen, X.Y.; Jiang, L.; Fu, C.H.; Hu, Y.B.; Lei, X.X.; Zhang, L.; Yu, X.; Yang, S.Y.; et al. Selaginellin Inhibits Melanogenesis via the MAPK Signaling Pathway. J. Nat. Prod. 2022, 85, 838–845. [Google Scholar] [CrossRef]
- Wusiman, Z.; Zhang, A.M.; Zhang, S.S.; Zhao, P.P.; Kang, Y.T.; Zhang, Y.; Li, Z.J.; Huo, S.X. Galangin ameliorates PTU-induced vitiligo in zebrafish and B16F10 cells by increasing melanogenesis through activation of the p38/JNK MAPK pathway. Front. Pharmacol. 2025, 16, 1521097. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Wang, H.; Li, Y.; Wu, H.; Guo, F. Germacrone, isolated from Curcuma wenyujin, inhibits melanin synthesis through the regulation of the MAPK signaling pathway. J. Nat. Med. 2024, 78, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, J.; Jung, J.; Kim, G.H.; Yun, C.Y.; Jung, S.H.; Hwang, B.Y.; Hong, J.T.; Han, S.B.; Jung, J.K.; et al. Interruption of p38MAPK-MSK1-CREB-MITF-M pathway to prevent hyperpigmentation in the skin. Int. J. Biol. Sci. 2024, 20, 1688–1704. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jian, M.; Teng, H.; Li, Z.; Xu, X.; Li, X.; Jiang, R.; Zhao, D.; Sun, L.; Liu, J. Ginsenoside Rf in wild ginseng adventitious roots extract inhibits melanogenesis via cAMP/PKA and NO/cGMP signalling pathways in α-melanocyte-stimulating hormone-stimulated B16F10 mouse melanoma cells and zebrafish. Nat. Prod. Res. 2025, 39, 2763–2770. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Lv, J.; Wang, F. Pterostilbene inhibits melanogenesis, melanocyte dendricity and melanosome transport through cAMP/PKA/CREB pathway. Eur. J. Pharmacol. 2022, 932, 175231. [Google Scholar] [CrossRef]
- Cao, Y.; Lv, J.; Tan, Y.; Chen, R.; Jiang, X.; Meng, D.; Zou, K.; Pan, M.; Tang, L. Tribuloside acts on the PDE/cAMP/PKA pathway to enhance melanogenesis, melanocyte dendricity and melanosome transport. J. Ethnopharmacol. 2024, 323, 117673. [Google Scholar] [CrossRef]



| No | Test Sample | No. of Responses | 1st Assessment | 2nd Assessment | Reaction Grade (R) * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| +1 | +2 | +3 | +4 | +1 | +2 | +3 | +4 | ||||
| 1 | D-cycloserine (50 μM) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | D-cycloserine (100 μM) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Hyun, C.-G. Repurposing the Antibiotic D-Cycloserine for the Treatment of Hyperpigmentation: Therapeutic Potential and Mechanistic Insights. Int. J. Mol. Sci. 2025, 26, 7721. https://doi.org/10.3390/ijms26167721
Lee Y-J, Hyun C-G. Repurposing the Antibiotic D-Cycloserine for the Treatment of Hyperpigmentation: Therapeutic Potential and Mechanistic Insights. International Journal of Molecular Sciences. 2025; 26(16):7721. https://doi.org/10.3390/ijms26167721
Chicago/Turabian StyleLee, Ye-Jin, and Chang-Gu Hyun. 2025. "Repurposing the Antibiotic D-Cycloserine for the Treatment of Hyperpigmentation: Therapeutic Potential and Mechanistic Insights" International Journal of Molecular Sciences 26, no. 16: 7721. https://doi.org/10.3390/ijms26167721
APA StyleLee, Y.-J., & Hyun, C.-G. (2025). Repurposing the Antibiotic D-Cycloserine for the Treatment of Hyperpigmentation: Therapeutic Potential and Mechanistic Insights. International Journal of Molecular Sciences, 26(16), 7721. https://doi.org/10.3390/ijms26167721







