Targeting pH Inversion in Prostate Cancer Cells: A Role for Systems of Molecules of Vegetal Origin
Abstract
1. Introduction
2. Results
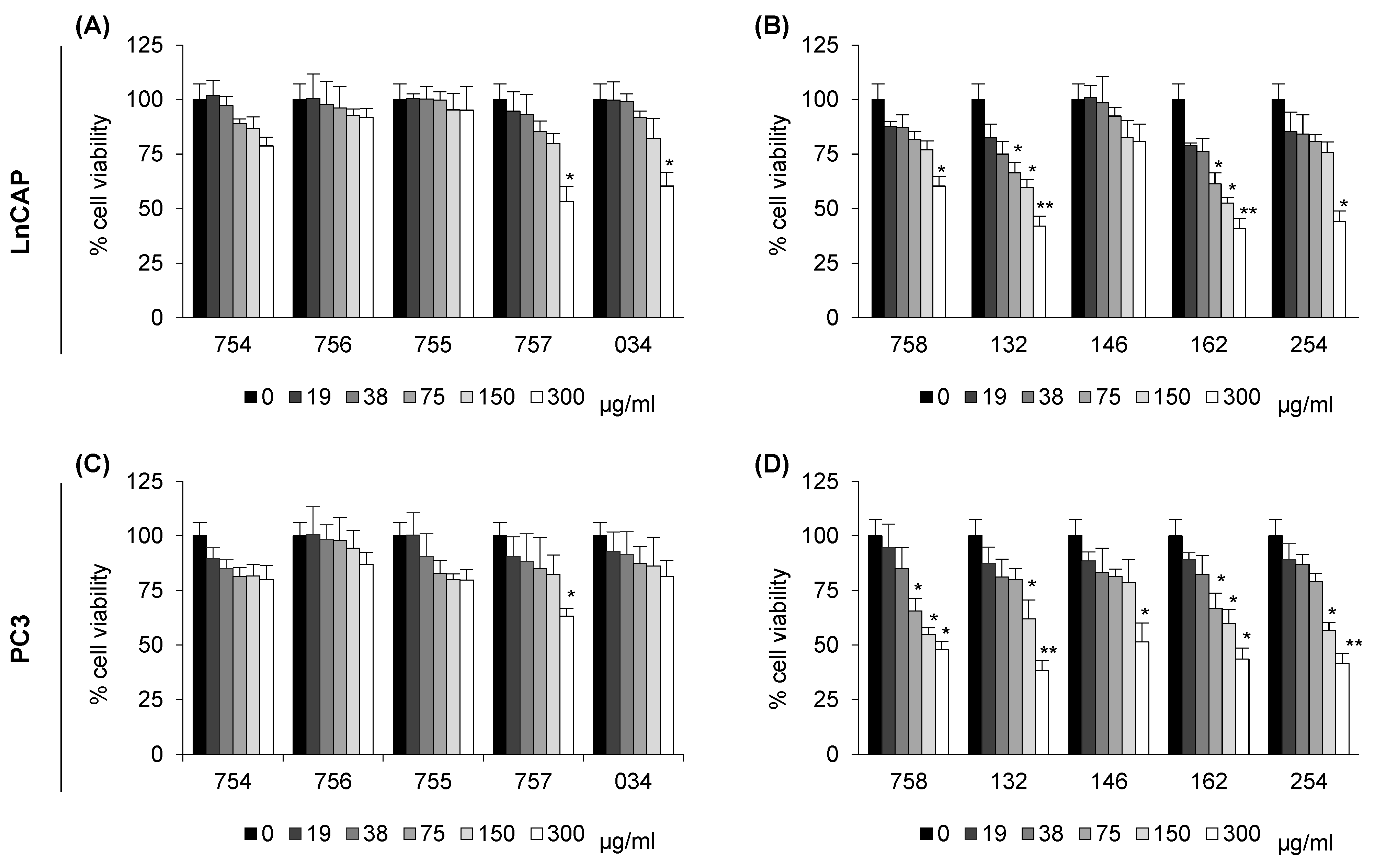
2.1. Analysis of Cell Viability
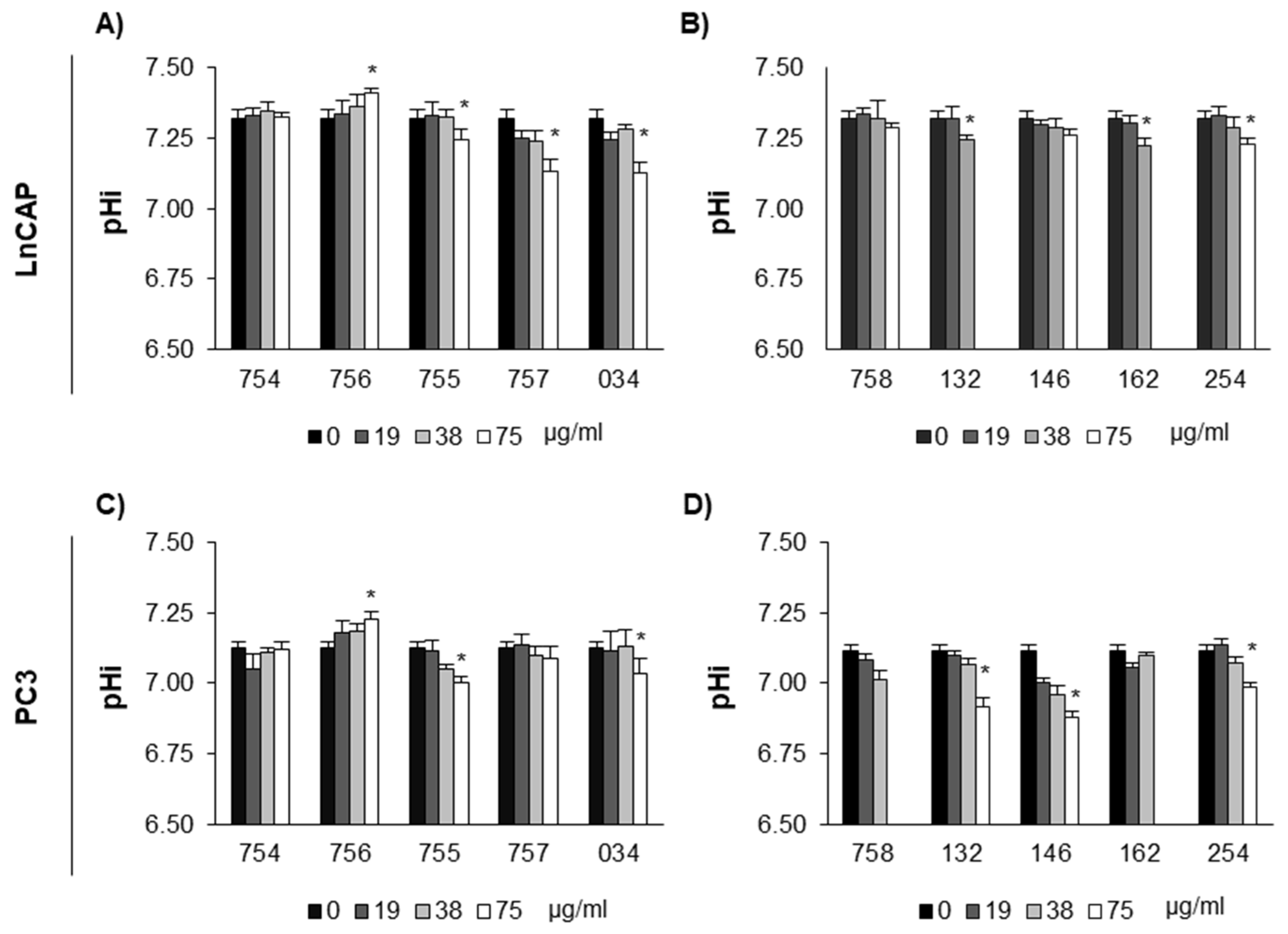
2.2. Measure of Intracellular pH
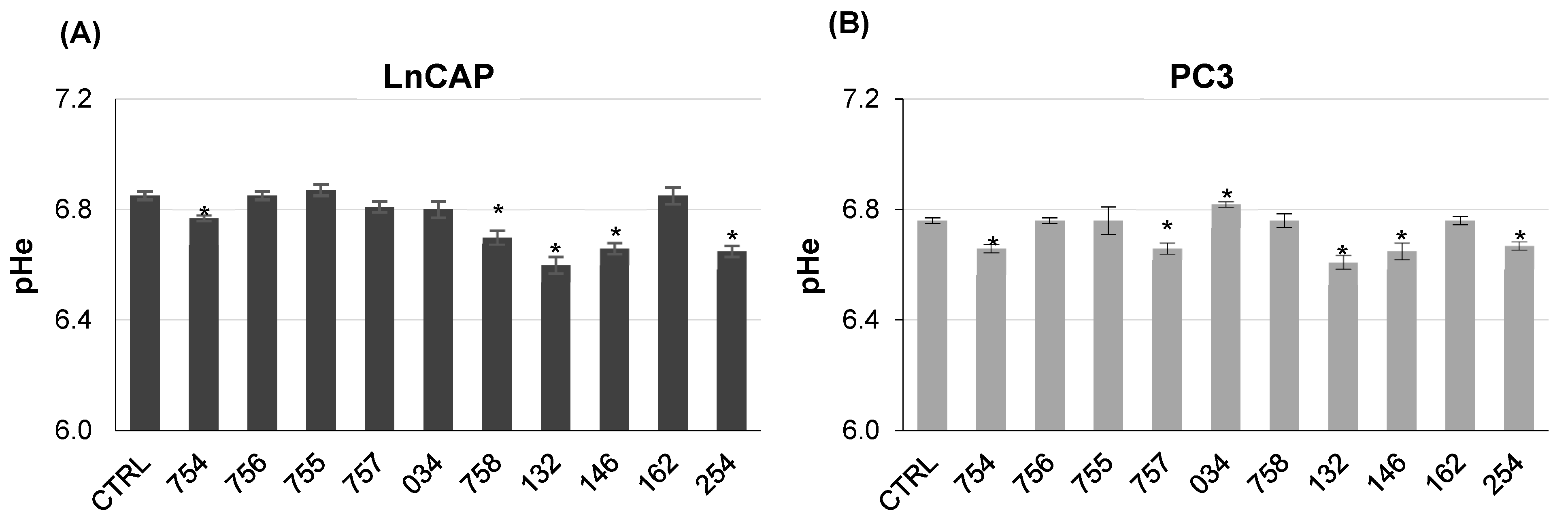
2.3. Measure of Extracellular pH
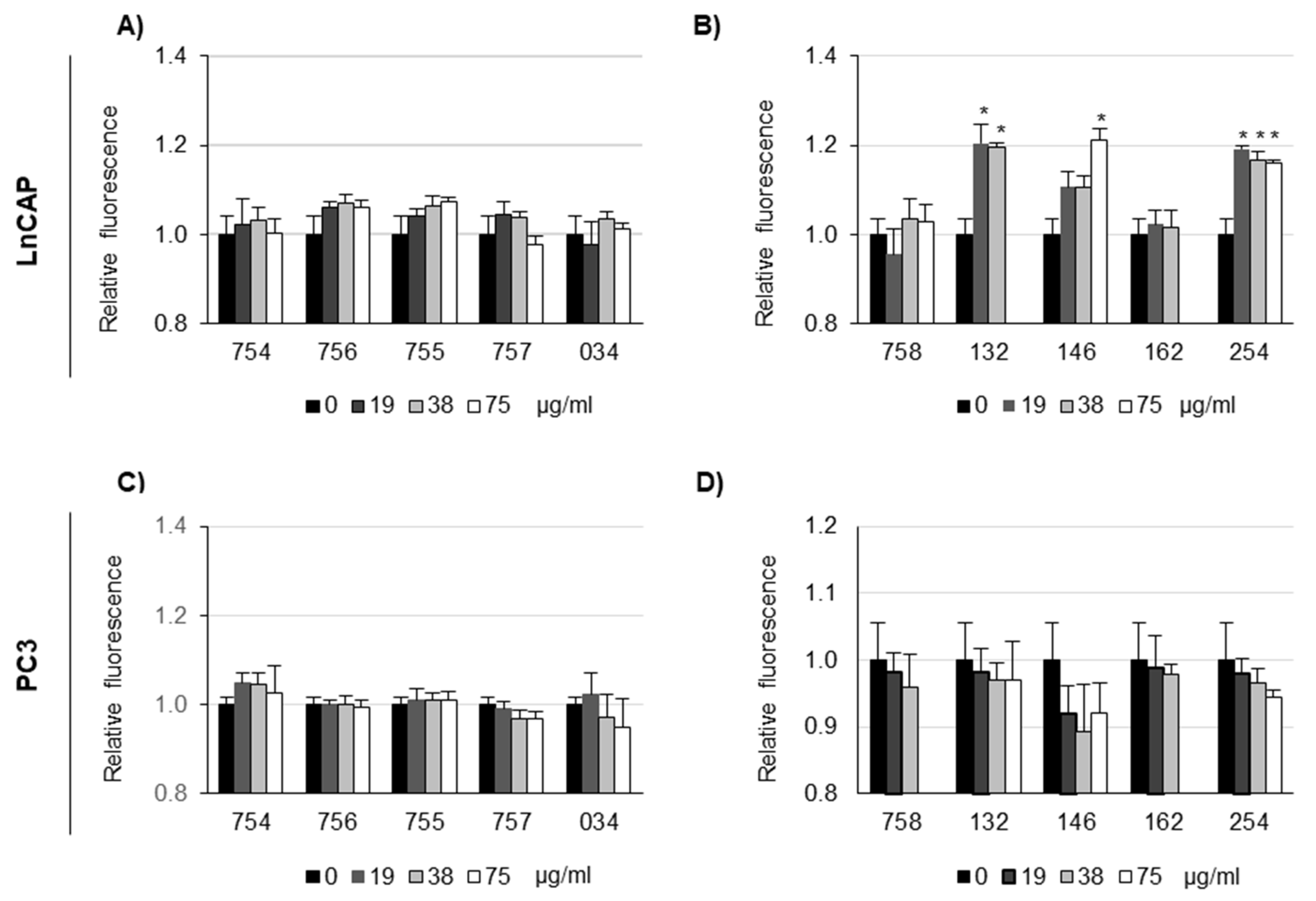
2.4. Measure of Organelle pH
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Freeze-Dried Herbal Extracts
4.3. Cell Culture and Treatments
4.4. MTT Assay
4.5. Measurement of Intracellular pH: BCECF-AM Assay
4.6. Measurement of Extracellular pH
4.7. Measurement of Organelle pH
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Gillies, R.J.; Raghunand, N.; Karczmar, G.S.; Bhujwalla, Z.M. MRI of the tumor microenvironment. J. Magn. Reson. Imaging 2002, 16, 430–450. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef]
- Helmlinger, G.; Sckell, A.; Dellian, M.; Forbes, N.S.; Jain, R.K. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin. Cancer Res. 2002, 8, 1284–1291. [Google Scholar]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.; Camisaschi, C.; Berzi, A.; Ferro, S.; Lugini, L.; Triulzi, T.; Tuccitto, A.; Tagliabue, E.; Castelli, C.; Rivoltini, L. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin. Cancer Biol. 2017, 43, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [CrossRef]
- Izumi, H.; Torigoe, T.; Ishiguchi, H.; Uramoto, H.; Yoshida, Y.; Tanabe, M.; Ise, T.; Murakami, T.; Yoshida, T.; Nomoto, M.; et al. Cellular pH regulators: Potentially promising molecular targets for cancer chemotherapy. Cancer Treat. Rev. 2003, 29, 541–549. [Google Scholar] [CrossRef]
- Mboge, M.; Mahon, B.; McKenna, R.; Frost, S. Carbonic Anhydrases: Role in pH Control and Cancer. Metabolites 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Alper, S.L. Molecular physiology of SLC4 anion exchangers. Exp. Physiol. 2006, 91, 153–161. [Google Scholar] [CrossRef]
- Alper, S.L.; Sharma, A.K. The SLC26 gene family of anion transporters and channels. Mol. Asp. Med. 2013, 34, 494–515. [Google Scholar] [CrossRef]
- Halestrap, A.P. The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life 2012, 64, 1–9. [Google Scholar] [CrossRef]
- Stock, C.; Pedersen, S.F. Roles of pH and the Na+/H+ exchanger NHE1 in cancer: From cell biology and animal models to an emerging translational perspective? Semin. Cancer Biol. 2017, 43, 5–16. [Google Scholar] [CrossRef]
- Brisson, L.; Driffort, V.; Benoist, L.; Poet, M.; Counillon, L.; Antelmi, E.; Rubino, R.; Besson, P.; Labbal, F.; Chevalier, S.; et al. NaV1.5 Na+ channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J. Cell Sci. 2013, 126, 4835–4842. [Google Scholar] [CrossRef]
- Pamarthy, S.; Kulshrestha, A.; Katara, G.K.; Beaman, K.D. The curious case of vacuolar ATPase: Regulation of signaling pathways. Mol. Cancer 2018, 17, 41. [Google Scholar] [CrossRef]
- Chen, R.; Jäättelä, M.; Liu, B. Lysosome as a Central Hub for Rewiring pH Homeostasis in Tumors. Cancers 2020, 12, 2437. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.P.; Forgac, M. Regulation and function of V-ATPases in physiology and disease. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183341. [Google Scholar] [CrossRef]
- Chiche, J.; Ilc, K.; Laferriere, J.; Trottier, E.; Dayan, F.; Mazure, N.M.; Brahimi-Horn, M.C.; Pouyssegur, J. Hypoxia-Inducible Carbonic Anhydrase IX and XII Promote Tumor Cell Growth by Counteracting Acidosis through the Regulation of the Intracellular pH. Cancer Res. 2009, 69, 358–368. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996, 56, 1194–1198. [Google Scholar] [PubMed]
- Parks, S.K.; Pouysségur, J. Targeting pH regulating proteins for cancer therapy-Progress and limitations. Semin. Cancer Biol. 2017, 43, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef]
- Koltai, T. Cancer: Fundamentals behind pH targeting and the double-edged approach. OncoTargets Ther. 2016, 9, 6343–6360. [Google Scholar] [CrossRef] [PubMed]
- Cerella, C.; Radogna, F.; Dicato, M.; Diederich, M. Natural compounds as regulators of the cancer cell metabolism. Int. J. Cell Biol. 2013, 2013, 639401. [Google Scholar] [CrossRef] [PubMed]
- Levraut, J.; Giunti, C.; Ciebiera, J.P.; de Sousa, G.; Ramhani, R.; Payan, P.; Grimaud, D. Initial effect of sodium bicarbonate on intracellular pH depends on the extracellular nonbicarbonate buffering capacity. Crit. Care Med. 2001, 29, 1033–1039. [Google Scholar] [CrossRef]
- Lin, H.J.; Herman, P.; Kang, J.S.; Lakowicz, J.R. Fluorescence lifetime characterization of novel low-pH probes. Anal. Biochem. 2001, 294, 118–125. [Google Scholar] [CrossRef]
- Pillai, S.R.; Damaghi, M.; Marunaka, Y.; Spugnini, E.P.; Fais, S.; Gillies, R.J. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev. 2019, 38, 205–222. [Google Scholar] [CrossRef]
- Bogdanov, A.; Bogdanov, A.; Chubenko, V.; Volkov, N.; Moiseenko, F.; Moiseyenko, V. Tumor acidity: From hallmark of cancer to target of treatment. Front. Oncol. 2022, 12, 979154. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Amaravadi, R.K. Targeting the lysosome in cancer. Ann. N. Y. Acad. Sci. 2016, 1371, 45–54. [Google Scholar] [CrossRef]
- Trybus, W.; Trybus, E.; Król, T. Lysosomes as a Target of Anticancer Therapy. Int. J. Mol. Sci. 2023, 24, 2176. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, G.; Bai, W.; Han, X.; Li, C.; Bian, S. The Role of Bioactive Compounds in Natural Products Extracted from Plants in Cancer Treatment and Their Mechanisms Related to Anticancer Effects. Oxid. Med. Cell Longev. 2022, 2022, 1429869. [Google Scholar] [CrossRef]
- Parisio, C.; Lucarini, E.; Micheli, L.; Toti, A.; Di Cesare Mannelli, L.; Antonini, G.; Panizzi, E.; Maidecchi, A.; Giovagnoni, E.; Lucci, J.; et al. Researching New Therapeutic Approaches for Abdominal Visceral Pain Treatment: Preclinical Effects of an Assembled System of Molecules of Vegetal Origin. Nutrients 2019, 12, 22. [Google Scholar] [CrossRef]
- Zheng, T.; Jäättelä, M.; Liu, B. pH gradient reversal fuels cancer progression. Int. J. Biochem. Cell Biol. 2020, 125, 105796. [Google Scholar] [CrossRef]
- Swietach, P.; Boedtkjer, E.; Pedersen, S.F. How protons pave the way to aggressive cancers. Nat. Rev. Cancer 2023, 23, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Ravenna, L.; Principessa, L.; Verdina, A.; Salvatori, L.; Russo, M.A.; Petrangeli, E. Distinct phenotypes of human prostate cancer cells associate with different adaptation to hypoxia and pro-inflammatory gene expression. PLoS ONE 2014, 9, e96250. [Google Scholar] [CrossRef]
- Ferreri, C.; Sansone, A.; Buratta, S.; Urbanelli, L.; Costanzi, E.; Emiliani, C.; Chatgilialoglu, C. The n-10 Fatty Acids Family in the Lipidome of Human Prostatic Adenocarcinoma Cell Membranes and Extracellular Vesicles. Cancers 2020, 12, 900. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, D. Naviglio’s Principle and Presentation of an Innovative Solid–Liquid Extraction Technology: Extractor Naviglio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Graber, M.L.; DiLillo, D.C.; Friedman, B.L.; Pastoriza-Munoz, E. Characteristics of fluoroprobes for measuring intracellular pH. Anal. Biochem. 1986, 156, 202–212. [Google Scholar] [CrossRef]
- Grant, R.L.; Acosta, D. Ratiometric measurement of intracellular pH of cultured cells with BCECF in a fluorescence multi-well plate reader. In Vitro Cell Dev. Biol. Anim. 1997, 33, 256–260. [Google Scholar] [CrossRef]
- Pi, H.; Li, M.; Tian, L.; Yang, Z.; Yu, Z.; Zhou, Z. Enhancing lysosomal biogenesis and autophagic flux by activating the transcription factor EB protects against cadmium-induced neurotoxicity. Sci. Rep. 2017, 7, 43466. [Google Scholar] [CrossRef]
| Description | Code | Binomial Name | Plant Part | Extraction Solvent | Plant-to-Solvent Ratio | Extraction Time | Extraction Temperature | Freeze-Dried Extract Yield |
|---|---|---|---|---|---|---|---|---|
| Long pepper | 2016-254 | Piper longum L. | Grains | Ethanol 70% | 1/2 | 7 h | 40 °C | 7% |
| Nutmeg | 2016-132 | Myristica fragrans Houtt | Seed | Ethanol 70% | 1/5 | 7 h | 40 °C | 4% |
| Feverfew | 2016-162 | Tanacetum parthenium L. | Flowers | Ethanol 70% | 1/8 | 7 h | 40 °C | 16% |
| Filipendula | 2016-757 | Filupendula vulgaris Hill | Flowered aerial parts | Ethanol 50% | 1/10 | 6 h | 40 °C | 18% |
| Laurel freeze | 2014-034 | Laurus Laurus nobilis L. | Leaf | Ethanol 70% | 1/6 | 7 h | 40 °C | 15% |
| Artichoke | 2016-758 | Cynara scolymus L. | Leaf | Ethanol 50% | 1/10 | 8 h | 50 °C | 17% |
| Turmeric | 2016-755 | Curcuma longa L. | Root | Ethanol 40% | 1/8 | 8 h | 50 °C | 7% |
| Poplar freeze | 2013-146 | Populus nigra L. | Buds | 1-Ethanol 85% 2-Ethanol 8% | 1–1/6 2–1/6 | 1–6 h 2–6 h | 50 °C | 12% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanelli, L.; Sagini, K.; Delo, F.; Buratta, S.; Lucci, J.; Mercati, V.; Emiliani, C. Targeting pH Inversion in Prostate Cancer Cells: A Role for Systems of Molecules of Vegetal Origin. Int. J. Mol. Sci. 2025, 26, 7700. https://doi.org/10.3390/ijms26167700
Urbanelli L, Sagini K, Delo F, Buratta S, Lucci J, Mercati V, Emiliani C. Targeting pH Inversion in Prostate Cancer Cells: A Role for Systems of Molecules of Vegetal Origin. International Journal of Molecular Sciences. 2025; 26(16):7700. https://doi.org/10.3390/ijms26167700
Chicago/Turabian StyleUrbanelli, Lorena, Krizia Sagini, Federica Delo, Sandra Buratta, Jacopo Lucci, Valentino Mercati, and Carla Emiliani. 2025. "Targeting pH Inversion in Prostate Cancer Cells: A Role for Systems of Molecules of Vegetal Origin" International Journal of Molecular Sciences 26, no. 16: 7700. https://doi.org/10.3390/ijms26167700
APA StyleUrbanelli, L., Sagini, K., Delo, F., Buratta, S., Lucci, J., Mercati, V., & Emiliani, C. (2025). Targeting pH Inversion in Prostate Cancer Cells: A Role for Systems of Molecules of Vegetal Origin. International Journal of Molecular Sciences, 26(16), 7700. https://doi.org/10.3390/ijms26167700










