How Actin Polymerization and Myosin II Activity Regulate Focal Adhesion Dynamics in Motile Cells
Abstract
1. Introduction
2. Results
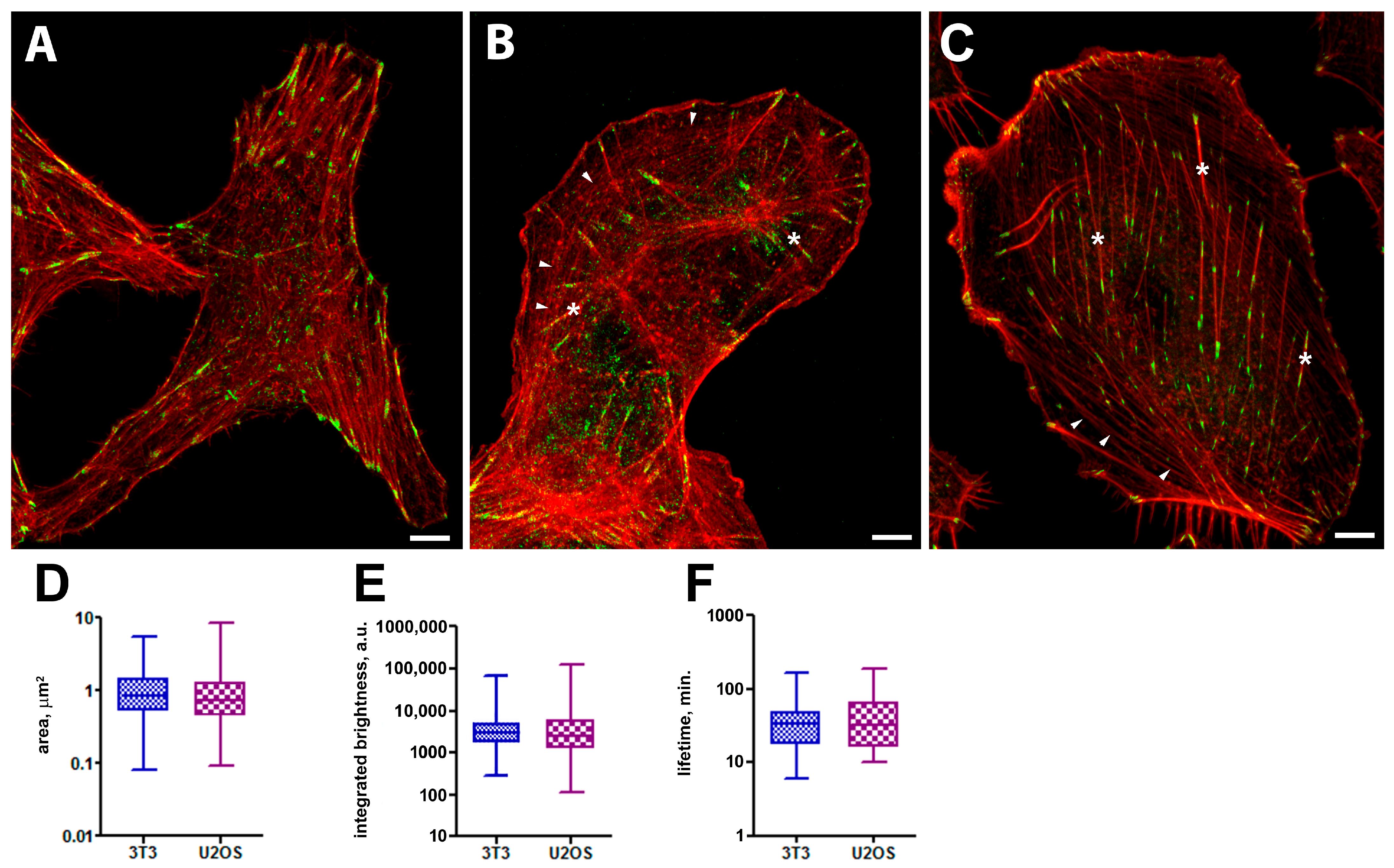
2.1. Morphology and Dynamics of FAs in Control Cells
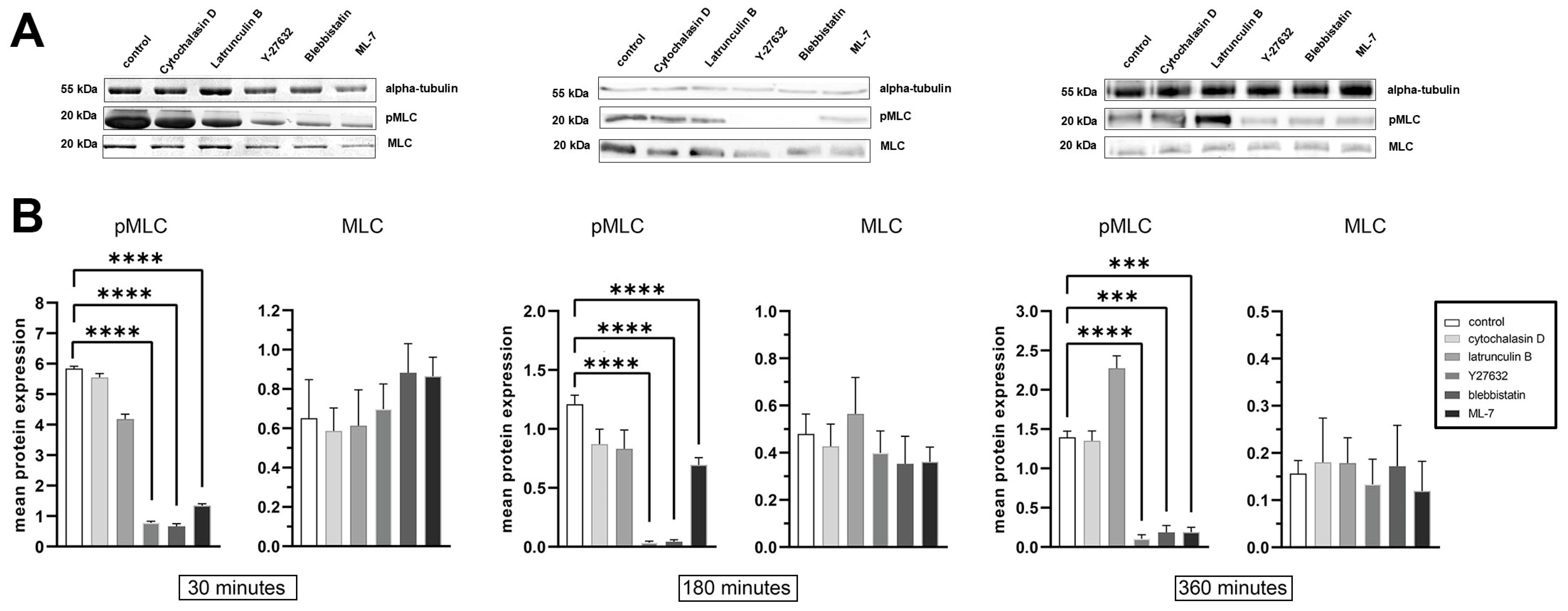
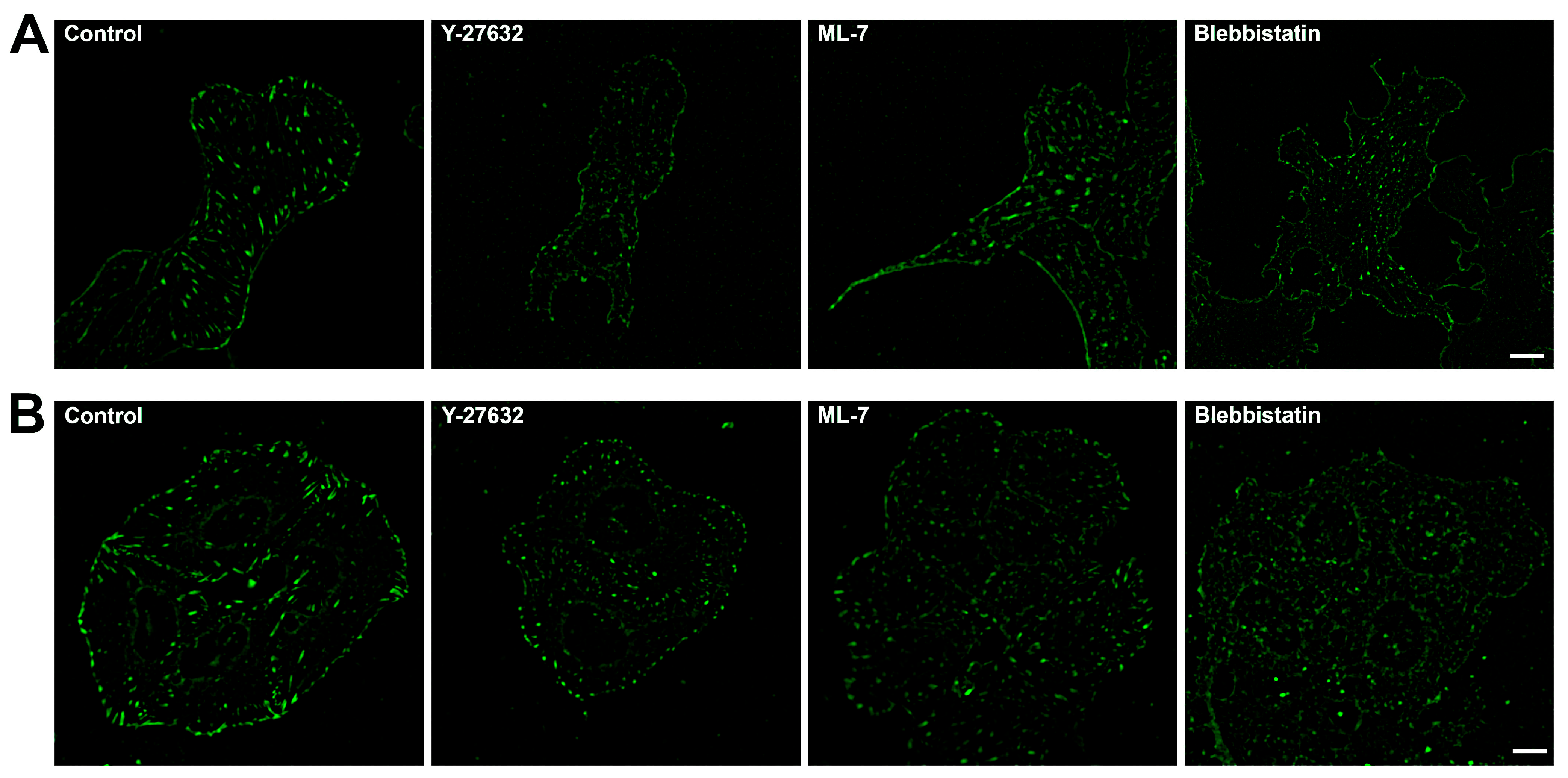
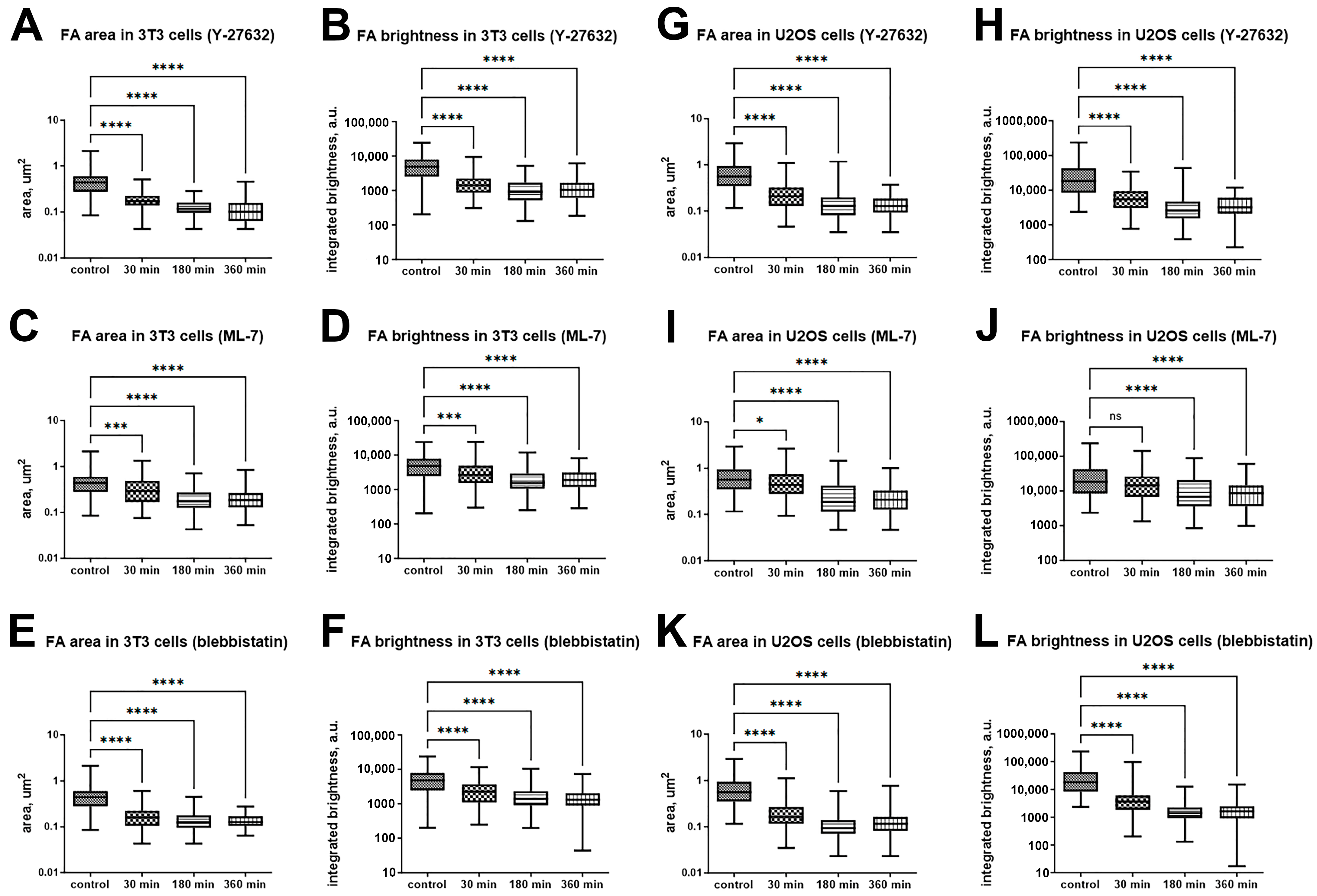
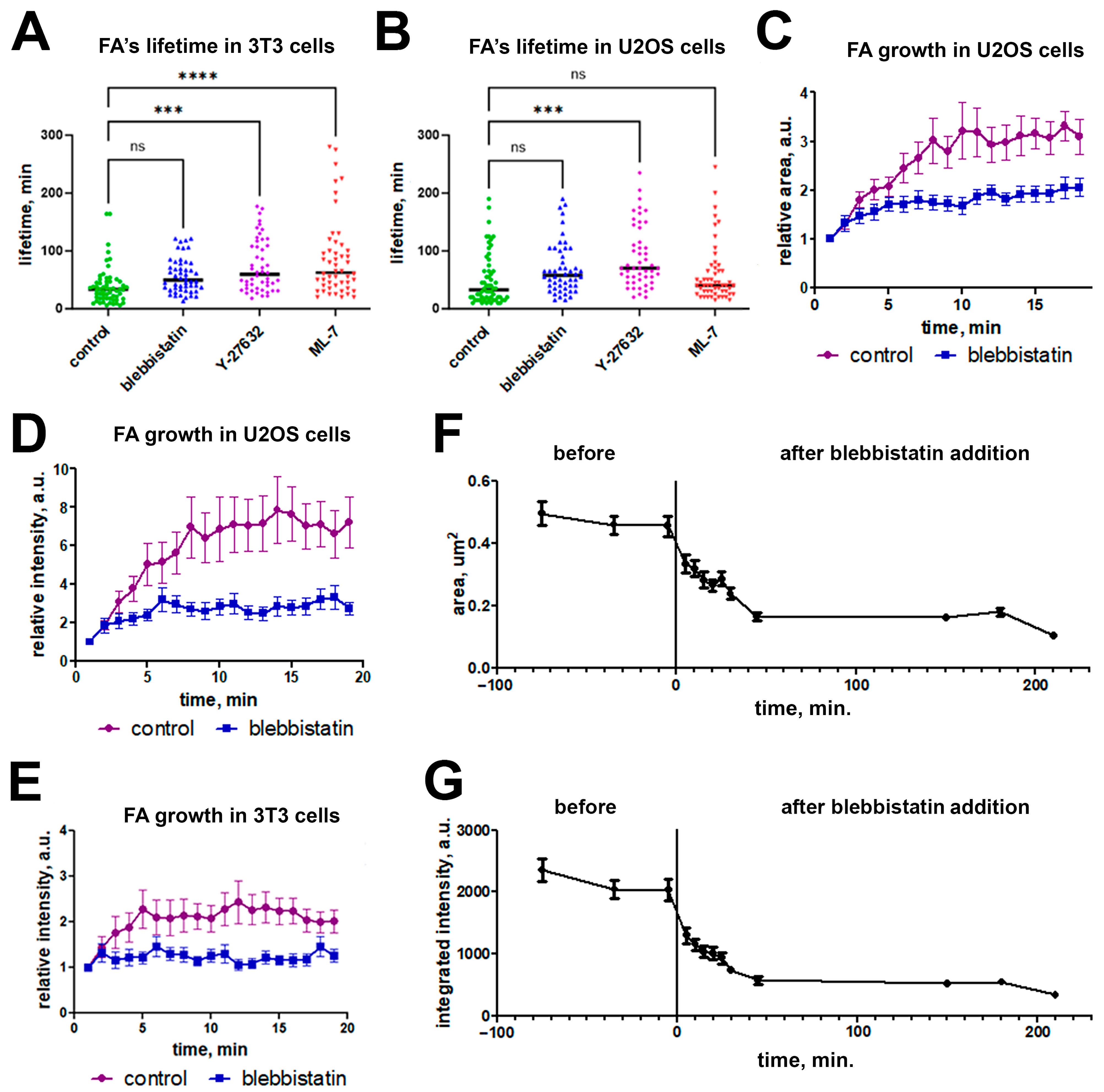
2.2. Focal Adhesions Decrease in Area and Brightness, but Do Not Completely Disappear After Inhibition of Myosin II Phosphorylation
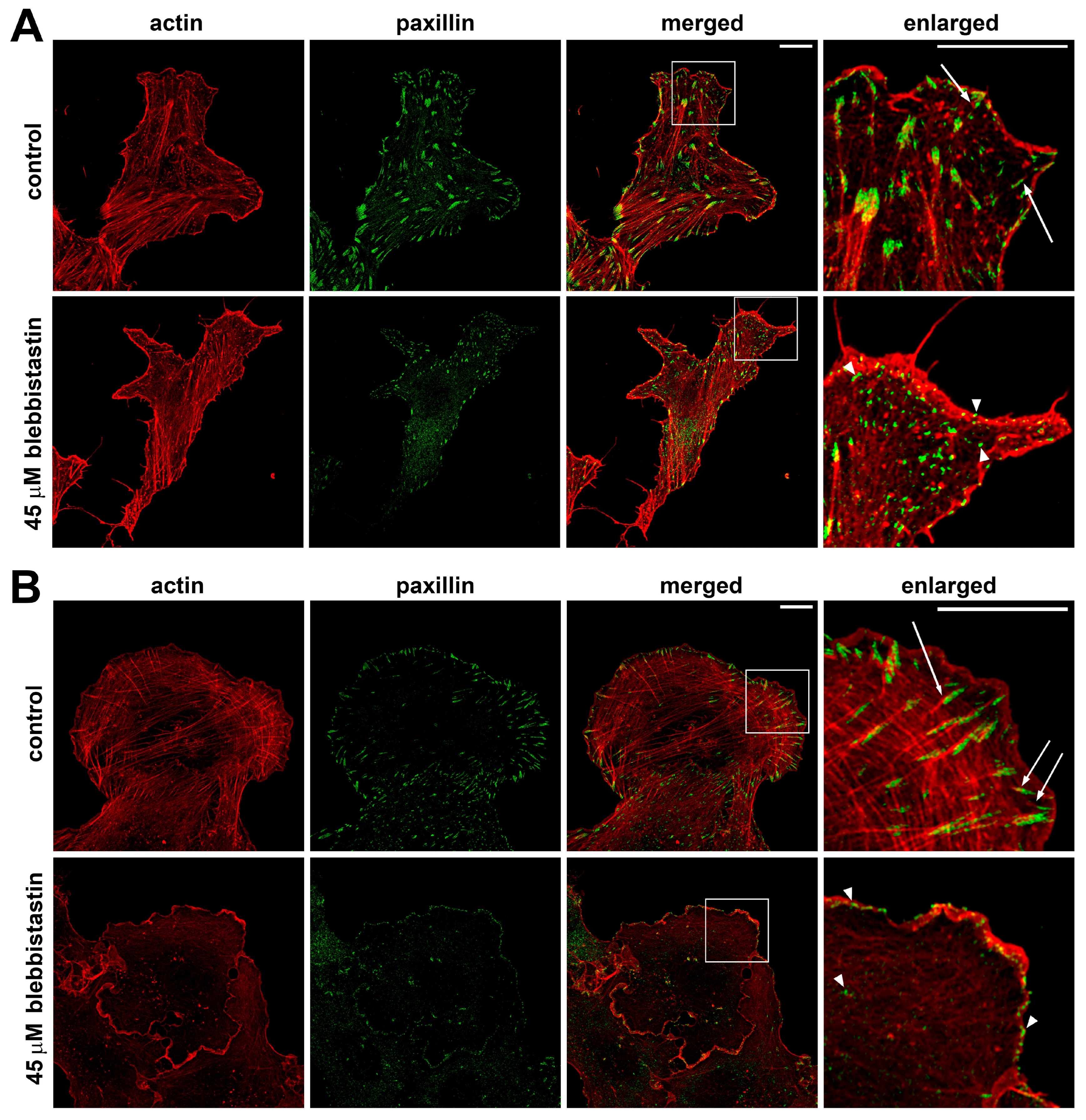
2.3. Blebbistatin Treatment Promotes Disassembly of Pre-Formed FAs and Inhibits Growth of New FAs
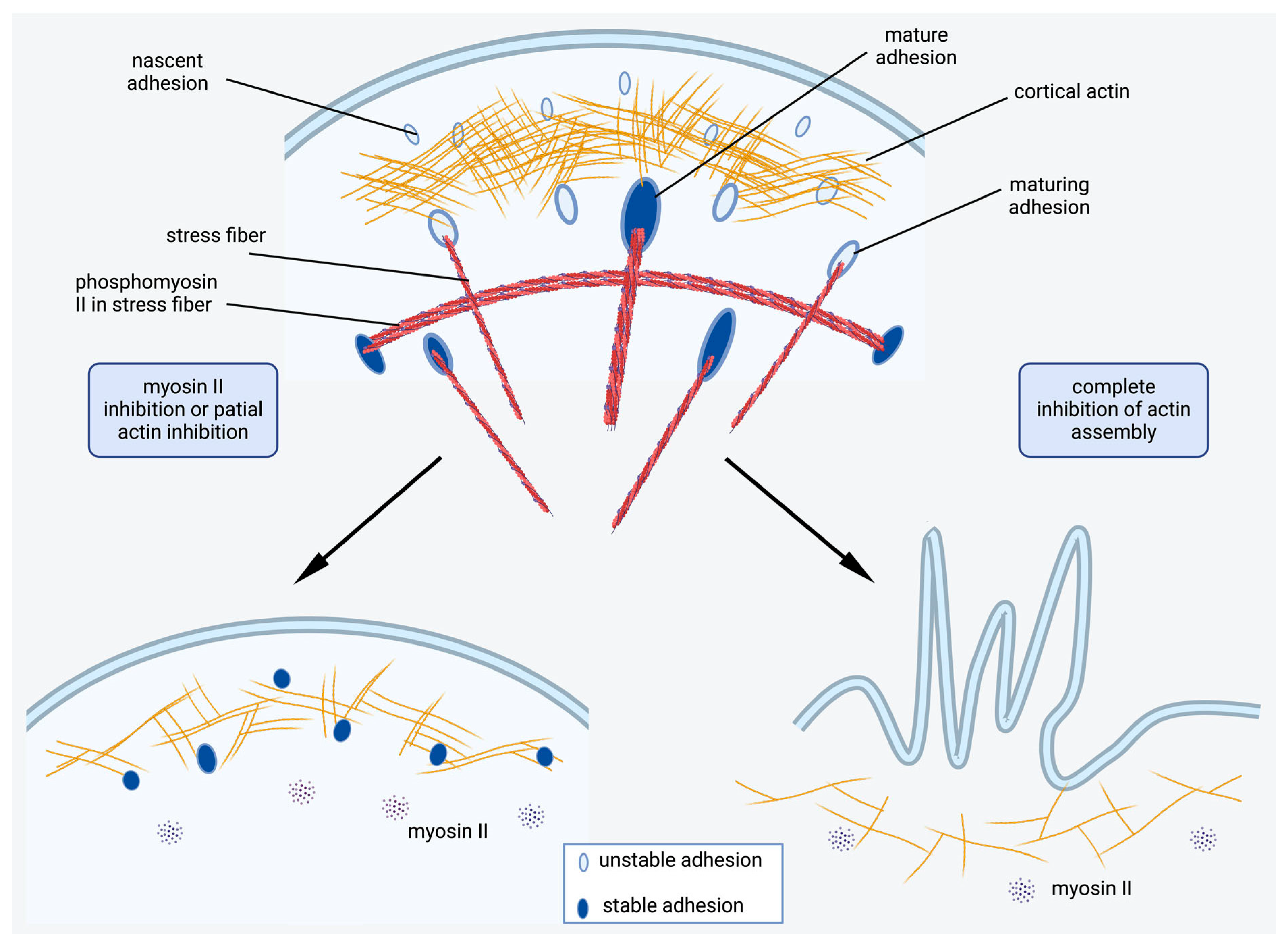
2.4. Actin Cytoskeleton Changes Under the Action of Myosin II Inhibitors
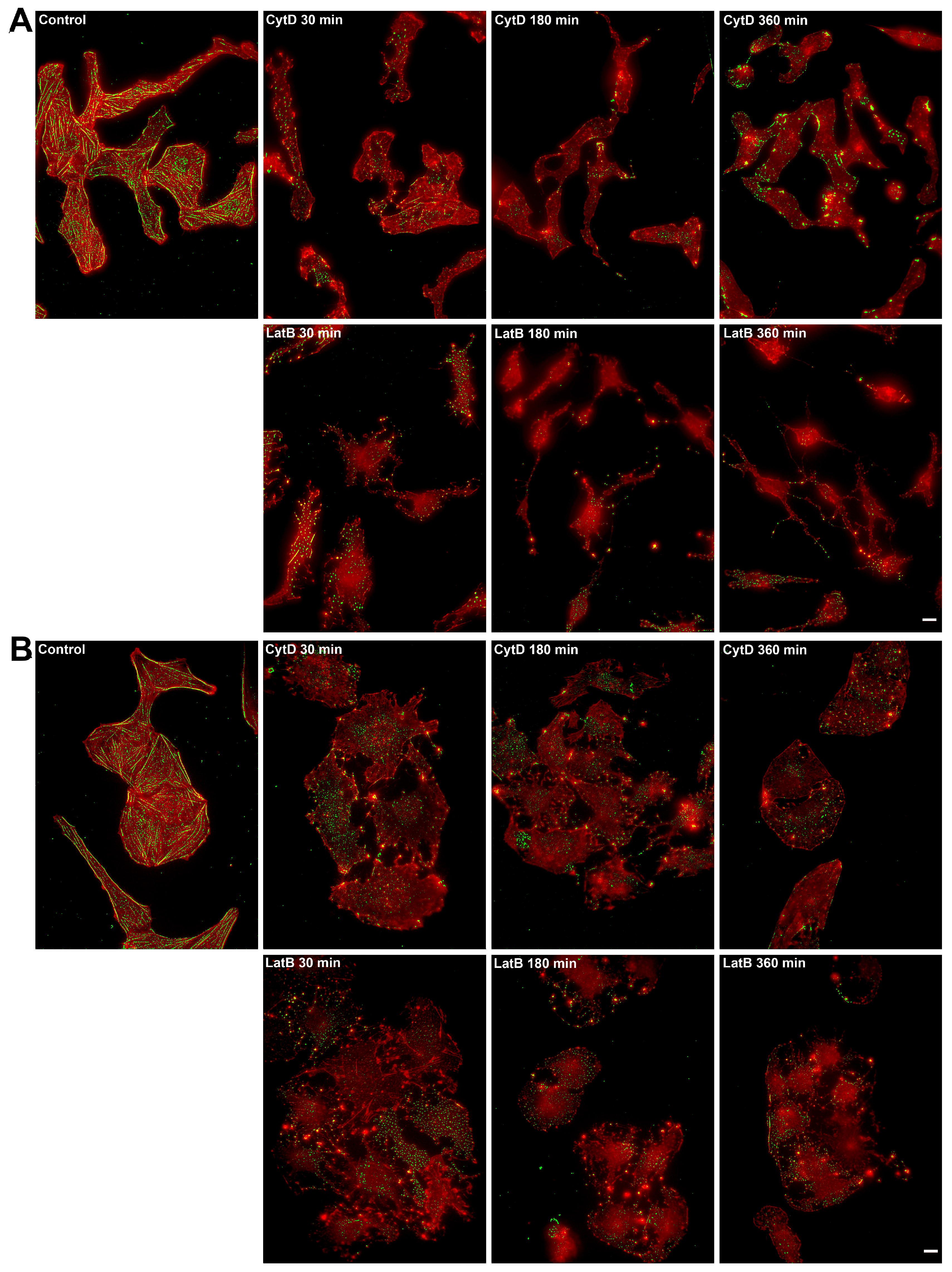
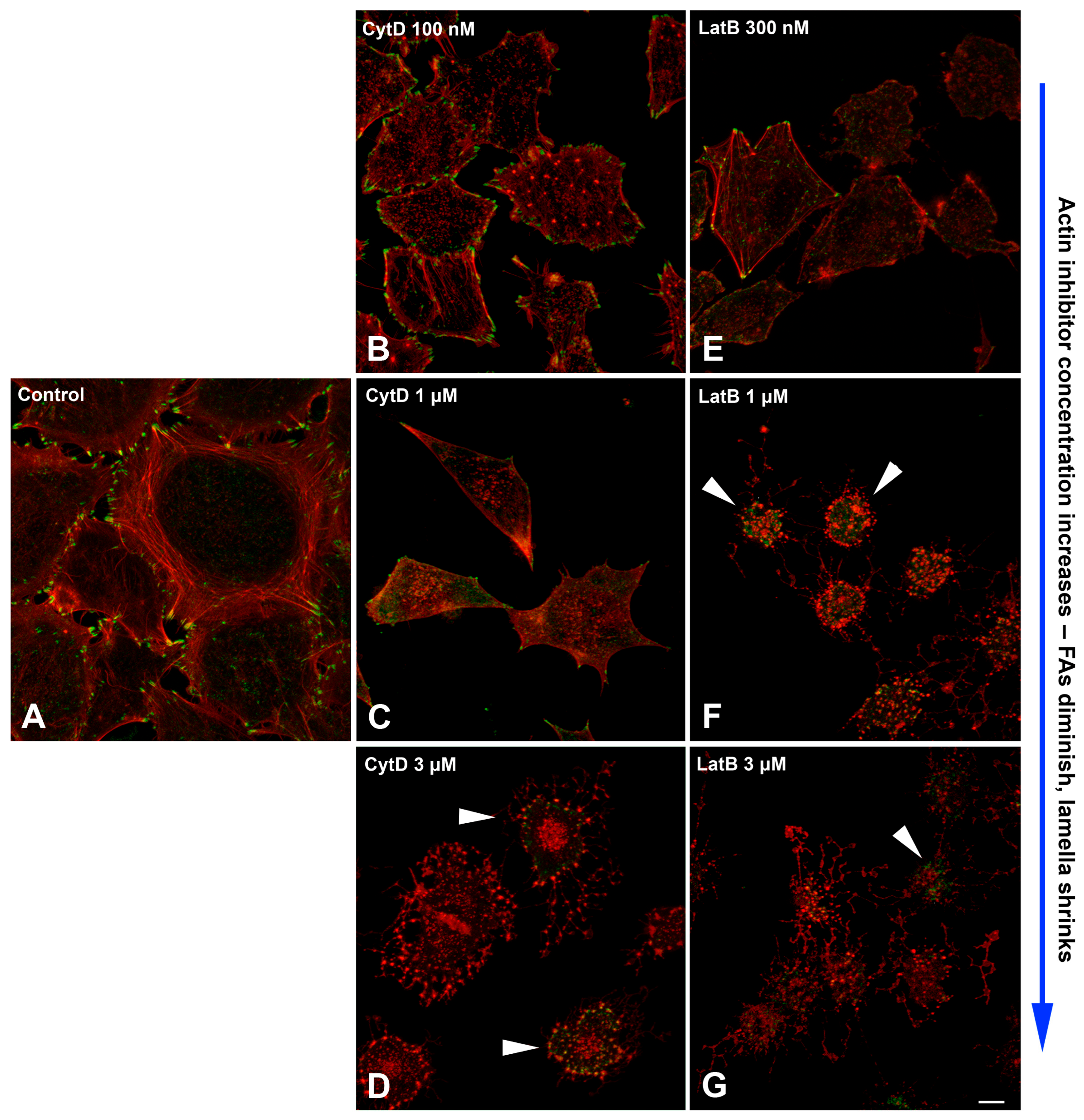
2.5. Actin Filaments Are Necessary for the Formation of New Dynamic FAs
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Production of 3T3 and U2OS Model Cell Lines with Stable Vinculin Expression
4.3. Live Cell Fluorescence Microscopy
4.4. Immunocytochemical Staining
4.5. siRNA Transfection and Validation
4.6. RTqPCR
4.7. Inhibitory Analysis
4.8. Confocal Microscopy with Airyscan Super-Resolution Module
4.9. Western Blot
4.10. Image Processing and Data Analysis
4.11. Statistical Analysis and Data Visualization
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cimmino, C.; Rossano, L.; Netti, P.A.; Ventre, M. Spatio-Temporal Control of Cell Adhesion: Toward Programmable Platforms to Manipulate Cell Functions and Fate. Front. Bioeng. Biotechnol. 2018, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Horwitz, A.R. Adhesion dynamics at a glance. J. Cell Sci. 2011, 124 Pt 23, 3923–3927. [Google Scholar] [CrossRef] [PubMed]
- Winograd-Katz, S.; Fässler, R.; Geiger, B.; Legate, K.R. The integrin adhesome: From genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Wehrle-Haller, B. Structure and function of focal adhesions. Curr. Opin. Cell Biol. 2012, 24, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Revach, O.Y.; Grosheva, I.; Geiger, B. Biomechanical regulation of focal adhesion and invadopodia formation. J. Cell Sci. 2020, 133, jcs244848. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, A.Y.; Arnold, K.; Schaub, S.; Vasiliev, J.M.; Meister, J.J.; Bershadsky, A.D.; Verkhovsky, A.B. Comparative dynamics of retrograde actin flow and focal adhesions: Formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE 2008, 3, e3234. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Ven, K.; Chastney, M.; Kokate, S.B.; Peränen, J.; Aaron, J.; Kogan, K.; Almeida-Souza, L.; Kremneva, E.; Poincloux, R.; et al. Focal adhesions contain three specialized actin nanoscale layers. Nat. Commun. 2024, 15, 2547. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal Crosstalk in Cell Migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Zaidel-Bar, R.; Cohen, M.; Addadi, L.; Geiger, B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 2004, 32 Pt 3, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Giannone, G.; Mège, R.M.; Thoumine, O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009, 19, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska-Wodnicka, M.; Burridge, K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996, 133, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Wehrle-Haller, B. Assembly and disassembly of cell matrix adhesions. Curr. Opin. Cell Biol. 2012, 24, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Newell-Litwa, K.; Bachir, A.I.; Whitmore, L.A.; Horwitz, A.R. Myosin IIA/IIB restrict adhesive and protrusive signaling to generate front-back polarity in migrating cells. J. Cell Biol. 2011, 193, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, H.; Bershadsky, A.; Henis, Y.I.; Geiger, B. Actomyosin-generated tension controls the molecular kinetics of focal adhesions. J. Cell Sci. 2011, 124 Pt 9, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Stricker, J.; Beckham, Y.; Davidson, M.W.; Gardel, M.L. Myosin II-mediated focal adhesion maturation is tension insensitive. PLoS ONE 2013, 8, e70652. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Azarova, E.V.; Whitewood, A.J.; Bachir, A.; Guttierrez, E.; Groisman, A.; Horwitz, A.R.; Goult, B.T.; Dean, K.M.; Danuser, G. Pre-complexation of talin and vinculin without tension is required for efficient nascent adhesion maturation. eLife 2021, 10, e66151. [Google Scholar] [CrossRef] [PubMed]
- Pasapera, A.M.; Schneider, I.C.; Rericha, E.; Schlaepfer, D.D.; Waterman, C.M. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol. 2010, 188, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Cuenca, R.; Llorente-González, C.; Chapman, J.R.; Talayero, V.C.; Garrido-Casado, M.; Delgado-Arévalo, C.; Millán-Salanova, M.; Shabanowitz, J.; Hunt, D.F.; Sellers, J.R.; et al. Tyrosine Phosphorylation of the Myosin Regulatory Light Chain Controls Non-muscle Myosin II Assembly and Function in Migrating Cells. Curr. Biol. 2020, 30, 2446–2458.e6. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Manzanares, M.; Zareno, J.; Whitmore, L.; Choi, C.K.; Horwitz, A.F. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 2007, 176, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Tatsumi, H.; Sokabe, M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J. Cell Sci. 2008, 121 Pt 17, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Chugh, P.; Paluch, E.K. The actin cortex at a glance. J. Cell Sci. 2018, 131, jcs186254. [Google Scholar] [CrossRef] [PubMed]
- Bershadsky, A.; Kozlov, M.; Geiger, B. Adhesion-mediated mechanosensitivity: A time to experiment, and a time to theorize. Curr. Opin. Cell Biol. 2006, 18, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Rossier, O.; Octeau, V.; Sibarita, J.B.; Leduc, C.; Tessier, B.; Nair, D.; Gatterdam, V.; Destaing, O.; Albigès-Rizo, C.; Tampé, R.; et al. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat. Cell Biol. 2012, 14, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Dumbauld, D.W.; Michael, K.E.; Hanks, S.K.; García, A.J. Focal adhesion kinase-dependent regulation of adhesive forces involves vinculin recruitment to focal adhesions. Biol. Cell 2010, 102, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.H.; Frey, M.T.; Burnham, N.A.; Wang, Y.L. Substrate rigidity regulates the formation and maintenance of tissues. Biophys. J. 2006, 90, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Casado, M.; Asensio-Juárez, G.; Vicente-Manzanares, M. Nonmuscle Myosin II Regulation Directs Its Multiple Roles in Cell Migration and Division. Annu. Rev. Cell Dev. Biol. 2021, 37, 285–310. [Google Scholar] [CrossRef] [PubMed]
- Holle, A.W.; McIntyre, A.J.; Kehe, J.; Wijesekara, P.; Young, J.L.; Vincent, L.G.; Engler, A.J. High content image analysis of focal adhesion-dependent mechanosensitive stem cell differentiation. Integr. Biol. 2016, 8, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, M.; Wehrle-Haller, B.; de Curtis, I. Paxillin: A Hub for Mechano-Transduction from the β3 Integrin-Talin-Kindlin Axis. Front. Cell Dev. Biol. 2022, 10, 852016. [Google Scholar] [CrossRef] [PubMed]
- Shoyer, T.C.; Gates, E.M.; Cabe, J.I.; Conway, D.E.; Hoffman, B.D. Coupling During Collective Cell Migration is Controlled by a Vinculin Mechanochemical Switch. bioRxiv 2023, 120, e2316456120. [Google Scholar] [CrossRef] [PubMed]
- Grandy, C.; Port, F.; Pfeil, J.; Oliva, M.A.G.; Vassalli, M.; Gottschalk, K.E. Cell shape and tension alter focal adhesion structure. Biomater. Adv. 2003, 145, 213277. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.K.; Vicente-Manzanares, M.; Zareno, J.; Whitmore, L.A.; Mogilner, A.; Horwitz, A.R. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008, 10, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Gardel, M.L.; Schneider, I.C.; Aratyn-Schaus, Y.; Waterman, C.M. Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 2010, 26, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Hotulainen, P.; Lappalainen, P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006, 173, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Cronin, N.M.; DeMali, K.A. Dynamics of the Actin Cytoskeleton at Adhesion Complexes. Biology 2021, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hosoya, H.; Yonemura, S. Regulation of myosin II dynamics by phosphorylation and dephosphorylation of its light chain in epithelial cells. Mol. Biol. Cell 2007, 18, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, C.; Zhu, C.; Salaita, K. DNA-based digital tension probes reveal integrin forces during early cell adhesion. Nat. Commun. 2014, 5, 5167. [Google Scholar] [CrossRef] [PubMed]
- Changede, R.; Xu, X.; Margadant, F.; Sheetz, M.P. Nascent Integrin Adhesions Form on All Matrix Rigidities after Integrin Activation. Dev. Cell 2015, 35, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Oakes, P.W.; Beckham, Y.; Stricker, J.; Gardel, M.L. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J. Cell Biol. 2012, 196, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Le Dévédec, S.E.; Geverts, B.; de Bont, H.; Yan, K.; Verbeek, F.J.; Houtsmuller, A.B.; van de Water, B. The residence time of focal adhesion kinase (FAK) and paxillin at focal adhesions in renal epithelial cells is determined by adhesion size, strength and life cycle status. J. Cell Sci. 2012, 125 Pt 19, 4498–4506. [Google Scholar] [CrossRef] [PubMed]
- Lavelin, I.; Wolfenson, H.; Patla, I.; Henis, Y.I.; Medalia, O.; Volberg, T.; Livne, A.; Kam, Z.; Geiger, B. Differential effect of actomyosin relaxation on the dynamic properties of focal adhesion proteins. PLoS ONE 2013, 8, e73549. [Google Scholar] [CrossRef] [PubMed]
- Küpper, K.; Lang, N.; Möhl, C.; Kirchgessner, N.; Born, S.; Goldmann, W.H.; Merkel, R.; Hoffmann, B. Tyrosine phosphorylation of vinculin at position 1065 modifies focal adhesion dynamics and cell tractions. Biochem. Biophys. Res. Commun. 2010, 399, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.; Lemière, J.; Faqir, F.; Manzi, J.; Blanchoin, L.; Plastino, J.; Sykes, C. Actin polymerization or myosin contraction: Two ways to build up cortical tension for symmetry breaking. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20130005. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Michels, E.B.; Massey, A.E.; Qiu, Y.; Royer-Weeden, S.P.; Smith, B.R.; Han, S.J. Myosin-independent stiffness sensing by fibroblasts is regulated by the viscoelasticity of flowing actin. Commun. Mater. 2024, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- DeMali, K.A.; Barlow, C.A.; Burridge, K. Recruitment of the Arp2/3 complex to vinculin: Coupling membrane protrusion to matrix adhesion. J. Cell Biol. 2002, 159, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Serrels, B.; Serrels, A.; Brunton, V.G.; Holt, M.; McLean, G.W.; Gray, C.H.; Frame, M.C. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat. Cell Biol. 2007, 9, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Bear, J.E.; Gertler, F.B. Ena/VASP: Towards resolving a pointed controversy at the barbed end. J. Cell Sci. 2009, 122 Pt 12, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Critchley, D.R. Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu. Rev. Biophys. 2009, 38, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, W.H.; Liddington, R.C.; Critchley, D.R. The structure and regulation of vinculin. Trends Cell Biol. 2006, 16, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Sjöblom, B.; Salmazo, A.; Djinović-Carugo, K. Alpha-actinin structure and regulation. Cell Mol. Life Sci. 2008, 65, 2688–2701. [Google Scholar] [CrossRef] [PubMed]
- Saphirstein, R.J.; Gao, Y.Z.; Lin, Q.Q.; Morgan, K.G. Cortical actin regulation modulates vascular contractility and compliance in veins. J. Physiol. 2015, 593, 3929–3941. [Google Scholar] [CrossRef] [PubMed]
- Berginski, M.E.; Vitriol, E.A.; Hahn, K.M.; Gomez, S.M. High-resolution quantification of focal adhesion spatiotemporal dynamics in living cells. PLoS ONE 2011, 6, e22025. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lin, K.H.; Chew, L.Y. Study on the regulation of focal adesions and cortical actin by matrix nanotopography in 3D environment. J. Phys. Condens. Matter 2017, 29, 455101. [Google Scholar] [CrossRef] [PubMed]
- Haviv, L.; Gillo, D.; Backouche, F.; Bernheim-Groswasser, A. A cytoskeletal demolition worker: Myosin II acts as an actin depolymerization agent. J. Mol. Biol. 2008, 375, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.A.; Tsuchida, M.A.; Allen, G.M.; Barnhart, E.L.; Applegate, K.T.; Yam, P.T.; Ji, L.; Keren, K.; Danuser, G.; Theriot, J.A. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature 2010, 465, 373–377. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovaleva, A.; Solomatina, E.; Tlegenova, M.; Saidova, A.; Vorobjev, I.A. How Actin Polymerization and Myosin II Activity Regulate Focal Adhesion Dynamics in Motile Cells. Int. J. Mol. Sci. 2025, 26, 7701. https://doi.org/10.3390/ijms26167701
Kovaleva A, Solomatina E, Tlegenova M, Saidova A, Vorobjev IA. How Actin Polymerization and Myosin II Activity Regulate Focal Adhesion Dynamics in Motile Cells. International Journal of Molecular Sciences. 2025; 26(16):7701. https://doi.org/10.3390/ijms26167701
Chicago/Turabian StyleKovaleva, Anastasiia, Evgeniya Solomatina, Madina Tlegenova, Aleena Saidova, and Ivan A. Vorobjev. 2025. "How Actin Polymerization and Myosin II Activity Regulate Focal Adhesion Dynamics in Motile Cells" International Journal of Molecular Sciences 26, no. 16: 7701. https://doi.org/10.3390/ijms26167701
APA StyleKovaleva, A., Solomatina, E., Tlegenova, M., Saidova, A., & Vorobjev, I. A. (2025). How Actin Polymerization and Myosin II Activity Regulate Focal Adhesion Dynamics in Motile Cells. International Journal of Molecular Sciences, 26(16), 7701. https://doi.org/10.3390/ijms26167701





