Coupled Bone–Muscle Degeneration in Chronic Pancreatitis: A Juvenile Porcine Model of Secondary Osteosarcopenia
Abstract
1. Introduction
2. Results
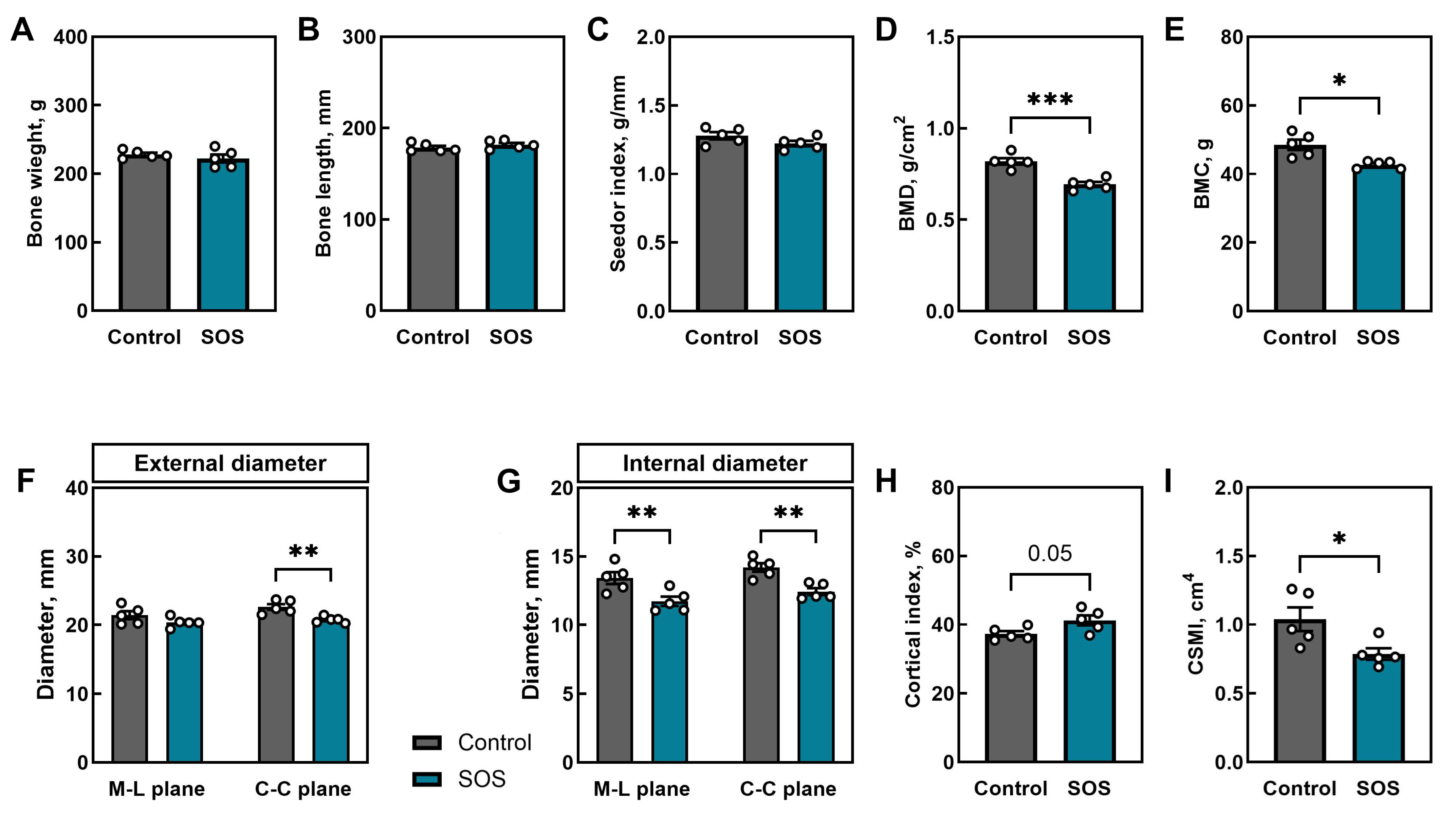
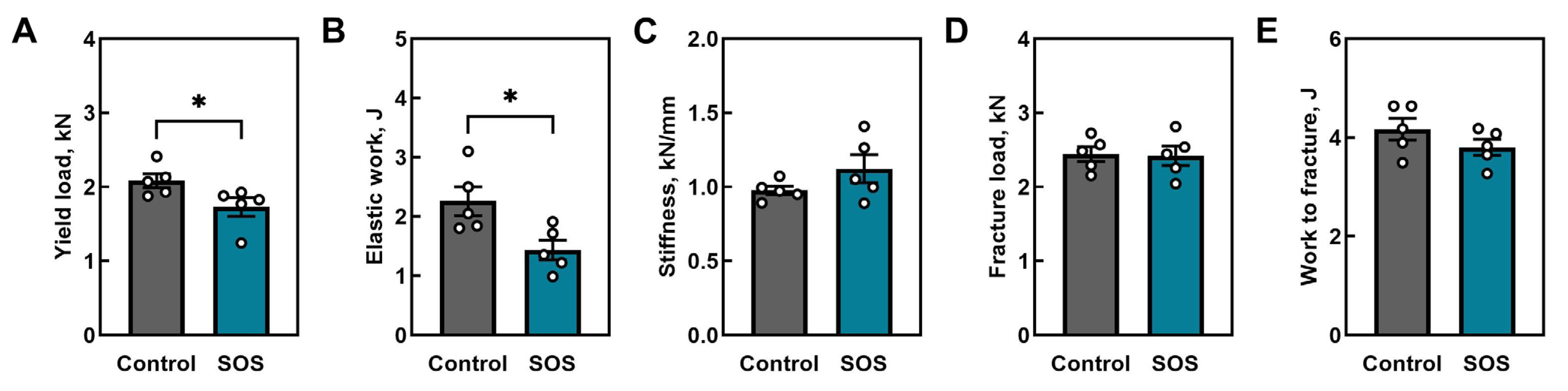
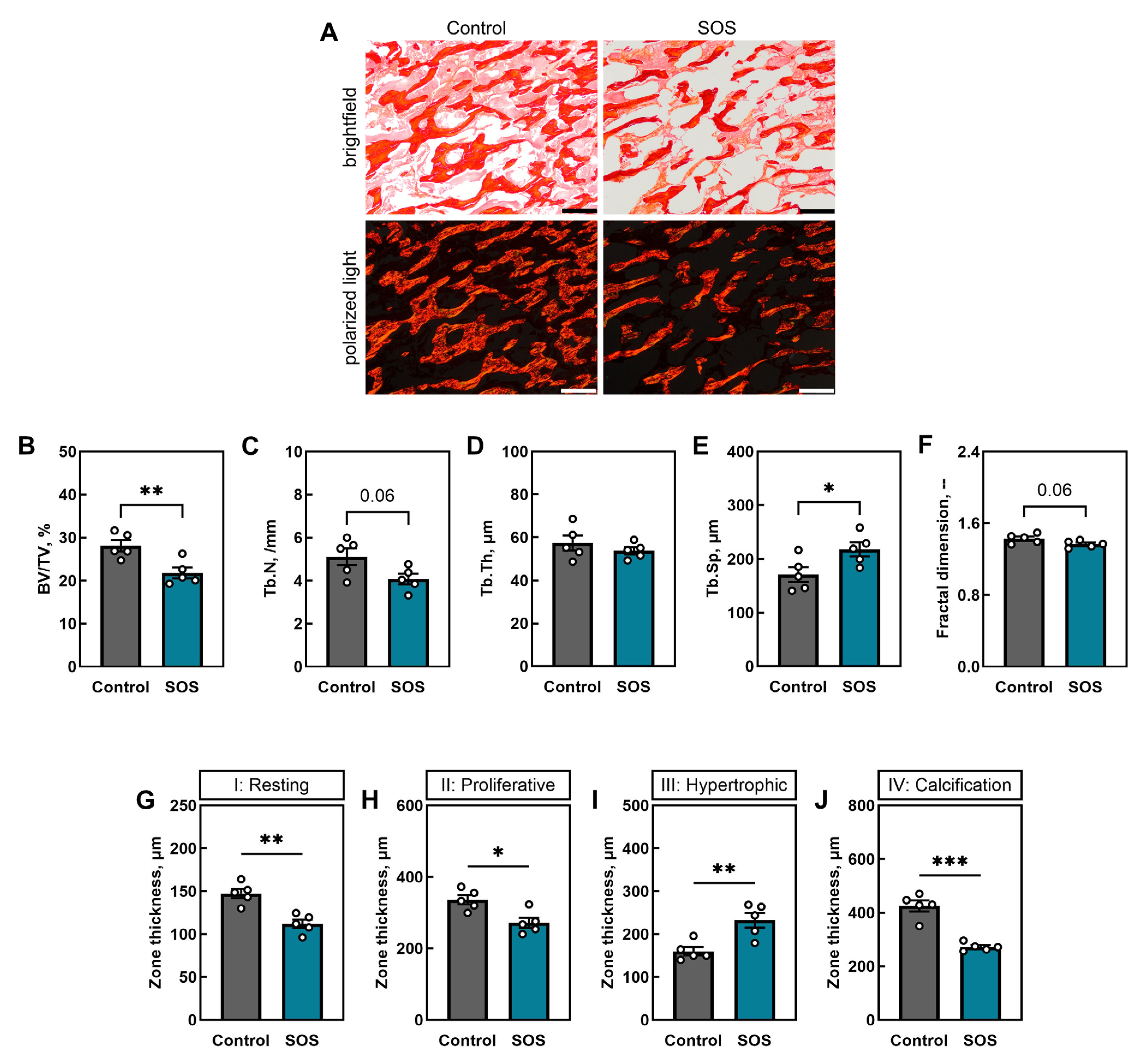
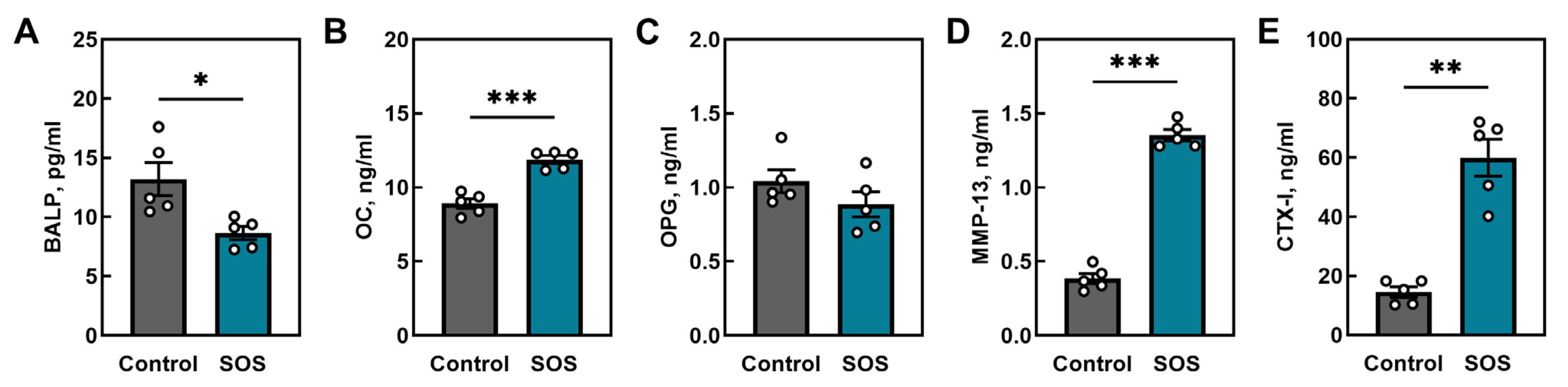
2.1. Bone Analysis
2.1.1. Femoral Morphometry and Densitometry
2.1.2. Mechanical Properties of Femora
2.1.3. Trabecular Bone Microarchitecture and Growth Plate Morphology
2.1.4. Bone Turnover Markers
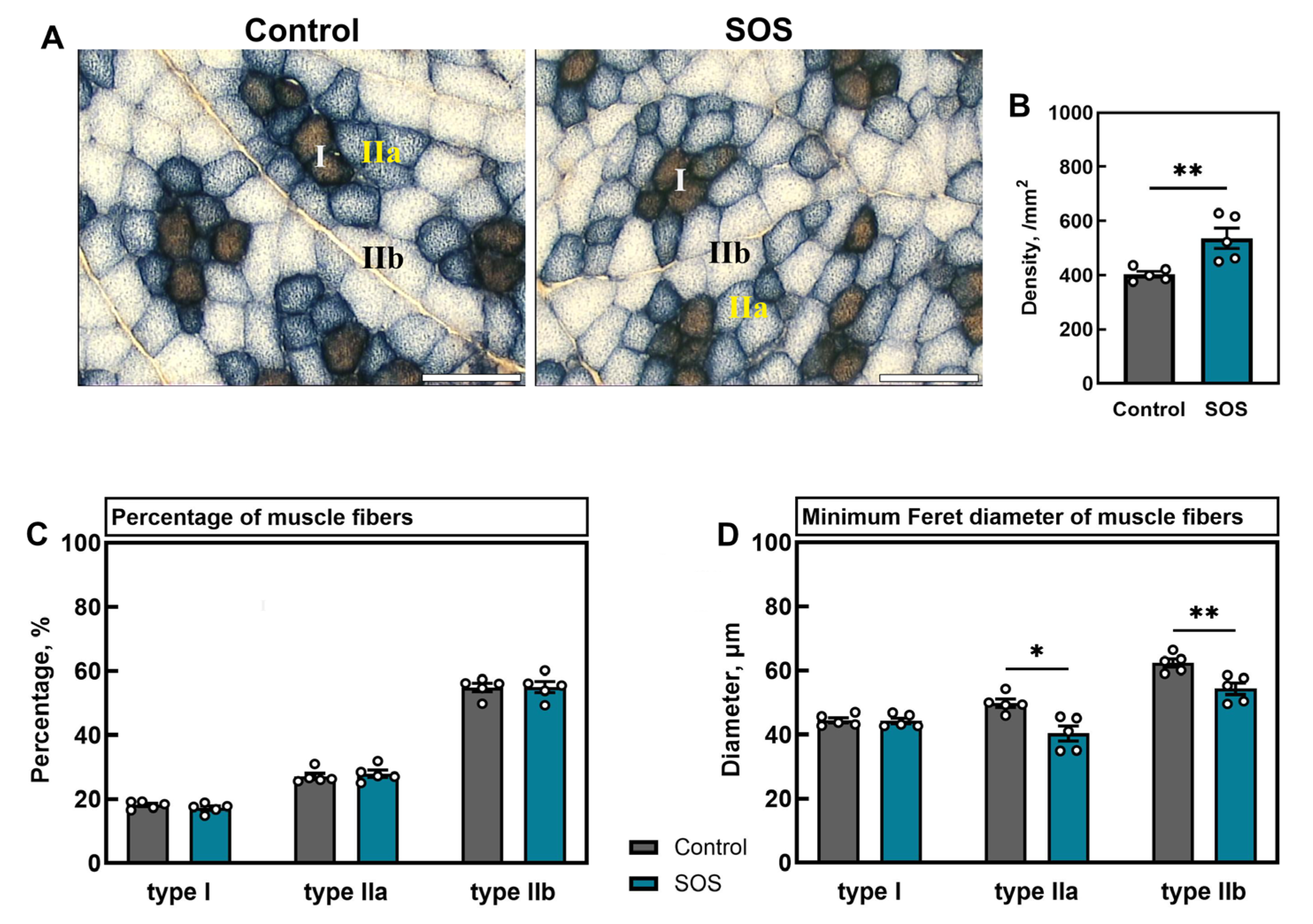
2.2. Muscle Analysis
2.2.1. Muscle Fiber Density, Composition, and Diameters
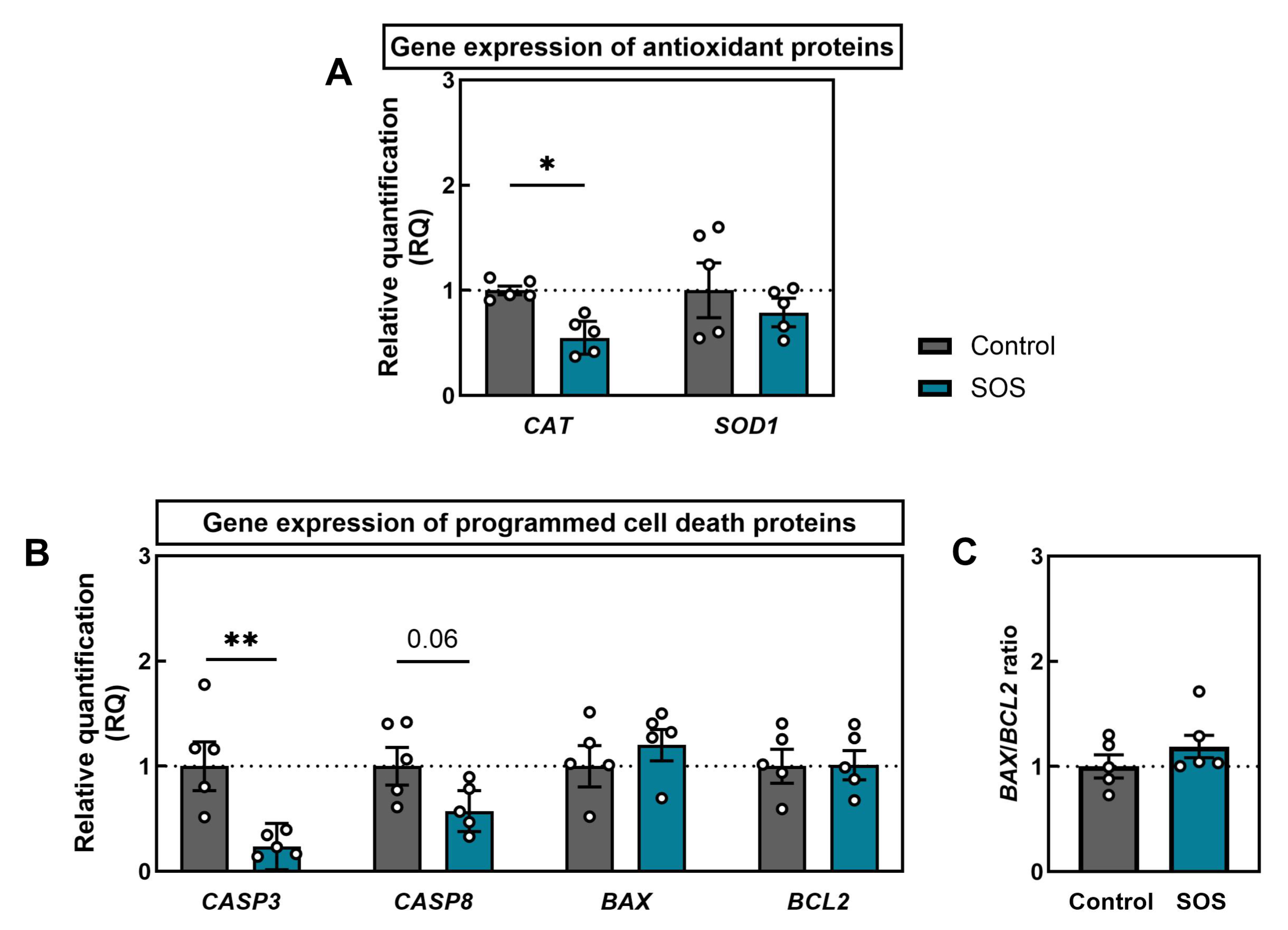
2.2.2. Gene Expression of Antioxidant Enzymes and Apoptotic Regulators
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment Groups
4.2. Sample Collection
4.3. Bone Densitometry, Osteometry, and Mechanical Testing
4.4. Bone Histomorphometry
4.5. Bone Turnover Markers
4.6. Muscle Histomorphometry
4.7. RT-qPCR Analysis of mRNA Expression of Antioxidant Enzymes and Apoptotic Regulators
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| b.w. | Body weight |
| BALP | Bone-specific alkaline phosphatase |
| BAX | apoptosis regulator BAX, BCL-2-like protein 4 |
| BCL2 | apoptosis regulator BCL2, B-cell lymphoma 2 |
| BMC | Bone mineral content |
| BMD | Bone mineral density |
| BV/TV | Bone volume fraction |
| CASP3 | Caspase-3 |
| CASP8 | Caspase-8 |
| CAT | Catalase |
| CI | Confidence interval |
| CP | Chronic pancreatitis |
| CSMI | Cross-sectional moment of inertia |
| CTX-I | C-terminal telopeptide of type I collagen |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GSE | Geometric standard error |
| MMP-13 | Matrix metallopeptidase 13 |
| OC | Osteocalcin |
| OPG | Osteoprotegerin |
| PBM | Peak bone mass |
| RANK | Receptor activator for nuclear factor κ B |
| RANKL | Receptor activator for nuclear factor κ B ligand |
| ROS | Reactive oxygen species |
| RQ | Relative quantification |
| SEM | Standard error of the mean |
| SOD1 | Superoxide dismutase-1 |
| SOS | Secondary osteosarcopenia |
| Tb.N | Trabecular number |
| Tb.Sp | Trabecular separation |
| Tb.Th | Trabecular thickness |
| UPS | Ubiquitin–proteasome system |
References
- DiGirolamo, D.J.; Clemens, T.L.; Kousteni, S. The Skeleton as an Endocrine Organ. Nat. Rev. Rheumatol. 2012, 8, 674–683. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, Diagnosis, and Treatment—Facts and Numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef]
- He, C.; He, W.; Hou, J.; Chen, K.; Huang, M.; Yang, M.; Luo, X.; Li, C. Bone and Muscle Crosstalk in Aging. Front. Cell Dev. Biol. 2020, 8, 585644. [Google Scholar] [CrossRef]
- Binkley, N.; Buehring, B. Beyond FRAX®: It’s Time to Consider “Sarco-Osteopenia”. J. Clin. Densitom. 2009, 12, 413–416. [Google Scholar] [CrossRef]
- Kirk, B.; Miller, S.; Zanker, J.; Duque, G. A Clinical Guide to the Pathophysiology, Diagnosis and Treatment of Osteosarcopenia. Maturitas 2020, 140, 27–33. [Google Scholar] [CrossRef]
- Huo, Y.R.; Suriyaarachchi, P.; Gomez, F.; Curcio, C.L.; Boersma, D.; Muir, S.W.; Montero-Odasso, M.; Gunawardene, P.; Demontiero, O.; Duque, G. Phenotype of Osteosarcopenia in Older Individuals with a History of Falling. J. Am. Med. Dir. Assoc. 2015, 16, 290–295. [Google Scholar] [CrossRef]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where Bone, Muscle, and Fat Collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef]
- Supriya, R.; Singh, K.P.; Gao, Y.; Gu, Y.; Baker, J.S. Effect of Exercise on Secondary Sarcopenia: A Comprehensive Literature Review. Biology 2021, 11, 51. [Google Scholar] [CrossRef]
- Silva, T.L.D.; Dos Santos Chiappetta Salgado Nogueira, V.; Mulder, A.P. Sarcopenia and Poor Muscle Quality Associated with Severe Obesity in Young Adults and Middle-Aged Adults. Clin. Nutr. ESPEN 2021, 45, 299–305. [Google Scholar] [CrossRef]
- Bundred, J.; Thakkar, R.G.; Pandanaboyana, S. Systematic Review of Sarcopenia in Chronic Pancreatitis: Prevalence, Impact on Surgical Outcomes, and Survival. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 665–672. [Google Scholar] [CrossRef]
- Khurmatullina, A.R.; Andreev, D.N.; Maev, I.V.; Kucheryavyy, Y.A.; BEliy, P.A.; Dzhafarova, A.R.; Cherenkova, V.V.; Sokolov, F.S. Prevalence and Risk of Sarcopenia in Patients with Chronic Pancreatitis: Systematic Review and Meta-Analysis. Nutrients 2025, 17, 870. [Google Scholar] [CrossRef]
- Kuan, L.L.; Dennison, A.R.; Garcea, G. Prevalence and Impact of Sarcopenia in Chronic Pancreatitis: A Review of the Literature. World J. Surg. 2021, 45, 590–597. [Google Scholar] [CrossRef]
- Olesen, S.S.; Büyükuslu, A.; Køhler, M.; Rasmussen, H.H.; Drewes, A.M. Sarcopenia Associates with Increased Hospitalization Rates and Reduced Survival in Patients with Chronic Pancreatitis. Pancreatology 2019, 19, 245–251. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Omer, E. Chronic Pancreatitis and Nutrition Therapy. Nutr. Clin. Prac. 2019, 34, S13–S26. [Google Scholar] [CrossRef]
- Pham, A.; Forsmark, C. Chronic Pancreatitis: Review and Update of Etiology, Risk Factors, and Management. F1000Res 2018, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Forsmark, C.E. Diagnosis and Management of Exocrine Pancreatic Insufficiency. Curr. Treat. Options Gastroenterol. 2018, 16, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.N.; Smyth, N.D.; Murphy, A.; MacNaughton, D.; O’Keefe, S.J.D.; Conlon, K.C. High Prevalence of Osteoporosis in Patients with Chronic Pancreatitis: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 219–228. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Świątkiewicz, M.; Muszyński, S.; Donaldson, J.; Ropka-Molik, K.; Arciszewski, M.B.; Murawski, M.; Schwarz, T.; Dobrowolski, P.; Szymańczyk, S.; et al. Repetitive Cerulein-Induced Chronic Pancreatitis in Growing Pigs—A Pilot Study. Int. J. Mol. Sci. 2023, 24, 7715. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Wojtysiak, D.; Grzegorzewska, A.; Świątkiewicz, M.; Donaldson, J.; Arciszewski, M.B.; Dresler, S.; Puzio, I.; Szymańczyk, S.; Dobrowolski, P.; et al. Understanding Secondary Sarcopenia Development in Young Adults Using Pig Model with Chronic Pancreatitis. Int. J. Mol. Sci. 2024, 25, 8735. [Google Scholar] [CrossRef]
- Barkin, J.A.; Barkin, J.S. Chronic Pancreatitis and Bone Disease. J. Clin. Densitom. 2020, 23, 237–243. [Google Scholar] [CrossRef]
- Fasullo, M.; Omer, E.; Kaspar, M. Sarcopenia in Chronic Pancreatitis—Prevalence, Diagnosis, Mechanisms and Potential Therapies. Curr. Gastroenterol. Rep. 2022, 24, 53–63. [Google Scholar] [CrossRef]
- Bieliuniene, E.; Brøndum Frøkjær, J.; Pockevicius, A.; Kemesiene, J.; Lukosevicius, S.; Basevicius, A.; Atstupenaite, V.; Barauskas, G.; Ignatavicius, P.; Gulbinas, A.; et al. CT- and MRI-Based Assessment of Body Composition and Pancreatic Fibrosis Reveals High Incidence of Clinically Significant Metabolic Changes That Affect the Quality of Life and Treatment Outcomes of Patients with Chronic Pancreatitis and Pancreatic Cancer. Medicina 2019, 55, 649. [Google Scholar] [CrossRef]
- Kozlova, I.V.; Bykova, A.P. Osteosarcopenia in Chronic Pancreatitis. Ter. Arkhiv 2021, 93, 869–875. [Google Scholar] [CrossRef]
- Takeda, T.; Sasaki, T.; Okamoto, T.; Ishitsuka, T.; Yamada, M.; Nakagawa, H.; Mie, T.; Furukawa, T.; Kasuga, A.; Matsuyama, M.; et al. Prognostic Impact of Osteosarcopenia in Patients with Advanced Pancreatic Cancer Receiving Gemcitabine plus Nab-Paclitaxel. Pancreatology 2023, 23, 275–282. [Google Scholar] [CrossRef]
- Sharma, P.; Parikh, N.D.; Yu, J.; Barman, P.; Derstine, B.A.; Sonnenday, C.J.; Wang, S.C.; Su, G.L. Bone Mineral Density Predicts Posttransplant Survival among Hepatocellular Carcinoma Liver Transplant Recipients. Liver Transplant. 2016, 22, 1092–1098. [Google Scholar] [CrossRef]
- Parhiala, M.; Ukkonen, M.; Sand, J.; Laukkarinen, J. Osteoporosis and Sarcopenia Are Common and Insufficiently Diagnosed among Chronic Pancreatitis Patients. BMC Gastroenterol. 2023, 23, 124. [Google Scholar] [CrossRef]
- Hart, P.A.; Yadav, D.; Li, L.; Appana, S.; Fisher, W.; Fogel, E.; Forsmark, C.E.; Park, W.G.; Pandol, S.; Topazian, M.D.; et al. High Prevalence of Osteopathy in Chronic Pancreatitis: A Cross-Sectional Analysis from the PROCEED Study. Clin. Gastroenterol. Hepatol. 2022, 20, 2005–2013. [Google Scholar] [CrossRef]
- Srivastava, A.; Saini, N.; Mathias, A.; Arya, A.; Jain, S.; Yachha, S.K. Prevalence and Predictive Factors of Undernutrition and Low Bone Mineral Density in Children with Chronic Pancreatitis. Pancreatology 2021, 21, 74–80. [Google Scholar] [CrossRef]
- Abu-El-Haija, M.; Hornung, L.; Ellery, K.; Fishman, D.S.; Gonska, T.Y.; Gariepy, C.; Lowe, M.; Larson Ode, K.; Maqbool, A.; Mascarenhas, M.; et al. Bone Health in Children with Recurrent and Chronic Pancreatitis: A Multi-Center Cross Sectional Analysis. Pancreatology 2023, 23, 755–760. [Google Scholar] [CrossRef]
- Jura, J. Animal Models for the Treatment of Human Diseases—A Review. Ann. Anim. Sci. 2024, 24, 1153–1159. [Google Scholar] [CrossRef]
- Sipos, W. Are Pigs a Suitable Model Species for Osteoporosis Research? J. Transl. Sci. 2020, 5, 1–2. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Wang, H.; Lu, J.; He, B. Application of Imaging Methods and the Latest Progress in Sarcopenia. Chin. J. Acad. Radiol. 2024, 7, 15–27. [Google Scholar] [CrossRef]
- Jarecki, J.; Tomczyk-Warunek, A.; Posturzyńska, A.; Warda, E.; Waśko, M.; Arciszewski, K.; Tomaszewska, E.; Muszyński, S.; Bieniaś, J.; Ostapiuk, M.; et al. Treatment of Ochronotic Osteoarthropathy and the Evaluation of Selected Lower Limb Muscle Properties, Including the Patellar Tendon: A Case Report and Mini Literature Review. J. Clin. Med. 2025, 14, 4413. [Google Scholar] [CrossRef]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone Modeling and Remodeling: Potential as Therapeutic Targets for the Treatment of Osteoporosis. Ther. Adv. Musculoskelet. 2016, 8, 225–235. [Google Scholar] [CrossRef]
- Martin, R.B. Determinants of the Mechanical Properties of Bones. J. Biomech. 1991, 24, 79–88. [Google Scholar] [CrossRef]
- Stover, K.K.; Weinreich, D.M.; Roberts, T.J.; Brainerd, E.L. Patterns of Musculoskeletal Growth and Dimensional Changes Associated with Selection and Developmental Plasticity in Domestic and Wild Strain Turkeys. Ecol. Evol. 2018, 8, 3229–3239. [Google Scholar] [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Martin, R.B.; Boardman, D.L. The Effects of Collagen Fiber Orientation, Porosity, Density, and Mineralization on Bovine Cortical Bone Bending Properties. J. Biomech. 1993, 26, 1047–1054. [Google Scholar] [CrossRef]
- Gürbüz, A.; Gür, M. Bilateral Tibial Stress Fractures and Osteoporosis in a Young Patient. Sports Health A Multidiscip. Approach 2022, 14, 440–443. [Google Scholar] [CrossRef]
- Chen, Q.; Bao, N.; Yao, Q.; Li, Z.-Y. Fractal Dimension: A Complementary Diagnostic Indicator of Osteoporosis to Bone Mineral Density. Med. Hypotheses 2018, 116, 136–138. [Google Scholar] [CrossRef]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone Mechanical Properties and Changes with Osteoporosis. Injury 2016, 47, S11–S20. [Google Scholar] [CrossRef]
- Burdan, F.; Szumiło, J.; Korobowicz, A.; Farooquee, R.; Patel, S.; Patel, A.; Dave, A.; Szumiło, M.; Solecki, M.; Klepacz, R.; et al. Morphology and Physiology of the Epiphyseal Growth Plate. Folia Histochem. Cytobiol. 2009, 47, 5–16. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Sävendahl, L. Promoting Growth in Chronic Inflammatory Disease: Lessons from Studies of the Growth Plate. Horm. Res. Paediatr. 2009, 72, 42–47. [Google Scholar] [CrossRef]
- Wong, S.C.; Dobie, R.; Altowati, M.A.; Werther, G.A.; Farquharson, C.; Ahmed, S.F. Growth and the Growth Hormone-Insulin Like Growth Factor 1 Axis in Children with Chronic Inflammation: Current Evidence, Gaps in Knowledge, and Future Directions. Endocr. Rev. 2016, 37, 62–110. [Google Scholar] [CrossRef]
- Cirillo, F.; Lazzeroni, P.; Sartori, C.; Street, M. Inflammatory Diseases and Growth: Effects on the GH–IGF Axis and on Growth Plate. Int. J. Mol. Sci. 2017, 18, 1878. [Google Scholar] [CrossRef]
- Sobol, M.; Skiba, G.; Kowalczyk, P.; Świątkiewicz, M.; Grela, E.R. Markers of Bone Turnover and Biomechanical Properties of the Third Metacarpal Bone of Growing Pigs Subjected to the Different Dietary Phosphorus and Calcium Content. Ann. Anim. Sci. 2024, 24, 479–490. [Google Scholar] [CrossRef]
- Sørensen, K.U.; Kruger, M.C.; Hansen-Møller, J.; Poulsen, H.D. Bone Biochemical Markers for Assessment of Bone Responses to Differentiated Phosphorus Supply in Growing-Finishing Pigs. J. Anim. Sci. 2018, 96, 4693–4703. [Google Scholar] [CrossRef]
- Hofbauer, L.C. Clinical Implications of the Osteoprotegerin/RANKL/RANK System for Bone and Vascular Diseases. JAMA 2004, 292, 490. [Google Scholar] [CrossRef]
- Turan, S.; Topcu, B.; Gökce, İ.; Güran, T.; Atay, Z.; Omar, A.; Akçay, T.; Bereket, A. Serum Alkaline Phosphatase Levels in Healthy Children and Evaluation of Alkaline Phosphatase Z-Scores in Different Types of Rickets. J. Clin. Res. Pediatr. Endocrinol. 2011, 3, 7–11. [Google Scholar] [CrossRef]
- Demiaux, B.; Arlot, M.E.; Chapuy, M.C.; Meunier, P.J.; Delmas, P.D. Serum Osteocalcin Is Increased in Patients with Osteomalacia: Correlations with Biochemical and Histomorphometric Findings. J. Clin. Endocrinol. Metab. 1992, 74, 1146–1151. [Google Scholar] [CrossRef]
- Mohammadi, S.M.; Saniee, N.; Mousaviasl, S.; Radmanesh, E.; Doustimotlagh, A.H. The Role of Osteocalcin in Patients with Osteoporosis: A Systematic Review. Iran. J. Public Health 2024, 53, 2432–2439. [Google Scholar] [CrossRef]
- Singh, S. Serum Osteocalcin as a Diagnostic Biomarker for Primary Osteoporosis in Women. J. Clin. Diagn. Res. 2015, 8, RC04–RC07. [Google Scholar] [CrossRef]
- Yu, X.-F.; Teng, B.; Li, J.-F.; Zhang, J.V.; Su, Z.; Ren, P.-G. Novel Function of Osteocalcin in Chondrocyte Differentiation and Endochondral Ossification Revealed on a CRISPR/Cas9 Bglap–Bglap2 Deficiency Mouse Model. Int. J. Mol. Sci. 2024, 25, 9945. [Google Scholar] [CrossRef]
- Jahangir, S.; Eglin, D.; Pötter, N.; Khozaei Ravari, M.; Stoddart, M.J.; Samadikuchaksaraei, A.; Alini, M.; Baghaban Eslaminejad, M.; Safa, M. Inhibition of Hypertrophy and Improving Chondrocyte Differentiation by MMP-13 Inhibitor Small Molecule Encapsulated in Alginate-Chondroitin Sulfate-Platelet Lysate Hydrogel. Stem Cell Res. Ther. 2020, 11, 436. [Google Scholar] [CrossRef]
- Chi, G.; Qiu, L.; Ma, J.; Wu, W.; Zhang, Y. The Association of Osteoprotegerin and RANKL with Osteoporosis: A Systematic Review with Meta-Analysis. J. Orthop. Surg. Res. 2023, 18, 839. [Google Scholar] [CrossRef]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular Mechanisms and Open Questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef]
- Meng, S.-J.; Yu, L.-J. Oxidative Stress, Molecular Inflammation and Sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef]
- Sullivan-Gunn, M.J.; Lewandowski, P.A. Elevated Hydrogen Peroxide and Decreased Catalase and Glutathione Peroxidase Protection Are Associated with Aging Sarcopenia. BMC Geriatr. 2013, 13, 104. [Google Scholar] [CrossRef]
- Foreman, N.A.; Hesse, A.S.; Ji, L.L. Redox Signaling and Sarcopenia: Searching for the Primary Suspect. Int. J. Mol. Sci. 2021, 22, 9045. [Google Scholar] [CrossRef]
- Du, J.; Wang, X.; Miereles, C.; Bailey, J.L.; Debigare, R.; Zheng, B.; Price, S.R.; Mitch, W.E. Activation of Caspase-3 Is an Initial Step Triggering Accelerated Muscle Proteolysis in Catabolic Conditions. J. Clin. Investig. 2004, 113, 115–123. [Google Scholar] [CrossRef]
- Jun, L.; Robinson, M.; Geetha, T.; Broderick, T.L.; Babu, J.R. Prevalence and Mechanisms of Skeletal Muscle Atrophy in Metabolic Conditions. Int. J. Mol. Sci. 2023, 24, 2973. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular Stress Responses: Cell Survival and Cell Death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef]
- Dupont-Versteegden, E.E. Apoptosis in Muscle Atrophy: Relevance to Sarcopenia. Exp. Gerontol. 2005, 40, 473–481. [Google Scholar] [CrossRef]
- Paul-Samojedny, M.; Kokocińska, D.; Samojedny, A.; Mazurek, U.; Partyka, R.; Lorenz, Z.; Wilczok, T. Expression of Cell Survival/Death Genes: Bcl-2 and Bax at the Rate of Colon Cancer Prognosis. Biochim. Biophys. Acta (BBA)–Mol. Basis Dis. 2005, 1741, 25–29. [Google Scholar] [CrossRef]
- Xia, Q.; Huang, X.; Huang, J.; Zheng, Y.; March, M.E.; Li, J.; Wei, Y. The Role of Autophagy in Skeletal Muscle Diseases. Front. Physiol. 2021, 12, 638983. [Google Scholar] [CrossRef]
- Sandri, M. Protein Breakdown in Muscle Wasting: Role of Autophagy-Lysosome and Ubiquitin-Proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef]
- Haberecht-Müller, S.; Krüger, E.; Fielitz, J. Out of Control: The Role of the Ubiquitin Proteasome System in Skeletal Muscle during Inflammation. Biomolecules 2021, 11, 1327. [Google Scholar] [CrossRef]
- Kitajima, Y.; Yoshioka, K.; Suzuki, N. The Ubiquitin–Proteasome System in Regulation of the Skeletal Muscle Homeostasis and Atrophy: From Basic Science to Disorders. J. Physiol. Sci. 2020, 70, 40. [Google Scholar] [CrossRef]
- Singh, A.; Phogat, J.; Yadav, A.; Dabur, R. The Dependency of Autophagy and Ubiquitin Proteasome System during Skeletal Muscle Atrophy. Biophys. Rev. 2021, 13, 203–219. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and Molecular Mechanisms of Muscle Atrophy. Dis. Models Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Lal, N.N.; Cornwall, J.; Sheard, P.W. Age-Related Structural Changes Show That Loss of Fibers Is Not a Significant Contributor to Muscle Atrophy in Old Mice. Exp. Gerontol. 2021, 156, 111618. [Google Scholar] [CrossRef]
- Frost, H.M. Bone’s Mechanostat: A 2003 Update. Anat. Rec. 2003, 275A, 1081–1101. [Google Scholar] [CrossRef]
- Clynes, M.A.; Gregson, C.L.; Bruyère, O.; Cooper, C.; Dennison, E.M. Osteosarcopenia: Where Osteoporosis and Sarcopenia Collide. Rheumatology 2021, 60, 529–537. [Google Scholar] [CrossRef]
- Galea, G.L.; Lanyon, L.E.; Price, J.S. Sclerostin’s Role in Bone’s Adaptive Response to Mechanical Loading. Bone 2017, 96, 38–44. [Google Scholar] [CrossRef]
- Wojtysiak, D.; Calik, J.; Leszczyński, B.; Tomaszewska, E.; Obrzut, J.; Krawczyk, J.; Panek, D.; Muszyński, S. Effect of Caponization on Blood Parameters and Tibia Characteristics in Crossbred Chickens Derived from Conserved Breed Hens and Meat Roosters. Ann. Anim. Sci. 2024, 24, 819–828. [Google Scholar] [CrossRef]
- Osiak-Wicha, C.; Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Andres, K.; Schwarz, T.; Świetlicki, M.; Mielnik-Błaszczak, M.; Arciszewski, M.B. Developmental Changes in Tibia and Humerus of Goose: Morphometric, Densitometric, and Mechanical Analysis. Animal 2023, 17, 100960. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Szulc, K.; Wojtysiak, D.; Migdał, Ł.; Migdał, W. The Muscle Fibre Characteristics and the Meat Quality of m. Longissimus Thoracis from Polish Native Złotnicka Spotted Pigs and the Crossbreed Fatteners from the Crossing of Duroc and Polish Large White Boars. Appl. Sci. 2022, 12, 3051. [Google Scholar] [CrossRef]
- Wojtysiak, D. Effect of Breed on Microstructure and Tenderness of Porcine Semimembranosus Muscle. Ann. Anim. Sci. 2014, 14, 697–705. [Google Scholar] [CrossRef]
- Briguet, A.; Courdier-Fruh, I.; Foster, M.; Meier, T.; Magyar, J.P. Histological Parameters for the Quantitative Assessment of Muscular Dystrophy in the Mdx-Mouse. Neuromuscul. Disord. 2004, 14, 675–682. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muszyński, S.; Świetlicki, M.; Wojtysiak, D.; Grzegorzewska, A.; Dobrowolski, P.; Świątkiewicz, M.; Arciszewski, M.B.; Puzio, I.; Bonior, J.; Tomczyk-Warunek, A.; et al. Coupled Bone–Muscle Degeneration in Chronic Pancreatitis: A Juvenile Porcine Model of Secondary Osteosarcopenia. Int. J. Mol. Sci. 2025, 26, 7690. https://doi.org/10.3390/ijms26167690
Muszyński S, Świetlicki M, Wojtysiak D, Grzegorzewska A, Dobrowolski P, Świątkiewicz M, Arciszewski MB, Puzio I, Bonior J, Tomczyk-Warunek A, et al. Coupled Bone–Muscle Degeneration in Chronic Pancreatitis: A Juvenile Porcine Model of Secondary Osteosarcopenia. International Journal of Molecular Sciences. 2025; 26(16):7690. https://doi.org/10.3390/ijms26167690
Chicago/Turabian StyleMuszyński, Siemowit, Michał Świetlicki, Dorota Wojtysiak, Agnieszka Grzegorzewska, Piotr Dobrowolski, Małgorzata Świątkiewicz, Marcin B. Arciszewski, Iwona Puzio, Joanna Bonior, Agnieszka Tomczyk-Warunek, and et al. 2025. "Coupled Bone–Muscle Degeneration in Chronic Pancreatitis: A Juvenile Porcine Model of Secondary Osteosarcopenia" International Journal of Molecular Sciences 26, no. 16: 7690. https://doi.org/10.3390/ijms26167690
APA StyleMuszyński, S., Świetlicki, M., Wojtysiak, D., Grzegorzewska, A., Dobrowolski, P., Świątkiewicz, M., Arciszewski, M. B., Puzio, I., Bonior, J., Tomczyk-Warunek, A., Mielnik-Błaszczak, M., & Tomaszewska, E. (2025). Coupled Bone–Muscle Degeneration in Chronic Pancreatitis: A Juvenile Porcine Model of Secondary Osteosarcopenia. International Journal of Molecular Sciences, 26(16), 7690. https://doi.org/10.3390/ijms26167690






