Computational Profiling of Monoterpenoid Phytochemicals: Insights for Medicinal Chemistry and Drug Design Strategies
Abstract
1. Introduction
2. Results
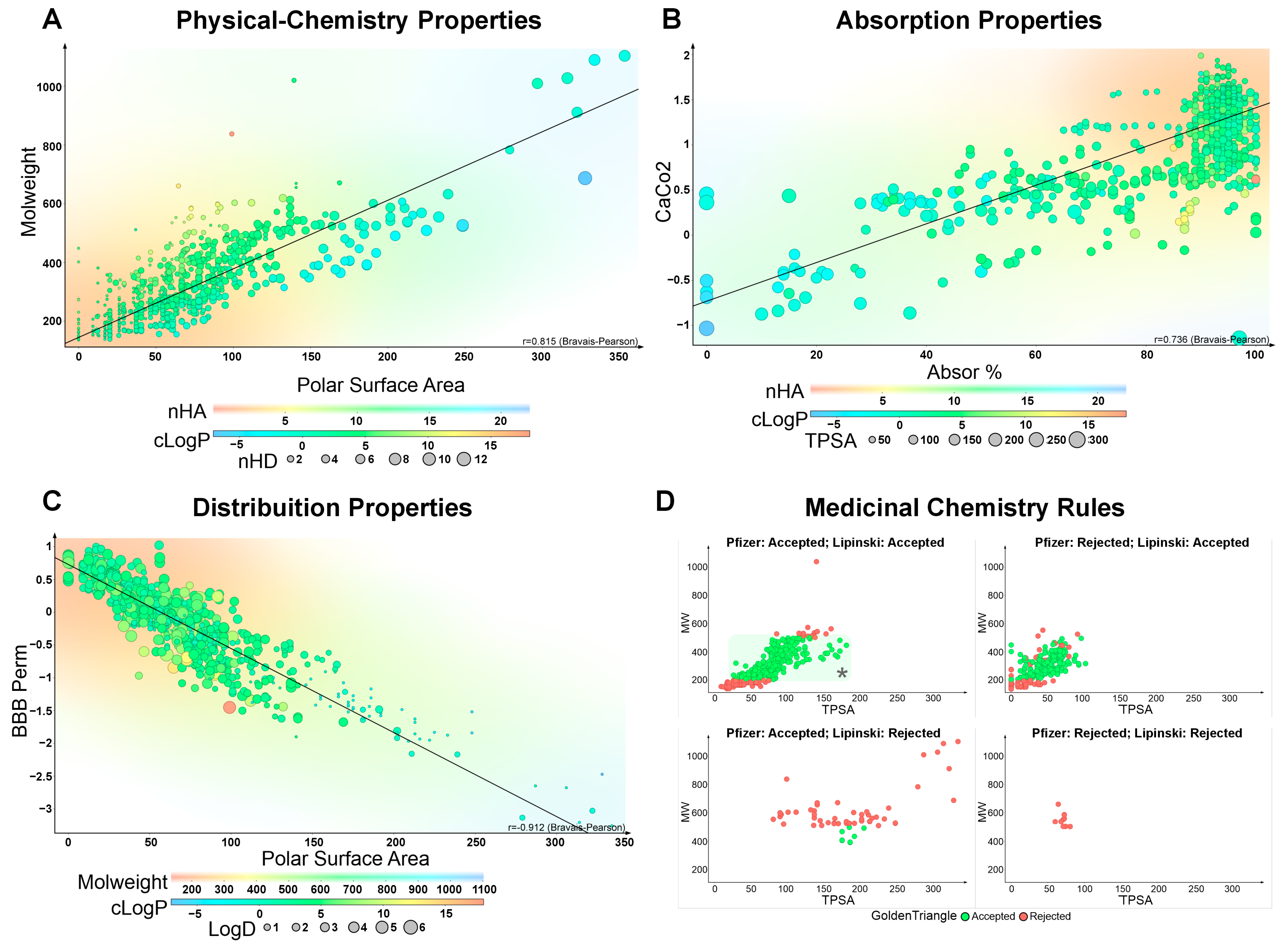
2.1. Absorption
2.2. Distribution
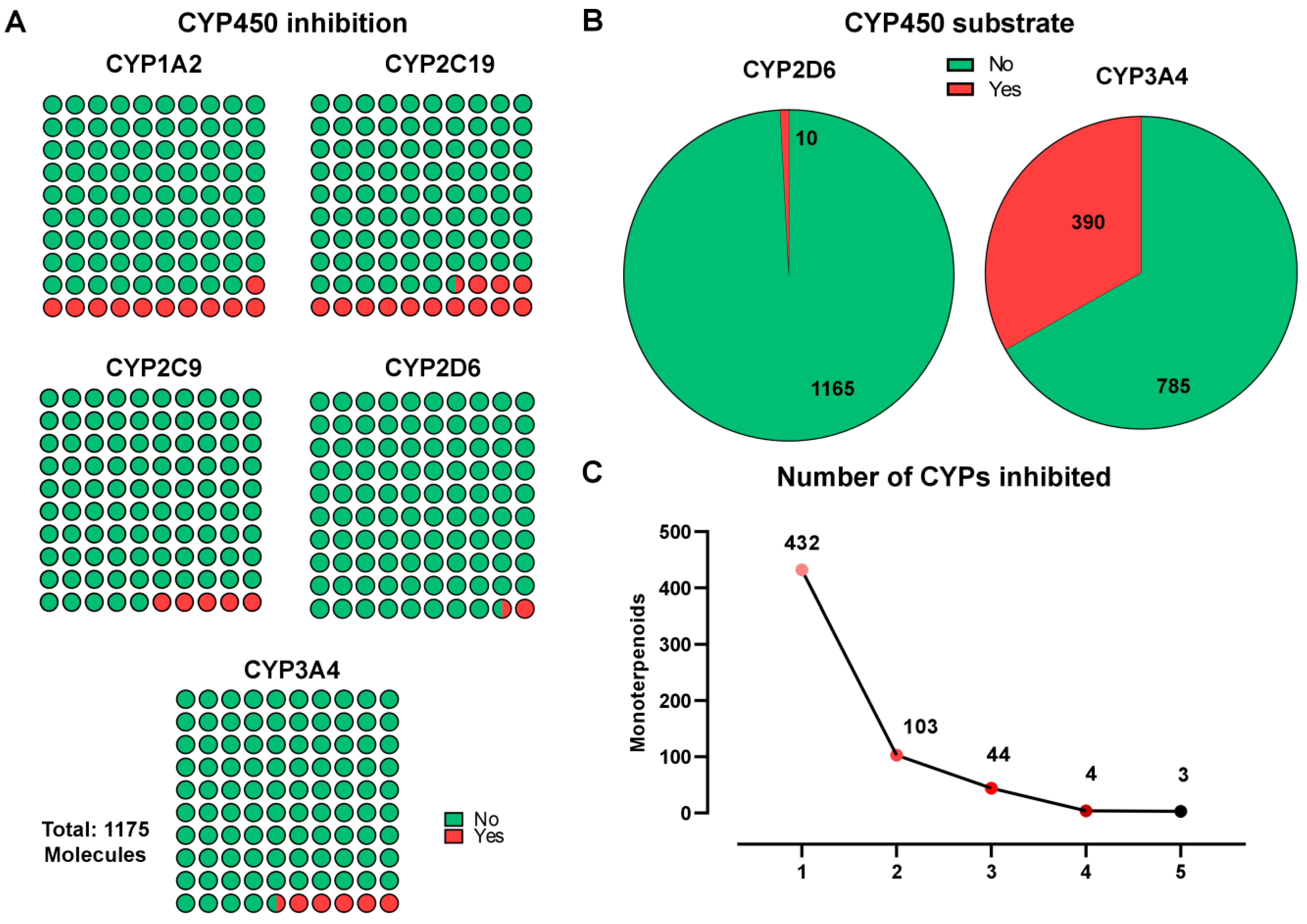
2.3. Metabolism
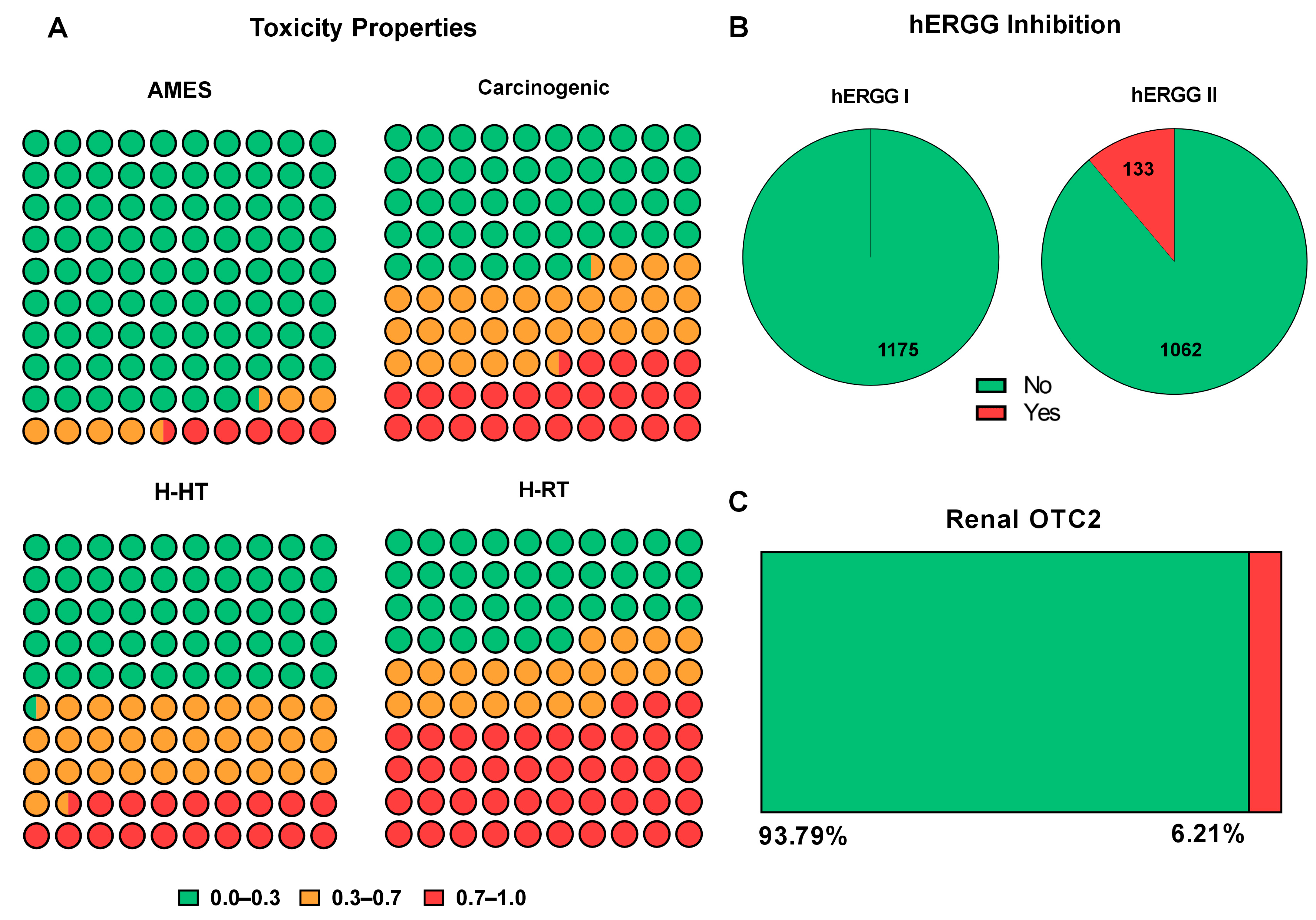
2.4. Excretion
2.5. Toxicity
2.6. Medicinal Chemistry Assessment
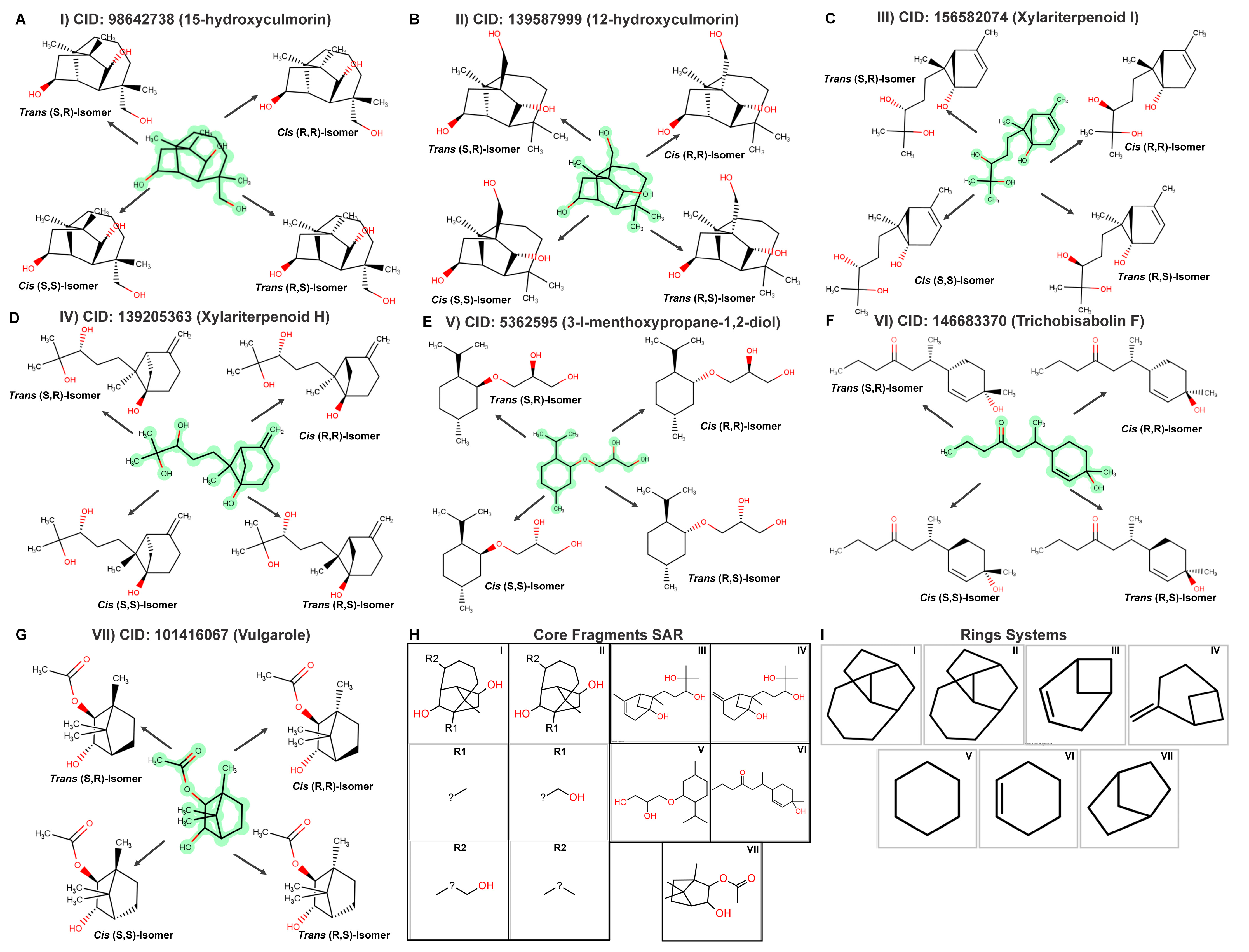
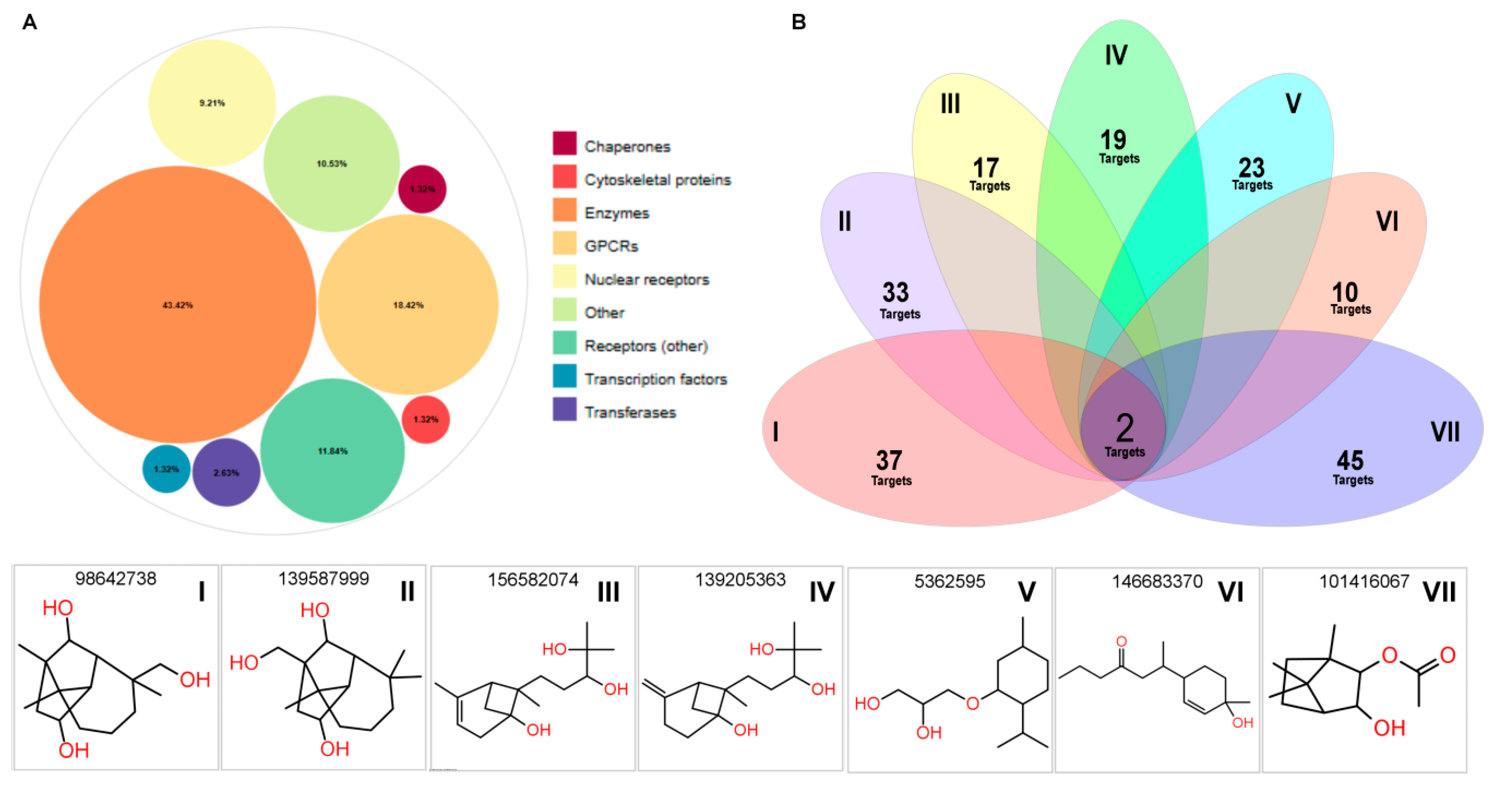
2.7. Top Monoterpenoid Molecules and SAR Analysis
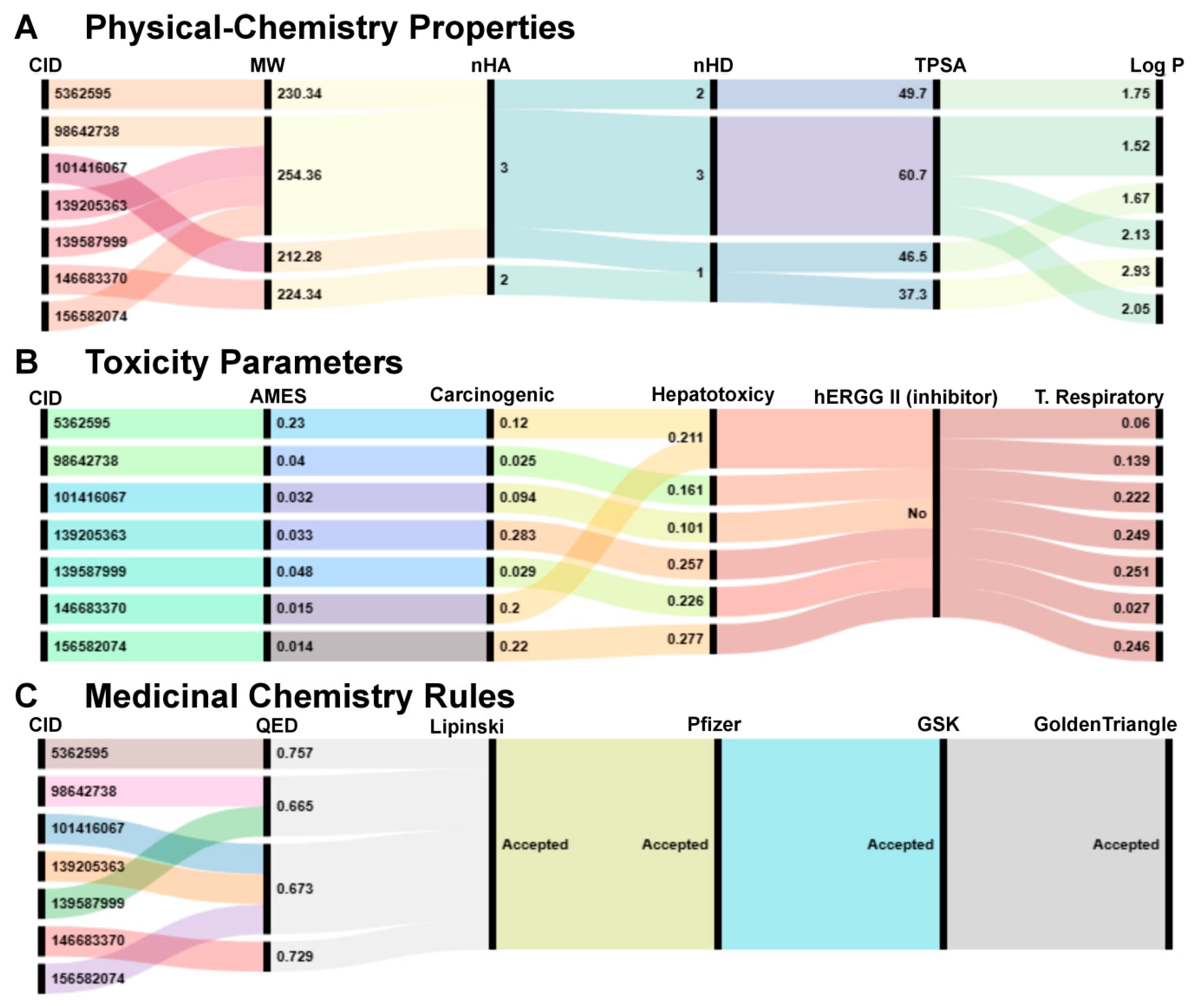
2.8. Physicochemical Properties, Toxicological Parameters, and Medicinal Chemistry Compliance of the Seven Final Compounds
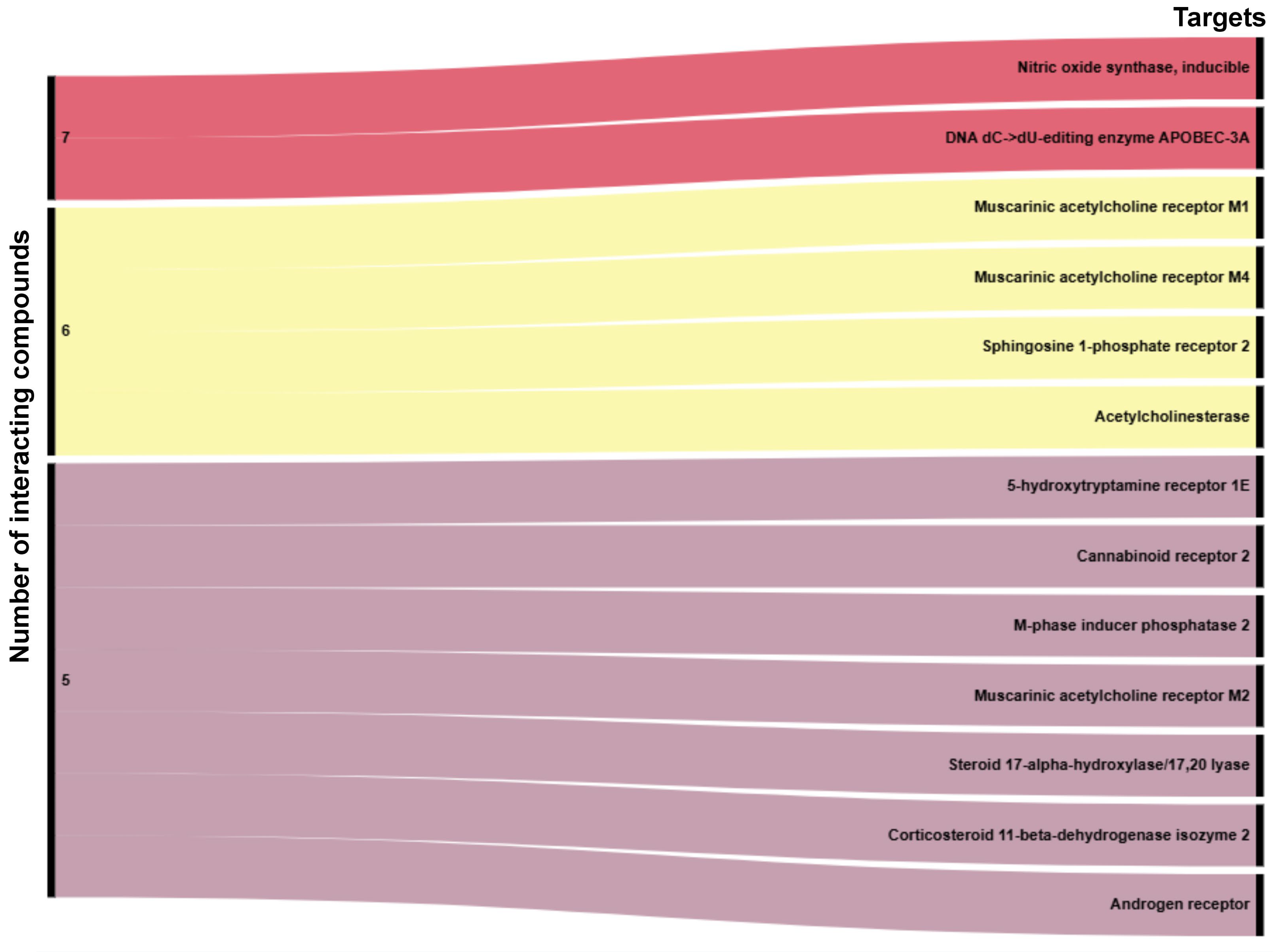
2.9. Computational Target Profiling and Shared Bioactivity Landscape
3. Discussion
4. Materials and Methods
4.1. Compound Retrieval and Dataset Preparation
4.2. Computational Tools and Platforms
4.3. Pharmacokinetic Profiling
4.4. Toxicological Risk Assessment, Drug-likeness, and Medicinal Chemistry Filters
4.5. Data Integration and Filtering Strategy
4.6. Target Bioactivity Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardeal dos Santos, A.N.; da Cruz Freiretos, J.E.; Rodrigues, B.F.; Ferreira-da-Silva, F.W.; Júnior, J.E.R.H.; Cardoso, J.H.L.; Coelho de Souza, A.N. Translational Perspectives on the Therapeutic Potential of Hyptis Crenata Essential Oil Terpenes in Smooth Muscle Function. Planta Med. 2024, 90, 1005–1014. [Google Scholar] [CrossRef]
- Begh, M.Z.A.; Khan, J.; Al Amin, M.; Sweilam, S.H.; Dharmamoorthy, G.; Gupta, J.K.; Sangeetha, J.; Lokeshvar, R.; Nafady, M.H.; Ahmad, I.; et al. Monoterpenoid Synergy: A New Frontier in Biological Applications. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Gutiérrez-Del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Périco, L.L.; Emílio-Silva, M.T.; Ohara, R.; Rodrigues, V.P.; Bueno, G.; Barbosa-Filho, J.M.; da Rocha, L.R.M.; Batista, L.M.; Hiruma-Lima, C.A. Systematic Analysis of Monoterpenes: Advances and Challenges in the Treatment of Peptic Ulcer Diseases. Biomolecules 2020, 10, 265. [Google Scholar] [CrossRef]
- Somade, O.T. Camphor Toxicity: A Review of Recent Findings. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 775–790. [Google Scholar] [CrossRef]
- Santos, C.D.; Cabot, J.C. Persistent Effects after Camphor Ingestion: A Case Report and Literature Review. J. Emerg. Med. 2015, 48, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.R.C.; de Lira, G.H.S.; de Oliveira Neto, R.M.; da Graça, J.R.V.; de Vasconcelos, P.R.L.; Nobre e Souza, M.A.; Magalhães, P.J.C.; Rola, F.H.; dos Santos, A.A. 1.8 Cineole Decreases Gastric Compliance in Anesthetized Rats. Acta Cir. Bras. 2007, 22, 63–67. [Google Scholar] [CrossRef][Green Version]
- Asthana, A.; Larson, R.A.; Marley, K.A.; Tuveson, R.W. Mechanisms of Citral Phototoxicity. Photochem. Photobiol. 1992, 56, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, E.M.; Shaver, R.L.; DeKoven, J.G.; Maibach, H.I.; Taylor, J.S.; Atwater, A.R.; Belsito, D.V.; Silverberg, J.I.; Reeder, M.J.; Zug, K.A.; et al. Patch Testing to Carvone: North American Contact Dermatitis Group Experience, 2009 to 2018. Dermatitis 2022, 33, 42–50. [Google Scholar] [CrossRef]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.M.; Olsson, S.B.; Ravikanth, G.; Uma Shaanker, R. Inhibition of Plant Pathogenic Fungi by Endophytic Trichoderma Spp. through Mycoparasitism and Volatile Organic Compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef] [PubMed]
- Schempp, F.M.; Hofmann, K.E.; Mi, J.; Kirchner, F.; Meffert, A.; Schewe, H.; Schrader, J.; Buchhaupt, M. Investigation of Monoterpenoid Resistance Mechanisms in Pseudomonas Putida and Their Consequences for Biotransformations. Appl. Microbiol. Biotechnol. 2020, 104, 5519–5533. [Google Scholar] [CrossRef] [PubMed]
- Atriya, A.; Majee, C.; Mazumder, R.; Choudhary, A.N.; Salahuddin; Mazumder, A.; Dahiya, A.; Priya, N. Insight into the Various Approaches for the Enhancement of Bioavailability and Pharmacological Potency of Terpenoids: A Review. Curr. Pharm. Biotechnol. 2023, 24, 1228–1244. [Google Scholar] [CrossRef] [PubMed]
- de Alvarenga, J.F.R.; Genaro, B.; Costa, B.L.; Purgatto, E.; Manach, C.; Fiamoncini, J. Monoterpenes: Current Knowledge on Food Source, Metabolism, and Health Effects. Crit. Rev. Food Sci. Nutr. 2023, 63, 1352–1389. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Takagi, A.; Kitazawa, H.; Kawakami, J.; Adachi, I. Effects of Citronellal, a Monoterpenoid in Zanthoxyli Fructus, on the Intestinal Absorption of Digoxin in Vitro and in Vivo. J. Pharm. Sci. 2006, 95, 552–560. [Google Scholar] [CrossRef]
- Kaur, D.K.K.; Allahbadia, D.G. Iridoids. Some Monotepenes and Other Natural Products for Development of Potential Therapies in Alzheimer’s Disease—A Review. Sci. Ric. 2019, 3, 741–756. [Google Scholar]
- Aziz, M.A.; Shehab, W.S.; Al-Karmalawy, A.A.; EL-Farargy, A.F.; Abdellattif, M.H. Design, Synthesis, Biological Evaluation, 2D-QSAR Modeling, and Molecular Docking Studies of Novel 1H-3-Indolyl Derivatives as Significant Antioxidants. Int. J. Mol. Sci. 2021, 22, 10396. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, X.; Wang, Z. A Review on Progress in QSPR Studies for Surfactants. Int. J. Mol. Sci. 2010, 11, 1020–1047. [Google Scholar] [CrossRef]
- Malallah, O.S.; Coleman, L.; Nasereddin, S.M.; Lockhat, M.; Chen, T.; Jones, S.A. Systematic Review and QSPR Analysis of Chemical Penetration through the Nail to Inform Onychomycosis Candidate Selection. Drug Discov. Today 2024, 29, 103844. [Google Scholar] [CrossRef]
- Jiang, S.; Liang, Y.; Shi, S.; Wu, C.; Shi, Z. Improving Predictions and Understanding of Primary and Ultimate Biodegradation Rates with Machine Learning Models. Sci. Total Environ. 2023, 904, 166623. [Google Scholar] [CrossRef]
- da Cruz Freire, J.E.; Cardeal dos Santos, A.N.; Coelho de Souza, A.N.; de Oliveira, A.C.; Nicolete, R.; de Sousa, B.L.; Martins da Silva, J.H.; de Abreu Gomes Vasconcelos, Y.; da Silva, I.N.G.; Soares, P.M.; et al. Molecular and Immunological Properties of a Chimeric Glycosyl Hydrolase 18 Based on Immunoinformatics Approaches: A Design of a New Anti-Leishmania Vaccine. ACS Pharmacol. Transl. Sci. 2025, 8, 78–96. [Google Scholar] [CrossRef]
- Fu, Y.; Ye, T.; Liu, Y.-X.; Wang, J.; Ye, F. Based on the Virtual Screening of Multiple Pharmacophores, Docking and Molecular Dynamics Simulation Approaches toward the Discovery of Novel HPPD Inhibitors. Int. J. Mol. Sci. 2020, 21, 5546. [Google Scholar] [CrossRef]
- Cardeal dos Santos, A.N.; da Cruz Freire, J.E.; Coelho-de-Souza, A.N. Pharmacokinetic and Toxicological Profile in Pharmaceutical Bioprospecting of Caryophyllene Molecules Using Computational Biology for Therapeutic Purposes. In Silico Pharmacol. 2025, 13, 105. [Google Scholar] [CrossRef]
- Araújo, M.C.; Pereira-Gonçalves, Á.; Cardeal dos Santos, A.N.; da Cruz Freire, J.E.; Peireira-de-Morais, L.; Henrique-Félix, F.S.; Sousa-Júlio, N.C.C.; Leal-Cardoso, J.H.; Coelho-de-Souza, A.N. Effect of Eugenol on Detrusor Muscle: Potential for Overactive Bladder Treatment. Int. Neurourol. J. 2024, 28, 253–263. [Google Scholar] [CrossRef]
- dos Cardeal, A.N.C.; da Cruz Freire, J.E.; de Abreu Gomes Vasconcelos, Y.; Honório, J.E.R.; Cardoso, J.H.L.; Cardeal dos Santos, A.N. Possible Adjuvant Role of Cineole in Controlling Vascular Smooth Muscle Dysfunction. Am. J. Biomed. Sci. Res. 2024, 21, 68–69. [Google Scholar] [CrossRef]
- Li-Zhulanov, N.S.; Zaikova, N.P.; Sari, S.; Gülmez, D.; Sabuncuoğlu, S.; Ozadali-Sari, K.; Arikan-Akdagli, S.; Nefedov, A.A.; Rybalova, T.V.; Volcho, K.P.; et al. Rational Design of New Monoterpene-Containing Azoles and Their Antifungal Activity. Antibiotics 2023, 12, 818. [Google Scholar] [CrossRef]
- Dong, J.; Wang, N.-N.; Yao, Z.-J.; Zhang, L.; Cheng, Y.; Ouyang, D.; Lu, A.-P.; Cao, D.-S. ADMETlab: A Platform for Systematic ADMET Evaluation Based on a Comprehensively Collected ADMET Database. J. Cheminform. 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Goo, S.; Hwang, T.; Lee, H.; Kim, Y.-K.; Chae, J.-W.; Yun, H.-Y.; Jung, S. Absorption Distribution Metabolism Excretion and Toxicity Property Prediction Utilizing a Pre-Trained Natural Language Processing Model and Its Applications in Early-Stage Drug Development. Pharmaceuticals 2024, 17, 382. [Google Scholar] [CrossRef] [PubMed]
- Omirin, E.S.; Aribisala, P.O.; Olugbogi, E.A.; Adeniran, O.Y.; Emaleku, S.A.; Saliu, J.A.; Fapohunda, O.; Omirin, A.K.; Gbadamosi, M.O.; Wopara, I. QSAR, Molecular Docking, MD Simulations, and ADMET Screening Identify Potential Heliotropium indicum Leads against Key Targets in Benign Prostatic Hyperplasia. In Silico Pharmacol. 2024, 12, 107. [Google Scholar] [CrossRef]
- Gayakvad, B.; Chauhan, K.; Bhatt, V.; Pandya, D.J.; Chauhan, S.; Khunt, D.; Vegad, U.G. In-Silico Screening, Molecular Dynamics Simulation and ADME Evaluation of Onosma bracteata Wall. for Antiviral Activity against Chandipura Virus. In Silico Pharmacol. 2025, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, C.I. Antioxidative, Metabolic and Vascular Medicinal Potentials of Natural Products in the Non-Edible Wastes of Fruits Belonging to the Citrus and Prunus Genera: A Review. Plants 2024, 13, 191. [Google Scholar] [CrossRef]
- Cai, F.; Wang, C. Comprehensive Review of the Phytochemistry, Pharmacology, Pharmacokinetics, and Toxicology of Alkamides (2016–2022). Phytochemistry 2024, 220, 114006. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Bukhari, I.; Albassam, A.; Alenazi, M. An Update on the Potential Role of Intestinal First-Pass Metabolism for the Prediction of Drug-Drug Interactions: The Role of PBPK Modeling. Expert Opin. Drug Metab. Toxicol. 2018, 14, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- Shi, Y.; Hua, Y.; Wang, B.; Zhang, R.; Li, X. In Silico Prediction and Insights Into the Structural Basis of Drug Induced Nephrotoxicity. Front. Pharmacol. 2021, 12, 793332. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, J.; Xu, Y.; Zhou, N.; Peng, J.; Xiong, Z.; Liu, X.; Luo, X.; Luo, C.; Chen, K.; et al. In Silico ADME/T Modelling for Rational Drug Design. Q. Rev. Biophys. 2015, 48, 488–515. [Google Scholar] [CrossRef]
- Yan, G.; Wang, X.; Chen, Z.; Wu, X.; Pan, J.; Huang, Y.; Wan, G.; Yang, Z. In-Silico ADME Studies for New Drug Discovery: From Chemical Compounds to Chinese Herbal Medicines. Curr. Drug Metab. 2017, 18, 535–539. [Google Scholar] [CrossRef]
- Guedes, I.A.; Pereira da Silva, M.M.; Galheigo, M.; Krempser, E.; de Magalhães, C.S.; Correa Barbosa, H.J.; Dardenne, L.E. DockThor-VS: A Free Platform for Receptor-Ligand Virtual Screening. J. Mol. Biol. 2024, 436, 168548. [Google Scholar] [CrossRef] [PubMed]
- Šegan, S.; Mosić, M.; Šukalović, V.; Jevtić, I. Experimental and Computational Analysis of Lipophilicity and Plasma Protein Binding Properties of Potent Tacrine Based Cholinesterase Inhibitors. J. Chromatogr. B 2025, 1253, 124481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, Y.; Zhang, R.; Chen, Z.; Hua, Y.; Zhang, P.; Guo, H.; Cui, X.; Huang, X.; Li, X. Machine Learning Modeling and Insights into the Structural Characteristics of Drug-Induced Neurotoxicity. J. Chem. Inf. Model. 2022, 62, 6035–6045. [Google Scholar] [CrossRef]
- Degtyarenko, K.; de Matos, P.; Ennis, M.; Hastings, J.; Zbinden, M.; McNaught, A.; Alcántara, R.; Darsow, M.; Guedj, M.; Ashburner, M. ChEBI: A Database and Ontology for Chemical Entities of Biological Interest. Nucleic Acids Res. 2008, 36, D344–D350. [Google Scholar] [CrossRef]
- Lehmann, A.; Haas, M.; Taenzer, J.; Hamscher, G.; Kloft, C.; These, A.; Hethey, C. Characterization of Lipophilicity and Blood Partitioning of Pyrrolizidine Alkaloids and Their N-Oxides In Vitro and In Silico for Toxicokinetic Modeling. Planta Med. 2025, 91, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, B.A.; Sher, P.M.; Wu, X.; Wu, G.; Sulsky, R.B.; Gu, Z.; Murugesan, N.; Zhu, Y.; Yu, G.; Sitkoff, D.F.; et al. Reductions in Log P Improved Protein Binding and Clearance Predictions Enabling the Prospective Design of Cannabinoid Receptor (CB1) Antagonists with Desired Pharmacokinetic Properties. J. Med. Chem. 2013, 56, 9586–9600. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.C. How Medicinal Chemists© Learned about Log P. J. Comput. Aided Mol. Des. 2018, 32, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Caron, G.; Digiesi, V.; Solaro, S.; Ermondi, G. Flexibility in Early Drug Discovery: Focus on the beyond-Rule-of-5 Chemical Space. Drug Discov. Today 2020, 25, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Ozgür, B.; Saaby, L.; Janfelt, C.; Langthaler, K.; Eneberg, E.; Jacobsen, A.-M.; Badolo, L.; Montanari, D.; Brodin, B. Screening Novel CNS Drug Candidates for P-Glycoprotein Interactions Using the Cell Line iP-Gp: In Vitro Efflux Ratios from iP-Gp and MDCK-MDR1 Monolayers Compared to Brain Distribution Data from Mice. Eur. J. Pharm. Biopharm. 2021, 169, 211–219. [Google Scholar] [CrossRef]
- Yoshida, N.; Takagi, A.; Kitazawa, H.; Kawakami, J.; Adachi, I. Inhibition of P-Glycoprotein-Mediated Transport by Extracts of and Monoterpenoids Contained in Zanthoxyli Fructus. Toxicol. Appl. Pharmacol. 2005, 209, 167–173. [Google Scholar] [CrossRef]
- Waring, M.J. Lipophilicity in Drug Discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef]
- Riccardi, K.; Cawley, S.; Yates, P.D.; Chang, C.; Funk, C.; Niosi, M.; Lin, J.; Di, L. Plasma Protein Binding of Challenging Compounds. J. Pharm. Sci. 2015, 104, 2627–2636. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiot. 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Scali, E.; Scumaci, D.; Pellegrino, M.; Aquaro, S.; Saturnino, C.; Sinicropi, M.S. Impact of Cytochrome P450 Enzymes on the Phase I Metabolism of Drugs. Appl. Sci. 2023, 13, 6045. [Google Scholar] [CrossRef]
- Ahmad, I.; Jadhav, H.; Shinde, Y.; Jagtap, V.; Girase, R.; Patel, H. Optimizing Bedaquiline for Cardiotoxicity by Structure Based Virtual Screening, DFT Analysis and Molecular Dynamic Simulation Studies to Identify Selective MDR-TB Inhibitors. In Silico Pharmacol. 2021, 9, 23. [Google Scholar] [CrossRef]
- Souza, A.C.S.; Silva, L.K.; Queiroz, T.B.; Marques, E.S.; Hiruma-Lima, C.A.; Gaivão, I.O.M.; Maistro, E.L. Citral Presents Cytotoxic and Genotoxic Effects in Human Cultured Cells. Drug Chem. Toxicol. 2020, 43, 435–440. [Google Scholar] [CrossRef]
- Caldwell, R.L.; Weerasinghe, P. Synergizing Nature and Medicine: The Anti- Inflammatory and Analgesic Potential of Essential Oils in Adjunctive Rheumatoid Arthritis Treatment. Adv. Complement. Alt. Med. 2024, 8, 837–848. [Google Scholar]
- Bennion, B.J.; Be, N.A.; McNerney, M.W.; Lao, V.; Carlson, E.M.; Valdez, C.A.; Malfatti, M.A.; Enright, H.A.; Nguyen, T.H.; Lightstone, F.C.; et al. Predicting a Drug’s Membrane Permeability: A Computational Model Validated with in Vitro Permeability Assay Data. J. Phys. Chem. B 2017, 121, 5228–5237. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y. Recent Advances of Chitosan-Based Nanoparticles for Biomedical and Biotechnological Applications. Int. J. Biol. Macromol. 2022, 203, 379–388. [Google Scholar] [CrossRef]
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-Score—A Comprehensive Scoring Function for Evaluation of Chemical Drug-Likeness. Med. Chem. Commun. 2018, 10, 148–157. [Google Scholar] [CrossRef]
- Williams, J.D.; Saleh, A.M.; Acharya, D.N. Composition of the Essential Oil of Wild Growing Artemisia Vulgaris from Erie, Pennsylvania. Nat. Prod. Commun. 2012, 7, 637–640. [Google Scholar] [CrossRef]
- Bogas, A.C.; Cruz, F.P.N.; Lacava, P.T.; Sousa, C.P. Endophytic Fungi: An Overview on Biotechnological and Agronomic Potential. Braz. J. Biol. 2022, 84, e258557. [Google Scholar] [CrossRef]
- Mulyani, Y.; Sinaga, S.E.; Supratman, U. Phytochemistry and Biological Activities of Endophytic Fungi from the Meliaceae Family. Molecules 2023, 28, 778. [Google Scholar] [CrossRef]
- Fujii, M.; Takeda, Y.; Yoshida, M.; Utoguchi, N.; Matsumoto, M.; Watanabe, Y. Comparison of Skin Permeation Enhancement by 3-l-Menthoxypropane-1,2-Diol and l-Menthol: The Permeation of Indomethacin and Antipyrine through Yucatan Micropig Skin and Changes in Infrared Spectra and X-Ray Diffraction Patterns of Stratum Corneum. Int. J. Pharm. 2003, 258, 217–223. [Google Scholar] [CrossRef]
- Shimomura, K.; Oikawa, H.; Hasobe, M.; Suzuki, N.; Yajima, S.; Tomizawa, M. Contact Repellency by L-Menthol Is Mediated by TRPM Channels in the Red Flour Beetle Tribolium Castaneum. Pest. Manag. Sci. 2021, 77, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Czyżnikowska, Ż.; Mysłek, M.; Marciniak, A.; Płaczek, R.; Kotynia, A.; Krzyżak, E. In Silico Approach to Design of New Multi-Targeted Inhibitors Based on Quinoline Ring with Potential Anticancer Properties. Int. J. Mol. Sci. 2025, 26, 4620. [Google Scholar] [CrossRef]
- Oh, J.; Cha, J.; Choi, S. Identification of Novel Genetic Variants and Food Intake Factors Associated with Type 2 Diabetes in South Korean Adults, Using an Illness–Death Model. Int. J. Mol. Sci. 2025, 26, 2597. [Google Scholar] [CrossRef]
- Hummadi, A.; Yafei, S.; Mutawwam, D.A.; Abutaleb, R.; Solan, Y.; Khawaji, A.; Alhagawy, A.J.; Algohani, T.; Khardali, M.; Hakami, M.; et al. Genomic and Bioinformatics Analysis of Familial Partial Lipodystrophy Type 3 Identified in a Patient with Novel PPARγ Mutation and Robust Response to Pioglitazone. Int. J. Mol. Sci. 2024, 25, 12060. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Elsaman, T.; Elderdery, A.Y.; Alsrhani, A.; Ghanem, H.B.; Alruwaili, M.M.; Hamza, S.M.A.; Mekki, S.E.I.; Alotaibi, H.A.; Mills, J. Unveiling the Anticancer Potential: Computational Exploration of Nitrogenated Derivatives of (+)-Pancratistatin as Topoisomerase I Inhibitors. Int. J. Mol. Sci. 2024, 25, 10779. [Google Scholar] [CrossRef] [PubMed]
- Alberti, T.B.; Barbosa, W.L.R.; Vieira, J.L.F.; Raposo, N.R.B.; Dutra, R.C. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef] [PubMed]
- Szczurowska, E.; Szánti-Pintér, E.; Randáková, A.; Jakubík, J.; Kudova, E. Allosteric Modulation of Muscarinic Receptors by Cholesterol, Neurosteroids and Neuroactive Steroids. Int. J. Mol. Sci. 2022, 23, 13075. [Google Scholar] [CrossRef]
- Gonzatti, M.B.; Júnior, J.E.M.; Rocha, A.J.; de Oliveira, J.S.; de Oliveira, J.S.; de Jesus Evangelista, A.J.; Fonseca, F.M.P.; Ceccatto, V.M.; de Oliveira, A.C.; da Cruz Freire, J.E. Mechanism of Molecular Interaction of Sitagliptin with Human DPP4 Enzyme—New Insights. Adv. Med. Sci. 2023, 68, 402–408. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, S.; Wang, F.; Jiang, R.; Yin, X.; Wang, S.; Jin, R.; Guo, H.; Tang, Y.; Wang, Y. 3D-QSAR, Scaffold Hopping, Virtual Screening, and Molecular Dynamics Simulations of Pyridin-2-One as mIDH1 Inhibitors. Int. J. Mol. Sci. 2024, 25, 7434. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Rajagopal, D.; Narayanaswamy, R.; Prabhakaran, V.-S. Sivelestat (Neutrophil Elastase Inhibitor) as an Anti-Inflammatory and Anti-Viral Agent: An In Silico Study. Cureus 2024, 16, e56846. [Google Scholar] [CrossRef]
- Tian, H.; Ketkar, R.; Tao, P. ADMETboost: A Web Server for Accurate ADMET Prediction. J. Mol. Model. 2022, 28, 408. [Google Scholar] [CrossRef]
- Yin, X.; Wang, X.; Li, Y.; Wang, J.; Wang, Y.; Deng, Y.; Hou, T.; Liu, H.; Luo, P.; Yao, X. CODD-Pred: A Web Server for Efficient Target Identification and Bioactivity Prediction of Small Molecules. J. Chem. Inf. Model. 2023, 63, 6169–6176. [Google Scholar] [CrossRef]
- Braga, R.C.; Alves, V.M.; Silva, M.F.B.; Muratov, E.; Fourches, D.; Lião, L.M.; Tropsha, A.; Andrade, C.H. Pred-hERG: A Novel Web-Accessible Computational Tool for Predicting Cardiac Toxicity. Mol. Inf. 2015, 34, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Matlock, M.K.; Hughes, T.B.; Swamidass, S.J. XenoSite Server: A Web-Available Site of Metabolism Prediction Tool. Bioinformatics 2015, 31, 1136–1137. [Google Scholar] [CrossRef] [PubMed]
- Schyman, P.; Liu, R.; Desai, V.; Wallqvist, A. vNN Web Server for ADMET Predictions. Front. Pharmacol. 2017, 8, 889. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Choudhury, S.; Arora, A.; Tijare, P.; Raghava, G.P.S. ToxinPred 3.0: An Improved Method for Predicting the Toxicity of Peptides. Comput. Biol. Med. 2024, 179, 108926. [Google Scholar] [CrossRef]
- Yang, H.; Sun, L.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. ADMETopt: A Web Server for ADMET Optimization in Drug Design via Scaffold Hopping. J. Chem. Inf. Model. 2018, 58, 2051–2056. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Chiodi, D.; Ishihara, Y. Tertiary Alcohol: Reaping the Benefits but Minimizing the Drawbacks of Hydroxy Groups in Drug Discovery. J. Med. Chem. 2025, 68, 7889–7913. [Google Scholar] [CrossRef] [PubMed]
- Kalash, L.N.; Cole, J.C.; Copley, R.C.B.; Edge, C.M.; Moldovan, A.A.; Sadiq, G.; Doherty, C.L. First Global Analysis of the GSK Database of Small Molecule Crystal Structures. CrystEngComm 2021, 23, 5430–5442. [Google Scholar] [CrossRef]
- Kaya, B.; Acar Çevik, U.; Necip, A.; Duran, H.E.; Çiftçi, B.; Işık, M.; Soyer, P.; Bostancı, H.E.; Kaplancıklı, Z.A.; Beydemir, Ş. Design, Synthesis, Biological Evaluation, and Molecular Docking Studies of Novel 1,3,4-Thiadiazole Derivatives Targeting Both Aldose Reductase and α-Glucosidase for Diabetes Mellitus. ACS Omega 2025, 10, 18812–18828. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Li, S.; Zhang, Y.; Guo, H.; Wu, L.; Liu, H.; Manyande, A.; Xu, F.; Wang, J. NMR Based Metabolomics Comparison of Different Blood Sampling Techniques in Awake and Anesthetized Rats. Molecules 2019, 24, 2542. [Google Scholar] [CrossRef]
- Ghaemi, Z.; Alberga, D.; Carloni, P.; Laio, A.; Lattanzi, G. Permeability Coefficients of Lipophilic Compounds Estimated by Computer Simulations. J. Chem. Theory Comput. 2016, 12, 4093–4099. [Google Scholar] [CrossRef]
- Duan, Y.-J.; Fu, L.; Zhang, X.-C.; Long, T.-Z.; He, Y.-H.; Liu, Z.-Q.; Lu, A.-P.; Deng, Y.-F.; Hsieh, C.-Y.; Hou, T.-J.; et al. Improved GNNs for Log D7.4 Prediction by Transferring Knowledge from Low-Fidelity Data. J. Chem. Inf. Model. 2023, 63, 2345–2359. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, R.B.; Li, Y. Caco-2 Cell Permeability Assays to Measure Drug Absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- Cheng, A.; Merz, K.M. Prediction of Aqueous Solubility of a Diverse Set of Compounds Using Quantitative Structure−Property Relationships. J. Med. Chem. 2003, 46, 3572–3580. [Google Scholar] [CrossRef]
- Soriano-Meseguer, S.; Fuguet, E.; Port, A.; Rosés, M. Ability of Biomimetic Chromatography and Physicochemical Systems to Predict the Skin Permeation of Neutral Compounds. A Comparison Study. Talanta 2024, 271, 125696. [Google Scholar] [CrossRef] [PubMed]
- Elmeliegy, M.; Vourvahis, M.; Guo, C.; Wang, D.D. Effect of P-Glycoprotein (P-Gp) Inducers on Exposure of P-Gp Substrates: Review of Clinical Drug-Drug Interaction Studies. Clin. Pharmacokinet. 2020, 59, 699–714. [Google Scholar] [CrossRef]
- Karsauliya, K.; Sonker, A.K.; Bhateria, M.; Taneja, I.; Srivastava, A.; Sharma, M.; Singh, S.P. Plasma Protein Binding, Metabolism, Reaction Phenotyping and Toxicokinetic Studies of Fenarimol after Oral and Intravenous Administration in Rats. Xenobiotica 2021, 51, 72–81. [Google Scholar] [CrossRef]
- Sodhi, J.K.; Huang, C.H.; Benet, L.Z. Volume of Distribution Is Unaffected by Metabolic Drug-Drug Interactions. Clin. Pharmacokinet. 2021, 60, 205–222. [Google Scholar] [CrossRef]
- Gabriel, L.; Tod, M.; Goutelle, S. Quantitative Prediction of Drug Interactions Caused by CYP1A2 Inhibitors and Inducers. Clin. Pharmacokinet. 2016, 55, 977–990. [Google Scholar] [CrossRef]
- Pelkonen, O.; Hakkola, J.; Hukkanen, J.; Turpeinen, M. CYP-Associated Drug-Drug Interactions: A Mission Accomplished? Arch. Toxicol. 2020, 94, 3931–3934. [Google Scholar] [CrossRef]
- Krishnan, S.; Ramsden, D.; Ferguson, D.; Stahl, S.H.; Wang, J.; McGinnity, D.F.; Hariparsad, N. Challenges and Opportunities for Improved Drug-Drug Interaction Predictions for Renal OCT2 and MATE1/2-K Transporters. Clin. Pharmacol. Ther. 2022, 112, 562–572. [Google Scholar] [CrossRef]
- Vijay, U.; Gupta, S.; Mathur, P.; Suravajhala, P.; Bhatnagar, P. Microbial Mutagenicity Assay: Ames Test. Bio-Protoc. J. 2018, 8, e2763. [Google Scholar] [CrossRef]
- Johnson, T.W.; Dress, K.R.; Edwards, M. Using the Golden Triangle to Optimize Clearance and Oral Absorption. Bioorg. Med. Chem. Lett. 2009, 19, 5560–5564. [Google Scholar] [CrossRef]
- Nicolov, M.; Cocora, M.; Buda, V.; Danciu, C.; Duse, A.O.; Watz, C.; Borcan, F. Hydrosoluble and Liposoluble Vitamins: New Perspectives through ADMET Analysis. Medicina 2021, 57, 1204. [Google Scholar] [CrossRef]
- Chakraborty, A.; Kumar, A. Computational Screening for Natural Compounds as Potential Immune Checkpoint Inhibitors against TIGIT, a New Avenue in Cancer Immunotherapy. Mol. Divers. 2025. [Google Scholar] [CrossRef]
- López-López, E.; Naveja, J.J.; Medina-Franco, J.L. DataWarrior: An Evaluation of the Open-Source Drug Discovery Tool. Expert Opin. Drug Discov. 2019, 14, 335–341. [Google Scholar] [CrossRef]
- Min, S.; Lee, B.; Yoon, S. TargetNet: Functional microRNA Target Prediction with Deep Neural Networks. Bioinformatics 2022, 38, 671–677. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardeal dos Santos, A.N.; Oliveira, P.E.G.d.; da Cruz Freire, J.E.; Araújo dos Santos, S.; Júnior, J.E.R.H.; Andrade, C.R.d.; Sousa, B.L.d.; Silva, W.M.B.d.; de Oliveira, A.C.; Ceccatto, V.M.; et al. Computational Profiling of Monoterpenoid Phytochemicals: Insights for Medicinal Chemistry and Drug Design Strategies. Int. J. Mol. Sci. 2025, 26, 7671. https://doi.org/10.3390/ijms26167671
Cardeal dos Santos AN, Oliveira PEGd, da Cruz Freire JE, Araújo dos Santos S, Júnior JERH, Andrade CRd, Sousa BLd, Silva WMBd, de Oliveira AC, Ceccatto VM, et al. Computational Profiling of Monoterpenoid Phytochemicals: Insights for Medicinal Chemistry and Drug Design Strategies. International Journal of Molecular Sciences. 2025; 26(16):7671. https://doi.org/10.3390/ijms26167671
Chicago/Turabian StyleCardeal dos Santos, André Nogueira, Paulo Elesson Guimarães de Oliveira, José Ednésio da Cruz Freire, Sara Araújo dos Santos, José Eduardo Ribeiro Honório Júnior, Claudia Roberta de Andrade, Bruno Lopes de Sousa, Wildson Max Barbosa da Silva, Ariclécio Cunha de Oliveira, Vânia Marilande Ceccatto, and et al. 2025. "Computational Profiling of Monoterpenoid Phytochemicals: Insights for Medicinal Chemistry and Drug Design Strategies" International Journal of Molecular Sciences 26, no. 16: 7671. https://doi.org/10.3390/ijms26167671
APA StyleCardeal dos Santos, A. N., Oliveira, P. E. G. d., da Cruz Freire, J. E., Araújo dos Santos, S., Júnior, J. E. R. H., Andrade, C. R. d., Sousa, B. L. d., Silva, W. M. B. d., de Oliveira, A. C., Ceccatto, V. M., Leal Cardoso, J. H., Aquino, A. J. A., & Coelho de Sousa, A. N. (2025). Computational Profiling of Monoterpenoid Phytochemicals: Insights for Medicinal Chemistry and Drug Design Strategies. International Journal of Molecular Sciences, 26(16), 7671. https://doi.org/10.3390/ijms26167671






