Circulating FGF-21 as a Disease-Modifying Factor Associated with Distinct Symptoms and Cognitive Profiles in Myalgic Encephalomyelitis and Fibromyalgia
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics and Questionnaire Validation
2.2. Circulating FGF-21 Levels Reveal Clinically Relevant Subtypes Across ME, FM, and ME + FM
2.3. Differential Clinical Consequences of Low Circulating FGF-21 Across FM, ME, and ME + FM
2.4. Low FGF-21 Is Associated with Acute Exertional Sensitivity and Cognitive Rigidity in FM
2.5. Low FGF-21 Reflects Delayed PEM and Neuroimmune Vulnerability in ME and ME + FM
2.6. High Circulating FGF-21 Levels Show Divergent Clinical Significance Across Diagnoses
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethical Approval
4.2. Study Population
4.3. Post-Exertional Malaise Provocation and Symptom Assessment
4.4. Blood Collection and Circulating FGF-21 Quantification
4.5. Symptom and Health Status Evaluation
4.6. FGF-21 Stratification
4.7. Cognitive Assessment at Baseline
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABR | advanced biomedical rehabilitation |
| ACR | American College of Rheumatology (ACR) |
| AKR-001 | Akero Therapeutics drug, also named Efruxufermin |
| Akt | family of serine/threonine protein kinases, also known as Protein Kinase B |
| AMP | adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| ANOVA | analysis of variance |
| AQEM | Association Québécoise de l’Encéphalomyélite Myalgique |
| Avimer | avidity multimers |
| BIO89-100 | pegozafermin |
| BMI | body mass index |
| C3201 | an avimer of FGF21 receptor agonist |
| CCC | Canadian Consensus Criteria |
| CHU | Centre hospitalier universitaire, university hospital center |
| DPEMQ | DePaul Post-Exertional Malaise Questionnaire |
| DSQ | DePaul Symptom Questionnaire |
| ELISA | enzyme-linked immunosorbent assay |
| F2/F3 | liver fibrosis, with the level/stage of the disease being moderate to severe |
| FAP | fibroblast activation protein |
| FGF-21 | fibroblast growth gactor 21 |
| FGFR1c | fibroblast growth factor receptor 1, variant c |
| FGFR3c | fibroblast growth factor receptor 3, isoform 3c |
| FM | fibromyalgia |
| HC | healthy control |
| Hz | hertz |
| IL-18 | interleukin-18 |
| IL-1β | interleukin-1 beta |
| K2-EDTA | dipotassium ethylenediamineteraacetic acid |
| kg/m2 | kilogram per quare meter |
| ME | myalgic encephalomyelitis |
| ME + FM | myalgic encephalomyelitis and fibromyalgia |
| MFI-20 | Multidimensional Fatigue Inventory |
| mimAb1 | monoclonal antibody designed to bind to β-Klotho/FGFR1c receptor complex effectively mimicking the action of FGF21 |
| mg | milligram |
| miRNA | microRNA |
| MS | multiple sclerosis |
| mTOR | mammalian target of rapamycin |
| n/a | not applicable |
| NAS | nonalcoholic steatohepatitis |
| NLRP3 | NOD-like receptor domain containing protein 3 |
| NOD | nucleotide-binding oligomerization domain |
| p70S6K | p70 ribosomal S6 kinase |
| PEM | post-exertional malaise |
| pg/mL | picogram per milliliter |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| psi | pounds per square inch |
| p-value | probability value |
| r | Pearson correlation coefficients |
| RNA | ribonucleic acid |
| SEM | standard error of the mean |
| SF-36 | 36-item Short Form Survey |
| SGLT2 | sodium–glucose cotransporter-2 |
References
- Meeus, M.; Nijs, J.; Meirleir, K.D. Chronic musculoskeletal pain in patients with the chronic fatigue syndrome: A systematic review. Eur. J. Pain 2007, 11, 377–386. [Google Scholar] [CrossRef]
- Deumer, U.S.; Varesi, A.; Floris, V.; Savioli, G.; Mantovani, E.; López-Carrasco, P.; Rosati, G.M.; Prasad, S.; Ricevuti, G. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): An Overview. J. Clin. Med. 2021, 10, 4786. [Google Scholar] [CrossRef]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Nacul, L.; O’Boyle, S.; Palla, L.; Nacul, F.E.; Mudie, K.; Kingdon, C.C.; Cliff, J.M.; Clark, T.G.; Dockrell, H.M.; Lacerda, E.M. How Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Progresses: The Natural History of ME/CFS. Front. Neurol. 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Hoel, F.; Hoel, A.; Pettersen, I.K.; Rekeland, I.G.; Risa, K.; Alme, K.; Sørland, K.; Fosså, A.; Lien, K.; Herder, I.; et al. A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. J. CI Insight. 2021, 6, e149217. [Google Scholar] [CrossRef] [PubMed]
- Buskila, D. Fibromyalgia, chronic fatigue syndrome, and myofascial pain syndrome. Curr. Opin. Rheumatol. 2001, 13, 117–127. [Google Scholar] [CrossRef]
- Aaron, L.A.; Buchwald, D. Chronic diffuse musculoskeletal pain, fibromyalgia and co-morbid unexplained clinical conditions. Best Pract. Res. Clin. Rheumatol. 2003, 17, 563–574. [Google Scholar] [CrossRef]
- Słomko, J.; Newton, J.L.; Kujawski, S.; Tafil-Klawe, M.; Klawe, J.; Staines, D.; Marshall-Gradisnik, S.; Zalewski, P. Prevalence and characteristics of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) in Poland: A cross-sectional study. BMJ Open. 2019, 9, e023955. [Google Scholar] [CrossRef]
- Lumley, M.A.; Schubiner, H.; Lockhart, N.A.; Kidwell, K.M.; Harte, S.E.; Clauw, D.J.; Williams, D.A. Emotional awareness and expression therapy, cognitive behavioral therapy, and education for fibromyalgia: A cluster-randomized controlled trial. Pain 2017, 158, 2354–2363. [Google Scholar] [CrossRef]
- Qureshi, A.G.; Jha, S.K.; Iskander, J.; Avanthika, C.; Jhaveri, S.; Patel, V.H.; Rasagna Potini, B.; Talha Azam, A. Diagnostic Challenges and Management of Fibromyalgia. Cureus 2021, 13, e18692. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Reyes Del Paso, G.A. Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives. J. Clin. Med. 2020, 9, 1219. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Myalgic Encephalomyelitis (or Encephalopathy)/Chronic Fatigue Syndrome: Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2021.
- Brurberg, K.G.; Fønhus, M.S.; Larun, L.; Flottorp, S.; Malterud, K. Case definitions for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): A systematic review. BMJ Open. 2014, 4, e003973. [Google Scholar] [CrossRef]
- Crofford, L.J.; Clauw, D.J. Fibromyalgia: Where are we a decade after the American College of Rheumatology classification criteria were developed? Arthritis Rheum. 2002, 46, 1136–1138. [Google Scholar] [CrossRef]
- Fall, E.A.; Chen, Y.; Lin, J.S.; Issa, A.; Brimmer, D.J.; Bateman, L.; Lapp, C.W.; Podell, R.N.; Natelson, B.H.; Kogelnik, A.M.; et al. Chronic Overlapping Pain Conditions in people with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A sample from the Multi-site Clinical Assessment of ME/CFS (MCAM) study. BMC Neurol. 2024, 24, 399. [Google Scholar] [CrossRef]
- Berkis, U.; Svirskis, S.; Krumina, A.; Gravelsina, S.; Vilmane, A.; Araja, D.; Nora-Krukle, Z.; Murovska, M. Exploring the joint potential of inflammation, immunity, and receptor-based biomarkers for evaluating ME/CFS progression. Front. Immunol. 2023, 14, 1294758. [Google Scholar] [CrossRef]
- Morris, G.; Anderson, G.; Maes, M. Hypothalamic-Pituitary-Adrenal Hypofunction in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS) as a Consequence of Activated Immune-Inflammatory and Oxidative and Nitrosative Pathways. Mol. Neurobiol. 2017, 54, 6806–6819. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, D.; Annesley, S.J.; Fisher, P.R. Pathological Mechanisms Underlying Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Diagnostics 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Velingkar, A.; Vuree, S.; Prabhakar, P.K.; Kalashikam, R.R.; Banerjee, A.; Kondeti, S. Fibroblast growth factor 21 as a potential master regulator in metabolic disorders. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E409–E424. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.C.; Xu, A.; Wang, Y.; Lam, K.S. Fibroblast growth factor 21 as an emerging metabolic regulator: Clinical perspectives. Clin. Endocrinol. 2013, 78, 489–496. [Google Scholar] [CrossRef]
- Szczepańska, E.; Gietka-Czernel, M. FGF21: A Novel Regulator of Glucose and Lipid Metabolism and Whole-Body Energy Balance. Horm. Metab. Res. 2022, 54, 203–211. [Google Scholar] [CrossRef]
- Cui, X.; Sun, Q.; Wang, H. Targeting fibroblast growth factor (FGF)-21: A promising strategy for metabolic dysfunction-associated steatotic liver disease treatment. Front. Pharmacol. 2025, 16, 1510322. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Wei, P.; Zou, T.; You, J. Fibroblast Growth Factor 21: A Fascinating Perspective on the Regulation of Muscle Metabolism. Int. J. Mol. Sci. 2023, 24, 16951. [Google Scholar] [CrossRef] [PubMed]
- Keipert, S.; Ost, M.; Johann, K.; Imber, F.; Jastroch, M.; van Schothorst, E.M.; Keijer, J.; Klaus, S. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E469–E482. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Farrar, J.S.; Vaitkus, J.A.; Celi, F.S. Metabolic Effects of FGF-21: Thermoregulation and Beyond. Front. Endocrinol. 2015, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Klein Hazebroek, M.; Keipert, S. Adapting to the Cold: A Role for Endogenous Fibroblast Growth Factor 21 in Thermoregulation? Front. Endocrinol. 2020, 11, 389. [Google Scholar] [CrossRef]
- Wang, D.; Liu, F.; Zhu, L.; Lin, P.; Han, F.; Wang, X.; Tan, X.; Lin, L.; Xiong, Y. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J. Neuroinflamm. 2020, 17, 257. [Google Scholar] [CrossRef]
- Scholle, L.M.; Lehmann, D.; Deschauer, M.; Kraya, T.; Zierz, S. FGF-21 as a Potential Biomarker for Mitochondrial Diseases. Curr. Med. Chem. 2018, 25, 2070–2081. [Google Scholar] [CrossRef]
- Nicolaisen, T.S.; Lyster, A.E.; Sjøberg, K.A.; Haas, D.T.; Voldstedlund, C.T.; Lundsgaard, A.M.; Jensen, J.K.; Madsen, E.M.; Nielsen, C.K.; Bloch-Ibenfeldt, M.; et al. Dietary protein restriction elevates FGF21 levels and energy requirements to maintain body weight in lean men. Nat. Metab. 2025, 7, 602–616. [Google Scholar] [CrossRef]
- Khalafi, M.; Alamdari, K.A.; Symonds, M.E.; Nobari, H.; Carlos-Vivas, J. Impact of acute exercise on immediate and following early post-exercise FGF-21 concentration in adults: Systematic review and meta-analysis. Hormones 2021, 20, 23–33. [Google Scholar] [CrossRef]
- Cheng, X.; Zhu, B.; Jiang, F.; Fan, H. Serum FGF-21 levels in type 2 diabetic patients. Endocr. Res. 2011, 36, 142–148. [Google Scholar] [CrossRef]
- Matuszek, B.; Lenart-Lipińska, M.; Duma, D.; Solski, J.; Nowakowski, A. Evaluation of concentrations of FGF-21—A new adipocytokine in type 2 diabetes. Endokrynol. Pol. 2010, 61, 50–54. [Google Scholar]
- Ritchie, M.; Hanouneh, I.A.; Noureddin, M.; Rolph, T.; Alkhouri, N. Fibroblast growth factor (FGF)-21 based therapies: A magic bullet for nonalcoholic fatty liver disease (NAFLD)? Expert. Opin. Investig. Drugs 2020, 29, 197–204. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Long, X.X.; Fang, Q.C.; Jia, W.P.; Li, H.T. The role of FGF21 in the pathogenesis of cardiovascular disease. Chin. Med. J. 2021, 34, 2931–2943. [Google Scholar] [CrossRef]
- Łukawska, A.; Mulak, A.J.P.H.M.D. Physiological and pathophysiological role of endocrine fibroblast growth factors. Postep. Hig. Med. 2022, 76, 39–53. [Google Scholar] [CrossRef]
- Soto Sauza, K.A.; Ryan, K.K. FGF21 mediating the Sex-dependent Response to Dietary Macronutrients. J. Clin. Endocrinol. Metab. 2024, 109, e1689–e1696. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, A.T.; Larson, K.R.; Huang, K.P.; Wu, C.T.; Godoroja, N.; Fang, Y.; Jayakrishnan, D.; Soto Sauza, K.A.; Sims, L.C.; Mohajerani, N.; et al. FGF21 controls hepatic lipid metabolism via sex-dependent interorgan crosstalk. JCI Insight 2022, 7, e155848. [Google Scholar] [CrossRef] [PubMed]
- Chee, Y.; Toh, G.L.; Lim, C.J.; Goh, L.L.; Dalan, R. Sex Modifies the Association of Fibroblast Growth Factor 21 With Subclinical Carotid Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 627691. [Google Scholar] [CrossRef]
- Domingo, J.C.; Cordobilla, B.; Ferrer, R.; Giralt, M.; Alegre-Martín, J.; Castro-Marrero, J. Are Circulating Fibroblast Growth Factor 21 and N-Terminal Prohormone of Brain Natriuretic Peptide Promising Novel Biomarkers in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Antioxid. Redox Signal. 2021, 34, 1420–1427. [Google Scholar] [CrossRef]
- Nepotchatykh, E.; Caraus, I.; Elremaly, W.; Leveau, C.; Elbakry, M.; Godbout, C.; Rostami-Afshari, B.; Petre, D.; Khatami, N.; Franco, A.; et al. Circulating microRNA expression signatures accurately discriminate myalgic encephalomyelitis from fibromyalgia and comorbid conditions. Sci. Rep. 2023, 13, 1896. [Google Scholar] [CrossRef]
- Wirth, K.J.; Scheibenbogen, C. Pathophysiology of skeletal muscle disturbances in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). J. Transl. Med. 2021, 19, 162. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab. Brain Dis. 2014, 29, 19–36. [Google Scholar] [CrossRef]
- Post, A.; Groothof, D.; Schutten, J.C.; Kelly, D.; Swarte, J.C.; Flores-Guerrero, J.L.; van der Veen, Y.; Kema, I.P.; Ozyilmaz, A.; Enya, A.; et al. Fibroblast growth factor 21 and protein energy wasting in hemodialysis patients. Clin. Nutr. 2021, 40, 4216–4224. [Google Scholar] [CrossRef] [PubMed]
- Dolegowska, K.; Marchelek-Mysliwiec, M.; Nowosiad-Magda, M.; Slawinski, M.; Dolegowska, B. FGF19 subfamily members: FGF19 and FGF21. J. Physiol. Biochem. 2019, 75, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Croon, M.; Szczepanowska, K.; Popovic, M.; Lienkamp, C.; Senft, K.; Brandscheid, C.P.; Bock, T.; Gnatzy-Feik, L.; Ashurov, A.; Acton, R.J.; et al. FGF21 modulates mitochondrial stress response in cardiomyocytes only under mild mitochondrial dysfunction. Sci. Adv. 2022, 8, eabn7105. [Google Scholar] [CrossRef] [PubMed]
- Giesecke, T.; Gracely, R.H.; Grant, M.A.; Nachemson, A.; Petzke, F.; Williams, D.A.; Clauw, D.J. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004, 50, 613–623. [Google Scholar] [CrossRef]
- Giesecke, T.; Williams, D.A.; Harris, R.E.; Cupps, T.R.; Tian, X.; Tian, T.X.; Gracely, R.H.; Clauw, D.J. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003, 48, 2916–2922. [Google Scholar] [CrossRef]
- Liu, C.; Yan, X.; Zong, Y.; He, Y.; Yang, G.; Xiao, Y.; Wang, S. The effects of exercise on FGF21 in adults: A systematic review and meta-analysis. Peer J. 2024, 12, e17615. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdés, P.; Meza-Arana, C.E.; Brito-Córdova, G.; Gómez-Pérez, F.J.; Mehta, R.; Oseguera-Moguel, J.; Aguilar-Salinas, C.A. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS ONE 2012, 7, e38022. [Google Scholar] [CrossRef]
- Ickmans, K.; Meeus, M.; De Kooning, M.; Lambrecht, L.; Pattyn, N.; Nijs, J. Associations Between Cognitive Performance and Pain in Chronic Fatigue Syndrome: Comorbidity with Fibromyalgia Does Matter. Pain Physician 2015, 18, E841–E852. [Google Scholar] [CrossRef]
- Tan, H.; Yue, T.; Chen, Z.; Wu, W.; Xu, S.; Weng, J. Targeting FGF21 in cardiovascular and metabolic diseases: From mechanism to medicine. Int. J. Biol. Sci. 2023, 19, 66–88. [Google Scholar] [CrossRef]
- Falamarzi, K.; Malekpour, M.; Tafti, M.F.; Azarpira, N.; Behboodi, M.; Zarei, M. The role of FGF21 and its analogs on liver associated diseases. Front. Med. 2022, 9, 967375. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Wang, Z.; Tsai, L.K.; Leeds, P.; Fessler, E.B.; Wang, J.; Chuang, D.M. FGF-21, a novel metabolic regulator, has a robust neuroprotective role and is markedly elevated in neurons by mood stabilizers. Mol. Psychiatry 2015, 20, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, W.; Deng, P.; Wang, X.; Zhu, L.; Zhao, L.; Li, C.; Gao, H. Fibroblast growth factor 21 ameliorates behavior deficits in Parkinson’s disease mouse model via modulating gut microbiota and metabolic homeostasis. CNS Neurosci. Ther. 2023, 29, 3815–3828. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhu, Z.; Wang, Y.; Qian, S.; Xu, C.; Zhang, B. Fibroblast growth factor-21 alleviates proteasome injury via activation of autophagy flux in Parkinson’s disease. Exp. Brain Res. 2024, 242, 25–32. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, H.; Ni, Z.; Shen, Y.; Wang, D.; Li, W.; Zhao, L.; Li, C.; Gao, H. Fibroblast growth factor 21 alleviates diabetes-induced cognitive decline. Cereb. Cortex 2024, 34, bhad502, Erratum in: Cereb. Cortex. 2024, 34, bhae091. https://doi.org/10.1093/cercor/bhae091. [Google Scholar] [CrossRef]
- Wang, D.X.; Huang, W.T.; Shi, J.F.; Liu, F.; Jiang, W.Y.; Chen, K.Y.; Zhang, S.Y.; Li, X.K.; Lin, L. FGF21, a modulator of astrocyte reactivity, protects against ischemic brain injury through anti-inflammatory and neurotrophic pathways. Acta Pharmacol. Sin. 2025, 46, 1834–1851. [Google Scholar] [CrossRef]
- Thiessen, S.E.; Vanhorebeek, I.; Derese, I.; Gunst, J.; Van den Berghe, G. FGF21 Response to Critical Illness: Effect of Blood Glucose Control and Relation with Cellular Stress and Survival. J. Clin. Endocrinol. Metab. 2015, 100, E1319–E1327. [Google Scholar] [CrossRef]
- Hendrix, J.; Fanning, L.; Wyns, A.; Ahmed, I.; Patil, M.S.; Richter, E.; Van Campenhout, J.; Ickmans, K.; Mertens, R.; Nijs, J.; et al. Adrenergic dysfunction in patients with myalgic encephalomyelitis/chronic fatigue syndrome and fibromyalgia: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2025, 55, e14318. [Google Scholar] [CrossRef]
- Lange, G.; Lin, J.S.; Chen, Y.; Fall, E.A.; Peterson, D.L.; Bateman, L.; Lapp, C.; Podell, R.N.; Natelson, B.H.; Kogelnik, A.M.; et al. Cognitive assessment in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A cognitive substudy of the multi-site clinical assessment of ME/CFS (MCAM). Front. Neurosci. 2024, 18, 1460157. [Google Scholar] [CrossRef]
- Coppage, A.L.; Heard, K.R.; DiMare, M.T.; Liu, Y.; Wu, W.; Lai, J.H.; Bachovchin, W.W. Human FGF-21 Is a Substrate of Fibroblast Activation Protein. PLoS ONE 2016, 11, e0151269. [Google Scholar] [CrossRef]
- Pedersen, A.K.N.; Gormsen, L.C.; Nielsen, S.; Jessen, N.; Bjerre, M. Metformin Improves the Prerequisites for FGF21 Signaling in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2024, 109, e552–e561. [Google Scholar] [CrossRef]
- AboTaleb, H.A.; Alghamdi, B.S. Metformin and fibromyalgia pathophysiology: Current insights and promising future therapeutic strategies. Mol. Biol. Rep. 2024, 52, 60. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Flores, G.D.C.; Guzmán-Priego, C.G.; Parra-Flores, L.I.; Murbartián, J.; Torres-López, J.E.; Granados-Soto, V. Metformin: A Prospective Alternative for the Treatment of Chronic Pain. Front. Pharmacol. 2020, 11, 558474. [Google Scholar] [CrossRef] [PubMed]
- Bullón, P.; Alcocer-Gómez, E.; Carrión, A.M.; Marín-Aguilar, F.; Garrido-Maraver, J.; Román-Malo, L.; Ruiz-Cabello, J.; Culic, O.; Ryffel, B.; Apetoh, L.; et al. AMPK Phosphorylation Modulates Pain by Activation of NLRP3 Inflammasome. Antioxid. Redox Signal. 2016, 24, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Frias, J.P.; Neff, G.; Abrams, G.A.; Lucas, K.J.; Sanchez, W.; Gogia, S.; Sheikh, M.Y.; Behling, C.; Bedossa, P.; et al. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): A multicentre, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1080–1093. [Google Scholar] [CrossRef]
- Zimodro, J.M.; Rizzo, M.; Gouni-Berthold, I. Current and Emerging Treatment Options for Hypertriglyceridemia: State-of-the-Art Review. Pharmaceuticals 2025, 18, 147. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. eBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Liu, S.Y.; Chen, L.K.; Li, P.H.; Wu, G.L.; Wu, T.H.; Yu, Y.B.; Lin, H.F.; Juan, C.C. Glucosamine induces hepatic FGF21 expression by activating the Akt/mTOR/p70S6K axis and driving PGC-1α activity. Sci. Rep. 2025, 15, 13096. [Google Scholar] [CrossRef]
- Basolo, A.; Begaye, B.; Hollstein, T.; Vinales, K.L.; Walter, M.; Santini, F.; Krakoff, J.; Piaggi, P. Effects of Short-Term Fasting and Different Overfeeding Diets on Thyroid Hormones in Healthy Humans. Thyroid 2019, 29, 1209–1219. [Google Scholar] [CrossRef]
- Laeger, T.; Henagan, T.M.; Albarado, D.C.; Redman, L.M.; Bray, G.A.; Noland, R.C.; Münzberg, H.; Hutson, S.M.; Gettys, T.W.; Schwartz, M.W.; et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Investig. 2014, 124, 3913–3922. [Google Scholar] [CrossRef]
- Post, A.; Dam, W.A.; Groothof, D.; Franssen, C.F.M.; Bakker, S.J.L.; Dullaart, R.P.F. Higher circulating FGF21, lower protein intake, and lower muscle mass: Associations with a higher risk of mortality. J. Intern. Med. 2025, 298, 2–15. [Google Scholar] [CrossRef]
- Suzuki, M.; Uehara, Y.; Motomura-Matsuzaka, K.; Oki, J.; Koyama, Y.; Kimura, M.; Asada, M.; Komi-Kuramochi, A.; Oka, S.; Imamura, T. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol. Endocrinol. 2008, 22, 1006–1014. [Google Scholar] [CrossRef]
- So, W.Y.; Cheng, Q.; Chen, L.; Evans-Molina, C.; Xu, A.; Lam, K.S.; Leung, P.S. High glucose represses β-klotho expression and impairs fibroblast growth factor 21 action in mouse pancreatic islets: Involvement of peroxisome proliferator-activated receptor γ signaling. Diabetes 2013, 62, 3751–3759. [Google Scholar] [CrossRef]
- Smith, R.; Duguay, A.; Bakker, A.; Li, P.; Weiszmann, J.; Thomas, M.R.; Alba, B.M.; Wu, X.; Gupte, J.; Yang, L.; et al. FGF21 can be mimicked in vitro and in vivo by a novel anti-FGFR1c/β-Klotho bispecific protein. PLoS ONE 2013, 8, e61432. [Google Scholar] [CrossRef]
- Roberti, A.; Chaffey, L.E.; Greaves, D.R. NF-κB Signaling and Inflammation-Drug Repurposing to Treat Inflammatory Disorders? Biology 2022, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Nepotchatykh, E.; Elremaly, W.; Caraus, I.; Godbout, C.; Leveau, C.; Chalder, L.; Beaudin, C.; Kanamaru, E.; Kosovskaia, R.; Lauzon, S.; et al. Profile of circulating microRNAs in myalgic encephalomyelitis and their relation to symptom severity, and disease pathophysiology. Sci. Rep. 2020, 10, 19620. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Holtzman, C.S.; Sunnquist, M.; Cotler, J. The development of an instrument to assess post-exertional malaise in patients with myalgic encephalomyelitis and chronic fatigue syndrome. J. Health Psychol. 2021, 26, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Cotler, J.; Holtzman, C.; Dudun, C.; Jason, L.A. A Brief Questionnaire to Assess Post-Exertional Malaise. Diagnostics 2018, 8, 66. [Google Scholar] [CrossRef]
- Ware, J.E., Jr. SF-36 health survey update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef]
- Smets, E.M.; Garssen, B.; Bonke, B.; De Haes, J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Jason, L.A.; Sunnquist, M.; Brown, A.; Furst, J.; Cid, M.; Farietta, J.; Kot, B.; Bloomer, C.; Nicholson, L.; Williams, Y.; et al. Factor Analysis of the DePaul Symptom Questionnaire: Identifying Core Domains. J. Neurol. Neurobiol. 2015, 1, 114. [Google Scholar] [CrossRef] [PubMed]
- Keuper, M.; Häring, H.U.; Staiger, H. Circulating FGF21 Levels in Human Health and Metabolic Disease. Exp. Clin. Endocrinol. Diabetes 2020, 128, 752–770. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, A.; Elo, J.M.; Pietiläinen, K.H.; Hakonen, A.H.; Sevastianova, K.; Korpela, M.; Isohanni, P.; Marjavaara, S.K.; Tyni, T.; Kiuru-Enari, S.; et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: A diagnostic study. Lancet Neurol. 2011, 10, 806–818. [Google Scholar] [CrossRef]
- Yang, S.; Flores, B.; Magal, R.; Harris, K.; Gross, J.; Ewbank, A.; Davenport, S.; Ormachea, P.; Nasser, W.; Le, W.; et al. Diagnostic accuracy of tablet-based software for the detection of concussion. PLoS ONE 2017, 12, e0179352. [Google Scholar] [CrossRef]
- Groppell, S.; Soto-Ruiz, K.M.; Flores, B.; Dawkins, W.; Smith, I.; Eagleman, D.M.; Katz, Y. A Rapid, Mobile Neurocognitive Screening Test to Aid in Identifying Cognitive Impairment and Dementia (BrainCheck): Cohort Study. JMIR Aging 2019, 2, e12615. [Google Scholar] [CrossRef]
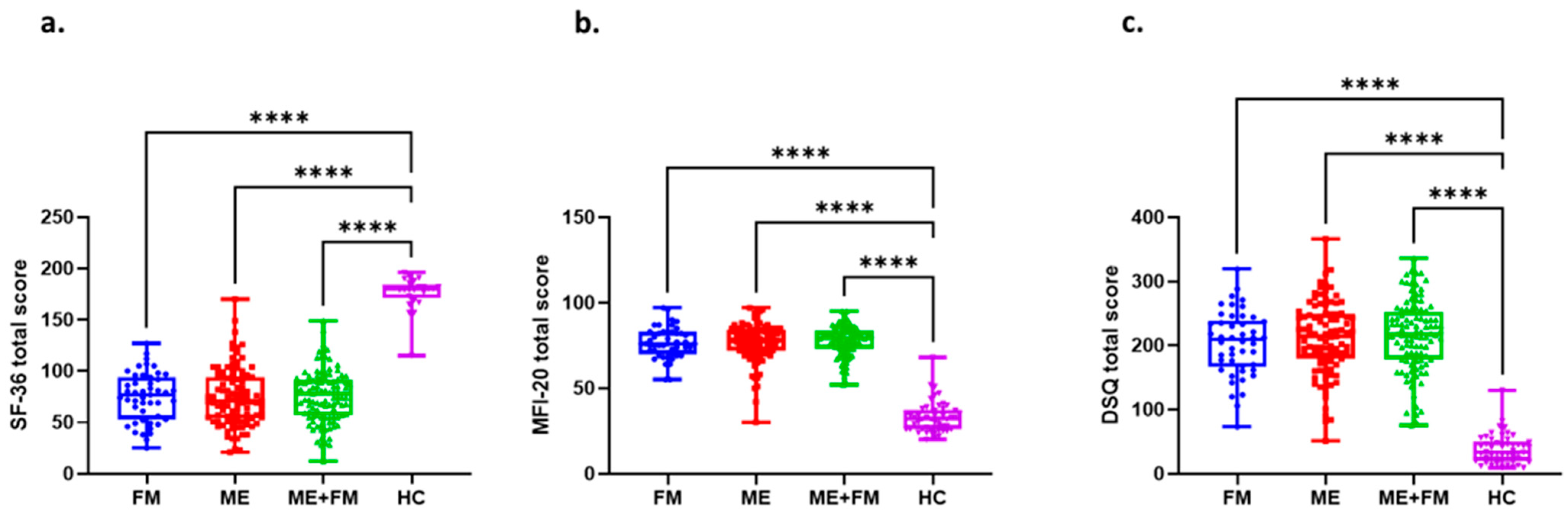
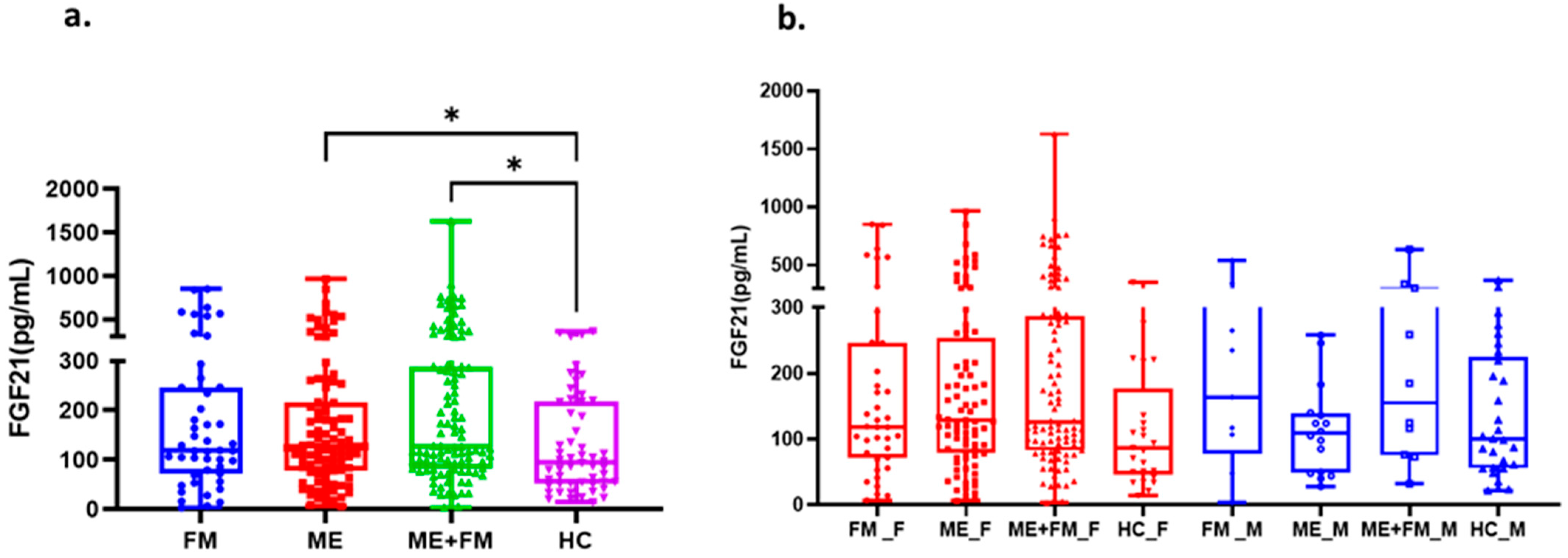

| FM | ME | ME + FM | HC | |
|---|---|---|---|---|
| n | 47 | 99 | 104 | 54 |
| Female/Male | 38/9 | 83/16 | 94/10 | 25/29 |
| Age (year) | 48 ± 1.7 | 47 ± 1.4 | 48 ± 1.3 | 47 ± 1.5 |
| BMI (kg/m2) | 26 ± 0.8 | 26 ± 0.7 | 26 ± 0.8 | 25 ± 0.6 |
| Illness duration (years) | 11 ± 1.6 | 12 ± 1.1 | 14 ± 1.3 | n/a |
| Severity Score vs. FGF-21 | FM (n = 8) (0–50 pg/mL) | ME (n = 17) (0–50 pg/mL) | ME + FM (n = 10) (0–50 pg/mL) | HC (n = 12) (0–50 pg/mL) |
|---|---|---|---|---|
| Autonomic/endocrine/ immunity (DSQ) | r = −0.19 p (raw) = 0.65 p (adj) = 0.65 | r = −0.52 p (raw) = 0.01 p (adj) = 0.03 * | r = −0.61 p (raw) = 0.037 p (adj) = 0.062 | r = 0.48 p (raw) = 0.04 p (adj) = 0.12 |
| Cognitive (DSQ) | r = −0.36 p (raw) = 0.23 p (adj) = 0.38 | r = −0.12 p (raw) = 0.65 p (adj) = 0.64 | r = −0.67 p (raw) = 0.01 p (adj) = 0.03 * | r = 0.021 p (raw) = 0.47 p (adj) = 0.94 |
| Post-exertional malaise (PEM) (DSQ) | r = −0.90 p (raw) = 0.001 p (adj) = 0.0023 ** | r = −0.001 p (raw) = 0.98 p (adj) = 0.97 | r = −0.39 p (raw) = 0.21 p (adj) = 0.26 | r = 0.32 p (raw) = 0.23 p (adj) = 0.31 |
| DPEMQ | r = −0.44 p (raw) = 0.3 p (adj) = 0.38 | r = −0.65 p (raw) = 0.01 p (adj) = 0.03 * | r = 0.36 p (raw) = 0.38 p (adj) = 0.38 | n/a |
| Mental score (SF-36) | r = −0.34 p (raw) = 0.073 p (adj) = 0.41 | r = −0.42 p (raw) = 0.098 p (adj) = 0.097 | r = 0.68 p (raw) = 0.01 p (adj) = 0.03 * | r = 0.34 p (raw) = 0.27 p (adj) = 0.27 |
| DSQ Questionnaire | FM (n = 8) (0–50 pg/mL) | ME (n = 17) (0–50 pg/mL) | ME + FM (n = 10) (0–50 pg/mL) |
|---|---|---|---|
| FGF-21 vs. Fatigue/extreme tiredness | r = −0.80 p (raw) = 0.01 p (adj) = 0.02 * | r = −0.04 p (raw) = 0.55 p (adj) = 0.89 | r = −0.29 p (raw) = 0.21 p (adj) = 0.42 |
| FGF-21 vs. Dead, heavy feeling after starting to exercise | r = −0.53 p (raw) = 0.018 p (adj) = 0.18 | r = −0.23 p (raw) = 0.05 p (adj) = 0.37 | r = −0.10 p (raw) = 0.79 p (adj) = 0.79 |
| FGF-21 vs. Next day soreness or fatigue after non-strenuous, everyday activities | r = −0.77 p (raw) = 0.02 p (adj) = 0.02 * | r = −0.14 p (raw) = 0.29 p (adj) = 0.59 | r = −0.29 p (raw) = 0.26 p (adj) = 0.42 |
| FGF-21 vs. Mentally tired after the slightest effort | r = −0.89 p (raw) = 0.001 p (adj) = 0.003 ** | r = 0.01 p (raw) = 0.84 p (adj) = 0.96 | r = −0.64 p (raw) = 0.01 p (adj) = 0.04 * |
| FGF-21 vs. Minimum exercise makes you physically tired | r = −0.83 p (raw) = 0.01 p (adj) = 0.012 * | r = 0.04 p (raw) = 0.66 p (adj) = 0.89 | r = −0.16 p (raw) = 0.48 p (adj) = 0.65 |
| FGF-21 vs. Physically drained or sick after mild activity | r = −0.65 p (raw) = 0.07 p (adj) = 0.08 | r = 0.15 p (raw) = 0.22 p (adj) = 0.58 | r = −0.48 p (raw) = 0.04 p (adj) = 0.16 |
| FGF-21 vs. Muscle weakness | r = −0.87 p (raw) = 0.002 p (adj) = 0.01 ** | r = 0.15 p (raw) = 0.14 p (adj) = 0.57 | r = 0.16 p (raw) = 0.56 p (adj) = 0.65 |
| FGF-21 vs. PEM | r = −0.90 p (raw) = 0.001 p (adj) = 0.005 ** | r = −0.01 p (raw) = 0.98 p (adj) = 0.98 | r = −0.39 p (raw) = 0.21 p (adj) = 0.27 |
| DPEMQ Questionnaires | FM (n = 8) (0–50 pg/mL) | ME (n = 17) (0–50 pg/mL) | ME + FM (n = 10) (0–50 pg/mL) |
|---|---|---|---|
| FGF-21 vs. 1. Endurance/ability to perform activities | r = 0.54 p (raw) = 0.10 p (adj) = 0.27 | r = −0.51 p (raw) = 0.05 p (adj) = 0.11 | r = −0.66 p (raw) = 0.01 p (adj) = 0.07 |
| FGF-21 vs. 2. Physical fatigue, mental overstimulation | r = −0.46 p (raw) = 0.18 p (adj) = 0.35 | r = 0.3435 p (raw) = 0.24 p (adj) = 0.301 | r = 0.3218 p (raw) = 0.25 p (adj) = 0.43 |
| FGF-21 vs. 3. Cognitive exhaustion | r = −0.83 p (raw) = 0.003 p (adj) = 0.043 * | r = −0.7631 p (raw) = 0.001 p (adj) = 0.006 ** | r = −0.6441 p (raw) = 0.02 p (adj) = 0.084 |
| FGF-21 vs. 4. Difficulty thinking | r = −0.56 p (raw) = 0.07 p (adj) = 0.24 | r = −0.62 p (raw) = 0.01 p (adj) = 0.040 * | r = −0.75 p (raw) = 0.002 p (adj) = 0.03 * |
| FGF-21 vs. 5. Unrestful sleep | r = 0.10 p (raw) = 0.79 p (adj) = 0.85 | r = −0.52 p (raw) = 0.04 p (adj) = 0.09 | r = −0.53 p (raw) = 0.052 p (adj) = 0.18 |
| FGF-21 vs. 6. Insomnia | r = −0.18 p (raw) = 0.52 p (adj) = 0.73 | r = −0.011 p (raw) = 0.97 p (adj) = 0.97 | r = 0.15 p (raw) = 0.56 p (adj) = 0.71 |
| FGF-21 vs. 7. Muscle pain | r = 0.31 p (raw) = 0.35 p (adj) = 0.54 | r = 0.018 p (raw) = 0.89 p (adj) = 0.95 | r = 0.49 p (raw) = 0.07 p (adj) = 0.21 |
| FGF-21 vs. 8. Muscle weakness | r = 0.17 p (raw) = 0.58 p (adj) = 0.74 | r = −0.48 p (raw) = 0.08 p (adj) = 0.13 | r = −0.08 p (raw) = 0.78 p (adj) = 0.84 |
| FGF-21 vs. 9. Pain all over the body | r = −0.09 p (raw) = 0.87 p (adj) = 0.86 | r = 0.28 p (raw) = 0.35 p (adj) = 0.40 | r = 0.10 p (raw) = 0.68 p (adj) = 0.80 |
| FGF-21 vs. 10. Dizziness | r = −0.64 p (raw) = 0.04 p (adj) = 0.172 | r = −0.37 p (raw) = 0.19 p (adj) = 0.26 | r = −0.05 p (raw) = 0.89 p (adj) = 0.88 |
| FGF-21 vs. 11. Flu-like symptoms | r = 0.15 p (raw) = 0.66 p (adj) = 0.77 | r = −0.76 p (raw) = 0.001 p (adj) = 0.007 ** | r = −0.29 p (raw) = 0.31 p (adj) = 0.48 |
| FGF-21 vs. 12. Temperature disturbances | r = −0.71 p (raw) = 0.02 p (adj) = 0.12 | r = −0.53 p (raw) = 0.03 p (adj) = 0.95 | r = −0.16 p (raw) = 0.50 p (adj) = 0.70 |
| FGF-21 vs. 13. Mental fog | r = −0.48 p (raw) = 0.14 p (adj) = 0.34 | r = −0.44 p (raw) = 0.11 p (adj) = 0.17 | r = −0.40 p (raw) = 0.14 p (adj) = 0.32 |
| FGF-21 vs. DPEMQ total score | r = −0.44 p (raw) = 0.3 p (adj) = 0.38 | r = −0.65 p (raw) = 0.01 p (adj) = 0.03 * | r = −0.36 p (raw) = 0.38 p (adj) = 0.38 |
| FGF-21 vs. | FM (n = 39) | ME (n = 62) | ME + FM (n = 56) |
|---|---|---|---|
| BrainCheck combined test population percentile | r = 0.12 p (raw) = 0.15 p (adj) = 0.45 | r = 0.26 p (raw) = 0.043 p (adj) = 0.043 * | r = 0.08 p (raw) = 0.18 p (adj) = 0.55 |
| Immediate recognition population percentile | r = 0.12 p (raw) = 0.51 p (adj) = 0.51 | r = 0.39 p (raw) = 0.001 p (adj) = 0.002 ** | r = 0.039 p (raw) = 0.51 p (adj) = 0.77 |
| Delayed recognition population percentile | r = 0.11 p (raw) = 0.33 p (adj) = 0.49 | r = 0.33 p (raw) = 0.006 p (adj) = 0.009 ** | r = 0.031 p (raw) = 0.81 p (adj) = 0.81 |
| Severity Score vs. FGF-21 | FM (n = 14) (>200 pg/mL) | ME (n = 27) (>200 pg/mL) | ME + FM (n = 38) (>200 pg/mL) | HC (n = 13) (>200 pg/mL) |
|---|---|---|---|---|
| Mental fatigue (MFI-20) | r = 0.13 p (raw) = 0.63 p (adj) = 0.63 | r = −0.53 p (raw) = 0.002 p (adj) = 0.004 ** | r = −0.02 p (raw) = 0.89 p (adj) = 0.89 | r = 0.13 p (raw) = 0.68 p (adj) = 0.68 |
| Physical fatigue (MFI-20) | r = 0.14 p (raw) = 0.31 p (adj) = 0.61 | r = −0.29 p (raw) = 0.14 p (adj) = 0.14 | r = 0.37 p (raw) = 0.01 p (adj) = 0.02 * | r = 0.21 p (raw) = 0.25 p (adj) = 0.50 |
| Study/Source | Disease Context | Key Findings on FGF-21 | Key Associations & Relevance to Fatigue/Cognition | Specific Method/Notes |
|---|---|---|---|---|
| Current study | ME, FM, ME + FM, HC | Elevated in ME & ME + FM vs. HC. FGF-21 is a biomarker that varies in concentration. | Low FGF-21: associated with worse PEM (FM), autonomic symptoms (ME), and cognitive deficits (ME + FM). High FGF-21: associated with less mental fatigue (ME) but more physical fatigue (ME + FM). | Large, well-characterized cohort; miRNA-based diagnosis; multi-modal symptom assessment including PEM provocation; FGF-21 stratification. |
| Domingo et al., 2021 [39] | ME | Elevated levels in ME patients vs. healthy controls. | FGF-21 is proposed as a biomarker candidate for ME. | Plasma FGF-21 by ELISA. |
| Hoel et al., 2021 [5] | ME-M2 subgroup (metabolic phenotype) | Higher FGF-21 in a specific ME subgroup with low serum fatty acid derivatives. | Links FGF-21 to a distinct metabolic disruption and expands the metabolic map of ME. | Subgroup analysis based on a specific metabolic profile. |
| Post et al., 2021 [43] | Hemodialysis patients | Higher FGF-21 was associated with markers of malnutrition and more fatigue. | FGF-21 levels reflect a state of metabolic stress and inflammation, which is common in chronic diseases. | Study in hemodialysis patients. |
| Dolegowska et al., 2019 [44] | Obesity, diabetes, and metabolic syndrome | Highlights the paradox of elevated endogenous FGF-21. | Elevated FGF-21 in these chronic metabolic diseases often reflects a state of FGF-21 resistance or metabolic overload. | Review article on FGF19/FGF21. |
| Tan et al., 2023 [51] | Cardiovascular disease (CAD), T2DM | Elevated FGF-21 predicts cardiovascular risk and diabetic complications. | Protective anti-inflammatory and antioxidative roles. High levels may reflect an overwhelmed compensatory response or resistance. | Review of FGF-21 in metabolic and cardiovascular diseases. |
| Falamarzi et al., 2022 [52] | Liver diseases (NAFLD, NASH) | FGF-21 regulates hepatic lipid and glucose metabolism. Therapeutic analogs are being developed. | Elevated levels are associated with liver stress and disease, but therapeutic FGF-21 analogs can still be beneficial. | Review of FGF-21 in liver diseases. |
| Leng et al., 2015 [53] | Neuronal cells (in vitro) | Induces complete neuronal protection against glutamate toxicity. | Acts via Akt-1 activation and GSK-3 inhibition, showing a key neuroprotective role. | In vitro study on neuronal culture. |
| Yang et al., 2023 [54] | Parkinson’s disease mouse model | Preserves neurons and improves motor/cognitive scores. | Modulates gut microbiota and metabolic homeostasis, linking FGF-21 to brain–gut axis and neuroprotection. | In vivo mouse model study. |
| Shen et al., 2024 [55] | Parkinson’s disease cellular models | Prevents dopaminergic neuron loss. | Shows beneficial effects against proteasome impairment-induced Parkinson’s disease syndrome. | Cellular models. |
| Zhang et al., 2024 [56] | Diabetes-induced cognitive decline | Remodels cerebral glucose metabolism and alleviates cognitive decline. | Activation of the PI3K/AKT/GSK-3β signaling pathway. | In vivo mouse model of diabetic cognitive decline. |
| Wang et al., 2025 [57] | Ischemic brain injury mouse model | Suppresses astrocyte activation and protects brain tissue. | Acts through anti-inflammatory and neurotrophic pathways, showing a protective role in neuroinflammation. | In vivo mouse model study. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azimi, G.; Elremaly, W.; Elbakry, M.; Franco, A.; Godbout, C.; Moreau, A. Circulating FGF-21 as a Disease-Modifying Factor Associated with Distinct Symptoms and Cognitive Profiles in Myalgic Encephalomyelitis and Fibromyalgia. Int. J. Mol. Sci. 2025, 26, 7670. https://doi.org/10.3390/ijms26167670
Azimi G, Elremaly W, Elbakry M, Franco A, Godbout C, Moreau A. Circulating FGF-21 as a Disease-Modifying Factor Associated with Distinct Symptoms and Cognitive Profiles in Myalgic Encephalomyelitis and Fibromyalgia. International Journal of Molecular Sciences. 2025; 26(16):7670. https://doi.org/10.3390/ijms26167670
Chicago/Turabian StyleAzimi, Ghazaleh, Wesam Elremaly, Mohamed Elbakry, Anita Franco, Christian Godbout, and Alain Moreau. 2025. "Circulating FGF-21 as a Disease-Modifying Factor Associated with Distinct Symptoms and Cognitive Profiles in Myalgic Encephalomyelitis and Fibromyalgia" International Journal of Molecular Sciences 26, no. 16: 7670. https://doi.org/10.3390/ijms26167670
APA StyleAzimi, G., Elremaly, W., Elbakry, M., Franco, A., Godbout, C., & Moreau, A. (2025). Circulating FGF-21 as a Disease-Modifying Factor Associated with Distinct Symptoms and Cognitive Profiles in Myalgic Encephalomyelitis and Fibromyalgia. International Journal of Molecular Sciences, 26(16), 7670. https://doi.org/10.3390/ijms26167670





