Molecular Mechanisms of Phytochemicals from Chaga Mushroom (Inonotus obliquus) Against Colorectal Cancer: Insights from Network Pharmacology, Molecular Docking, and Bioinformatics
Abstract
1. Introduction
2. Results
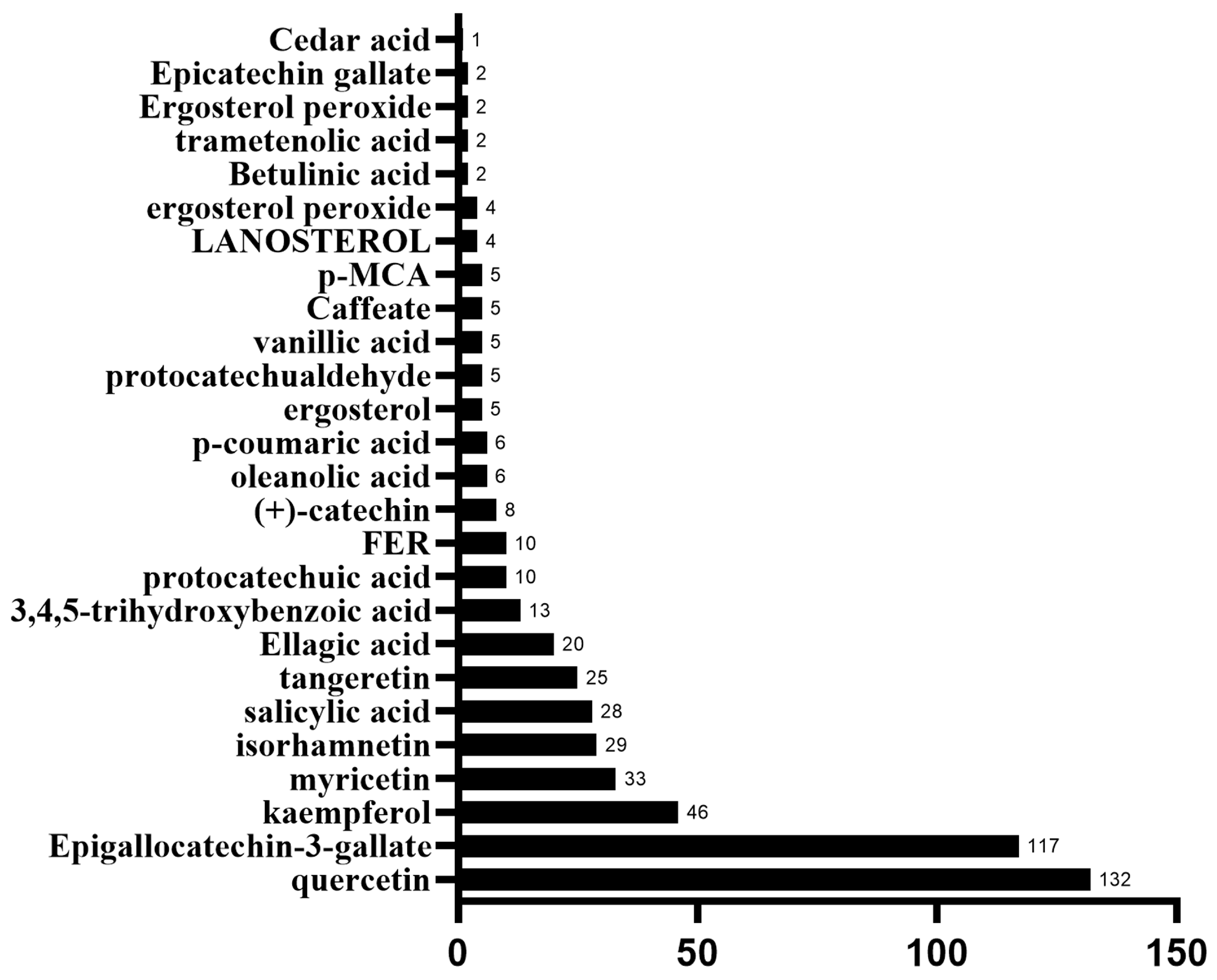
2.1. The Bioactive Compounds of Chaga Mushroom and Their Target Proteins
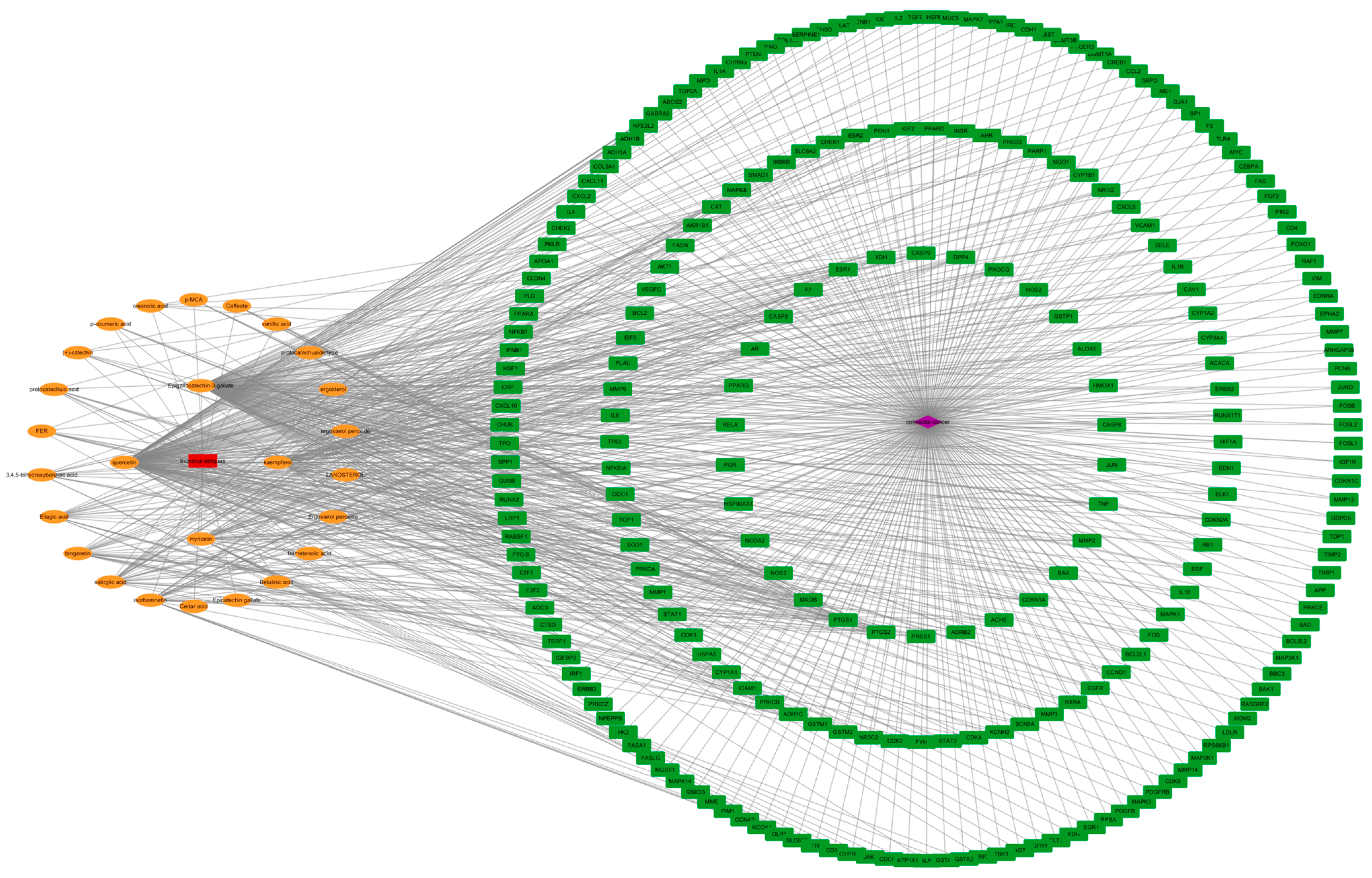
2.2. Construction of the “Drug Active Ingredient Target” Interaction Network of Chaga Mushroom
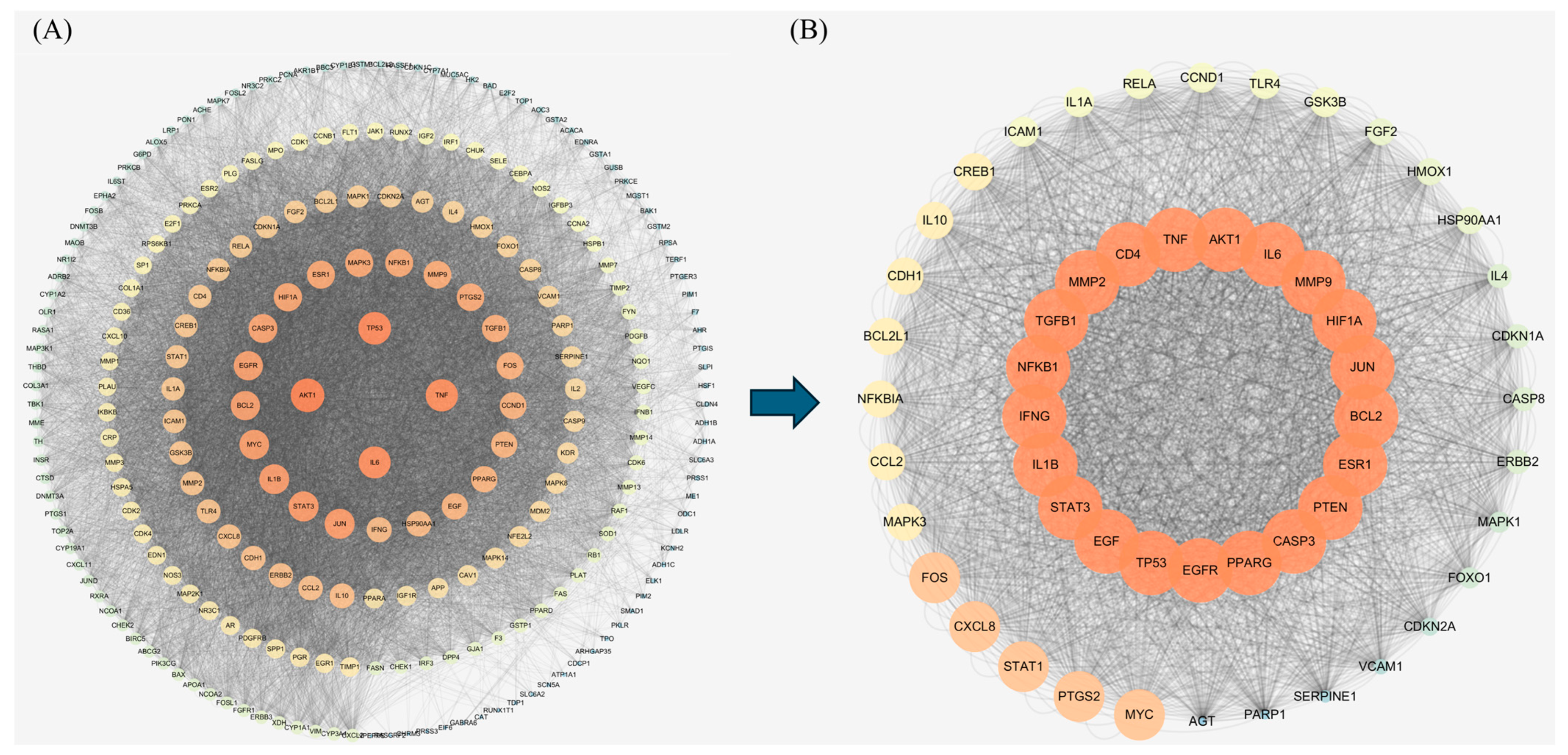
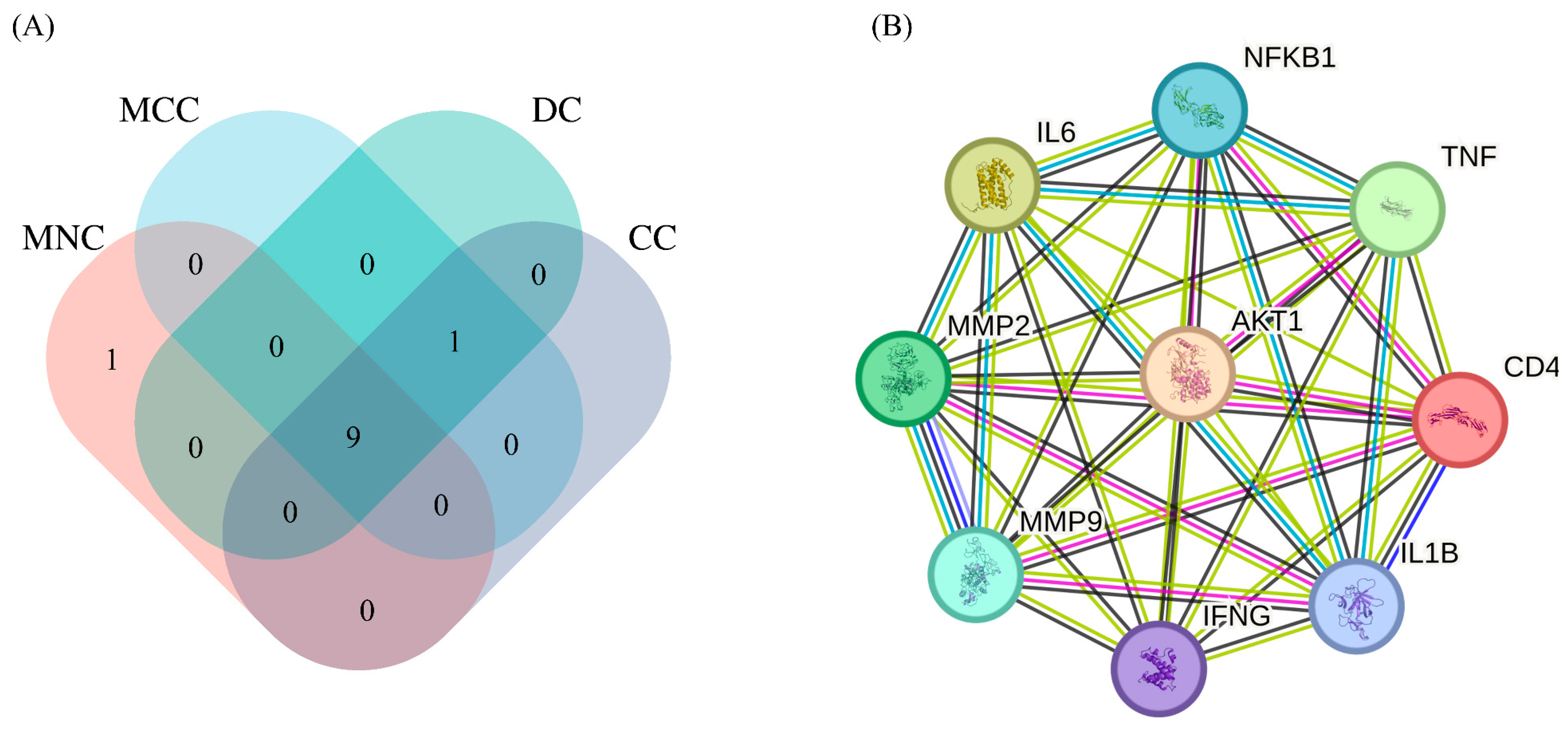
2.3. The Protein–Protein Interaction (PPI) Analysis and Target Screening
2.4. Results of KEGG and GO Enrichment Analysis
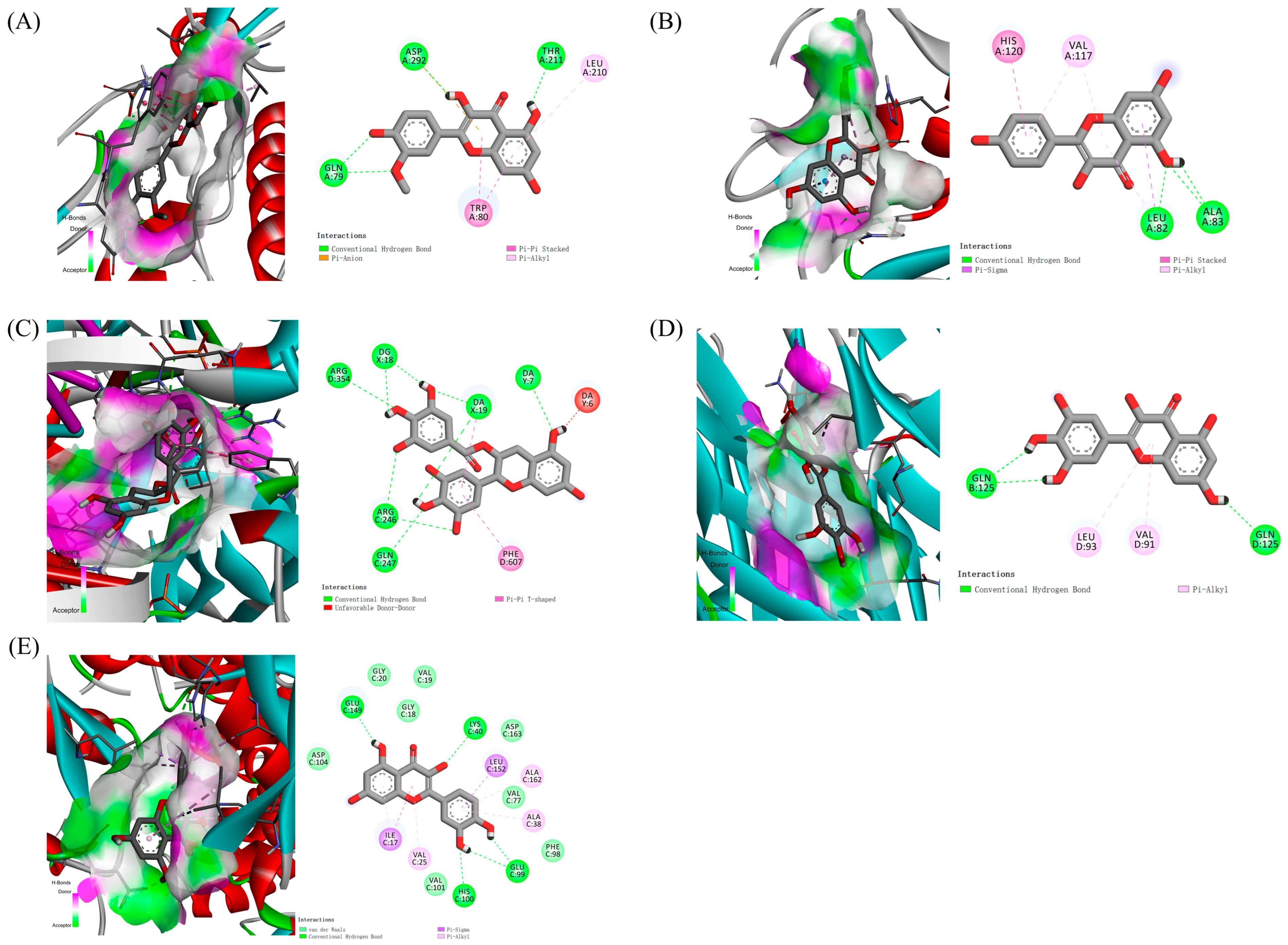
2.5. Results of Molecular Docking
2.6. Results of Bioinformatical Study
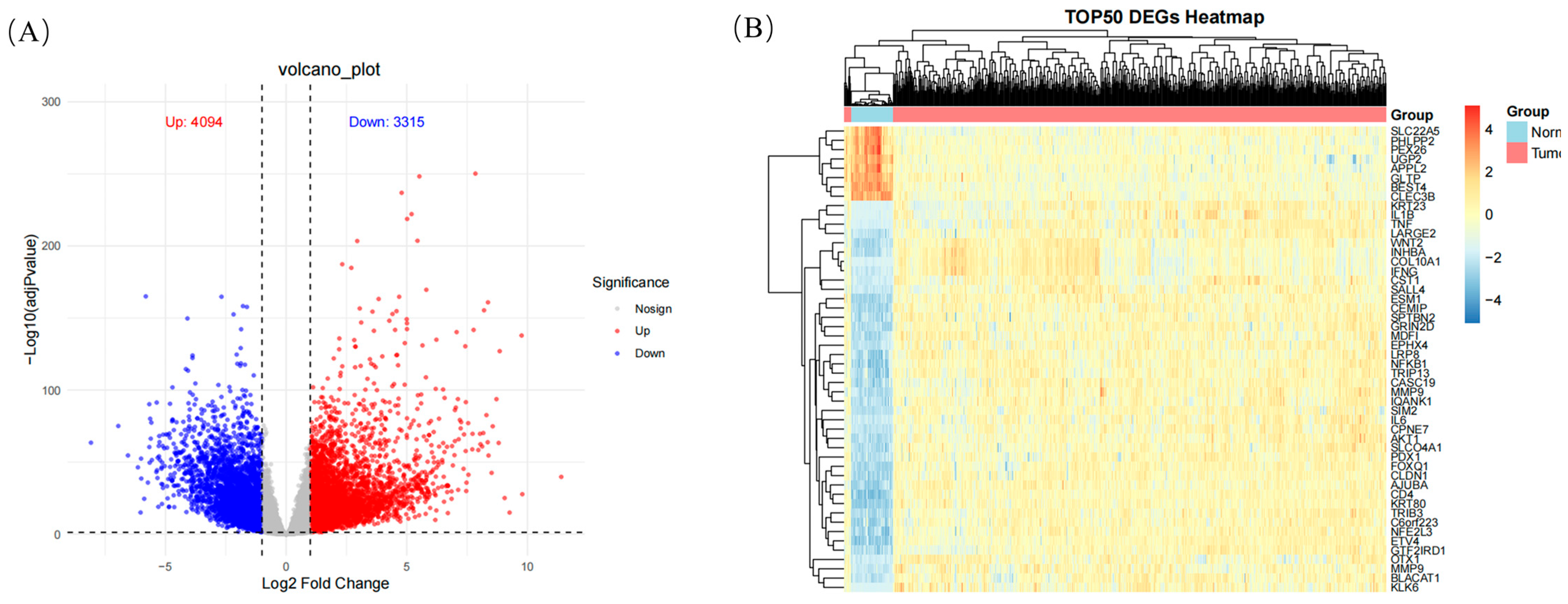
2.6.1. Differential Expression Analysis
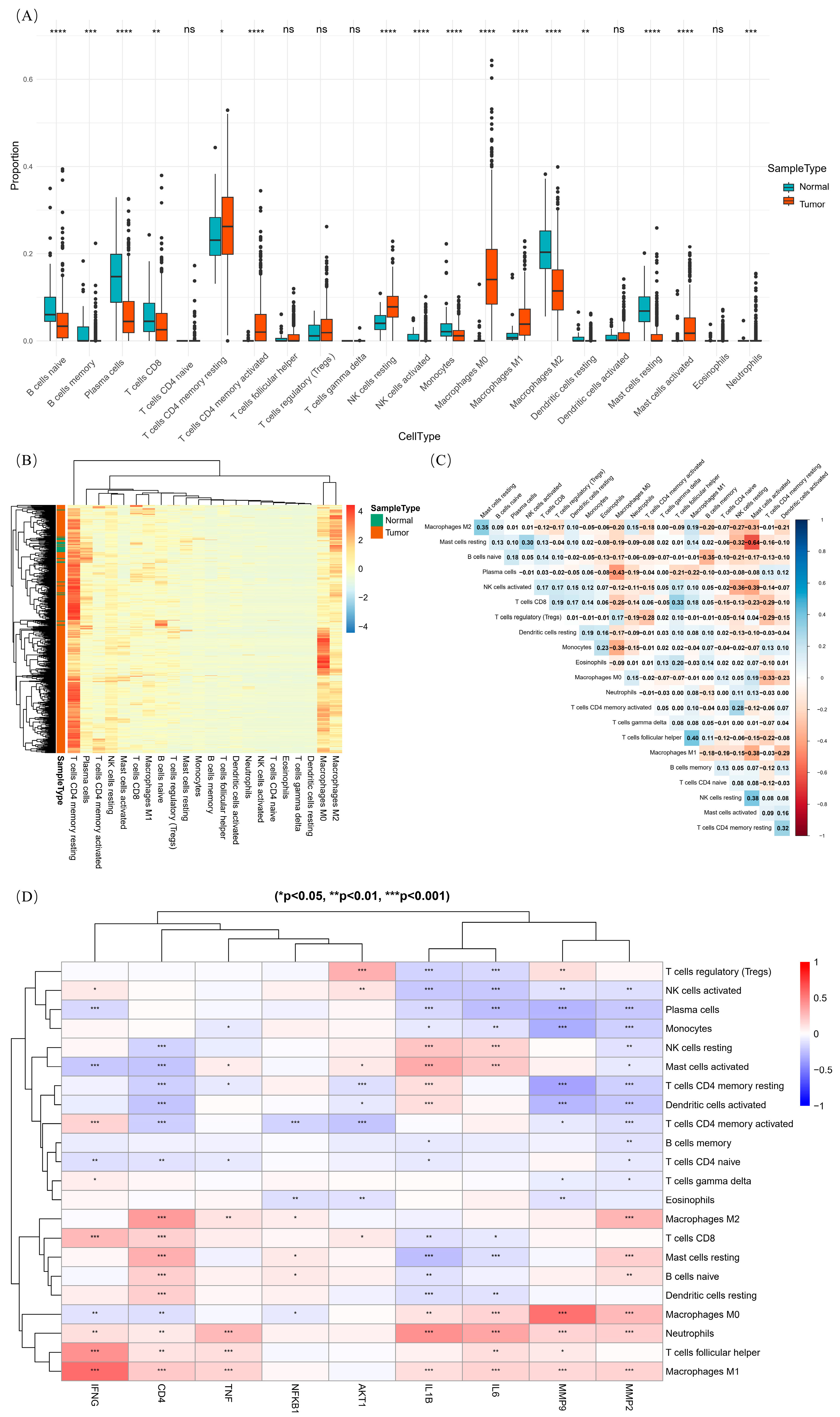
2.6.2. Immune Infiltration Analysis of Core Gene
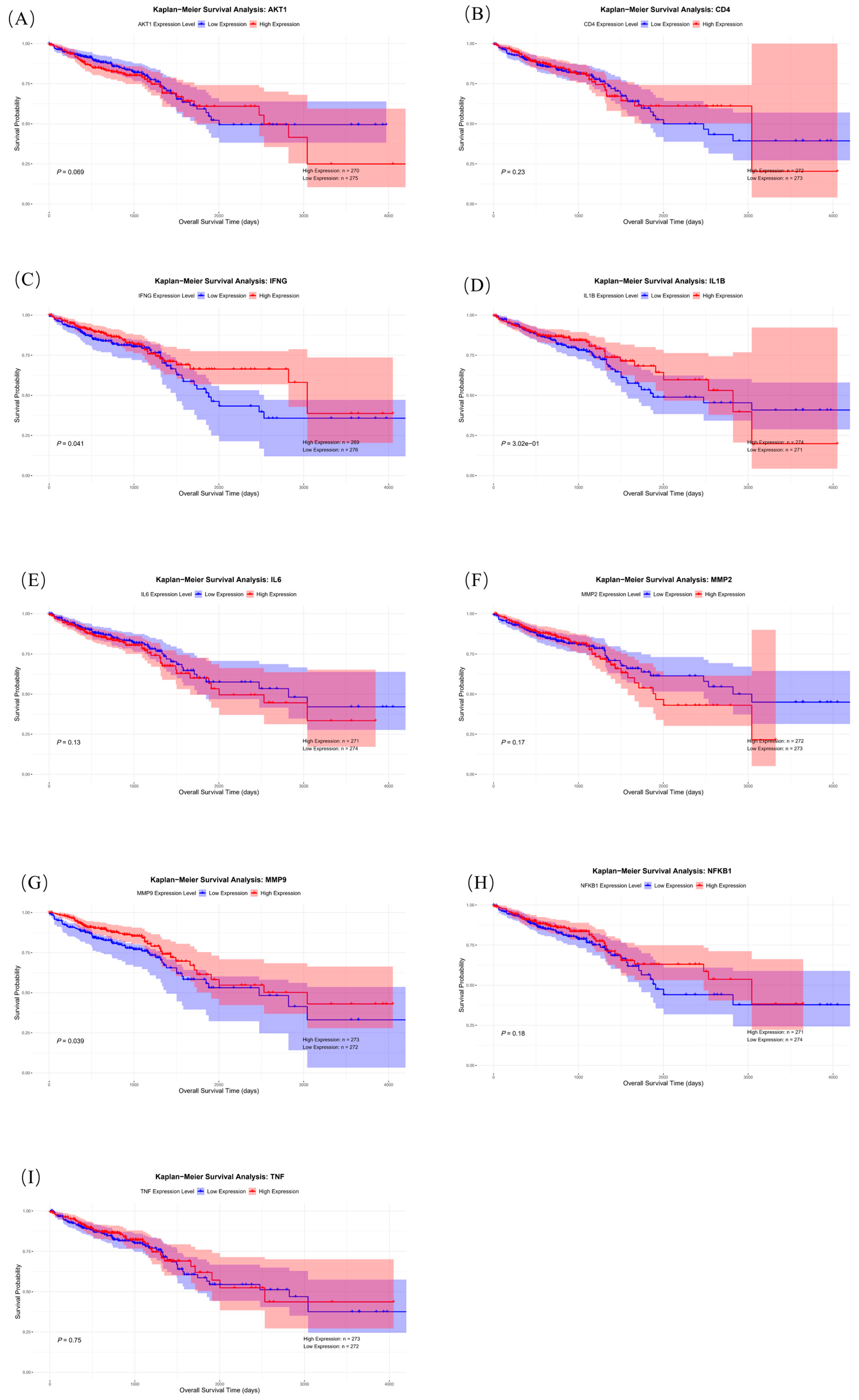
2.6.3. Survival Analysis of Core Genes
3. Discussion
4. Materials and Methods
4.1. Collection of Active Components and Targets of Chaga Mushroom (Inonotus obliquus)
4.2. Collection of Colorectal Cancer Targets
4.3. Collection of Targets for the Treatment of Colorectal Cancer by Chaga Mushroom
4.4. Protein–Protein Interaction (PPI) Analysis of the Anti-Colorectal Cancer Targets of Chaga Mushroom
4.5. The Screening of the Core Targets and Central Targets
4.6. The KEGG and GO Analysis
4.7. Molecular Docking
4.8. Bioinformatical Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Betweenness centrality |
| BP | Biological process |
| CC | Cellular component |
| CRC | Colorectal cancer |
| DC | Degree centrality |
| DEGs | Differential expression genes |
| DL | Drug-likeness |
| ECM | Extracellular matrix |
| GO | Gene Ontology |
| IL1B | Interleukin lB |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MF | Molecular function |
| MCC | Maximum Clique Centrality |
| MMP | Matrix metalloprotease 9 |
| MNC | Maximum Neighborhood Component |
| PDB | Protein Data Bank |
| PPI | Protein–protein Interaction |
| TME | Tumor microenvironment |
References
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.-Q.; Luo, Q.; Wang, L.; Song, G.-B.; Sheng, J.-P.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Abedizadeh, R.; Majidi, F.; Khorasani, H.R.; Abedi, H.; Sabour, D. Colorectal cancer: A comprehensive review of carcinogenesis, diagnosis, and novel strategies for classified treatments. Cancer Metastasis Rev. 2024, 43, 729–753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, L.; Chen, X.; Yin, Q. Natural compounds targeting ferroptosis in ovarian cancer: Research progress and application potential. Pharmacol. Res. 2025, 215, 107729. [Google Scholar] [CrossRef] [PubMed]
- Kurl, S.; Kaur, S.; Mittal, N.; Kaur, G. Mushrooms and colorectal cancer: Unveiling mechanistic insights and therapeutic innovations. Phytother. Res. 2025, 39, 480–493. [Google Scholar] [CrossRef]
- Camilleri, E.; Blundell, R.; Baral, B.; Karpinski, T.M.; Aruci, E.; Atrooz, O.M. A brief overview of the medicinal and nutraceutical importance of Inonotus obliquus (Chaga) mushrooms. Heliyon 2024, 10, e35638. [Google Scholar] [CrossRef]
- Bentharavithana, J.; Islam, T.; Xu, B. Medicinal mushrooms in colon cancer therapy: Mechanisms of action of bioactive compounds and therapeutic potential. Int. J. Mol. Sci. 2025, 26, 5304. [Google Scholar] [CrossRef]
- Frost, M. Three Popular Medicinal Mushroom Supplements: A Review of Human Clinical Trials. 2016. Available online: https://scholarsarchive.byu.edu/facpub/1609/ (accessed on 6 May 2025).
- Rajadnya, R.; Sharma, N.; Mahajan, A.; Ulhe, A.; Patil, R.; Hegde, M.; Mali, A. Novel systems biology experimental pipeline reveals matairesinol’s antimetastatic potential in prostate cancer: An integrated approach of network pharmacology, bioinformatics, and experimental validation. Brief. Bioinform. 2024, 25, bbae466. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar]
- Li, T.; Guo, R.; Zong, Q.; Ling, G. Application of molecular docking in elaborating molecular mechanisms and interactions of supramolecular cyclodextrin. Carbohydr. Polym. 2022, 276, 118644. [Google Scholar] [CrossRef]
- Baxevanis, A.D.; Bader, G.D.; Wishart, D.S. Bioinformatics; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Yang, A.; Liu, H.; Yang, Y. Study on the mechanism of action of Scutellaria barbata on hepatocellular carcinoma based on network pharmacology and bioinformatics. Front. Pharmacol. 2023, 13, 1072547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ouyang, F.; Teng, C.; Qu, J. Optimization for the extraction of polyphenols from Inonotus obliquus and its antioxidation activity. Prep. Biochem. Biotechnol. 2021, 51, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, W.; Shen, M. Antioxidant activity of liquid cultured Inonotus obliquus polyphenols using tween-20 as a stimulatory agent: Correlation of the activity and the phenolic profiles. J. Taiwan Inst. Chem. Eng. 2016, 69, 41–47. [Google Scholar] [CrossRef]
- Wold, C.W.; Gerwick, W.H.; Wangensteen, H.; Inngjerdingen, K.T. Bioactive triterpenoids and water-soluble melanin from Inonotus obliquus (Chaga) with immunomodulatory activity. J. Funct. Foods 2020, 71, 104025. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Critch, A.L.; Manful, C.F.; Rajakaruna, A.; Vidal, N.P.; Pham, T.H.; Cheema, M.; Thomas, R. Effects of pH and temperature on water under pressurized conditions in the extraction of nutraceuticals from chaga (Inonotus obliquus) mushroom. Antioxidants 2021, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-S.; Wang, Y.; Dai, L.-J.; He, F.-X.; Zhang, J.-L.; Zhou, Q. Triterpenoid acids from medicinal mushroom Inonotus obliquus (Chaga) alleviate hyperuricemia and inflammation in hyperuricemic mice: Possible inhibitory effects on xanthine oxidase activity. J. Food Biochem. 2022, 46, e13932. [Google Scholar] [CrossRef]
- Hwang, B.S.; Lee, I.-K.; Yun, B.-S. Phenolic compounds from the fungus Inonotus obliquus and their antioxidant properties. J. Antibiot. 2016, 69, 108–110. [Google Scholar] [CrossRef]
- Hao, R.; Li, Y.; Shan, S.; Xu, H.; Li, J.; Li, Z.; Li, R. Antioxidant potential of styrene pyrone polyphenols from Inonotus obliquus: A combined experimental, density functional theory (DFT) approach and molecular dynamic (MD) simulation. Saudi Chem. Soc. 2023, 27, 101652. [Google Scholar] [CrossRef]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef]
- Kang, J.-H.; Jang, J.-E.; Mishra, S.K.; Lee, H.-J.; Nho, C.W.; Shin, D.; Jin, M.; Kim, M.K.; Choi, C.; Oh, S.H. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the β-catenin pathway in colorectal cancer. J. Ethnopharmacol. 2015, 173, 303–312. [Google Scholar] [CrossRef]
- Luo, J.; Wu, Y.; Moussa, A.Y.; Huang, Y.; Fu, Y.; Wei, Y.; Luo, J.; Xu, B. Unveiling the molecular mechanisms of adzuki bean (Vigna angularis) derived bioactive phytochemicals in combating obesity. Food Biosci. 2024, 62, 105515. [Google Scholar] [CrossRef]
- Li, X.; Xiang, L.; Lin, Y.; Tang, Q.; Meng, F.; Chen, W. Computational analysis illustrates the mechanism of qingfei paidu decoction in blocking the transition of COVID-19 patients from mild to severe stage. Curr. Gene Ther. 2022, 22, 277–289. [Google Scholar] [CrossRef]
- Pradiba, D.; Aarthy, M.; Shunmugapriya, V.; Singh, S.K.; Vasanthi, M. Structural insights into the binding mode of flavonols with the active site of matrix metalloproteinase-9 through molecular docking and molecular dynamic simulations studies. J. Biomol. Struct. Dyn. 2018, 36, 3718–3739. [Google Scholar] [CrossRef]
- Guo, J.; Hu, M.; Yang, M.; Cao, H.; Li, H.; Zhu, J.; Li, S.; Zhang, J. Inhibition mechanism of theaflavins on matrix metalloproteinase-2: Inhibition kinetics, multispectral analysis, molecular docking and molecular dynamics simulation. Food Funct. 2024, 15, 7452–7467. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Xu, B. Elucidation of anti-obesity mechanisms of phenolics in Artemisiae argyi Folium (Aiye) by integrating LC-MS, network pharmacology, and molecular docking. Life 2024, 14, 656. [Google Scholar] [CrossRef]
- Chen, D.; Chen, Y.; Huang, F.; Zhang, X.; Zhou, Y.; Xu, L. The underlying regulatory mechanisms of colorectal carcinoma by combining Vitexin and Aspirin: Based on systems biology, molecular docking, molecular dynamics simulation, and in vitro study. Front. Endocrinol. 2023, 14, 1147132. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, J.; Xu, B. Network pharmacology and bioinformatics study of geniposide regulating oxidative stress in colorectal cancer. Int. J. Mol. Sci. 2023, 24, 15222. [Google Scholar] [CrossRef] [PubMed]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P. The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phytother. Res. 2019, 33, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Margina, D.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Saloustros, E.; Fenga, C.; Spandidos, D.; Libra, M.; Tsatsakis, A.M. Akt inhibitors in cancer treatment: The long journey from drug discovery to clinical use (Review). Int. J. Oncol. 2016, 48, 869–885. [Google Scholar] [CrossRef]
- Mirza, Z.; Karim, S. Structure-based profiling of potential phytomolecules with AKT1 a key cancer drug target. Molecules 2023, 28, 2597. [Google Scholar] [CrossRef]
- Unnikrishnan Meenakshi, D.; Narde, G.K.; Ahuja, A.; Al Balushi, K.; Francis, A.P.; Khan, S.A. Therapeutic applications of nanoformulated resveratrol and quercetin phytochemicals in colorectal cancer-An updated review. Pharmaceutics 2024, 16, 761. [Google Scholar] [CrossRef]
- Li, D.; Cao, D.; Sun, Y.; Cui, Y.; Zhang, Y.; Jiang, J.; Cao, X. The roles of epigallocatechin gallate in the tumor microenvironment, metabolic reprogramming, and immunotherapy. Front. Immunol. 2024, 15, 1331641. [Google Scholar] [CrossRef]
- Singh, T.; Sharma, D.; Sharma, R.; Tuli, H.S.; Haque, S.; Ramniwas, S.; Mathkor, D.M.; Yadav, V. The role of phytonutrient kaempferol in the prevention of gastrointestinal cancers: Recent trends and future perspectives. Cancers 2024, 16, 1711. [Google Scholar] [CrossRef]
- Zhu, M.-L.; Zhang, P.-M.; Jiang, M.; Yu, S.-W.; Wang, L. Myricetin induces apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signalling in human colon cancer cells. BMC Complement. Med. Ther. 2020, 20, 209. [Google Scholar] [CrossRef]
- Saud, S.M.; Young, M.R.; Jones-Hall, Y.L.; Ileva, L.; Evbuomwan, M.O.; Wise, J.; Colburn, N.H.; Kim, Y.S.; Bobe, G. Chemopreventive activity of plant flavonoid isorhamnetin in colorectal cancer is mediated by oncogenic Src and β-catenin. Cancer Res. 2013, 73, 5473–5484. [Google Scholar] [CrossRef] [PubMed]
- Curio, S.; Lin, W.; Bromley, C.; McGovern, J.; Triulzi, C.; Jonsson, G.; Ghislat, G.; Zelenay, S.; Guerra, N. NKG2D fine-tunes thelocal inflammatory response in colorectal cancer. Cancers 2023, 15, 1792. [Google Scholar] [CrossRef]
- Lee, C.-J.; Jang, T.-Y.; Jeon, S.-E.; Yun, H.-J.; Cho, Y.-H.; Lim, D.-Y.; Nam, J.-S. The dysadherin/MMP9 axis modifies the extracellular matrix to accelerate colorectal cancer progression. Nat. Commun. 2024, 15, 10422. [Google Scholar] [CrossRef] [PubMed]
- Ern, P.T.Y.; Quan, T.Y.; Yee, F.S.; Yin, A.C.Y. Therapeutic properties of Inonotus obliquus (Chaga mushroom): A review. Mycology 2024, 15, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Han, B.; Luo, J.; Xu, B. Revealing molecular mechanisms of the bioactive saponins from edible root of Platycodon grandiflorum in combating obesity. Plants 2024, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Randeni, N.; Luo, J.; Wu, Y.; Xu, B. Elucidating the anti-diabetic mechanisms of mushroom Chaga (Inonotus obliquus) by integrating LC-MS, network pharmacology, molecular docking, and bioinformatics. Int. J. Mol. Sci. 2025, 26, 5202. [Google Scholar] [CrossRef]
- Blum, A.; Wang, P.; Zenklusen, J.C. Snapshot: TCGA-analyzed tumors. Cell 2018, 173, 530. [Google Scholar] [CrossRef]
- Zhang, H.G.; Yao, W.R.; Zhou, Z.Y.; Liu, L. The immunosuppressive role of VSIG4 in colorectal cancer and its interaction with the tumor microenvironment. Discov. Oncol. 2025, 16, 664. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, J.; Xu, B. Network pharmacology and bioinformatics to identify the molecular mechanisms of Gleditsiae Spina against colorectal cancer. Curr. Res. Toxicol. 2023, 5, 100139. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, B.; Chen, J.; Huang, X. Novel prognostic genes of diffuse large B-cell lymphoma revealed by survival analysis of gene expression data. Onco Targets Ther. 2015, 8, 3407–3413. [Google Scholar] [CrossRef] [PubMed][Green Version]



| PubChem Name | CID Number | Molecular Formula | Structure | References |
|---|---|---|---|---|
| Quercetin | 5280343 | C15H10O7 | 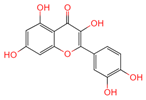 | [13] |
| Epigallocatechin-3-gallate | 65064 | C22H18O11 |  | [14] |
| Kaempferol | 5280863 | C15H10O6 | 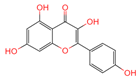 | [15] |
| Myricetin | 5281672 | C15H10O8 | 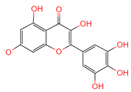 | [16] |
| Isorhamnetin | 5281654 | C16H12O7 | 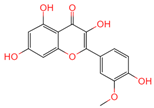 | [16] |
| Salicylic acid | 338 | C7H6O3 | 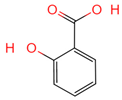 | [16] |
| Tangeretin | 68077 | C20H20O7 | 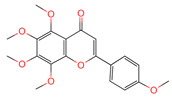 | [15] |
| Betulinic acid | 64971 | C30H48O3 | 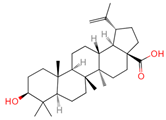 | [15] |
| Oleanolic acid | 10494 | C30H48O3 | 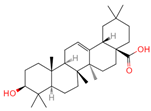 | [17] |
| Ellagic acid | 5281855 | C14H6O8 | 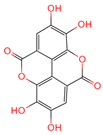 | [16] |
| 3,4,5-Trihydroxybenzoic acid | 370 | C7H26O5 | 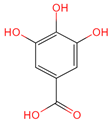 | [18] |
| Protocatechuic aldehyde | 8768 | C7H6O3 |  | [19] |
| Ferulic acid | 445858 | C10H10O4 |  | [14] |
| Catechin | 9064 | C15H14O6 | 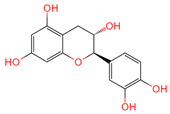 | [19] |
| Protocatechuic acid | 72 | C7H6O4 |  | [16] |
| p-Coumaric acid | 637542 | C9H8O3 | 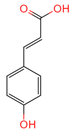 | [15] |
| Ergosterol | 444679 | C28H44O | 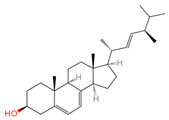 | [20] |
| Vanillic acid | 8468 | C8H8O4 |  | [16] |
| Caffeate | 689043 | C9H8O4 | 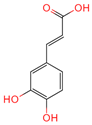 | [15] |
| p-MCA | 699414 | C10H10O3 |  | [16] |
| Lanosterol | 246983 | C30H50O |  | [20] |
| Ergosterol peroxide | 5351516 | C28H44O3 | 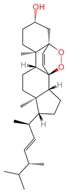 | [21] |
| Trametenolic acid | 12309443 | C30H48O3 |  | [15] |
| Epicatechin gallate | 107905 | C22H18O10 | 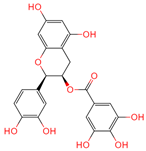 | [14] |
| Cedar acid | 10742 | C9H10O5 |  | [18] |
| Aspect | Key Findings | Implications |
|---|---|---|
| Differential gene expression | 7409 DEGs identified, including 3315 upregulated and 4094 downregulated genes | Core genes (e.g., IFNG, IL1B, MMP9) are upregulated in tumor samples, indicating their role in cancer progression. |
| Immune cell infiltration | Significant differences in immune cell infiltration between tumor and normal tissues | Immune cells such as CD4+ T cells, M0 macrophages, and M2 macrophages show higher infiltration in tumor tissues, correlating with core genes like IFNG and MMP9. |
| Survival analysis | High expression of IFNG, MMP9, and IL1B indicates shorter survival time in colorectal cancer patients | These core genes are potential biomarkers for prognosis and therapeutic targets. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Liu, J.; Luo, J.; Xu, B. Molecular Mechanisms of Phytochemicals from Chaga Mushroom (Inonotus obliquus) Against Colorectal Cancer: Insights from Network Pharmacology, Molecular Docking, and Bioinformatics. Int. J. Mol. Sci. 2025, 26, 7664. https://doi.org/10.3390/ijms26167664
Wu Y, Liu J, Luo J, Xu B. Molecular Mechanisms of Phytochemicals from Chaga Mushroom (Inonotus obliquus) Against Colorectal Cancer: Insights from Network Pharmacology, Molecular Docking, and Bioinformatics. International Journal of Molecular Sciences. 2025; 26(16):7664. https://doi.org/10.3390/ijms26167664
Chicago/Turabian StyleWu, Yingzi, Jiayin Liu, Jinhai Luo, and Baojun Xu. 2025. "Molecular Mechanisms of Phytochemicals from Chaga Mushroom (Inonotus obliquus) Against Colorectal Cancer: Insights from Network Pharmacology, Molecular Docking, and Bioinformatics" International Journal of Molecular Sciences 26, no. 16: 7664. https://doi.org/10.3390/ijms26167664
APA StyleWu, Y., Liu, J., Luo, J., & Xu, B. (2025). Molecular Mechanisms of Phytochemicals from Chaga Mushroom (Inonotus obliquus) Against Colorectal Cancer: Insights from Network Pharmacology, Molecular Docking, and Bioinformatics. International Journal of Molecular Sciences, 26(16), 7664. https://doi.org/10.3390/ijms26167664








