Molecular Crosstalk Between RUNX2 and HIF-1α in Osteosarcoma: Implications for Angiogenesis, Metastasis, and Therapy Resistance
Abstract
1. Introduction
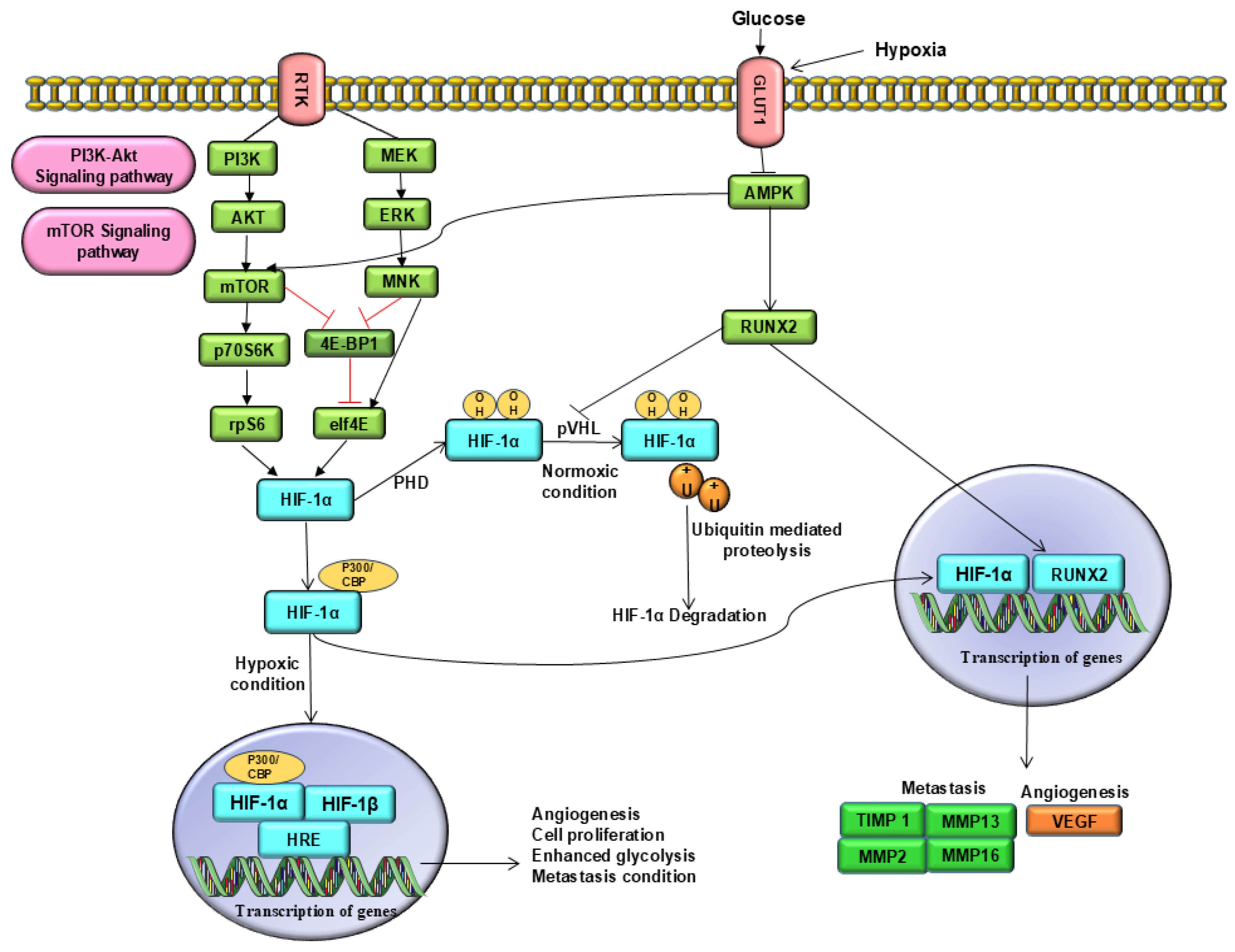
2. Molecular Cross-Talk Between RUNX2 and HIF-1α
Role of RUNX2 and HIF-1α in Osteogenic and Angiogenic Signalling Pathways
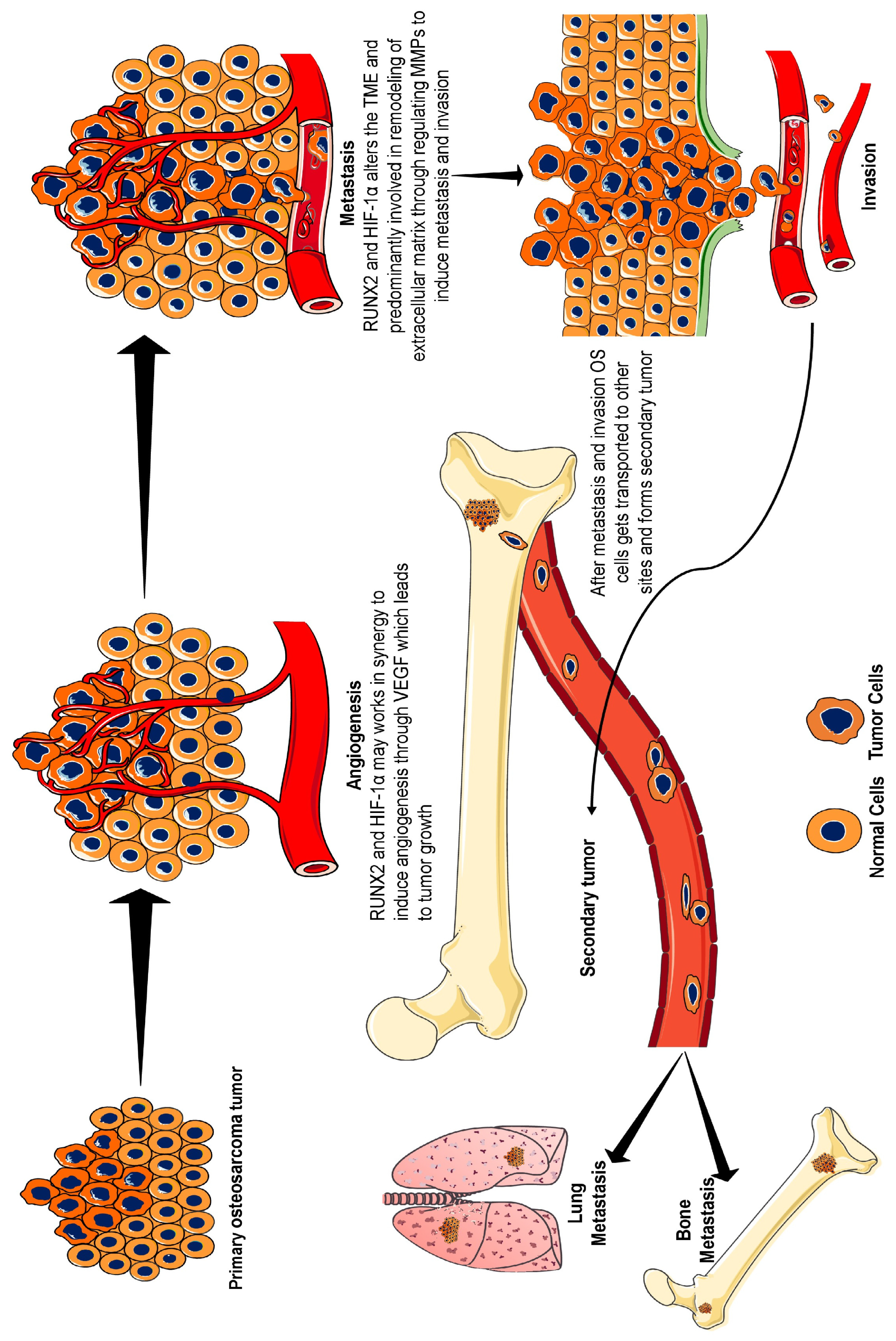
3. RUNX2–HIF-1α Role in Osteosarcoma Progression
3.1. Tumour Microenvironment
3.2. Angiogenesis
3.3. Metastasis
3.4. Chemotherapy Resistance
3.5. Radiotherapy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | adenosine monophosphate-activated protein kinase |
| EMT | Epithelial–mesenchymal transition |
| GLUT1 | Glucose transporter 1 |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| MMP | Matrix metalloproteinase |
| mTOR | mammalian target of rapamycin |
| Mxd1 | MAX dimerization protein 1 |
| NOXA | phorbol-12-myristate-13-acetate-induced protein 1 |
| PTEN | Phosphatase and tensin homologue |
| PHDs | Prolyl hydroxylase domain |
| PI3K/Akt | Phosphatidylinositol 3-kinase/protein kinase B |
| RUNX2 | Runt-related transcription factor 2 |
| SKA1 | Spindle and kinetochore-associated complex subunit 1 |
| VEGF | Vascular endothelial growth factor |
| pVHL | von Hippel Lindau tumour suppressor protein |
| ZEB | Zinc finger E-box-binding homeobox |
References
- Idoate, M.Á.; Aquerreta, J.D.; Lamo-Espinosa, J.M.; San-Julian, M. A Reassessment of the Barrier Effect of the Physis against Metaphyseal Osteosarcoma: A Comprehensive Pathological Study with Its Radiological and Clinical Follow-Up Correlations. Diagnostics 2022, 12, 450. [Google Scholar] [CrossRef]
- Prabowo, Y.; Primaputra, M.R.A.; Kodrat, E. Reconstruction of osteosarcoma of the proximal tibia using bone on polyethylene hemiarthroplasty knee joint system: A case report. Int. J. Surg. Case Rep. 2020, 72, 188–196. [Google Scholar] [CrossRef]
- Chen, C.; Xie, L.; Ren, T.; Huang, Y.; Xu, J.; Guo, W. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021, 500, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eaton, B.R.; Schwarz, R.; Vatner, R.; Yeh, B.; Claude, L.; Indelicato, D.J.; Laack, N. Osteosarcoma. Pediatr. Blood Cancer 2021, 68, e28352. [Google Scholar] [CrossRef] [PubMed]
- Tuck, S.P.; Walker, J. Adult Paget’s disease of bone. Clin. Med. 2020, 20, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A review of current and future therapeutic approaches. Biomed. Eng. Online 2021, 20, 24. [Google Scholar] [CrossRef]
- Meltzer, P.S.; Helman, L.J. New Horizons in the Treatment of Osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef]
- Chicón-Bosch, M.; Tirado, O.M. Exosomes in Bone Sarcomas: Key Players in Metastasis. Cells 2020, 9, 241. [Google Scholar] [CrossRef]
- Shoaib, Z.; Fan, T.M.; Irudayaraj, J.M.K. Osteosarcoma mechanobiology and therapeutic targets. Br. J. Pharmacol. 2022, 179, 201–217. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [Google Scholar] [CrossRef] [PubMed]
- Jonason, J.H.; Xiao, G.; Zhang, M.; Xing, L.; Chen, D. Post-translational Regulation of Runx2 in Bone and Cartilage. J. Dent. Res. 2009, 88, 693–703. [Google Scholar] [CrossRef]
- Van der Deen, M.; Taipaleenmäki, H.; Zhang, Y.; Teplyuk, N.M.; Gupta, A.; Cinghu, S.; Shogren, K.; Maran, A.; Yaszemski, M.J.; Ling, L.; et al. MicroRNA-34c inversely couples the biological functions of the runt-related transcription factor RUNX2 and the tumor suppressor p53 in osteosarcoma. J. Biol. Chem. 2013, 288, 21307–21319. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Zielenska, M.; Stein, G.S.; van Wijnen, A.J.; Squire, J.A. The Role of RUNX2 in Osteosarcoma Oncogenesis. Sarcoma 2011, 2011, 282745. [Google Scholar] [CrossRef]
- Leonardi, L.; Manuali, E.; Bufalari, A.; Porcellato, I. Canine soft tissue sarcomas: The expression of RUNX2 and karyopherin alpha-2 in extraskeletal (soft tissues) and skeletal osteosarcomas. Front. Vet. Sci. 2024, 11, 1292852. [Google Scholar] [CrossRef]
- Roos, A.; Satterfield, L.; Zhao, S.; Fuja, D.; Shuck, R.; Hicks, M.J.; Donehower, L.A.; Yustein, J.T. Loss of Runx2 sensitises osteosarcoma to chemotherapy-induced apoptosis. Br. J. Cancer 2015, 113, 1289–1297. [Google Scholar] [CrossRef]
- Guo, M.; Cai, C.; Zhao, G.; Qiu, X.; Zhao, H.; Ma, Q.; Tian, L.; Li, X.; Hu, Y.; Liao, B. Hypoxia promotes migration and induces CXCR4 expression via HIF-1α activation in human osteosarcoma. PLoS ONE 2014, 9, e90518. [Google Scholar] [CrossRef]
- Shen, S.; Yao, T.; Xu, Y.; Zhang, D.; Fan, S.; Ma, J. CircECE1 activates energy metabolism in osteosarcoma by stabilizing c-Myc. Mol. Cancer 2020, 19, 151. [Google Scholar] [CrossRef]
- Zhou, D.; Duan, Z.; Li, Z.; Ge, F.; Wei, R.; Kong, L. The significance of glycolysis in tumor progression and its relationship with the tumor microenvironment. Front Pharmacol. 2022, 13, 1091779. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.Y.; Zhang, Y.H.; Li, H.Y.; Xie, T.; Sun, L.L.; Zhu, T.; Wang, S.D.; Ye, Z.M. Prognostic role of hypoxia-inducible factor-1 alpha expression in osteosarcoma: A meta-analysis. Onco Targets Ther. 2016, 9, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Xu, Y.; Gong, Z.; Yao, T.; Qiao, D.; Huang, Y.; Zhang, Z.; Gao, J.; Ni, H.; Jin, Z.; et al. Positive Feedback Regulation of Circular RNA Hsa_circ_0000566 and HIF-1α promotes Osteosarcoma Progression and Glycolysis Metabolism. Aging Dis. 2023, 14, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.G.; Zhao, X.; Yang, Q.; Li, Y.; Ge, C.; Zhao, G.; Franceschi, R.T. Physical and functional interactions between Runx2 and HIF-1α induce vascular endothelial growth factor gene expression. J. Cell. Biochem. 2011, 112, 3582–3593. [Google Scholar] [CrossRef]
- Lee, Y.M. RUNX Family in Hypoxic Microenvironment and Angiogenesis in Cancers. Cells 2022, 11, 3098. [Google Scholar] [CrossRef]
- Kong, P.; Chen, R.; Zou, F.Q.; Wang, Y.; Liu, M.C.; Wang, W.G. HIF-1α repairs degenerative chondrocyte glycolytic metabolism by the transcriptional regulation of Runx2. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1206–1214. [Google Scholar] [CrossRef]
- Lee, S.H.; Che, X.; Jeong, J.H.; Choi, J.Y.; Lee, Y.J.; Lee, Y.H.; Bae, S.C.; Lee, Y.M. Runx2 protein stabilizes hypoxia-inducible factor-1α through competition with von Hippel-Lindau protein (pVHL) and stimulates angiogenesis in growth plate hypertrophic chondrocytes. J. Biol. Chem. 2012, 287, 14760–14771. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakamura, M.; Ogata, T.; Sang, M.; Shimozato, O. The functional interplay between pro-oncogenic RUNX2 and hypoxia-inducible factor-1α (HIF-1α) during hypoxia-mediated tumor progression. In Regulation of Signal Transduction in Human Cell Research; Springer: Singapore, 2018; pp. 85–98. [Google Scholar]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.J.; Park, K.H.; Lee, G.; Oh, Y.; Ryu, J.H.; Huh, Y.H. Differential but complementary roles of HIF-1α and HIF-2α in the regulation of bone homeostasis. Commun. Biol. 2024, 7, 892. [Google Scholar] [CrossRef]
- Jia, Y.; Li, R.; Li, Y.; Kachler, K.; Meng, X.; Gießl, A.; Qin, Y.; Zhang, F.; Liu, N.; Andreev, D.; et al. Melanoma bone metastasis-induced osteocyte ferroptosis via the HIF1α-HMOX1 axis. Bone Res. 2025, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Solal, K.A.; Boregowda, R.K.; Lasfar, A. RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression. Mol. Cancer 2015, 14, 137. [Google Scholar] [CrossRef]
- Chava, S.; Chennakesavulu, S.; Gayatri, B.M.; Reddy, A.B.M. A novel phosphorylation by AMP-activated kinase regulates RUNX2 from ubiquitination in osteogenesis over adipogenesis. Cell Death Dis. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.T.; Naumova, N.; Marchais, A.; Gaspar, N.; Geoerger, B.; Brenner, C. Insight into the interplay between mitochondria-regulated cell death and energetic metabolism in osteosarcoma. Front. Cell Dev. Biol. 2022, 10, 948097. [Google Scholar] [CrossRef]
- Tandon, M.; Chen, Z.; Othman, A.H.; Pratap, J. Role of Runx2 in IGF-1Rβ/Akt- and AMPK/Erk-dependent growth, survival and sensitivity towards metformin in breast cancer bone metastasis. Oncogene 2016, 35, 4730–4740. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ueharu, H.; Mishina, Y. Energy metabolism: A newly emerging target of BMP signaling in bone homeostasis. Bone 2020, 138, 115467. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.M.; Yang, J.; Shen, M.H.; Sampson, J.R.; Tee, A.R. mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene 2015, 34, 2239–2250. [Google Scholar] [CrossRef] [PubMed]
- Land, S.C.; Tee, A.R. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J. Biol. Chem. 2007, 282, 20534–20543. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Lawlor, E.R.; Lyssiotis, C.A. Amino acid metabolism in primary bone sarcomas. Front. Oncol. 2022, 12, 1001318. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xia, L.; Liang, J.; Han, Y.; Wang, H.; Oyang, L.; Tan, S.; Tian, Y.; Rao, S.; Chen, X.; et al. The roles of glucose metabolic reprogramming in chemo- and radio-resistance. J. Exp. Clin. Cancer Res. 2019, 38, 218. [Google Scholar] [CrossRef]
- Magar, A.G.; Morya, V.K.; Kwak, M.K.; Oh, J.U.; Noh, K.C. A Molecular Perspective on HIF-1α and Angiogenic Stimulator Networks and Their Role in Solid Tumors: An Update. Int. J. Mol. Sci. 2024, 25, 3313. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, S.H.; Wang, R.Y.; Ye, S.N.; Xia, T.; Ma, D.Z. Effect of silencing HIF-1alpha by RNA interference on expression of vascular endothelial growth factor in osteosarcoma cell line SaOS-2 under hypoxia. Ai Zheng 2005, 24, 531–535. (In Chinese) [Google Scholar]
- Lee, S.H.; Golinska, M.; Griffiths, J.R. HIF-1-Independent Mechanisms Regulating Metabolic Adaptation in Hypoxic Cancer Cells. Cells 2021, 10, 2371. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Yang, M.; Zhao, W.; Tu, J.; Liu, B.; Yuan, X. RUNX transcription factors: Biological functions and implications in cancer. Clin. Exp. Med. 2024, 24, 50. [Google Scholar] [CrossRef]
- Zhu, W.J.; Chang, B.Y.; Wang, X.F.; Zang, Y.F.; Zheng, Z.X.; Zhao, H.J.; Cui, Q.D. FBW7 regulates HIF-1α/VEGF pathway in the IL-1β induced chondrocytes degeneration. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5914–5924. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, X. RUNX2 RNA interference inhibits the invasion of osteosarcoma. Oncol. Lett. 2015, 9, 2455–2458. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Gao, Y.; Yang, Y.; Wu, H. Osteosarcoma and Metastasis. Front. Oncol. 2021, 11, 780264. [Google Scholar] [CrossRef] [PubMed]
- Del Mare, S.; Aqeilan, R.I. Tumor Suppressor WWOX inhibits osteosarcoma metastasis by modulating RUNX2 function. Sci. Rep. 2015, 5, 12959. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Manner, P.A.; Horner, A.; Shum, L.; Tuan, R.S.; Nuckolls, G.H. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthr. Cartil. 2004, 12, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Rici, R.E.G.; Will, S.E.A.L.; Luna, A.C.L.; Melo, L.F.; Santos, A.C.; Rodrigues, R.F.; Leandro, R.M.; Maria, D.A. Combination therapy of canine osteosarcoma with canine bone marrow stem cells, bone morphogenetic protein and carboplatin in an in vivo model. Vet. Comp. Oncol. 2018, 16, 478–488. [Google Scholar] [CrossRef]
- Won, K.Y.; Park, H.R.; Park, Y.K. Prognostic implication of immunohistochemical Runx2 expression in osteosarcoma. Tumori 2009, 95, 311–316. [Google Scholar] [CrossRef]
- Kurek, K.C.; Del Mare, S.; Salah, Z.; Abdeen, S.; Sadiq, H.; Lee, S.H.; Gaudio, E.; Zanesi, N.; Jones, K.B.; DeYoung, B.; et al. Frequent attenuation of the WWOX tumor suppressor in osteosarcoma is associated with increased tumorigenicity and aberrant RUNX2 expression. Cancer Res. 2010, 70, 5577–5586. [Google Scholar] [CrossRef]
- Zhou, J.; Lan, F.; Liu, M.; Wang, F.; Ning, X.; Yang, H.; Sun, H. Hypoxia inducible factor-1ɑ as a potential therapeutic target for osteosarcoma metastasis. Front. Pharmacol. 2024, 15, 1350187. [Google Scholar] [CrossRef]
- Fujiwara, S.; Kawamoto, T.; Kawakami, Y.; Koterazawa, Y.; Hara, H.; Takemori, T.; Kitayama, K.; Yahiro, S.; Kakutani, K.; Matsumoto, T.; et al. Acquisition of cancer stem cell properties in osteosarcoma cells by defined factors. Stem Cell Res. Ther. 2020, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; He, L. Sauchinone inhibits hypoxia-induced invasion and epithelial-mesenchymal transition in osteosarcoma cells via inactivation of the sonic hedgehog pathway. J. Recept. Signal Transduct. Res. 2022, 42, 173–179. [Google Scholar] [CrossRef]
- Yang, D.C.; Yang, M.H.; Tsai, C.C.; Huang, T.F.; Chen, Y.H.; Hung, S.C. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS ONE 2011, 6, e23965. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, H.; Chai, J.; Xing, L. RUNX2 as a promising therapeutic target for malignant tumors. Cancer Manag. Res. 2021, 13, 2539–2548. [Google Scholar] [CrossRef] [PubMed]
- Sadikovic, B.; Thorner, P.; Chilton-Macneill, S.; Martin, J.W.; Cervigne, N.K.; Squire, J.; Zielenska, M. Expression analysis of genes associated with human osteosarcoma tumors shows correlation of RUNX2 overexpression with poor response to chemotherapy. BMC Cancer 2010, 10, 202. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Zawacka-Pankau, J.E. The Role of p53 Family in Cancer. Cancers 2022, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Wu, D.; Sugimoto, H.; Nagase, H.; Nakagawara, A. Runt-related transcription factor 2 (RUNX2) inhibits p53-dependent apoptosis through the collaboration with HDAC6 in response to DNA damage. Cell Death Dis. 2013, 4, e610. [Google Scholar] [CrossRef]
- Nathan, S.S.; Pereira, B.P.; Zhou, Y.F.; Gupta, A.; Dombrowski, C.; Soong, R.; Pho, R.W.; Stein, G.S.; Salto-Tellez, M.; Cool, S.M.; et al. Elevated expression of Runx2 as a key parameter in the etiology of osteosarcoma. Mol. Biol. Rep. 2009, 36, 153–158. [Google Scholar] [CrossRef]
- Ozaki, T.; Sugimoto, H.; Nakamura, M.; Hiraoka, K.; Yoda, H.; Sang, M.; Fujiwara, K.; Nagase, H. Runt-related transcription factor 2 attenuates the transcriptional activity as well as DNA damage-mediated induction of pro-apoptotic TAp73 to regulate chemosensitivity. FEBS J. 2015, 282, 114–128. [Google Scholar] [CrossRef][Green Version]
- Sugimoto, H.; Nakamura, M.; Yoda, H.; Hiraoka, K.; Shinohara, K.; Sang, M.; Fujiwara, K.; Shimozato, O.; Nagase, H.; Ozaki, T. Silencing of RUNX2 enhances gemcitabine sensitivity of p53-deficient human pancreatic cancer AsPC-1 cells through the stimulation of TAp63-mediated cell death. Cell Death Discov. 2015, 1, 15010. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, L.; Liu, L.; Wang, L.; Lin, W.; Zhu, X.; Su, W.; Lv, C. Knockdown of microRNA-203 reduces cisplatin chemo-sensitivity to osteosarcoma cell lines MG63 and U2OS in vitro by targeting RUNX2. J. Chemother. 2021, 33, 328–341. [Google Scholar] [CrossRef]
- Lin, W.; Zhu, X.; Yang, S.; Chen, X.; Wang, L.; Huang, Z.; Ding, Y.; Huang, L.; Lv, C. MicroRNA-203 inhibits proliferation and invasion, and promotes apoptosis of osteosarcoma cells by targeting Runt-related transcription factor 2. Biomed. Pharmacother. 2017, 91, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, Y.; Liu, T.; Jiang, K.; Wen, Y.; Fan, Q.; Qiu, X. Hypoxia promotes chemotherapy resistance by down-regulating SKA1 gene expression in human osteosarcoma. Cancer Biol. Ther. 2017, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Chen, X.; Xiang, F.; Deng, Y.; Li, Z.; Wei, D. TGF-β protects osteosarcoma cells from chemotherapeutic cytotoxicity in a SDH/HIF1α dependent manner. BMC Cancer 2021, 21, 1200. [Google Scholar] [CrossRef]
- Keremu, A.; Aini, A.; Maimaitirexiati, Y.; Liang, Z.; Aila, P.; Xierela, P.; Tusun, A.; Moming, H.; Yusufu, A. Overcoming cisplatin resistance in osteosarcoma through the miR-199a-modulated inhibition of HIF-1α. Biosci. Rep. 2019, 39, BSR20170080. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wu, W.; Dong, N.; Jiang, X.; Xu, J.; Zhan, X.; Zhang, Z.; Hu, Z. Mxd1 mediates hypoxia-induced cisplatin resistance in osteosarcoma cells by repression of the PTEN tumor suppressor gene. Mol. Carcinog. 2017, 56, 2234–2244. [Google Scholar] [CrossRef]
- Chen, H.H.; Zhang, T.N.; Zhang, F.Y.; Zhang, T. Non-coding RNAs in drug and radiation resistance of bone and soft-tissue sarcoma: A systematic review. Elife 2022, 11, e79655. [Google Scholar] [CrossRef]
- Spałek, M.J.; Poleszczuk, J.; Czarnecka, A.M.; Dudzisz-Śledź, M.; Napieralska, A.; Matysiakiewicz, J.; Chojnacka, M.; Raciborska, A.; Sztuder, A.; Maciejczyk, A.; et al. Radiotherapy in the Management of Pediatric and Adult Osteosarcomas: A Multi-Institutional Cohort Analysis. Cells 2021, 10, 366. [Google Scholar] [CrossRef]
- Schwarz, R.; Bruland, O.; Cassoni, A.; Schomberg, P.; Bielack, S. The role of radiotherapy in oseosarcoma. Cancer Treat. Res. 2009, 152, 147–164. [Google Scholar] [CrossRef]
- Nisar, H.; Labonté, F.M.; Roggan, M.D.; Schmitz, C.; Chevalier, F.; Konda, B.; Diegeler, S.; Baumstark-Khan, C.; Hellweg, C.E. Hypoxia Modulates Radiosensitivity and Response to Different Radiation Qualities in A549 Non-Small Cell Lung Cancer (NSCLC) Cells. Int. J. Mol. Sci. 2024, 25, 1010. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, J.; Chen, W.; Shan, B.; Guo, Y.; Xu, J.; Wan, L.; Guo, P.; Zhang, Y. Hypoxia-induced autophagy as an additional mechanism in human osteosarcoma radioresistance. J. Bone Oncol. 2016, 5, 67–73. [Google Scholar] [CrossRef] [PubMed]
- AL-Hamad, K.A. Role of RUNX2 in oral squamous cell carcinoma (OSCC): A systematic scoping review. Eur. J. Cancer Care 2024, 2024, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chen, Y.K.; Lo, S.; Chi, T.C.; Chen, Y.H.; Hu, S.C.; Chen, Y.W.; Jiang, S.S.; Tsai, F.Y.; Liu, W.; et al. MRE11 promotes oral cancer progression through RUNX2/CXCR4/AKT/FOXA2 signaling in a nuclease-independent manner. Oncogene 2021, 40, 3510–3532. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magar, A.G.; Morya, V.K.; Noh, K.-C. Molecular Crosstalk Between RUNX2 and HIF-1α in Osteosarcoma: Implications for Angiogenesis, Metastasis, and Therapy Resistance. Int. J. Mol. Sci. 2025, 26, 7642. https://doi.org/10.3390/ijms26157642
Magar AG, Morya VK, Noh K-C. Molecular Crosstalk Between RUNX2 and HIF-1α in Osteosarcoma: Implications for Angiogenesis, Metastasis, and Therapy Resistance. International Journal of Molecular Sciences. 2025; 26(15):7642. https://doi.org/10.3390/ijms26157642
Chicago/Turabian StyleMagar, Anuja Gajanan, Vivek Kumar Morya, and Kyu-Cheol Noh. 2025. "Molecular Crosstalk Between RUNX2 and HIF-1α in Osteosarcoma: Implications for Angiogenesis, Metastasis, and Therapy Resistance" International Journal of Molecular Sciences 26, no. 15: 7642. https://doi.org/10.3390/ijms26157642
APA StyleMagar, A. G., Morya, V. K., & Noh, K.-C. (2025). Molecular Crosstalk Between RUNX2 and HIF-1α in Osteosarcoma: Implications for Angiogenesis, Metastasis, and Therapy Resistance. International Journal of Molecular Sciences, 26(15), 7642. https://doi.org/10.3390/ijms26157642





