Alterations in the Platelet Transcriptome Mediate Prenatal Thirdhand Smoke Exposure Associated Thrombogenicity via Integrated miRNA-mRNA Regulatory Networks
Abstract
1. Introduction
2. Results
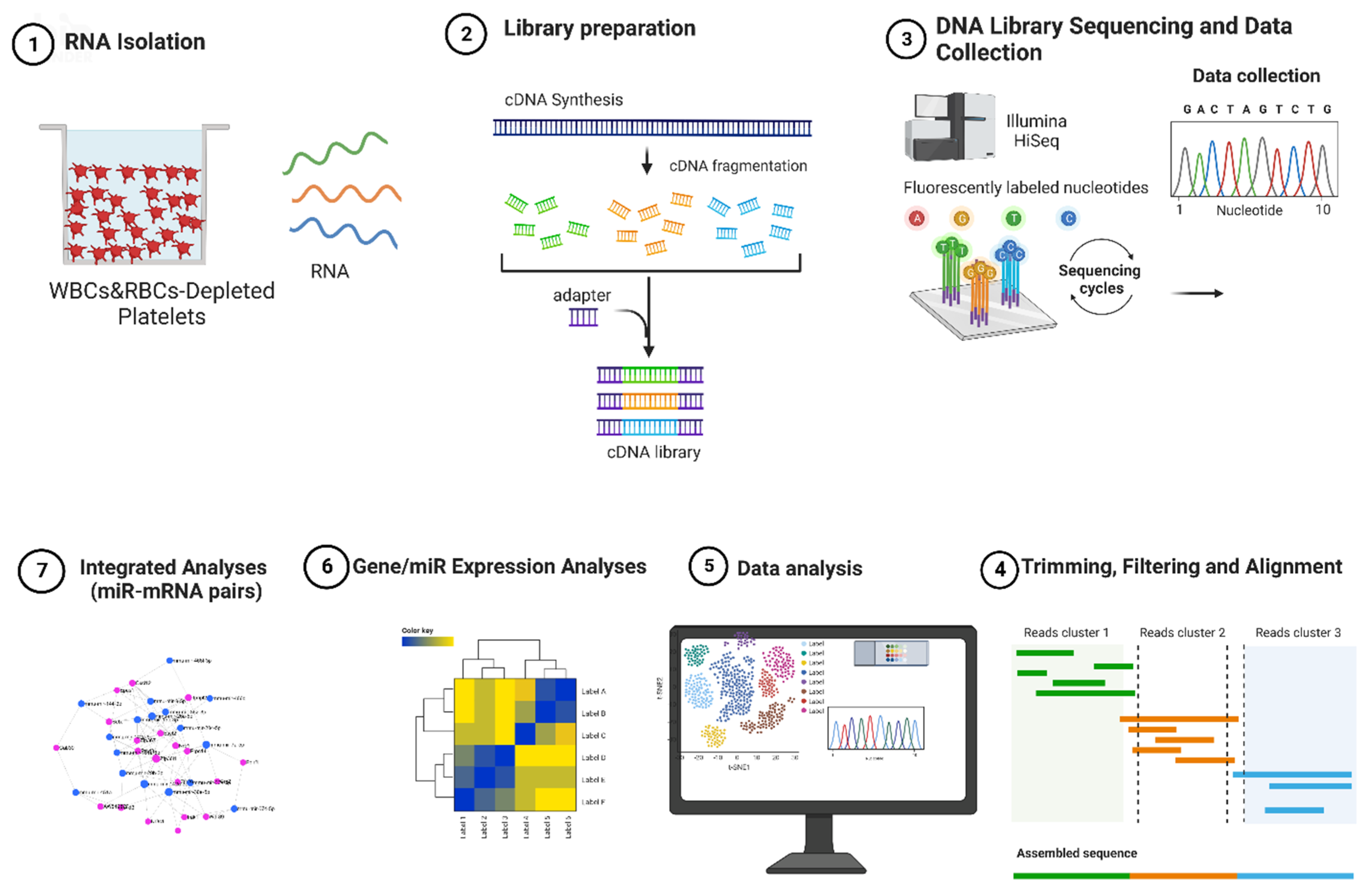
2.1. Experimental Design
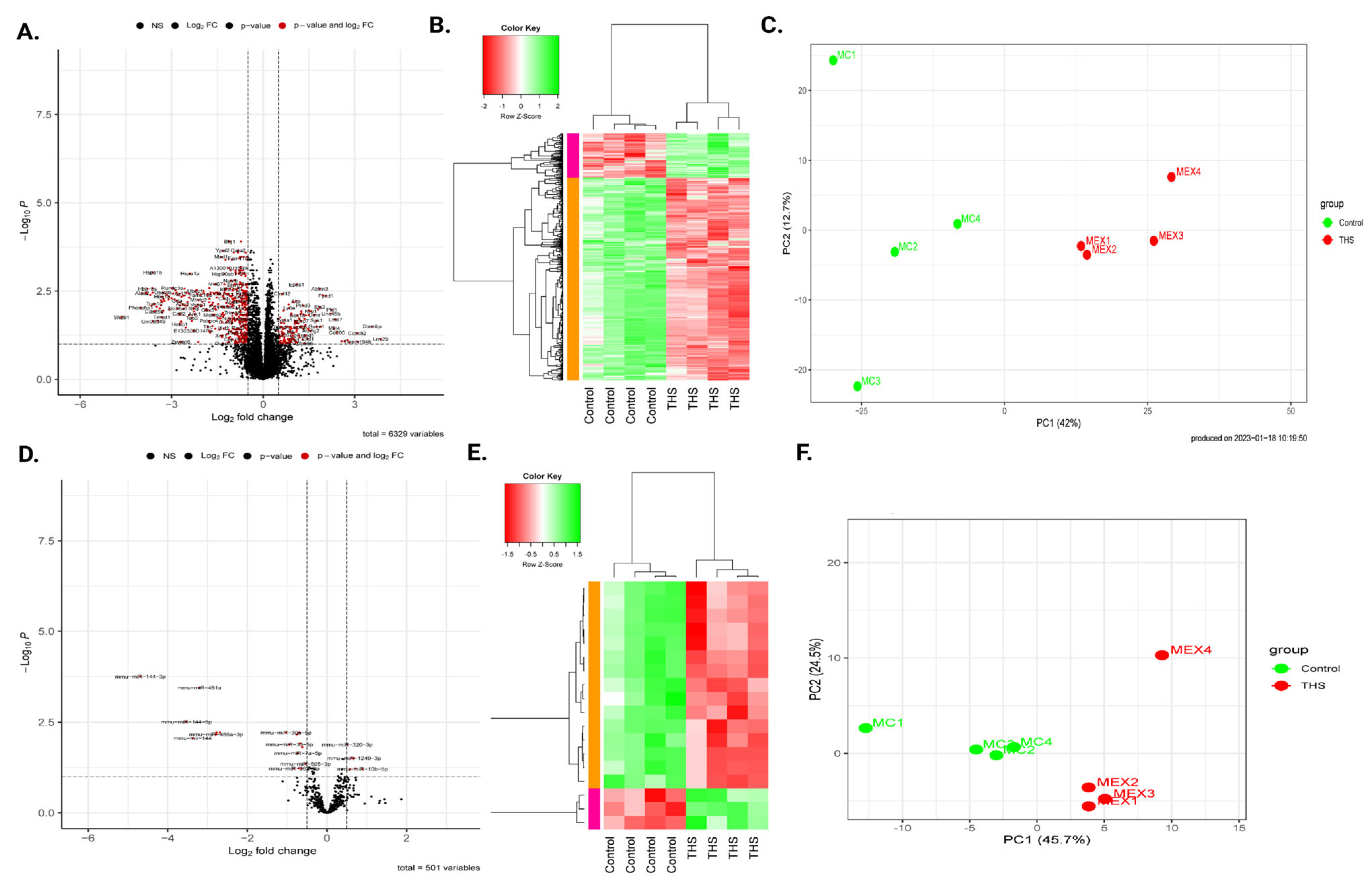
2.2. Prenatal THS Exposure Triggers Changes in Gene Expression in the Platelets of the Offspring
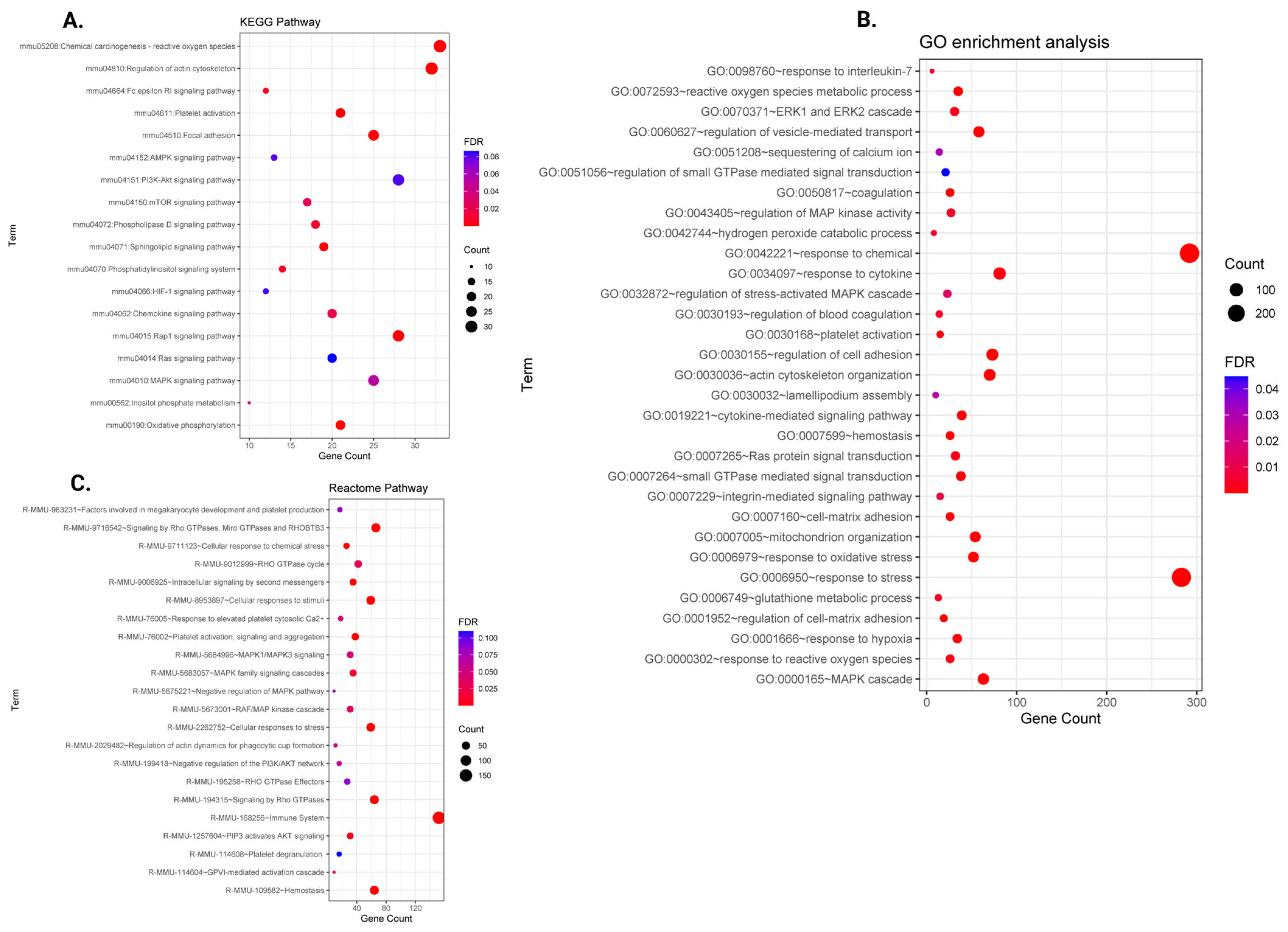
2.3. Functional Enrichment Analysis of Differentially Expressed Genes
2.4. Prenatal THS Modifies Platelet miRNA
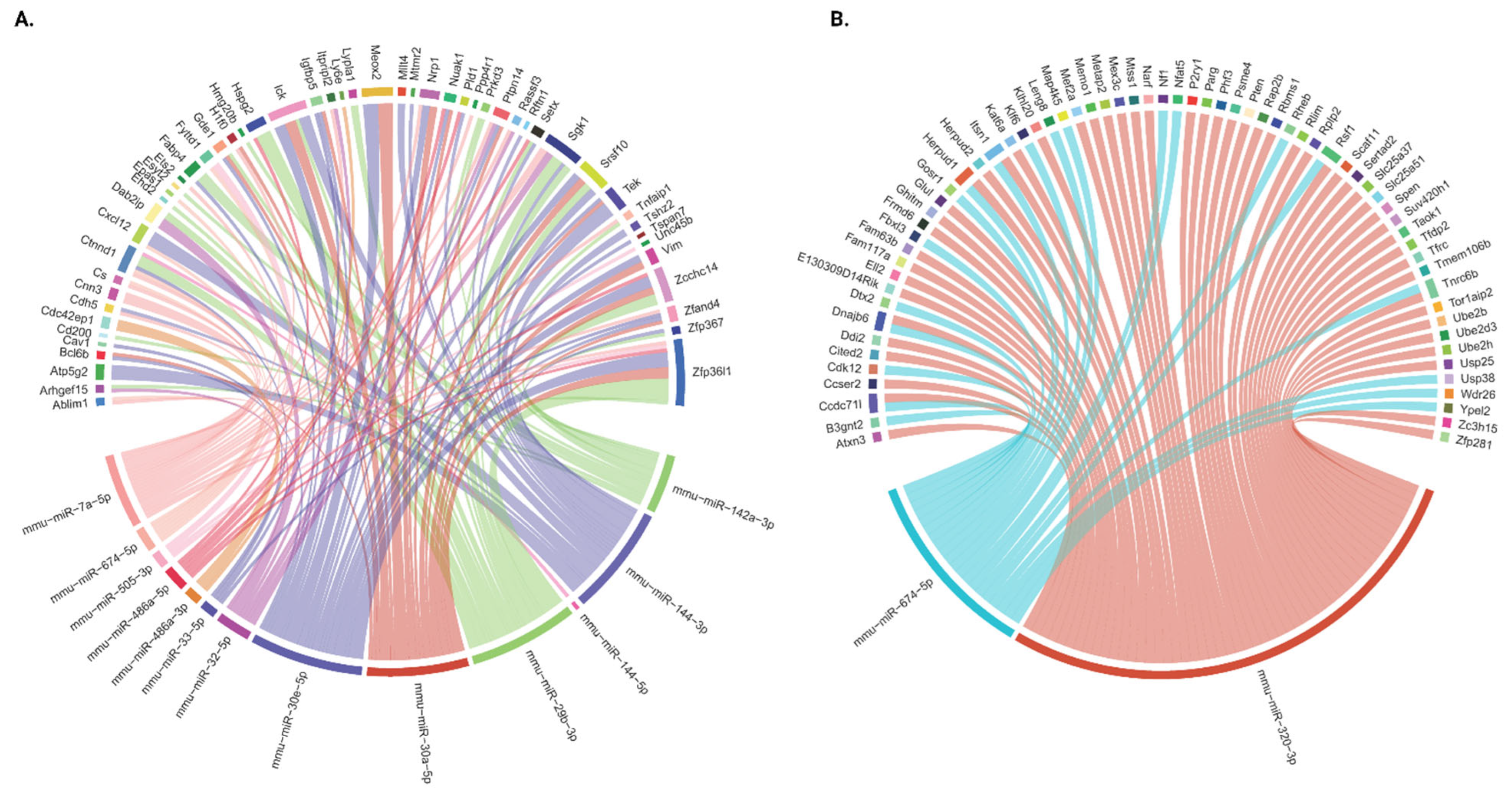
2.5. Integration of miRNA and mRNA Expression Data
2.6. The miRNA–mRNA-Regulated Functional Pathways
2.7. Q-PCR Validation of Prenatal THS-Associated Transcripts
2.8. Gene–Chemical Component Enrichment Using the Comparative Toxicogenomic Database
3. Discussion
4. Materials and Methods
4.1. Animals and Exposure
4.2. Platelet Purification and RNA Extraction
4.3. mRNA Sequencing and Analysis
4.4. miRNA Sequencing and Analysis
4.5. Functional Enrichment Analysis
4.6. Identification of miRNA-Targeted Genes and the mRNA–miRNA Regulatory Network
4.7. Validation of DEGs and DE miRs by Quantitative RT-qPCR
4.8. Toxic Chemical–Gene Interaction Enrichment Analysis Using the Toxicogenomics Database
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| THS | Thirdhand smoke |
| CA | Clean air |
| GPRP | Gly-Pro-Arg-Pro |
| DEG | Differentially Expressed Gene |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| CTD | Comparative Toxicogenomics Database |
| PCA | Principal Component Analysis |
References
- Davis, J.W.; Shelton, L.; Watanabe, I.S.; Arnold, J. Passive smoking affects endothelium and platelets. Arch. Intern. Med. 1989, 149, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Vitali, M. The New Danger of Thirdhand Smoke: Why Passive Smoking Does Not Stop at Secondhand Smoke. Environ. Health Perspect. 2011, 119, a422. [Google Scholar] [CrossRef]
- Sleiman, M.; Gundel, L.A.; Pankow, J.F.; Jacob, P.; Singer, B.C.; Destaillats, H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc. Natl. Acad. Sci. USA 2010, 107, 6576–6581. [Google Scholar] [CrossRef]
- Sleiman, M.; Logue, J.M.; Luo, W.; Pankow, J.F.; Gundel, L.A.; Destaillats, H. Inhalable Constituents of Thirdhand Tobacco Smoke: Chemical Characterization and Health Impact Considerations. Environ. Sci. Technol. 2014, 48, 13093–13101. [Google Scholar] [CrossRef] [PubMed]
- Becquemin, M.H.; Bertholon, J.F.; Bentayeb, M.; Attoui, M.; Ledur, D.; Roy, F.; Roy, M.; Annesi-Maesano, I.; Dautzenberg, B. Third-hand smoking: Indoor measurements of concentration and sizes of cigarette smoke particles after resuspension. Tob. Control 2010, 19, 347–348. [Google Scholar] [CrossRef]
- Matt, G.E.; Quintana, P.J.E.; Hovell, M.F.; Chatfield, D.; Ma, D.S.; Romero, R.; Uribe, A. Residual tobacco smoke pollution in used cars for sale: Air, dust, and surfaces. Nicotine Tob. Res. 2008, 10, 1467–1475. [Google Scholar] [CrossRef]
- Matt, G.E.; Quintana, P.J.E.; Zakarian, J.M.; Fortmann, A.L.; Chatfield, D.A.; Hoh, E.; Uribe, A.M.; Hovell, M.F. When smokers move out and non-smokers move in: Residential thirdhand smoke pollution and exposure. Tob. Control 2010, 20, e1. [Google Scholar] [CrossRef]
- Schick, S. Thirdhand smoke: Here to stay. Tob. Control 2010, 20, 1–3. [Google Scholar] [CrossRef]
- Winickoff, J.P.; Friebely, J.; Tanski, S.E.; Sherrod, C.; Matt, G.E.; Hovell, M.F.; McMillen, R.C. Beliefs About the Health Effects of “Thirdhand” Smoke and Home Smoking Bans. Pediatrics 2009, 123, e74–e79. [Google Scholar] [CrossRef]
- Destaillats, H.; Singer, B.C.; Gundel, L.A. Evidence of acid-base interactions between amines and model indoor surfaces by ATR-FTIR spectroscopy. Atmos. Environ. 2007, 41, 3177. [Google Scholar] [CrossRef]
- Sleiman, M.; Destaillats, H.; Smith, J.D.; Liu, C.L.; Ahmed, M.; Wilson, K.R.; Gundel, L.A. Secondary organic aerosol formation from ozone-initiated reactions with nicotine and secondhand tobacco smoke. Atmos. Environ. 2010, 44, 4191. [Google Scholar] [CrossRef]
- Kuschner, W.G.; Reddy, S.; Mehrotra, N.; Paintal, H.S. Electronic cigarettes and thirdhand tobacco smoke: Two emerging health care challenges for the primary care provider. Int. J. Gen. Med. 2011, 4, 115–120. [Google Scholar] [CrossRef]
- Matt, G.E.; Quintana, P.J.E.; Hovell, M.F.; Bernert, J.T.; Song, S.; Novianti, N.; Juarez, T.; Floro, J.; Gehrman, C.; Garcia, M.; et al. Households contaminated by environmental tobacco smoke: Sources of infant exposures. Tob. Control 2004, 13, 29–37. [Google Scholar] [CrossRef]
- Adhami, N.; Starck, S.R.; Flores, C.; Martins Green, M. A Health Threat to Bystanders Living in the Homes of Smokers: How Smoke Toxins Deposited on Surfaces Can Cause Insulin Resistance. PLoS ONE 2016, 11, e0149510. [Google Scholar]
- Prignot, J.J. Recent Contributions of Air- and Biomarkers to the Control of Secondhand Smoke (SHS): A Review. Int. J. Environ. Res. Public Health 2011, 8, 648–682. [Google Scholar] [CrossRef]
- Martins-Green, M.; Adhami, N.; Frankos, M.; Valdez, M.; Goodwin, B.; Lyubovitsky, J.; Dhall, S.; Garcia, M.; Egiebor, I.; Martinez, B.; et al. Cigarette Smoke Toxins Deposited on Surfaces: Implications for Human Health. PLoS ONE 2014, 9, e86391. [Google Scholar] [CrossRef]
- Burton, A. Does the Smoke Ever Really Clear? Thirdhand Smoke Exposure Raises New Concerns. Environ. Health Perspect. 2011, 119, A70–A74. [Google Scholar] [CrossRef]
- Chaouachi, K. Hookah (Shisha, Narghile) Smoking and Environmental Tobacco Smoke (ETS). A critical review of the relevant literature and the public health consequences. Int. J. Environ. Res. Public Health 2009, 6, 798–843. [Google Scholar] [CrossRef] [PubMed]
- Drehmer, J.E.; Ossip, D.J.; Rigotti, N.A.; Nabi-Burza, E.; Woo, H.; Wasserman, R.C.; Chang, Y.; Winickoff, J.P. Pediatrician Interventions and Thirdhand Smoke Beliefs of Parents. Am. J. Prev. Med. 2012, 43, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Whitlatch, A.; Schick, S. Thirdhand Smoke at Philip Morris. Nicotine Tob. Res. 2018, 21, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.L.; Hecht, S.S.; Luo, X.; Ming, X.; Ahluwalia, J.S.; Carmella, S.G. Thirdhand Tobacco Smoke: A Tobacco-Specific Lung Carcinogen on Surfaces in Smokers’ Homes. Nicotine Tob. Res. 2013, 16, 26–32. [Google Scholar] [CrossRef]
- Karim, Z.A.; Alshbool, F.Z.; Vemana, H.P.; Adhami, N.; Dhall, S.; Espinosa, E.V.; Martins-Green, M.; Khasawneh, F.T. Third-hand Smoke: Impact on Hemostasis and Thrombogenesis. J. Cardiovasc. Pharmacol. 2015, 66, 177–182. [Google Scholar] [CrossRef]
- Godwin, M.D.; Aggarwal, A.; Hilt, Z.; Shah, S.; Gorski, J.; Cameron, S.J. Sex-Dependent Effect of Platelet Nitric Oxide: Production and Platelet Reactivity in Healthy Individuals. JACC Basic Transl. Sci. 2022, 7, 14–25. [Google Scholar] [CrossRef]
- Snijders, A.M.; Zhou, M.; Whitehead, T.P.; Fitch, B.; Pandey, P.; Hechmer, A.; Huang, A.; Schick, S.F.; de Smith, A.J.; Olshen, A.B.; et al. In utero and early-life exposure to thirdhand smoke causes profound changes to the immune system. Clin. Sci. 2021, 135, 1053–1063. [Google Scholar] [CrossRef]
- Ali, H.E.; Alarabi, A.B.; Karim, Z.A.; Rodriguez, V.; Hernandez, K.R.; Lozano, P.A.; El-Halawany, M.S.; Alshbool, F.Z.; Khasawneh, F.T. In utero thirdhand smoke exposure modulates platelet function in a sex-dependent manner. Haematologica 2021, 107, 312–315. [Google Scholar] [CrossRef]
- Sakamaki-Ching, S.; Schick, S.; Grigorean, G.; Li, J.; Talbot, P. Dermal thirdhand smoke exposure induces oxidative damage, initiates skin inflammatory markers, and adversely alters the human plasma proteome. EBioMedicine 2022, 84, 104256. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-García, D.; Ali, H.E.A.; Alarabi, A.B.; El-Halawany, M.S.; Alshbool, F.Z.; Khasawneh, F.T. Exposure of Mice to Thirdhand Smoke Modulates In Vitro and In Vivo Platelet Responses. Int. J. Mol. Sci. 2022, 23, 5595. [Google Scholar] [CrossRef] [PubMed]
- Bahl, V.; Shim, H.J.; Jacob, P., 3rd; Dias, K.; Schick, S.F.; Talbot, P. Thirdhand smoke: Chemical dynamics, cytotoxicity, and genotoxicity in outdoor and indoor environments. Toxicol. In Vitro 2016, 32, 220–231. [Google Scholar] [CrossRef]
- Xu, B.; Chen, M.; Yao, M.; Ji, X.; Mao, Z.; Tang, W.; Qiao, S.; Schick, S.F.; Mao, J.-H.; Hang, B.; et al. Metabolomics reveals metabolic changes in male reproductive cells exposed to thirdhand smoke. Sci. Rep. 2015, 5, 15512. [Google Scholar] [CrossRef]
- Brody, J.S. Transcriptome alterations induced by cigarette smoke. Int. J. Cancer 2012, 131, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.O.; Gümüş, Z.H.; Kacker, A.; Choksi, V.L.; Bocker, J.M.; Zhou, X.K.; Yantiss, R.K.; Hughes, D.B.; Du, B.; Judson, B.L.; et al. Effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev. Res. 2010, 3, 266–278. [Google Scholar] [CrossRef]
- Harvey, B.-G.; Heguy, A.; Leopold, P.L.; Carolan, B.J.; Ferris, B.; Crystal, R.G. Modification of gene expression of the small airway epithelium in response to cigarette smoking. J. Mol. Med. 2007, 85, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Huan, T.; Joehanes, R.; Schurmann, C.; Schramm, K.; Pilling, L.C.; Peters, M.J.; Mägi, R.; DeMeo, D.; O’Connor, G.T.; Ferrucci, L.; et al. A whole-blood transcriptome meta-analysis identifies gene expression signatures of cigarette smoking. Hum. Mol. Genet. 2016, 25, 4611–4623. [Google Scholar] [CrossRef] [PubMed]
- Pickett, G.; Seagrave, J.; Boggs, S.; Polzin, G.; Richter, P.; Tesfaigzi, Y. Effects of 10 cigarette smoke condensates on primary human airway epithelial cells by comparative gene and cytokine expression studies. Toxicol. Sci. 2010, 114, 79–89. [Google Scholar] [CrossRef] [PubMed]
- López-Boado, Y.S.; Li, J.U.; Clayton, C.L.; Wright, J.L.; Churg, A. Modification of the rat airway explant transcriptome by cigarette smoke. Inhal. Toxicol. 2010, 22, 234–244. [Google Scholar] [CrossRef]
- Bahl, V.; Johnson, K.; Phandthong, R.; Zahedi, A.; Schick, S.F.; Talbot, P. From the Cover: Thirdhand Cigarette Smoke Causes Stress-Induced Mitochondrial Hyperfusion and Alters the Transcriptional Profile of Stem Cells. Toxicol. Sci. 2016, 153, 55–69. [Google Scholar] [CrossRef]
- Quelhas, D.; Kompala, C.; Wittenbrink, B.; Han, Z.; Parker, M.; Shapiro, M.; Downs, S.; Kraemer, K.; Fanzo, J.; Morris, S.; et al. The association between active tobacco use during pregnancy and growth outcomes of children under five years of age: A systematic review and meta-analysis. BMC Public Health 2018, 18, 1372. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Spindel, E.R. Pulmonary Effects of Maternal Smoking on the Fetus and Child: Effects on Lung Development, Respiratory Morbidities, and Life Long Lung Health. Paediatr. Respir. Rev. 2017, 21, 27–33. [Google Scholar] [CrossRef]
- Noakes, P.S.; Hale, J.; Thomas, R.; Lane, C.; Devadason, S.G.; Prescott, S.L. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. Eur. Respir. J. 2006, 28, 721–729. [Google Scholar] [CrossRef]
- Polańska, K.; Jurewicz, J.; Hanke, W. Smoking and alcohol drinking during pregnancy as the risk factors for poor child neurodevelopment—A review of epidemiological studies. Int. J. Occup. Med. Environ. Health 2015, 28, 419–443. [Google Scholar] [CrossRef]
- Wiklund, P.; Karhunen, V.; Richmond, R.C.; Parmar, P.; Rodriguez, A.; De Silva, M.; Wielscher, M.; Rezwan, F.I.; Richardson, T.G.; Veijola, J.; et al. DNA methylation links prenatal smoking exposure to later life health outcomes in offspring. Clin. Epigenetics 2019, 11, 97. [Google Scholar] [CrossRef]
- Joubert, B.R.; Felix, J.F.; Yousefi, P.; Bakulski, K.M.; Just, A.C.; Breton, C.; Reese, S.E.; Markunas, C.A.; Richmond, R.C.; Xu, C.-J.; et al. DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. Am. J. Hum. Genet. 2016, 98, 680–696. [Google Scholar] [CrossRef]
- Everson, T.M.; Vives-Usano, M.; Seyve, E.; Cardenas, A.; Lacasaña, M.; Craig, J.M.; Lesseur, C.; Baker, E.R.; Fernandez-Jimenez, N.; Heude, B.; et al. Placental DNA methylation signatures of maternal smoking during pregnancy and potential impacts on fetal growth. Nat. Commun. 2021, 12, 5095. [Google Scholar] [CrossRef]
- Chhabra, D.; Sharma, S.; Kho, A.T.; Gaedigk, R.; Vyhlidal, C.A.; Leeder, J.S.; Morrow, J.; Carey, V.J.; Weiss, S.T.; Tantisira, K.G.; et al. Fetal lung and placental methylation is associated with in utero nicotine exposure. Epigenetics 2014, 9, 1473–1484. [Google Scholar] [CrossRef]
- Breton, C.V.; Byun, H.-M.; Wenten, M.; Pan, F.; Yang, A.; Gilliland, F.D. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am. J. Respir. Crit. Care Med. 2009, 180, 462–467. [Google Scholar] [CrossRef]
- Rauschert, S.; Melton, P.E.; Burdge, G.; Craig, J.M.; Godfrey, K.M.; Holbrook, J.D.; Lillycrop, K.; Mori, T.A.; Beilin, L.J.; Oddy, W.H.; et al. Maternal Smoking During Pregnancy Induces Persistent Epigenetic Changes into Adolescence, Independent of Postnatal Smoke Exposure and Is Associated with Cardiometabolic Risk. Front. Genet. 2019, 10, 770. [Google Scholar] [CrossRef]
- Bray, P.F.; McKenzie, S.E.; Edelstein, L.C.; Nagalla, S.; Delgrosso, K.; Ertel, A.; Kupper, J.; Jing, Y.; Londin, E.; Loher, P.; et al. The complex transcriptional landscape of the anucleate human platelet. BMC Genom. 2013, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.W.; Green, R.; Ingolia, N.T. Slowed decay of mRNAs enhances platelet specific translation. Blood 2017, 129, e38–e48. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Narayanan, P.; Li, W.; Silverstein, R.L.; McIntyre, T.M. Lipopolysaccharide stimulates platelets through an IL-1β autocrine loop. J. Immunol. 2013, 191, 5196–5203. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S.; Novelli, E.M.; Bullock, G.C.; Kenny, E.; Hill, G.; Corey, C.G. Mitochondrial Uncoupling Protein 2 Is Present in Human Platelets and Regulates Platelet Activation. Blood 2014, 124, 4147. [Google Scholar] [CrossRef]
- Englert, M.; Aurbach, K.; Becker, I.C.; Gerber, A.; Heib, T.; Wackerbarth, L.M.; Kusch, C.; Mott, K.; Araujo, G.H.M.; Baig, A.A.; et al. Impaired microtubule dynamics contribute to microthrombocytopenia in RhoB-deficient mice. Blood Adv. 2022, 6, 5184–5197. [Google Scholar] [CrossRef]
- Mangin, P.; Ohlmann, P.; Eckly, A.; Cazenave, J.; Lanza, F.; Gachet, C. The P2Y1 receptor plays an essential role in the platelet shape change induced by collagen when TxA2 formation is prevented. J. Thromb. Haemost. 2004, 2, 969–977. [Google Scholar] [CrossRef]
- Weng, Z.; Li, D.; Zhang, L.; Chen, J.; Ruan, C.; Chen, G.; Gartner, T.K.; Liu, J. PTEN regulates collagen-induced platelet activation. Blood 2010, 116, 2579–2581. [Google Scholar] [CrossRef]
- Poisson, C.; Rollin, S.; Véronneau, S.; Bousquet, S.M.; Larrivée, J.-F.; Le Gouill, C.; Boulay, G.; Stankova, J.; Rola-Pleszczynski, M. Caveolae Facilitate but Are Not Essential for Platelet-Activating Factor-Mediated Calcium Mobilization and Extracellular Signal-Regulated Kinase Activation. J. Immunol. 2009, 183, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Leberzammer, J.; Agten, S.M.; Blanchet, X.; Duan, R.; Ippel, H.; Megens, R.T.A.; Schulz, C.; Aslani, M.; Duchene, J.; Döring, Y.; et al. Targeting platelet derived CXCL12 impedes arterial thrombosis. Blood 2022, 139, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- Nagalla, S.; Shaw, C.; Kong, X.; Kondkar, A.A.; Edelstein, L.C.; Ma, L.; Chen, J.; McKnight, G.S.; López, J.A.; Yang, L.; et al. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood 2011, 117, 5189–5197. [Google Scholar] [CrossRef]
- Csordas, A.; Bernhard, D. The biology behind the atherothrombotic effects of cigarette smoke. Nat. Rev. Cardiol. 2013, 10, 219–230. [Google Scholar] [CrossRef]
- Malyla, V.; Paudel, K.R.; Shukla, S.D.; Donovan, C.; Wadhwa, R.; Pickles, S.; Chimankar, V.; Sahu, P.; Bielefeldt-Ohmann, H.; Bebawy, M.; et al. Recent advances in experimental animal models of lung cancer. Future Med. Chem. 2020, 12, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, S.; Prabhakaran, D.; Roy, A. Pathophysiological Mechanisms of Tobacco-Related CVD. Glob. Heart 2012, 7, 113–120. [Google Scholar] [CrossRef]
- Schaeffer, G.; Wascher, T.C.; Kostner, G.M.; Graier, W.F. Alterations in platelet Ca2+ signalling in diabetic patients is due to increased formation of superoxide anions and reduced nitric oxide production. Diabetologia 1999, 42, 167–176. [Google Scholar] [CrossRef]
- Spinetti, G.; Kraenkel, N.; Emanueli, C.; Madeddu, P. Diabetes and vessel wall remodelling: From mechanistic insights to regenerative therapies. Cardiovasc. Res. 2008, 78, 265–273. [Google Scholar] [CrossRef]
- Adhami, N.; Chen, Y.; Martins-Green, M. Biomarkers of disease can be detected in mice as early as 4 weeks after initiation of exposure to third-hand smoke levels equivalent to those found in homes of smokers. Clin. Sci. 2017, 131, 2409–2426. [Google Scholar] [CrossRef]
- Remenyi, G.; Szasz, R.; Friese, P.; Dale, G.L. Role of mitochondrial permeability transition pore in coated-platelet formation. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 467–471. [Google Scholar] [CrossRef]
- Choo, H.-J.; Saafir, T.B.; Mkumba, L.; Wagner, M.B.; Jobe, S.M. Mitochondrial calcium and reactive oxygen species regulate agonist-initiated platelet phosphatidylserine exposure. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Panth, N.; Manandhar, B.; Singh, S.K.; Gupta, G.; Wich, P.R.; Nammi, S.; MacLoughlin, R.; Adams, J.; Warkiani, M.E.; et al. Attenuation of Cigarette-Smoke-Induced Oxidative Stress, Senescence, and Inflammation by Berberine-Loaded Liquid Crystalline Nanoparticles: In Vitro Study in 16HBE and RAW264.7 Cells. Antioxidants 2022, 11, 873. [Google Scholar] [CrossRef]
- James, R.W.; Leviev, I.; Righetti, A. Smoking is associated with reduced serum paraoxonase activity and concentration in patients with coronary artery disease. Circulation 2000, 101, 2252–2257. [Google Scholar] [CrossRef]
- Yasue, H.; Hirai, N.; Mizuno, Y.; Harada, E.; Itoh, T.; Yoshimura, M.; Kugiyama, K.; Ogawa, H. Low-grade inflammation, thrombogenicity, and atherogenic lipid profile in cigarette smokers. Circ. J. 2006, 70, 8–13. [Google Scholar] [CrossRef]
- FitzGerald, G.A.; Oates, J.A.; Nowak, J. Cigarette smoking and hemostatic function. Am. Heart J. 1988, 115, 267–271. [Google Scholar] [CrossRef]
- Li, Z.; Delaney, M.K.; O’BRien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, J.R.; Bergmeier, W.; Zhang, Y.; Piffath, C.L.; Liang, Y.; Wagner, D.D.; Housman, D.E.; Graybiel, A.M. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat. Med. 2004, 10, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, T.; Kareem, K.; Tran, D.; Griffith, B.P.; Wu, Z.J. The role of PI3K/Akt signaling pathway in non-physiological shear stress-induced platelet activation. Artif. Organs 2019, 43, 897–908. [Google Scholar] [CrossRef]
- Börsch-Haubold, A.G.; Kramer, R.M.; Watson, S.P. Inhibition of mitogen-activated protein kinase kinase does not impair primary activation of human platelets. Biochem. J. 1996, 318 Pt 1, 207–212. [Google Scholar] [CrossRef]
- Papkoff, J.; Chen, R.-H.; Blenis, J.; Forsman, J. p42 mitogen-activated protein kinase and p90 ribosomal S6 kinase are selectively phosphorylated and activated during thrombin-induced platelet activation and aggregation. Mol. Cell Biol. 1994, 14, 463–472. [Google Scholar] [CrossRef][Green Version]
- Adam, F.; Kauskot, A.; Nurden, P.; Sulpice, E.; Hoylaerts, M.F.; Davis, R.J.; Rosa, J.-P.; Bryckaert, M. Platelet JNK1 is involved in secretion and thrombus formation. Blood 2010, 115, 4083–4092. [Google Scholar] [CrossRef]
- Aslan, J.E.; Mccarty, O.J.T. Rho GTPases in platelet function. J. Thromb. Haemost. 2013, 11, 35–46. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, F.; Honda, T.; Greis, K.D.; Redington, A.N. Ablation of miR-144 increases vimentin expression and atherosclerotic plaque formation. Sci. Rep. 2020, 10, 6127. [Google Scholar] [CrossRef] [PubMed]
- Madè, A.; Greco, S.; Vausort, M.; Miliotis, M.; Schordan, E.; Baksi, S.; Zhang, L.; Baryshnikova, E.; Ranucci, M.; Cardani, R.; et al. Association of miR-144 levels in the peripheral blood with COVID-19 severity and mortality. Sci. Rep. 2022, 12, 20048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Zhang, C.; Hu, L.; Liu, C.; Pan, N.; Li, M.; Han, H.; Zhou, Y.; Li, J.; Zhao, L.-Y.; et al. Platelet CFTR inhibition enhances arterial thrombosis via increasing intracellular Cl− concentration and activation of SGK1 signaling pathway. Acta Pharmacol. Sin. 2022, 43, 2596–2608. [Google Scholar] [CrossRef]
- Elvers, M.; Stegner, D.; Hagedorn, I.; Kleinschnitz, C.; Braun, A.; Kuijpers, M.E.J.; Boesl, M.; Chen, Q.; Heemskerk, J.W.M.; Stoll, G.; et al. Impaired alpha(IIb)beta(3) integrin activation and shear-dependent thrombus formation in mice lacking phospholipase D1. Sci Signal. 2010, 3, ra1. [Google Scholar] [CrossRef]
- Graff, J.W.; Powers, L.S.; Dickson, A.M.; Kim, J.; Reisetter, A.C.; Hassan, I.H.; Kremens, K.; Gross, T.J.; Wilson, M.E.; Monick, M.M.; et al. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS ONE 2012, 7, e44066. [Google Scholar] [CrossRef]
- Izzotti, A.; Calin, G.A.; Steele, V.E.; Croce, C.M.; De Flora, S. Relationships of microRNA expression in mouse lung with age and exposure to cigarette smoke and light. FASEB J. 2009, 23, 3243–3250. [Google Scholar] [CrossRef]
- Espinosa-Parrilla, Y.; Gonzalez-Billault, C.; Fuentes, E.; Palomo, I.; Alarcón, M. Decoding the Role of Platelets and Related MicroRNAs in Aging and Neurodegenerative Disorders. Front. Aging Neurosci. 2019, 11, 151. [Google Scholar] [CrossRef]
- Donato, A.J.; Walker, A.E.; Magerko, K.A.; Bramwell, R.C.; Black, A.D.; Henson, G.D.; Lawson, B.R.; Lesniewski, L.A.; Seals, D.R. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 2013, 12, 772–783. [Google Scholar] [CrossRef]
- Yanbaeva, D.G.; Dentener, M.A.; Creutzberg, E.C.; Wesseling, G.; Wouters, E.F.M. Systemic effects of smoking. Chest 2007, 131, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Pergoli, L.; Cantone, L.; Favero, C.; Angelici, L.; Iodice, S.; Pinatel, E.; Hoxha, M.; Dioni, L.; Letizia, M.; Albetti, B.; et al. Extracellular vesicle-packaged miRNA release after short-term exposure to particulate matter is associated with increased coagulation. Part. Fibre Toxicol. 2017, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Omidian, K.; Rafiei, H.; Bandy, B. Polyphenol inhibition of benzo[a]pyrene-induced oxidative stress and neoplastic transformation in an in vitro model of carcinogenesis. Food Chem. Toxicol. 2017, 106, 165–174. [Google Scholar] [CrossRef]
- Liamin, M.; Le Mentec, H.; Evrard, B.; Huc, L.; Chalmel, F.; Boutet-Robinet, E.; Le Ferrec, E.; Sparfel, L. Genome-Wide Transcriptional and Functional Analysis of Human T Lymphocytes Treated with Benzo[α]pyrene. Int. J. Mol. Sci. 2018, 19, 3626. [Google Scholar] [CrossRef]
- Gall, S.; Huynh, Q.L.; Magnussen, C.G.; Juonala, M.; Viikari, J.S.; Kähönen, M.; Dwyer, T.; Raitakari, O.T.; Venn, A. Exposure to parental smoking in childhood or adolescence is associated with increased carotid intima-media thickness in young adults: Evidence from the Cardiovascular Risk in Young Finns study and the Childhood Determinants of Adult Health Study. Eur. Hear. J. 2014, 35, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Narciso, M.G.; Nasimuzzaman, M. Purification of Platelets from Mouse Blood. J. Vis. Exp. 2019, 147, e59803. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Croft, D.; O’Kelly, G.; Wu, G.; Haw, R.; Gillespie, M.; Matthews, L.; Caudy, M.; Garapati, P.; Gopinath, G.; Jassal, B.; et al. Reactome: A database of reactions, pathways and biological processes. Nucleic Acids Res. 2011, 39, D691–D697. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA–target interactions along with their disease and drug associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Wiegers, T.C.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Mattingly, C.J. Comparative Toxicogenomics Database (CTD): Update 2021. Nucleic Acids Res. 2021, 49, D1138–D1143. [Google Scholar] [CrossRef]

| Category | Term | FDR |
|---|---|---|
| REACTOME_PATHWAY | R-MMU-168256~Immune System | 1.9 × 10−5 |
| REACTOME_PATHWAY | R-MMU-109582~Hemostasis | 1.9 × 10−5 |
| REACTOME_PATHWAY | R-MMU-2262752~Cellular responses to stress | 1.9 × 10−5 |
| REACTOME_PATHWAY | R-MMU-8953897~Cellular responses to stimuli | 1.9 × 10−5 |
| REACTOME_PATHWAY | R-MMU-76002~Platelet activation, signaling and aggregation | 1.0 × 10−4 |
| REACTOME_PATHWAY | R-MMU-9716542~Signaling by Rho GTPases, Miro GTPases and RHOBTB3 | 6.3 × 10−4 |
| REACTOME_PATHWAY | R-MMU-194315~Signaling by Rho GTPases | 6.4 × 10−4 |
| REACTOME_PATHWAY | R-MMU-9711123~Cellular response to chemical stress | 1.1 × 10−3 |
| REACTOME_PATHWAY | R-MMU-9006925~Intracellular signaling by second messengers | 1.9 × 10−3 |
| REACTOME_PATHWAY | R-MMU-1257604~PIP3 activates AKT signaling | 5.8 × 10−3 |
| REACTOME_PATHWAY | R-MMU-5683057~MAPK family signaling cascades | 1.5 × 10−2 |
| REACTOME_PATHWAY | R-MMU-114604~GPVI-mediated activation cascade | 3.2 × 10−2 |
| REACTOME_PATHWAY | R-MMU-9012999~RHO GTPase cycle | 3.2 × 10−2 |
| REACTOME_PATHWAY | R-MMU-5673001~RAF/MAP kinase cascade | 3.6 × 10−2 |
| REACTOME_PATHWAY | R-MMU-76005~Response to elevated platelet cytosolic Ca2+ | 4.3 × 10−2 |
| REACTOME_PATHWAY | R-MMU-5684996~MAPK1/MAPK3 signaling | 4.5 × 10−2 |
| REACTOME_PATHWAY | R-MMU-2029482~Regulation of actin dynamics for phagocytic cup formation | 5.1 × 10−2 |
| REACTOME_PATHWAY | R-MMU-199418~Negative regulation of the PI3K/AKT network | 5.9 × 10−2 |
| REACTOME_PATHWAY | R-MMU-5675221~Negative regulation of MAPK pathway | 7.4 × 10−2 |
| REACTOME_PATHWAY | R-MMU-983231~Factors involved in megakaryocyte development and platelet production | 8.4 × 10−2 |
| REACTOME_PATHWAY | R-MMU-195258~RHO GTPase Effectors | 8.7 × 10−2 |
| REACTOME_PATHWAY | R-MMU-114608~Platelet degranulation | 1.1 × 10−1 |
| # | Gene ID | RNA SEQ | Q-PCR | |||||
|---|---|---|---|---|---|---|---|---|
| Log2FC | Trend | p Value | Log2FC | Trend | p Value | SIG | ||
| 1 | PLD1 | 2.25 | UP | 0.0107 | 2.14 | UP | 0.00022 | S |
| 2 | MEOX2 | 1.89 | UP | 0.0316 | 1.00 | UP | 0.07618 | NS ↑ |
| 3 | EPAS1 | 1.09 | UP | 0.0021 | 0.72 | UP | 0.07022 | NS ↑ |
| 4 | ABLIM1 | 1.18 | UP | 0.0187 | 0.75 | UP | 0.05965 | NS ↑ |
| 5 | CNN3 | 1.03 | UP | 0.0267 | 1.45 | UP | 0.01718 | S |
| 6 | VIM | 0.98 | UP | 0.0065 | 1.08 | UP | 0.00311 | S |
| 7 | ARHGEF15 | 0.92 | UP | 0.0137 | 1.65 | UP | 0.01669 | S |
| 8 | CXCL12 | 0.62 | UP | 0.0037 | 1.26 | UP | 0.00109 | S |
| 9 | GDE1 | 0.50 | UP | 0.0448 | 0.53 | UP | 0.01594 | S |
| 10 | PTEN | −0.74 | Down | 0.0114 | −0.89 | Down | 0.00118 | S |
| 11 | KLF6 | −0.80 | Down | 0.0046 | −0.88 | Down | 0.00321 | S |
| 12 | TRAF5 | −1.32 | Down | 0.0130 | −1.57 | Down | 0.00305 | S |
| 13 | GPX1 | −1.62 | Down | 0.0037 | −1.67 | Down | 0.00130 | S |
| 14 | TFRC | −1.82 | Down | 0.0326 | −1.87 | Down | 0.00104 | S |
| 15 | UCP2 | −2.59 | Down | 0.0042 | −2.05 | Down | 0.00030 | S |
| 16 | CD82 | −2.76 | Down | 0.0139 | −0.25 | Down | 0.26002 | NS |
| 17 | TMOD1 | −3.32 | Down | 0.0174 | −2.33 | Down | 0.00916 | S |
| 18 | TSPAN33 | −3.39 | Down | 0.0097 | −3.27 | Down | 0.00024 | S |
| # | Gene ID | RNA SEQ | Q-PCR | |||||
|---|---|---|---|---|---|---|---|---|
| Log2FC | Trend | p Value | Log2FC | Trend | p Value | SIG | ||
| 1 | mmu-miR-320-3p | 0.51 | UP | 0.0132 | 0.96 | UP | 0.0205 | S |
| 2 | mmu-miR-674-5p | 0.61 | UP | 0.0313 | 1.26 | UP | 0.00326 | S |
| 3 | mmu-miR-7a-5p | −0.73 | Down | 0.0227 | −1.46 | Down | 0.0027 | S |
| 4 | mmu-miR-30a-5p | −1.03 | Down | 0.0061 | −1.15 | Down | 0.0713 | NS ↓ |
| 5 | mmu-miR-30e-5p | −0.70 | Down | 0.0061 | −1.10 | Down | 0.1053 | NS ↓ |
| 6 | mmu-miR-33-5p | −0.94 | Down | 0.0130 | −1.70 | Down | 0.0146 | S |
| 7 | mmu-miR-144-3p | −4.69 | Down | 0.0002 | −3.96 | Down | 1.6 × 10−6 | S |
| 8 | mmu-miR-144-5p | −3.53 | Down | 0.0031 | −3.24 | Down | 2.7 × 10−5 | S |
| 9 | mmu-miR-486a-3p | −2.79 | Down | 0.0061 | −1.53 | Down | 0.0015 | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, H.E.A.; Alarabi, A.B.; Alshbool, F.Z.; Khasawneh, F.T. Alterations in the Platelet Transcriptome Mediate Prenatal Thirdhand Smoke Exposure Associated Thrombogenicity via Integrated miRNA-mRNA Regulatory Networks. Int. J. Mol. Sci. 2025, 26, 7633. https://doi.org/10.3390/ijms26157633
Ali HEA, Alarabi AB, Alshbool FZ, Khasawneh FT. Alterations in the Platelet Transcriptome Mediate Prenatal Thirdhand Smoke Exposure Associated Thrombogenicity via Integrated miRNA-mRNA Regulatory Networks. International Journal of Molecular Sciences. 2025; 26(15):7633. https://doi.org/10.3390/ijms26157633
Chicago/Turabian StyleAli, Hamdy E. A., Ahmed B. Alarabi, Fatima Z. Alshbool, and Fadi T. Khasawneh. 2025. "Alterations in the Platelet Transcriptome Mediate Prenatal Thirdhand Smoke Exposure Associated Thrombogenicity via Integrated miRNA-mRNA Regulatory Networks" International Journal of Molecular Sciences 26, no. 15: 7633. https://doi.org/10.3390/ijms26157633
APA StyleAli, H. E. A., Alarabi, A. B., Alshbool, F. Z., & Khasawneh, F. T. (2025). Alterations in the Platelet Transcriptome Mediate Prenatal Thirdhand Smoke Exposure Associated Thrombogenicity via Integrated miRNA-mRNA Regulatory Networks. International Journal of Molecular Sciences, 26(15), 7633. https://doi.org/10.3390/ijms26157633





