GM1 Oligosaccharide Modulates Microglial Activation and α-Synuclein Clearance in a Human In Vitro Model
Abstract
1. Introduction
2. Results
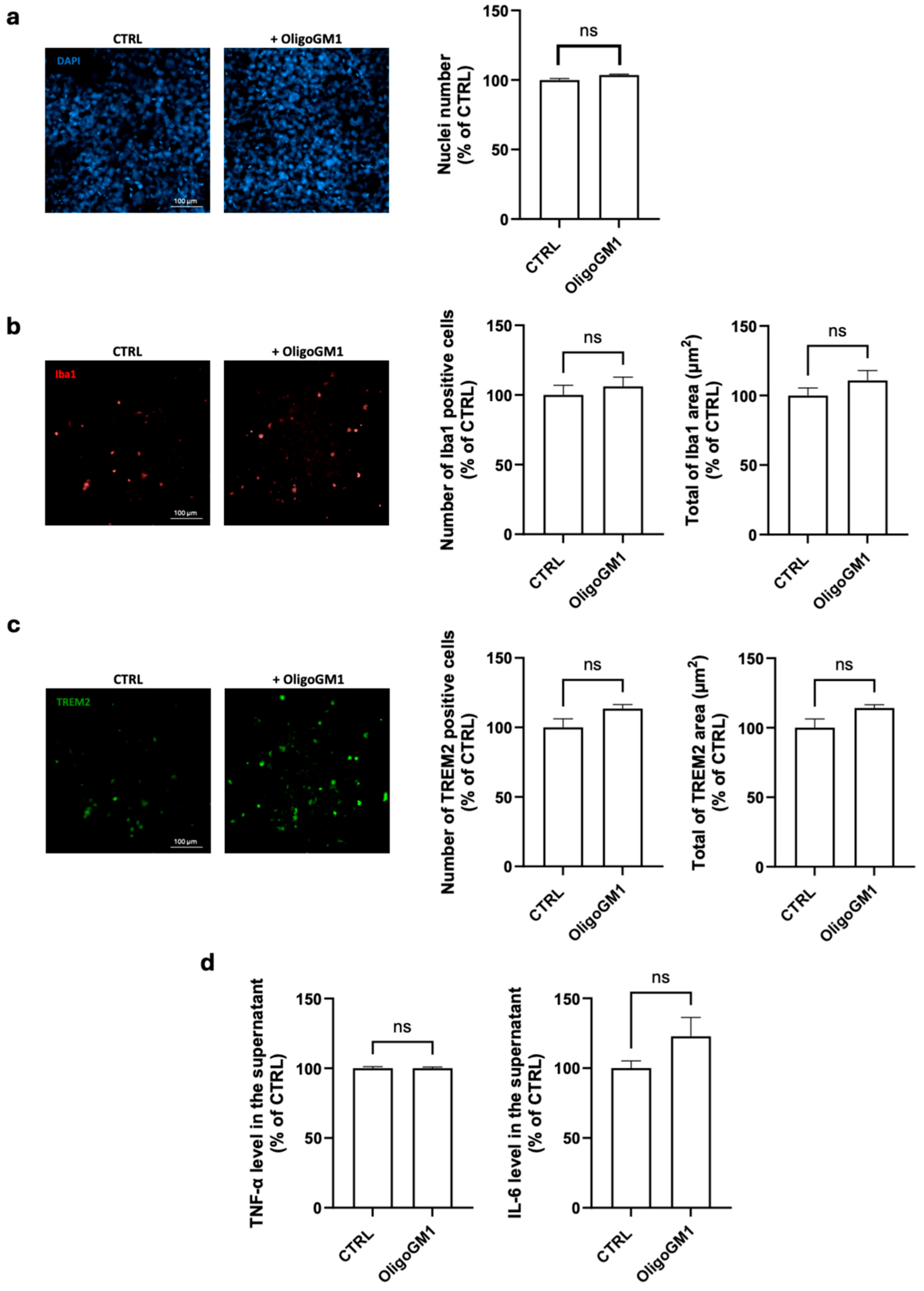
2.1. Maintenance of Microglial Resting Conditions upon OligoGM1 Treatment
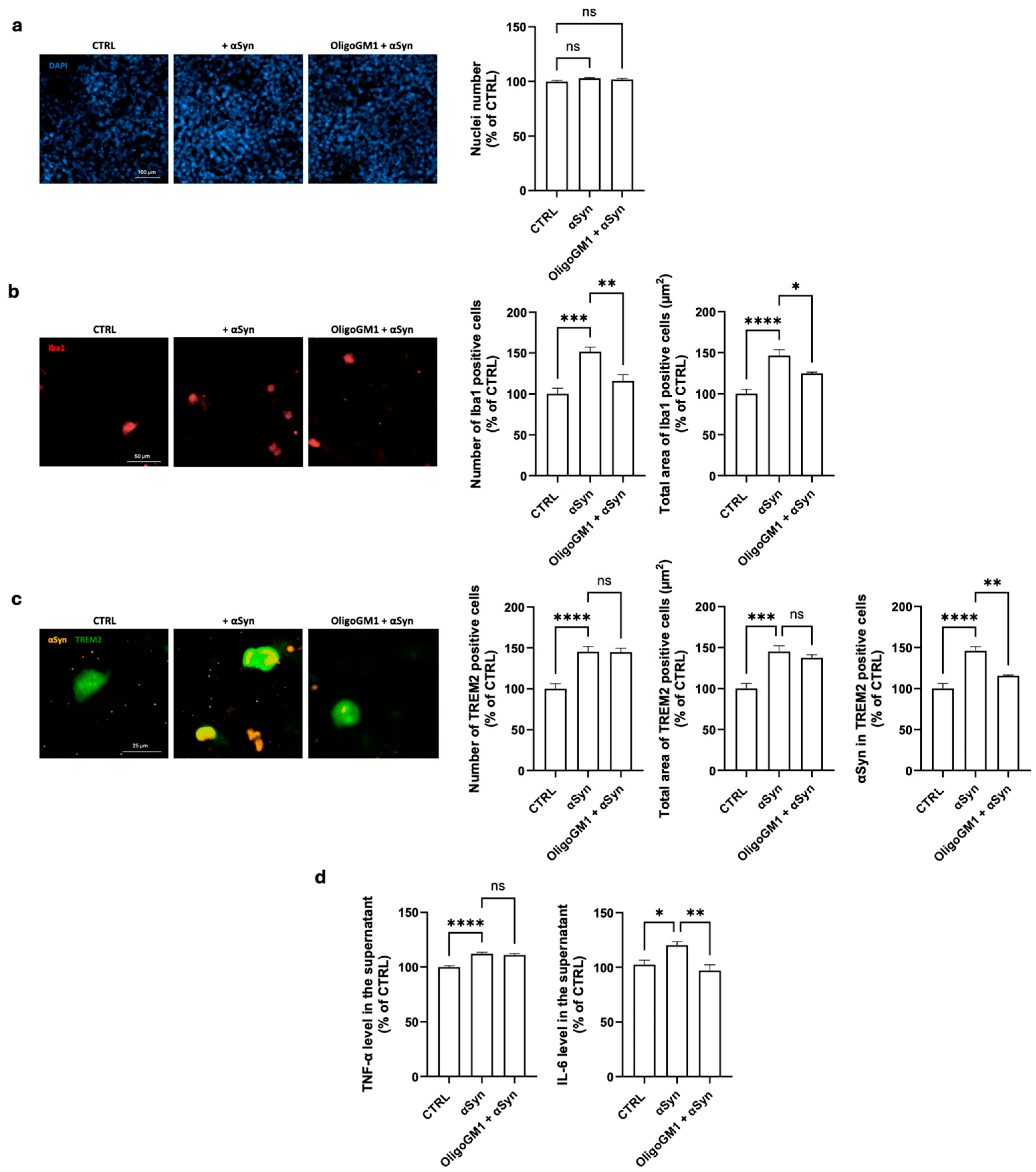
2.2. Protective Effects of OligoGM1 on αSyn-Induced Microglia Activation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Antibodies
4.3. Culture of HMC3 Cell Line
4.4. Cell Treatments
4.5. Cell Immunostaining
4.6. Quantification of TNF-α and IL-6
4.7. OligoGM1 Preparation
4.8. αSyn Preparation
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ganglioside nomenclature is in accordance with IUPAC-IUBB recommendations [41] | |
| BSA | bovine serum albumin |
| CTRL | control |
| EDTA | ethylenediaminetetraacetic acid |
| EMEM | Eagle’s minimum essential medium |
| FBS | fetal bovine serum |
| GM1 | II3Neu5Ac-GG4Cer, β-Gal-(1-3)-β-GalNac-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal-(1-4)-β-Glc-Cer |
| HMC3 | human embryonic microglial cells |
| HPTLC | high-performance thin layer chromatography |
| Iba1 | ionized calcium-binding adaptor molecule 1 |
| IL-6 | interleukin 6 |
| NaCl | sodium chloride |
| ns | not significative |
| OligoGM1 | GM1-oligosaccharide, II3Neu5Ac-GG4, β-Gal-(1-3)-β-GalNac-(1-4)-[α-Neu5Ac-(2-3)]-β-Gal-(1-4)-Glc |
| PBS | phosphate-buffered saline |
| PCR | polymerase chain reaction |
| PD | Parkinson’s disease |
| PFA | paraformaldehyde |
| PFFs | pre-formed fibrils |
| RRID | research resource identifier |
| SDS | sodium dodecyl sulfate |
| SEM | standard error of the mean |
| αSyn | α-synuclein |
| TNF-α | tumor necrosis factor alpha |
| TREM2 | triggering receptor expressed on myeloid cells 2 |
References
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S. GM1 Ganglioside as a Disease-Modifying Therapeutic for Parkinson’s Disease: A Multi-Functional Glycosphingolipid That Targets Multiple Parkinson’s Disease-Relevant Pathogenic Mechanisms. Int. J. Mol. Sci. 2023, 24, 9183. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R.; Brundin, P.; Langston, J.W. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Schneider, J.S.; Singh, G.; Williams, C.K.; Singh, V. GM1 ganglioside modifies microglial and neuroinflammatory responses to alpha-synuclein in the rat AAV-A53T alpha-synuclein model of Parkinson’s disease. Mol. Cell. Neurosci. 2022, 120, 103729. [Google Scholar] [CrossRef]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef]
- Badanjak, K.; Fixemer, S.; Smajić, S.; Skupin, A.; Grünewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Putnam, G.L.; Maitta, R.W. Alpha synuclein and inflammaging. Heliyon 2025, 11, e41981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, H.; Feng, J. Emerging role of microglia in inter-cellular transmission of alpha-synuclein in Parkinson’s disease. Front. Aging Neurosci. 2024, 16, 1411104. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Y.; Xie, X.X.; Liu, R.T. The Role of alpha-Synuclein Oligomers in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8645. [Google Scholar] [CrossRef]
- Guo, Z. Ganglioside GM1 and the Central Nervous System. Int. J. Mol. Sci. 2023, 24, 9558. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, E.; Di Biase, E.; Lunghi, G.; Fazzari, M.; Loberto, N.; Aureli, M.; Mauri, L.; Sonnino, S. Turning the spotlight on the oligosaccharide chain of GM1 ganglioside. Glycoconj. J. 2021, 38, 101–117. [Google Scholar] [CrossRef]
- Lunghi, G.; Pedroli, C.; Tagliabue, I.; Dobi, D.; Ciampa, M.G.; Mauri, L.; Rouvière, L.; Henriques, A.; Callizot, N.; Sonnino, S.; et al. GM1 oligosaccharide-mediated rescue in GBA-linked Parkinson’s disease via modulation of lysosomal and mitochondrial dysfunctions. Glycoconj. J. 2025. [Google Scholar] [CrossRef] [PubMed]
- Fazzari, M.; Di Biase, E.; Zaccagnini, L.; Henriques, A.; Callizot, N.; Ciampa, M.G.; Mauri, L.; Carsana, E.V.; Loberto, N.; Aureli, M.; et al. GM1 oligosaccharide efficacy against alpha-synuclein aggregation and toxicity in vitro. Biochim Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159350. [Google Scholar]
- Fazzari, M.; Lunghi, G.; Henriques, A.; Callizot, N.; Ciampa, M.G.; Mauri, L.; Prioni, S.; Carsana, E.V.; Loberto, N.; Aureli, M.; et al. GM1 Oligosaccharide Efficacy in Parkinson’s Disease: Protection against MPTP. Biomedicines 2023, 11, 1305. [Google Scholar] [CrossRef]
- Abou-Sleiman, P.M.; Muqit, M.M.; Wood, N.W. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat. Rev. Neurosci. 2006, 7, 207–219. [Google Scholar] [CrossRef]
- Dello Russo, C.; Cappoli, N.; Coletta, I.; Mezzogori, D.; Paciello, F.; Pozzoli, G.; Navarra, P.; Battaglia, A. The human microglial HMC3 cell line: Where do we stand? A systematic literature review. J. Neuroinflamm. 2018, 15, 259. [Google Scholar] [CrossRef]
- Janabi, N.; Peudenier, S.; Héron, B.; Ng, K.H.; Tardieu, M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci. Lett. 1995, 195, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.; Rouvière, L.; Giorla, E.; Farrugia, C.; El Waly, B.; Poindron, P.; Callizot, N. Alpha-Synuclein: The Spark That Flames Dopaminergic Neurons, In Vitro and In Vivo Evidence. Int. J. Mol. Sci. 2022, 23, 9864. [Google Scholar] [CrossRef]
- Ballabio, A.; Gieselmann, V. Lysosomal disorders: From storage to cellular damage. Biochim. Biophys. Acta 2009, 1793, 684–696. [Google Scholar] [CrossRef]
- Nixon, R.A. The role of autophagy in neurodegenerative disease. Nat. Med. 2013, 19, 983–997. [Google Scholar] [CrossRef]
- Nixon, R.A.; Yang, D.S.; Lee, J.H. Neurodegenerative lysosomal disorders: A continuum from development to late age. Autophagy 2008, 4, 590–599. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.-S.; et al. Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005, 19, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wei, X.; Yan, H.; Qin, Y.; Yan, S.; Liu, J.; Zhao, Y.; Jiang, F.; Lou, H. TREM2 deficiency aggravates alpha-synuclein-induced neurodegeneration and neuroinflammation in Parkinson’s disease models. FASEB J. 2019, 33, 12164–12174. [Google Scholar] [CrossRef]
- Cui, W.; Sun, C.; Ma, Y.; Wang, S.; Wang, X.; Zhang, Y. Inhibition of TLR4 Induces M2 Microglial Polarization and Provides Neuroprotection via the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Neurosci. 2020, 14, 444. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Feng, S.; Nie, K.; Li, Y.; Gao, Y.; Gan, R.; Wang, L.; Li, B.; Sun, X.; et al. TREM2 modulates microglia phenotypes in the neuroinflammation of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2018, 499, 797–802. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Maggioni, M.; DI Biase, E.; Lunghi, G.; Fazzari, M.; Loberto, N.; Elisa, M.; Scalvini Grassi, F.; Tedeschi, G.; Sonnino, S. The Neuroprotective Role of the GM1 Oligosaccharide, II(3)Neu5Ac-Gg4, in Neuroblastoma Cells. Mol. Neurobiol. 2019, 56, 6673–6702. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, E.; Chiricozzi, E.; Mauri, L.; Lunghi, G.; Di Biase, E.; Fazzari, M.; Maggioni, M.; Valsecchi, M.; Prioni, S.; Loberto, N.; et al. Parkinson’s disease recovery by GM1 oligosaccharide treatment in the B4galnt1(+/−) mouse model. Sci. Rep. 2019, 9, 19330. [Google Scholar] [CrossRef] [PubMed]
- Koronyo-Hamaoui, M.; Gaire, B.P.; Frautschy, S.A.; Alvarez, J.I. Editorial: Role of Inflammation in Neurodegenerative Diseases. Front. Immunol. 2022, 13, 958487. [Google Scholar] [CrossRef]
- Shi, Q.; Gutierrez, R.A.; Bhat, M.A. Microglia, Trem2, and Neurodegeneration. Neuroscientist 2025, 31, 159–176. [Google Scholar] [CrossRef]
- Rayaprolu, S.; Rayaprolu, S.; Mullen, B.; Baker, M.; Lynch, T.; Finger, E.; Seeley, W.W.; Hatanpaa, K.J.; Lomen-Hoerth, C.; Kertesz, A.; et al. TREM2 in neurodegeneration: Evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol. Neurodegener. 2013, 8, 19. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, H.; Zhang, J.; Huang, K.; Chen, Y.; Jing, X.; Tao, E. alpha-Synuclein Induces Neuroinflammation Injury through the IL6ST-AS/STAT3/HIF-1alpha Axis. Int. J. Mol. Sci. 2023, 24, 1436. [Google Scholar] [CrossRef]
- Lindberg, C.; Hjorth, E.; Post, C.; Winblad, B.; Schultzberg, M. Cytokine production by a human microglial cell line: Effects of beta-amyloid and alpha-melanocyte-stimulating hormone. Neurotox. Res. 2005, 8, 267–276. [Google Scholar] [CrossRef]
- Ambrosius, B.; Faissner, S.; Guse, K.; von Lehe, M.; Grunwald, T.; Gold, R.; Grewe, B.; Chan, A. Teriflunomide and monomethylfumarate target HIV-induced neuroinflammation and neurotoxicity. J. Neuroinflamm. 2017, 14, 51. [Google Scholar] [CrossRef]
- Rajalakshmy, A.R.; Malathi, J.; Madhavan, H.N.; Srinivasan, B.; Iyer, G.K. Hepatitis C virus core and NS3 antigens induced conjunctival inflammation via toll-like receptor-mediated signaling. Mol. Vis. 2014, 20, 1388–1397. [Google Scholar]
- Hjorth, E.; Zhu, M.; Toro, V.C.; Vedin, I.; Palmblad, J.; Cederholm, T.; Freund-Levi, Y.; Faxen-Irving, G.; Wahlund, L.-O.; Basun, H.; et al. Omega-3 fatty acids enhance phagocytosis of Alzheimer’s disease-related amyloid-beta42 by human microglia and decrease inflammatory markers. J. Alzheimer’s Dis. 2013, 35, 697–713. [Google Scholar] [CrossRef]
- Liu, J.; Hjorth, E.; Zhu, M.; Calzarossa, C.; Samuelsson, E.; Schultzberg, M.; Åkesson, E. Interplay between human microglia and neural stem/progenitor cells in an allogeneic co-culture model. J. Cell. Mol. Med. 2013, 17, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Akhter, R.; Shao, Y.; Formica, S.; Khrestian, M.; Bekris, L.M. TREM2 alters the phagocytic, apoptotic and inflammatory response to Abeta(42) in HMC3 cells. Mol. Immunol. 2021, 131, 171–179. [Google Scholar] [CrossRef]
- Lawrimore, C.J.; Coleman, L.G.; Zou, J.; Crews, F.T. Ethanol Induction of Innate Immune Signals Across BV2 Microglia and SH-SY5Y Neuroblastoma Involves Induction of IL-4 and IL-13. Brain Sci. 2019, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Lunghi, G.; Fazzari, M.; Di Biase, E.; Mauri, L.; Sonnino, S.; Chiricozzi, E. Modulation of calcium signaling depends on the oligosaccharide of GM1 in Neuro2a mouse neuroblastoma cells. Glycoconj. J. 2020, 37, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Chester, M.A. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids--recommendations 1997. Eur. J. Biochem. 1998, 257, 293–298. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunghi, G.; Pedroli, C.; Ciampa, M.G.; Mauri, L.; Rouvière, L.; Henriques, A.; Callizot, N.; Savino, B.; Fazzari, M. GM1 Oligosaccharide Modulates Microglial Activation and α-Synuclein Clearance in a Human In Vitro Model. Int. J. Mol. Sci. 2025, 26, 7634. https://doi.org/10.3390/ijms26157634
Lunghi G, Pedroli C, Ciampa MG, Mauri L, Rouvière L, Henriques A, Callizot N, Savino B, Fazzari M. GM1 Oligosaccharide Modulates Microglial Activation and α-Synuclein Clearance in a Human In Vitro Model. International Journal of Molecular Sciences. 2025; 26(15):7634. https://doi.org/10.3390/ijms26157634
Chicago/Turabian StyleLunghi, Giulia, Carola Pedroli, Maria Grazia Ciampa, Laura Mauri, Laura Rouvière, Alexandre Henriques, Noelle Callizot, Benedetta Savino, and Maria Fazzari. 2025. "GM1 Oligosaccharide Modulates Microglial Activation and α-Synuclein Clearance in a Human In Vitro Model" International Journal of Molecular Sciences 26, no. 15: 7634. https://doi.org/10.3390/ijms26157634
APA StyleLunghi, G., Pedroli, C., Ciampa, M. G., Mauri, L., Rouvière, L., Henriques, A., Callizot, N., Savino, B., & Fazzari, M. (2025). GM1 Oligosaccharide Modulates Microglial Activation and α-Synuclein Clearance in a Human In Vitro Model. International Journal of Molecular Sciences, 26(15), 7634. https://doi.org/10.3390/ijms26157634






