Molecular Links Between Metabolism and Mental Health: Integrative Pathways from GDF15-Mediated Stress Signaling to Brain Energy Homeostasis
Abstract
1. Introduction
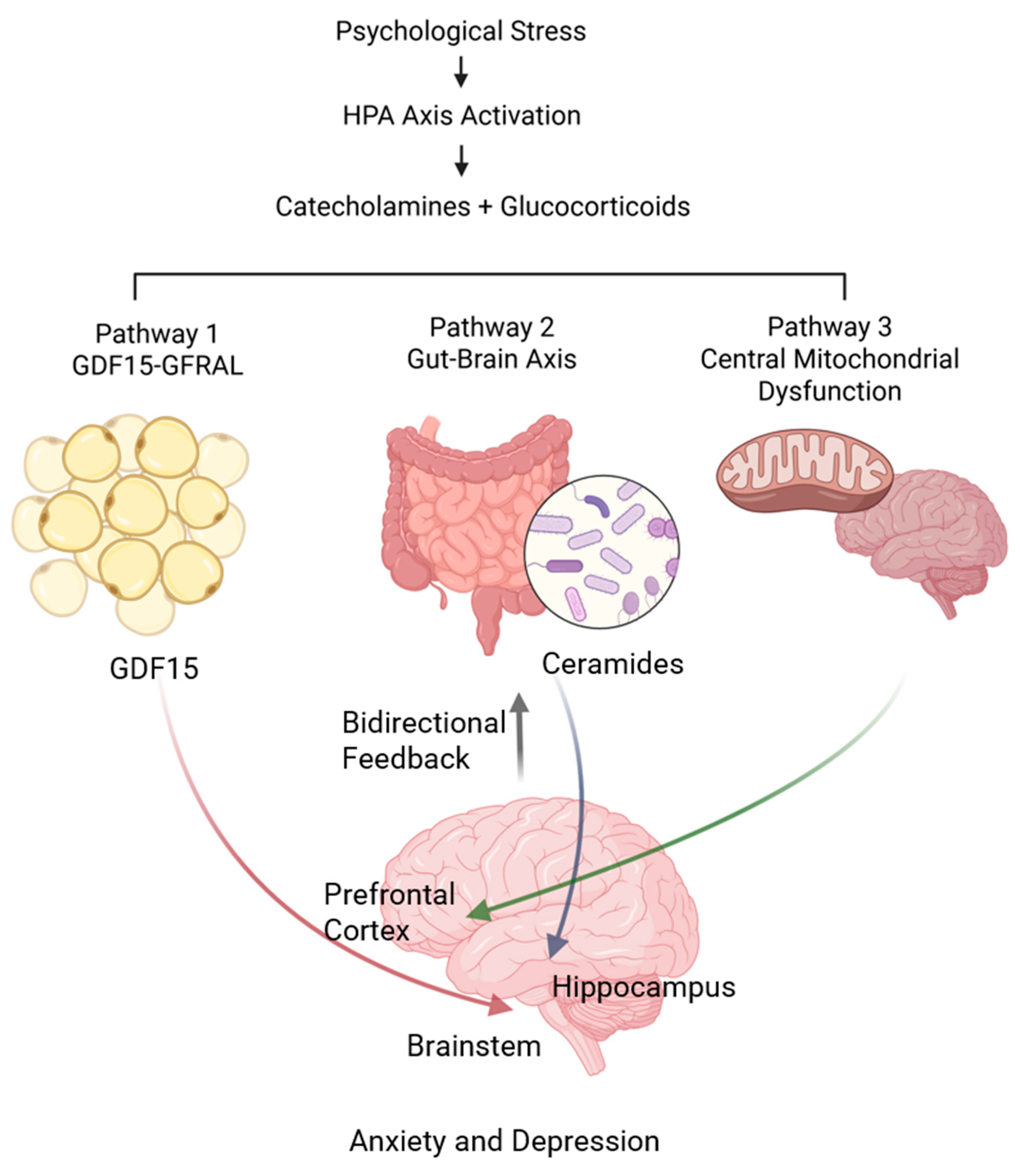
2. Integrative Model: Convergent Pathways Linking Metabolism and Mental Health
2.1. Conceptual Framework
2.1.1. Primary Pathway: Stress-Induced GDF15 Signaling
2.1.2. Secondary Pathway: Gut–Brain Axis Dysregulation
2.1.3. Tertiary Pathway: Central Mitochondrial Dysfunction
2.2. Pathway Integration and Bidirectional Feedback Mechanisms
3. Peripheral Stress Signaling: The GDF15-GFRAL Pathway
3.1. Stress-Induced Hormonal Cascades and Metabolic Signaling
3.2. GDF15: A Dynamic Biomarker of Energetic Stress
3.3. Mechanistic Insights: From Lipolysis to GDF15 Production
3.4. GDF15-GFRAL Signaling and Behavioral Regulation
3.5. Clinical Implications and Therapeutic Potential
4. Central Energy Metabolism and Mitochondrial Function
4.1. Brain Energy Requirements and Neuronal Vulnerability
4.2. Mitochondrial Dynamics and Stress Vulnerability
4.3. Mitophagy and Quality Control in Mental Health
4.4. Clinical Evidence for Mitochondrial Dysfunction in Mental Health
4.5. Therapeutic Targeting of Mitochondrial Function
5. Gut–Brain Axis: Microbiota-Mediated Metabolic Signaling
5.1. Microbiota Composition and Metabolite Production
5.2. Ceramides: A Critical Link Between Gut Dysbiosis and Depression
5.3. Therapeutic Targeting of the Gut–Brain Axis
6. Sex Differences, Age-Related Changes, and Genetic Modulation
6.1. Sexual Dimorphism in Metabolic–Psychiatric Connections
6.1.1. Sex-Specific Stress Response Patterns
Acute vs. Chronic Stress Responses
Hormonal and Neural Mechanisms
6.1.2. Metabolic–Psychiatric Comorbidity Patterns
Clinical Manifestations
Underlying Mechanisms
6.1.3. Mitochondrial Function and Sex-Specific Responses
6.2. Age-Related Changes in Metabolism–Mental Health Connections
6.2.1. Peripheral Stress Signaling and Aging
6.2.2. Mitochondrial Function and Aging
6.2.3. Social and Environmental Aging Effects
6.3. Genetic Modulation of Pathway Function
6.3.1. Genetic Variants in Stress-Related Pathways
Stress Resilience Genetics
6.3.2. Mitochondrial Genetic Variants
6.3.3. Metabolic Syndrome Genetics
6.4. Environmental Modulation of Metabolic–Psychiatric Connections
6.4.1. Socioeconomic Factors
Stress Hormone Regulation
Developmental Effects
6.4.2. Social and Work Environment
Work–Life Balance
Chronic Social Stress
6.4.3. Epigenetic Environmental Effects
Gene–Environment Interactions
6.5. Integrative Framework: Multi-Level Interactions
7. Clinical Implications and Therapeutic Strategies
7.1. Novel Therapeutic Modalities
7.2. Precision Medicine Applications
7.3. Biomarker Development and Clinical Translation
8. Future Research Directions and Clinical Translation
8.1. Advanced Biomarker Development
8.2. Technology Integration and Digital Health
9. Limitations and Critical Considerations
9.1. Translational Challenges
9.2. Methodological Considerations
9.3. Ethical and Safety Considerations
10. Conclusions and Clinical Implications
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| eCB | Endocannabinoid |
| HPA | Hypothalamic–pituitary–adrenal |
| mPFC | Medial prefrontal cortex |
| UCMS | Unpredictable chronic mild stress |
References
- Arnold, M.; Buyukozkan, M.; Doraiswamy, P.M.; Nho, K.; Wu, T.; Gudnason, V.; Launer, L.J.; Wang-Sattler, R.; Adamski, J.; Alzheimer’s Disease Neuroimaging, I.; et al. Individual bioenergetic capacity as a potential source of resilience to Alzheimer’s disease. medRxiv 2024. [Google Scholar] [CrossRef]
- Mokhtari, A.; Ibrahim, E.C.; Gloaguen, A.; Barrot, C.C.; Cohen, D.; Derouin, M.; Vachon, H.; Charbonnier, G.; Loriod, B.; Decraene, C.; et al. Using multiomic integration to improve blood biomarkers of major depressive disorder: A case-control study. EBioMedicine 2025, 113, 105569. [Google Scholar] [CrossRef]
- Picard, M.; McManus, M.J.; Gray, J.D.; Nasca, C.; Moffat, C.; Kopinski, P.K.; Seifert, E.L.; McEwen, B.S.; Wallace, D.C. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. Proc. Natl. Acad. Sci. USA 2015, 112, E6614–E6623. [Google Scholar] [CrossRef]
- Hollis, F.; van der Kooij, M.A.; Zanoletti, O.; Lozano, L.; Canto, C.; Sandi, C. Mitochondrial function in the brain links anxiety with social subordination. Proc. Natl. Acad. Sci. USA 2015, 112, 15486–15491. [Google Scholar] [CrossRef]
- Crosswell, A.D.; Mayer, S.E.; Whitehurst, L.N.; Picard, M.; Zebarjadian, S.; Epel, E.S. Deep rest: An integrative model of how contemplative practices combat stress and enhance the body’s restorative capacity. Psychol. Rev. 2024, 131, 247–270. [Google Scholar] [CrossRef]
- Maitra, M.; Mitsuhashi, H.; Rahimian, R.; Chawla, A.; Yang, J.; Fiori, L.M.; Davoli, M.A.; Perlman, K.; Aouabed, Z.; Mash, D.C.; et al. Cell type specific transcriptomic differences in depression show similar patterns between males and females but implicate distinct cell types and genes. Nat. Commun. 2023, 14, 2912. [Google Scholar] [CrossRef]
- Tavenier, J.; Rasmussen, L.J.H.; Andersen, A.L.; Houlind, M.B.; Langkilde, A.; Andersen, O.; Petersen, J.; Nehlin, J.O. Association of GDF15 with Inflammation and Physical Function During Aging and Recovery After Acute Hospitalization: A Longitudinal Study of Older Patients and Age-Matched Controls. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 964–974. [Google Scholar] [CrossRef] [PubMed]
- van Rensburg, D.J.; Lindeque, Z.; Harvey, B.H.; Steyn, S.F. Ndufs4 KO mice: A model to study comorbid mood disorders associated with mitochondrial dysfunction. Pharmacol. Biochem. Behav. 2024, 234, 173689. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Nie, L.; Ali, T.; Liu, Z.; Li, W.; Gao, R.; Zhang, Z.; Liu, J.; Dai, Z.; Xie, Y.; et al. Adiponectin deficiency accelerates brain aging via mitochondria-associated neuroinflammation. Immun. Ageing 2023, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.K.; Wang, D.; Knuth, C.M.; Fayyazi, R.; Mohammad, A.; Becker, L.J.; Tsakiridis, E.E.; Desjardins, E.M.; Patel, Z.; Valvano, C.M.; et al. GDF15 links adipose tissue lipolysis with anxiety. Nat. Metab. 2025, 7, 1004–1017. [Google Scholar] [CrossRef]
- Huang, Q.; Monzel, A.S.; Rausser, S.; Haahr, R.; Indik, C.E.; Savin, M.J.; Bobba-Alves, N.; Liu, C.C.; Devine, J.; Thompson, E.; et al. The energetic stress cytokine GDF15 is elevated in the context of chronic and acute psychosocial stress. bioRxiv 2025. [Google Scholar] [CrossRef]
- Liu, C.C.; Trumpff, C.; Huang, Q.; Juster, R.P.; Picard, M. Biopsychosocial Correlates of Resting and Stress-Reactive Salivary GDF15: Preliminary Findings. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, L.; Li, S.; Zhang, M.; Li, Y.; Duan, J.; Li, Y.; Hu, Z.; Wu, J.; Ni, J.; et al. Gut microbiota dysbiosis-mediated ceramides elevation contributes to corticosterone-induced depression by impairing mitochondrial function. NPJ Biofilms Microbiomes 2024, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Wu, P.F.; He, J.G.; Li, Y.K.; Long, L.H.; Yao, X.P.; Yang, J.H.; Chen, H.S.; Zhang, X.N.; Hu, Z.L.; et al. BNIP3L/NIX-mediated mitophagy alleviates passive stress-coping behaviors induced by tumor necrosis factor-alpha. Mol. Psychiatry 2023, 28, 5062–5076. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Xu, B.; Li, S.; Qiu, Y.; Chen, J.; Wu, H.; Cai, H. Bioenergetic biomarkers as predictive indicators and their relationship with cognitive function in newly diagnosed, drug-naive patients with bipolar disorder. Transl. Psychiatry 2025, 15, 148. [Google Scholar] [CrossRef]
- Igual Gil, C.; Coull, B.M.; Jonas, W.; Lippert, R.N.; Klaus, S.; Ost, M. Mitochondrial stress-induced GFRAL signaling controls diurnal food intake and anxiety-like behavior. Life Sci. Alliance 2022, 5, e202201495. [Google Scholar] [CrossRef]
- Cao, Y.; Fan, X.; Zang, T.; Li, Y.; Tu, Y.; Wei, Y.; Bai, J.; Liu, Y. Gut microbiota causes depressive phenotype by modulating glycerophospholipid and sphingolipid metabolism via the gut-brain axis. Psychiatry Res. 2025, 346, 116392. [Google Scholar] [CrossRef]
- Dong, W.T.; Long, L.H.; Deng, Q.; Liu, D.; Wang, J.L.; Wang, F.; Chen, J.G. Mitochondrial fission drives neuronal metabolic burden to promote stress susceptibility in male mice. Nat. Metab. 2023, 5, 2220–2236. [Google Scholar] [CrossRef]
- Du, N.; Xie, Y.; Geng, D.; Li, J.; Xu, H.; Wang, Y.; Gou, J.; Tan, X.; Xu, X.; Shi, L.; et al. Restoration of mitochondrial energy metabolism by electroconvulsive therapy in adolescent and juvenile mice. Front. Psychiatry 2025, 16, 1555144. [Google Scholar] [CrossRef]
- de Camargo, R.W.; Joaquim, L.; Machado, R.S.; de Souza Ramos, S.; da Rosa, L.R.; de Novais Junior, L.R.; Mathias, K.; Maximiano, L.; Strickert, Y.R.; Nord, R.; et al. Ayahuasca Pretreatment Prevents Sepsis-Induced Anxiety-Like Behavior, Neuroinflammation, and Oxidative Stress, and Increases Brain-Derived Neurotrophic Factor. Mol. Neurobiol. 2025, 62, 5695–5719. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Jiang, M.; Wang, M.; Ji, M.; Xie, X.; Sheng, H. Chronic stress induces depression-like behavior in rats through affecting brain mitochondrial function and inflammation. Psychoneuroendocrinology 2025, 172, 107261. [Google Scholar] [CrossRef]
- Teratani, T.; Mikami, Y.; Nakamoto, N.; Suzuki, T.; Harada, Y.; Okabayashi, K.; Hagihara, Y.; Taniki, N.; Kohno, K.; Shibata, S.; et al. The liver-brain-gut neural arc maintains the T(reg) cell niche in the gut. Nature 2020, 585, 591–596. [Google Scholar] [CrossRef]
- Ma, X.; Park, H.S.; Shin, Y.J.; Kim, J.K.; Hong, J.K.; Han, S.W.; Yoon, I.Y.; Kim, D.H. The extracellular vesicle of depressive patient-derived Escherichia fergusonii induces vagus nerve-mediated neuroinflammation in mice. J. Neuroinflamm. 2024, 21, 224. [Google Scholar] [CrossRef]
- Leonard, B.; Maes, M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 2012, 36, 764–785. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kan, W.; Zhang, Y.; Wang, T.; Yang, F.; Ji, T.; Wang, G.; Du, J. Quantitative proteomics combined independent PRM analysis reveals the mitochondrial and synaptic mechanism underlying norisoboldine’s antidepressant effects. Transl. Psychiatry 2024, 14, 400. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Shire, D.; Hollis, F.; Abuaish, S.; Picard, M.; Monk, C.; Duman, E.A.; Trumpff, C. Mitochondrial health, prenatal distress, and gestational age: Investigation of cf-mtDNA and GDF15 in two pregnancy studies from the USA and Turkey. Mitochondrion 2025, 84, 102057. [Google Scholar] [CrossRef] [PubMed]
- Mulderrig, L.; Garaycoechea, J.I.; Tuong, Z.K.; Millington, C.L.; Dingler, F.A.; Ferdinand, J.R.; Gaul, L.; Tadross, J.A.; Arends, M.J.; O’Rahilly, S.; et al. Aldehyde-driven transcriptional stress triggers an anorexic DNA damage response. Nature 2021, 600, 158–163. [Google Scholar] [CrossRef]
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K.; et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 2017, 23, 1215–1219. [Google Scholar] [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Yang, L.; Chang, C.C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjaer, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef]
- Worth, A.A.; Shoop, R.; Tye, K.; Feetham, C.H.; D’Agostino, G.; Dodd, G.T.; Reimann, F.; Gribble, F.M.; Beebe, E.C.; Dunbar, J.D.; et al. The cytokine GDF15 signals through a population of brainstem cholecystokinin neurons to mediate anorectic signalling. Elife 2020, 9, e55164. [Google Scholar] [CrossRef] [PubMed]
- Day, E.A.; Ford, R.J.; Smith, B.K.; Mohammadi-Shemirani, P.; Morrow, M.R.; Gutgesell, R.M.; Lu, R.; Raphenya, A.R.; Kabiri, M.; McArthur, A.G.; et al. Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. Nat. Metab. 2019, 1, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Karhunen, V.; Larsson, S.C.; Gill, D. Genetically proxied growth-differentiation factor 15 levels and body mass index. Br. J. Clin. Pharmacol. 2021, 87, 4036–4039. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Mannan, A.; Singh, S.; Singh, T.G. Unlocking the cellular mystery: How proton pump inhibitors may alter the dementia landscape. Brain Res. 2025, 1861, 149702. [Google Scholar] [CrossRef]
- Cao, G.; Chen, B.; Sun, Y.; Qiao, J.; Liu, T.; Hou, J.; Han, X.; Tang, Y.; Fu, Y.; Ye, J.H.; et al. Plasma metabolic profiles in alcohol use disorder: Diagnostic role of arginine and emotional implications of N6-acetyl-lysine and succinic acid. BMC Psychiatry 2025, 25, 563. [Google Scholar] [CrossRef]
- Shil, R.S.K.; Seed, A.; Franklyn, N.E.; Sargent, B.F.; Wood, G.K.; Huang, Y.; Dodd, K.C.; Lilleker, J.B.; Pollak, T.A.; Defres, S.; et al. Patients with neurological or psychiatric complications of COVID-19 have worse long-term functional outcomes: COVID-CNS-A multicentre case-control study. Sci. Rep. 2025, 15, 3443. [Google Scholar] [CrossRef]
- Traa, A.; Keil, A.; AlOkda, A.; Jacob-Tomas, S.; Tamez Gonzalez, A.A.; Zhu, S.; Rudich, Z.; Van Raamsdonk, J.M. Overexpression of mitochondrial fission or mitochondrial fusion genes enhances resilience and extends longevity. Aging Cell 2024, 23, e14262. [Google Scholar] [CrossRef]
- Shekhar, N.; Thakur, A.K. Investigating the Effect of Capric Acid on Antibiotic-Induced Autism-Like Behavior in Rodents. Dev. Neurobiol. 2025, 85, e22959. [Google Scholar] [CrossRef]
- Yang, L.; Ao, Y.; Li, Y.; Dai, B.; Li, J.; Duan, W.; Gao, W.; Zhao, Z.; Han, Z.; Guo, R. Morinda officinalis oligosaccharides mitigate depression-like behaviors in hypertension rats by regulating Mfn2-mediated mitophagy. J. Neuroinflamm. 2023, 20, 31. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Li, J.; Rao, H.; Sun, J.; Xiu, J.; Wu, N. Gypenosides alleviate oxidative stress in the hippocampus, promote mitophagy, and mitigate depressive-like behaviors induced by CUMS via SIRT1. J. Ethnopharmacol. 2025, 337, 118823. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Yu, Z.; Chen, X.; Lan, T.; Wang, M.; Yu, S. Agomelatine Alleviates Depressive-like Behaviors by Suppressing Hippocampal Oxidative Stress in the Chronic Social Defeat Stress Model. Antioxidants 2025, 14, 410. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, C.; Huang, P.; Cheng, Y.; Ma, Y.; Gao, J.; Ding, H. Diosmetin Ameliorates HFD-induced Cognitive Impairments via Inhibiting Metabolic Disorders, Mitochondrial Dysfunction and Neuroinflammation in Male SD Rats. Mol. Neurobiol. 2024, 61, 8069–8085. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, W.; Guo, Q.; Hu, Y.; Hu, H.; Zheng, Y.; Chen, H.; Liu, C.; Tang, X.; Wei, Y.; et al. Attenuated frontotemporal brain activation during cognitive tasks is associated with lower succinate dehydrogenase protein levels in patients with major depressive disorder. J. Affect. Disord. 2024, 363, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cheng, B.; Zhao, Y.; He, D.; Chu, X.; Qin, X.; Zhang, N.; Shi, S.; Cai, Q.; Hui, J.; et al. Exploring the Interplay between Mitochondrial DNA and Lifestyle Factors in the Pathogenesis of Psychiatric Disorders. Depress. Anxiety 2024, 2024, 4914777. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, M.; Leccisotti, I.; Mollica, A.; Berardino, G.; Moretti, M.C.; Altamura, M.; Bellomo, A.; Daniele, A.; Dibello, V.; Solfrizzi, V.; et al. Neuropsychiatric symptoms and apolipoprotein E genotypes in neurocognitive disorders. Neural Regen. Res. 2026, 21, 1528–1541. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, M.; Ito, M.; Huang, Z.; Nishiyama, Y.; Masuda, A.; Mino, H.; Tachibana, M.; Inada, T.; Ohno, K. Extremely Low-Frequency Electromagnetic Field (ELF-EMF) Increases Mitochondrial Electron Transport Chain Activities and Ameliorates Depressive Behaviors in Mice. Int. J. Mol. Sci. 2024, 25, 11315. [Google Scholar] [CrossRef]
- Rezin, G.T.; Goncalves, C.L.; Daufenbach, J.F.; Fraga, D.B.; Santos, P.M.; Ferreira, G.K.; Hermani, F.V.; Comim, C.M.; Quevedo, J.; Streck, E.L. Acute administration of ketamine reverses the inhibition of mitochondrial respiratory chain induced by chronic mild stress. Brain Res. Bull. 2009, 79, 418–421. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, H.S.; Park, S.S.; Kim, C.J.; Kim, D.H.; Kim, T.W. Physical exercise ameliorates psychiatric disorders and cognitive dysfunctions by hippocampal mitochondrial function and neuroplasticity in post-traumatic stress disorder. Exp. Neurol. 2019, 322, 113043. [Google Scholar] [CrossRef]
- Aguiar, A.S., Jr.; Stragier, E.; da Luz Scheffer, D.; Remor, A.P.; Oliveira, P.A.; Prediger, R.D.; Latini, A.; Raisman-Vozari, R.; Mongeau, R.; Lanfumey, L. Effects of exercise on mitochondrial function, neuroplasticity and anxio-depressive behavior of mice. Neuroscience 2014, 271, 56–63. [Google Scholar] [CrossRef]
- de Melo Reis, R.A.; Isaac, A.R.; Freitas, H.R.; de Almeida, M.M.; Schuck, P.F.; Ferreira, G.C.; Andrade-da-Costa, B.; Trevenzoli, I.H. Quality of Life and a Surveillant Endocannabinoid System. Front. Neurosci. 2021, 15, 747229. [Google Scholar] [CrossRef]
- Du, J.; Zhu, M.; Bao, H.; Li, B.; Dong, Y.; Xiao, C.; Zhang, G.Y.; Henter, I.; Rudorfer, M.; Vitiello, B. The Role of Nutrients in Protecting Mitochondrial Function and Neurotransmitter Signaling: Implications for the Treatment of Depression, PTSD, and Suicidal Behaviors. Crit. Rev. Food Sci. Nutr. 2016, 56, 2560–2578. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, Z.; Sun, L.; He, Z.; Li, J.; Geng, J.; Zong, Y.; Chen, W.; Du, R. 20 (S)-Protopanaxadiol Alleviates DRP1-Mediated Mitochondrial Dysfunction in a Depressive Model In Vitro and In Vivo via the SIRT1/PGC-1alpha Signaling Pathway. Molecules 2024, 29, 5085. [Google Scholar] [CrossRef]
- Xu, W.; Gao, W.; Guo, Y.; Xue, F.; Di, L.; Fang, S.; Fan, L.; He, Y.; Zhou, Y.; Xie, X.; et al. Targeting mitophagy for depression amelioration: A novel therapeutic strategy. Front. Neurosci. 2023, 17, 1235241. [Google Scholar] [CrossRef] [PubMed]
- Assies, J.; Mocking, R.J.; Lok, A.; Ruhe, H.G.; Pouwer, F.; Schene, A.H. Effects of oxidative stress on fatty acid- and one-carbon-metabolism in psychiatric and cardiovascular disease comorbidity. Acta Psychiatr. Scand. 2014, 130, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G.; Siopi, E.; Guenin-Mace, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef]
- Rathore, K.; Shukla, N.; Naik, S.; Sambhav, K.; Dange, K.; Bhuyan, D.; Imranul Haq, Q.M. The Bidirectional Relationship Between the Gut Microbiome and Mental Health: A Comprehensive Review. Cureus 2025, 17, e80810. [Google Scholar] [CrossRef]
- Lu, C.Y.; Yuan, X.M.; He, L.H.; Mao, J.R.; Chen, Y.G. Mechanism of total flavone of Abelmoschus manihot in treating ulcerative colitis and depression via intestinal flora-glycerophospholipid metabolism- macrophage polarization pathway. Zhongguo Zhong Yao Za Zhi 2025, 50, 1286–1297. [Google Scholar] [CrossRef]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef]
- Torrisi, S.A.; Rizzo, S.; Laudani, S.; Ieraci, A.; Drago, F.; Leggio, G.M. Acute stress alters recognition memory and AMPA/NMDA receptor subunits in a sex-dependent manner. Neurobiol. Stress. 2023, 25, 100545. [Google Scholar] [CrossRef]
- Andersen, E.; Fiacco, S.; Gordon, J.; Kozik, R.; Baresich, K.; Rubinow, D.; Girdler, S. Methods for characterizing ovarian and adrenal hormone variability and mood relationships in peripubertal females. Psychoneuroendocrinology 2022, 141, 105747. [Google Scholar] [CrossRef]
- McDowell, A.L.; Fransen, K.M.; Elliott, K.S.; Elghouche, A.; Kostylev, P.V.; O’Dea, P.K.; Garraghty, P.E. Sex Differences and the Impact of Chronic Stress and Recovery on Instrumental Learning. Neurosci. J. 2015, 2015, 697659. [Google Scholar] [CrossRef]
- Lin, Y.; Ter Horst, G.J.; Wichmann, R.; Bakker, P.; Liu, A.; Li, X.; Westenbroek, C. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb. Cortex 2009, 19, 1978–1989. [Google Scholar] [CrossRef] [PubMed]
- Traustadottir, T.; Bosch, P.R.; Matt, K.S. The HPA axis response to stress in women: Effects of aging and fitness. Psychoneuroendocrinology 2005, 30, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.A.; Lemmens, S.G.; Adam, T.C.; Westerterp-Plantenga, M.S. Sex differences in HPA axis activity in response to a meal. Physiol. Behav. 2012, 106, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zheng, Y.; Lang, X.; Fu, Z.; Zhang, P.; Jiang, G.; Zhang, X. Prevalence and correlates of dyslipidemia in first-episode and drug-naive major depressive disorder patients with comorbid abnormal glucose metabolism: Sex differences. Front. Psychiatry 2023, 14, 1101865. [Google Scholar] [CrossRef]
- Chen, Q.; Hartman, C.A.; Haavik, J.; Harro, J.; Klungsoyr, K.; Hegvik, T.A.; Wanders, R.; Ottosen, C.; Dalsgaard, S.; Faraone, S.V.; et al. Common psychiatric and metabolic comorbidity of adult attention-deficit/hyperactivity disorder: A population-based cross-sectional study. PLoS ONE 2018, 13, e0204516. [Google Scholar] [CrossRef]
- Johansen, I.T.; Steen, N.E.; Rodevand, L.; Werner, M.C.F.; Lunding, S.H.; Hjell, G.; Ormerod, M.; Agartz, I.; Melle, I.; Lagerberg, T.V.; et al. Sex-specific associations between metabolic hormones, severe mental disorders and antipsychotic treatment. Psychoneuroendocrinology 2022, 146, 105927. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, X.; Guo, Y.; Lang, X.; Li, Z.; Zhang, X.Y. Sex differences in metabolic disorder patterns of first-episode drug-naive patients with schizophrenia. Psychoneuroendocrinology 2021, 124, 105061. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, J.; Wang, D.; Li, C.; Liu, B.; Fang, X.; You, J.; Guo, M.; Lu, X.Y. SIRT1 in forebrain excitatory neurons produces sexually dimorphic effects on depression-related behaviors and modulates neuronal excitability and synaptic transmission in the medial prefrontal cortex. Mol. Psychiatry 2020, 25, 1094–1111. [Google Scholar] [CrossRef]
- Alipour, P.; Azizi, Z.; Raparelli, V.; Norris, C.M.; Kautzky-Willer, A.; Kublickiene, K.; Herrero, M.T.; Emam, K.E.; Vollenweider, P.; Preisig, M.; et al. Role of sex and gender-related variables in development of metabolic syndrome: A prospective cohort study. Eur. J. Intern. Med. 2024, 121, 63–75. [Google Scholar] [CrossRef]
- Meier, S.M.; Trontti, K.; Purves, K.L.; Als, T.D.; Grove, J.; Laine, M.; Pedersen, M.G.; Bybjerg-Grauholm, J.; Baekved-Hansen, M.; Sokolowska, E.; et al. Genetic Variants Associated with Anxiety and Stress-Related Disorders: A Genome-Wide Association Study and Mouse-Model Study. JAMA Psychiatry 2019, 76, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, G.; Hariri, A.R.; Enoch, M.A.; Scott, D.; Sinha, R.; Virkkunen, M.; Mash, D.C.; Lipsky, R.H.; Hu, X.Z.; et al. Genetic variation in human NPY expression affects stress response and emotion. Nature 2008, 452, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Czarny, P.; Ziolkowska, S.; Kolodziej, L.; Watala, C.; Wigner-Jeziorska, P.; Blizniewska-Kowalska, K.; Wachowska, K.; Galecka, M.; Synowiec, E.; Galecki, P.; et al. Single-Nucleotide Polymorphisms in Genes Maintaining the Stability of Mitochondrial DNA Affect the Occurrence, Onset, Severity and Treatment of Major Depressive Disorder. Int. J. Mol. Sci. 2023, 24, 14752. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Wang, B.; Shi, S.; Chu, X.; Liu, C.; Kang, M.; Hui, J.; Gou, Y.; Zhou, R.; Liu, Y.; et al. Assessing the joint effects of mitochondrial genes and physical activity on the psychiatric phenotype of subjective well-being based on the UK Biobank data. Eur. Arch. Psychiatry Clin. Neurosci. 2025, 275, 667–678. [Google Scholar] [CrossRef]
- Sriretnakumar, V.; Harripaul, R.; Vincent, J.B.; Kennedy, J.L.; So, J. Enrichment of pathogenic variants in genes associated with inborn errors of metabolism in psychiatric populations. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2019, 180, 46–54. [Google Scholar] [CrossRef]
- Cohen, S.; Schwartz, J.E.; Epel, E.; Kirschbaum, C.; Sidney, S.; Seeman, T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom. Med. 2006, 68, 41–50. [Google Scholar] [CrossRef]
- Chen, E.; Matthews, K.A. Cognitive appraisal biases: An approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Ann. Behav. Med. 2001, 23, 101–111. [Google Scholar] [CrossRef]
- Kim, Y.M.; Cho, S.I. Socioeconomic status, work-life conflict, and mental health. Am. J. Ind. Med. 2020, 63, 703–712. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Xue, J.; Zhao, L.; Tang, P.; Tian, Y.; Fan, H.; Hao, M.; Zhao, X.; Geng, F.; et al. Associations between appetite loss and clinical features as well as inflammatory cytokines in adolescents with major depressive disorder. Front. Psychiatry 2025, 16, 1583060. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, H.; Ren, Q.; Yin, L.; Zhao, J.; Yang, F.; Li, Z.; Ran, J.; Liu, H.; Li, W.; et al. Association between dietary inflammatory index and the risk of postpartum depression in China. J. Affect. Disord. 2025, 384, 135–143. [Google Scholar] [CrossRef]
- Bot, M.; Milaneschi, Y.; Al-Shehri, T.; Amin, N.; Garmaeva, S.; Onderwater, G.L.J.; Pool, R.; Thesing, C.S.; Vijfhuizen, L.S.; Vogelzangs, N.; et al. Metabolomics Profile in Depression: A Pooled Analysis of 230 Metabolic Markers in 5283 Cases with Depression and 10,145 Controls. Biol. Psychiatry 2020, 87, 409–418. [Google Scholar] [CrossRef]
- Hallab, A.; The Health and Aging Brain Study (HABS-HD) Study Team. Mediating effect of pro-inflammatory cytokines in the association between depression, anxiety, and cardiometabolic disorders in an ethnically diverse community-dwelling middle-aged and older US population. medRxiv 2025. [Google Scholar] [CrossRef]

| Biomarker | Origin/Source | Link to Mental Health | Link to Metabolic Health | Potential Clinical Application | Relevant Section(s) |
|---|---|---|---|---|---|
| GDF15 | Adipose tissue macrophages (stress-induced lipolysis); muscle tissue (mitochondrial stress) | Stress-responsive biomarker, links to anxiety circuits; circadian waking response similar to cortisol; linked to prenatal stress | Linked to peripheral metabolism; Chronic increases linked to weight loss and metabolic issues; levels rise with age, linked to frailty and inflammation | Non-invasive stress monitoring (saliva); early intervention for at-risk individuals | Section 3.1, Section 3.2, Section 3.3, Section 3.5, Section 6.2, Section 7.3, Section 8.1, Section 9.2, Section 10 |
| Ceramides | Gut bacteria (gut dysbiosis); Sphingolipid metabolism | Directly impair hippocampal mitochondrial function; linked to corticosterone-induced depression; linked to prenatal depression | Involved in glycerophospholipid and sphingolipid metabolism | Targeting with probiotics to reduce depressive-like behaviors | Section 2.1, Section 5.2, Section 8.1 and Section 10 |
| Succinate Dehydrogenase (SDH) | Mitochondrial enzyme | Lower serum levels correlated with attenuated frontal-temporal brain activation in MDD patients | Key mitochondrial enzyme | Potential peripheral biomarker of mitochondrial dysfunction in psychiatric disorders | Section 4.4 and Section 7.3 |
| NIX (BNIP3L) | Mitophagy receptor (outer mitochondrial membrane protein) | Degradation linked to accumulation of damaged mitochondria, synaptic defects, and passive stress-coping behaviors in depression models; lower levels in MDD patients; restoration by ketamine and TNF-α blockers reverses behavioral problems | Essential for maintaining neuron health during stress (mitophagy) | Therapeutic target for depression (promoting mitophagy) | Section 4.3 and Section 10 |
| Acylcarnitines | Involved in energy processes | Changes in short-chain acylcarnitine levels relate to presence and severity of depression, especially energy imbalance symptoms; profiles can predict treatment responses | Measures energy capacity; reflect issues with cellular metabolism | Metabolic resilience biomarkers to identify at-risk individuals; guiding treatment options for depression | Section 7.2, Section 8.1 and Section 10 |
| Circulating Mitochondrial DNA (cf-mtDNA) | Mitochondria | Linked to prenatal stress and pregnancy outcomes; potential sign of mitochondrial dysfunction in psychiatric disorders, especially in older adults with mild cognitive impairment and remitted MDD | Mitochondrial health marker | Potential peripheral biomarker of mitochondrial function for brain health and treatment response | Section 3.2 and Section 7.3 |
| Therapeutic Approach | Primary Target | Mechanism of Action | Examples/Key Findings | Relevant Section(s) |
|---|---|---|---|---|
| Mitochondrial Function Enhancement | Brain Mitochondria | Improving mitochondrial electron transport chain activities; restoring mitochondrial function; promoting mitophagy; reducing oxidative stress | Extremely low-frequency electromagnetic field therapy (activates Sirt3-FoxO3a-SOD2 pathway); ketamine (restores respiratory chain activity); natural products (e.g., 20(S)-Protopanaxadiol, Morinda officinalis oligosaccharides, gypenosides, diosmetin); Agomelatine (suppresses hippocampal oxidative stress) | Section 4.5, Section 7.1 and Section 10 |
| Gut Microbiota Modulation | Gut Microbiota | Normalizing gut microbiota composition; lowering ceramide levels; restoring microbial balance; influencing glycerophospholipid metabolism | Probiotics (e.g., Bifidobacterium pseudolongum, Lactobacillus reuteri); synbiotic interventions; dietary changes; fecal microbiota transplantation; traditional medicine compounds (e.g., total flavone of Abelmoschus manihot) | Section 5.3, Section 7.1 and Section 10 |
| Stress Signaling Pathway Targeting (GDF15/GFRAL) | GDF15-GFRAL Pathway | Modulating GDF15 levels or GFRAL receptor activity | Targeting GFRAL for new anxiety treatments; careful consideration of GDF15 metabolic roles (weight loss) | Section 3.5 and Section 10 |
| Lifestyle Interventions | Multiple Pathways (Mitochondria, Neuroplasticity, Overall Health) | Boosting mitochondrial function; improving neuroplasticity; reducing psychiatric disorders; enhancing resistance to inhibitors; regulating BDNF | Physical exercise (aerobic exercise) | Section 4.5 and Section 7.1 |
| Nutritional Approaches | Mitochondrial Function, Neurotransmitter Signaling, Oxidative Damage | Protecting mitochondria and membrane lipids; supporting neurotransmitter signaling | omega-3 fatty acids; antioxidants; vitamin B compounds; magnesium; anti-inflammatory diets | Section 4.5 and Section 7.1 |
| Inflammation Targeting | Inflammatory Pathways | Reducing pro-inflammatory cytokines; addressing neuroinflammation | Theobromine (suppresses neuroinflammation related to nicotine withdrawal); traditional medicine approaches; Infliximab (blocks TNF-α) | Section 4.4 and Section 10 |
| Precision Medicine/Biomarker Guided | Individual Pathways/Patient Profile | Identifying individual metabolic patterns; tailoring therapies; predicting treatment response | Metabolomic profiling (acylcarnitine profiles); multi-omic integration for blood biomarkers; circulating mitochondrial DNA; genetic profiling (mitochondrial SNPs, stress response genes, metabolic capacity) | Section 7.2, Section 7.3, Section 8.1 and Section 10 |
| Category | Specific Consideration | Description/Challenge | Relevant Section(s) |
|---|---|---|---|
| Translational Challenges | Species differences | Rodent findings may not fully apply to humans due to metabolic, stress response, and brain structure differences. Human mental health is more complex. | Section 9.1 |
| Variability in compound absorption/processing | Pharmacokinetics can differ significantly between controlled lab conditions and clinical settings. | Section 9.1 | |
| Timing and duration of interventions | Unclear optimal timing, treatment duration, and long-term effects of pathway changes. | Section 9.1 | |
| Methodological Considerations | Confounding variables | Diet, physical activity, sleep, medication, and other medical conditions can influence both metabolic and mental health outcomes. | Section 9.2 |
| Biomarker variability | The dynamic nature of many biomarkers requires clear protocols for sample collection and interpretation. | Section 9.2 | |
| Reproducibility issues | Concerns in metabolomics and microbiome research due to analytical method differences and population variations. Standardization and multi-group validation are crucial. | Section 9.2 | |
| Ethical and Safety Considerations | Long-term effects of interventions | Unknown long-term consequences of targeting fundamental metabolic pathways (e.g., GDF15 signaling, mitochondrial function) could lead to unexpected problems. | Section 9.3 |
| Fair access to personalized therapies | Individual variability in treatment response raises ethical questions about equitable access to tailored interventions. | Section 9.3 | |
| Risks of early/preventive intervention | Weighing benefits of early intervention in high-risk individuals against risks of unnecessary treatment. | Section 9.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.; Pyeon, S.Y.; Kim, M.S. Molecular Links Between Metabolism and Mental Health: Integrative Pathways from GDF15-Mediated Stress Signaling to Brain Energy Homeostasis. Int. J. Mol. Sci. 2025, 26, 7611. https://doi.org/10.3390/ijms26157611
Seo M, Pyeon SY, Kim MS. Molecular Links Between Metabolism and Mental Health: Integrative Pathways from GDF15-Mediated Stress Signaling to Brain Energy Homeostasis. International Journal of Molecular Sciences. 2025; 26(15):7611. https://doi.org/10.3390/ijms26157611
Chicago/Turabian StyleSeo, Minju, Seung Yeon Pyeon, and Man S. Kim. 2025. "Molecular Links Between Metabolism and Mental Health: Integrative Pathways from GDF15-Mediated Stress Signaling to Brain Energy Homeostasis" International Journal of Molecular Sciences 26, no. 15: 7611. https://doi.org/10.3390/ijms26157611
APA StyleSeo, M., Pyeon, S. Y., & Kim, M. S. (2025). Molecular Links Between Metabolism and Mental Health: Integrative Pathways from GDF15-Mediated Stress Signaling to Brain Energy Homeostasis. International Journal of Molecular Sciences, 26(15), 7611. https://doi.org/10.3390/ijms26157611






