Which Approach to Choose to Counteract Musculoskeletal Aging? A Comprehensive Review on the Multiple Effects of Exercise
Abstract
1. Introduction
2. Literature Search Strategy
3. Natural Compounds
| Natural Compound | Chemical Class | Molecular Target | Study Population | Evidence | Reference |
|---|---|---|---|---|---|
| Resveratrol | Polyphenolic phytoalexin | SIRT1/AMPK | MSCs isolated from 12-month-old Kunming mice treated with resveratrol for 48 h | - Reduced ROS - Improved cell viability - Promoted extracellular matrix calcification - Increased expression of ALP, OCN, OPN, Col-I, RUNX2 | [36] |
| SIRT1/FoxO1 | - 8-week-old ovariectomized female mice treated with resveratrol intraperitoneally (40 mg/kg body weight) once daily for 8 weeks - Murine osteoblasts subjected to H2O2-induced oxidative stress, treated with 0.1 mM resveratrol for 1, 3, and 5 days | In vivo: - Improved bone microarchitecture - Increased expression of ALP, RUNX2, Osx In vitro: - Promoted cell proliferation - Increased ALP activity - Increased SOD expression - Reduced ROS | [12] | ||
| SIRT1 | 25-month-old Sprague-Dawley rats treated with oral resveratrol (150 mg/kg/day) for 6 weeks | - Increased AMPK, SIRT1, and Bcl-2 - Reduced Bax and p53 | [37] | ||
| Rapamycin | Macrocyclic lactone | mTOR | 30-month-old female HET3 mice treated with rapamycin intraperitoneally (6 mg/kg body weight) every other day for 8 weeks | - mTORC1 inhibition - Reduced GDF15 and STAT3 expression - Attenuation of oxidative stress - Reduced muscle atrophy | [42] |
| 15 wild-type female mice aged 8 weeks treated with 4 mg/kg rapamycin intraperitoneally every other day for 12 weeks | - Reduced femoral bone mineral content - Reduced BMD - Increased serum CTX-1 - Increased bone resorption | [43] | |||
| - Chondrocytes isolated from osteoarthritic patients treated with 1 µM RPMs for 48 h - Wild-type C57BL/6 male mice with post-traumatic osteoarthritis treated with 1.8 µg RPMs by intramuscular injection on days 7, 24 and 42 in the prophylactic study, and on days 24 and 42 in the therapeutic study | In vitro: - Autophagy induction - Reduced cellular senescence - Maintenance of sulphated glycosaminoglycan production In vivo: - Reduction in cartilage damage and inflammation in both preventive and therapeutic phases | [44] | |||
| Quercetin | Flavonoid | NF-κB/β-catenin | - 8–10-week-old ovariectomized female Sprague Dawley rats treated with 50 mg/kg quercetin per day for 8 weeks - BMSCs treated with 1 µM quercetin for 24 h after stimulation with 5 ng/mL TNF-α | In vivo: - Improved trabecular microarchitecture and bone biomechanical properties In vitro: - Increased cell viability - Promoted bone mineralization - Increased RUNX2, Osx and β-catenin expression | [50] |
| ERK/p38 | BMSCs isolated from 4-week-old male rats treated with quercetin at concentrations of 0, 1, 2, 5, and 10 μM for 7 days | Optimal results at 2 μM concentration: - Increased cell proliferation - Increased ALP activity - Promoted calcium deposition and osteogenesis - Increased VEGF and ANG-1 expression | [51] | ||
| ITGB1/FAK/paxillin, IGF-1R/Akt/mTOR | C2C12 mouse myoblasts treated with quercetin at concentrations of 0, 2.5, 12.5, 25 and 50 µM for 7 days every 48 h | Optimal results at 12.5 µM concentration: - Increased cell fusion and migration - Increased myogenic differentiation - Promoted muscle regeneration | [53] | ||
| Curcumin | Polyphenol | NRF2 | 18 male F344xBN rats aged 32 months fed with 0.2% curcumin for 4 months | - Increased muscle mass and contractile function - Increased NRF2 expression - Reduced oxidative damage | [10] |
| Vitamin E | Tocopherols/Tocotrienols | NF-kB/STAT3 | Human myoblasts treated with 10 µM TRF, 10 µM ATF, or 5 µM NAC for 24 h | - Improved cell morphology and reduced senescence after NAC treatment - Reduced ROS production and lipid peroxidation after RTF and ATF treatments - Increased SOD activity and GSH/GSSG ratio after TRF treatment | [58] |
| Genistein | Isoflavone | ERRα | BMSCs isolated from 12-week-old ovariectomized female Sprague-Dawley rats treated with genistein at doses of 1 μM and 10−2 μM for 3 days | - Reduced p53, p16 and p21 expression - Improved mitochondrial function - Reduced oxidative damage - Increased SIRT3 and PGC-1α expression - Reduced bone loss | [14] |
| Fisetin | Flavonol | SIRT1 | - Primary human chondrocytes pretreated with fisetin at doses of 1, 5, 10 μM for 2 h, followed by stimulation with or without 10 ng/mL IL-1β for 24 h - 10-week-old male wild-type C57BL/6 mice treated with 20 mg/kg fisetin orally every day for 8 weeks | In vitro: - Reduced expression of NO, PGE2, TNF-α, IL-6, COX-2, iNOS, MMP-3, MMP-13 and ADAMTS-5 - Greater increase in SIRT1 expression at a concentration of 10 μM fisetin In vivo: - Attenuation of joint degeneration - Reduced subchondral bone plaque thickness - Reduced severity of synovitis | [60] |
| Epicatechin | Flavonol | Akt/mTOR | 23-month-old male Sprague Dawley rats treated with 1 mg/kg/day oral epicatechin for 8 weeks | - Improved strength, endurance, and muscle mass - Increased IGF-1 and follistatin - Reduced TNF-α, myostatin - Inhibited NF-κB signaling, MuRF1 and Atrogin-1 | [61] |
4. Synthetic Compounds
| Synthetic Compound | Molecular Target | Study Population | Evidence | Reference |
|---|---|---|---|---|
| Dasatinib | NF-κB | 12-month-old female ovariectomized rats treated orally with 5 mg/kg dasatinib and 50 mg/kg quercetin for 2 and 4 months | - Reduced bone loss - Reduced senescent cell accumulation - Restoration of MSCs functionality - Reduced NF-kB expression | [6] |
| - Male C57BL/6 mice with facet joint osteoarthritis aged between 8 and 10 weeks treated orally with 5 mg/kg dasatinib and 50 mg/kg quercetin for 10 weeks - Primary mouse chondrocytes treated with 200 nM dasatinib and 10 μm quercetin for 48 h | In vivo: - Improvement in joint degeneration - Reduced expression of p16, p21, p53, IL-1β, IL-6 In vitro: - Attenuation of senescence and release of SASPs | [64] | ||
| Navitoclax | Bcl-2/Bcl-xL | - 20 male (n = 10) and female (n = 10) 24-month-old C57BL/6 mice treated orally with 50 mg/kg navitoclax once daily for 2 weeks - Mouse BMSCs treated with 5 μM navitoclax for 5 days | In vivo: - Trabecular bone loss - Reduced mineralized matrix production - Reduced Osx expression In vitro: - Reduced cellular senescence - Compromised bone formation - Increased cytotoxicity | [7] |
| UBX0101 | p53/p21 | 10-week-old or 19-month-old male C57BL/6 mice with induced osteoarthritis treated with 1 mM UBX0101 via six intra-articular injections every two days | Reduction in the presence of oxidized proteins in cartilage and synovial fluid in 19-month-old mice | [8] |
| Panobinostat | FoxO | - Primary murine chondrocytes and synoviocytes treated with panobinostat at doses of 10, 50, and 100 nM for 24 h after stimulation with 5 ng/mL IL-1β - C57BL/6J mice treated with panobinostat at doses of 100 μg/kg and 2.5 mg/kg intraperitoneally 3 times a week for 11 weeks | In vitro: - Increased FoxO1, PRG4, ACAN expression - Reduced NOS2 expression In vivo: - Attenuated histopathological alterations in cartilage, synovium and subchondral bone - Improved pain-related behavioral parameters | [15] |
| Metformin | AMPK/SIRT1 | - Mouse osteoblasts and MC3T3-E1 cell line treated with metformin at concentrations of 0–800 µM for 0–72 h - 45 female Sprague–Dawley rats aged 8–10 weeks, ovariectomized and treated with 100 mg/kg metformin orally every day for two months | In vitro: - Stimulated OPG and reduced RANKL mRNA and protein expression In vivo: - Increased BMD - Prevented bone loss - Increased OPG expression - Decreased RANKL expression | [67] |
| - 15 male C57BL/6 mice aged 8 weeks treated with 16.5 mg/mL metformin intra-articularly every three days for 8 weeks - Chondrocytes isolated from 6 to 8-week-old male C57BL/6 mice treated with 1 mM metformin administered one hour before stimulation with 10 ng/mL IL-1β | In vivo: - Restored overexpression of MMP-13 and underexpression of Col-II in articular cartilage In vitro: - Increased phosphorylation of AMPK and SIRT1 - Promoted autophagy - Reduced catabolism and apoptosis | [68] | ||
| C2C12 myoblasts at advanced passages treated with metformin at doses of 75 µM and 500 µM every 24 h for 2 days | Optimal results at 75 µM dose: - Reduced cellular senescence - Improved autophagy - Promoted myogenic differentiation | [16] |
5. Physical Exercise as a Non-Pharmacological Intervention Against Musculoskeletal Aging
| Molecular Target | Study Population | Evidence | Reference |
|---|---|---|---|
| AMPK | - 9-week-old and 19-month-old male C57/BL6 mice subjected to moderate treadmill running (8 m/min) for 2 days, followed by 4 weeks of exercise training, 30 min twice a day with 1 h of rest, 5 days/week - Differentiated C2C12 myoblasts pretreated with 100 μM AICAR for 30 min prior to administration of 1 μM doxorubicin for 15 min | In vivo: - Reduced cellular senescence and p16 and p21 expression in 19-month-old male In vitro: - Pretreatment with AICAR attenuates doxorubicin-induced AMPK phosphorylation and prevents the cellular senescence phenotype | [24] |
| AMPK/FOXO3a | 4-month-old male Sprague-Dawley rats: - Group D: treated with D-gal (200 mg/kg/day) - Group DS: D-gal + spermidine (5 mg/kg/day) - Group DE: D-gal + exercise (60 min of swimming per day, 5 days per week, for 6 weeks) - Group DES: D-gal + exercise + spermidine | Optimal results in the DES group: - Reduced SA-β-gal - Increased SOD activity - Reduced MDA levels - Promoted autophagy | [76] |
| SIRT1/PGC-1α | 3-, 12- and 18-month-old female Sprague-Dawley rats subjected to swimming training for 40 min/day, 5 days/week for 12 weeks | - Upregulation of SIRT1, PGC-1α, and AMPK in the gastrocnemius and soleus muscles - Improved muscle function | [83] |
| SIRT1 | Male Wistar rats aged 3 and 26 months subjected to treadmill running for 2 weeks at a speed of 10 m/min and a 5% incline for 30 min, progressively increased to 60% of VO2max | - Increased SIRT1 activity - Restored NAD+ and NAMPT levels - Increased UCP3 expression - Reduced HIF-1α and VEGF expression - Reduced oxidative stress and mitochondrial damage | [84] |
| NOX4/SIRT1/FNDC5 | Wild-type BALB/c male mice aged 4, 12 and 24 months subjected to a WBV protocol consisting of 3 series of 2 min and 30 s each, with 2 min and 30 s of recovery, 3 days a week for 12 weeks | - Increase bone volume and trabecular thickness - Up-regulation of SIRT1 and FNDC5 - Down-regulation of NOX4 | [85] |
| SIRT3 | 23 young subjects (aged 18–30, 11 women and 12 men) and 20 elderly subjects (aged ≥65, 9 women and 11 men) underwent 8 weeks of cycling at 65% of peak oxygen consumption for 60 min | - Reduction in oxidative damage in the skeletal muscle of young subjects - Increased SIRT3, CAT and SOD2 in the skeletal muscle of elderly subjects | [91] |
| Akt/PGC-1α/FoxO3a | 7-week-old male Wistar rats subjected to treadmill running for 60 min a day, 4 times a week for 8 weeks | - Increase in the phosphorylation of Akt, FoxO3a and PGC-1α in the gastrocnemius and soleus muscles - Reduced MuRF1 and Atrogin-1 expression - Attenuation of muscle atrophy | [98] |
| Akt/mTOR, Akt/FoxO3a | 21-month-old male Wistar rats subjected to resistance exercise, which consisted of climbing an inclined ladder with progressive loads applied to their tails until reaching 80% of their body weight | - Reduced Atrogin-1, MuRF1, p62 and Bax expression - Promoted autophagy - Increased expression of Beclin1, Bcl-2 and LC3-II/LC3-I ratio - Improved mitochondrial function - Increased PGC-1α, Mfn2, Drp1 and PINK1 expression | [99] |
| NRF2 | 22-month-old wild-type C57/BL6/J male mice and 22-month-old NRF2 knockout mice subjected to 20 min of treadmill running per day for 8 weeks, with 1 day of rest per week Senescent C2C12 myoblasts treated with sulforaphane at different concentrations (0, 1, 2, 3, 4, 5 and 6 μM) for 20 h | In vivo (wild-type mice): - Improved muscle function - Reverted sarcopenic phenotype - Increased NRF2 expression In vitro: - Increased NRF2 and Drp1 expression - Promoted mitochondrial fission - Reduced cellular senescence | [100] |
6. Clinical Evidence on the Efficacy and Safety of Senotherapeutics and/or Combined with Physical Exercise
| Compound | Study Type | Study Population | Evidence | Adverse Events | Reference |
|---|---|---|---|---|---|
| Resveratrol | Double-blind RCT | n = 30 older adults aged 65–80 years underwent to resistance training for 12 weeks: - Placebo group (n = 6 males, n = 9 females) - Treated group (n = 6 males, n = 9 females): 500 mg/day resveratrol for 12 weeks | In the treated group: - Improved mitochondrial density and muscle fatigue resistance - Increased fiber area and number of myonuclei in the vastus lateralis muscle | No adverse events reported during the study | [102] |
| Three-arm, two-site pilot RCT | n = 60 older adults ≥ 65 years with physical limitations underwent to two sessions a week for 12 weeks of center-based walking and whole-body resistance training: - EX0 group (n = 6 males, n = 14 females): placebo for 12 weeks - EX500 group (n = 7 males, n = 13 females): 500 mg/day resveratrol for 12 weeks - EX1000 group (n = 2 males, n = 18 females): 1000 mg/day resveratrol for 12 weeks | In the EX1000 group: - Clinically significant improvement in the walking endurance - Increased citrate synthase | 27 adverse events (EX0, n = 8; EX500, n = 12; EX1000, n = 7) related to gastrointestinal or musculoskeletal problems | [103] | |
| Double-blind crossover RCT | n = 128 postmenopausal women ≥ 65 years - Placebo group (n = 65) - Treated group (n = 63): 75 mg/day resveratrol for 12 months | In the treated group: - Increased BMD in the lumbar spine, femoral neck and total hip - Reduced plasma CTX-1 | Treated group: 4 subjects developed itching, menses, prolapsed bladder, prescheduled eye operation | [104] | |
| Double-blind RCT | n = 92 knee osteoarthritic patients, average age 58 years - Placebo group (n = 10 males, n = 32 females): 15 mg meloxicam + placebo, once daily for 90 days - Treated group (n = 13 males, n = 37 females): 15 mg meloxicam + 500 mg resveratrol, once daily for 90 days | In the treated group: - Improvement in pain and physical function | No serious adverse events reported during the study | [106] | |
| Rapamycin | Double-blind RCT | n = 114 healthy individuals aged 50–85 years: - Placebo group (n = 24 males, n = 15 females) - 5 mg/week of rapamycin (n = 23 males, n = 17 females) for 48 weeks - 10 mg/week of rapamycin (n = 27 males, n = 8 females) for 48 weeks | - Improvement in lean body mass and self-reported pain in the 10 mg/week rapamycin group - Improvement in self-reported emotional well-being and overall health in the 5 mg/week rapamycin group | Similar adverse events between groups, with a higher frequency of gastrointestinal symptoms in the treated groups | [108] |
| Double-blind RCT | 25 healthy older adults aged 70–95 years Phase 1: - Placebo group (n = 4 males) - Treated group (n = 4 males): 1 mg/die rapamycin for 4 months Phase 2: - Placebo group (n = 5 males, n = 5 females) - Treated group (n = 5 males, n = 2 females): 1 mg/die rapamycin for 8 weeks | No significant differences in cognitive function, physical performance, or self-perceived health status between the experimental groups | - Phase 1: one subject treated with rapamycin developed nocturnal diarrhea after 11 weeks of treatment Phase 2 - Placebo group: one subject with self-limiting stomatitis - Treated group: three subjects developed, respectively, self-limiting stomatitis, diarrhea, and facial rash | [109] | |
| Dasatinib Quercetin | Double-blind RCT | n = 60 postmenopausal women aged 60–90 years: - Control group (n = 30) - Treated group (n = 30): 100 mg/day dasatinib for two consecutive days + 1000 mg/day quercetin for three consecutive days orally with an intermittent schedule repeated every 28 days for 20 weeks | - Increased serum P1NP at 2 and 4 weeks in the treated group - No significant differences in serum CTX-1 between the two groups | Headaches and gastrointestinal events were more frequent in the treated group | [113] |
| Quercetin | Double-blind RCT | n = 33 postmenopausal women aged 45–75 years: - Placebo group (n = 18) - Treated group (n = 15): 500 mg/day quercetin for 90 days | In the treated group: - Increased serum levels of osteocalcin, P1NP and CTX-1 - Reduced serum levels of IL-6 and TNF-α | No adverse events reported during the study | [114] |
| Double-blind RCT | n = 26 older adults aged 65–82 years undergoing to isometric knee extension resistance training on both legs three times a week for 6 weeks: - Placebo group (n = 5 males, n = 8 females) - Treated group (n = 6 males, n = 7 females): 200 mg/day quercetin glycosides for 6 weeks | In the treated group: - Increased MVF of the knee extensor muscle - Increased motor unit firing rates with recruitment thresholds between 20 and 40% of MVF and between 40 and 60% of MVF | No adverse events reported during the study | [115] | |
| Curcumin | Double-blind RCT | n = 30 healthy older adults ≥ 65 years (n = 13 males, n = 17 females) - Placebo group (n = 15) - Treated group (n = 15): 500 mg/day curcumin for 3 months | In the treated group: - Increased handgrip strength and weight lift strength | No serious adverse events reported during the study | [117] |
| Triple-blind RCT | n = 120 postmenopausal women aged 50–65 years treated with 70 mg alendronate: - Placebo group (n = 30): placebo of nanomicelle curcumin + placebo of Nigella sativa oil once a day for 6 months - CUR group (n = 30): 80 mg nanomicelle curcumin + placebo of Nigella sativa oil once a day for 6 months - NS group (n = 30): placebo of nanomicelle curcumin + 1000 mg Nigella sativa oil once a day for 6 months - CUR-NS group (n = 30): 80 mg nanomicelle curcumin + 1000 mg Nigella sativa oil once a day for 6 months | - Reduced serum ALP in the CUR-NS group | No adverse events reported during the study | [118] | |
| Vitamin E | Double-blind RCT | n = 52 postmenopausal osteopenic women aged over 45-year-old: - Placebo group (n = 26) - Treated group (n = 26): 400 IU/day mixed-tocopherol for 12 weeks | - Significant increase of 35.3% in CTX-1 at 12 weeks of supplementation compared to baseline in the placebo group - No significant change in CTX-1 and P1NP levels in the treated group | Postmenopausal bleeding after 10 weeks of supplementation in one participant, with spontaneous relief | [120] |
| Double-blind RCT | n = 60 sarcopenic older adult subjects aged 60–85 years: - Placebo group (n = 14 males, n = 16 females) - Treated group (n = 13 males, n = 17 females): 57.5% whey protein, 702 IU vitamin D and 109 mg vitamin E for 6 months | In the treated group: - Improved RSMI and handgrip strength - Reduced serum levels of IL-2 - Improved quality of life | - Difficulty defecating in 3 subjects in the treated group - Pain during urination in one subject in the placebo group and one subject in the treated group | [121] | |
| Genistein | Post hoc analysis using data from a multicenter RCT | n = 121 postmenopausal osteoporotic women, average age 54 years: - Placebo group (n = 59) - Treated group (n = 62): 54 mg/day genistein for 24 months | In the treated group: - Increased BMD in the femoral neck, lumbar spine, and total hip at 1 year and 2 years - Increased ALP level | Gastrointestinal problems were the most common adverse event after treatment | [126,127] |
| Epicatechin | Double-blind RCT | n = 62 sarcopenic elderly males aged 65–75 years: - Placebo group (n = 15) - RT group (n = 14): 3 sets of 8–12 repetitions consisting of 45 min of leg press, leg extension, leg curl, chest press, shoulder press, rowing, biceps curl, and sit-ups, with a 90 s rest between sets, for 8 weeks - EP group (n = 17): 1 mg/kg/day epicatechin for 8 weeks - RT + EP group (n = 15) | In the RT, EP, and RT + EP groups: - Reduced time to complete the TUG test - Increased plasma follistatin levels - Increased follistatin/myostatin ratio, with higher values in the RT + EP group In the RT and RT + EP groups: - Increased maximum strength of chest press and leg press - Reduced plasma myostatin levels | No adverse events reported during the study | [132] |
| Metformin | RCT | n = 132 older adult males ≥ 70 years - Placebo group (n = 70) - Treated group (n = 62): 1700 mg metformin twice a day for 16 weeks | In the treated group: - Increased handgrip strength, gait speed, and physical performance - Reduced plasma levels of CAF22 and NfL | Treated group: - Metallic taste (n = 5) - Soft stools/diarrhea (n = 9) - Flatulence (n = 7) | [134] |
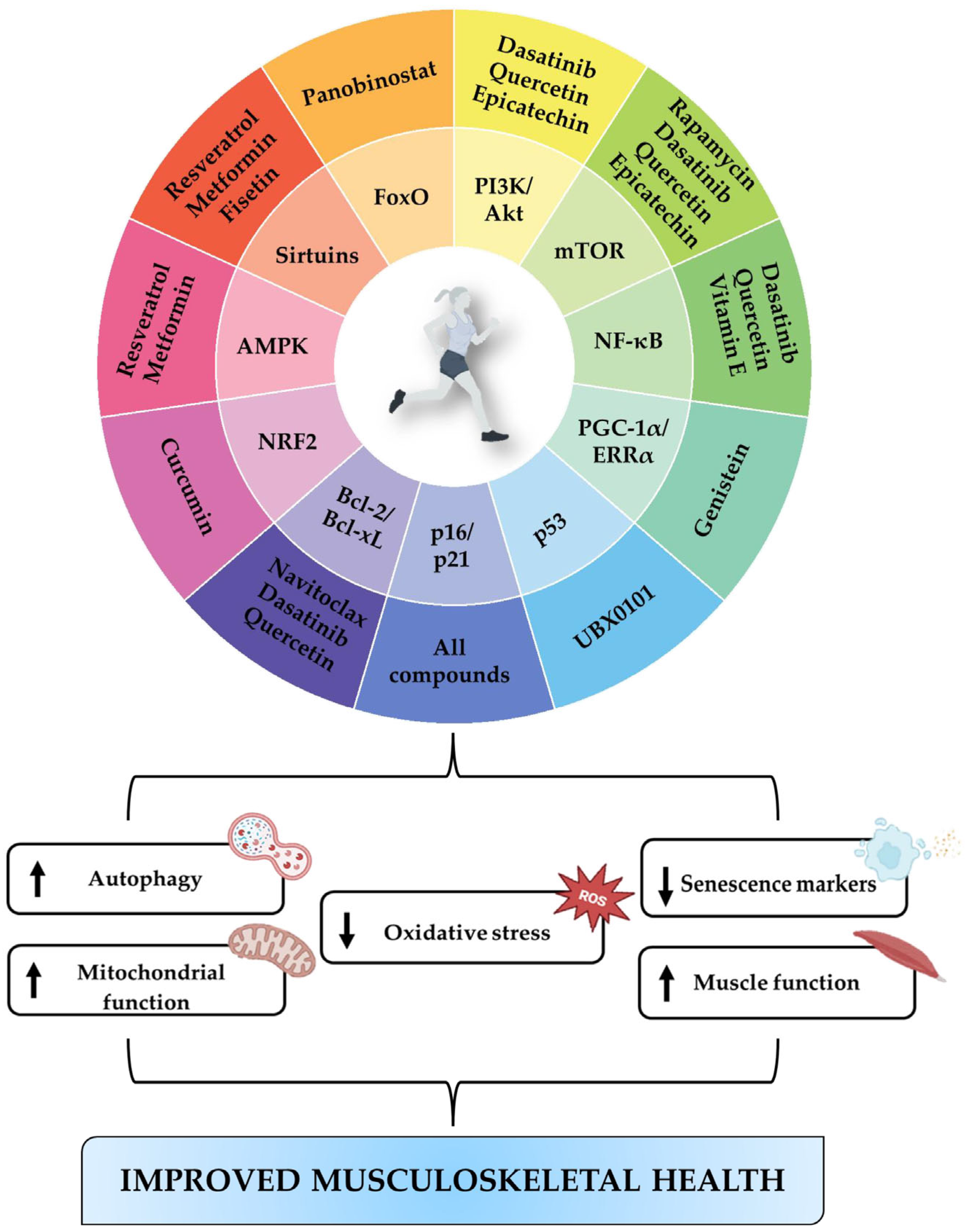
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SASP | Senescence-associated secretory phenotype |
| mTOR | Mechanistic target of rapamycin |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| SIRT1 | Sirtuin 1 |
| ERRα | Estrogen-related receptor alpha |
| FoxO1 | Forkhead box O1 |
| MSCs | Mesenchymal stem cells |
| AICAR | 5-aminoimidazole-4-carboxamide ribonucleotide |
| TNF-α | Tumor necrosis factor-alfa |
| AMPK | AMP-activated protein kinase |
| RUNX2 | Runt-related transcription factor 2 |
| FoxO3 | Forkhead box O3 |
| ROS | Reactive oxygen species |
| ALP | Alkaline phosphatase |
| OCN | Osteocalcin |
| OPN | Osteopontin |
| Col-I | Type I collagen |
| Osx | Osterix |
| SOD | Superoxide dismutase |
| Bcl-2 | B-cell lymphoma 2 |
| Bax | Bcl-2-associated X protein |
| mTORC1 | mTOR complex 1 |
| GDF15 | Growth differentiation factor 15 |
| STAT3 | Signal transduced and activator of transcription 3 |
| BMD | Bone mineral density |
| CTX-1 | C-terminal telopeptide of type 1 collagen |
| RPMs | Rapamycin-loaded poly(lactic-co-glycolic acid) microparticles |
| BMSCs | Bone marrow stem cells |
| VEGF | Vascular endothelial growth factor |
| ANG-1 | Angiopoietin-1 |
| ERK | Extracellular signal-regulated kinase |
| ITGB1 | Integrin subunit beta 1 |
| FAK | Focal adhesion kinase |
| IGF-1R | Insulin-like growth factor 1 receptor |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| IL-4 | Interleukin-4 |
| IL-10 | Interleukin-10 |
| CD206 | Cluster of differentiation 206 |
| IL-1β | Interleukin-1 beta |
| CCR7 | C-C motif chemokine receptor 7 |
| iNOS | Inducible nitric oxide synthase |
| ATF | α-tocopherol |
| TRF | Tocotrienol-rich fraction |
| NAC | N-acetylcysteine |
| GSH/GSSG | Reduced/oxidized glutathione |
| SIRT3 | Sirtuin 3 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| IL-6 | Interleukin-6 |
| TGF-β | Transforming growth factor beta |
| NO | Nitric oxide |
| PGE2 | Prostaglandin E2 |
| COX-2 | Cyclooxygenase-2 |
| MMP-3 | Matrix metalloproteinase-3 |
| MMP-13 | Matrix metalloproteinase-13 |
| ADAMTS-5 | A disintegrin and metalloproteinase with thrombospondin motifs 5 |
| IGF-1 | Insulin-like growth factor 1 |
| MuRF1 | Muscle ring-finger protein-1 |
| SMAD | Suppressor of mothers against decapentaplegic homolog |
| PI3K | Phosphoinositide 3-kinase |
| Bcl-xL | B-cell lymphoma-extra large |
| BMP-2 | Morphogenetic protein 2 |
| PRG4 | Proteoglycan 4 |
| ACAN | Aggrecan |
| NOS2 | Nitric oxide synthase |
| OPG | Osteoprotegerin |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| NAD+/NADH | Nicotinamide adenine dinucleotide/reduced NAD+ |
| SA-β-gal | Senescence-associated β-galactosidase |
| MDA | Malondialdehyde |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| UCP3 | Uncoupling protein 3 |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| WBV | Whole body vibration |
| FNDC5 | Fibronectin type III domain-containing protein 5 |
| NOX4 | NADPH oxidase 4 |
| CAT | Catalase |
| SOD2 | Superoxide dismutase 2 |
| BAIBA | β-aminoisobutyric acid |
| BDNF | Brain-derived neurotrophic factor |
| EGF | Epidermal growth factor |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 |
| Mfn2 | Mitofusin 2 |
| Drp1 | Dynamin-related protein 1 |
| PINK1 | PTEN-induced kinase 1 |
| RCT | Randomized controlled trial |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| P1NP | Procollagen type 1 N-terminal propeptide |
| MVF | Maximal voluntary force |
| RSMI | Relative skeletal mass index |
| IL-2 | Interleukin-2 |
| TUG | Timed up and go |
| SPPB | Short physical performance battery |
| CAF22 | C-terminal agrin-fragment-22 |
| NfL | Neurofilament light chain |
References
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting Cellular Senescence with Senotherapeutics: Senolytics and Senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Cariati, I.; Scimeca, M.; Bonanni, R.; Triolo, R.; Naldi, V.; Toro, G.; Marini, M.; Tancredi, V.; Iundusi, R.; Gasbarra, E.; et al. Role of Myostatin in Muscle Degeneration by Random Positioning Machine Exposure: An in Vitro Study for the Treatment of Sarcopenia. Front. Physiol. 2022, 13, 782000. [Google Scholar] [CrossRef]
- Tarantino, U.; Cariati, I.; Greggi, C.; Iundusi, R.; Gasbarra, E.; Iolascon, G.; Kurth, A.; Akesson, K.E.; Bouxsein, M.; Tranquilli Leali, P.; et al. Gaps and Alternative Surgical and Non-Surgical Approaches in the Bone Fragility Management: An Updated Review. Osteoporos. Int. 2022, 33, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Kletsas, D. Plant-Derived Senotherapeutics for the Prevention and Treatment of Intervertebral Disc Degeneration and Aging. Metabolites 2024, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Gil, J. Senescence and the SASP: Many Therapeutic Avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Che, L.; Chen, X.; He, Z.; Song, D.; Yuan, Y.; Liu, C. Repurpose Dasatinib and Quercetin: Targeting Senescent Cells Ameliorates Postmenopausal Osteoporosis and Rejuvenates Bone Regeneration. Bioact. Mater. 2023, 25, 13–28. [Google Scholar] [CrossRef]
- Sharma, A.K.; Roberts, R.L.; Benson, R.D.J.; Pierce, J.L.; Yu, K.; Hamrick, M.W.; McGee-Lawrence, M.E. The Senolytic Drug Navitoclax (ABT-263) Causes Trabecular Bone Loss and Impaired Osteoprogenitor Function in Aged Mice. Front. Cell Dev. Biol. 2020, 8, 354. [Google Scholar] [CrossRef]
- Chin, A.F.; Han, J.; Clement, C.C.; Choi, Y.; Zhang, H.; Browne, M.; Jeon, O.H.; Elisseeff, J.H. Senolytic Treatment Reduces Oxidative Protein Stress in an Aging Male Murine Model of Post-Traumatic Osteoarthritis. Aging Cell 2023, 22, e13979. [Google Scholar] [CrossRef]
- Ham, D.J.; Börsch, A.; Chojnowska, K.; Lin, S.; Leuchtmann, A.B.; Ham, A.S.; Thürkauf, M.; Delezie, J.; Furrer, R.; Burri, D.; et al. Distinct and Additive Effects of Calorie Restriction and Rapamycin in Aging Skeletal Muscle. Nat. Commun. 2022, 13, 2025. [Google Scholar] [CrossRef]
- Receno, C.N.; Liang, C.; Korol, D.L.; Atalay, M.; Heffernan, K.S.; Brutsaert, T.D.; DeRuisseau, K.C. Effects of Prolonged Dietary Curcumin Exposure on Skeletal Muscle Biochemical and Functional Responses of Aged Male Rats. Int. J. Mol. Sci. 2019, 20, 1178. [Google Scholar] [CrossRef]
- Falvino, A.; Gasperini, B.; Cariati, I.; Bonanni, R.; Chiavoghilefu, A.; Gasbarra, E.; Botta, A.; Tancredi, V.; Tarantino, U. Cellular Senescence: The Driving Force of Musculoskeletal Diseases. Biomedicines 2024, 12, 1948. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, W.; Wang, B.; Wang, X.; Gong, P.; Xiong, Y. Resveratrol Promotes Osteogenesis via Activating SIRT1/FoxO1 Pathway in Osteoporosis Mice. Life Sci. 2020, 246, 117422. [Google Scholar] [CrossRef] [PubMed]
- Hambright, W.S.; Mu, X.; Gao, X.; Guo, P.; Kawakami, Y.; Mitchell, J.; Mullen, M.; Nelson, A.-L.; Bahney, C.; Nishimura, H.; et al. The Senolytic Drug Fisetin Attenuates Bone Degeneration in the Zmpste24(−/−) Progeria Mouse Model. J. Osteoporos. 2023, 2023, 5572754. [Google Scholar] [CrossRef]
- Li, M.; Yu, Y.; Xue, K.; Li, J.; Son, G.; Wang, J.; Qian, W.; Wang, S.; Zheng, J.; Yang, C.; et al. Genistein Mitigates Senescence of Bone Marrow Mesenchymal Stem Cells via ERRα-Mediated Mitochondrial Biogenesis and Mitophagy in Ovariectomized Rats. Redox Biol. 2023, 61, 102649. [Google Scholar] [CrossRef]
- Ohzono, H.; Hu, Y.; Nagira, K.; Kanaya, H.; Okubo, N.; Olmer, M.; Gotoh, M.; Kurakazu, I.; Akasaki, Y.; Kawata, M.; et al. Targeting FoxO Transcription Factors with HDAC Inhibitors for the Treatment of Osteoarthritis. Ann. Rheum. Dis. 2023, 82, 262–271. [Google Scholar] [CrossRef]
- Bang, S.; Kim, D.-E.; Kang, H.-T.; Lee, J.H. Metformin Restores Autophagic Flux and Mitochondrial Function in Late Passage Myoblast to Impede Age-Related Muscle Loss. Biomed. Pharmacother. 2024, 180, 116981. [Google Scholar] [CrossRef]
- Farr, J.N.; Khosla, S. Cellular Senescence in Bone. Bone 2019, 121, 121–133. [Google Scholar] [CrossRef]
- Ansari, M.M.; Ghosh, M.; Lee, D.-S.; Son, Y.-O. Senolytic Therapeutics: An Emerging Treatment Modality for Osteoarthritis. Ageing Res. Rev. 2024, 96, 102275. [Google Scholar] [CrossRef]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise Attenuates the Major Hallmarks of Aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Cariati, I.; Scimeca, M.; Pallone, G.; Bonanno, E.; Tancredi, V.; D’Arcangelo, G.; Frank, C. Effects of Short-Term Aerobic Exercise in a Mouse Model of Niemann-Pick Type C Disease on Synaptic and Muscle Plasticity. Ann. Ist. Super. Sanita 2019, 55, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Cariati, I.; Bonanni, R.; Pallone, G.; Scimeca, M.; Frank, C.; Tancredi, V.; D’Arcangelo, G. Hippocampal Adaptations to Continuous Aerobic Training: A Functional and Ultrastructural Evaluation in a Young Murine Model. J. Funct. Morphol. Kinesiol. 2021, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- McGregor, N.E.; Walker, E.C.; Chan, A.S.; Poulton, I.J.; Cho, E.H.-J.; Windahl, S.H.; Sims, N.A. STAT3 Hyperactivation Due to SOCS3 Deletion in Murine Osteocytes Accentuates Responses to Exercise- and Load-Induced Bone Formation. J. Bone Miner. Res. 2022, 37, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Sherk, V.D.; Rosen, C.J. Senescent and Apoptotic Osteocytes and Aging: Exercise to the Rescue? Bone 2019, 121, 255–258. [Google Scholar] [CrossRef]
- Yoon, K.J.; Zhang, D.; Kim, S.-J.; Lee, M.-C.; Moon, H.Y. Exercise-Induced AMPK Activation Is Involved in Delay of Skeletal Muscle Senescence. Biochem. Biophys. Res. Commun. 2019, 512, 604–610. [Google Scholar] [CrossRef]
- Falvino, A.; Bonanni, R.; Gasperini, B.; Cariati, I.; Chiavoghilefu, A.; Smakaj, A.; Visconti, V.V.; Botta, A.; Iundusi, R.; Gasbarra, E.; et al. Altered Expression of Cell Cycle Regulators and Factors Released by Aged Cells in Skeletal Muscle of Patients with Bone Fragility: A Pilot Study on the Potential Role of SIRT1 in Muscle Atrophy. Biomedicines 2025, 13, 1350. [Google Scholar] [CrossRef]
- Englund, D.A.; Sakamoto, A.E.; Fritsche, C.M.; Heeren, A.A.; Zhang, X.; Kotajarvi, B.R.; Lecy, D.R.; Yousefzadeh, M.J.; Schafer, M.J.; White, T.A.; et al. Exercise Reduces Circulating Biomarkers of Cellular Senescence in Humans. Aging Cell 2021, 20, e13415. [Google Scholar] [CrossRef]
- Gurău, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafè, M.; Olivieri, F. Anti-Senescence Compounds: A Potential Nutraceutical Approach to Healthy Aging. Ageing Res. Rev. 2018, 46, 14–31. [Google Scholar] [CrossRef]
- Mechchate, H.; El Allam, A.; El Omari, N.; El Hachlafi, N.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Bouyahya, A. Vegetables and Their Bioactive Compounds as Anti-Aging Drugs. Molecules 2022, 27, 2316. [Google Scholar] [CrossRef]
- Mobasheri, A.; Shakibaei, M. Osteogenic Effects of Resveratrol in Vitro: Potential for the Prevention and Treatment of Osteoporosis. Ann. N. Y. Acad. Sci. 2013, 1290, 59–66. [Google Scholar] [CrossRef]
- Hosoda, R.; Nakashima, R.; Yano, M.; Iwahara, N.; Asakura, S.; Nojima, I.; Saga, Y.; Kunimoto, R.; Horio, Y.; Kuno, A. Resveratrol, a SIRT1 Activator, Attenuates Aging-Associated Alterations in Skeletal Muscle and Heart in Mice. J. Pharmacol. Sci. 2023, 152, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and Aging Related Signaling Pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS Signaling under Metabolic Stress: Cross-Talk between AMPK and AKT Pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.-C.; Hou, S.-M.; Chen, R.-J.; Peng, H.-W.; Hsieh, C.-F.; Kuo, M.-L.; Yen, M.-L. Resveratrol Promotes Osteogenesis of Human Mesenchymal Stem Cells by Upregulating RUNX2 Gene Expression via the SIRT1/FOXO3A Axis. J. Bone Miner. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef]
- Bäckesjö, C.-M.; Li, Y.; Lindgren, U.; Haldosén, L.-A. Activation of Sirt1 Decreases Adipocyte Formation during Osteoblast Differentiation of Mesenchymal Stem Cells. Cells Tissues Organs 2009, 189, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Knutson, M.D.; Leeuwenburgh, C. Resveratrol and Novel Potent Activators of SIRT1: Effects on Aging and Age-Related Diseases. Nutr. Rev. 2008, 66, 591–596. [Google Scholar] [CrossRef]
- Zhou, T.; Yan, Y.; Zhao, C.; Xu, Y.; Wang, Q.; Xu, N. Resveratrol Improves Osteogenic Differentiation of Senescent Bone Mesenchymal Stem Cells through Inhibiting Endogenous Reactive Oxygen Species Production via AMPK Activation. Redox Rep. 2019, 24, 62–69. [Google Scholar] [CrossRef]
- Liao, Z.-Y.; Chen, J.-L.; Xiao, M.-H.; Sun, Y.; Zhao, Y.-X.; Pu, D.; Lv, A.-K.; Wang, M.-L.; Zhou, J.; Zhu, S.-Y.; et al. The Effect of Exercise, Resveratrol or Their Combination on Sarcopenia in Aged Rats via Regulation of AMPK/Sirt1 Pathway. Exp. Gerontol. 2017, 98, 177–183. [Google Scholar] [CrossRef]
- Selvarani, R.; Mohammed, S.; Richardson, A. Effect of Rapamycin on Aging and Age-Related Diseases-Past and Future. GeroScience 2021, 43, 1135–1158. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Fernández-Fernández, C.; López-Pereiro, Y.; Castro-Calvo, I.; Carneiro-Freire, N. The Effects of Glucagon and the Target of Rapamycin (TOR) on Skeletal Muscle Protein Synthesis and Age-Dependent Sarcopenia in Humans. Clin. Nutr. ESPEN 2021, 44, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Shrager, J.B.; Goldman, D. Rapamycin Protects Aging Muscle. Aging 2019, 11, 5868–5870. [Google Scholar] [CrossRef]
- Lamming, D.W. Inhibition of the Mechanistic Target of Rapamycin (MTOR)-Rapamycin and Beyond. Cold Spring Harb. Perspect. Med. 2016, 6, a025924. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Inoki, K.; Brooks, S.V.; Okazawa, H.; Lee, M.; Wang, J.; Kim, M.; Kennedy, C.L.; Macpherson, P.C.D.; Ji, X.; et al. MTORC1 Underlies Age-Related Muscle Fiber Damage and Loss by Inducing Oxidative Stress and Catabolism. Aging Cell 2019, 18, e12943. [Google Scholar] [CrossRef]
- Martin, S.A.; Riordan, R.T.; Wang, R.; Yu, Z.; Aguirre-Burk, A.M.; Wong, C.P.; Olson, D.A.; Branscum, A.J.; Turner, R.T.; Iwaniec, U.T.; et al. Rapamycin Impairs Bone Accrual in Young Adult Mice Independent of Nrf2. Exp. Gerontol. 2021, 154, 111516. [Google Scholar] [CrossRef]
- Dhanabalan, K.M.; Dravid, A.A.; Agarwal, S.; Sharath, R.K.; Padmanabhan, A.K.; Agarwal, R. Intra-Articular Injection of Rapamycin Microparticles Prevent Senescence and Effectively Treat Osteoarthritis. Bioeng. Transl. Med. 2023, 8, e10298. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence. Int. J. Mol. Sci. 2020, 21, 6448. [Google Scholar] [CrossRef]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin Attenuates Mitochondrial Dysfunction and Biogenesis via Upregulated AMPK/SIRT1 Signaling Pathway in OA Rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. Quercetin, a Potent Suppressor of NF-ΚB and Smad Activation in Osteoblasts. Int. J. Mol. Med. 2011, 28, 521–525. [Google Scholar] [CrossRef]
- Feng, Y.; Dang, X.; Zheng, P.; Liu, Y.; Liu, D.; Che, Z.; Yao, J.; Lin, Z.; Liao, Z.; Nie, X.; et al. Quercetin in Osteoporosis Treatment: A Comprehensive Review of Its Mechanisms and Therapeutic Potential. Curr. Osteoporos. Rep. 2024, 22, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Min, J.; Zhao, Y.; Cheng, Q.; Wang, K.; Lin, S.; Luo, J.; Liu, H. Quercetin Rescued TNF-Alpha-Induced Impairments in Bone Marrow-Derived Mesenchymal Stem Cell Osteogenesis and Improved Osteoporosis in Rats. Am. J. Transl. Res. 2018, 10, 4313–4321. [Google Scholar] [PubMed]
- Zhou, Y.; Wu, Y.; Jiang, X.; Zhang, X.; Xia, L.; Lin, K.; Xu, Y. The Effect of Quercetin on the Osteogenesic Differentiation and Angiogenic Factor Expression of Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0129605. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Sharma, A.R.; Lee, Y.-H.; Chatterjee, S.; Choi, Y.J.; Rajvansh, R.; Chakraborty, C.; Lee, S.-S. Therapeutic Potential of Quercetin as an Antioxidant for Bone-Muscle-Tendon Regeneration and Aging. Aging Dis. 2024, 16, 1414–1437. [Google Scholar] [CrossRef] [PubMed]
- Hour, T.-C.; Vo, T.C.T.; Chuu, C.-P.; Chang, H.-W.; Su, Y.-F.; Chen, C.-H.; Chen, Y.-K. The Promotion of Migration and Myogenic Differentiation in Skeletal Muscle Cells by Quercetin and Underlying Mechanisms. Nutrients 2022, 14, 4106. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liang, H.; Ji, Y.; Kou, H.; Zhang, C.; Shang, G.; Shang, C.; Song, Z.; Yang, L.; Liu, L.; et al. Curcumin Modulates the Crosstalk Between Macrophages and Bone Mesenchymal Stem Cells to Ameliorate Osteogenesis. Front. Cell Dev. Biol. 2021, 9, 634650. [Google Scholar] [CrossRef]
- Khor, S.C.; Abdul Karim, N.; Ngah, W.Z.W.; Yusof, Y.A.M.; Makpol, S. Vitamin E in Sarcopenia: Current Evidences on Its Role in Prevention and Treatment. Oxidative Med. Cell. Longev. 2014, 2014, 914853. [Google Scholar] [CrossRef]
- Howard, A.C.; McNeil, A.K.; McNeil, P.L. Promotion of Plasma Membrane Repair by Vitamin E. Nat. Commun. 2011, 2, 597. [Google Scholar] [CrossRef]
- Lim, J.J.; Ngah, W.Z.W.; Mouly, V.; Abdul Karim, N. Reversal of Myoblast Aging by Tocotrienol Rich Fraction Posttreatment. Oxidative Med. Cell. Longev. 2013, 2013, 978101. [Google Scholar] [CrossRef]
- Khor, S.C.; Wan Ngah, W.Z.; Mohd Yusof, Y.A.; Abdul Karim, N.; Makpol, S. Tocotrienol-Rich Fraction Ameliorates Antioxidant Defense Mechanisms and Improves Replicative Senescence-Associated Oxidative Stress in Human Myoblasts. Oxidative Med. Cell. Longev. 2017, 2017, 3868305. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, Z.; You, S.; Zhang, H.; Tao, Z.; Wang, Q.; Chen, H.; Wu, Y. Fisetin Inhibits IL-1β-Induced Inflammatory Response in Human Osteoarthritis Chondrocytes through Activating SIRT1 and Attenuates the Progression of Osteoarthritis in Mice. Int. Immunopharmacol. 2017, 45, 135–147. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Navarrete-Yañez, V.; Ramirez, L.; Galera, L.; Mendez-Bolaina, E.; Najera, V.; Ceballos, G.; Villarreal, F. Restorative Effects of (+)-Epicatechin in a Rodent Model of Aging Induced Muscle Atrophy: Underlying Mechanisms. Food Funct. 2024, 15, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ Heel of Senescent Cells: From Transcriptome to Senolytic Drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Deng, B.; Lin, A.; Zhang, G.; Ma, M.; Wang, Y.; Yang, Y.; Kang, X. The PI3K/AKT Signalling Pathway in Inflammation, Cell Death and Glial Scar Formation after Traumatic Spinal Cord Injury: Mechanisms and Therapeutic Opportunities. Cell Prolif. 2022, 55, e13275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zheng, L.; Dai, G.; Sun, Y.; He, R.; Liu, Z.; Jin, Y.; Wu, T.; Hu, J.; Cao, Y.; et al. Senolytics Cocktail Dasatinib and Quercetin Alleviate Chondrocyte Senescence and Facet Joint Osteoarthritis in Mice. Spine J. 2025, 25, 184–198. [Google Scholar] [CrossRef]
- Yue, J.; Aobulikasimu, A.; Sun, W.; Liu, S.; Xie, W.; Sun, W. Targeted Regulation of FoxO1 in Chondrocytes Prevents Age-Related Osteoarthritis via Autophagy Mechanism. J. Cell. Mol. Med. 2022, 26, 3075–3082. [Google Scholar] [CrossRef]
- Cheng, F.-F.; Liu, Y.-L.; Du, J.; Lin, J.-T. Metformin’s Mechanisms in Attenuating Hallmarks of Aging and Age-Related Disease. Aging Dis. 2022, 13, 970–986. [Google Scholar] [CrossRef]
- Mai, Q.-G.; Zhang, Z.-M.; Xu, S.; Lu, M.; Zhou, R.-P.; Zhao, L.; Jia, C.-H.; Wen, Z.-H.; Jin, D.-D.; Bai, X.-C. Metformin Stimulates Osteoprotegerin and Reduces RANKL Expression in Osteoblasts and Ovariectomized Rats. J. Cell. Biochem. 2011, 112, 2902–2909. [Google Scholar] [CrossRef]
- Wang, C.; Yao, Z.; Zhang, Y.; Yang, Y.; Liu, J.; Shi, Y.; Zhang, C. Metformin Mitigates Cartilage Degradation by Activating AMPK/SIRT1-Mediated Autophagy in a Mouse Osteoarthritis Model. Front. Pharmacol. 2020, 11, 1114. [Google Scholar] [CrossRef]
- Chen, X.-K.; Yi, Z.-N.; Wong, G.T.-C.; Hasan, K.M.M.; Kwan, J.S.-K.; Ma, A.C.-H.; Chang, R.C.-C. Is Exercise a Senolytic Medicine? A Systematic Review. Aging Cell 2021, 20, e13294. [Google Scholar] [CrossRef]
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef]
- Zhang, X.; Englund, D.A.; Aversa, Z.; Jachim, S.K.; White, T.A.; LeBrasseur, N.K. Exercise Counters the Age-Related Accumulation of Senescent Cells. Exerc. Sport Sci. Rev. 2022, 50, 213–221. [Google Scholar] [CrossRef]
- Mora, J.C.; Valencia, W.M. Exercise and Older Adults. Clin. Geriatr. Med. 2018, 34, 145–162. [Google Scholar] [CrossRef]
- Spaulding, H.R.; Yan, Z. AMPK and the Adaptation to Exercise. Annu. Rev. Physiol. 2022, 84, 209–227. [Google Scholar] [CrossRef]
- White, A.T.; Schenk, S. NAD+/NADH and Skeletal Muscle Mitochondrial Adaptations to Exercise. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E308–E321. [Google Scholar] [CrossRef]
- Pyla, R.; Hartney, T.J.; Segar, L. AICAR Promotes Endothelium-Independent Vasorelaxation by Activating AMP-Activated Protein Kinase via Increased ZMP and Decreased ATP/ADP Ratio in Aortic Smooth Muscle. J. Basic Clin. Physiol. Pharmacol. 2022, 33, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yang, X.; Li, J.; Shu, Z.; Dai, J.; Liu, X.; Li, B.; Jia, S.; Kou, X.; Yang, Y.; et al. Spermidine Coupled with Exercise Rescues Skeletal Muscle Atrophy from D-Gal-Induced Aging Rats through Enhanced Autophagy and Reduced Apoptosis via AMPK-FOXO3a Signal Pathway. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Suzuki, K.; Posa, A.; Petrovszky, Z.; Koltai, E.; Boldogh, I. The Systemic Role of SIRT1 in Exercise Mediated Adaptation. Redox Biol. 2020, 35, 101467. [Google Scholar] [CrossRef]
- Gurd, B.J. Deacetylation of PGC-1α by SIRT1: Importance for Skeletal Muscle Function and Exercise-Induced Mitochondrial Biogenesis. Appl. Physiol. Nutr. Metab. 2011, 36, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; de Souza, C.T. Multi-Regulatory Network of ROS: The Interconnection of ROS, PGC-1 Alpha, and AMPK-SIRT1 during Exercise. J. Physiol. Biochem. 2017, 73, 487–494. [Google Scholar] [CrossRef]
- Brown, E.L.; Foletta, V.C.; Wright, C.R.; Sepulveda, P.V.; Konstantopoulos, N.; Sanigorski, A.; Della Gatta, P.; Cameron-Smith, D.; Kralli, A.; Russell, A.P. PGC-1α and PGC-1β Increase Protein Synthesis via ERRα in C2C12 Myotubes. Front. Physiol. 2018, 9, 1336. [Google Scholar] [CrossRef]
- Billon, C.; Sitaula, S.; Banerjee, S.; Welch, R.; Elgendy, B.; Hegazy, L.; Oh, T.G.; Kazantzis, M.; Chatterjee, A.; Chrivia, J.; et al. Synthetic ERRα/β/γ Agonist Induces an ERRα-Dependent Acute Aerobic Exercise Response and Enhances Exercise Capacity. ACS Chem. Biol. 2023, 18, 756–771. [Google Scholar] [CrossRef]
- Bonanni, R.; Falvino, A.; Matticari, A.; Rinaldi, A.M.; D’Arcangelo, G.; Cifelli, P.; Iundusi, R.; Gasbarra, E.; Tancredi, V.; Cariati, I.; et al. Targeting ERRs to Counteract Age-Related Muscle Atrophy Associated with Physical Inactivity: A Pilot Study. Front. Physiol. 2025, 16, 1616693. [Google Scholar] [CrossRef]
- Huang, C.-C.; Wang, T.; Tung, Y.-T.; Lin, W.-T. Effect of Exercise Training on Skeletal Muscle SIRT1 and PGC-1α Expression Levels in Rats of Different Age. Int. J. Med. Sci. 2016, 13, 260–270. [Google Scholar] [CrossRef]
- Koltai, E.; Szabo, Z.; Atalay, M.; Boldogh, I.; Naito, H.; Goto, S.; Nyakas, C.; Radak, Z. Exercise Alters SIRT1, SIRT6, NAD and NAMPT Levels in Skeletal Muscle of Aged Rats. Mech. Ageing Dev. 2010, 131, 21–28. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Romagnoli, C.; Caprioli, L.; D’Arcangelo, G.; Tancredi, V.; Annino, G. Bone Adaptations to a Whole Body Vibration Protocol in Murine Models of Different Ages: A Preliminary Study on Structural Changes and Biomarker Evaluation. J. Funct. Morphol. Kinesiol. 2025, 10, 26. [Google Scholar] [CrossRef]
- Cariati, I.; Bonanni, R.; Rinaldi, A.M.; Marini, M.; Iundusi, R.; Gasbarra, E.; Tancredi, V.; Tarantino, U. Recombinant Irisin Prevents Cell Death and Mineralization Defects Induced by Random Positioning Machine Exposure in Primary Cultures of Human Osteoblasts: A Promising Strategy for the Osteoporosis Treatment. Front. Physiol. 2023, 14, 1107933. [Google Scholar] [CrossRef] [PubMed]
- Jing, E.; Emanuelli, B.; Hirschey, M.D.; Boucher, J.; Lee, K.Y.; Lombard, D.; Verdin, E.M.; Kahn, C.R. Sirtuin-3 (Sirt3) Regulates Skeletal Muscle Metabolism and Insulin Signaling via Altered Mitochondrial Oxidation and Reactive Oxygen Species Production. Proc. Natl. Acad. Sci. USA 2011, 108, 14608–14613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Pinho, R.; Gu, Y.; Radak, Z. The Role of SIRT3 in Exercise and Aging. Cells 2022, 11, 2596. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Qin, A.; Liu, D.; Ruan, R.; Wang, Q.; Yuan, J.; Cheng, T.S.; Filipovska, A.; Papadimitriou, J.M.; Dai, K.; et al. Endoplasmic Reticulum Mediates Mitochondrial Transfer within the Osteocyte Dendritic Network. Sci. Adv. 2019, 5, eaaw7215. [Google Scholar] [CrossRef]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 Maintains Osteoblast Differentiation and Bone Formation by Regulating Mitochondrial Stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef]
- Johnson, M.L.; Irving, B.A.; Lanza, I.R.; Vendelbo, M.H.; Konopka, A.R.; Robinson, M.M.; Henderson, G.C.; Klaus, K.A.; Morse, D.M.; Heppelmann, C.; et al. Differential Effect of Endurance Training on Mitochondrial Protein Damage, Degradation, and Acetylation in the Context of Aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2015, 70, 1386–1393. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, R.; Feng, Y.; Cheng, L. Molecular Mechanisms of Exercise Contributing to Tissue Regeneration. Signal Transduct. Target. Ther. 2022, 7, 383. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via AV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef]
- Kitase, Y.; Vallejo, J.A.; Gutheil, W.; Vemula, H.; Jähn, K.; Yi, J.; Zhou, J.; Brotto, M.; Bonewald, L.F. β-Aminoisobutyric Acid, l-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor. Cell Rep. 2018, 22, 1531–1544. [Google Scholar] [CrossRef]
- Chen, Z.; Li, L.; Wu, W.; Liu, Z.; Huang, Y.; Yang, L.; Luo, Q.; Chen, J.; Hou, Y.; Song, G. Exercise Protects Proliferative Muscle Satellite Cells against Exhaustion via the Igfbp7-Akt-MTOR Axis. Theranostics 2020, 10, 6448–6466. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Chen, J. Mammalian Target of Rapamycin (MTOR) Signaling Network in Skeletal Myogenesis. J. Biol. Chem. 2012, 287, 43928–43935. [Google Scholar] [CrossRef] [PubMed]
- Mannick, J.B.; Lamming, D.W. Targeting the Biology of Aging with MTOR Inhibitors. Nat. Aging 2023, 3, 642–660. [Google Scholar] [CrossRef]
- Bae, J.H.; Seo, D.Y.; Lee, S.H.; Shin, C.; Jamrasi, P.; Han, J.; Song, W. Effects of Exercise on AKT/PGC1-α/FOXO3a Pathway and Muscle Atrophy in Cisplatin-Administered Rat Skeletal Muscle. Korean J. Physiol. Pharmacol. 2021, 25, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liang, J.; Wu, L.; Zhang, H.; Lv, J.; Chen, N. Exercise-Induced Autophagy Suppresses Sarcopenia Through Akt/MTOR and Akt/FoxO3a Signal Pathways and AMPK-Mediated Mitochondrial Quality Control. Front. Physiol. 2020, 11, 583478. [Google Scholar] [CrossRef]
- Yan, X.; Shen, Z.; Yu, D.; Zhao, C.; Zou, H.; Ma, B.; Dong, W.; Chen, W.; Huang, D.; Yu, Z. Nrf2 Contributes to the Benefits of Exercise Interventions on Age-Related Skeletal Muscle Disorder via Regulating Drp1 Stability and Mitochondrial Fission. Free Radic. Biol. Med. 2022, 178, 59–75. [Google Scholar] [CrossRef]
- Huang, D.-D.; Fan, S.-D.; Chen, X.-Y.; Yan, X.-L.; Zhang, X.-Z.; Ma, B.-W.; Yu, D.-Y.; Xiao, W.-Y.; Zhuang, C.-L.; Yu, Z. Nrf2 Deficiency Exacerbates Frailty and Sarcopenia by Impairing Skeletal Muscle Mitochondrial Biogenesis and Dynamics in an Age-Dependent Manner. Exp. Gerontol. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol Enhances Exercise-Induced Cellular and Functional Adaptations of Skeletal Muscle in Older Men and Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef]
- Harper, S.A.; Bassler, J.R.; Peramsetty, S.; Yang, Y.; Roberts, L.M.; Drummer, D.; Mankowski, R.T.; Leeuwenburgh, C.; Ricart, K.; Patel, R.P.; et al. Resveratrol and Exercise Combined to Treat Functional Limitations in Late Life: A Pilot Randomized Controlled Trial. Exp. Gerontol. 2021, 143, 111111. [Google Scholar] [CrossRef]
- Wong, R.H.; Thaung Zaw, J.J.; Xian, C.J.; Howe, P.R. Regular Supplementation With Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef]
- Bo, S.; Gambino, R.; Ponzo, V.; Cioffi, I.; Goitre, I.; Evangelista, A.; Ciccone, G.; Cassader, M.; Procopio, M. Effects of Resveratrol on Bone Health in Type 2 Diabetic Patients. A Double-Blind Randomized-Controlled Trial. Nutr. Diabetes 2018, 8, 51. [Google Scholar] [CrossRef]
- Hussain, S.A.; Marouf, B.H.; Ali, Z.S.; Ahmmad, R.S. Efficacy and Safety of Co-Administration of Resveratrol with Meloxicam in Patients with Knee Osteoarthritis: A Pilot Interventional Study. Clin. Interv. Aging 2018, 13, 1621–1630. [Google Scholar] [CrossRef]
- Vesely, O.; Baldovska, S.; Kolesarova, A. Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids. Nutrients 2021, 13, 3095. [Google Scholar] [CrossRef]
- Moel, M.; Harinath, G.; Lee, V.; Nyquist, A.; Morgan, S.L.; Isman, A.; Zalzala, S. Influence of Rapamycin on Safety and Healthspan Metrics after One Year: PEARL Trial Results. Aging 2025, 17, 908–936. [Google Scholar] [CrossRef] [PubMed]
- Kraig, E.; Linehan, L.A.; Liang, H.; Romo, T.Q.; Liu, Q.; Wu, Y.; Benavides, A.D.; Curiel, T.J.; Javors, M.A.; Musi, N.; et al. A Randomized Control Trial to Establish the Feasibility and Safety of Rapamycin Treatment in an Older Human Cohort: Immunological, Physical Performance, and Cognitive Effects. Exp. Gerontol. 2018, 105, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Lamming, D.W. Blazing a Trail for the Clinical Use of Rapamycin as a GeroprotecTOR. GeroScience 2023, 45, 2769–2783. [Google Scholar] [CrossRef] [PubMed]
- Mannick, J.B.; Del Giudice, G.; Lattanzi, M.; Valiante, N.M.; Praestgaard, J.; Huang, B.; Lonetto, M.A.; Maecker, H.T.; Kovarik, J.; Carson, S.; et al. MTOR Inhibition Improves Immune Function in the Elderly. Sci. Transl. Med. 2014, 6, 268ra179. [Google Scholar] [CrossRef] [PubMed]
- Harinath, G.; Lee, V.; Nyquist, A.; Moel, M.; Wouters, M.; Hagemeier, J.; Verkennes, B.; Tacubao, C.; Nasher, S.; Kauppi, K.; et al. The Bioavailability and Blood Levels of Low-Dose Rapamycin for Longevity in Real-World Cohorts of Normative Aging Individuals. GeroScience 2025, 1–14. [Google Scholar] [CrossRef]
- Farr, J.N.; Atkinson, E.J.; Achenbach, S.J.; Volkman, T.L.; Tweed, A.J.; Vos, S.J.; Ruan, M.; Sfeir, J.; Drake, M.T.; Saul, D.; et al. Effects of Intermittent Senolytic Therapy on Bone Metabolism in Postmenopausal Women: A Phase 2 Randomized Controlled Trial. Nat. Med. 2024, 30, 2605–2612. [Google Scholar] [CrossRef]
- Bailly, A.R.; Hester, G.M.; Alesi, M.G.; Buresh, R.J.; Feito, Y.; Mermier, C.M.; Ducharme, J.B.; VanDusseldorp, T.A. Quercetins Efficacy on Bone and Inflammatory Markers, Body Composition, and Physical Function in Postmenopausal Women. J. Bone Miner. Metab. 2025, 43, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Takeda, R.; Ueda, S.; Igawa, K.; Hirono, T.; Okudaira, M.; Mita, Y.; Ohya, T.; Watanabe, K. Quercetin Ingestion Alters Motor Unit Behavior and Enhances Improvement in Muscle Strength Following Resistance Training in Older Adults: A Randomized, Double-Blind, Controlled Trial. Eur. J. Nutr. 2025, 64, 117. [Google Scholar] [CrossRef]
- Georgiou, N.; Kakava, M.G.; Routsi, E.A.; Petsas, E.; Stavridis, N.; Freris, C.; Zoupanou, N.; Moschovou, K.; Kiriakidi, S.; Mavromoustakos, T. Quercetin: A Potential Polydynamic Drug. Molecules 2023, 28, 8141. [Google Scholar] [CrossRef]
- Varma, K.; Amalraj, A.; Divya, C.; Gopi, S. The Efficacy of the Novel Bioavailable Curcumin (Cureit) in the Management of Sarcopenia in Healthy Elderly Subjects: A Randomized, Placebo-Controlled, Double-Blind Clinical Study. J. Med. Food 2021, 24, 40–49. [Google Scholar] [CrossRef]
- Kheiridoost, H.; Shakouri, S.K.; Shojaei-Zarghani, S.; Dolatkhah, N.; Farshbaf-Khalili, A. Efficacy of Nanomicelle Curcumin, Nigella Sativa Oil, and Their Combination on Bone Turnover Markers and Their Safety in Postmenopausal Women with Primary Osteoporosis and Osteopenia: A Triple-Blind Randomized Controlled Trial. Food Sci. Nutr. 2022, 10, 515–524. [Google Scholar] [CrossRef]
- Hatamipour, M.; Sahebkar, A.; Alavizadeh, S.H.; Dorri, M.; Jaafari, M.R. Novel Nanomicelle Formulation to Enhance Bioavailability and Stability of Curcuminoids. Iran. J. Basic Med. Sci. 2019, 22, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Vallibhakara, S.A.-O.; Nakpalat, K.; Sophonsritsuk, A.; Tantitham, C.; Vallibhakara, O. Effect of Vitamin E Supplement on Bone Turnover Markers in Postmenopausal Osteopenic Women: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients 2021, 13, 4226. [Google Scholar] [CrossRef]
- Bo, Y.; Liu, C.; Ji, Z.; Yang, R.; An, Q.; Zhang, X.; You, J.; Duan, D.; Sun, Y.; Zhu, Y.; et al. A High Whey Protein, Vitamin D and E Supplement Preserves Muscle Mass, Strength, and Quality of Life in Sarcopenic Older Adults: A Double-Blind Randomized Controlled Trial. Clin. Nutr. 2019, 38, 159–164. [Google Scholar] [CrossRef]
- Borel, P.; Preveraud, D.; Desmarchelier, C. Bioavailability of Vitamin E in Humans: An Update. Nutr. Rev. 2013, 71, 319–331. [Google Scholar] [CrossRef]
- Squadrito, F.; Imbalzano, E.; Rottura, M.; Arcoraci, V.; Pallio, G.; Catalano, A.; Atteritano, M.; Irrera, N.; Mannino, F.; Squadrito, G.; et al. Effects of Genistein Aglycone in Glucocorticoid Induced Osteoporosis: A Randomized Clinical Trial in Comparison with Alendronate. Biomed. Pharmacother. 2023, 163, 114821. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; D’Anna, R.; et al. Breast Safety and Efficacy of Genistein Aglycone for Postmenopausal Bone Loss: A Follow-up Study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef]
- Lappe, J.; Kunz, I.; Bendik, I.; Prudence, K.; Weber, P.; Recker, R.; Heaney, R.P. Effect of a Combination of Genistein, Polyunsaturated Fatty Acids and Vitamins D3 and K1 on Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled, Double-Blind Pilot Study. Eur. J. Nutr. 2013, 52, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the Phytoestrogen Genistein on Bone Metabolism in Osteopenic Postmenopausal Women: A Randomized Trial. Ann. Intern. Med. 2007, 146, 839–847. [Google Scholar] [CrossRef]
- Arcoraci, V.; Atteritano, M.; Squadrito, F.; D’Anna, R.; Marini, H.; Santoro, D.; Minutoli, L.; Messina, S.; Altavilla, D.; Bitto, A. Antiosteoporotic Activity of Genistein Aglycone in Postmenopausal Women: Evidence from a Post-Hoc Analysis of a Multicenter Randomized Controlled Trial. Nutrients 2017, 9, 179. [Google Scholar] [CrossRef]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and Pharmacokinetics of Genistein: Mechanistic Studies on Its ADME. Anti-Cancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef]
- Anupongsanugool, E.; Teekachunhatean, S.; Rojanasthien, N.; Pongsatha, S.; Sangdee, C. Pharmacokinetics of Isoflavones, Daidzein and Genistein, after Ingestion of Soy Beverage Compared with Soy Extract Capsules in Postmenopausal Thai Women. BMC Clin. Pharmacol. 2005, 5, 2. [Google Scholar] [CrossRef]
- Krishnakumar, I.M.; Jaja-Chimedza, A.; Joseph, A.; Balakrishnan, A.; Maliakel, B.; Swick, A. Enhanced Bioavailability and Pharmacokinetics of a Novel Hybrid-Hydrogel Formulation of Fisetin Orally Administered in Healthy Individuals: A Randomised Double-Blinded Comparative Crossover Study. J. Nutr. Sci. 2022, 11, e74. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, J.; Cielecka-Piontek, J. Fisetin-In Search of Better Bioavailability-From Macro to Nano Modifications: A Review. Int. J. Mol. Sci. 2023, 24, 14158. [Google Scholar] [CrossRef] [PubMed]
- Mafi, F.; Biglari, S.; Ghardashi Afousi, A.; Gaeini, A.A. Improvement in Skeletal Muscle Strength and Plasma Levels of Follistatin and Myostatin Induced by an 8-Week Resistance Training and Epicatechin Supplementation in Sarcopenic Older Adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Ottaviani, J.I.; van der Hooft, J.J.J.; Schroeter, H.; Crozier, A. Absorption, Metabolism, Distribution and Excretion of (-)-Epicatechin: A Review of Recent Findings. Mol. Asp. Med. 2018, 61, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Javed, M.; Khan, I.M.; Ahmad, F.; Karim, A. Metformin Improves Skeletal Muscle and Physical Capacity by Stabilizing Neuromuscular Junction in Older Adults. Arch. Gerontol. Geriatr. 2024, 127, 105587. [Google Scholar] [CrossRef]
- Hegazy, S.K. Evaluation of the Anti-Osteoporotic Effects of Metformin and Sitagliptin in Postmenopausal Diabetic Women. J. Bone Miner. Metab. 2015, 33, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Spielmann, G.; Yang, S.; Compton, S.L.E.; Jones, L.W.; Irwin, M.L.; Ligibel, J.A.; Meyerhardt, J.A. Effects of Exercise or Metformin on Myokine Concentrations in Patients with Breast and Colorectal Cancer: A Phase II Multi-Centre Factorial Randomized Trial. J. Cachexia Sarcopenia Muscle 2024, 15, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Wook Huh, H.; Na, Y.-G.; Kang, H.; Kim, M.; Han, M.; Mai Anh Pham, T.; Lee, H.; Baek, J.-S.; Lee, H.-K.; Cho, C.-W. Novel Self-Floating Tablet for Enhanced Oral Bioavailability of Metformin Based on Cellulose. Int. J. Pharm. 2021, 592, 120113. [Google Scholar] [CrossRef]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and Therapeutic Implications of Cellular Senescence in Osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and Age-Related Diseases: From Mechanisms to Therapeutic Strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantó, C. The Molecular Targets of Resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, J.; Li, L.; Lin, S.; Yang, Y.; Liu, T.; Zhang, T.; Xie, G.; Wu, D.; Xu, Y. Quercetin Alleviates Lipopolysaccharide-induced Acute Lung Injury by Inhibiting Ferroptosis via the Sirt1/Nrf2/Gpx4 Pathway. Int. J. Mol. Med. 2023, 52, 118. [Google Scholar] [CrossRef]
- Wang, K. The Potential Therapeutic Role of Curcumin in Osteoporosis Treatment: Based on Multiple Signaling Pathways. Front. Pharmacol. 2024, 15, 1446536. [Google Scholar] [CrossRef]
- Lu, R.; Zheng, Z.; Yin, Y.; Jiang, Z. Genistein Prevents Bone Loss in Type 2 Diabetic Rats Induced by Streptozotocin. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Yamaura, K.; Nelson, A.L.; Nishimura, H.; Rutledge, J.C.; Ravuri, S.K.; Bahney, C.; Philippon, M.J.; Huard, J. The Effects of Fisetin on Bone and Cartilage: A Systematic Review. Pharmacol. Res. 2022, 185, 106504. [Google Scholar] [CrossRef]
- Xing, H.; Liang, C.; Wang, C.; Xu, X.; Hu, Y.; Qiu, B. Metformin Mitigates Cholesterol Accumulation via the AMPK/SIRT1 Pathway to Protect Osteoarthritis Chondrocytes. Biochem. Biophys. Res. Commun. 2022, 632, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Jean, W.-H.; Hsieh, Y.-W.; Lai, L.-F.; Dewi, L.; Liao, Y.-C.; Ye, M.; Yu, S.-H.; Kao, C.-L.; Huang, C.-Y.; Kuo, C.-H. Senolytic Effect of High Intensity Interval Exercise on Human Skeletal Muscle. Aging 2023, 15, 765–776. [Google Scholar] [CrossRef]
- Jean, W.-H.; Lin, Y.-C.; Ang, P.-Y.; Goto, K.; Lin, C.-A.; Dewi, L.; Liao, Y.-C.; Huang, C.-Y.; Kuo, C.-H. Senolytic Effects of Exercise in Human Muscles Require Acute Inflammation. Aging 2024, 16, 8599–8610. [Google Scholar] [CrossRef] [PubMed]
- Elliehausen, C.J.; Anderson, R.M.; Diffee, G.M.; Rhoads, T.W.; Lamming, D.W.; Hornberger, T.A.; Konopka, A.R. Geroprotector Drugs and Exercise: Friends or Foes on Healthy Longevity? BMC Biol. 2023, 21, 287. [Google Scholar] [CrossRef]
- Saito, Y.; Chikenji, T.S.; Matsumura, T.; Nakano, M.; Fujimiya, M. Exercise Enhances Skeletal Muscle Regeneration by Promoting Senescence in Fibro-Adipogenic Progenitors. Nat. Commun. 2020, 11, 889. [Google Scholar] [CrossRef] [PubMed]
- Keizer, H.G.; Brands, R.; Seinen, W. An AMP Kinase-Pathway Dependent Integrated Stress Response Regulates Ageing and Longevity. Biogerontology 2023, 24, 443–455. [Google Scholar] [CrossRef]
- Juan, C.G.; Matchett, K.B.; Davison, G.W. A Systematic Review and Meta-Analysis of the SIRT1 Response to Exercise. Sci. Rep. 2023, 13, 14752. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.L.; Snow, R.J.; Wright, C.R.; Cho, Y.; Wallace, M.A.; Kralli, A.; Russell, A.P. PGC-1α and PGC-1β Increase CrT Expression and Creatine Uptake in Myotubes via ERRα. Biochim. Biophys. Acta 2014, 1843, 2937–2943. [Google Scholar] [CrossRef]
- de Smalen, L.M.; Börsch, A.; Leuchtmann, A.B.; Gill, J.F.; Ritz, D.; Zavolan, M.; Handschin, C. Impaired Age-Associated Mitochondrial Translation Is Mitigated by Exercise and PGC-1α. Proc. Natl. Acad. Sci. USA 2023, 120, e2302360120. [Google Scholar] [CrossRef]
- Xia, Y.; Yao, B.; Fu, Z.; Li, L.; Jin, S.; Qu, B.; Huang, Y.; Ding, H. Clock Genes Regulate Skeletal Muscle Energy Metabolism through NAMPT/NAD(+)/SIRT1 Following Heavy-Load Exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 325, R490–R503. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-H.; Luo, Y.-X.; Yao, X.-Q. Exercise-Driven Cellular Autophagy: A Bridge to Systematic Wellness. J. Adv. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guan, X.; Huang, Y.; Qu, B.; Yao, B.; Ding, H. Identification of Key Genes and Signaling Pathways Based on Transcriptomic Studies of Aerobic and Resistance Training Interventions in Sarcopenia in SAMP8 Mice. Sports Med. Health Sci. 2024, 6, 358–369. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, Y.; Wang, H.; Chen, L.; Yu, C.; Zhang, X.; Yang, L.; Zhang, X.; Wu, A. Exercise-Induced FNDC5/Irisin Protects Nucleus Pulposus Cells against Senescence and Apoptosis by Activating Autophagy. Exp. Mol. Med. 2022, 54, 1038–1048. [Google Scholar] [CrossRef]
- Fiorenza, M.; Gunnarsson, T.P.; Hostrup, M.; Iaia, F.M.; Schena, F.; Pilegaard, H.; Bangsbo, J. Metabolic Stress-Dependent Regulation of the Mitochondrial Biogenic Molecular Response to High-Intensity Exercise in Human Skeletal Muscle. J. Physiol. 2018, 596, 2823–2840. [Google Scholar] [CrossRef]
- Nieman, D.C.; Kay, C.D.; Rathore, A.S.; Grace, M.H.; Strauch, R.C.; Stephan, E.H.; Sakaguchi, C.A.; Lila, M.A. Increased Plasma Levels of Gut-Derived Phenolics Linked to Walking and Running Following Two Weeks of Flavonoid Supplementation. Nutrients 2018, 10, 1718. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falvino, A.; Bonanni, R.; Tarantino, U.; Tancredi, V.; Cariati, I. Which Approach to Choose to Counteract Musculoskeletal Aging? A Comprehensive Review on the Multiple Effects of Exercise. Int. J. Mol. Sci. 2025, 26, 7573. https://doi.org/10.3390/ijms26157573
Falvino A, Bonanni R, Tarantino U, Tancredi V, Cariati I. Which Approach to Choose to Counteract Musculoskeletal Aging? A Comprehensive Review on the Multiple Effects of Exercise. International Journal of Molecular Sciences. 2025; 26(15):7573. https://doi.org/10.3390/ijms26157573
Chicago/Turabian StyleFalvino, Angela, Roberto Bonanni, Umberto Tarantino, Virginia Tancredi, and Ida Cariati. 2025. "Which Approach to Choose to Counteract Musculoskeletal Aging? A Comprehensive Review on the Multiple Effects of Exercise" International Journal of Molecular Sciences 26, no. 15: 7573. https://doi.org/10.3390/ijms26157573
APA StyleFalvino, A., Bonanni, R., Tarantino, U., Tancredi, V., & Cariati, I. (2025). Which Approach to Choose to Counteract Musculoskeletal Aging? A Comprehensive Review on the Multiple Effects of Exercise. International Journal of Molecular Sciences, 26(15), 7573. https://doi.org/10.3390/ijms26157573







