Rapid Change in FcεRI Occupancy on Basophils After Venom Immunotherapy Induction
Abstract
1. Introduction
2. Results
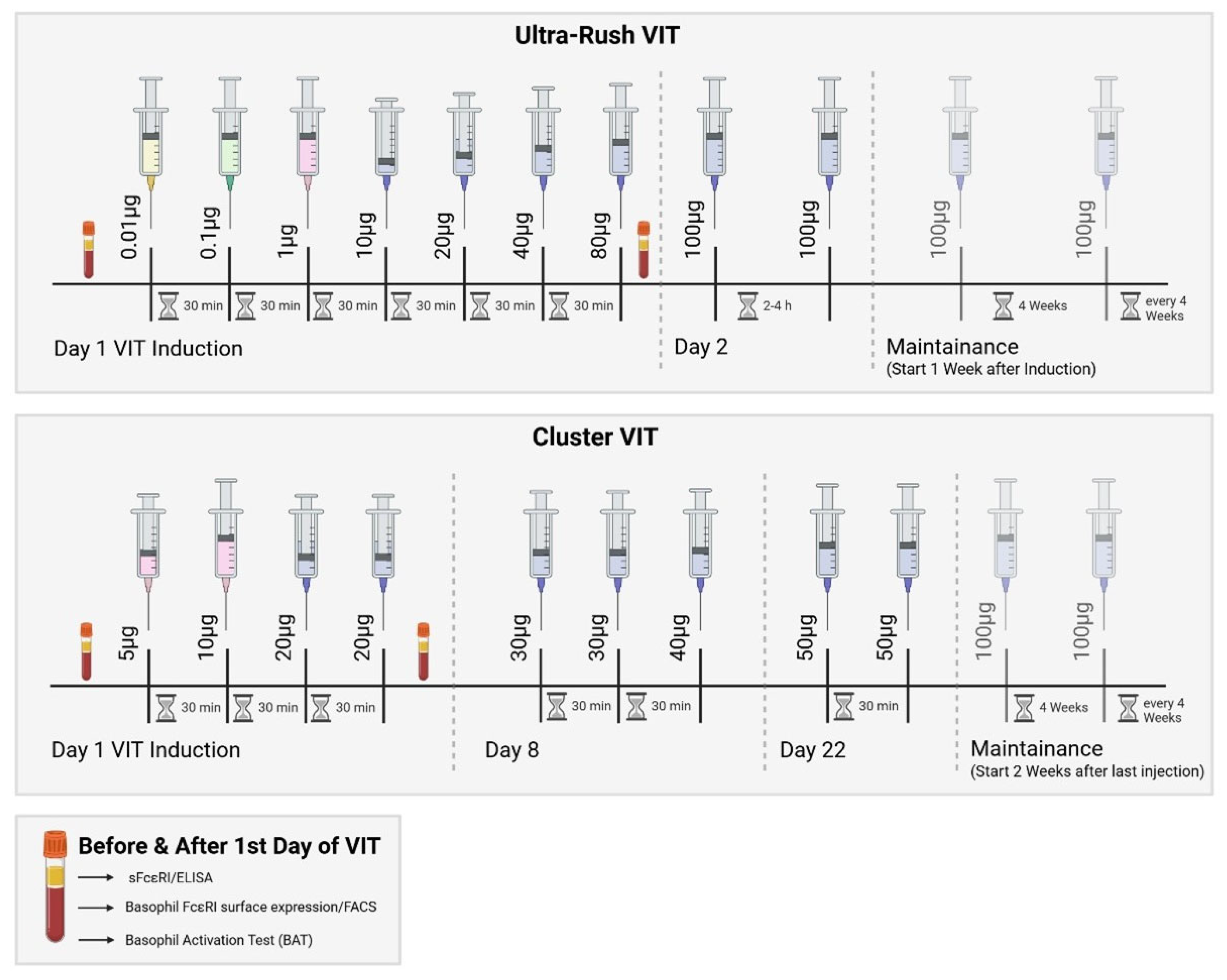
2.1. VIT Induction Is Well-Tolerated
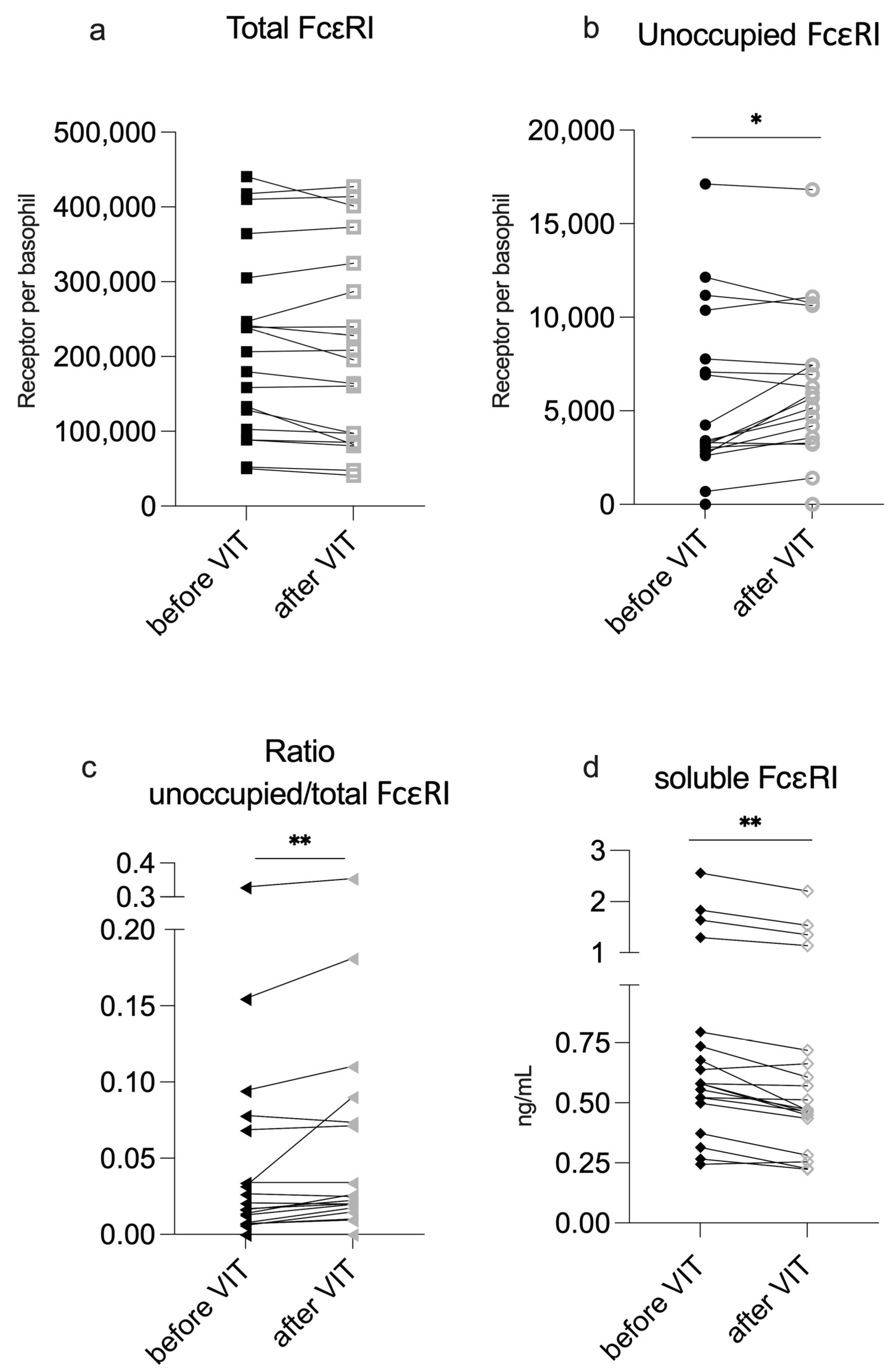
2.2. VIT Induction Reduces IgE Occupancy on Basophils but Does Not Increase sFcεRI Serum Levels
| n = 19 | Before VIT | After VIT Induction | p-Value |
|---|---|---|---|
| Tryptase (µg/L) | 5.6 (4.5) | 5.5 (4.6) | 0.07 |
| Total FcεRI | 215,426.0 ± 124,803.3 | 208,180.9 ± 130,439.8 | 0.17 |
| Unoccupied FcεRI | 5360.2 ± 4588.1 | 6028.2 ± 4163.8 | 0.04 * |
| Ratio of unoccupied/total FcεRI | 0.017 (0.06) | 0.023 (0.06) | <0.01 ** |
| sFcεRI (ng/mL) | 0.58 (0.3) | 0.47 (0.28) | <0.01 ** |
2.3. Significant Changes in Unoccupied FcεRI Density Is Primarily Seen in Patients with Low Baseline Numbers of Unoccupied FcεRI on Their Basophils
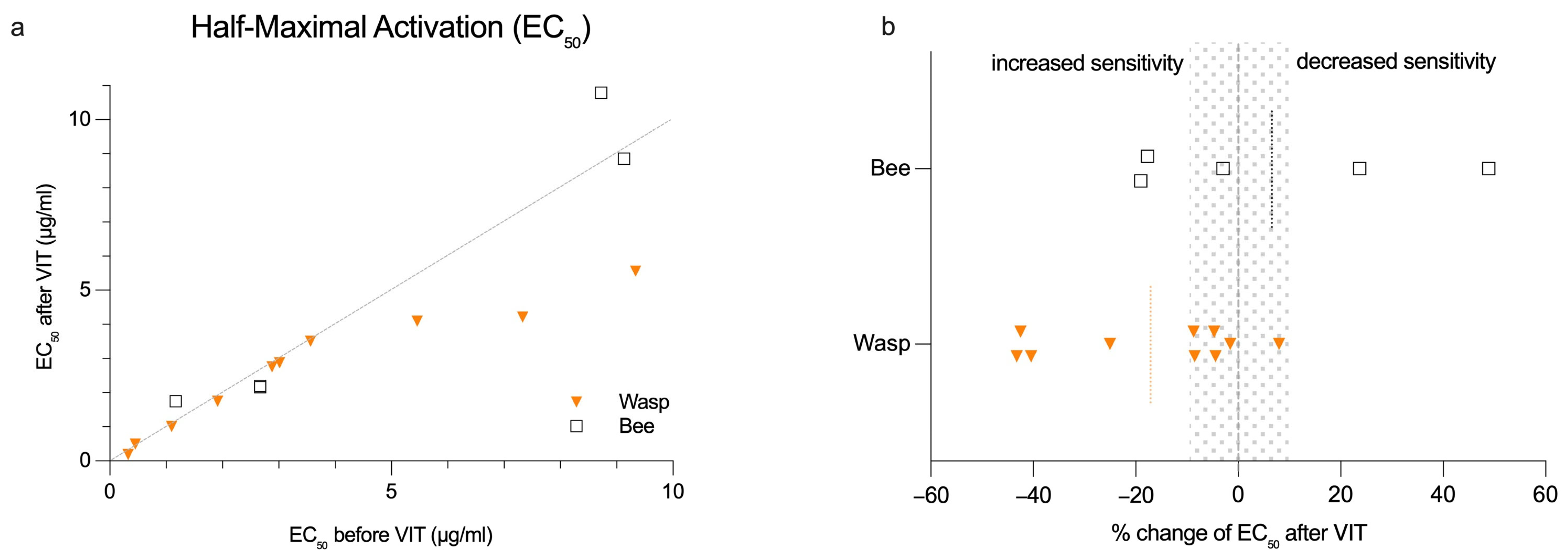
2.4. BAT Sensitivity Does Not Significantly Change During VIT Induction for Most Patients
2.5. Changes in BAT Sensitivity Correlate with Total FcεRI Expression on Basophils
3. Discussion
4. Materials and Methods
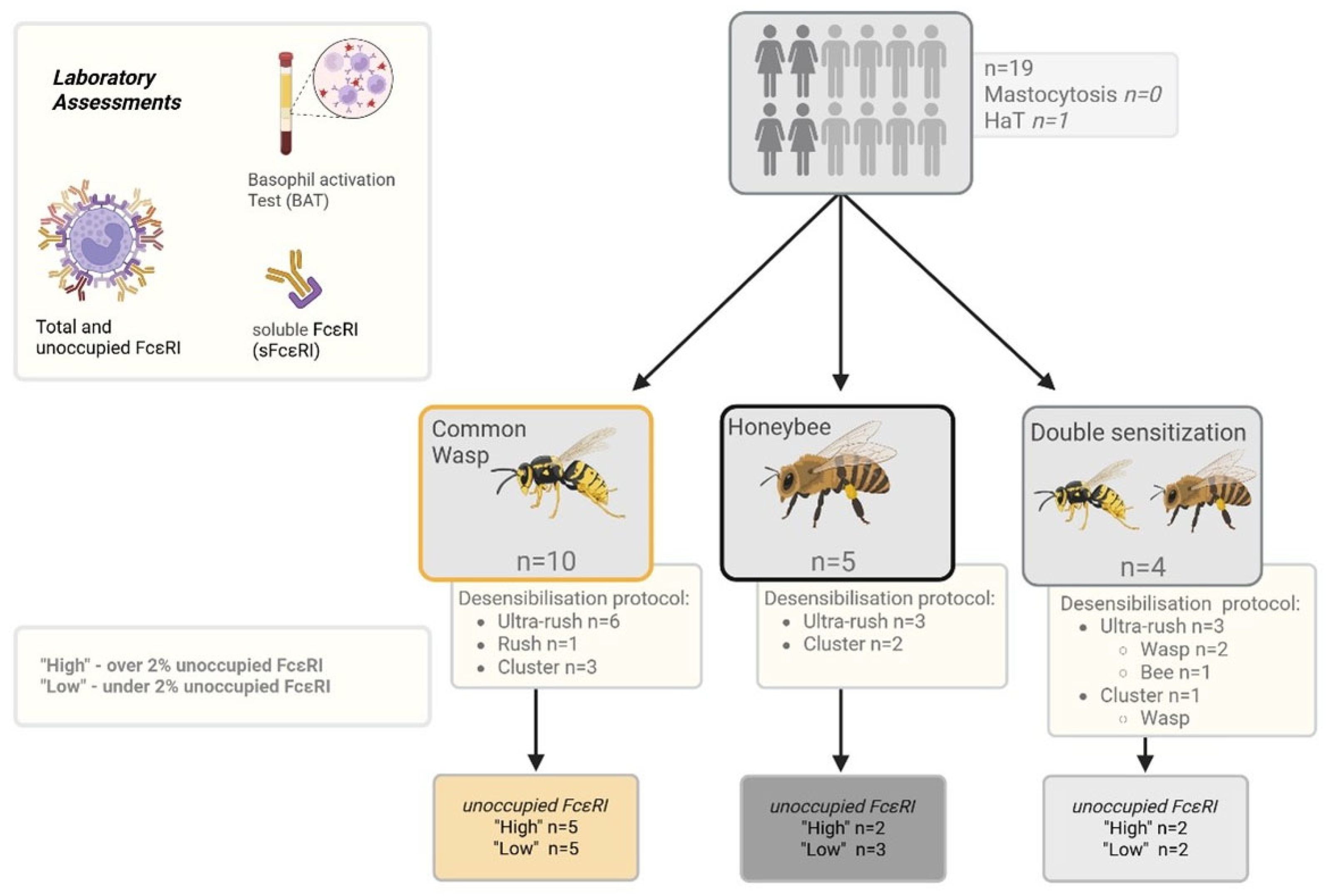
4.1. Patients
4.2. Clinical and Laboratory Assessments
4.3. Patient Grouping
4.4. Basophil Activation Test (BAT) and Flow Cytometry
4.5. FcεRI Expression of Basophil Profile Measurements
4.6. Soluble FcεRI Measurement
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| BAT | Basophil activation test |
| EC50 | Half-maximum basophil activation |
| HaT | Hereditary alpha tryptasemia |
| HVA | Hymenopter venom allergy |
| SCIT | Subcutaneous specific immunotherapy |
| VIT | Venom immunotherapy |
References
- Müller, U.; Helbling, A.; Berchtold, E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. J. Allergy Clin. Immunol. 1992, 89, 529–535. [Google Scholar] [CrossRef]
- Ruëff, F.; Vos, B.; Oude Elberink, J.; Bender, A.; Chatelain, R.; Dugas-Breit, S.; Horny, H.-P.; Küchenhoff, H.; Linhardt, A.; Mastnik, S.; et al. Predictors of clinical effectiveness of Hymenoptera venom immunotherapy. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2014, 44, 736–746. [Google Scholar] [CrossRef]
- Patella, V.; Florio, G.; Giuliano, A.; Oricchio, C.; Spadaro, G.; Marone, G.; Genovese, A. Hymenoptera Venom Immunotherapy: Tolerance and Efficacy of an Ultrarush Protocol versus a Rush and a Slow Conventional Protocol. J. Allergy 2012, 2012, 192192. [Google Scholar] [CrossRef]
- Bousquet, J.; Müller, U.R.; Dreborg, S.; Jarisch, R.; Malling, H.J.; Mosbech, H.; Urbanek, R.; Youlten, L. Immunotherapy with Hymenoptera venoms. Position paper of the Working Group on Immunotherapy of the European Academy of Allergy and Clinical Immunology. Allergy 1993, 48, 36–46. [Google Scholar]
- Sturm, G.J.; Varga, E.-M.; Roberts, G.; Mosbech, H.; Bilò, M.B.; Akdis, C.A.; Antolín-Amérigo, D.; Cichocka-Jarosz, E.; Gawlik, R.; Jakob, T.; et al. EAACI guidelines on allergen immunotherapy: Hymenoptera venom allergy. Allergy 2018, 73, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Plewako, H.; Wosińska, K.; Arvidsson, M.; Bjorkander, J.; Skov, P.S.; Håkansson, L.; Rak, S. Basophil interleukin 4 and interleukin 13 production is suppressed during the early phase of rush immunotherapy. Int. Arch. Allergy Immunol. 2006, 141, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Demšar Luzar, A.; Korošec, P.; Košnik, M.; Zidarn, M.; Rijavec, M. Hymenoptera Venom Immunotherapy: Immune Mechanisms of Induced Protection and Tolerance. Cells 2021, 10, 1575. [Google Scholar] [CrossRef]
- MacGlashan, D. IgE receptor and signal transduction in mast cells and basophils. Curr. Opin. Immunol. 2008, 20, 717–723. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Bjørklund, G.; Sboarina, A.; Vella, A. The role of basophils as innate immune regulatory cells in allergy and immunotherapy. Hum. Vaccines Immunother. 2018, 14, 815–831. [Google Scholar] [CrossRef]
- Novak, N.; Mete, N.; Bussmann, C.; Maintz, L.; Bieber, T.; Akdis, M.; Zumkehr, J.; Jutel, M.; Akdis, C. Early suppression of basophil activation during allergen-specific immunotherapy by histamine receptor 2. J. Allergy Clin. Immunol. 2012, 130, 1153–1158.e2. [Google Scholar] [CrossRef]
- Čelesnik, N.; Vesel, T.; Rijavec, M.; Šilar, M.; Eržen, R.; Košnik, M.; Žitnik, S.E.K.; Avčin, T.; Korošec, P. Short-term venom immunotherapy induces desensitization of FcεRI-mediated basophil response. Allergy 2012, 67, 1594–1600. [Google Scholar] [CrossRef]
- Flicker, S.; Valenta, R. Renaissance of the blocking antibody concept in type I allergy. Int. Arch. Allergy Immunol. 2003, 132, 13–24. [Google Scholar] [CrossRef]
- Dehlink, E.; Platzer, B.; Baker, A.H.; Larosa, J.; Pardo, M.; Dwyer, P.; Yen, E.H.; Szépfalusi, Z.; Nurko, S.; Fiebiger, E. A soluble form of the high affinity IgE receptor, Fc-epsilon-RI, circulates in human serum. PLoS ONE 2011, 6, e19098. [Google Scholar] [CrossRef]
- Moñino-Romero, S.; Erkert, L.; Schmidthaler, K.; Diesner, S.C.; Sallis, B.F.; Pennington, L.; Jardetzky, T.; Oettgen, H.C.; Bohle, B.; Fiebiger, E.; et al. The soluble isoform of human FcɛRI is an endogenous inhibitor of IgE-mediated mast cell responses. Allergy 2019, 74, 236–245. [Google Scholar] [CrossRef]
- Moñino-Romero, S.; Kolkhir, P.; Ohanyan, T.; Szépfalusi, Z.; Weller, K.; Metz, M.; Scheffel, J.; Maurer, M.; Altrichter, S. Elevated baseline soluble FcεRI may be linked to early response to omalizumab treatment in chronic spontaneous urticaria. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, S.M.; Sachs, B.; Kwiecien, R.; Moll-Slodowy, S.; Sauer, I.; Merk, H.F. The basophil activation test in wasp venom allergy: Sensitivity, specificity and monitoring specific immunotherapy. Allergy 2004, 59, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Korosec, P.; Erzen, R.; Silar, M.; Bajrovic, N.; Kopac, P.; Kosnik, M. Basophil responsiveness in patients with insect sting allergies and negative venom-specific immunoglobulin E and skin prick test results. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2009, 39, 1730–1737. [Google Scholar] [CrossRef]
- Santos, A.F.; Alpan, O.; Hoffmann, H.-J. Basophil activation test: Mechanisms and considerations for use in clinical trials and clinical practice. Allergy 2021, 76, 2420–2432. [Google Scholar] [CrossRef]
- Arzt, L.; Bokanovic, D.; Schrautzer, C.; Laipold, K.; Möbs, C.; Pfützner, W.; Herzog, S.A.; Vollmann, J.; Reider, N.; Bohle, B.; et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy 2018, 73, 1223–1231. [Google Scholar] [CrossRef]
- Žitnik, S.E.K.; Vesel, T.; Avčin, T.; Šilar, M.; Košnik, M.; Korošec, P. Monitoring honeybee venom immunotherapy in children with the basophil activation test. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2012, 23, 166–172. [Google Scholar] [CrossRef]
- Lockey, R.F.; Turkeltaub, P.C.; Olive, E.S.; Hubbard, J.M.; Baird-Warren, I.A.; Bukantz, S.C. The Hymenoptera venom study. III: Safety of venom immunotherapy. J. Allergy Clin. Immunol. 1990, 86, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Chovanec, J.; O’Connell, M.P.; Liu, Y.; Šelb, J.; Zanotti, R.; Bai, Y.; Kim, J.; Le, Q.T.; DiMaggio, T.; et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J. Allergy Clin. Immunol. 2021, 147, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, P.; Perbellini, O.; Passalacqua, G.; Caruso, B.; Colarossi, S.; Dal Fior, D.; Castellani, L.; Bonetto, C.; Frattini, F.; Dama, A.; et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J. Allergy Clin. Immunol. 2009, 123, 680–686. [Google Scholar] [CrossRef]
- Ruëff, F.; Przybilla, B.; Biló, M.B.; Müller, U.; Scheipl, F.; Aberer, W.; Birnbaum, J.; Bodzenta-Lukaszyk, A.; Bonifazi, F.; Bucher, C.; et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: Importance of baseline serum tryptase-a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. J. Allergy Clin. Immunol. 2009, 124, 1047–1054. [Google Scholar] [CrossRef]
- Broesby-Olsen, S.; Farkas, D.K.; Vestergaard, H.; Hermann, A.P.; Møller, M.B.; Mortz, C.G.; Kristensen, T.K.; Bindslev-Jensen, C.; Sørensen, H.T.; Frederiksen, H. Risk of solid cancer, cardiovascular disease, anaphylaxis, osteoporosis and fractures in patients with systemic mastocytosis: A nationwide population-based study. Am. J. Hematol. 2016, 91, 1069–1075. [Google Scholar] [CrossRef]
- Golden, D.B.K.; Wang, J.; Waserman, S.; Akin, C.; Campbell, R.L.; Ellis, A.K.; Greenhawt, M.; Lang, D.M.; Ledford, D.K.; Lieberman, J.; et al. Anaphylaxis: A 2023 practice parameter update. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2024, 132, 124–176. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, P.; Gonzalez-de-Olano, D.; Zanotti, R.; Riccio, A.; De Ferrari, L.; Lombardo, C.; Rogkakou, A.; Escribano, L.; Alvarez-Twose, I.; Matito, A.; et al. Venom immunotherapy in patients with clonal mast cell disorders: Efficacy, safety, and practical considerations. J. Allergy Clin. Immunol. Pract. 2013, 1, 474–478. [Google Scholar] [CrossRef]
- Moñino-Romero, S.; de las Vecillas, L.; Alenazy, L.A.; Labella, M.; Szépfalusi, Z.; Fiebiger, E.; Castells, M.C. Soluble FcεRI, IgE, and tryptase as potential biomarkers of rapid desensitizations for platin IgE sensitized cancer patients. J. Allergy Clin. Immunol. Pract. 2020, 8, 2085–2088.e10. [Google Scholar] [CrossRef]
- Szymański, Ł.; Urbańska, W.; Ciepielak, M.; Cios, A.; Stankiewicz, W.; Stelmasiak, M.; Rzeszotarska, A.; Korsak, J.; Lewicki, S.; Chciałowski, A. Time-dependent effect of desensitization with wasp venom on selected parameters of the immune system. Sci. Rep. 2022, 12, 7206. [Google Scholar] [CrossRef]
- Molfetta, R.; Gasparrini, F.; Santoni, A.; Paolini, R. Ubiquitination and endocytosis of the high affinity receptor for IgE. Mol. Immunol. 2010, 47, 2427–2434. [Google Scholar] [CrossRef]
- Fattakhova, G.; Masilamani, M.; Borrego, F.; Gilfillan, A.M.; Metcalfe, D.D.; Coligan, J.E. The High-Affinity Immunoglobulin-E Receptor (FceRI) is Endocytosed by an AP-2/Clathrin-Independent, Dynamin-Dependent Mechanism. Traffic 2006, 7, 673–685. [Google Scholar] [CrossRef]
- Zaidi, A.K.; MacGlashan, D. IgE-dependent and IgE-independent stimulation of human basophils increases the presence of immature FcεRIα by reversing degradative pathways. Int. Arch. Allergy Immunol. 2011, 154, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rajakulasingam, K.; Durham, S.R.; O’Brien, F.; Humbert, M.; Barata, L.T.; Reece, L.; Kay, A.B.; Grant, J.A. Enhanced expression of high-affinity IgE receptor (Fc epsilon RI) alpha chain in human allergen-induced rhinitis with co-localization to mast cells, macrophages, eosinophils, and dendritic cells. J. Allergy Clin. Immunol. 1997, 100, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.S.; Klion, A.D.; Holland, S.M.; Hamilton, R.G.; Bochner, B.S.; Macglashan, D.W. The relationship between serum IgE and surface levels of FcepsilonR on human leukocytes in various diseases: Correlation of expression with FcepsilonRI on basophils but not on monocytes or eosinophils. J. Allergy Clin. Immunol. 2000, 106, 514–520. [Google Scholar] [CrossRef]
- Mirzahosseini, A.; Dalmadi, B.; Csutora, P. Histamine receptor H4 regulates mast cell degranulation and IgE induced FcεRI upregulation in murine bone marrow-derived mast cells. Cell. Immunol. 2013, 283, 38–44. [Google Scholar] [CrossRef]
- Ring, J.; Messmer, K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet 1977, 1, 466–469. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Metcalfe, D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 2017, 129, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Yu, X.; Hughes, J.D.; Le, Q.T.; Jamil, A.; Bai, Y.; Ho, N.; Zhao, M.; Liu, Y.; O’Connell, M.P.; et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat. Genet. 2016, 48, 1564–1569. [Google Scholar] [CrossRef]
- EXBIO Praha, a.s. BasoFlowEx Kit (ED7043)—Instructions for Use; Version 7 [PDF]; EXBIO Praha: Vestec, Czech Republic, 2025; Available online: https://www.exbio.cz/getattachment/dc5e9246-9959-4ed1-9a99-f04a1aa98117/ED7043_IFU_v7_EN.pdf.aspx (accessed on 24 July 2025).
- Charpy, J.; Barbier, O.; Le Mauff, B.; Sarrat, A.; Roland-Nicaise, P.; Goret, J. Basophil activation test: Feedback and lessons from routine practice in French clinical laboratories. Clin. Exp. Allergy 2023, 53, 1055–1058. [Google Scholar] [CrossRef]
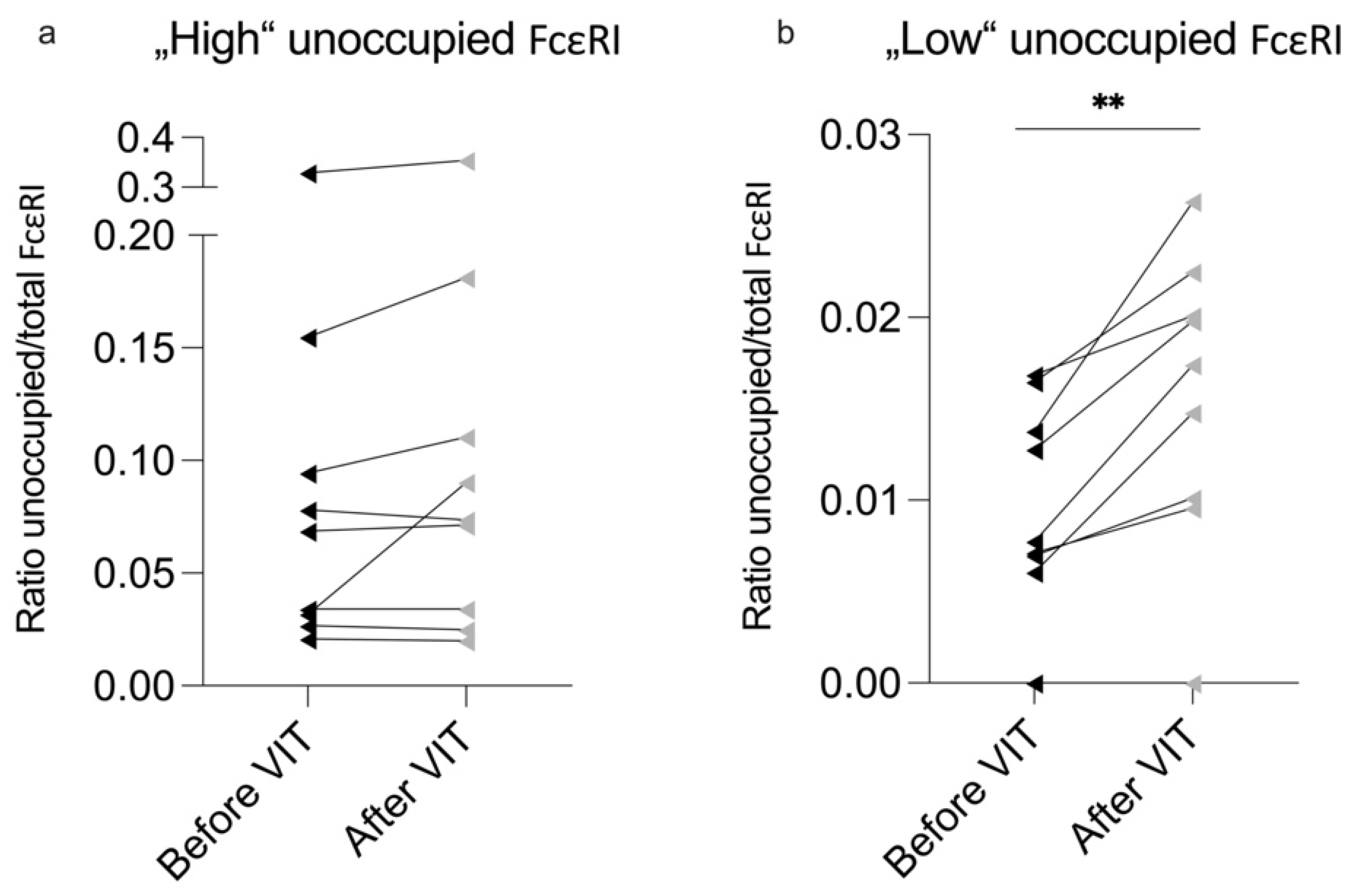
| High Unoccupied FcεRI (n = 9) | Low Unoccupied FcεRI (n = 10) | p-Value | |
|---|---|---|---|
| Total IgE (U/mL) | 37 (49) | 98.5 (126) | 0.01 ** |
| Wasp sIgE (U/mL) a | 2.0 (1) (n = 6) | 2.0 (2) (n = 7) | 0.39 |
| Bee sIgE (U/mL) a | 10.5 (-) (n = 3) | 6.7 (-) (n = 3) | 0.83 |
| ∆ Before–After VIT | High Unoccupied FcεRI (n = 9) | p-Value (Before/After) | Low Unoccupied FcεRI (n = 10) | p-Value (Before/After) | p-Value (Between the Groups) |
|---|---|---|---|---|---|
| ∆ Tryptase (µg/L) | 0.3 (1.35) | 0.63 | 0.42 ± 0.52 | 0.03 * | 0.55 |
| ∆ Total FcεRI | 8212.97 ± 21,146.54 | 0.28 | 6373.87 ± 23,995.24 | 0.42 | 0.86 |
| ∆ Unoccupied FcεRI | 299.27 (909.7) | 0.44 | −1224.43 ± 1083.77 | <0.01 ** | 0.05 * |
| ∆ Ratio unoccupied/total FcεRI | −0.003 (0.03) | 0.14 | −0.005 ± 0.004 | <0.01 ** | 1.00 |
| ∆ sFcεRI (ng/mL) | 0.138 ± 0.12 | <0.01 ** | 0.093 ± 0.96 | 0.01 * | 0.19 |
| Correlation Coefficients | Wasp (n = 10) | Bee (n = 5) | All (n = 15) | |||
|---|---|---|---|---|---|---|
| ∆ EC50 (µg/mL) | p-Value | ∆ EC50 (µg/mL) | p-Value | ∆ EC50 (µg/mL) | p-Value | |
| ∆ Total FcεRI | −0.733 | 0.02 * | −0.800 | 0.10 | −0.768 | <0.01 ** |
| ∆ Unoccupied FcεRI | −0.742 | 0.01 * | 0.600 | 0.29 | −0.104 | 0.71 |
| ∆ Ratio of unoccupied/total FcεRI | −0.292 | 0.41 | 0.800 | 0.10 | 0.268 | 0.33 |
| HVA Patients (n = 19) | |
|---|---|
| Age (y) | 46.32 ± 15.1 |
| Sex (f, %) | 7 (36.8) |
| BMI (kg/m2) | 26.53 ± 3.36 |
| Mastocytosis (pos., %) | 0 (0) |
| HaT (pos., %) | 1 (5.3) |
| Other concomitant allergies (pos., %) | 5 (35.71) |
| Total IgE (U/mL) | 46.0 (76) |
| Hymenoptera sensibilization (n, %) | |
| Wasp | 10 (52.6) |
| Bee | 5 (26.3) |
| Both | 4 (21.1) |
| Anaphylaxis (grade, %) | |
| I | 2 (10.5) |
| II | 14 (73.7) |
| III | 3 (15.8) |
| IV | 0 (0) |
| Desensitized venom (n, %) | |
| Wasp | 13 (68.4) |
| Bee | 6 (31.6) |
| Desensitization Protocol (n, %) | |
| Ultra-rush | 12 (63.2) |
| Rush | 1 (5.3) |
| Cluster | 6 (31.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puxkandl, V.; Aigner, S.; Burner, T.; Lackner, A.; Moñino-Romero, S.; Kimeswenger, S.; Hoetzenecker, W.; Altrichter, S. Rapid Change in FcεRI Occupancy on Basophils After Venom Immunotherapy Induction. Int. J. Mol. Sci. 2025, 26, 7511. https://doi.org/10.3390/ijms26157511
Puxkandl V, Aigner S, Burner T, Lackner A, Moñino-Romero S, Kimeswenger S, Hoetzenecker W, Altrichter S. Rapid Change in FcεRI Occupancy on Basophils After Venom Immunotherapy Induction. International Journal of Molecular Sciences. 2025; 26(15):7511. https://doi.org/10.3390/ijms26157511
Chicago/Turabian StylePuxkandl, Viktoria, Stefan Aigner, Teresa Burner, Angelika Lackner, Sherezade Moñino-Romero, Susanne Kimeswenger, Wolfram Hoetzenecker, and Sabine Altrichter. 2025. "Rapid Change in FcεRI Occupancy on Basophils After Venom Immunotherapy Induction" International Journal of Molecular Sciences 26, no. 15: 7511. https://doi.org/10.3390/ijms26157511
APA StylePuxkandl, V., Aigner, S., Burner, T., Lackner, A., Moñino-Romero, S., Kimeswenger, S., Hoetzenecker, W., & Altrichter, S. (2025). Rapid Change in FcεRI Occupancy on Basophils After Venom Immunotherapy Induction. International Journal of Molecular Sciences, 26(15), 7511. https://doi.org/10.3390/ijms26157511






