Abstract
Specific venom immunotherapy (VIT) in patients with hymenoptera venom allergy (HVA) represents a well-studied approach to reduce the severity of a possible anaphylactic reaction. Currently, data on mechanisms of tolerance induction at the cellular level within the first hours of therapy are lacking. To address this, total and unoccupied high-affinity IgE receptor (FcεRI) numbers per basophil, soluble FcεRI (sFcεRI) and serum tryptase levels were measured before and after the first day of VIT induction in HVA patients. Additionally, basophil activation tests (BATs) were performed at those time points. In the early phase of VIT induction, no significant change in total FcεRI receptor density on basophils was observed, but a significant increase in unoccupied FcεRI was noticeable, predominantly in patients with high total IgE and low baseline unoccupied FcεRI density. No meaningful difference in serum tryptase levels or sFcεRI levels was observed after VIT induction. BATs showed heterogeneous results, often unchanged before and after VIT (in 47% of the cases), sometimes increased (in 40%) and only rarely decreased EC50 sensitivity (in 13%). Changes in the BAT EC50 correlated with FcεRI receptor density changes in basophils. In summary, VIT induction led to an increased ratio of unoccupied-to-total FcεRI without notable tryptase or sFcεRI serum elevation, pointing towards subthreshold cell activation with receptor internalization and recycling. However, the mostly unchanged or even increased basophil sensitivity in EC50 calls for further research to clarify the clinical relevance of these rapid receptor modulations.
1. Introduction
Specific venom immunotherapy (VIT) for patients with hymenoptera venom allergy (HVA) is a well-established approach to reduce the severity of potential anaphylactic reactions after re-exposure to the culprit insect’s venom [1,2].
In subcutaneous specific immunotherapy (SCIT), patients receive gradually increasing doses of insect venom through subcutaneous injections, starting with a very small amount. The dosage is incrementally increased until it reaches the equivalent of one to two insect stings. Depending on the patient’s medical history and the severity of the previous anaphylactic reactions, the most accelerated form of SCIT—known as the ultra-rush protocol—can be administered over just two days to quickly reach the target dose [3,4] (Materials and Methods Section 4.2). As maintenance therapy, patients receive follow-up injections every 4 to 8 weeks for at least 3 to 5 years, according to current guidelines [5].
To date, the full mechanism behind tolerance induction within the first few hours of venom immunotherapy remains unclear. Basophils and mast cells—key drivers of anaphylaxis—also likely play a central role in the early desensitization process [6,7]. Both cell types express the high-affinity IgE receptor FcεRI on their surface, making them essential components in Type-I/IgE-driven allergies [8,9]. One study has shown that within the first 6 h of the induction phase, mRNA levels of histamine receptor 2 (H2R) are upregulated in basophils. H2R-mediated ex vivo stimulation resulted in basophil activation test (BAT) inhibition [10]. However, in vivo confirmation of this pathway is still lacking. Furthermore, previous studies suggested a desensitization of basophils due to the loss of FcεRI expression in the first week(s) between VIT induction and before the first maintenance dose [11]. Plewako et al. [6] even showed a decreased number of circulating basophils during the first days of VIT. Later in the course of VIT—over the first weeks and months—antigen-specific blocking antibodies such as IgA, IgG1 and IgG4 are produced. These antibodies compete with IgE for antigen binding, thereby inhibiting IgE–antigen crosslinking [12] and contributing to long-term suppression of Type-I anaphylactic responses.
The soluble FcεRI alpha chain (sFcεRI) could also play a role in the desensitization process. This truncated molecule emerges following IgE crosslinking of the FcεRI of effector cells after antigen exposure. It is believed to be part of a negative feedback loop, acting as an endogenous inhibitor of FcεRI on basophils and mast cells, thereby preventing further IgE binding [13,14]. Patients with higher serum sFcεRI levels (>2 ng/mL) have been shown to better tolerate drug desensitization protocols [15].
The basophil activation test (BAT) is an ex vivo test that measures the degree of degranulation of basophils following allergen stimulation. It is currently considered to be the gold standard for evaluating hymenoptera venom allergy/anaphylaxis [16,17]. By assessing basophil reactivity, the BAT provides insights into a patient’s clinical sensitivity to allergen exposure and is, therefore, regarded as a useful tool for monitoring the desensitization process during venom immunotherapy [18]. However, it has been reported that after successful VIT (confirmed by sting provocation), the BAT showed inconsistent results [16]. Arzt et al. [19] reported significantly reduced BAT activity in patients who underwent vespid VIT, but not bee VIT, compared with hymenoptera-allergic patients without VIT. Additionally, there are reports that the BAT only decreased after 6 months of VIT; before that, activation seems to be comparable with before VIT [20]. Notably, data on immediate changes in BAT responses—within just a few hours following VIT induction—are lacking in the current literature.
Despite the high efficacy of VIT [1,2], a small percentage of patients do not achieve protection by this therapy. Also, a small proportion of patients encounter significant side effects, up to severe anaphylactic reactions, upon therapy initiation. Currently, we cannot reliably identify patients at risk of severe reactions or therapy failure because we do not completely understand the biological processes at the early stages of VIT.
Therefore, we aimed to investigate if the induction of hymenoptera VIT leads to alterations in the surface expression levels of FcεRI on effector cells to create an immediate change in the release of sFcεRI and changes in BAT responses in order to gain insights into the modulation of allergic effector mechanisms in the very early stages of VIT.
2. Results
2.1. VIT Induction Is Well-Tolerated
Of the 19 patients (for clinical features, see Materials and Methods Section 4.2) undergoing ultra-rush or cluster VIT, 18 tolerated the protocol well. Local reactions at the injection site were common (78%). Blood samples were collected before and immediately after the final dose on the first day of VIT induction for further analysis.
During VIT induction, one patient (undergoing wasp-venom rush VIT induction) reported a sensation of facial swelling, the feeling of a lump in the throat and chest tightness on the third day (after 10 µg wasp venom). No objective signs of oral or facial swelling were observed and vital parameters remained within normal limits. Serum tryptase (BST) did not exceed baseline levels during this episode. Consequently, up-dosing was continued the next day with the last tolerated dose (8 µg). The subsequent increasing doses were well-tolerated until reaching the maintenance dose (Table S1). Due to elevated BST (21.1 µg/L), further diagnostic testing was performed. Hereditary alpha tryptasemia (HaT) (TPSAB1 triplication; Genotype baa:baa) was detected, but no cKIT mutation.
Overall, serum tryptase levels did not significantly change during VIT induction (Table 1).
2.2. VIT Induction Reduces IgE Occupancy on Basophils but Does Not Increase sFcεRI Serum Levels
The mean total FcεRI density on basophils slightly decreased by 3% following VIT although this was not statistically significant. However, the mean unoccupied FcεRI significantly increased by an average of 21% during VIT and the ratio of unoccupied/total FcεRI also increased from a median of 1.69% to 2.25% (Table 1; individual changes are depicted in Figure 1a–c).

Table 1.
Values before and after VIT.
Table 1.
Values before and after VIT.
| n = 19 | Before VIT | After VIT Induction | p-Value |
|---|---|---|---|
| Tryptase (µg/L) | 5.6 (4.5) | 5.5 (4.6) | 0.07 |
| Total FcεRI | 215,426.0 ± 124,803.3 | 208,180.9 ± 130,439.8 | 0.17 |
| Unoccupied FcεRI | 5360.2 ± 4588.1 | 6028.2 ± 4163.8 | 0.04 * |
| Ratio of unoccupied/total FcεRI | 0.017 (0.06) | 0.023 (0.06) | <0.01 ** |
| sFcεRI (ng/mL) | 0.58 (0.3) | 0.47 (0.28) | <0.01 ** |
Statistical analysis was performed with a paired t-test or Wilcoxon test (if abnormally distributed). Normally distributed values are depicted as median ± standard deviation; abnormally distributed values are displayed as median (interquartile range). Total and unoccupied FcεRI are expressed as receptors per basophil. VIT—venom immunotherapy; sFcεRI—soluble FcεRI. Significant difference before and after VIT is indicated as * (p-value <0.5) or ** (p-value < 0.01).
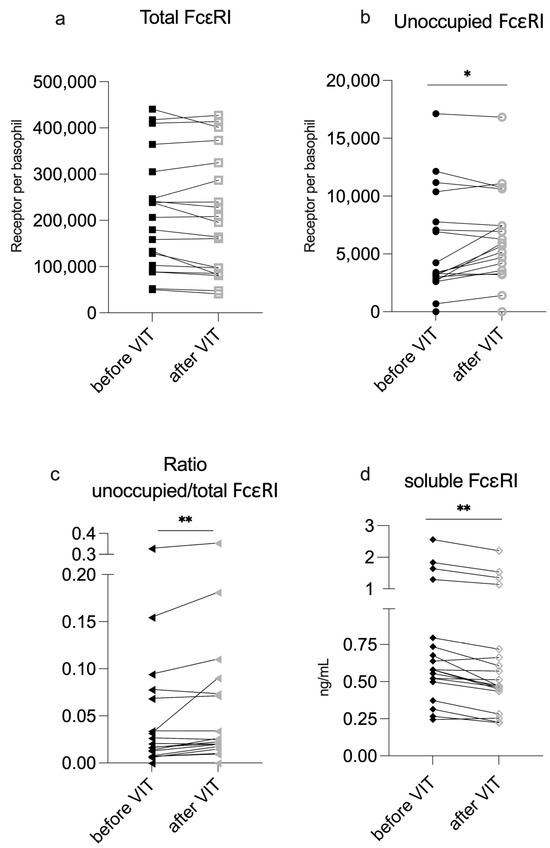
Figure 1.
Changes after VIT Induction. Total and unoccupied FcεRI are shown as receptors per basophil. sFcεRI is shown as ng/mL. VIT—venom immunotherapy. Significant difference before and after VIT is indicated as * (p-value < 0.5) or ** (p-value < 0.01).
When grouping the patients regarding the desensitized venom, significant results comparable with the overall cohort were seen in the wasp-sensitized group, while only a trend was observed in the bee-sensitized group (Table S2). No difference was observed regarding the change in FcεRI before and after induction when comparing the different induction protocols.
Serum sFcεRI levels were very low overall and did not correlate with total IgE levels (Figure S1). Only one of the patients presented with predefined clinically relevant levels (>2 ng/mL) [15]. Surprisingly, following VIT induction, the median sFcεRI concentration in patient sera was not elevated; instead, it was slightly, but significantly, reduced (Table 1; Figure 1d).
2.3. Significant Changes in Unoccupied FcεRI Density Is Primarily Seen in Patients with Low Baseline Numbers of Unoccupied FcεRI on Their Basophils
In our cohort, about half of the patients (53%) presented with a very low average number (<2%) of unoccupied FcεRI on their basophils. These patients had a significantly higher total serum IgE, but not specific IgE (Table 2). There was no difference between them in their sensitization to the culprit venom.

Table 2.
Total and specific IgE comparisons for the “High” and “Low” unoccupied FcεRI groups.
When dividing our cohort into “High” (over 2%) and “Low” (under 2%) unoccupied FcεRI groups, a significant increase (unoccupied FcεRI before VIT minus after VIT = mean −1222.43 receptors per basophil) in unoccupied FcεRI was observed; the ratio of unoccupied/total was only observed within the “Low” group (Table 3; Figure 2b). This indicates that more unoccupied FcεRI was present in the ‘Low’ group after VIT.

Table 3.
Delta of values before and after VIT for individuals with “High” (over 2%) or “Low” (under 2%) unoccupied FcεRI on basophils.
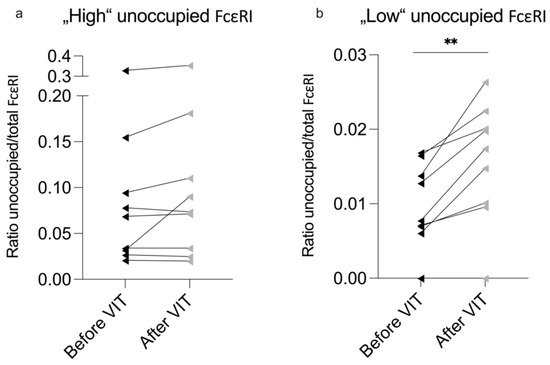
Figure 2.
Comparison of ‘High’ and ‘Low’ unoccupied FcεRI groups. The ratio of total-to-unoccupied FcεRI in the (a) ‘High’ (over 2%) and (b) ‘Low’ (under 2%) unoccupied FcεRI groups. VIT—venom immunotherapy. Significant difference before and after VIT is indicated as ** (p-value < 0.01).
Further, this group showed a minimal, but significant, decrease in tryptase levels before and after VIT. However, a minimal decrease in soluble FcεRI was noticeable in both groups.
2.4. BAT Sensitivity Does Not Significantly Change During VIT Induction for Most Patients
In the BAT, wasp-sensitized individuals showed a higher mean percentage of maximum-activated basophils (mean >80% activated basophils) compared with bee-sensitized individuals (mean <60% activated basophils) (p = 0.07) (Figure S2).
Changes greater than 10% in the maximum-activated basophils from the BAT were considered to be potentially meaningful and were observed in 3 (15%) of the 19 patients. In two patients (one allergic to wasps, one to bees) the maximum activation increased and the maximum activation decreased only in one (wasp-sensitive) patient. The remaining patients showed only minimal changes in the number of maximally activated basophils (Figure S3).
We did not observe a significant change in overall BAT sensitivity when assessing the venom concentration at the EC50 level during VIT induction (Figure 3a). In 7 out of 15 patients, EC50 changes of more than 10% were not detectable. Only two patients (13%) had a detectable decrease in BAT EC50, while six patients (40%) exhibited an increase in EC50 (Figure 3b). An increased basophil sensitivity during wasp VIT was observed in 3 out of 4 cases. These patients initially showed a high threshold for venom concentration. Although sensitivity increased by more than 40% in some cases, these changes remained within the same order of magnitude of wasp venom dilution (Figure S4a). Only one patient undergoing bee VIT demonstrated a decrease in EC50 of about 24%, and shifted the venom dilution category to the highest venom concentration (Figure S4b).
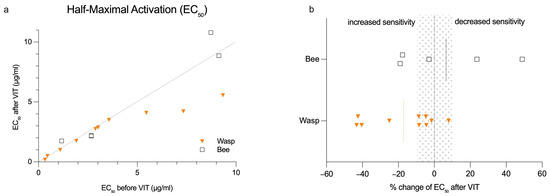
Figure 3.
Changes in half-maximal activation (EC50) for BATs before/after VIT induction in bee- and wasp-allergic patients. (a) Absolute change in EC50 for BATs; (b) relative change in EC50 for BATs. Orange and black dotted line represent the mean. Gray dotted area represents ±10%.
2.5. Changes in BAT Sensitivity Correlate with Total FcεRI Expression on Basophils
The shift in EC50, predominantly towards increased basophil sensitivity, indicated by a lower venom concentration required to reach half-maximal activation, showed a highly significant correlation with the overall change in total FcεRI expression (Table 4). Despite the significance, total FcεRI changes after VIT induction in the wasp-allergic patient group were mostly subtle, except for one patient with a strong increase who also had a marked decrease in EC50 sensitivity (Table 4; Figure S5a). A trend towards a correlation with lower total FcεRI in patients with decreased EC50 sensitivity was also seen in the bee-venom-allergic group (Table 4; Figure S5a). When looking at the relative change in EC50 before and after VIT, the correlation of EC50 with total FcεRI was significant in the bee VIT group and trending in the wasp VIT group (Table S3; Figure S5c).

Table 4.
Changes in BAT sensitivity correlated with FcεRI changes before/after VIT induction.
Regarding the correlation of the BAT EC50 with unoccupied FcεRI density, the picture was mixed. In the wasp VIT group, increased unoccupied FcεRI positively correlated with EC50 (Table 4; Figure S5b,d), whereas in the bee VIT group, an increase in unoccupied FcεRI was seen in all patients, which negatively correlated with the relative EC50 (Table S3; Figure S5b,d).
3. Discussion
This study demonstrates that FcεRI occupancy on basophils can rapidly change within the first hours of hymenoptera VIT induction.
Individuals undergoing ultra-rush or cluster VIT induction protocols did not experience any major complications. These initiation protocols, therefore, appear to be highly reliable, as reflected in the literature [5,21]. Only one patient undergoing the ultra-rush induction protocol (with diagnosed HaT) reported subjective, but not measurable systemic symptoms during initiation; however, they were still able to complete VIT. HaT has been associated with severe anaphylactic reactions [22], although up to now, no issues have been reported for the initiation of VIT in individuals with HaT. Nevertheless, elevated BST has been reported to be a risk factor for severe reactions during VIT initiation [23,24]. In mastocytosis, which is associated with severe anaphylaxis [25,26], especially upon contact with hymenoptera venom, VIT is still recommended as the treatment of choice (in addition to an emergency kit) [5,27].
After the first day of VIT, a significant increase of over 20% in the mean unoccupied FcεRI (reduced IgE occupancy on basophils) was observed. The mean total FcεRI showed a minimal, though not significant, difference, resulting in a highly significant difference in the ratio of unoccupied-to-total FcεRI before and after VIT induction. Čelesnik et al. [11] also reported fewer FcεRI on basophils after VIT induction; however, in that study, the examination did not take place until at least five days after induction. The tryptase levels did not significantly change during this period, suggesting no significantly increase in MC degranulation.
It has been shown that sFcεRI can block the binding of IgE to free FcεRI, potentially reducing the activation of basophils and MCs, and patients with higher serum sFcεRI levels (>2 ng/mL) were less prone to reactions during desensitization [14]. Surprisingly, we did not observe an increase in sFcεRI levels, as previously described [15]. Instead, we observed a significant decrease in sFcεRI a few hours after VIT induction. However, the serum sFcεRI levels in our cohort were generally very low, similar to those of non-atopic individuals where changes are less pronounced overall. The observed decrease of 0.11 ng/mL may be biologically irrelevant, given that previous desensitization studies have reported changes of several ng/mL [28]. The reduced levels of sFcεRI and tryptase in the serum may simply be a result of the saline drip that patients receive during the process of tolerance induction for safety-measure reasons. According to the literature, a stable tryptase level is expected in patients tolerating VIT [29].
An increase in the ratio of unoccupied-to-total FcεRI without a meaningful tryptase elevation or an increase in sFcεRI may suggest a subthreshold activation of cells with IgE receptor complex internalization [30,31] and FcεRI recycling to the cell surface [32]. However, our study does not present direct experimental evidence to support this mechanism. De novo expression of FcεRI is assumed to take several hours after allergen exposure [33], so this seems an unlikely explanation for the rapid change. The suggested mechanism is supported by the results of the detailed analysis, revealing that the increase in unoccupied FcεRI was more pronounced in the “Low” (<2%) unoccupied FcεRI baseline group. This group also had higher total IgE levels overall, indicating a possible role for IgE-mediated processes. Previous studies have shown a significant correlation between unoccupied FcεRI and circulating IgE levels, supporting the notion that FcεRI expression is modulated by IgE levels [34]. Other mechanisms—such as histamine 4 receptor activation, which has been shown to regulate surface FcεRI expression in MCs—could also play a role, specifically in an autocrine feedback loop [35]. Other previously described studies focused on mechanisms such as the rapid upregulation of histamine receptor 2 on basophils within the first six hours of the VIT build-up phase. However, these studies did not examine changes in FcεRI expression on basophils in relation to suppressed FcεRI-mediated cell activation and mediator release [10]. Nevertheless, it is conceivable that unoccupied FcεRI may play a role in monitoring the efficacy of VIT.
During VIT induction, the median BAT sensitivity (max. activation and EC50) did not significantly change. Surprisingly, an increase in BAT sensitivity with an EC50 occurring at lower venom concentrations was more frequent (40%) than a decrease (13%), which would have been expected in tolerance induction. An increase in sensitivity correlated with an increase in total FcεRI expression on basophils and vice versa. However, the overall higher numbers of unoccupied FcεRI did not stringently correlate with an increase or decrease in sensitivity. In addition to IgE–FcεRI-mediated basophil activation, other mechanisms could increase the expression of surface activation markers (CD63 and CD203c) during the BAT, which could result in an earlier EC50 being reached at lower venom concentrations. The main question remains whether basophils play a central role in the induction of tolerance, or if MCs are the key players in both anaphylaxis and tolerance induction. Unfortunately, the tissue residency of these cells makes studies on them difficult.
A main limitation of the study is the small number of patients, and further subgrouping reduces the statistical power even more, affecting the generalizability of the results. Therefore, studies with a greater sample size are needed.
Another major limitation is the heterogeneity of the SIT protocols in this trial. Ideally, further studies should include basophil count in peripheral blood, which was not assessed in this study, as prior findings indicate a reduction in basophil numbers during the build-up phase of immunotherapy [6,10]. Furthermore, the aim should also be to assess MCs and MC-derived markers in these patients in order to determine if the observed phenomena have a further clinical impact. Understanding these mechanisms would help to identify the low percentage of patients at risk of tolerance induction failure.
4. Materials and Methods
4.1. Patients
This study analyzed patients with suspected hymenoptera venom allergy who presented at the allergy outpatient clinic of the Department of Dermatology and Venereology, Comprehensive Allergy Centre, Kepler University Hospital, between May 2022 and May 2023, and who received specific hymenoptera venom immunotherapy. In total, 19 patients agreed to participate in this study.
Ethical approval was obtained from the local ethics committee (ECS No. 1026/2022). All patient records were handled in a pseudonymized manner, following data protection and local ethics considerations. Patient data on clinical data correlation are published elsewhere.
4.2. Clinical and Laboratory Assessments
Patient data, including age, sex, documented allergies and concomitant diseases, were obtained from the patient charts. Hymenoptera venom allergies were graded according to Ring and Messmer’s criteria [36].
Patients were screened for mastocytosis, which was defined as prevalent if patients had a KIT-D816V mutation (and elevated tryptase >11.4 µg/L) [37]. None of the patients met the criteria for systemic mastocytosis. Additionally, each patient was screened for hereditary alpha tryptasemia (HaT) using whole blood samples at an external laboratory (MVZ Martinsried GmbH, Planegg, Germany; https://www.medicover-diagnostics.de/leistungsverzeichnis/humangenetik/hereditare-alpha-tryptasamie-hat-labor-diagnostik (accessed on 3 August 2025)) via digital droplet PCR (ddPCR) [38]. HaT was diagnosed if there was evidence of a TPSAB1 gene number variation, such as an additional copy of the gene.
All patients underwent either the ultra-rush or cluster VIT induction protocol (bee or wasp venom) (Figure 4). On the first day of the ultra-rush protocol, patients received seven subcutaneous injections every thirty minutes, starting with a concentration of 0.01 µg of the respective venom. Patients undergoing the cluster VIT protocol only received four injections on the first day of induction, starting with a venom concentration of 5 µg. The patient’s blood was first drawn immediately before the first SCIT injection (before) and after the last injection on the first day (after); this was three hours for the ultra-rush protocol and one-and-a-half hours for the cluster protocol. A cumulative dose of 151.11 µg of the respective venom was reached at that point during the ultra-rush induction, or 55 µg during the cluster induction. Only one patient underwent the rush induction protocol (Table S1). The decision for each desensitization protocol was made according to the patient’s history and preference.
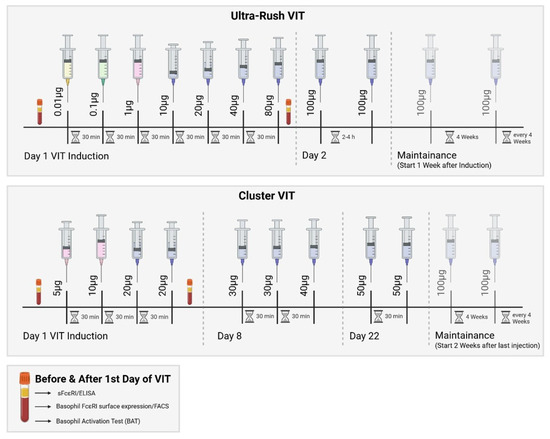
Figure 4.
Ultra-rush and cluster VIT induction protocols with the corresponding maintenance doses. SCIT—subcutaneous specific immunotherapy. Created using https://BioRender.com. In this study, blood was drawn from the patient according to this protocol.
Total IgE and tryptase serum levels were assessed in the central nuclear laboratory of the Kepler University Hospital, Linz, Austria, using the ImmunoCAP System® (Phadia Laboratory Systems, Thermo Fisher Scientific Inc, Uppsala, Sweden).
The characteristics of the patients are shown in Table 5.

Table 5.
Cohort characteristics.
4.3. Patient Grouping
Patients were grouped according to desensitized hymenoptera and protocols. The percentage of unoccupied FcεRI was calculated for each patient based on their FcεRI levels prior to VIT induction. In a further analysis, patients were grouped into unoccupied FcεRI ‘High’ and ‘Low’ groups, where the ‘High’ group was defined as an amount of unoccupied FcεRI over two percent and the ‘Low’ group had correspondingly lower values (Figure 5).
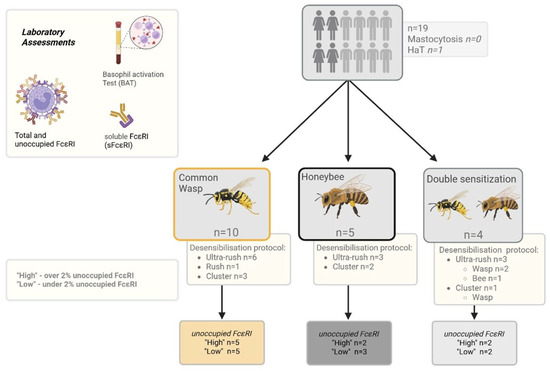
Figure 5.
Patient grouping flowchart. HaT—hereditary alpha tryptasemia. Created using https://BioRender.com.
4.4. Basophil Activation Test (BAT) and Flow Cytometry
A BAT was performed using the allergen to which the patient was known to be sensitized, both before and after VIT induction. Double-sensitized patients received a BAT with only the allergen undergoing VIT.
In the study, a standardized BAT kit from EXIBO© (BasoFlowEx Kit Ref. ED7043) was used with bee and wasp allergens obtained from ALK (Hørsholm, Denmark) (100 µg/mL ALK wässerig SQ© 801 Bienengift and 100 µg/mL ALK wässerig SQ© 802 Wespengift). The respective venom dilutions with the ALK diluent were produced by serial dilution at the following concentrations: 1, 10−1, 10−2, 10−3, 10−4 and 10−5. Patients’ heparinized whole blood was stimulated with serial dilutions of the venom and processed according to the basophil test kit instructions. The probes were analyzed by flow cytometry (Beckman coulter DxFlex©, Brea, CA, USA). The gating strategy used is shown in Figure S6a. A positive control used monoclonal anti-IgE from the basophil test kit.
A positive and negative control was performed for each case. As there was no cut-off given for the negative control in the manufacturer manual [39], and no generally accepted cut-offs are established for the BAT, it has been suggested that the cut-off for the negative control and its coefficient of variation should be adopted by each laboratory [40]. In the study lab, the mean ± SD of the negative control (max. activation) was 13.5% ± 8.5% and, therefore, the cut-off was set at 21%. If the negative control of the BAT reached ≥21% activation, the patient was excluded from further analyses due to a too-high preactivation.
The proposed cut-off for the positive control in the manufacturer manual [39] was given as >20%. However, as our laboratory’s set negative control was higher and the study aimed at following changes in the BAT over time (not just identifying sensitization), the cut-off was set higher, at 40%, to better monitor potentially meaningful changes. Overall, two patients were excluded due to negative control exclusions and two due to positive control exclusions.
With the created FACS data, an MS Excel calculation tool was implemented to calculate the dilution interval at which the maximal and half-maximum basophil activation (EC50) occurred. Furthermore, it allowed the allergen concentration at half-maximum and maximum basophil activation levels to be calculated. The highest activation level reached throughout the serial dilution was marked and showed the maximum activation level. For the MS Excel calculation tool, the activation level was plotted on the y-axis and the serial dilution on the x-axis. The different activations at each serial dilution point were connected with lines. A horizontal bar was then drawn for the half-maximum activation. The two dilution points that created a linear line, which crossed this half-maximum activation bar, were used in order to calculate the half-maximum activation via a linear function. Due to observed fluctuations at high basophil activation levels, the concentration at which maximum activation was first achieved in the BAT was determined with a 10% range tolerance. The highest activation was used as the reference point and the concentration at which >90% of the highest activation was observed was used as the calculated value for maximum BAT activation. As no meaningful differences for the BAT have been established in the literature, we considered a change of more than 10% to be potentially meaningful.
EC50 is given as the calculated concentration (µg/mL) at which the half-maximum activation of basophils is reached, or as a concentration step/range in which the respective calculated EC50 falls within [<1:10,000, 1:1000–1:10,000, 1:100–1:1000, 1:10–1:100, or 1–1:10]. A change in a shift of one concentration step (10-1) was considered to be meaningful.
4.5. FcεRI Expression of Basophil Profile Measurements
To evaluate FcεRI density on basophil granulocytes, patients’ heparinized whole blood was first blocked with human immunoglobulin (50g/L IG VENA (Kedrion Biopharma S.p.A., Bologna, Italy)), of which 1 µL IgG was diluted in 500 µL PBS. Anti-human CD193-APC (5E8; BD. Ref. 558208) and anti-human CD123-PE (9F5; BD. Ref. 555644) were used to identify basophils. FcεRI on basophils were stained with CRA1-BV421 (334624; BioLegend®, San Diego, CA, USA) for total FcεRI and CRA2-FITC (GTX00853; GeneTex©, Inc., Irvine, CA, USA GeneTex) for unoccupied FcεRI antibodies. The isotype control antibodies were IgG2b-BV421 (MPC-11; BioLegend®, San Diego, CA, USA. Ref. 400307) and IgG1-FITC (R&D Systems, Minneapolis, MN, USA, IC002F). The gating strategy is shown in Figure S6b. MFI of CRA1 (total FcεRI) and CRA2 (unoccupied FcεRI) were assessed.
Furthermore, quantification beads (QuantumTM Simply Cellular® anti-Mouse IgG; Bangs Laboratories, Inc., Fishers, IN, USA. Cat.#: 815B) were used together with the provided calculation tool (QuickCal® v 2.3) to transform MFI into the “receptors per basophil granulocyte” unit. In total, seven receptor quantifications via beads and FACS were performed parallel to the acquisition of patient FACS, representing data over a timespan of about one year. The mean of these seven bead measurements was taken to transform MFI into receptors/cell. Five patients showed a slightly negative MFI in the FACS, which could not be transformed into receptors/cell with the provided calculation tool; therefore, the receptors/cell value was defined as zero for a close and standardized approximation.
4.6. Soluble FcεRI Measurement
After clotting and centrifugation, the patient serum was aliquoted and stored frozen at −20°C. An ELISA Kit (Invitrogen; Thermo Fisher Scientific Inc., Waltham, MA, USA. Ref.: BMS2101-2) was used for the measurement of soluble FcεRI in patient sera according to the manufacturer’s protocol. Using the provided standard, the sample duplicates were converted into concentration values (given as ng/mL).
4.7. Statistical Analyses
Statistical analyses were performed using ISM SPSS Statistics (Version 30.0.0.0). Normal distribution was determined by the Shapiro–Wilk test. Variables that were normally distributed were given as the median ± SD, and others as the median and interquartile range (IQR). A paired comparison was performed using a paired t-test (if normally distributed) or a Wilcoxon test (if abnormally distributed). A Mann–Whitney U test was used for unpaired group comparisons if they were abnormally distributed. Correlations were calculated using Spearman’s rho. A p-value ≤ 0.05 was considered to indicate statistical significance.
5. Conclusions
VIT induction was tolerated well and resulted in a measurable reduction in IgE occupancy on basophils without a corresponding increase in sFcεRI serum levels. Significant changes in the density of unoccupied FcεRI levels were primarily observed in patients with low baseline levels of unoccupied receptors, indicating a differential immunological response based on initial receptor status. Although BAT sensitivity was inconsistent during the induction phase for most patients, individual variations in BAT sensitivity correlated with changes in FcεRI surface expression.
These early immunological shifts highlight the dynamics of effector cell regulation during VIT, suggesting that FcεRI expression patterns could serve as both mechanistic markers and potential tools for monitoring treatment responses in the management of hymenoptera venom allergy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26157511/s1.
Author Contributions
Conceptualization, S.A. (Sabine Altrichter); methodology, S.A. (Sabine Altrichter) and S.M.-R.; validation, V.P. and S.A. (Stefan Aigner); formal analysis, V.P. and S.A. (Stefan Aigner); investigation, S.A. (Stefan Aigner) and V.P.; resources, S.A. (Sabine Altrichter); data curation, S.A. (Stefan Aigner), T.B. and A.L.; writing—original draft preparation, V.P.; writing—review and editing, S.A. (Sabine Altrichter), S.A. (Stefan Aigner) and W.H.; visualization, V.P.; supervision, S.A. (Sabine Altrichter), W.H. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Impetus I-05-22 METOI fund of the Johannes Kepler University Linz.
Institutional Review Board Statement
Ethic approval was obtained from the local ethic committee (Ethics Committee of the Johannes Kepler University Faculty of Medicine, Linz, Austria: ECS Nr. 1026/2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank our colleagues at the Comprehensive Allergy Center, Kepler University Hospital, Linz, Austria, for their exceptional work and dedicated patient care. For their support in providing resources and infrastructure, we would like to thank the Department for Dermatology at the Kepler University Hospital, Linz, Austria; the ZMF (Zentrum für Medizinische Forschung), Johannes Kepler University, Linz, Austria; and the Institute for Nuclear Medicine and Endocrinology, Kepler University Hospital, Linz, Austria. We would also like to express our sincere thanks to Antonia Currie for proofreading the manuscript.
Conflicts of Interest
WH has conducted studies for/was an advisor for/was a speaker for Novartis, Eli Lilly, Bencard, ALK, Leo Pharma, Kyowa Kirin, Takeda, Sanofi-Aventis and AbbVie. SA has conducted studies for/was an advisor for/was a speaker for AstraZeneca, Allakos, ALK, Biocryst, Blueprint, CSLBehring, HAL Allergy Group, Kalvista, LeoPharma, Phavaris, Moxie, Novartis, Sanofi, Takeda and Thermofisher. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Statement
This article has been republished with a minor correction to an author’s ORCID. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| BAT | Basophil activation test |
| EC50 | Half-maximum basophil activation |
| HaT | Hereditary alpha tryptasemia |
| HVA | Hymenopter venom allergy |
| SCIT | Subcutaneous specific immunotherapy |
| VIT | Venom immunotherapy |
References
- Müller, U.; Helbling, A.; Berchtold, E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. J. Allergy Clin. Immunol. 1992, 89, 529–535. [Google Scholar] [CrossRef]
- Ruëff, F.; Vos, B.; Oude Elberink, J.; Bender, A.; Chatelain, R.; Dugas-Breit, S.; Horny, H.-P.; Küchenhoff, H.; Linhardt, A.; Mastnik, S.; et al. Predictors of clinical effectiveness of Hymenoptera venom immunotherapy. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2014, 44, 736–746. [Google Scholar] [CrossRef]
- Patella, V.; Florio, G.; Giuliano, A.; Oricchio, C.; Spadaro, G.; Marone, G.; Genovese, A. Hymenoptera Venom Immunotherapy: Tolerance and Efficacy of an Ultrarush Protocol versus a Rush and a Slow Conventional Protocol. J. Allergy 2012, 2012, 192192. [Google Scholar] [CrossRef]
- Bousquet, J.; Müller, U.R.; Dreborg, S.; Jarisch, R.; Malling, H.J.; Mosbech, H.; Urbanek, R.; Youlten, L. Immunotherapy with Hymenoptera venoms. Position paper of the Working Group on Immunotherapy of the European Academy of Allergy and Clinical Immunology. Allergy 1993, 48, 36–46. [Google Scholar]
- Sturm, G.J.; Varga, E.-M.; Roberts, G.; Mosbech, H.; Bilò, M.B.; Akdis, C.A.; Antolín-Amérigo, D.; Cichocka-Jarosz, E.; Gawlik, R.; Jakob, T.; et al. EAACI guidelines on allergen immunotherapy: Hymenoptera venom allergy. Allergy 2018, 73, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Plewako, H.; Wosińska, K.; Arvidsson, M.; Bjorkander, J.; Skov, P.S.; Håkansson, L.; Rak, S. Basophil interleukin 4 and interleukin 13 production is suppressed during the early phase of rush immunotherapy. Int. Arch. Allergy Immunol. 2006, 141, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Demšar Luzar, A.; Korošec, P.; Košnik, M.; Zidarn, M.; Rijavec, M. Hymenoptera Venom Immunotherapy: Immune Mechanisms of Induced Protection and Tolerance. Cells 2021, 10, 1575. [Google Scholar] [CrossRef]
- MacGlashan, D. IgE receptor and signal transduction in mast cells and basophils. Curr. Opin. Immunol. 2008, 20, 717–723. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Bjørklund, G.; Sboarina, A.; Vella, A. The role of basophils as innate immune regulatory cells in allergy and immunotherapy. Hum. Vaccines Immunother. 2018, 14, 815–831. [Google Scholar] [CrossRef]
- Novak, N.; Mete, N.; Bussmann, C.; Maintz, L.; Bieber, T.; Akdis, M.; Zumkehr, J.; Jutel, M.; Akdis, C. Early suppression of basophil activation during allergen-specific immunotherapy by histamine receptor 2. J. Allergy Clin. Immunol. 2012, 130, 1153–1158.e2. [Google Scholar] [CrossRef]
- Čelesnik, N.; Vesel, T.; Rijavec, M.; Šilar, M.; Eržen, R.; Košnik, M.; Žitnik, S.E.K.; Avčin, T.; Korošec, P. Short-term venom immunotherapy induces desensitization of FcεRI-mediated basophil response. Allergy 2012, 67, 1594–1600. [Google Scholar] [CrossRef]
- Flicker, S.; Valenta, R. Renaissance of the blocking antibody concept in type I allergy. Int. Arch. Allergy Immunol. 2003, 132, 13–24. [Google Scholar] [CrossRef]
- Dehlink, E.; Platzer, B.; Baker, A.H.; Larosa, J.; Pardo, M.; Dwyer, P.; Yen, E.H.; Szépfalusi, Z.; Nurko, S.; Fiebiger, E. A soluble form of the high affinity IgE receptor, Fc-epsilon-RI, circulates in human serum. PLoS ONE 2011, 6, e19098. [Google Scholar] [CrossRef]
- Moñino-Romero, S.; Erkert, L.; Schmidthaler, K.; Diesner, S.C.; Sallis, B.F.; Pennington, L.; Jardetzky, T.; Oettgen, H.C.; Bohle, B.; Fiebiger, E.; et al. The soluble isoform of human FcɛRI is an endogenous inhibitor of IgE-mediated mast cell responses. Allergy 2019, 74, 236–245. [Google Scholar] [CrossRef]
- Moñino-Romero, S.; Kolkhir, P.; Ohanyan, T.; Szépfalusi, Z.; Weller, K.; Metz, M.; Scheffel, J.; Maurer, M.; Altrichter, S. Elevated baseline soluble FcεRI may be linked to early response to omalizumab treatment in chronic spontaneous urticaria. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, S.M.; Sachs, B.; Kwiecien, R.; Moll-Slodowy, S.; Sauer, I.; Merk, H.F. The basophil activation test in wasp venom allergy: Sensitivity, specificity and monitoring specific immunotherapy. Allergy 2004, 59, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Korosec, P.; Erzen, R.; Silar, M.; Bajrovic, N.; Kopac, P.; Kosnik, M. Basophil responsiveness in patients with insect sting allergies and negative venom-specific immunoglobulin E and skin prick test results. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2009, 39, 1730–1737. [Google Scholar] [CrossRef]
- Santos, A.F.; Alpan, O.; Hoffmann, H.-J. Basophil activation test: Mechanisms and considerations for use in clinical trials and clinical practice. Allergy 2021, 76, 2420–2432. [Google Scholar] [CrossRef]
- Arzt, L.; Bokanovic, D.; Schrautzer, C.; Laipold, K.; Möbs, C.; Pfützner, W.; Herzog, S.A.; Vollmann, J.; Reider, N.; Bohle, B.; et al. Immunological differences between insect venom-allergic patients with and without immunotherapy and asymptomatically sensitized subjects. Allergy 2018, 73, 1223–1231. [Google Scholar] [CrossRef]
- Žitnik, S.E.K.; Vesel, T.; Avčin, T.; Šilar, M.; Košnik, M.; Korošec, P. Monitoring honeybee venom immunotherapy in children with the basophil activation test. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2012, 23, 166–172. [Google Scholar] [CrossRef]
- Lockey, R.F.; Turkeltaub, P.C.; Olive, E.S.; Hubbard, J.M.; Baird-Warren, I.A.; Bukantz, S.C. The Hymenoptera venom study. III: Safety of venom immunotherapy. J. Allergy Clin. Immunol. 1990, 86, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Chovanec, J.; O’Connell, M.P.; Liu, Y.; Šelb, J.; Zanotti, R.; Bai, Y.; Kim, J.; Le, Q.T.; DiMaggio, T.; et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J. Allergy Clin. Immunol. 2021, 147, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, P.; Perbellini, O.; Passalacqua, G.; Caruso, B.; Colarossi, S.; Dal Fior, D.; Castellani, L.; Bonetto, C.; Frattini, F.; Dama, A.; et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J. Allergy Clin. Immunol. 2009, 123, 680–686. [Google Scholar] [CrossRef]
- Ruëff, F.; Przybilla, B.; Biló, M.B.; Müller, U.; Scheipl, F.; Aberer, W.; Birnbaum, J.; Bodzenta-Lukaszyk, A.; Bonifazi, F.; Bucher, C.; et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: Importance of baseline serum tryptase-a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. J. Allergy Clin. Immunol. 2009, 124, 1047–1054. [Google Scholar] [CrossRef]
- Broesby-Olsen, S.; Farkas, D.K.; Vestergaard, H.; Hermann, A.P.; Møller, M.B.; Mortz, C.G.; Kristensen, T.K.; Bindslev-Jensen, C.; Sørensen, H.T.; Frederiksen, H. Risk of solid cancer, cardiovascular disease, anaphylaxis, osteoporosis and fractures in patients with systemic mastocytosis: A nationwide population-based study. Am. J. Hematol. 2016, 91, 1069–1075. [Google Scholar] [CrossRef]
- Golden, D.B.K.; Wang, J.; Waserman, S.; Akin, C.; Campbell, R.L.; Ellis, A.K.; Greenhawt, M.; Lang, D.M.; Ledford, D.K.; Lieberman, J.; et al. Anaphylaxis: A 2023 practice parameter update. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2024, 132, 124–176. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, P.; Gonzalez-de-Olano, D.; Zanotti, R.; Riccio, A.; De Ferrari, L.; Lombardo, C.; Rogkakou, A.; Escribano, L.; Alvarez-Twose, I.; Matito, A.; et al. Venom immunotherapy in patients with clonal mast cell disorders: Efficacy, safety, and practical considerations. J. Allergy Clin. Immunol. Pract. 2013, 1, 474–478. [Google Scholar] [CrossRef]
- Moñino-Romero, S.; de las Vecillas, L.; Alenazy, L.A.; Labella, M.; Szépfalusi, Z.; Fiebiger, E.; Castells, M.C. Soluble FcεRI, IgE, and tryptase as potential biomarkers of rapid desensitizations for platin IgE sensitized cancer patients. J. Allergy Clin. Immunol. Pract. 2020, 8, 2085–2088.e10. [Google Scholar] [CrossRef]
- Szymański, Ł.; Urbańska, W.; Ciepielak, M.; Cios, A.; Stankiewicz, W.; Stelmasiak, M.; Rzeszotarska, A.; Korsak, J.; Lewicki, S.; Chciałowski, A. Time-dependent effect of desensitization with wasp venom on selected parameters of the immune system. Sci. Rep. 2022, 12, 7206. [Google Scholar] [CrossRef]
- Molfetta, R.; Gasparrini, F.; Santoni, A.; Paolini, R. Ubiquitination and endocytosis of the high affinity receptor for IgE. Mol. Immunol. 2010, 47, 2427–2434. [Google Scholar] [CrossRef]
- Fattakhova, G.; Masilamani, M.; Borrego, F.; Gilfillan, A.M.; Metcalfe, D.D.; Coligan, J.E. The High-Affinity Immunoglobulin-E Receptor (FceRI) is Endocytosed by an AP-2/Clathrin-Independent, Dynamin-Dependent Mechanism. Traffic 2006, 7, 673–685. [Google Scholar] [CrossRef]
- Zaidi, A.K.; MacGlashan, D. IgE-dependent and IgE-independent stimulation of human basophils increases the presence of immature FcεRIα by reversing degradative pathways. Int. Arch. Allergy Immunol. 2011, 154, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rajakulasingam, K.; Durham, S.R.; O’Brien, F.; Humbert, M.; Barata, L.T.; Reece, L.; Kay, A.B.; Grant, J.A. Enhanced expression of high-affinity IgE receptor (Fc epsilon RI) alpha chain in human allergen-induced rhinitis with co-localization to mast cells, macrophages, eosinophils, and dendritic cells. J. Allergy Clin. Immunol. 1997, 100, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.S.; Klion, A.D.; Holland, S.M.; Hamilton, R.G.; Bochner, B.S.; Macglashan, D.W. The relationship between serum IgE and surface levels of FcepsilonR on human leukocytes in various diseases: Correlation of expression with FcepsilonRI on basophils but not on monocytes or eosinophils. J. Allergy Clin. Immunol. 2000, 106, 514–520. [Google Scholar] [CrossRef]
- Mirzahosseini, A.; Dalmadi, B.; Csutora, P. Histamine receptor H4 regulates mast cell degranulation and IgE induced FcεRI upregulation in murine bone marrow-derived mast cells. Cell. Immunol. 2013, 283, 38–44. [Google Scholar] [CrossRef]
- Ring, J.; Messmer, K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet 1977, 1, 466–469. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Metcalfe, D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 2017, 129, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Yu, X.; Hughes, J.D.; Le, Q.T.; Jamil, A.; Bai, Y.; Ho, N.; Zhao, M.; Liu, Y.; O’Connell, M.P.; et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat. Genet. 2016, 48, 1564–1569. [Google Scholar] [CrossRef]
- EXBIO Praha, a.s. BasoFlowEx Kit (ED7043)—Instructions for Use; Version 7 [PDF]; EXBIO Praha: Vestec, Czech Republic, 2025; Available online: https://www.exbio.cz/getattachment/dc5e9246-9959-4ed1-9a99-f04a1aa98117/ED7043_IFU_v7_EN.pdf.aspx (accessed on 24 July 2025).
- Charpy, J.; Barbier, O.; Le Mauff, B.; Sarrat, A.; Roland-Nicaise, P.; Goret, J. Basophil activation test: Feedback and lessons from routine practice in French clinical laboratories. Clin. Exp. Allergy 2023, 53, 1055–1058. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).