Metabolic Interactions in the Tumor Microenvironment of Classical Hodgkin Lymphoma: Implications for Targeted Therapy
Abstract
1. Introduction
2. Tumor Microenvironment (TME) in cHL
2.1. Composition of the TME
2.2. Immune Evasion and Inflammation in cHL Progression
3. Metabolic Alterations in the cHL Microenvironment
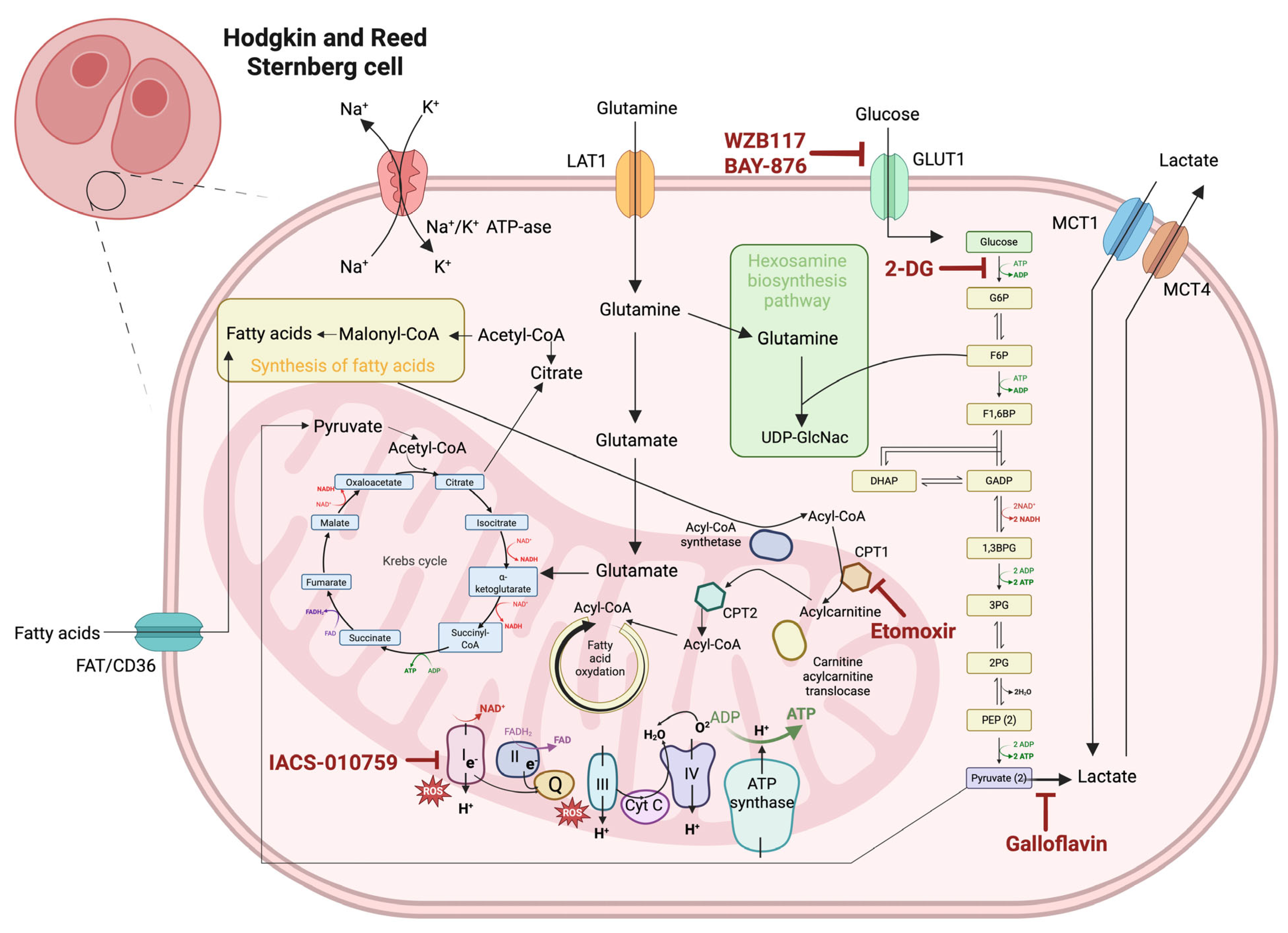
3.1. Warburg Effect: Glycolysis vs. Oxidative Phosphorylation in HRS Cells
3.2. Lipid and Amino Acid Metabolism in Tumor Survival
3.3. Oxidative Stress in HL
3.4. Hypoxia and Metabolic Stress Response
4. Therapeutic Targeting of Glycolytic Metabolism in cHL
4.1. Glycolysis Inhibitors
4.2. Targeting Glucose Transporters
4.3. Lactate Dehydrogenase Inhibition
4.4. Altering Mitochondrial Metabolism
5. Exploiting Fatty Acid and Amino Acid Metabolism in cHL
5.1. Disrupting Lipid-Fueled Tumor Growth
5.2. Starving Tumors of Critical Amino Acids
6. Immunometabolism-Based Therapies
6.1. Immunometabolic Modulators
6.2. Epigenetic Modulators
6.3. Checkpoint Inhibitors
6.4. Bispecific Antibodies and Bifunctional Fusion Proteins
6.5. Antibody–Drug Conjugates (ADCs)
6.6. CAR-T and CAR-NK Approaches
| (A) Preclinical Studies | ||
|---|---|---|
| Therapeutic Modality | Mechanism of Action | References |
| AMPK activator—metformin | Activates AMPK pathway; reduces PD-1 expression; enhances effector function | [94] |
| mTOR inhibitor—rapamycin | Inhibits mTORC1–S6K pathway; promotes CD8+ memory T-cell differentiation | [93] |
| CD39/CD73 inhibitors | Inhibit adenosine production; restore T-cell and NK cell cytotoxicity | [95,96] |
| CSF1R inhibitors | Reprogram TAMs; enhance inflammatory and anti-tumor immune responses | [97] |
| Glycolysis inhibitor—2-deoxy-D-glucose (2-DG) | Reduces lactate-mediated immunosuppression; downregulates PD-L1 expression | [103] |
| Anti-CD70 CAR-NK cells with IL-15 expression | CD70-directed cytotoxicity enhanced by IL-15-mediated NK-cell proliferation and persistence | [129] |
| Anti-CD86 CAR-T-cell therapy | Targets CD86 on HRS cells and TAMs to overcome CTLA-4:CD86 immune suppression | [128] |
| (B) Clinical Trials | ||
| Therapeutic Modality | Mechanism of Action | References |
| EZH2 inhibitor—SHR2554 | Reduces H3K27me3 levels; reactivates silenced tumor suppressor genes; inhibits tumor proliferation | [98] |
| PD-1 inhibitors—nivolumab, pembrolizumab | Block PD-1 receptor on T cells; restore—cell activity and cytotoxicity by overcoming immune exhaustion in the TME | [100,101] |
| DNMT inhibitor—CC-486 + nivolumab | Reverses PD-1 resistance by modifying TME and enhancing antigen presentation | [99] |
| JAK-STAT pathway inhibitor | Modulates PD-L1 expression and inflammatory milieu in TME; enhances PD-1 blockade efficacy | [104] |
| CTLA-4 inhibitor | Disrupts CTLA-4:CD86 immunosuppressive axis; restores T-cell activation | [8,105,107] |
| TIGIT inhibitor—vibostolimab | Blocks TIGIT–CD155 interaction; enhances T- and NK-cell responses | [108] |
| LAG-3 inhibitor—favezelimab | Boosts T-cell proliferation and cytokine production | [109] |
| CCR4-targeting agents—mogamulizumab, FLX475, CCR4-351 | Blocks Treg recruitment via CCL17/CCL22–CCR4 axis; restores anti-tumor immunity | [110] |
| CD47 inhibitor | Disrupts CD47-SIRPα interaction; promotes macrophage-mediated phagocytosis | [111,112,113] |
| Bispecific antibody—AFM13 (CD30/CD16A) | Mediates NK-cell ADCC against HRS cells | [114] |
| Bispecific antibody—IBI322 (CD47/PD-L1) | Blocks innate and adaptive immune evasion by inhibiting CD47 and PD-L1; promotes phagocytosis and T-cell activation | [115] |
| Bispecific antibody—MGD024 (CD123 x CD3) | Redirects T cells to CD123-expressing tumor cells | [116,117] |
| Bifunctional fusion protein—SHR-1701 (PD-L1 + TGF-β) | Simultaneous blockade of PD-L1 and TGF-β pathways; modulates immunosuppressive TME | [119,120] |
| Antibody–drug conjugate—brentuximab vedotin (BV) | Anti-CD30 antibody linked to MMAE; induces apoptosis in HRS cells | [122,123,124] |
| Anti-CD30 CAR-T-cell therapy | HLA-independent CD30 recognition and T cell-mediated cytotoxicity | [126,127] |
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2-DG | 2-deoxy-D-glucose |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| BV | Brentuximab vedotin |
| CAR-NK | Chimeric antigen receptor-engineered natural killer cells |
| CAR-T | Chimeric antigen receptor-engineered T cells |
| cHL | Classical Hodgkin Lymphoma |
| CPIs | Immune checkpoint inhibitors |
| CPT-1 | Carnitine palmitoyltransferase 1 |
| CRS | Cytokine release syndrome |
| EBV | Epstein–Barr Virus |
| EZH2 | Enhancer of zeste homolog 2 |
| FAO | Fatty acid β-oxidation |
| FASN | Fatty acid synthase |
| HIF-1 | Hypoxia-inducible factor 1 |
| HRS | Reed–Sternberg cell |
| LDHA | Lactate dehydrogenase A |
| MCT | Monocarboxylate transporter |
| NHL | Non-Hodgkin lymphoma |
| NK | Natural Killer |
| ORR | Overall response rate |
| OXPHOS | Oxidative phosphorylation |
| PD-1 | Programmed death receptor 1 |
| PIM | Provirus Integration site for Moloney leukemia virus |
| TAMs | Tumor-associated macrophages |
| TIGIT | T-cell immunoreceptor with Ig and ITIM domains |
| TME | Tumor microenvironment |
| TOMM20 | Translocase Of Outer Mitochondrial Membrane 20 |
| Tregs | Regulatory T cells |
References
- Wang, H.W.; Balakrishna, J.P.; Pittaluga, S.; Jaffe, E.S. Diagnosis of Hodgkin Lymphoma in the Modern Era. Br. J. Haematol. 2019, 184, 45–59. [Google Scholar] [CrossRef]
- Aggarwal, P.; Limaiem, F. Reed-Sternberg Cells. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2025; pp. 113–115. [Google Scholar] [CrossRef]
- Dawson, P.J. Whatever Happened to Dorothy Reed? Ann. Diagn. Pathol. 2003, 7, 195–203. [Google Scholar] [CrossRef]
- Steidl, C.; Connors, J.M.; Gascoyne, R.D. Molecular Pathogenesis of Hodgkin’s Lymphoma: Increasing Evidence of the Importance of the Microenvironment. J. Clin. Oncol. 2011, 29, 1812–1826. [Google Scholar] [CrossRef]
- Opinto, G.; Agostinelli, C.; Ciavarella, S.; Guarini, A.; Maiorano, E.; Ingravallo, G. Hodgkin Lymphoma: A Special Microenvironment. J. Clin. Med. 2021, 10, 4665. [Google Scholar] [CrossRef] [PubMed]
- Marafioti, T.; Hummel, M.; Foss, H.D.; Laumen, H.; Korbjuhn, P.; Anagnostopoulos, I.; Lammert, H.; Demel, G.; Theil, J.; Wirth, T.; et al. Hodgkin and Reed-Sternberg Cells Represent an Expansion of a Single Clone Originating from a Germinal Center B-Cell with Functional Immunoglobulin Gene Rearrangements but Defective Immunoglobulin Transcription. Blood 2000, 95, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Bräuninger, A.; Wacker, H.-H.; Rajewsky, K.; Kü, R.; Hansmann, M.-L. Typing the Histogenetic Origin of the Tumor Cells of Lymphocyte-Rich Classical Hodgkin’s Lymphoma in Relation to Tumor Cells of Classical and Lymphocyte-Predominance Hodgkin’s Lymphoma 1. Cancer Res. 2003, 63, 1644–1651. [Google Scholar] [PubMed]
- Patel, S.S.; Weirather, J.L.; Lipschitz, M.; Lako, A.; Chen, P.H.; Griffin, G.K.; Armand, P.; Shipp, M.A.; Rodig, S.J. The Microenvironmental Niche in Classic Hodgkin Lymphoma Is Enriched for CTLA-4-Positive T Cells That Are PD-1-Negative. Blood 2019, 134, 2059–2069. [Google Scholar] [CrossRef]
- Weniger, M.A.; Küppers, R. Molecular Biology of Hodgkin Lymphoma. Leukemia 2021, 35, 968–981. [Google Scholar] [CrossRef]
- Shishodia, S.; Aggarwal, B.B. Nuclear Factor-KappaB Activation Mediates Cellular Transformation, Proliferation, Invasion Angiogenesis and Metastasis of Cancer. Cancer Treat. Res. 2004, 119, 139–173. [Google Scholar] [CrossRef]
- Grover, N.S.; Dittus, C.; Thakkar, A.; Beaven, A.W. The Optimal Management of Relapsed and Refractory Hodgkin Lymphoma: Post–Brentuximab and Checkpoint Inhibitor Failure. Hematology 2023, 2023, 510–518. [Google Scholar] [CrossRef]
- Masel, R.; Roche, M.E.; Martinez-Outschoorn, U. Hodgkin Lymphoma: A Disease Shaped by the Tumor Micro- and Macroenvironment. Best Pract. Res. Clin. Haematol. 2023, 36, 101514. [Google Scholar] [CrossRef]
- Pang, Y.; Lu, T.; Xu-Monette, Z.Y.; Young, K.H. Metabolic Reprogramming and Potential Therapeutic Targets in Lymphoma. Int. J. Mol. Sci. 2023, 24, 5493. [Google Scholar] [CrossRef] [PubMed]
- Diehl, V.; Thomas, R.K.; Re, D. Part II: Hodgkin’s Lymphoma—Diagnosis and Treatment. Lancet Oncol. 2004, 5, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Momotow, J.; Borchmann, S.; Eichenauer, D.A.; Engert, A.; Sasse, S. Hodgkin Lymphoma-Review on Pathogenesis, Diagnosis, Current and Future Treatment Approaches for Adult Patients. J. Clin. Med. 2021, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Engert, A.; Plütschow, A.; Eich, H.T.; Lohri, A.; Dörken, B.; Borchmann, P.; Berger, B.; Greil, R.; Willborn, K.C.; Wilhelm, M.; et al. Reduced Treatment Intensity in Patients with Early-Stage Hodgkin’s Lymphoma. N. Engl. J. Med. 2010, 363, 640–652. [Google Scholar] [CrossRef]
- Bertuzzi, C.; Sabattini, E.; Agostinelli, C. Immune Microenvironment Features and Dynamics in Hodgkin Lymphoma. Cancers 2021, 13, 3634. [Google Scholar] [CrossRef]
- Aldinucci, D.; Celegato, M.; Casagrande, N. Microenvironmental Interactions in Classical Hodgkin Lymphoma and Their Role in Promoting Tumor Growth, Immune Escape and Drug Resistance. Cancer Lett. 2016, 380, 243–252. [Google Scholar] [CrossRef]
- Aldinucci, D.; Gloghini, A.; Pinto, A.; De Filippi, R.; Carbone, A. The Classical Hodgkin’s Lymphoma Microenvironment and Its Role in Promoting Tumour Growth and Immune Escape. J. Pathol. 2010, 221, 248–263. [Google Scholar] [CrossRef]
- Veldman, J.; Rodrigues Plaça, J.; Chong, L.; Terpstra, M.M.; Mastik, M.; van Kempen, L.C.; Kok, K.; Aoki, T.; Steidl, C.; van den Berg, A.; et al. CD4+ T Cells in Classical Hodgkin Lymphoma Express Exhaustion Associated Transcription Factors TOX and TOX2: Characterizing CD4+ T Cells in Hodgkin Lymphoma. Oncoimmunology 2022, 11, 2033433. [Google Scholar] [CrossRef]
- Karihtala, K.; Leivonen, S.K.; Karjalainen-Lindsberg, M.L.; Chan, F.C.; Steidl, C.; Pellinen, T.; Leppä, S. Checkpoint Protein Expression in the Tumor Microenvironment Defines the Outcome of Classical Hodgkin Lymphoma Patients. Blood Adv. 2022, 6, 1919–1931. [Google Scholar] [CrossRef]
- Roemer, M.G.M.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J.; et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J. Clin. Oncol. 2016, 34, 2697. [Google Scholar] [CrossRef]
- Weber, J. Immune Checkpoint Proteins: A New Therapeutic Paradigm for Cancer—Preclinical Background: CTLA-4 and PD-1 Blockade. Semin. Oncol. 2010, 37, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Roemer, M.G.M.; Advani, R.H.; Redd, R.A.; Pinkus, G.S.; Natkunam, Y.; Ligon, A.H.; Connelly, C.F.; Pak, C.J.; Carey, C.D.; Daadi, S.E.; et al. Classical Hodgkin Lymphoma with Reduced Β2M/MHC Class I Expression Is Associated with Inferior Outcome Independent of 9p24.1 Status. Cancer Immunol. Res. 2016, 4, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Wein, F.; Küppers, R. The Role of T Cells in the Microenvironment of Hodgkin Lymphoma. J. Leukoc. Biol. 2015, 99, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, I.; Yoshino, T.; Omonishi, K.; Nishiuchi, R.; Teramoto, N.; Yanai, H.; Kawahara, K.; Kubonishi, I.; Matsuo, Y.; Akagi, T. CD95 Ligand Is Expressed in Reed-Sternberg Cells of Hodgkin’s Disease. Pathol. Int. 1999, 49, 103–109. [Google Scholar] [CrossRef]
- Alibrahim, M.N.; Gloghini, A.; Carbone, A. Immune Deficiency/Dysregulation-Associated EBV-Positive Classic Hodgkin Lymphoma. Cancers 2025, 17, 1433. [Google Scholar] [CrossRef]
- Vaysberg, M.; Lambert, S.L.; Krams, S.M.; Martinez, O.M. Activation of The JAK/STAT Pathway in Epstein Barr Virus+ Associated Post Transplant Lymphoproliferative Disease: Role of Interferon-γ. Am. J. Transplant. 2009, 9, 2292–2302. [Google Scholar] [CrossRef]
- Kume, A.; Shinozaki-Ushiku, A.; Kunita, A.; Kondo, A.; Ushiku, T. Enhanced PD-L1 Expression in LMP1-Positive Cells of Epstein-Barr Virus-Associated Malignant Lymphomas and Lymphoproliferative Disorders: A Single-Cell Resolution Analysis With Multiplex Fluorescence Immunohistochemistry and In Situ Hybridization. Am. J. Surg. Pathol. 2022, 46, 1386–1396. [Google Scholar] [CrossRef]
- Georgoulis, V.; Papoudou-Bai, A.; Makis, A.; Kanavaros, P.; Hatzimichael, E. Unraveling the Immune Microenvironment in Classic Hodgkin Lymphoma: Prognostic and Therapeutic Implications. Biology 2023, 12, 862. [Google Scholar] [CrossRef]
- Aoki, T.; Wierzbicki, K.; Sun, S.; Steidl, C.; Giulino-Roth, L.; Merli, P.; Chabay, P. Tumor-Microenvironment and Molecular Biology of Classic Hodgkin Lymphoma in Children, Adolescents, and Young Adults. Front. Oncol. 2025, 15, 1515250. [Google Scholar] [CrossRef]
- Romano, A.; Parrinello, N.L.; Chiarenza, A.; Motta, G.; Tibullo, D.; Giallongo, C.; La Cava, P.; Camiolo, G.; Puglisi, F.; Palumbo, G.A.; et al. Immune Off-Target Effects of Brentuximab Vedotin in Relapsed/Refractory Hodgkin Lymphoma. Br. J. Haematol. 2019, 185, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, C.; Gallamini, A.; Stracqualursi, L.; Agati, P.; Tripodo, C.; Fuligni, F.; Sista, M.T.; Fanti, S.; Biggi, A.; Vitolo, U.; et al. The Combined Role of Biomarkers and Interim PET Scan in Prediction of Treatment Outcome in Classical Hodgkin’s Lymphoma: A Retrospective, European, Multicentre Cohort Study. Lancet Haematol. 2016, 3, e467–e479. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.; Newport, E.; Morten, K.J. The Warburg Effect: 80 Years On. Biochem. Soc. Trans. 2016, 44, 1499–1505. [Google Scholar] [CrossRef]
- Mikkilineni, L.; Whitaker-Menezes, D.; Domingo-Vidal, M.; Sprandio, J.; Avena, P.; Cotzia, P.; Dulau-Florea, A.; Gong, J.; Uppal, G.; Zhan, T.; et al. Hodgkin Lymphoma: A Complex Metabolic Ecosystem with Glycolytic Reprogramming of the Tumor Microenvironment. Semin. Oncol. 2017, 44, 218–225. [Google Scholar] [CrossRef]
- Barbato, A.; Scandura, G.; Puglisi, F.; Cambria, D.; La Spina, E.; Palumbo, G.A.; Lazzarino, G.; Tibullo, D.; Di Raimondo, F.; Giallongo, C.; et al. Mitochondrial Bioenergetics at the Onset of Drug Resistance in Hematological Malignancies: An Overview. Front. Oncol. 2020, 10, 604143. [Google Scholar] [CrossRef]
- Weniger, M.A.; Küppers, R. NF-ΚB Deregulation in Hodgkin Lymphoma. Semin. Cancer Biol. 2016, 39, 32–39. [Google Scholar] [CrossRef]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-ΚB) Doing in and to the Mitochondrion? Front. Cell Dev. Biol. 2019, 7, 470308. [Google Scholar] [CrossRef]
- Ravi, D.; Kritharis, A.; Evens, A.M. Deciphering the Metabolic Basis and Molecular Circuitry of the Warburg Paradox in Lymphoma. Cancers 2024, 16, 3606. [Google Scholar] [CrossRef]
- Akter, R.; Awais, M.; Boopathi, V.; Ahn, J.C.; Yang, D.C.; Kang, S.C.; Yang, D.U.; Jung, S.K. Inversion of the Warburg Effect: Unraveling the Metabolic Nexus between Obesity and Cancer. ACS Pharmacol. Transl. Sci. 2024, 7, 560–569. [Google Scholar] [CrossRef]
- Zhao, Z.; Mei, Y.; Wang, Z.; He, W. The Effect of Oxidative Phosphorylation on Cancer Drug Resistance. Cancers 2023, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Martin-Perez, M.; Urdiroz-Urricelqui, U.; Bigas, C.; Benitah, S.A. The Role of Lipids in Cancer Progression and Metastasis. Cell Metab. 2022, 34, 1675–1699. [Google Scholar] [CrossRef] [PubMed]
- Kloo, B.; Nagel, D.; Pfeifer, M.; Grau, M.; Düwel, M.; Vincendeau, M.; Dörken, B.; Lenz, P.; Lenz, G.; Krappmann, D. Critical Role of PI3K Signaling for NF-ΚB-Dependent Survival in a Subset of Activated B-Cell-like Diffuse Large B-Cell Lymphoma Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Lam, P.Y.; Jiang, H.; Bednarska, K.; Gloury, R.; Murigneux, V.; Tay, J.; Jacquelot, N.; Li, R.; Tuong, Z.K.; et al. Increased Lipid Metabolism Impairs NK Cell Function and Mediates Adaptation to the Lymphoma Environment. Blood 2020, 136, 3004–3017. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.M. Lipid Metabolism in Cancer: New Perspectives and Emerging Mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef]
- Liao, P.; Chang, N.; Xu, B.; Qiu, Y.; Wang, S.; Zhou, L.; He, Y.; Xie, X.; Li, Y. Amino Acid Metabolism: Challenges and Opportunities for the Therapeutic Treatment of Leukemia and Lymphoma. Immunol. Cell Biol. 2022, 100, 507–528. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, A.; Li, M.; Niu, T. Amino Acids in Hematologic Malignancies: Current Status and Future Perspective. Front. Nutr. 2023, 10, 1113228. [Google Scholar] [CrossRef]
- Romano, A.; Scandura, G.; Tibullo, D.; Amorini, A.M.; La Spina, E.; Barbato, A.; Giallongo, C.; Cerchione, C.; Simonetti, G.; Martinelli, G.; et al. P1288: Altered Amino Acid Homeostasis Can Elicit an Adaptive Response Via Upregulation of Ufmylation in Hodgkin Lymphoma. Hemasphere 2022, 6, 1173–1174. [Google Scholar] [CrossRef]
- Barberini, L.; Noto, A.; Fattuoni, C.; Satta, G.; Zucca, M.; Cabras, M.G.; Mura, E.; Cocco, P. The Metabolomic Profile of Lymphoma Subtypes: A Pilot Study. Molecules 2019, 24, 2367. [Google Scholar] [CrossRef]
- Cabrera-Serrano, A.J.; Sánchez-Maldonado, J.M.; González-Olmedo, C.; Carretero-Fernández, M.; Díaz-Beltrán, L.; Gutiérrez-Bautista, J.F.; García-Verdejo, F.J.; Gálvez-Montosa, F.; López-López, J.A.; García-Martín, P.; et al. Crosstalk Between Autophagy and Oxidative Stress in Hematological Malignancies: Mechanisms, Implications, and Therapeutic Potential. Antioxidants 2025, 14, 264. [Google Scholar] [CrossRef]
- Bur, H.; Haapasaari, K.M.; Turpeenniemi-Hujanen, T.; Kuittinen, O.; Auvinen, P.; Marin, K.; Koivunen, P.; Sormunen, R.; Soini, Y.; Karihtala, P. Oxidative Stress Markers and Mitochondrial Antioxidant Enzyme Expression Are Increased in Aggressive Hodgkin Lymphomas. Histopathology 2014, 65, 319–327. [Google Scholar] [CrossRef]
- Marini, C.; Cossu, V.; Lanfranchi, F.; Carta, S.; Vitale, F.; D’Amico, F.; Bauckneht, M.; Morbelli, S.; Donegani, M.I.; Chiola, S.; et al. Divergent Oxidative Stress in Normal Tissues and Inflammatory Cells in Hodgkin and Non-Hodgkin Lymphoma. Cancers 2023, 15, 3533. [Google Scholar] [CrossRef]
- Sousa-Pimenta, M.; Estevinho, M.M.; Sousa Dias, M.; Martins, Â.; Estevinho, L.M. Oxidative Stress and Inflammation in B-Cell Lymphomas. Antioxidants 2023, 12, 936. [Google Scholar] [CrossRef]
- Wein, F.; Otto, T.; Lambertz, P.; Fandrey, J.; Hansmann, M.L.; Küppers, R. Potential Role of Hypoxia in Early Stages of Hodgkin Lymphoma Pathogenesis. Haematologica 2015, 100, 1320–1326. [Google Scholar] [CrossRef]
- Wei, J.; Hu, M.; Du, H. Improving Cancer Immunotherapy: Exploring and Targeting Metabolism in Hypoxia Microenvironment. Front. Immunol. 2022, 13, 845923. [Google Scholar] [CrossRef]
- Chen, G.; Wu, K.; Li, H.; Xia, D.; He, T. Role of Hypoxia in the Tumor Microenvironment and Targeted Therapy. Front. Oncol. 2022, 12, 961637. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Tang, Y.Q.; Miao, H. Metabolism in Tumor Microenvironment: Implications for Cancer Immunotherapy. MedComm 2020, 1, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R.; Engert, A.; Hansmann, M.L. Hodgkin Lymphoma. J. Clin. Investig. 2012, 122, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Löser, P.; Mathas, S.; Krappmann, D.; Dörken, B.; Scheidereit, C. Constitutive NF-ΚB Maintains High Expression of a Characteristic Gene Network, Including CD40, CD86, and a Set of Antiapoptotic Genes in Hodgkin/Reed-Sternberg Cells. Blood 2001, 97, 2798–2807. [Google Scholar] [CrossRef]
- Marxsen, J.H.; Stengel, P.; Doege, K.; Heikkinen, P.; Jokilehto, T.; Wagner, T.; Jelkmann, W.; Jaakkola, P.; Metzen, E. Hypoxia-Inducible Factor-1 (HIF-1) Promotes Its Degradation by Induction of HIF-α-Prolyl-4-Hydroxylases. Biochem. J. 2004, 381, 761–767. [Google Scholar] [CrossRef]
- Basheeruddin, M.; Qausain, S. Hypoxia-Inducible Factor 1-Alpha (HIF-1α): An Essential Regulator in Cellular Metabolic Control. Cureus 2024, 16, e63852. [Google Scholar] [CrossRef] [PubMed]
- Paraskeva, E.; Haj Zen, A.; Zuheir Bakleh, M.; Al Haj Zen, A. The Distinct Role of HIF-1α and HIF-2α in Hypoxia and Angiogenesis. Cells 2025, 14, 673. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of Glycolysis by the Hypoxia-Inducible Factor (HIF): Implications for Cellular Physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 Regulatory Pathway and Its Potential for Therapeutic Intervention in Malignancy and Ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar]
- Mele, L.; del Vecchio, V.; Liccardo, D.; Prisco, C.; Schwerdtfeger, M.; Robinson, N.; Desiderio, V.; Tirino, V.; Papaccio, G.; La Noce, M. The Role of Autophagy in Resistance to Targeted Therapies. Cancer Treat. Rev. 2020, 88, 102043. [Google Scholar] [CrossRef]
- Hu, Y.L.; DeLay, M.; Jahangiri, A.; Molinaro, A.M.; Rose, S.D.; Carbonell, W.S.; Aghi, M.K. Hypoxia-Induced Autophagy Promotes Tumor Cell Survival and Adaptation to Anti-Angiogenic Treatment in Glioblastoma. Cancer Res. 2012, 72, 1773–1783. [Google Scholar] [CrossRef]
- Hill, R.M.; Fok, M.; Grundy, G.; Parsons, J.L.; Rocha, S. The Role of Autophagy in Hypoxia-Induced Radioresistance. Radiother. Oncol. 2023, 189, 109951. [Google Scholar] [CrossRef]
- Baldi, A.; Jalouli, M. Emerging Role of Hypoxia-Inducible Factors (HIFs) in Modulating Autophagy: Perspectives on Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 1752. [Google Scholar] [CrossRef]
- Hersey, P.; Zhang, X.D. Resistance of Follicular Lymphoma Cells to Chemotherapy Is More than Just BCL-2. Cancer Biol. Ther. 2003, 2, 541–543. [Google Scholar] [CrossRef]
- Shanmugam, M.; McBrayer, S.K.; Rosen, S.T. Targeting the Warburg Effect in Hematological Malignancies: From PET to Therapy. Curr. Opin. Oncol. 2009, 21, 531–536. [Google Scholar] [CrossRef][Green Version]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/MTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- 2-Deoxy-d-Glucose-Induced Cytotoxicity and Radiosensitization in Tumor Cells Is Mediated via Disruptions in Thiol Metabolism. Cancer Res. 2003, 63, 3413–3417. Available online: https://aacrjournals.org/cancerres/article/63/12/3413/510064/2-Deoxy-d-Glucose-induced-Cytotoxicity-and (accessed on 10 June 2025).
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Mediani, L.; Gibellini, F.; Bertacchini, J.; Frasson, C.; Bosco, R.; Accordi, B.; Basso, G.; Bonora, M.; Calabrò, M.L.; Mattiolo, A.; et al. Reversal of the Glycolytic Phenotype of Primary Effusion Lymphoma Cells by Combined Targeting of Cellular Metabolism and PI3K/Akt/ MTOR Signaling. Oncotarget 2015, 7, 5521–5537. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.Y.; Yang, J.L.; Lai, R.; Zhou, Z.J.; Tang, D.; Hu, L.; Zhao, L.J. Impact of Lactate on Immune Cell Function in the Tumor Microenvironment: Mechanisms and Therapeutic Perspectives. Front. Immunol. 2025, 16, 1563303. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Yang, K.; Wang, S.; Zhang, T.; Hui, F.; Zheng, F.; Geng, H.; Xu, C.; Xun, F.; et al. The Efficacy and Safety of PI3K and AKT Inhibitors for Patients with Cancer: A Systematic Review and Network Meta-Analysis. Eur. J. Pharmacol. 2024, 983, 176952. [Google Scholar] [CrossRef]
- Chang, C.H.; Curtis, J.D.; Maggi, L.B.; Faubert, B.; Villarino, A.V.; O’Sullivan, D.; Huang, S.C.C.; Van Der Windt, G.J.W.; Blagih, J.; Qiu, J.; et al. Posttranscriptional Control of T Cell Effector Function by Aerobic Glycolysis. Cell 2013, 153, 1239–1251. [Google Scholar] [CrossRef]
- Sukumar, M.; Liu, J.; Ji, Y.; Subramanian, M.; Crompton, J.G.; Yu, Z.; Roychoudhuri, R.; Palmer, D.C.; Muranski, P.; Karoly, E.D.; et al. Inhibiting Glycolytic Metabolism Enhances CD8+ T Cell Memory and Antitumor Function. J. Clin. Investig. 2013, 123, 4479–4488. [Google Scholar] [CrossRef]
- de-Brito, N.M.; Duncan-Moretti, J.; da-Costa, H.C.; Saldanha-Gama, R.; Paula-Neto, H.A.; Dorighello, G.G.; Simões, R.L.; Barja-Fidalgo, C. Aerobic Glycolysis Is a Metabolic Requirement to Maintain the M2-like Polarization of Tumor-Associated Macrophages. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118604. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chakraborty, P.; Daenthanasanmak, A.; Iamsawat, S.; Andrejeva, G.; Luevano, L.A.; Wolf, M.; Baliga, U.; Krieg, C.; Beeson, C.C.; et al. Targeting PIM Kinase with PD1 Inhibition Improves Immunotherapeutic Antitumor T-Cell Response. Clin. Cancer Res. 2019, 25, 1036–1049. [Google Scholar] [CrossRef]
- Cader, F.Z.; Hu, X.; Goh, W.L.; Wienand, K.; Ouyang, J.; Mandato, E.; Redd, R.; Lawton, L.N.; Chen, P.H.; Weirather, J.L.; et al. A Peripheral Immune Signature of Responsiveness to PD-1 Blockade in Patients with Classical Hodgkin Lymphoma. Nat. Med. 2020, 26, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Vaeth, M.; Eckstein, M.; Delgobo, M.; Ramos, G.; Frantz, S.; Hofmann, U.; Gladow, N. Characterization of the Effect of the GLUT-1 Inhibitor BAY-876 on T Cells and Macrophages. Eur. J. Pharmacol. 2023, 945, 175552. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; ba-alawi, W.; Deblois, G.; Cruickshank, J.; Duan, S.; Lima-Fernandes, E.; Haight, J.; Tonekaboni, S.A.M.; Fortier, A.M.; Kuasne, H.; et al. GLUT1 Inhibition Blocks Growth of RB1-Positive Triple Negative Breast Cancer. Nat. Commun. 2020, 11, 4205. [Google Scholar] [CrossRef]
- Doherty, J.R.; Cleveland, J.L. Targeting Lactate Metabolism for Cancer Therapeutics. J. Clin. Investig. 2013, 123, 3685–3692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lou, J.; Tian, Y.; Ding, J.; Wang, X.; Tang, B. How Lactate Affects Immune Strategies in Lymphoma. Front. Mol. Biosci. 2024, 11, 1480884. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, D.; Bankhead, A.; Neamati, N. Why All the Fuss about Oxidative Phosphorylation (OXPHOS)? J. Med. Chem. 2020, 63, 14276–14307. [Google Scholar] [CrossRef]
- Bi, C.; Fu, K.; Jiang, C.; Huang, X.; Chan, W.C.; McKeithan, T. The Combination Of 2-DG and Metformin Inhibits The MTORC1 Pathway and Suppresses Aggressive B Cell Lymphoma Growth and Survival. Blood 2013, 122, 1665. [Google Scholar] [CrossRef]
- Wang, M.; Wang, K.; Liao, X.; Hu, H.; Chen, L.; Meng, L.; Gao, W.; Li, Q. Carnitine Palmitoyltransferase System: A New Target for Anti-Inflammatory and Anticancer Therapy? Front. Pharmacol. 2021, 12, 760581. [Google Scholar] [CrossRef]
- Vanauberg, D.; Schulz, C.; Lefebvre, T. Involvement of the Pro-Oncogenic Enzyme Fatty Acid Synthase in the Hallmarks of Cancer: A Promising Target in Anti-Cancer Therapies. Oncogenesis 2023, 12, 16. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Chen, Y.; Zhai, Y.; Li, J.; Zhang, N.; Yin, J.; Wang, L. Metabolomics for Hematologic Malignancies: Advances and Perspective. Medicine 2024, 103, e39782. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Fan, N.; Zhou, C.; Li, D.; Macvicar, T.; Dong, Q.; Bruns, C.J.; Zhao, Y. Targeting Glutaminolysis: New Perspectives to Understand Cancer Development and Novel Strategies for Potential Target Therapies. Front. Oncol. 2020, 10, 589508. [Google Scholar] [CrossRef]
- Ara, A.; Xu, A.; Ahmed, K.A.; Leary, S.C.; Islam, M.F.; Wu, Z.; Chibbar, R.; Xiang, J. The Energy Sensor AMPKα1 Is Critical in Rapamycin-Inhibition of MTORC1-S6K-Induced T-Cell Memory. Int. J. Mol. Sci. 2021, 23, 37. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, F.; Tian, Y.; Cao, L.; Gao, Q.; Zhang, C.; Zhang, K.; Shen, C.; Ping, Y.; Maimela, N.R.; et al. Metformin Enhances the Antitumor Activity of CD8+ T Lymphocytes via the AMPK-MiR-107-Eomes-PD-1 Pathway. J. Immunol. 2020, 204, 2575–2588. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, C.; Kauer, J.; Brinkmann, B.; Dreger, P.; Huber, W.; Müller-Tidow, C.; Dietrich, S.; Roider, T. Blocking the CD39/CD73 Pathway Synergizes with Anti-CD20 Bispecific Antibody in Nodal B-Cell Lymphoma. J. Immunother. Cancer 2025, 13, e009245. [Google Scholar] [CrossRef] [PubMed]
- Grund, J.; Iben, K.; Reinke, S.; Bühnen, I.; Plütschow, A.; Müller-Meinhard, B.; Marquez, M.A.G.; Schlößer, H.A.; Tresckow, B.V.; Kellermeier, F.; et al. Low B-Cell Content Is Associated with a CD73-Low Tumour Microenvironment and Unfavourable Prognosis in Classic Hodgkin Lymphoma. Br. J. Haematol. 2023, 201, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Cannarile, M.A.; Weisser, M.; Jacob, W.; Jegg, A.M.; Ries, C.H.; Rüttinger, D. Colony-Stimulating Factor 1 Receptor (CSF1R) Inhibitors in Cancer Therapy. J. Immunother. Cancer 2017, 5, 53. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Li, Z.M.; Li, L.; Su, H.; Jin, Z.; Zuo, X.; Wu, J.; Zhou, H.; Li, K.; et al. SHR2554, an EZH2 Inhibitor, in Relapsed or Refractory Mature Lymphoid Neoplasms: A First-in-Human, Dose-Escalation, Dose-Expansion, and Clinical Expansion Phase 1 Trial. Lancet Haematol. 2022, 9, e493–e503. [Google Scholar] [CrossRef]
- Mei, M.G.; Chen, L.; Puverel, S.; Budde, L.E.; Kambhampati, S.; Daniels, S.; Dunning, B.; Banez, M.; Kwak, L.W.; Herrera, A.F. The Combination of Nivolumab and CC-486 Is Active in Hodgkin Lymphoma Refractory to PD-1 Blockade. Blood 2023, 142, 1697. [Google Scholar] [CrossRef]
- Heger, J.-M.; Mammadova, L.; Mattlener, J.; Sobesky, S.; Cirillo, M.; Altmüller, J.; Kirst, E.; Reinke, S.; Klapper, W.; Bröckelmann, P.J.; et al. Circulating Tumor DNA Sequencing for Biologic Classification and Individualized Risk Stratification in Patients With Hodgkin Lymphoma. J. Clin. Oncol. 2024, 42, 4218–4230. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.P.; Liaskas, A.; Pereyra, P.; Panayiotidis, P.; Angelopoulou, M.K.; Gallamini, A. Incorporating Monoclonal Antibodies into the First-Line Treatment of Classical Hodgkin Lymphoma. Int. J. Mol. Sci. 2023, 24, 13187. [Google Scholar] [CrossRef]
- Rossi, C.; Gilhodes, J.; Maerevoet, M.; Herbaux, C.; Morschhauser, F.; Brice, P.; Garciaz, S.; Borel, C.; Ysebaert, L.; Obéric, L.; et al. Efficacy of Chemotherapy or Chemo-Anti-PD-1 Combination after Failed Anti-PD-1 Therapy for Relapsed and Refractory Hodgkin Lymphoma: A Series from Lysa Centers. Am. J. Hematol. 2018, 93, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Mao, G.; Tang, Y.; Li, C.; Gao, Y.; Nie, W.; Song, T.; Liu, S.; Zhang, P.; Tao, K.; et al. Inhibition of Glycolysis Enhances the Efficacy of Immunotherapy via PDK-Mediated Upregulation of PD-L1. Cancer Immunol. Immunother. 2024, 73, 151. [Google Scholar] [CrossRef] [PubMed]
- Zak, J.; Pratumchai, I.; Marro, B.S.; Marquardt, K.L.; Zavareh, R.B.; Lairson, L.L.; Oldstone, M.B.A.; Varner, J.A.; Hegerova, L.; Cao, Q.; et al. JAK Inhibition Enhances Checkpoint Blockade Immunotherapy in Patients with Hodgkin Lymphoma. Science 2024, 384, eade8520. [Google Scholar] [CrossRef]
- Ansell, S.; Gutierrez, M.E.; Shipp, M.A.; Gladstone, D.; Moskowitz, A.; Borello, I.; Popa-Mckiver, M.; Farsaci, B.; Zhu, L.; Lesokhin, A.M.; et al. A Phase 1 Study of Nivolumab in Combination with Ipilimumab for Relapsed or Refractory Hematologic Malignancies (CheckMate 039). Blood 2016, 128, 183. [Google Scholar] [CrossRef]
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.-B.; McSweeney, P.; et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016, 375, 143–153. [Google Scholar] [CrossRef]
- Study Details. Ipilimumab With or Without Nivolumab in Relapsed/Refractory CHL. ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04938232 (accessed on 23 July 2025).
- Yusuf, R.; Jemielita, T.; Marinello, P. Safety and Efficacy of Vibostolimab and Pembrolizumab in Patients with Relapsed or Refractory Hematologic Malignancies: A Multicohort, Open-Label, Phase 2 Study. Blood 2021, 138, 2484. [Google Scholar] [CrossRef]
- Timmerman, J.; Lavie, D.; Johnson, N.A.; Avigdor, A.; Borchmann, P.; Andreadis, C.; Bazargan, A.; Gregory, G.P.; Keane, C.; Tzoran, I.; et al. Favezelimab in Combination with Pembrolizumab in Patients with Heavily Pretreated Anti-PD-1-Refractory Classical Hodgkin Lymphoma: Updated Analysis of an Open-Label Phase 1/2 Study. Blood 2023, 142, 4440. [Google Scholar] [CrossRef]
- Zou, S.; Liu, B.; Feng, Y. CCL17, CCL22 and Their Receptor CCR4 in Hematologic Malignancies. Discov. Oncol. 2024, 15, 412. [Google Scholar] [CrossRef]
- Benevolo Savelli, C.; Bisio, M.; Legato, L.; Fasano, F.; Santambrogio, E.; Nicolosi, M.; Morra, D.; Boccomini, C.; Freilone, R.; Botto, B.; et al. Advances in Hodgkin Lymphoma Treatment: From Molecular Biology to Clinical Practice. Cancers 2024, 16, 1830. [Google Scholar] [CrossRef]
- Schroers-Martin, J.; Spinner, M.; Merryman, R.; Chang, C.; Yeung, A.; Silin, C.; Powell, M.; Armand, P.; Shipp, M.A.; Advani, R.H. P127: Phase II Trial Evaluating the Anti-CD47 Antibody Magrolimab in Combination with Pembrolizumab in Patients with Relapsed/Refractory Classic Hodgkin Lymphoma. Hemasphere 2024, 8 (Suppl. 1). [Google Scholar] [CrossRef]
- Study Details. Study of Magrolimab and Pembrolizumab in Relapsed or Refractory Classic Hodgkin Lymphoma. ClinicalTrials. Available online: https://clinicaltrials.gov/study/NCT04788043 (accessed on 23 July 2025).
- Bartlett, N.L.; Herrera, A.F.; Domingo-Domenech, E.; Mehta, A.; Forero-Torres, A.; Garcia-Sanz, R.; Armand, P.; Devata, S.; Izquierdo, A.R.; Lossos, I.S.; et al. A Phase 1b Study of AFM13 in Combination with Pembrolizumab in Patients with Relapsed or Refractory Hodgkin Lymphoma. Blood 2020, 136, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, J.; LI, H.; Qian, W.; Xiao, X.; Cai, Q.; Liu, Y.; Zhang, Y.; Zhang, L.; Qin, L.; et al. S216: CD47/PD-L1 Bispecific Antibody (IBI322) in Anti-PD-1 or PD-L1 Treatment-Resistant Classical Hodgkin Lymphoma: A Phase I Study. Hemasphere 2023, 7, e8102841. [Google Scholar] [CrossRef]
- Winer, E.S.; Maris, M.; Sharma, M.R.; Kaminker, P.; Zhao, E.; Ward, A.; Sochacki, A.L. A Phase 1, First-in-Human, Dose-Escalation Study of MGD024, a CD123 x CD3 Bispecific Dart® Molecule, in Patients with Relapsed or Refractory CD123-Positive (+) Hematologic Malignancies. Blood 2022, 140, 11753–11754. [Google Scholar] [CrossRef]
- Study Details. A Study of MGD024 in Patients With Relapsed or Refractory Hematologic Malignancies. ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/study/NCT05362773?cond=Systemic%20mastocytosis%20OR%20%22SMCD%20-%20systemic%20mast%20cell%20disease%22%20OR%20%22%20systemic%20tissue%20mast%20cell%20disease%22&aggFilters=status:not%20rec&viewType=Table&rank=10 (accessed on 23 July 2025).
- Shi, M.; Chen, J.; Li, K.; Fang, Y.; Wen, G.; Li, X.; Liu, Y.; Sun, Y.; Zhu, B.; Lin, L.; et al. SHR-1701, a Bifunctional Fusion Protein Targeting PD-L1 and TGF-β, for Advanced NSCLC with EGFR Mutations: Data from a Multicenter Phase 1 Study. J. Clin. Oncol. 2021, 39, 9055. [Google Scholar] [CrossRef]
- Study Details. Phase I/II Study of SHR2554 in Combination With SHR1701 in Patients With Advanced Solid Tumors and B-Cell Lymphomas. ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04407741 (accessed on 23 July 2025).
- Feng, K.; Liu, Y.; Wang, C.; Nie, J.; Xia, Y.; Jiang, J.; Han, W. Phase I Study of the Bifunctional Anti-PD-L1/TGF-ΒRII Agent SHR-1701 Combined with SHR2554, an EZH2 Inhibitor, in Patients with Previously Treated Advanced Lymphoma and Solid Tumors. J. Clin. Oncol. 2023, 41, 2507. [Google Scholar] [CrossRef]
- Borchmann, P.; Ferdinandus, J.; Schneider, G.; Moccia, A.; Greil, R.; Hertzberg, M.; Schaub, V.; Hüttmann, A.; Keil, F.; Dierlamm, J.; et al. Assessing the Efficacy and Tolerability of PET-Guided BrECADD versus EBEACOPP in Advanced-Stage, Classical Hodgkin Lymphoma (HD21): A Randomised, Multicentre, Parallel, Open-Label, Phase 3 Trial. Lancet 2024, 404, 341–352. [Google Scholar] [CrossRef]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illés, Á.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 378, 331–344. [Google Scholar] [CrossRef]
- Kahn, J.M.; Mauz-Korholz, C.; Hernandez, T.; Milgrom, S.A.; Castellino, S.M. Pediatric and Adolescent Hodgkin Lymphoma: Paving the Way for Standards of Care and Shared Decision Making. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e432420. [Google Scholar] [CrossRef]
- Driessen, J.; de Wit, F.; Herrera, A.F.; Zinzani, P.L.; LaCasce, A.S.; Cole, P.D.; Moskowitz, C.H.; García-Sanz, R.; Fuchs, M.; Müller, H.; et al. Brentuximab Vedotin and Chemotherapy in Relapsed/Refractory Hodgkin Lymphoma: A Propensity Score-Matched Analysis. Blood Adv. 2024, 8, 2740–2752. [Google Scholar] [CrossRef]
- Bowers, J.T.; Anna, J.; Bair, S.M.; Annunzio, K.; Epperla, N.; Pullukkara, J.J.; Gaballa, S.; Spinner, M.A.; Li, S.; Messmer, M.R.; et al. Brentuximab Vedotin plus AVD for Hodgkin Lymphoma: Incidence and Management of Peripheral Neuropathy in a Multisite Cohort. Blood Adv. 2023, 7, 6630–6638. [Google Scholar] [CrossRef]
- Ramos, C.A.; Grover, N.S.; Beaven, A.W.; Lulla, P.D.; Wu, M.F.; Ivanova, A.; Wang, T.; Shea, T.C.; Rooney, C.M.; Dittus, C.; et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J. Clin. Oncol. 2020, 38, 3794–3804. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Natrakul, D.A.; Karrs, J.; Patel, N.; Maass-Moreno, R.; Ahlman, M.A.; Mikkilineni, L.; Mann, J.; Stroncek, D.F.; Highfill, S.L.; et al. Transient Responses and Significant Toxicities of Anti-CD30 CAR T Cells for CD30+ Lymphomas: Results of a Phase 1 Trial. Blood Adv. 2024, 8, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Gottschlich, A.; Grünmeier, R.; Hoffmann, G.V.; Nandi, S.; Kavaka, V.; Müller, P.J.; Jobst, J.; Oner, A.; Kaiser, R.; Gärtig, J.; et al. Dissection of Single-Cell Landscapes for the Development of Chimeric Antigen Receptor T Cells in Hodgkin Lymphoma. Blood 2025, 145, 1536–1552. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lei, W.; Jin, X.; Liu, H.; Wang, J.Q.; Deng, W.; Qian, W. CD70-Specific CAR NK Cells Expressing IL-15 for the Treatment of CD19-Negative B-Cell Malignancy. Blood Adv. 2024, 8, 2635–2645. [Google Scholar] [CrossRef]
- Sanz-Ortega, L.; Leijonhufvud, C.; Schoutens, L.; Lambert, M.; Levy, E.; Andersson, A.; Wahlin, B.E.; Carlsten, M. Redirecting NK Cells to the Lymph Nodes to Augment Their Lymphoma-Targeting Capacity. NPJ Precis. Oncol. 2024, 8, 108. [Google Scholar] [CrossRef]
- Ansell, S.M. Hodgkin Lymphoma: 2025 Update on Diagnosis, Risk-Stratification, and Management. Am. J. Hematol. 2024, 99, 2367–2378. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurlapski, M.; Braczko, A.; Dubiela, P.; Walczak, I.; Kutryb-Zając, B.; Zaucha, J.M. Metabolic Interactions in the Tumor Microenvironment of Classical Hodgkin Lymphoma: Implications for Targeted Therapy. Int. J. Mol. Sci. 2025, 26, 7508. https://doi.org/10.3390/ijms26157508
Kurlapski M, Braczko A, Dubiela P, Walczak I, Kutryb-Zając B, Zaucha JM. Metabolic Interactions in the Tumor Microenvironment of Classical Hodgkin Lymphoma: Implications for Targeted Therapy. International Journal of Molecular Sciences. 2025; 26(15):7508. https://doi.org/10.3390/ijms26157508
Chicago/Turabian StyleKurlapski, Michał, Alicja Braczko, Paweł Dubiela, Iga Walczak, Barbara Kutryb-Zając, and Jan Maciej Zaucha. 2025. "Metabolic Interactions in the Tumor Microenvironment of Classical Hodgkin Lymphoma: Implications for Targeted Therapy" International Journal of Molecular Sciences 26, no. 15: 7508. https://doi.org/10.3390/ijms26157508
APA StyleKurlapski, M., Braczko, A., Dubiela, P., Walczak, I., Kutryb-Zając, B., & Zaucha, J. M. (2025). Metabolic Interactions in the Tumor Microenvironment of Classical Hodgkin Lymphoma: Implications for Targeted Therapy. International Journal of Molecular Sciences, 26(15), 7508. https://doi.org/10.3390/ijms26157508







