Biomarker Correlations in PTSD: IL-18, IRE1, pERK, and ATF6 via Courtauld Emotional Control Scale (CECS)
Abstract
1. Introduction
2. Results
2.1. Sociodemographic, Biomarker, and Emotional Control Profiles Across PTSD Status Groups
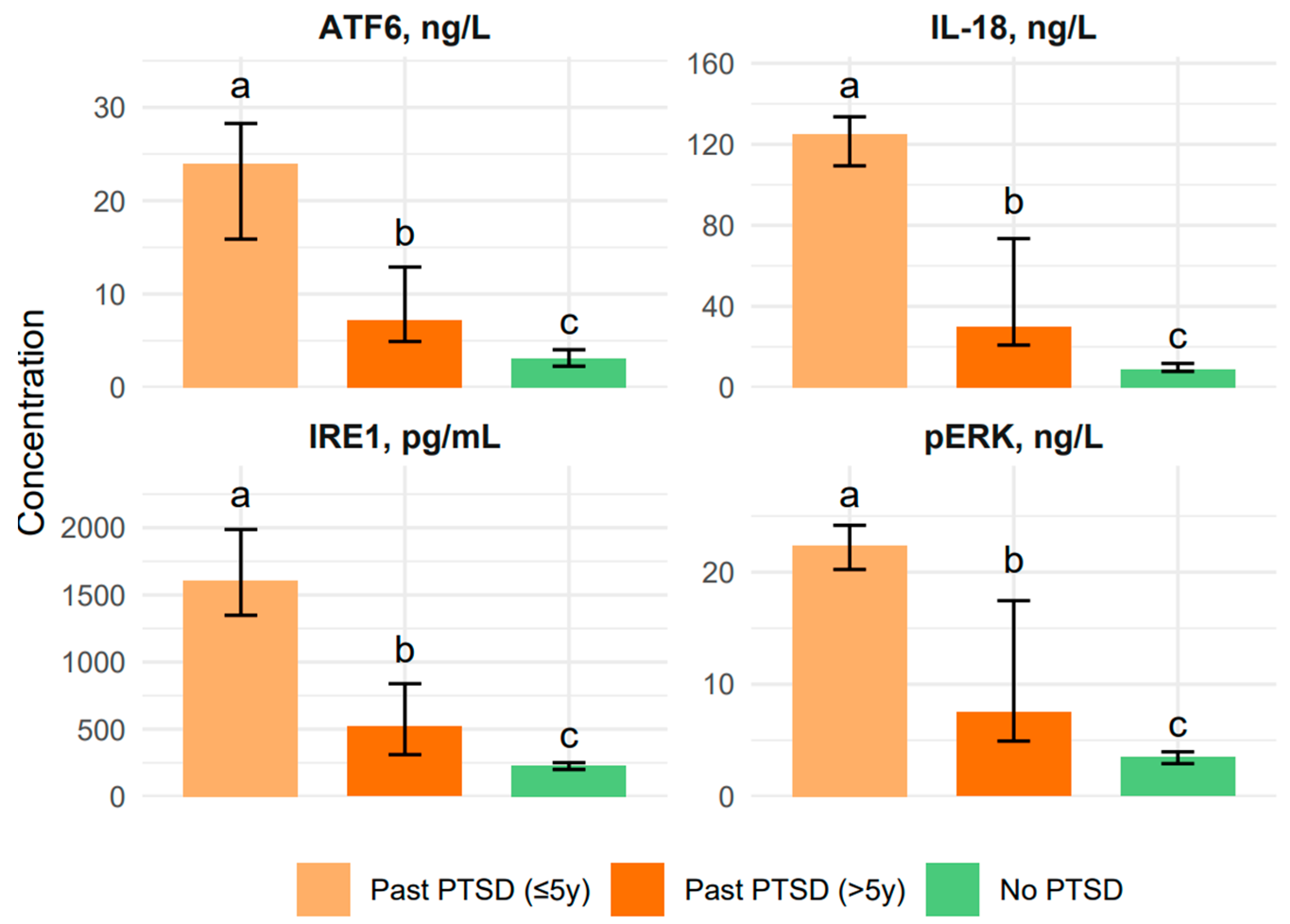
2.1.1. Impact of PTSD on Biomarker Levels and Emotional Control Profile in Patients
2.1.2. Influence of PTSD Duration on Biomarker Levels and Emotional Control Profile in Patients
2.2. Estimation of Correlations Between the Biomarker Levels and CECS Scores Across PTSD Status Groups
3. Discussion
4. Material and Methods
4.1. Characteristics of the Participants
4.2. Courtauld Emotional Control Scale
4.3. Blood Sampling and Serum Preparation
4.4. Biomarker Analysis Procedure
4.5. Assay Sensitivity and Standardization
4.6. Statistical Analysis
4.7. Data Collection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTSD | Post-Traumatic Stress Disorder |
| IL-18 | Interleukin 18 |
| IL-18BP | Interleukin-18 Binding Protein |
| IL-18RAP | Interleukin-18 Receptor Accessory Protein |
| IL-18R1 | interleukin-18 Receptor 1 |
| IRE1 | Inositol-Requiring Enzyme 1 |
| pERK | Phosphorylated Extracellular Signal-Regulated Kinase |
| ATF6 | Activating Transcription Factor6 |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| CECS | Courtauld Emotional Control Scale |
| CNS | Central Nervous System |
| Th1 | T-helper type 1 lymphocytes |
| NLR | NOD-like Receptor NLR |
| NLRP3 | NOD-like Receptor NLR (NLR) Family Pyrin Domain Containing 3 |
| IL-1β | Interleukin 1 beta |
| TLRs | Toll-Like Receptors |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| PAMPs | Pathogen-Associated Molecular Patterns |
| DAMPs | Danger-Associated Molecular Patterns |
| ASC | Apoptosis-Associated Speck-Like Protein Containing a CARD |
| CARD | Caspase Recruitment Domain |
| ER | Endoplasmic Reticulum |
| UPR | Unfolded Protein Response |
| XBP1 | X-box Binding Protein 1 |
| JNK | c-Jun N-terminal kinase |
| RIDD | Regulated IRE1-Dependent Decay |
| IRE1α | Inositol-requiring enzyme 1 alpha |
| PERK | Protein kinase R-like endoplasmic reticulum kinase |
| eIF2α | Eukaryotic initiation factor 2 alpha |
| ATF4 | Activating transcription factor 4 |
| CHOP | C/EBP homologous protein (pro-apoptotic factor) |
| S1P | Site-1 protease |
| S2P | Site-2 protease |
| ERAD | Endoplasmic reticulum-associated degradation |
| HPA axis | Hypothalamic–pituitary–adrenal axis |
| IQR | Interquartile Range |
| ELISA | Protein measurement assay |
| HRP | Enzyme used in ELISA signal detection |
References
- Lee, D.J.; Crowe, M.L.; Weathers, F.W.; Bovin, M.J.; Ellickson, S.; Sloan, D.M.; Schnurr, P.; Keane, T.M.; Marx, B.P. An item response theory analysis of the Clinician-Administered PTSD Scale for DSM-5 among veterans. Assess 2024, 31, 1262–1269. [Google Scholar] [CrossRef]
- Dieujuste, N.; Petri, J.M.; Mekawi, Y.; Lathan, E.C.; Carter, S.; Bradley, B.; Fani, N.; Powers, A. Investigating associations between emotion dysregulation and DSM-5 posttraumatic stress disorder (PTSD) using network analysis. J. Affect. Disord. 2025, 377, 106–115. [Google Scholar] [CrossRef]
- Szeifert, N.M.; Oláh, B.; Gonda, X. The mediating role of adult attachment styles between early traumas and suicidal behaviour. Sci. Rep. 2025, 15, 15855. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Dehghani, M.; Fathali Lavasani, F.; Farahani, H.; Ashouri, A. A network analysis of ICD-11 Complex PTSD, emotional processing, and dissociative experiences in the context of psychological trauma at different developmental stages. Front. Psychiatry 2024, 15, 1372620. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Zhu, S.; Tang, E.; Xue, C.; Li, K.; Yu, H.; Zhong, T.; Li, T.; Chen, H.; Deng, W. Neural correlates of emotional processing in trauma-related narratives. Psychol. Med. 2025, 55, e33. [Google Scholar] [CrossRef]
- Colvonen, P.J.; Straus, L.D.; Acheson, D.; Gehrman, P. A review of the relationship between emotional learning and memory, sleep, and PTSD. Curr. Psychiatry Rep. 2019, 21, 2. [Google Scholar] [CrossRef]
- Chung, M.C.; Shakra, M.; AlQarni, N.; AlMazrouei, M.; Al Mazrouei, S.; Al Hashimi, S. Posttraumatic stress among Syrian refugees: Trauma exposure characteristics, trauma centrality, and emotional suppression. Psychiatry 2018, 81, 54–70. [Google Scholar] [CrossRef]
- Han, F.; Yan, S.; Shi, Y. Single-prolonged stress induces endoplasmic reticulum-dependent apoptosis in the hippocampus in a rat model of post-traumatic stress disorder. PLoS ONE 2013, 8, e69340. [Google Scholar] [CrossRef]
- Xiao, B.; Yu, B.; Liu, D.J.; Han, F.; Shi, Y.X. Single prolonged stress induces dysfunction of endoplasmic reticulum in a rat model of post-traumatic stress disorder. Mol. Med. Rep. 2015, 12, 2015–2020. [Google Scholar] [CrossRef]
- Ogłodek, E.A. The role of PON-1, GR, IL-18, and OxLDL in depression with and without posttraumatic stress disorder. Pharmacol. Rep. 2017, 69, 837–845. [Google Scholar] [CrossRef]
- Watson, M.; Greer, S. Development of a questionnaire measure of emotional control. J. Psychosom. Res. 1983, 27, 299–305. [Google Scholar] [CrossRef]
- Borgonetti, V.; Cruz, B.; Vozella, V.; Khom, S.; Steinman, M.Q.; Bullard, R.; D’Ambrosio, S.; Oleata, C.S.; Vlkolinsky, R.; Bajo, M.; et al. IL-18 signaling in the rat central amygdala is disrupted in a comorbid model of post-traumatic stress and alcohol use disorder. Cells 2023, 12, 1943. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5166 patients and 5083 controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Deng, J.; Gao, H.; Song, Y.; Zhang, Y.; Sun, J.; Zhai, J. Involvement of the SIRT1-NLRP3 pathway in the inflammatory response. Cell Commun. Signal. 2023, 21, 185. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, G.; Zhao, C.; Yang, Y.; Miao, Z.; Xu, X. Interleukin-18 from neurons and microglia mediates depressive behaviors in mice with post-stroke depression. Brain Behav. Immun. 2020, 88, 411–420. [Google Scholar] [CrossRef]
- Kouba, B.R.; de Araujo Borba, L.; Borges de Souza, P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Role of inflammatory mechanisms in major depressive disorder: From etiology to potential pharmacological targets. Cells 2024, 13, 423. [Google Scholar] [CrossRef]
- Pandey, A.; Li, Z.; Gautam, M.; Ghosh, A.; Man, S.M. Molecular mechanisms of emerging inflammasome complexes and their activation and signaling in inflammation and pyroptosis. Immunol. Rev. 2025, 329, e13406. [Google Scholar] [CrossRef]
- Alboni, S.; Tascedda, F.; Uezato, A.; Sugama, S.; Chen, Z.; Marcondes, M.G.C.; Conti, B. Interleukin-18 and the brain: Neuronal functions, neuronal survival and psychoneuroimmunology during stress. Mol. Psychiatry 2025, 30, 3197–3208. [Google Scholar] [CrossRef]
- Mustafa, M.A.; Bansal, P.; Pallavi, M.S.; Panigrahi, R.; Nathiya, D.; Kumar, S.; Al-Hasnaawei, S.; Chauhan, A.S.; Singla, S. Exploring the role of NLRP3 in neurodegeneration: Cutting-edge therapeutic strategies and inhibitors. Dev. Neurobiol. 2025, 85, e22982. [Google Scholar] [CrossRef]
- Xiang, C.; Wang, Y.; Zhang, H.; Han, F. The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis 2017, 22, 1–26. [Google Scholar] [CrossRef]
- Tang, J.; Yu, W.; Chen, S.; Gao, Z.; Xiao, B. Microglia polarization and endoplasmic reticulum stress in chronic social defeat stress-induced depression mouse. Neurochem. Res. 2018, 43, 985–994. [Google Scholar] [CrossRef]
- Walter, N.S.; Gorki, V.; Bhardwaj, R.; Punnakkal, P. Endoplasmic Reticulum Stress: Implications in Diseases. Protein J. 2025, 44, 147–161. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Khair, M. Endoplasmic Reticulum Stress and Unfolded Protein Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 6127. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, R.L.; Mesgarzadeh, J.S.; Hendershot, L.M. Reshaping Endoplasmic Reticulum Quality Control through the Unfolded Protein Response. Mol. Cell 2022, 82, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Han, F.; Xu, Y.; Shi, Y. Molecular mechanisms of IRE1α-ASK1 pathway reactions to unfolded protein response in DRN neurons of post-traumatic stress disorder rats. J. Mol. Neurosci. 2017, 61, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, F.; Shi, Y. IRE1α-XBP1 pathway is activated upon induction of single-prolonged stress in rat neurons of the medial prefrontal cortex. J. Mol. Neurosci. 2015, 57, 63–72. [Google Scholar] [CrossRef]
- Peng, C.; Wang, J.; Wang, S.; Zhao, Y.; Wang, H.; Wang, Y.; Ma, Y.; Yang, J. Endoplasmic Reticulum Stress: Triggers Microenvironmental Regulation and Drives Tumor Evolution. Cancer Med. 2025, 14, e70684. [Google Scholar] [CrossRef]
- Wen, L.; Xiao, B.; Shi, Y.; Han, F. PERK signalling pathway mediates single prolonged stress-induced dysfunction of medial prefrontal cortex neurons. Apoptosis 2017, 22, 753–768. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, G.; Lang, J.; Sun, B.; Feng, S.; Li, D.; Sun, G. Astragaloside IV ameliorated neuroinflammation and improved neurological functions in mice exposed to traumatic brain injury by modulating the PERK-eIF2α-ATF4 signaling pathway. J. Investig. Med. 2024, 72, 747–762. [Google Scholar] [CrossRef]
- Huang, G.; Iqbal, J.; Shen, D.; Xue, Y.X.; Yang, M.; Jia, X. MicroRNA expression profiles of stress susceptibility and resilience in the prelimbic and infralimbic cortex of rats after single prolonged stress. Front. Psychiatry. 2023, 14, 1247714. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.M.; Perumal, N.; Manicam, C.; Basoglu, M.; Eimer, S.C.; Fuhrmann, D.C.; Pietrzik, C.U.; Clement, A.M.; Körschgen, H.; Schepers, J.; et al. Adaptive Responses of Neuronal Cells to Chronic Endoplasmic Reticulum (ER) Stress. Redox Biol. 2023, 67, 102943. [Google Scholar] [CrossRef]
- Yu, B.; Wen, L.; Xiao, B.; Han, F.; Shi, Y. Single prolonged stress induces ATF6 alpha-dependent endoplasmic reticulum stress and the apoptotic process in medial frontal cortex neurons. BMC Neurosci. 2014, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xu, Y.; Shi, Y. Molecular mechanism of the ATF6α/S1P/S2P signaling pathway in hippocampal neuronal apoptosis in SPS rats. J. Mol. Neurosci. 2021, 71, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.; Ragetli, R.; Mnich, K.; Doble, B.W.; Kammouni, W.; Logue, S.E. IRE1 signaling increases PERK expression during chronic ER stress. Cell Death Dis. 2024, 15, 276. [Google Scholar] [CrossRef]
- Tanaka, T.; Nguyen, D.T.; Kwankaew, N.; Sumizono, M.; Shinoda, R.; Ishii, H.; Takarada-Iemata, M.; Hattori, T.; Oyadomari, S.; Kato, N.; et al. ATF6β Deficiency Elicits Anxiety-like Behavior and Hyperactivity under Stress Conditions. Neurochem. Res. 2023, 48, 2175–2186. [Google Scholar] [CrossRef]
- Wen, L.; Han, F.; Shi, Y.; Li, X. Role of the Endoplasmic Reticulum Pathway in the Medial Prefrontal Cortex in Post-Traumatic Stress Disorder Model Rats. J. Mol. Neurosci. 2016, 59, 471–482. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, H.; Meng, D.; Fa, Z.; Yao, C.; Lin, X.; Schick, J.; Jin, X. Stress Management: How the Endoplasmic Reticulum Mitigates Protein Misfolding and Oxidative Stress by the Dual Role of Glutathione Peroxidase 8. Biomolecules 2025, 15, 847. [Google Scholar] [CrossRef]
- Le, Q.G.; Kimata, Y. Multiple Ways for Stress Sensing and Regulation of the Endoplasmic Reticulum-stress Sensors. Cell Struct. Funct. 2021, 46, 37–49. [Google Scholar] [CrossRef]
- Xue, M.; Irshad, Z.; Rabbani, N.; Thornalley, P.J. Increased Cellular Protein Modification by Methylglyoxal Activates Endoplasmic Reticulum-based Sensors of the Unfolded Protein Response. Redox Biol. 2024, 69, 103025. [Google Scholar] [CrossRef]
- Iwata, M.; Ota, K.T.; Duman, R.S. The Inflammasome: Pathways Linking Psychological Stress, Depression, and Systemic Illnesses. Brain Behav. Immun. 2016, 57, 17–24. [Google Scholar] [CrossRef]
- Kim, T.K.; Kim, J.E.; Choi, J.; Park, J.Y.; Lee, J.E.; Lee, E.H.; Lee, Y.J.; Kim, B.Y.; Oh, Y.J.; Han, P.L. Local Interleukin-18 System in the Basolateral Amygdala Regulates Susceptibility to Chronic Stress. Mol. Neurobiol. 2017, 54, 5347–5358. [Google Scholar] [CrossRef]
- Lisboa, S.F.; Issy, A.C.; Biojone, C.; Montezuma, K.; Fattori, V.; Del-Bel, E.A.; Guimaraes, F.S.; Cunha, F.Q.; Verri, W.A.; Joca, S.R.L. Mice Lacking Interleukin-18 Gene Display Behavioral Changes in Animal Models of Psychiatric Disorders: Possible Involvement of Immunological Mechanisms. J. Neuroimmunol. 2018, 314, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Aman, Y.; Ahmed, R.; Borsini, A. The Role of NLRP3 Inflammasome in Stress-induced Depression. Front. Immunol. 2015, 6, 290. [Google Scholar] [CrossRef]
- Sekiyama, A.; Ueda, H.; Kashiwamura, S.; Nishida, K.; Kawai, K.; Teshimakondo, S.; Rokutan, K.; Okamura, H. IL-18; a Cytokine Translates a Stress into Medical Science. J. Med. Investig. 2005, 52, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Zengeler, K.E.; Hollis, A.; Deutsch, T.C.J.; Samuels, J.D.; Ennerfelt, H.; Moore, K.A.; Steacy, E.J.; Sabapathy, V.; Sharma, R.; Patel, M.K.; et al. Inflammasome signaling in astrocytes modulates hippocampal plasticity. Immunity 2025, 58, 1519–1535.e11. [Google Scholar] [CrossRef]
- Hao, W.; Feng, C. Research Progress on Pyroptosis and Its Effect on the Central Nervous System. Neurobiol. Dis. 2023, 188, 106333. [Google Scholar] [CrossRef]
- Luhong, L.; Zhou, H.M.; Tang, X.H.; Chen, J.; Zhang, A.M.; Zhou, C.L.; Li, S.Y.; Wen Yu, C.; Liyan, H.; Xiang, Y.Y.; et al. PERK Inhibitor (ISRIB) Improves Depression-like Behavior by Inhibitions of HPA-axis Over-activation in Mice Exposed to Chronic Restraint Stress. Behav. Brain Res. 2024, 471, 115122. [Google Scholar] [CrossRef]
- Ghosh, R.; Wang, L.; Wang, E.S.; Perera, B.G.; Igbaria, A.; Morita, S.; Prado, K.; Thamsen, M.; Caswell, D.; Macias, H.; et al. Allosteric Inhibition of the IRE1α RNase Preserves Cell Viability and Function during Endoplasmic Reticulum Stress. Cell 2014, 158, 534–548. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, J.W.; Wang, Z.Y.; Liao, S.P.; Zhu, L.; Wang, X.; Wan, L.H. Protective Effect of Rosmarinic Acid on Endotoxin-Induced Neuronal Damage Through Modulating GRP78/PERK/MANF Pathway. Drug Des. Devel. Ther. 2025, 19, 39–50. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, N.; Choubey, V.; Dhingra, N.; Kaur, T. Endoplasmic Reticulum Stress and Its Role in Various Neurodegenerative Diseases. Brain Res. 2024, 1826, 148742. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Dwivedi, Y. Elevated Expression of Unfolded Protein Response Genes in the Prefrontal Cortex of Depressed Subjects: Effect of Suicide. J. Affect. Disord. 2020, 262, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef]
- Martínez, G.; Duran-Aniotz, C.; Cabral-Miranda, F.; Vivar, J.P.; Hetz, C. Endoplasmic Reticulum Proteostasis Impairment in Aging. Aging Cell 2018, 16, 615–623. [Google Scholar] [CrossRef]
- Wilker, S.; Vukojevic, V.; Schneider, A.; Pfeiffer, A.; Inerle, S.; Pauly, M.; Elbert, T.; Papassotiropoulos, A.; de Quervain, D.; Kolassa, I.T.; et al. Epigenetics of traumatic stress: The association of NR3C1 methylation and posttraumatic stress disorder symptom changes in response to narrative exposure therapy. Transl. Psychiatry 2023, 13, 14. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Yan, G.; Luo, Y. ATF6 Mediates Chronic Stress-induced Synaptic Plasticity Impairment and Memory Deficits. Neurobiol. Stress 2021, 14, 100319. [Google Scholar] [CrossRef]


| Characteristic | Total (N = 92) | Past PTSD (≤5y) (N = 33) | Past PTSD (>5y) (N = 31) | No PTSD (Control) (N = 28) | p-Value | Post hoc |
| Demographic characteristics, median (IQR) | ||||||
| Age, years | 34.0 (28.8–41.0) | 34.0 (31.0–41.0) | 36.0 (29.5–41.0) | 33.5 (24.3–41.5) | 0.524 | - |
| Employment in hazardous conditions, years | 10.0 (6.0–14.25) | 11.0 (7.0–14.0) | 10.0 (7.5–15.0) | 10.0 (3.0–14.0) | 0.418 | - |
| Biomarker Levels, median (IQR) | ||||||
| IL-18, ng/L | 36.2 (11.1–112.1) | 125.2 (109.4–133.6) a | 30.15 (20.8–73.4) b | 9.0 (7.9–11.8) c | <0.001 | a > b > c |
| IRE1, pg/mL | 577.3 (246.8–1379.0) | 1608.0 (1348.0–1987.0) a | 520.9 (310.7–838.9) b | 232.3 (199.9–251.0) c | <0.001 | a > b > c |
| pERK, ng/L | 10.0 (3.8–21.7) | 22.4 (20.2–24.2) a | 7.5 (4.9–17.5) b | 3.5 (2.9–4.0) c | <0.001 | a > b > c |
| ATF6, ng/L | 9.5 (3.6–17.5) | 24.0 (15.9–28.3) a | 7.2 (4.9–12.9) b | 3.05 (2.3–4.0) c | <0.001 | a > b > c |
| Questionnaire (c) parameters, median (IQR) | ||||||
| CECS Anger subscale | 16.5 (9.0–22.3) | 16.0 (13.0–19.0) b | 25.0 (22.0–27.0) a | 7.0 (7.0–9.0) c | <0.001 | a > b > c |
| CECS Depression subscale | 14.0 (9.0–26.0) | 10.0 (9.0–16.0) b | 28.0 (26.0–28.0) a | 9.0 (7.0–14.0) c | <0.001 | a > b > c |
| CECS Anxiety subscale | 16.50 (10.9–25.0) | 16.0 (13.0–19.0) b | 27.0 (24.5–28.0) a | 7.0 (7.0–9.0) c | <0.001 | a > b > c |
| CECS total score | 43.0 (30.0–72.0) | 42.0 (38.0–48.0) b | 79.0 (72.0–82.5) a | 25.0 (22.8–29.0) c | <0.001 | a > b > c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźny-Rasała, I.; Ogłodek, E.A. Biomarker Correlations in PTSD: IL-18, IRE1, pERK, and ATF6 via Courtauld Emotional Control Scale (CECS). Int. J. Mol. Sci. 2025, 26, 7506. https://doi.org/10.3390/ijms26157506
Woźny-Rasała I, Ogłodek EA. Biomarker Correlations in PTSD: IL-18, IRE1, pERK, and ATF6 via Courtauld Emotional Control Scale (CECS). International Journal of Molecular Sciences. 2025; 26(15):7506. https://doi.org/10.3390/ijms26157506
Chicago/Turabian StyleWoźny-Rasała, Izabela, and Ewa Alicja Ogłodek. 2025. "Biomarker Correlations in PTSD: IL-18, IRE1, pERK, and ATF6 via Courtauld Emotional Control Scale (CECS)" International Journal of Molecular Sciences 26, no. 15: 7506. https://doi.org/10.3390/ijms26157506
APA StyleWoźny-Rasała, I., & Ogłodek, E. A. (2025). Biomarker Correlations in PTSD: IL-18, IRE1, pERK, and ATF6 via Courtauld Emotional Control Scale (CECS). International Journal of Molecular Sciences, 26(15), 7506. https://doi.org/10.3390/ijms26157506






