Genetic Associations of ITGB3, FGG, GP1BA, PECAM1, and PEAR1 Polymorphisms and the Platelet Activation Pathway with Recurrent Pregnancy Loss in the Korean Population
Abstract
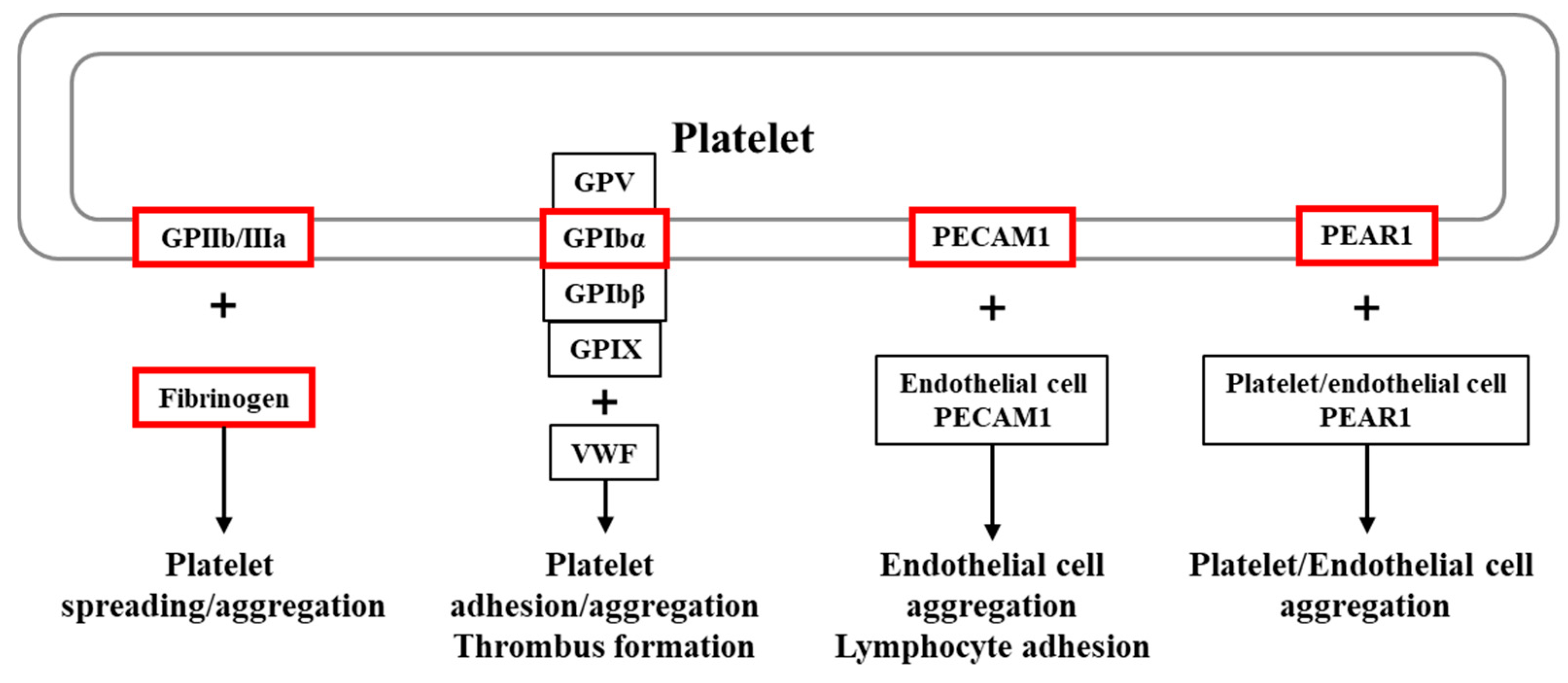
1. Introduction
2. Results
2.1. Clinical Characteristics of the Study Participants
2.2. Genotype and Allele Frequencies of the ITGB3, FGG, GP1BA, PECAM1, and PEAR1 Gene Polymorphisms
2.3. Allele Combination and Haplotype Analysis of the ITGB3, FGG, GP1BA, PECAM1, and PEAR1 Gene Polymorphisms
2.4. Genotype Combination Analysis of the ITGB3, FGG, GP1BA, PECAM1, and PEAR1 Gene Polymorphisms
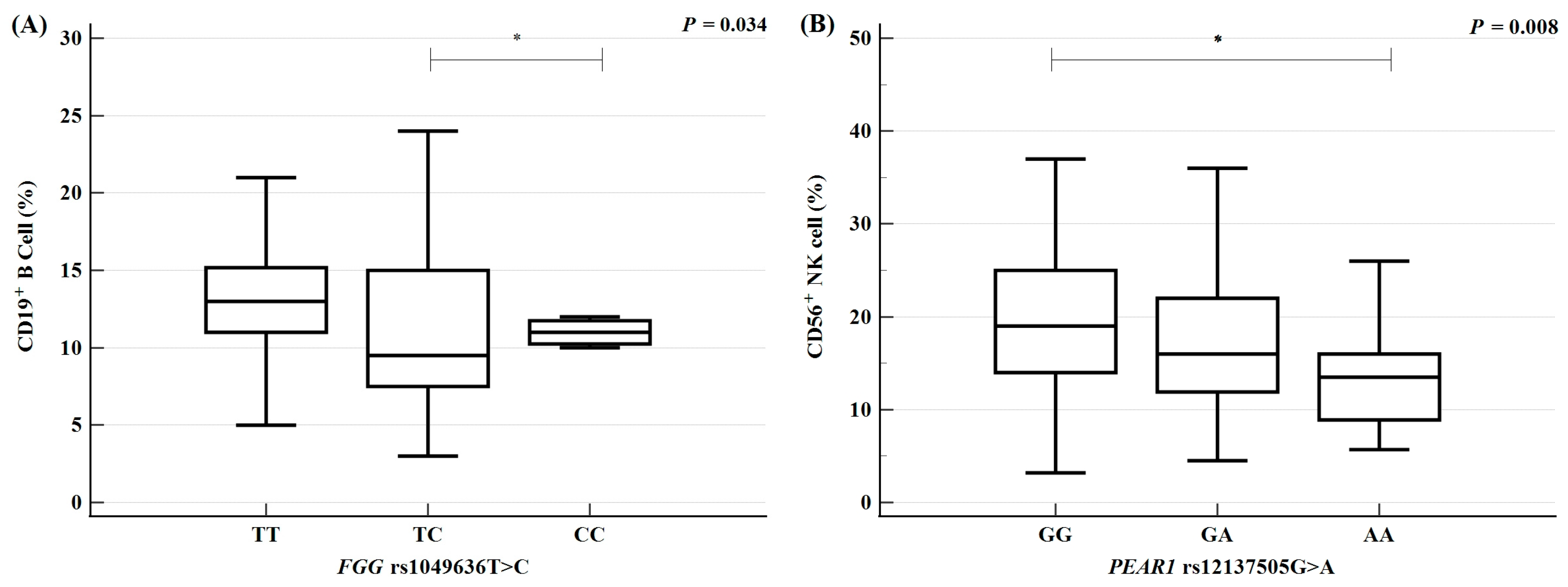
2.5. Variations in Clinical Parameters According to Polymorphism
2.6. Synergistic Interactions Between Polymorphisms and Clinical Parameters
3. Discussion
4. Materials and Methods
4.1. Study Approval
4.2. Study Population
4.3. Estimation of Biochemical Factor Concentrations
4.4. Flow Cytometry Analysis of Immune Cell Proportions
4.5. Hormone Assays
4.6. SNP Selection and Genetic Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, E.; Yi, J.S. Determinants of Fertility Intentions among South Koreans: Systematic Review and Meta-Analysis. Behav. Sci. 2024, 14, 939. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Reproductive health status and policy. Health Welf. Policy Forum 2022, 308, 94–104. [Google Scholar] [CrossRef]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef]
- RCOG. Green-Top Guideline No. 17 the Investigation and Treatment of Couples with Recurrent First-Trimester and Second-Trimester Miscarriage; RCOG: London, UK, 2011. [Google Scholar]
- Turesheva, A.; Aimagambetova, G.; Ukybassova, T.; Marat, A.; Kanabekova, P.; Kaldygulova, L.; Amanzholkyzy, A.; Ryzhkova, S.; Nogay, A.; Khamidullina, Z.; et al. Recurrent Pregnancy Loss Etiology, Risk Factors, Diagnosis, and Management. Fresh Look into a Full Box. J. Clin. Med. 2023, 12, 4074. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef]
- Kwak-Kim, J. Immunology of Recurrent Pregnancy Loss and Implantation Failure; Academic Press: London, UK, 2022; pp. 9–100. [Google Scholar]
- Smyth, S.S.; McEver, R.P.; Weyrich, A.S.; Morrell, C.N.; Hoffman, M.R.; Arepally, G.M.; French, P.A.; Dauerman, H.L.; Becker, R.C.; Platelet Colloquium, P. Platelet functions beyond hemostasis. J. Thromb. Haemost. 2009, 7, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Kovacs, K.; Sulyok, E.; Varnagy, A.; Bodis, J. Thrombocytes and Platelet-Rich Plasma as Modulators of Reproduction and Fertility. Int. J. Mol. Sci. 2023, 24, 17336. [Google Scholar] [CrossRef]
- Mouanness, M.; Ali-Bynom, S.; Jackman, J.; Seckin, S.; Merhi, Z. Use of Intra-uterine Injection of Platelet-rich Plasma (PRP) for Endometrial Receptivity and Thickness: A Literature Review of the Mechanisms of Action. Reprod. Sci. 2021, 28, 1659–1670. [Google Scholar] [CrossRef]
- Cakiroglu, Y.; Saltik, A.; Yuceturk, A.; Karaosmanoglu, O.; Kopuk, S.Y.; Scott, R.T.; Tiras, B.; Seli, E. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging 2020, 12, 10211–10222. [Google Scholar] [CrossRef]
- Nazari, L.; Salehpour, S.; Hoseini, S.; Zadehmodarres, S.; Azargashb, E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: A double-blind RCT. Int. J. Reprod. Biomed. 2019, 17, 443–448. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Haseefa, F.; Peyton, L.; Shadmehr, M.; Niyas, A.M.; Patel, A.; Krdi, G.; Movahed, M.R. A comprehensive review of the ten main platelet receptors involved in platelet activity and cardiovascular disease. Am. J. Blood Res. 2023, 13, 168–188. [Google Scholar] [CrossRef]
- Sivaraman, B.; Latour, R.A. Delineating the roles of the GPIIb/IIIa and GP-Ib-IX-V platelet receptors in mediating platelet adhesion to adsorbed fibrinogen and albumin. Biomaterials 2011, 32, 5365–5370. [Google Scholar] [CrossRef] [PubMed]
- Nanda, N.; Bao, M.; Lin, H.; Clauser, K.; Komuves, L.; Quertermous, T.; Conley, P.B.; Phillips, D.R.; Hart, M.J. Platelet endothelial aggregation receptor 1 (PEAR1), a novel epidermal growth factor repeat-containing transmembrane receptor, participates in platelet contact-induced activation. J. Biol. Chem. 2005, 280, 24680–24689. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Ren, L.; Ren, C.; Liu, X.; Dong, C.; Zhang, X. Platelet endothelial cell adhesion molecule-1 (PECAM1) plays a critical role in the maintenance of human vascular endothelial barrier function. Cell Biochem. Funct. 2015, 33, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.; Dhillon-Smith, R.; Islam, M.A.; Devall, A.; Coomarasamy, A. Genetic causes of sporadic and recurrent miscarriage. Fertil. Steril. 2023, 120, 940–944. [Google Scholar] [CrossRef]
- Wilkie, G.S.; Dickson, K.S.; Gray, N.K. Regulation of mRNA translation by 5′- and 3′-UTR-binding factors. Trends Biochem. Sci. 2003, 28, 182–188. [Google Scholar] [CrossRef]
- Uitte de Willige, S.; Rietveld, I.M.; De Visser, M.C.; Vos, H.L.; Bertina, R.M. Polymorphism 10034C>T is located in a region regulating polyadenylation of FGG transcripts and influences the fibrinogen gamma’/gammaA mRNA ratio. J. Thromb. Haemost. 2007, 5, 1243–1249. [Google Scholar] [CrossRef]
- Behforouz, A.; Dastgheib, S.A.; Abbasi, H.; Karimi-Zarchi, M.; Javaheri, A.; Hadadan, A.; Tabatabaei, R.S.; Meibodi, B.; Neamatzadeh, H. Association of MMP-2, MMP-3, and MMP-9 Polymorphisms with Susceptibility to Recurrent Pregnancy Loss. Fetal Pediatr. Pathol. 2021, 40, 378–386. [Google Scholar] [CrossRef]
- Middeldorp, S.; Naue, C.; Kohler, C. Thrombophilia, Thrombosis and Thromboprophylaxis in Pregnancy: For What and in Whom? Hamostaseologie 2022, 42, 54–64. [Google Scholar] [CrossRef]
- Sehring, J.; Beltsos, A.; Jeelani, R. Human implantation: The complex interplay between endometrial receptivity, inflammation, and the microbiome. Placenta 2022, 117, 179–186. [Google Scholar] [CrossRef]
- La Farina, F.; Raparelli, V.; Napoleone, L.; Guadagni, F.; Basili, S.; Ferroni, P. Inflammation and Thrombophilia in Pregnancy Complications: Implications for Risk Assessment and Clinical Management. Cardiovasc. Hematol. Disord. Drug Targets 2016, 15, 187–203. [Google Scholar] [CrossRef]
- Pabinger, I.; Grafenhofer, H.; Kaider, A.; Ilic, A.; Eichinger, S.; Quehenberger, P.; Husslein, P.; Mannhalter, C.; Lechner, K. Preeclampsia and fetal loss in women with a history of venous thromboembolism. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 874–879. [Google Scholar] [CrossRef]
- Cotechini, T.; Graham, C.H. Aberrant maternal inflammation as a cause of pregnancy complications: A potential therapeutic target? Placenta 2015, 36, 960–966. [Google Scholar] [CrossRef]
- Jenne, C.N.; Urrutia, R.; Kubes, P. Platelets: Bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 2013, 35, 254–261. [Google Scholar] [CrossRef]
- Blomqvist, L.R.F.; Strandell, A.M.; Baghaei, F.; Hellgren, M.S.E. Platelet aggregation in healthy women during normal pregnancy—A longitudinal study. Platelets 2019, 30, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.; Isermann, B. Crosstalk between inflammation and coagulation: Focus on pregnancy related complications. Thromb. Update 2021, 5, 100072. [Google Scholar] [CrossRef]
- Forstner, D.; Guettler, J.; Gauster, M. Changes in Maternal Platelet Physiology during Gestation and Their Interaction with Trophoblasts. Int. J. Mol. Sci. 2021, 22, 10732. [Google Scholar] [CrossRef] [PubMed]
- Philipp, C.S.; Dilley, A.; Miller, C.H.; Evatt, B.; Baranwal, A.; Schwartz, R.; Bachmann, G.; Saidi, P. Platelet functional defects in women with unexplained menorrhagia. J. Thromb. Haemost. 2003, 1, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Falcinelli, E.; Bury, L. Inherited platelet disorders in women. Thromb. Res. 2019, 181 (Suppl. S1), S54–S59. [Google Scholar] [CrossRef]
- Yagmur, E.; Bast, E.; Muhlfeld, A.S.; Koch, A.; Weiskirchen, R.; Tacke, F.; Neulen, J. High Prevalence of Sticky Platelet Syndrome in Patients with Infertility and Pregnancy Loss. J. Clin. Med. 2019, 8, 1328. [Google Scholar] [CrossRef]
- Ding, D.; Liu, X.; Duan, J.; Guo, S.W. Platelets are an unindicted culprit in the development of endometriosis: Clinical and experimental evidence. Hum. Reprod. 2015, 30, 812–832. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Huang, Y.; Xu, H.; Cen, X.; Zhang, Y.; Xu, Y.; Luo, Z.; Li, C.; Chen, C.; Shi, S.; et al. Comparing the coagulation and platelet parameters of women with premature ovarian insufficiency with those of age-matched controls: A case-control study. Int. J. Gynaecol. Obstet. 2025, 169, 232–239. [Google Scholar] [CrossRef]
- Sonehara, K.; Yano, Y.; Naito, T.; Goto, S.; Yoshihara, H.; Otani, T.; Ozawa, F.; Kitaori, T.; Biobank Japan, P.; Matsuda, K.; et al. Common and rare genetic variants predisposing females to unexplained recurrent pregnancy loss. Nat. Commun. 2024, 15, 5744. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; He, H.; Zhao, K. Thrombophilic gene polymorphisms and recurrent pregnancy loss: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2023, 40, 1533–1558. [Google Scholar] [CrossRef]
- Han, S.H.; Seo, J.J.; Kim, E.S.; Ryu, J.S.; Hong, S.H.; Hwang, S.Y. First Korean case of factor V Leiden mutation in pregnant woman with a history of recurrent pregnancy loss. J. Genet. Med. 2019, 16, 23–26. [Google Scholar] [CrossRef]
- Hwang, K.R.; Choi, Y.M.; Kim, J.J.; Lee, S.K.; Yang, K.M.; Paik, E.C.; Jeong, H.J.; Jun, J.K.; Yoon, S.H.; Hong, M.A. Methylenetetrahydrofolate Reductase Polymorphisms and Risk of Recurrent Pregnancy Loss: A Case-Control Study. J. Korean Med. Sci. 2017, 32, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Ahn, E.H.; Kim, J.O.; Ryu, C.S.; Park, H.S.; Cho, S.H.; Kim, J.H.; Lee, W.S.; Lee, J.R.; Kim, Y.R.; et al. Association between Platelet-Specific Collagen Receptor Glycoprotein 6 Gene Variants, Selected Biomarkers, and Recurrent Pregnancy Loss in Korean Women. Genes 2020, 11, 862. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, L.; Zhen, J.; Gu, Y.; Liu, Y.; Huang, S.; Wei, Y.; Zheng, H.; Guo, X.; Chen, G.B.; et al. Genetic basis of pregnancy-associated decreased platelet counts and gestational thrombocytopenia. Blood 2024, 143, 1528–1538. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Fiore, M.; Sentilhes, L.; d’Oiron, R. How I manage pregnancy in women with Glanzmann thrombasthenia. Blood 2022, 139, 2632–2641. [Google Scholar] [CrossRef]
- Miyashita, N.; Onozawa, M.; Hayasaka, K.; Yamada, T.; Migita, O.; Hata, K.; Okada, K.; Goto, H.; Nakagawa, M.; Hashimoto, D.; et al. A novel heterozygous ITGB3 p.T720del inducing spontaneous activation of integrin alphaIIbbeta3 in autosomal dominant macrothrombocytopenia with aggregation dysfunction. Ann. Hematol. 2018, 97, 629–640. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Y.; Dong, L.; Yu, H.; Cheng, L.; Zhao, X.; Ding, M. Genetic variation of ITGB3 is associated with asthma in Chinese Han children. PLoS ONE 2013, 8, e56914. [Google Scholar] [CrossRef]
- Frank, J.W.; Steinhauser, C.B.; Wang, X.; Burghardt, R.C.; Bazer, F.W.; Johnson, G.A. Loss of ITGB3 in ovine conceptuses decreases conceptus expression of NOS3 and SPP1: Implications for the developing placental vasculaturedagger. Biol. Reprod. 2021, 104, 657–668. [Google Scholar] [CrossRef]
- Changaei, M.; Javidan, M.; Ramezani Tehrani, F.; Mosaffa, N.; Noroozzadeh, M.; Hosseinzadeh, R.; Rajaei, S. Reduced expression of Il10, Stat3, Hoxa10, and Itgb3 in the embryo implantation site of rat model with prenatal androgen-induced polycystic ovary syndrome. Am. J. Reprod. Immunol. 2023, 90, e13702. [Google Scholar] [CrossRef]
- He, D.; Zeng, H.; Chen, J.; Xiao, L.; Zhao, Y.; Liu, N. H19 regulates trophoblastic spheroid adhesion by competitively binding to let-7. Reproduction 2019, 157, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, T.; Sandoval-Cooper, M.J.; Paiva, M.; Kobayashi, T.; Ploplis, V.A.; Castellino, F.J. Fibrinogen stabilizes placental-maternal attachment during embryonic development in the mouse. Am. J. Pathol. 2002, 160, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Snir, A.; Brenner, B.; Paz, B.; Ohel, G.; Lanir, N. The role of fibrin matrices and tissue factor in early-term trophoblast proliferation and spreading. Thromb. Res. 2013, 132, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Kamimoto, Y.; Wada, H.; Ikejiri, M.; Nakatani, K.; Sugiyama, T.; Osato, K.; Murabayashi, N.; Habe, K.; Mizutani, H.; Matsumoto, T.; et al. Hypofibrinogenemia and the alpha-Fibrinogen Thr312Ala Polymorphism may be Risk Factors for Early Pregnancy Loss. Clin. Appl. Thromb. Hemost. 2017, 23, 52–57. [Google Scholar] [CrossRef]
- Atallah, A.; Piccin, G.; Dubernard, G.; Abdul-Hay, M.J.; Cortet, M.; Huissoud, C. Fibrinogen for the prediction of severe maternal complications in placental abruption with fetal death after 24 weeks of gestation. Int. J. Gynaecol. Obstet. 2023, 160, 900–905. [Google Scholar] [CrossRef]
- Kotze, R.C.; Nienaber-Rousseau, C.; De Lange, Z.; De Maat, M.P.; Hoekstra, T.; Pieters, M. Genetic polymorphisms influencing total and gamma’ fibrinogen levels and fibrin clot properties in Africans. Br. J. Haematol. 2015, 168, 102–112. [Google Scholar] [CrossRef]
- Uitte de Willige, S.; Pyle, M.E.; Vos, H.L.; de Visser, M.C.; Lally, C.; Dowling, N.F.; Hooper, W.C.; Bertina, R.M.; Austin, H. Fibrinogen gamma gene 3′-end polymorphisms and risk of venous thromboembolism in the African-American and Caucasian population. Thromb. Haemost. 2009, 101, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Angelo-Dias, M.; Martins, C.; Dias, S.S.; Borrego, L.M.; Lima, J. Association of B Cells with Idiopathic Recurrent Pregnancy Loss: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 15200. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, B.; Hassouneh, F.; Delgado, E.; Casado, J.G.; Tarazona, R. Natural killer cells in recurrent miscarriage: An overview. J. Reprod. Immunol. 2020, 142, 103209. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Schettini, J.A.; Gomes, T.V.; Junior, C.; Heraclio, S.A.; Coelho, I.; Torres, L.C. Evaluation of Immunological Parameters in Pregnant Women: Low Levels of B and NK Cells. Rev. Bras. Ginecol. Obstet. 2019, 41, 213–219. [Google Scholar] [CrossRef]
- Liu, J.C.; Zeng, Q.; Duan, Y.G.; Yeung, W.S.B.; Li, R.H.W.; Ng, E.H.Y.; Cheung, K.W.; Zhang, Q.; Chiu, P.C.N. B cells: Roles in physiology and pathology of pregnancy. Front. Immunol. 2024, 15, 1456171. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef]
- Quan, Q.; Gu, H.; Wang, Y.; Yu, M. Immune micro-environment analysis and drug screening for ovarian endometriosis. Genes Genomics 2024, 46, 803–815. [Google Scholar] [CrossRef]
- Habets, D.H.J.; Al-Nasiry, S.; Nagelkerke, S.Q.; Voorter, C.E.M.; Spaanderman, M.E.A.; Kuijpers, T.W.; Wieten, L. Analysis of FCGR3A-p.176Val variants in women with recurrent pregnancy loss and the association with CD16a expression and anti-HLA antibody status. Sci. Rep. 2023, 13, 5232. [Google Scholar] [CrossRef]
- Li, J.; Liu, A.; Liu, H.; Li, C.; Wang, W.; Han, C.; Wang, X.; Zhang, Y.; Teng, W.; Shan, Z. Maternal TSH levels at first trimester and subsequent spontaneous miscarriage: A nested case-control study. Endocr. Connect. 2019, 8, 1288–1293. [Google Scholar] [CrossRef]
- Ebrahim, A.; Rienhardt, G.; Morris, S.; Kruger, T.F.; Lombard, C.J.; Van der Merwe, J.P. Follicle stimulating hormone levels on cycle day 3 predict ovulation stimulation response. J. Assist. Reprod. Genet. 1993, 10, 130–136. [Google Scholar] [CrossRef]
- Alsulaim, A.Y.; Azam, F.; Sebastian, T.; Mahdi Hassan, F.; AbdulAzeez, S.; Borgio, J.F.; Alzahrani, F.M. The association between two genetic polymorphisms in ITGB3 and increase risk of venous thromboembolism in cancer patients in Eastern Province of Saudi Arabia. Saudi J. Biol. Sci. 2022, 29, 183–189. [Google Scholar] [CrossRef]
- Yi, X.; Lin, J.; Luo, H.; Zhou, J.; Zhou, Q.; Wang, Y.; Wang, C. Interactions among variants in TXA2R, P2Y12 and GPIIIa are associated with carotid plaque vulnerability in Chinese population. Oncotarget 2018, 9, 17597–17607. [Google Scholar] [CrossRef][Green Version]
- Schwedler, C.; Heymann, G.; Bukreeva, L.; Hoppe, B. Association of Genetic Polymorphisms of Fibrinogen, Factor XIII A-Subunit and alpha(2)-Antiplasmin with Fibrinogen Levels in Pregnant Women. Life 2021, 11, 1340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, K.; Zou, J.; Ma, H.; Yang, H.; Zhang, X.; Jiao, Y. Associations between polymorphisms in coagulation-related genes and venous thromboembolism: A meta-analysis with trial sequential analysis. Medicine 2017, 96, e6537. [Google Scholar] [CrossRef] [PubMed]
- Pina-Cabral, L.B.; Carvalhais, V.; Mesquita, B.; Escorcio, C.; Silva, P.F.; Pinto, P.; Napoleao, P.; Pinheiro, T.; Monteiro, M.C.; Almeida-Dias, A.; et al. Myocardial infarction before and after the age of 45: Possible role of platelet receptor polymorphisms. Rev. Port. Cardiol. 2018, 37, 727–735. [Google Scholar] [CrossRef]
- Yuan, D.; Shi, X.; Guo, L.; Wang, G.; Zhao, Y.; Yang, Y.; Zhang, H.; Huang, Q.; Yuan, Y. Lower Platelet Aggregation Is a Risk Factor for Dual Antiplatelet Therapy-Associated Bleeding: A Preliminary Retrospective Study with Genotype Analysis. Med. Sci. Monit. 2020, 26, e923758. [Google Scholar] [CrossRef]
- Mao, X.J.; Zhang, Q.; Xu, F.; Gao, P.; Sun, N.; Wang, B.; Tang, Q.X.; Hao, Y.B.; Sun, C.Q. Improved detection of common variants in coronary artery disease and blood pressure using a pleiotropy cFDR method. Sci. Rep. 2019, 9, 10340. [Google Scholar] [CrossRef]
- Nie, X.Y.; Li, J.L.; Qin, S.B.; Fu, Y.; Liang, G.K.; Shi, L.W.; Shao, H.; Liu, J.; Lu, Y. Genetic mutations in PEAR1 associated with cardiovascular outcomes in Chinese patients with acute coronary syndrome. Thromb. Res. 2018, 163, 77–82. [Google Scholar] [CrossRef]
- Kavosh, Z.; Mohammadzadeh, Z.; Alizadeh, S.; Sharifi, M.J.; Hajizadeh, S.; Choobineh, H.; Omidkhoda, A. Factor VII R353Q (rs6046), FGA A6534G (rs6050), and FGG C10034T (rs2066865) Gene Polymorphisms and Risk of Recurrent Pregnancy Loss in Iranian Women. Indian. J. Hematol. Blood Transfus. 2024, 40, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Tosteson, T.D.; Borsuk, M. Weighted multiple testing procedures for genomic studies. BioData Min. 2012, 5, 4. [Google Scholar] [CrossRef]

| Characteristic | Controls (n = 375) | RPL Patients (n = 389) | p |
|---|---|---|---|
| Age | 32.85 ± 4.18 | 33.42 ± 4.35 | 0.275 * |
| Previous pregnancy losses (n, %) | N/A | 2.98 ± 1.48 | N/A |
| Live births (n, %) | 1.25 ± 0.03 | N/A | N/A |
| Mean gestational age (weeks) | 38.81 ± 1.44 | 7.44 ± 1.88 | <0.0001 * |
| BMI | 21.66 ± 3.08 | 21.42 ± 2.78 | 0.906 * |
| FSH (mIU/mL) | 8.52 ± 4.43 | 7.90 ± 11.50 | <0.0001 * |
| E2 (pg/mL) | 29.71 ± 28.56 | 37.09 ± 29.77 | 0.005 * |
| LH (mIU/mL) | 3.91 ± 4.57 | 6.37 ± 11.82 | <0.0001 * |
| TSH (uIU/mL) | 1.54 ± 1.12 | 2.13 ± 1.41 | <0.0001 * |
| Prolactin (ng/mL) | 11.33 ± 6.99 | 15.20 ± 11.85 | 0.123 * |
| AMH (ng/mL) | 4.20 ± 2.89 | 3.64 ± 4.05 | 0.163 * |
| DHEA-S (ug/dL) | 201.83 ± 72.45 | 148.40 ± 75.34 | 0.007 * |
| VEGF (pg/mL) | N/A | 166.19 ± 130.03 | N/A |
| PAI-1 (ng/mL) | N/A | 11.22 ± 7.69 | N/A |
| Anti-TPO (IU/mL) | 11.54 ± 17.57 | 52.27 ± 250.60 | 0.0001 * |
| Thyroglobulin-Ab (IU/mL) | 20.90 ± 18.17 | 62.00 ± 211.30 | 0.361 * |
| PT (sec) | 10.60 ± 1.41 | 11.45 ± 1.66 | <0.0001 * |
| aPTT (sec) | 29.01 ± 3.39 | 32.25 ± 4.31 | <0.0001 * |
| Protein C activity (%) | 102.50 ± 24.75 | 98.55 ± 23.61 | 0.817 |
| Protein S activity (%) | 93.00 ± 46.67 | 69.40 ± 23.09 | 0.168 |
| Homocysteine (umol/L) | 7.56 ± 4.82 | 6.89 ± 1.96 | 0.914 * |
| Folate (ng/mL) | 16.51 ± 12.86 | 15.62 ± 16.09 | 0.343 * |
| Hgb A1c (%) | 5.41 ± 0.42 | 5.40 ± 0.32 | 0.813 * |
| Glucose (mg/dL) | 96.56 ± 19.28 | 97.76 ± 16.67 | 0.049 * |
| BUN (mg/dL) | 8.76 ± 2.77 | 10.39 ± 2.85 | <0.0001 * |
| Creatinine (mg/dL) | 0.60 ± 0.14 | 0.74 ± 0.12 | <0.0001 * |
| Uric acid (mg/dL) | 3.86 ± 0.97 | 3.83 ± 0.91 | 0.689 * |
| Total cholesterol (mg/dL) | 232.87 ± 56.06 | 182.11 ± 46.82 | <0.0001 * |
| Triglyceride (mg/dL) | 224.10 ± 237.10 | 161.19 ± 137.74 | <0.0001 * |
| LDL (mg/dL) | 127.76 ± 40.59 | 109.57 ± 34.71 | 0.225 * |
| HDL (mg/dl) | 74.62 ± 21.30 | 68.50 ± 19.05 | 0.427 * |
| WBC (103/uL) | 7.24 ± 2.48 | 6.93 ± 2.43 | 0.038 * |
| RBC (106/uL) | 4.13 ± 0.38 | 4.25 ± 0.63 | 0.002 * |
| Hgb (g/dL) | 12.39 ± 1.93 | 12.68 ± 2.00 | 0.0003 * |
| Hct (%) | 36.55 ± 3.19 | 37.57 ± 3.52 | <0.0001 * |
| MCV (fL) | 88.13 ± 6.71 | 88.97 ± 6.69 | 0.107 * |
| MCH (pg) | 30.64 ± 14.83 | 29.92 ± 2.40 | 0.480 * |
| MCHC (g/dL) | 33.68 ± 1.20 | 33.54 ± 1.42 | 0.307 * |
| RDW (%) | 13.35 ± 1.20 | 13.12 ± 1.34 | <0.0001 * |
| PLT (103/ul) | 236.47 ± 60.12 | 253.62 ± 59.25 | 0.0001 * |
| PDW (fL) | 12.57 ± 2.43 | 15.59 ± 10.21 | 0.0001 * |
| MPV (fL) | 9.06 ± 4.62 | 8.51 ± 5.06 | 0.001 * |
| Seg (%) | 70.23 ± 8.53 | 62.65 ± 11.98 | <0.0001 * |
| Lym (%) | 21.40 ± 7.18 | 28.44 ± 10.60 | <0.0001 * |
| Mono (%) | 5.29 ± 1.66 | 5.64 ± 2.69 | 0.272 * |
| Eo (%) | 1.59 ± 1.30 | 2.12 ± 1.76 | 0.0002 * |
| Baso (%) | 0.35 ± 0.24 | 0.44 ± 0.31 | 0.001 * |
| CD56 NK cell (%) | N/A | 17.37 ± 7.79 | N/A |
| CD3 (pan T) (%) | N/A | 67.01 ± 0.89 | N/A |
| CD4 (helper T) (%) | N/A | 36.39 ± 7.41 | N/A |
| CD8 (suppressor T) (%) | N/A | 27.33 ± 0.79 | N/A |
| CD19 (B-cell) (%) | N/A | 12.66 ± 4.88 | N/A |
| Genotypes | Controls (n = 375) | RPL (n = 389) | AOR (95% CI) | p | FDR-p | PL ≥ 3 (n = 205) | AOR (95% CI) | p | FDR-p | PL ≥ 4 (n = 76) | AOR (95% CI) | p | FDR-p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITGB3 rs3809865A > T | |||||||||||||
| AA | 233 (62.1) | 218 (56.0) | 1.000 (reference) | 112 (54.6) | 1.000 (reference) | 40 (52.6) | 1.000 (reference) | ||||||
| AT | 129 (34.4) | 142 (36.5) | 1.170 (0.865–1.584) | 0.309 | 0.745 | 73 (35.6) | 1.158 (0.802–1.672) | 0.435 | 0.670 | 28 (36.8) | 1.248 (0.735–2.120) | 0.413 | 0.990 |
| TT | 13 (3.5) | 29 (7.5) | 2.505 (1.262–4.969) | 0.009 | 0.081 | 20 (9.8) | 3.255 (1.551–6.830) | 0.002 | 0.018 | 8 (10.5) | 3.613 (1.403–9.307) | 0.008 | 0.072 |
| Dominant (AA vs. AT + TT) | 1.285 (0.962–1.718) | 0.090 | 0.288 | 1.345 (0.950–1.903) | 0.094 | 0.306 | 1.461 (0.889–2.402) | 0.135 | 0.608 | ||||
| Recessive (AA + AT vs. TT) | 2.302 (1.176–4.508) | 0.015 | 0.135 | 3.050 (1.479–6.292) | 0.003 | 0.027 | 3.301 (1.316–8.280) | 0.011 | 0.099 | ||||
| HWE-p | 0.342 | 0.382 | |||||||||||
| FGG rs1049636T > C | |||||||||||||
| TT | 234 (62.4) | 265 (68.1) | 1.000 (reference) | 145 (70.7) | 1.000 (reference) | 56 (73.7) | 1.000 (reference) | ||||||
| TC | 127 (33.9) | 107 (27.5) | 0.738 (0.540–1.009) | 0.057 | 0.513 | 54 (26.3) | 0.673 (0.460–0.987) | 0.043 | 0.387 | 17 (22.4) | 0.556 (0.310–0.997) | 0.049 | 0.441 |
| CC | 14 (3.7) | 17 (4.4) | 1.065 (0.513–2.210) | 0.866 | 0.926 | 6 (2.9) | 0.650 (0.243–1.740) | 0.391 | 0.790 | 3 (3.9) | 0.880 (0.244–3.172) | 0.845 | 0.845 |
| Dominant (TT vs. TC + CC) | 0.771 (0.571–1.040) | 0.088 | 0.288 | 0.669 (0.463–0.968) | 0.033 | 0.297 | 0.588 (0.339–1.022) | 0.060 | 0.540 | ||||
| Recessive (TT + TC vs. CC) | 1.173 (0.569–2.417) | 0.666 | 0.797 | 0.734 (0.276–1.950) | 0.534 | 0.939 | 1.046 (0.293–3.737) | 0.945 | 0.945 | ||||
| HWE-p | 0.525 | 0.149 |
| Allele Combination | Controls (2n = 750) | RPL (2n = 778) | OR (95% CI) | p | FDR-p |
|---|---|---|---|---|---|
| ITGB3 rs2317676 A > G/ITGB3 rs3809865 A > T/FGG rs1049636 T > C/FGG rs2066865 T > C/GP1BA rs2243093 T > C/GP1BA rs6065 C > T/PECAM1 rs2812 C > T/PEAR1 rs822442 C > A/PEAR1 rs12137505 G > A | |||||
| A.A.T.T.T.C.C.C.G | 43 (5.7) | 55 (7.1) | 1.000 (reference) | ||
| A.A.T.T.T.C.T.A.A | 17 (2.3) | 0 (0.0) | 0.022 (0.001–0.383) | 0.0001 | 0.008 |
| A.A.T.C.C.C.C.A.A | 30 (4.0) | 4 (0.6) | 0.104 (0.034–0.319) | 0.0001 | 0.008 |
| A.T.T.T.T.C.C.C.G | 36 (4.8) | 11 (1.4) | 0.239 (0.109–0.524) | 0.0002 | 0.010 |
| A.T.T.C.T.C.C.C.G | 0 (0.0) | 17 (2.2) | 27.430 (1.603–469.400) | 0.001 | 0.023 |
| A.T.C.C.C.C.C.C.G | 9 (1.1) | 0 (0.0) | 0.041 (0.002–0.729) | 0.001 | 0.030 |
| ITGB3 rs2317676 A > G/ITGB3 rs3809865 A > T/FGG rs1049636 T > C/PEAR1 rs12137505 G > A | |||||
| A.A.T.G | 196 (26.2) | 209 (26.8) | 1.000 (reference) | ||
| A.T.C.G | 29 (3.9) | 12 (1.5) | 0.388 (0.193–0.782) | 0.006 | 0.045 |
| G.T.C.A | 0 (0.0) | 9 (1.2) | 17.820 (1.030–308.400) | 0.004 | 0.045 |
| ITGB3 rs2317676 A > G/ITGB3 rs3809865 A > T/GP1BA rs6065 C > T | |||||
| A.A.C | 433 (57.7) | 382 (49.1) | 1.000 (reference) | ||
| A.A.T | 32 (4.3) | 51 (6.5) | 1.807 (1.137–2.870) | 0.011 | 0.023 |
| A.T.C | 129 (17.2) | 169 (21.8) | 1.485 (1.137–1.940) | 0.004 | 0.011 |
| G.T.C | 0 (0.0) | 12 (1.6) | 28.330 (1.671–480.500) | 0.0003 | 0.002 |
| ITGB3 rs3809865 A > T/GP1BA rs6065 C > T | |||||
| A.C | 553 (73.71) | 518 (66.64) | 1.000 (reference) | ||
| A.T | 42 (5.62) | 60 (7.65) | 1.525 (1.010–2.303) | 0.044 | 0.066 |
| T.C | 129 (17.22) | 183 (23.46) | 1.514 (1.173–1.955) | 0.001 | 0.003 |
| ITGB3 rs2317676 A > G/ITGB3 rs3809865 A > T | |||||
| A.A | 465 (62.0) | 432 (55.6) | 1.000 (reference) | ||
| A.T | 155 (20.7) | 188 (24.1) | 1.306 (1.017–1.676) | 0.036 | 0.054 |
| G.A | 130 (17.3) | 146 (18.7) | 1.209 (0.923–1.584) | 0.169 | 0.169 |
| G.T | 0 (0.0) | 12 (1.6) | 26.910 (1.587–456.200) | 0.0004 | 0.001 |
| FGG rs1049636 T > C/FGG rs2066865 T > C | |||||
| T.T | 384 (51.1) | 384 (49.4) | 1.000 (reference) | ||
| T.C | 212 (28.2) | 253 (32.5) | 5.486 (0.276–109.100) | 0.259 | 0.267 |
| C.T | 2 (0.2) | 9 (1.1) | 4.500 (0.966–20.970) | 0.036 | 0.108 |
| C.C | 154 (20.5) | 132 (17.0) | 0.857 (0.653–1.125) | 0.267 | 0.267 |
| GP1BA rs2243093 T > C/GP1BA rs6065 C > T | |||||
| T.C | 489 (65.3) | 511 (65.7) | 1.000 (reference) | ||
| T.T | 64 (8.5) | 71 (9.2) | 1.062 (0.741–1.522) | 0.784 | 0.784 |
| C.C | 193 (25.7) | 190 (24.5) | 0.942 (0.744–1.192) | 0.62 | 0.784 |
| C.T | 4 (0.6) | 6 (0.7) | 1.435 (0.403–5.119) | 0.753 | 0.784 |
| PEAR1 rs822442 C > A/PEAR1 rs12137505 G > A | |||||
| C.G | 419 (55.91) | 416 (53.46) | 1.000 (reference) | ||
| C.A | 105 (13.95) | 94 (12.09) | 0.902 (0.662–1.229) | 0.512 | 0.512 |
| A.G | 5 (0.62) | 24 (3.09) | 4.835 (1.827–12.800) | 0.001 | 0.003 |
| A.A | 221 (29.51) | 244 (31.36) | 1.112 (0.886–1.395) | 0.359 | 0.512 |
| Genotype Combination | Controls (n = 375) | RPL Patients (n = 389) | AOR (95% CI) | p | FDR-p |
|---|---|---|---|---|---|
| ITGB3 rs3809865 A > T/FGG rs1049636 T > C/PECAM1 rs2812 C > T/PEAR1 rs12137505 G > A | |||||
| AA/TT/CC/GG | 19 (5.1) | 28 (7.2) | 1.000 (Reference) | ||
| AA/TT/CT/GG | 22 (5.9) | 11 (2.8) | 0.350 (0.137–0.893) | 0.028 | 0.581 |
| AT/TC/CT/GG | 12 (3.2) | 3 (0.8) | 0.170 (0.042–0.683) | 0.013 | 0.580 |
| AT/TC/CT/GA | 15 (4.0) | 7 (1.8) | 0.319 (0.109–0.938) | 0.038 | 0.581 |
| FGG rs1049636 T > C/PECAM1 rs2812 C > T/PEAR1 rs12137505 G > A | |||||
| TT/CC/GG | 27 (7.2) | 46 (11.8) | 1.000 (Reference) | ||
| TT/CC/GA | 67 (17.9) | 55 (14.1) | 0.485 (0.267–0.880) | 0.017 | 0.130 |
| TC/CT/GG | 24 (6.4) | 14 (3.6) | 0.341 (0.151–0.769) | 0.010 | 0.130 |
| TC/CT/GA | 32 (8.5) | 22 (5.7) | 0.404 (0.196–0.833) | 0.014 | 0.130 |
| ITGB3 rs3809865 A > T/GP1BA rs6065 C > T | |||||
| AA/CC | 202 (53.9) | 174 (44.7) | 1.000 (reference) | ||
| TT/CC | 9 (2.4) | 22 (5.7) | 3.107 (1.382–6.987) | 0.006 | 0.036 |
| PEAR1 rs822442 C > A/PEAR1 rs12137505 G > A | |||||
| CC/GG | 115 (30.7) | 110 (28.3) | 1.000 (reference) | ||
| AA/GA | 1 (0.3) | 12 (3.1) | 12.661 (1.618–99.085) | 0.016 | 0.112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, E.J.; Ahn, E.H.; Park, H.W.; Lee, J.H.; Kim, D.H.; Kim, Y.R.; Kim, J.H.; Kim, N.K. Genetic Associations of ITGB3, FGG, GP1BA, PECAM1, and PEAR1 Polymorphisms and the Platelet Activation Pathway with Recurrent Pregnancy Loss in the Korean Population. Int. J. Mol. Sci. 2025, 26, 7505. https://doi.org/10.3390/ijms26157505
Ko EJ, Ahn EH, Park HW, Lee JH, Kim DH, Kim YR, Kim JH, Kim NK. Genetic Associations of ITGB3, FGG, GP1BA, PECAM1, and PEAR1 Polymorphisms and the Platelet Activation Pathway with Recurrent Pregnancy Loss in the Korean Population. International Journal of Molecular Sciences. 2025; 26(15):7505. https://doi.org/10.3390/ijms26157505
Chicago/Turabian StyleKo, Eun Ju, Eun Hee Ahn, Hyeon Woo Park, Jae Hyun Lee, Da Hwan Kim, Young Ran Kim, Ji Hyang Kim, and Nam Keun Kim. 2025. "Genetic Associations of ITGB3, FGG, GP1BA, PECAM1, and PEAR1 Polymorphisms and the Platelet Activation Pathway with Recurrent Pregnancy Loss in the Korean Population" International Journal of Molecular Sciences 26, no. 15: 7505. https://doi.org/10.3390/ijms26157505
APA StyleKo, E. J., Ahn, E. H., Park, H. W., Lee, J. H., Kim, D. H., Kim, Y. R., Kim, J. H., & Kim, N. K. (2025). Genetic Associations of ITGB3, FGG, GP1BA, PECAM1, and PEAR1 Polymorphisms and the Platelet Activation Pathway with Recurrent Pregnancy Loss in the Korean Population. International Journal of Molecular Sciences, 26(15), 7505. https://doi.org/10.3390/ijms26157505





