Abstract
Cervical cancer (CC) is the gynecological cancer with the highest incidence and mortality worldwide. High-risk oncogenic human papillomaviruses (HPV) genotypes 16 and 18 are the primary risk factors for developing this female neoplasm, with them being the etiological agents of 70% of cervical cancers. Despite the availability of various prevention strategies, laboratory tests capable of detecting the disease in its previous and early stages, and multiple treatment schemes, CC incidence and mortality rates remain high, due in part to the population’s rejection or disinterest in the current type of sampling. An alternative that could encourage women to take better care of their gynecological health is the availability of tests that detect biomarkers in non-cervical samples with high sensitivity and specificity. The detection of biomarkers in non-cervical samples (blood, serum, plasma, urine, and vaginal fluids) may help reduce the discomfort associated with cervical sampling in patients, therefore promoting gynecological healthcare. This review discusses current diagnostic methods and recent advances in CC biomarkers detected in non-cervical samples, emphasizing their potential for diagnosis, prognosis, and patient monitoring. We further discuss the challenges and future perspectives of applying these biomarkers in clinical practice. The results of this review show that there is a considerable range of biomarkers proposed as alternative tools with high efficacy. Their identification in previous stages of the disease and routinely in non-cervical samples could help reduce the incidence and mortality rates of CC.
1. Introduction
Cervical cancer (CC) is the gynecological neoplasia with the highest incidence and mortality worldwide, with 662,301 new cases and 348,874 deaths reported in 2022 [1,2]. The primary risk factor for CC is the human papillomavirus (HPV), which infects immature squamous cells of the transformation zone of the cervix, or the basal cells of the mature squamous epithelium [3] (Figure 1), and disrupts the regulation of cell proliferation by blocking the activity of proteins essential for proper control of cell replication, such as p53 and pRb. Viral proteins E6 and E7 inhibit p53 and Rb, respectively, promoting the development of CC [4,5,6]. HPVs are classified into two major groups based on their oncogenic potential: low-risk and high-risk viruses [7]. The primary viruses responsible for CC are in the high-risk group, with HPV16 and HPV18 being the etiological agents in approximately 70% of cases [8,9].
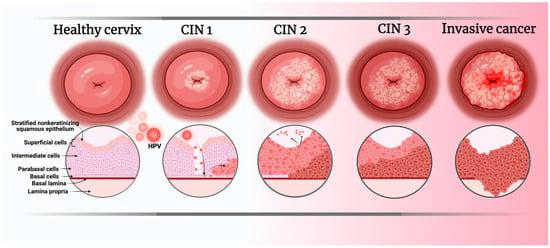
Figure 1.
Stages of HPV infection development leading to the appearance of CC. CIN: Cervical intraepithelial neoplasm. Image created in BioRender.com/bszht4w (accessed on 17 June 2025).
Currently, various prophylactic measures exist to prevent HPV infections, including vaccination campaigns against some high-risk oncogenic HPV types, correct condom use, and raising awareness among the youth population about sexually transmitted infections. However, several factors contribute to the persistently high morbidity and mortality rates of CC worldwide. These include early sexual activity, multiple sexual partners, lack of awareness among young people, alcohol consumption, smoking, resistance to vaccination, and the circulation of HPV strains not covered by the vaccine [1,2] (Figure 2).
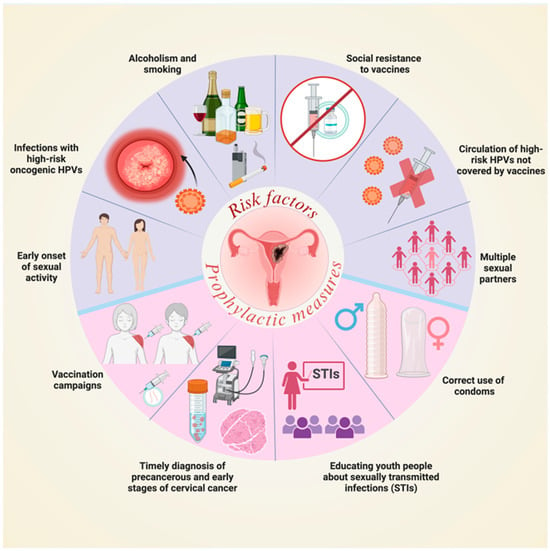
Figure 2.
Risk factors for CC and prophylactic measures against HPV infections. STIs: Sexually transmitted infections. Image created in BioRender.com/b71m613 (accessed on 1 July 2025).
2. Laboratory Tests for the Diagnosis of Cervical Cancer
Currently, there are laboratory tests that are very effective at diagnosing CC, even in its early stages [10,11] (Table 1); these involve visual and tactile examinations, while others require the collection of cells from the cervix to identify abnormalities. Histopathological examinations typically involve biopsies, while molecular tests generally detect the presence of viral nucleic acids in cells collected via cervical brushing [10]. However, the general population does not have the habit of regularly going for gynecological check-ups, probably due to the different degrees of discomfort generated by cervical sampling. Notably, the presence of gynecological pathologies at the time of sampling, such as endometriosis, bleeding, dryness or vaginal atrophy, increased body mass index, musculoskeletal problems, advanced age, or menopause, can result in sample collection being quite painful [12,13]. Additionally, the taboo generated by cultural, religious, social, and demographic aspects also limits women from going to the gynecologist [14].

Table 1.
Current tests and procedures for the diagnosis of cervical cancer [10,11].
3. Biomarkers in Non-Cervical Samples
Multiple strategies are currently being investigated to identify non-cervical samples that aid in the diagnosis and prognosis of CC, including the use of trained dogs capable of detecting CC-specific volatile organic compounds and discriminating CC from control samples [15], as well as the ongoing search for biomarkers in non-cervical samples [16,17,18,19,20]. Molecular biomarkers in non-cervical samples are emerging as an alternative to cervical sampling to avoid the discomfort, pain, and social taboo generated by cervical sampling performed for current tests. Identification and use of biomarkers in non-cervical samples could help reduce the high incidence and mortality of CC (Table 2).

Table 2.
Novel biomarkers detected in non-cervical samples for the diagnosis, prognosis, and prediction of cervical cancer.
3.1. ncRNAs
3.1.1. miRNAs
Several studies suggest using blood samples to obtain serum or plasma to analyze the presence of microRNAs (miRNAs) [39] since this type of sample causes less discomfort to patients as it only involves venipuncture. Using microRNA array assays, elevated levels of miRNA-1290, miRNA-146a-5p, miRNA-151a-3p, miRNA-2110, and miRNA-21-5p have been found in patients with CC compared to healthy individuals. Due to their high accuracy, these miRNAs are proposed as candidates for the diagnosis of CC [21,22].
A serum miRNA panel consisting of miR-16-2, miR-195, miR-2861, and miR-497 has been proposed to discriminate with high accuracy CC patients from cervical intraepithelial neoplasia (CIN) and healthy subjects. Intriguingly, miRNA-497 has been shown to promote apoptosis through caspase-3/7 activity and also inhibit cell proliferation in HeLa cells, and in a murine model, it was observed that overexpression of miR-497 significantly reduced tumor volume, tumor weight, and tumor formation [23]. Further studies are needed to confirm the pro-tumor role of miR-497 and the clinical relevance of its overexpression in the serum of patients with CC.
To prevent the development of CC, it is very important to diagnose the disease in its early stages [40]. Xin et al. found that miR-9, miR-10a, miR-20a, and miR-196a were upregulated in serum samples derived from patients with CIN compared with serum from healthy controls, and due to their high accuracy, they have suggested that these miRNAs could be useful and novel non-invasive biomarkers for the early detection of CIN [24]. Urine is another type of non-cervical and non-invasive sample that has been proposed as reliable in the early diagnosis of CC. A panel of miRNAs in urine, consisting of miR-145-5p, miR-218-5p, and miR-34a-5p, achieved 100% sensitivity and 92.8% specificity to distinguish precancer and cancer patients from healthy subjects, correlating with the levels found of these miRNAs in serum and tumor tissues. Additionally, miR-34a-5p and miR-218-5p have been proposed as independent prognostic factors for the overall survival of CC patients [25]. The expression characteristics of this panel of miRNAs highlight its usefulness as a diagnostic and prognostic CC biomarker.
The prognosis of cancer patients is crucial for designing the appropriate treatments, planning care, and estimating life expectancy. In this sense, miRNA-142-5p has been proposed as a biomarker of progression and poor clinical outcome, with it being overexpressed in the serum in the late stages of CC (III-IV) compared to the early stages (I-II) of this neoplasia. Evidence indicates that this miRNA is secreted in exosomes by cervical squamous cell carcinoma cells and is internalized by lymphatic endothelial cells. It activates the expression of the enzyme Indoleamine 2,3-dioxygenase (IDO), facilitating the immune escape of cancer cells via CD8+ T cell exhaustion through the conversion of tryptophan to kynurenine. This event is associated with a decrease in tumor-infiltrating lymphocytes (TILs) and poor clinical outcomes in multiple cancers [26].
Biomarkers can also indicate a good prognosis. For example, miRNA-651 is associated with a positive response to cisplatin. It is found at low levels in the plasma of CC patients and at elevated levels in healthy individuals. In vitro assays have demonstrated that exosomes loaded with miRNA-651 restrain cisplatin resistance and proliferation and facilitate apoptosis in CC cells. Therefore, miRNA-651 has been proposed as a biomarker of good prognosis and a potential therapeutic agent, capable of improving treatment response by downregulating ATG3 (Autophagy Related 3) [27].
3.1.2. lncRNAs
Long non-coding RNAs (lncRNAs) are another type of ncRNA proposed as diagnostic biomarkers for CC patients [41]. Wang et al. (2020) [28] analyzed the presence of serum lncRNAs in CC and CIN patients and healthy controls. They found that CCAT2, LINC01133, and LINC00511 are highly expressed in the serum of patients with CC, and that, together with the squamous cell carcinoma antigen, they suggest these lncRNAs as effective diagnostic biomarkers. Liu et al. (2023) [42] statistically analyzed a database of 304 CC tumor tissues and three cancer-free tissues. They found that lncRNA AC023043.1 was highly expressed in patients with poor overall survival, while the lncRNAs ZSCAN16-AS1, AC083799.1, AL021707.6, and LINC02356 were associated with a lower risk of death, increased sensitivity to chemotherapy drugs, better overall survival, and better progression-free survival. The authors propose this panel of lncRNAs of prognostic and predictive utility for patients with CC.
3.2. RNAs
CC generates differential gene expression even in the early stages of the disease. This has been demonstrated by Sheng and Zhang, who, using oligonucleotide microarrays, analyzed total RNA from peripheral blood lymphocytes. They found significant upregulation of tenasin-c (TNC), nucleolin (NCL), and enolase 2 (ENO2) in the blood of patients with early-stage CC (IB-IIA) compared to healthy controls [29], identifying these RNAs as potential tools for non-invasive diagnosis.
3.3. Cell Proteins
Characterizing the tumor protein profile is very important to define the prognosis of patients with CC. Studies conducted with CC cell lines have shown that high-risk HPVs can promote the expression of cellular proteins such as CD55 and CD59 [43]. Both complement regulatory proteins physiologically interfere with the assembly of the membrane attack complex [44,45,46] and have been observed pathologically to generate chemoresistance in patients with breast cancer [47], suggesting that these proteins could potentially be used as prognostic tools for CC patients. Another protein proposed as a diagnostic and prognostic biomarker is tropomyosin 3 (TPM3), which is overexpressed in tumor tissue from CC patients compared to healthy tissue. In both in vitro and in vivo assays, its presence has been shown to favor cell proliferation, migration, and invasion, as well as tumor growth in animal models. Furthermore, CC patients with high TPM3 expression had poorer overall survival compared to those with low levels of this protein [30]. FBXO5 promotes similar pro-tumor events to those exerted by TPM3, making it another candidate as a dual diagnostic and prognostic biomarker [31].
Other proteins proposed as biomarkers of poor prognosis include cleft lip and palate transmembrane protein 1-like protein (CLPTM1L) and lumican (LUM). Overexpression of either protein in tumor tissue leads to recurrence in CC patients by promoting resistance to chemotherapy, specifically cisplatin. In the particular case of CLPTM1L, this expression is associated with reduced overall survival [32,48]. In addition, it has been shown that chaperonin containing TCP1 subunit 3 (CCT3) expression is involved in the development of CC. This protein is part of the chaperonin-containing TCP1 complex (CCT), which folds various proteins in an ATP-dependent manner [49]. Due to its overexpression in other types of cancers [50,51,52,53], its ability to promote cisplatin resistance [54], and the evidence that CC patients with tumors overexpressing this protein exhibit lower overall survival [55], CCT3 has been proposed as a diagnostic and poor prognostic biomarker.
3.4. Biomarkers of Viral Origin
Biomarkers are not necessarily molecules expressed by human cells; there are also biomarkers of viral origin. Specifically, HPV16/18 E7 circulating cell-free DNA (cfDNA) has been proposed as a potential tumor marker with high specificity for minimally invasive CC monitoring. It is differentially expressed in the serum of patients with CC compared to patients with CIN and controls, and it is detected in less than 30 min through sensitive isothermal detection recombinase polymerase amplification combined with a lateral flow strip (RPA-LF) [33]. In pursuit of non-cervical samples, urine has also been proposed as a non-invasive liquid biopsy useful for detecting DNA of viral origin [56]. Lee et al. have proposed a versatile nanowire platform for highly efficient isolation and direct PCR-free colorimetric differential detection of HPV16 and HPV18 DNA from unprocessed urine with high sensitivity and specificity, even when using a small volume of urine (300 µL) [34]. Furthermore, the Cobas® 6800 automated system has been proposed as a next-generation device capable of detecting DNA from HPV16, HPV18, and HPV68 in urine samples [57]. This automated system, based on real-time polymerase chain reaction (PCR), has proven to be highly sensitive (93%, 94%, and 90% for HPV16, HPV18, and HPV68, respectively), 100% specific, and fully reproducible. These characteristics position urine as an excellent non-cervical sample for the detection of HPV.
Other non-cervical samples proposed for the identification of viral DNA are blood samples [18]. It has been shown that HPV16, HPV18, HPV31, HPV33, HPV35, HPV45, HPV52, HPV56, HPV59, HPV58, and HPV73 DNA can be detected in serum and plasma [35,36,37,58] samples from patients with CC, with these DNAs being identified as accurate, non-invasive, inexpensive, and easily accessible biomarkers for the diagnosis, prognosis, therapeutic monitoring and follow-up of patients with CC.
3.5. Miscellaneous Biomarkers
In addition to molecules of a biological nature, volatile organic compounds have also been proposed as biomarkers of CC. Rodríguez-Esquivel et al. described the volatolome of the female genitourinary area using Gas Chromatography–Mass Spectrometry. They differentially identified various alkanes with a sensitivity and specificity of 93% in samples from pads used by patients with CC and healthy controls [38]. Another proposed biomarker is the aberrant methylation of Septin9 DNA in plasma. This has shown high discriminatory power in predicting pelvic nodal metastasis of CC, with an optimal specificity of 81.48%. Consequently, aberrant methylation of Septin9 DNA in plasma has been proposed as an innovative and non-invasive biomarker with predictive potential for pelvic lymph node metastasis in CC [59]. Research continues on identifying other biomarkers in non-cervical and non-invasive samples for the diagnosis of CC. The detection of metabolites in plasma [16] and urine [60], free fatty acids in serum [61], and tumor DNA in urine [62] is of considerable interest.
4. Characteristics of miRNAs and lncRNAs as Effective Biomarkers of CC in Non-Cervical Samples
As discussed in Section 3, numerous reports highlight the usefulness of biomarkers in non-cervical samples for CC diagnosis, prognosis, prediction, disease monitoring, and patient follow-up, and as shown in Table 2, many of these proposed biomarkers are miRNAs and lncRNAs detected in liquid biopsies (serum, plasma, and urine). The strength of these ncRNAs lies in several qualities they possess: (i) miRNAs/lncRNAs are expressed at disproportionately higher levels compared to other types of biomarkers, such as proteins and mRNAs, since their transcriptional regulatory function requires these elevated expression levels to maintain control of gene expression [63,64]; (ii) they exhibit more tissue- and disease-specific expression patterns, whereas mRNA and protein expression is usually broader and shared among different tissues [65]; (iii) miRNAs/lncRNAs have greater stability in liquid biopsies, as they are usually protected in exosomes or protein complexes [66,67], while mRNAs degrade more easily, a characteristic that makes them less practical for detection in plasma, serum, or urine; (iv) deregulation of miRNAs caused by HPV is evidenced by the fact that miR-15a-5p, miR-17-5p, miR-20a-5p, miR-21-5p, miR-96, miR-106b-5p, and miR-365a are upregulated, while miR-497-5p is downregulated in HPV-infected cell models compared to healthy tissues [68]. Additionally, it has been demonstrated that HPV oncoproteins E6 and E7 can downregulate miRNAs (miR-3156-3p, miR-6779-3p, miR-4779-3p, miR-6811-3p, miR-545-5p, and miR-656-5p) in in vitro assays [69] and (v) current research trends highlight ncRNAs as novel and promising elements in various pathologies, particularly cancer [70,71,72,73,74]. These characteristics highlight the utility and relevance of miRNAs and lncRNAs over other types of biomarkers as diagnostic, prognostic, predictive, and monitoring tools in the context of CC.
5. Challenges and Future Perspectives of Cervical Cancer Biomarkers in Non-Cervical Samples
The use of CC biomarkers in non-cervical samples—such as blood, plasma, serum, urine, and vaginal fluids—represents a promising alternative to conventional diagnostic methods. Among its main advantages are minimal invasiveness, the possibility of repeated sampling, dynamic disease monitoring, and a more comprehensive assessment of tumor heterogeneity [60,75,76,77]. These features facilitate longitudinal monitoring of patients and can improve early detection, prognosis, and treatment personalization.
The integration of biomarkers into clinical practice faces significant challenges that extend beyond their scientific identification. The transition from discovery to clinical application is a complex and prolonged process that requires close and coordinated collaboration among researchers, clinicians, developers, and statisticians. This multidisciplinary interaction is essential to validate the sensitivity and specificity of biomarkers, yet it is often hindered by reluctance to share data and the need for verification by independent laboratories. Moreover, the high costs associated with advanced technologies, specialized equipment, and clinical trials further complicate widespread implementation, particularly in healthcare systems with limited resources. Collaborative networks such as the Early Detection Research Network (EDRN) promote an integrative model that facilitates access to resources and expertise, thereby accelerating the validation and clinical implementation of biomarkers. However, upon reviewing the EDRN database, a notable absence of reported advances in biomarkers for CC becomes evident, highlighting a significant gap in translational research for this malignancy [78]. This lack of representation may be attributed to the prioritization of other tumors with greater funding and a higher number of validated biomarkers, as well as the availability of effective screening methods for CC, such as cytology and HPV testing. Overcoming these challenges can lead to advancements in the research and development of CC-specific biomarkers, as well as foster multidisciplinary collaboration and investment in clinical validation to overcome the economic and structural barriers that continue to hinder the widespread adoption of these promising tools [79].
Within the clinical implementation of biomarkers in cancer, there are also other significant technical challenges, such as the lack of standardization in collection, processing, and analysis procedures, which limits the reproducibility and comparability of results between different laboratories [57,62,76,80]. Furthermore, the sensitivity and specificity of biomarkers in non-cervical samples have not yet reached the optimal levels required for routine use, which can lead to false-positive or -negative results [35,37,56,57]. Other challenges include the low concentration of biomarkers in peripheral fluids, the need for highly sensitive detection technologies, and the influence of biological and preanalytical factors that can affect the interpretation of results [35,37,56,57,62,76].
Despite the challenges of clinically implementing biomarkers in non-cervical samples, such as the low abundance of tumor genetic material, variability in collection and analysis methods, and the risk of false-positive or false-negative results, advances in ultrasensitive technologies, artificial intelligence, and multi-omics analysis have begun to transform this landscape. In particular, artificial intelligence has become an emerging tool with great potential to improve diagnostic and prognostic accuracy; for example, the use of machine learning algorithms integrated into the analysis of proteomic data derived from mass spectrometry has demonstrated an improved ability to predict CC from serum samples, supporting the identification of discriminating molecular signatures with high sensitivity and specificity [81]. These developments allow for better discrimination between patients and controls, the identification of more robust molecular signatures, and dynamic disease monitoring, which is especially useful for the early detection of recurrences and the evaluation of treatment response [35,37,56,57]. However, to translate these findings into real clinical benefits, it is essential to establish standardized protocols, promote well-designed multicenter studies with external validation, and foster interdisciplinary collaboration between oncologists, laboratory scientists, and biomedical sector stakeholders [18,82,83]. In this sense, although the vast majority of clinical trials related to CC analyze therapeutic regimens [84,85,86], assess the effectiveness of different vaccination schemes [87,88,89], identify biomarkers in cervical samples [90,91,92], promote public awareness regarding cervical screening [93,94,95], and propose imaging studies as preventive and diagnostic tools [96,97,98], the few clinical studies based on CC biomarkers in non-cervical samples are encouraging.
Clinical studies with CC patients are currently being conducted that (i) measure circulating levels of deoxyribonucleotides, HPV DNA, and tumor cells in blood samples of CC patients treated with radiochemotherapy followed by brachytherapy [99]; (ii) measure high risk HPV DNA in the urine of CIN 2/3 patients [100]; (iii) measure circulating HPV DNA in plasma from women with CIN 1-3 lesions [101]; (iv) measure circulating cell-free tumor tissue modified viral (TTMV)-HPV DNA in blood samples of women with genital dysplasia [102]; and (v) measure aurora kinase A and ninein interacting protein (AUNIP: a protein involved in DNA double-stand break repair) in serum samples of CC patients [103]. These clinical trials are designed to demonstrate the practical utility of these non-cervical samples for diagnosis, prognosis, disease monitoring, and patient follow-up. Among the interventional clinical trials, high-risk HPV DNA and DNA methylation are the main biomarkers analyzed in urine, via multiplex real-time polymerase chain reaction and multiplex methylation-specific quantitative PCR, to establish the diagnosis of CC and precursor lesions. Several trials are being conducted, designed to demonstrate the effectiveness of self-collection of samples for the identification of high-risk HPV DNA [100,104,105,106]. Self-collection of urine samples is ideal due to the favorable perception participants have regarding self-sampling and the non-invasive nature of sample collection [107,108]. The development of clinical trials is important to consolidate the diagnostic and prognostic utility of CC biomarkers in non-cervical samples in the comprehensive management of CC.
6. Conclusions
Despite various prophylactic measures against CC, such as vaccination campaigns, information campaigns against HPV infections, and promotion of condom use, among others, this neoplasm remains the leading cause of incidence and mortality among gynecological cancers worldwide. Fortunately, technological innovation in the diagnosis and prognosis of CC is constantly evolving. The detection of molecular biomarkers with high specificity and sensitivity in non-cervical samples emerges as an excellent alternative in the diagnosis and prognosis of CC. Its advantages over classical sampling could favor the reduction in the high incidence (when they are detected in previous stages of CC) and mortality rates of the most frequent gynecological neoplasia worldwide. Non-cervical sampling is a great alternative to cervical sampling because, being minimally invasive or non-invasive, it avoids the pain caused by current sampling, which is uncomfortable in the presence of gynecological morbidities, increased body mass index, musculoskeletal problems, advanced age, or menopause. In addition, it eliminates the taboo generated by cultural, religious, and social aspects that limit women from going to the gynecologist. This type of sampling can improve patient adherence to routine gynecological screening tests.
In conjunction with the hard work carried out in the laboratory, it is necessary that the geographical areas most affected by CC have the infrastructure, economic resources, and personnel necessary to carry out these innovative techniques. Other key strategies include increasing public awareness of sexually transmitted infections, continuing with CC prevention campaigns, and promoting gynecological health.
Biomarkers in non-cervical samples represent accessible tools that can significantly contribute to reducing the incidence and mortality of CC. The biomarkers proposed to date still require routine implementation in laboratory tests for validation and reliable use. Furthermore, the search for new biomarkers must continue to expand the repertoire of molecules that contribute to the diagnosis and prognosis of patients with CC.
Author Contributions
Conceptualization, J.C.V.-P. and A.L.P.-S.; writing—original draft preparation, M.d.R.L.-T.; writing—review and editing, M.G.-R., D.N.-A., A.R.-d.-A., A.L.P.-S., and J.C.V.-P.; visualization, M.d.R.L.-T.; supervision, J.C.V.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today, (Version 1.1); International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 14 March 2025).
- Woodman, C.B.J.; Collins, S.I.; Young, L.S. The Natural History of Cervical HPV Infection: Unresolved Issues. Nat. Rev. Cancer 2007, 7, 11–22. [Google Scholar] [CrossRef]
- Thomas, M.; Pim, D.; Banks, L. The Role of the E6-P53 Interaction in the Molecular Pathogenesis of HPV. Oncogene 1999, 18, 7690–7700. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Partricia, S.; Mathan, G. Overview of High-Risk HPV’s 16 and 18 Infected Cervical Cancer: Pathogenesis to Prevention. Biomed. Pharmacother. 2015, 70, 103–110. [Google Scholar] [CrossRef]
- Vats, A.; Trejo-Cerro, O.; Massimi, P.; Banks, L. Regulation of HPV E7 Stability by E6-Associated Protein (E6AP). J. Virol. 2022, 96, e0066322. [Google Scholar] [CrossRef]
- An, H.J.; Cho, N.H.; Lee, S.Y.; Kim, I.H.; Lee, C.; Kim, S.J.; Mun, M.S.; Kim, S.H.; Jeong, J.K. Correlation of Cervical Carcinoma and Precancerous Lesions with Human Papillomavirus (HPV) Genotypes Detected with the HPV DNA Chip Microarray Method. Cancer 2003, 97, 1672–1680. [Google Scholar] [CrossRef]
- Mayo, T.T.; Imtiaz, R.; Doan, H.Q.; Sambrano, B.L.; Gordon, R.; Ramirez-Fort, M.K.; Tyring, S.K. Human Papillomavirus: Epidemiology and Clinical Features of Related Cancer. In Viruses and Human Cancer; Springer: New York, NY, USA, 2014; pp. 199–228. [Google Scholar]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- American Society of Clinical Oncology (ASCO). Cervical Cancer: Diagnosis. Available online: https://www.cancer.net/cancer-types/cervical-cancer/diagnosis (accessed on 1 July 2024).
- National Cancer Institute (NCI). Cervical Cancer Diagnosis. Available online: https://www.cancer.gov/types/cervical/diagnosis (accessed on 1 September 2024).
- Landy, R.; Hollingworth, T.; Waller, J.; Marlow, L.A.; Rigney, J.; Round, T.; Sasieni, P.D.; Lim, A.W. Non-Speculum Sampling Approaches for Cervical Screening in Older Women: Randomised Controlled Trial. Br. J. Gen. Pr. 2022, 72, e26–e33. [Google Scholar] [CrossRef]
- Ørnskov, D.; Jochumsen, K.; Steiner, P.H.; Grunnet, I.M.; Lykkebo, A.W.; Waldstrøm, M. Clinical Performance and Acceptability of Self-Collected Vaginal and Urine Samples Compared with Clinician-Taken Cervical Samples for HPV Testing among Women Referred for Colposcopy. A Cross-Sectional Study. BMJ Open 2021, 11, e041512. [Google Scholar] [CrossRef]
- Afsah, Y.R.; Kaneko, N. Barriers to Cervical Cancer Screening Faced by Immigrant Muslim Women: A Systematic Scoping Review. BMC Public Health 2023, 23, 2375. [Google Scholar] [CrossRef]
- Guerrero-Flores, H.; Apresa-García, T.; Garay-Villar, Ó.; Sánchez-Pérez, A.; Flores-Villegas, D.; Bandera-Calderón, A.; García-Palacios, R.; Rojas-Sánchez, T.; Romero-Morelos, P.; Sánchez-Albor, V.; et al. A Non-Invasive Tool for Detecting Cervical Cancer Odor by Trained Scent Dogs. BMC Cancer 2017, 17, 79. [Google Scholar] [CrossRef]
- Khan, I.; Nam, M.; Kwon, M.; Seo, S.; Jung, S.; Han, J.S.; Hwang, G.-S.; Kim, M.K. LC/MS-Based Polar Metabolite Profiling Identified Unique Biomarker Signatures for Cervical Cancer and Cervical Intraepithelial Neoplasia Using Global and Targeted Metabolomics. Cancers 2019, 11, 511. [Google Scholar] [CrossRef]
- Nakabayashi, M.; Kawashima, A.; Yasuhara, R.; Hayakawa, Y.; Miyamoto, S.; Iizuka, C.; Sekizawa, A. Massively Parallel Sequencing of Cell-Free DNA in Plasma for Detecting Gynaecological Tumour-Associated Copy Number Alteration. Sci. Rep. 2018, 8, 11205. [Google Scholar] [CrossRef]
- Andrioaie, I.M.; Luchian, I.; Dămian, C.; Nichitean, G.; Andrese, E.P.; Pantilimonescu, T.F.; Trandabăț, B.; Prisacariu, L.J.; Budală, D.G.; Dimitriu, D.C.; et al. The Clinical Utility of Circulating HPV DNA Biomarker in Oropharyngeal, Cervical, Anal, and Skin HPV-Related Cancers: A Review. Pathogens 2023, 12, 908. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Chae, D.-K.; Lee, S.-H.; Lim, Y.; An, J.; Chae, C.H.; Kim, B.C.; Bhak, J.; Bolser, D.; Cho, D.-H. Efficient Mutation Screening for Cervical Cancers from Circulating Tumor DNA in Blood. BMC Cancer 2020, 20, 694. [Google Scholar] [CrossRef]
- Ning, R.; Meng, S.; Wang, L.; Jia, Y.; Tang, F.; Sun, H.; Zhang, Z.; Zhang, C.; Fan, X.; Xiao, B.; et al. 6 Circulating MiRNAs Can Be Used as Non-Invasive Biomarkers for the Detection of Cervical Lesions. J. Cancer 2021, 12, 5106–5113. [Google Scholar] [CrossRef]
- Nagamitsu, Y.; NishiI, H.; Sasaki, T.; Takaesu, Y.; Terauchi, F.; Isaka, K. Profiling Analysis of Circulating MicroRNA Expression in Cervical Cancer. Mol. Clin. Oncol. 2016, 5, 189–194. [Google Scholar] [CrossRef]
- Ma, G.; Song, G.; Zou, X.; Shan, X.; Liu, Q.; Xia, T.; Zhou, X.; Zhu, W. Circulating Plasma MicroRNA Signature for the Diagnosis of Cervical Cancer. Cancer Biomark. 2019, 26, 491–500. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Wang, F.; Xu, D.; Guo, Y.; Cui, W. Serum MiRNAs Panel (MiR-16-2*, MiR-195, MiR-2861, MiR-497) as Novel Non-Invasive Biomarkers for Detection of Cervical Cancer. Sci. Rep. 2015, 5, 17942. [Google Scholar] [CrossRef]
- Xin, F.; Liu, P.; Ma, C.-F. A Circulating Serum MiRNA Panel as Early Detection Biomarkers of Cervical Intraepithelial Neoplasia. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4846–4851. [Google Scholar]
- Aftab, M.; Poojary, S.S.; Seshan, V.; Kumar, S.; Agarwal, P.; Tandon, S.; Zutshi, V.; Das, B.C. Urine miRNA Signature as a Potential Non-Invasive Diagnostic and Prognostic Biomarker in Cervical Cancer. Sci. Rep. 2021, 11, 10323. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Yan, R.; Huang, L.; Mellor, A.L.; Yang, Y.; Chen, X.; Wei, W.; Wu, X.; Yu, L.; et al. Exosome-Derived MiR-142-5p Remodels Lymphatic Vessels and Induces IDO to Promote Immune Privilege in the Tumour Microenvironment. Cell Death Differ. 2021, 28, 715–729. [Google Scholar] [CrossRef]
- Zhu, X.; Long, L.; Xiao, H.; He, X. Cancer-Derived Exosomal MiR-651 as a Diagnostic Marker Restrains Cisplatin Resistance and Directly Targets ATG3 for Cervical Cancer. Dis. Markers 2021, 2021, 1544784. [Google Scholar] [CrossRef]
- Wang, W.-J.; Wang, D.; Zhao, M.; Sun, X.-J.; Li, Y.; Lin, H.; Che, Y.-Q.; Huang, C.-Z. Serum LncRNAs (CCAT2, LINC01133, LINC00511) with Squamous Cell Carcinoma Antigen Panel as Novel Non-Invasive Biomarkers for Detection of Cervical Squamous Carcinoma. Cancer Manag. Res. 2020, 12, 9495–9502. [Google Scholar] [CrossRef]
- Sheng, J.; Zhang, W. Identification of Biomarkers for Cervical Cancer in Peripheral Blood Lymphocytes Using Oligonucleotide Microarrays. Chin. Med. J. 2010, 123, 1000–1005. [Google Scholar]
- Zhao, Y.-C.; Wang, T.-J.; Qu, G.-H.; She, L.-Z.; Cui, J.; Zhang, R.-F.; Qu, H.-D. TPM3: A Novel Prognostic Biomarker of Cervical Cancer That Correlates with Immune Infiltration and Promotes Malignant Behavior In Vivo and In Vitro. Am. J. Cancer Res. 2023, 13, 3123–3139. [Google Scholar]
- Jiang, S.; Zheng, J.; Cui, Z.; Li, Y.; Wu, Q.; Cai, X.; Zheng, C.; Sun, Y. FBXO5 Acts as a Novel Prognostic Biomarker for Patients with Cervical Cancer. Front. Cell Dev. Biol. 2023, 11, 1200197. [Google Scholar] [CrossRef]
- Awazu, Y.; Fukuda, T.; Noda, T.; Uchikura, E.; Nanno, S.; Imai, K.; Yamauchi, M.; Yasui, T.; Sumi, T. CLPTM1L Expression Predicts Recurrence of Patients with Intermediate- and High-risk Stage IB-IIB Cervical Cancer Undergoing Radical Hysterectomy Followed by TP as Adjuvant Chemotherapy. Oncol. Lett. 2023, 26, 353. [Google Scholar] [CrossRef]
- Rungkamoltip, P.; Temisak, S.; Piboonprai, K.; Japrung, D.; Thangsunan, P.; Chanpanitkitchot, S.; Chaowawanit, W.; Chandeying, N.; Tangjitgamol, S.; Iempridee, T. Rapid and Ultrasensitive Detection of Circulating Human Papillomavirus E7 Cell-Free DNA as a Cervical Cancer Biomarker. Exp. Biol. Med. 2021, 246, 654–666. [Google Scholar] [CrossRef]
- Lee, H.; Choi, M.; Hwang, S.-H.; Cho, Y. A Versatile Nanowire Platform for Highly Efficient Isolation and Direct PCR-Free Colorimetric Detection of Human Papillomavirus DNA from Unprocessed Urine. Theranostics 2018, 8, 399–409. [Google Scholar] [CrossRef]
- Mittelstadt, S.; Kelemen, O.; Admard, J.; Gschwind, A.; Koch, A.; Wörz, S.; Oberlechner, E.; Engler, T.; Bonzheim, I.; Staebler, A.; et al. Detection of Circulating Cell-Free HPV DNA of 13 HPV Types for Patients with Cervical Cancer as Potential Biomarker to Monitor Therapy Response and to Detect Relapse. Br. J. Cancer 2023, 128, 2097–2103. [Google Scholar] [CrossRef]
- Cabel, L.; Bonneau, C.; Bernard-Tessier, A.; Héquet, D.; Tran-Perennou, C.; Bataillon, G.; Rouzier, R.; Féron, J.-G.; Fourchotte, V.; Le Brun, J.-F.; et al. HPV CtDNA Detection of High-Risk HPV Types during Chemoradiotherapy for Locally Advanced Cervical Cancer. ESMO Open 2021, 6, 100154. [Google Scholar] [CrossRef]
- Jeannot, E.; Latouche, A.; Bonneau, C.; Calméjane, M.-A.; Beaufort, C.; Ruigrok-Ritstier, K.; Bataillon, G.; Chérif, L.L.; Dupain, C.; Lecerf, C.; et al. Circulating HPV DNA as a Marker for Early Detection of Relapse in Patients with Cervical Cancer. Clin. Cancer Res. 2021, 27, 5869–5877. [Google Scholar] [CrossRef]
- Rodríguez-Esquivel, M.; Rosales, J.; Castro, R.; Apresa-García, T.; Garay, Ó.; Romero-Morelos, P.; Marrero-Rodríguez, D.; Taniguchi-Ponciano, K.; López-Romero, R.; Guerrero-Flores, H.; et al. Volatolome of the Female Genitourinary Area: Toward the Metabolome of Cervical Cancer. Arch. Med. Res. 2018, 49, 27–35. [Google Scholar] [CrossRef]
- do Nascimento Medeiros, J.A.; Sarmento, A.C.A.; Bernardes-Oliveira, E.; de Oliveira, R.; Lima, M.E.G.B.; Gonçalves, A.K.; de Souza Dantas, D.; de Oliveira Crispim, J.C. Evaluation of Exosomal MiRNA as Potential Biomarkers in Cervical Cancer. Epigenomes 2023, 7, 16. [Google Scholar] [CrossRef]
- Zheng, M.; Hou, L.; Ma, Y.; Zhou, L.; Wang, F.; Cheng, B.; Wang, W.; Lu, B.; Liu, P.; Lu, W.; et al. Exosomal Let-7d-3p and MiR-30d-5p as Diagnostic Biomarkers for Non-Invasive Screening of Cervical Cancer and Its Precursors. Mol. Cancer 2019, 18, 76. [Google Scholar] [CrossRef]
- Iempridee, T.; Wiwithaphon, S.; Piboonprai, K.; Pratedrat, P.; Khumkhrong, P.; Japrung, D.; Temisak, S.; Laiwejpithaya, S.; Chaopotong, P.; Dharakul, T. Identification of Reference Genes for Circulating Long Noncoding RNA Analysis in Serum of Cervical Cancer Patients. FEBS Open Bio 2018, 8, 1844–1854. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Lyu, Q.; Huang, J.; Chen, Y.; Feng, C.; Liu, Y.; Chen, F.; Wang, Z. Disulfidptosis-Associated LncRNAs Index Predicts Prognosis and Chemotherapy Drugs Sensitivity in Cervical Cancer. Sci. Rep. 2023, 13, 12470. [Google Scholar] [CrossRef]
- Khan, A.; Hussain, S.; Iyer, J.K.; Kaul, A.; Bonnewitz, M.; Kaul, R. Human Papillomavirus-Mediated Expression of Complement Regulatory Proteins in Human Cervical Cancer Cells. Eur. J. Obs. Gynecol. Reprod. Biol. 2023, 288, 222–228. [Google Scholar] [CrossRef]
- Detsika, M.G.; Palamaris, K.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. The Complement Cascade in Lung Injury and Disease. Respir. Res. 2024, 25, 20. [Google Scholar] [CrossRef]
- Lukacik, P.; Roversi, P.; White, J.; Esser, D.; Smith, G.P.; Billington, J.; Williams, P.A.; Rudd, P.M.; Wormald, M.R.; Harvey, D.J.; et al. Complement Regulation at the Molecular Level: The Structure of Decay-Accelerating Factor. Proc. Natl. Acad. Sci. USA 2004, 101, 1279–1284. [Google Scholar] [CrossRef]
- Couves, E.C.; Gardner, S.; Voisin, T.B.; Bickel, J.K.; Stansfeld, P.J.; Tate, E.W.; Bubeck, D. Structural Basis for Membrane Attack Complex Inhibition by CD59. Nat. Commun. 2023, 14, 890. [Google Scholar] [CrossRef]
- Montalvo-Castro, R.E.; Salinas-Jazmín, N. Relationship between the Expression of Complement Inhibitory Proteins and Therapeutic Efficacy of Antibodies in Breast Cancer. Gac. Med. Mex. 2022, 158, 141–149. [Google Scholar] [CrossRef]
- Hu, G.; Xiao, Y.; Ma, C.; Wang, J.; Qian, X.; Wu, X.; Zhu, F.; Sun, S.; Qian, J. Lumican Is a Potential Predictor on the Efficacy of Concurrent Chemoradiotherapy in Cervical Squamous Cell Carcinoma. Heliyon 2023, 9, e18011. [Google Scholar] [CrossRef]
- National Library of Medicine (NLM). CCT3 Chaperonin Containing TCP1 Subunit 3 [Homo Sapiens (Human)], Gene ID: 7203; NLM: Bethesda, MA, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/gene/7203 (accessed on 8 September 2024).
- Wang, K.; He, J.; Tu, C.; Xu, H.; Zhang, X.; Lv, Y.; Song, C. Upregulation of CCT3 Predicts Poor Prognosis and Promotes Cell Proliferation via Inhibition of Ferroptosis and Activation of AKT Signaling in Lung Adenocarcinoma. BMC Mol. Cell Biol. 2022, 23, 25. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Zhang, Z.; Wang, J.; Cheng, Z.; Fan, C. Identification of CCT3 as a Prognostic Factor and Correlates with Cell Survival and Invasion of Head and Neck Squamous Cell Carcinoma. Biosci. Rep. 2021, 41, BSR20211137. [Google Scholar] [CrossRef]
- Qian, T.; Cui, L.; Liu, Y.; Cheng, Z.; Quan, L.; Zeng, T.; Huang, W.; Dai, Y.; Chen, J.; Liu, L.; et al. High Expression of Chaperonin-Containing TCP1 Subunit 3 May Induce Dismal Prognosis in Multiple Myeloma. Pharmgenom. J. 2020, 20, 563–573. [Google Scholar] [CrossRef]
- Cui, X. Overexpression of Chaperonin Containing TCP1, Subunit 3 Predicts Poor Prognosis in Hepatocellular Carcinoma. World J. Gastroenterol. 2015, 21, 8588. [Google Scholar] [CrossRef]
- Danni, X.; Jiangzheng, Z.; Huamao, S.; Yinglian, P.; Changcheng, Y.; Yanda, L. Chaperonin Containing TCP1 Subunit 3 (CCT3) Promotes Cisplatin Resistance of Lung Adenocarcinoma Cells through Targeting the Janus Kinase 2/Signal Transducers and Activators of Transcription 3 (JAK2/STAT3) Pathway. Bioengineered 2021, 12, 7335–7347. [Google Scholar] [CrossRef]
- Li, M.; Zeng, J.; Chang, Y.; Lv, L.; Ye, G. CCT3 as a Diagnostic and Prognostic Biomarker in Cervical Cancer. Crit. Rev. Eukaryot. Gene Expr. 2023, 33, 17–28. [Google Scholar] [CrossRef]
- Téblick, L.; Van Keer, S.; De Smet, A.; Van Damme, P.; Laeremans, M.; Cortes, A.R.; Beyers, K.; Vankerckhoven, V.; Matheeussen, V.; Mandersloot, R.; et al. Impact of Collection Volume and DNA Extraction Method on the Detection of Biomarkers and HPV DNA in First-Void Urine. Molecules 2021, 26, 1989. [Google Scholar] [CrossRef]
- Hajjar, B.; Raheel, U.; Manina, R.; Simpson, J.; Irfan, M.; Waheed, Y. Clinical Performance of Cobas 6800 for the Detection of High-Risk Human Papillomavirus in Urine Samples. Vaccines 2023, 11, 1071. [Google Scholar] [CrossRef]
- Galati, L.; Combes, J.-D.; Le Calvez-Kelm, F.; McKay-Chopin, S.; Forey, N.; Ratel, M.; McKay, J.; Waterboer, T.; Schroeder, L.; Clifford, G.; et al. Detection of Circulating HPV16 DNA as a Biomarker for Cervical Cancer by a Bead-Based HPV Genotyping Assay. Microbiol. Spectr. 2022, 10, e0148021. [Google Scholar] [CrossRef]
- Bu, Q.; Luo, X.; He, L.; Ma, J.; He, S.; Lei, W.; Zhou, W.; Deng, H.; Lin, Y.; Zhang, L.; et al. Septin9 DNA Methylation as a Promising Biomarker for Cervical Cancer. J. Obstet. Gynaecol. 2023, 43, 2151356. [Google Scholar] [CrossRef]
- Cao, Y.; Qin, Y.; Cheng, Q.; Zhong, J.; Han, B.; Li, Y. Bifunctional Nanomaterial Enabled High-Specific Isolation of Urinary Exosomes for Cervical Cancer Metabolomics Analysis and Biomarker Discovery. Talanta 2025, 285, 127280. [Google Scholar] [CrossRef]
- Katoh, Y.; Kubo, A.; Hayashi, N.; Sugi, T.; Katoh, K.; Udagawa, S.; Ogawa, T.; Iwata, T.; Nishio, H.; Sugawara, M.; et al. Serum Levels of Stearic and Dihomo-γ-Linolenic Acids Can Be Used to Diagnose Cervical Cancer and Cervical Intraepithelial Neoplasia. Sci. Rep. 2024, 14, 20833. [Google Scholar] [CrossRef]
- Hentschel, A.E.; van den Helder, R.; van Trommel, N.E.; van Splunter, A.P.; van Boerdonk, R.A.A.; van Gent, M.D.J.M.; Nieuwenhuijzen, J.A.; Steenbergen, R.D.M. The Origin of Tumor DNA in Urine of Urogenital Cancer Patients: Local Shedding and Transrenal Excretion. Cancers 2021, 13, 535. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Esteller, M. Non-Coding RNAs in Human Disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of MiRNA Expression across Human Tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key Players in Cancer and Potential Therapeutic Strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating MicroRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Zhu, M.; Liu, S.; Wang, X. MiRNA Expression Profiles of HPV-Infected Patients with Cervical Cancer in the Uyghur Population in China. PLoS ONE 2016, 11, e0164701. [Google Scholar] [CrossRef]
- Xia, Y.-F.; Pei, G.-H.; Wang, N.; Che, Y.-C.; Yu, F.-S.; Yin, F.-F.; Liu, H.-X.; Luo, B.; Wang, Y.-K. MiR-3156-3p Is Downregulated in HPV-Positive Cervical Cancer and Performs as a Tumor-Suppressive MiRNA. Virol. J. 2017, 14, 20. [Google Scholar] [CrossRef]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-Coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Brillante, S.; Volpe, M.; Indrieri, A. Advances in MicroRNA Therapeutics: From Preclinical to Clinical Studies. Hum. Gene Ther. 2024, 35, 628–648. [Google Scholar] [CrossRef]
- Bayraktar, E.; Bayraktar, R.; Oztatlici, H.; Lopez-Berestein, G.; Amero, P.; Rodriguez-Aguayo, C. Targeting MiRNAs and Other Non-Coding RNAs as a Therapeutic Approach: An Update. Noncoding RNA 2023, 9, 27. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Z.; Lin, Q.; Luo, Q.; Cen, Y.; Li, J.; Fang, X.; Gong, C. MiRNAs and Cancer: Key Link in Diagnosis and Therapy. Genes 2021, 12, 1289. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA Therapeutics: Towards a New Era for the Management of Cancer and Other Diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Armakolas, A.; Kotsari, M.; Koskinas, J. Liquid Biopsies, Novel Approaches and Future Directions. Cancers 2023, 15, 1579. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid Biopsy in Cancer: Current Status, Challenges and Future Prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Heidrich, I.; Ačkar, L.; Mossahebi Mohammadi, P.; Pantel, K. Liquid Biopsies: Potential and Challenges. Int. J. Cancer 2021, 148, 528–545. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). Early Detection Research Network. Available online: https://edrn.nci.nih.gov/ (accessed on 22 June 2025).
- Wagner, P.D.; Srivastava, S. New Paradigms in Translational Science Research in Cancer Biomarkers. Transl. Res. 2012, 159, 343–353. [Google Scholar] [CrossRef]
- Weaver, C.; Nam, A.; Settle, C.; Overton, M.; Giddens, M.; Richardson, K.P.; Piver, R.; Mysona, D.P.; Rungruang, B.; Ghamande, S.; et al. Serum Proteomic Signatures in Cervical Cancer: Current Status and Future Directions. Cancers 2024, 16, 1629. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, L.; Guo, B.; Guo, A.; Ding, J.; Tong, D.; Wang, B.; Zhou, Z. Integrated Machine Learning Algorithms-Enhanced Predication for Cervical Cancer from Mass Spectrometry-Based Proteomics Data. Bioengineering 2025, 12, 269. [Google Scholar] [CrossRef]
- Kori, M.; Arga, K.Y. Potential Biomarkers and Therapeutic Targets in Cervical Cancer: Insights from the Meta-Analysis of Transcriptomics Data within Network Biomedicine Perspective. PLoS ONE 2018, 13, e0200717. [Google Scholar] [CrossRef]
- Martinelli, C.; Ercoli, A.; Vizzielli, G.; Burk, S.R.; Cuomo, M.; Satasiya, V.; Kacem, H.; Braccia, S.; Mazzarotti, G.; Miriello, I.; et al. Liquid Biopsy in Gynecological Cancers: A Translational Framework from Molecular Insights to Precision Oncology and Clinical Practice. J. Exp. Clin. Cancer Res. 2025, 44, 140. [Google Scholar] [CrossRef]
- Fracasso, P.M.; Duska, L.R.; Thaker, P.H.; Gao, F.; Zoberi, I.; Dehdashti, F.; Siegel, B.A.; Uliel, L.; Menias, C.O.; Rehm, P.K.; et al. An Exploratory Study of Neoadjuvant Cetuximab Followed by Cetuximab and Chemoradiotherapy in Women With Newly Diagnosed Locally Advanced Cervical Cancer. Am. J. Clin. Oncol. 2022, 45, 286–293. [Google Scholar] [CrossRef]
- Obstetrics & Gynecology Hospital of Fudan University. Iparomlimab and Tuvonralimab Combined with Paclitaxel and Cisplatin as Neoadjuvant Therapy for CC (ClinicalTrials.gov Identifier: NCT06878222). Available online: https://clinicaltrials.gov/study/NCT06878222 (accessed on 22 June 2025).
- Women’s Hospital School Of Medicine Zhejiang University. A Clinical Study Comparing Chemotherapy Combined with PD-1 Inhibitor Versus Concurrent Chemoradiotherapy in Cervical Cancer Patients with Positive Lymph Nodes After Surgery: A Multicenter Randomized Controlled Clinical Trial (ClinicalTrials.gov Identifier: NCT06866951). Available online: https://clinicaltrials.gov/study/NCT06866951 (accessed on 22 June 2025).
- Wu, Q.; Qian, M.; Welby, S.; Guignard, A.; Rosillon, D.; Gopala, K.; Xu, Y.; Liu, K.; He, Y.; Jiang, N.; et al. Prospective, Multi-Center Post-Marketing Surveillance Cohort Study to Monitor the Safety of the Human Papillomavirus-16/18 AS04-Adjuvanted Vaccine in Chinese Girls and Women Aged 9 to 45 Years, 2018–2020. Hum. Vaccines Immunother. 2023, 19, 2283912. [Google Scholar] [CrossRef]
- Charles University, Czech Republic. The Adjuvant Effect of HPV Vaccination on Recurrence of Cervical Precancer or Carcinoma in Women Undergoing Conization (ClinicalTrials.gov Identifier: NCT06258564). Available online: https://clinicaltrials.gov/study/NCT06258564 (accessed on 22 June 2025).
- Salmerón, J.; Torres-Ibarra, L.; Bosch, F.X.; Cuzick, J.; Lörincz, A.; Wheeler, C.M.; Castle, P.E.; Robles, C.; Lazcano-Ponce, E. HPV vaccination impact on a cervical cancer screening program: Methods of the FASTER-Tlalpan Study in Mexico. Salud Publica Mex. 2016, 58, 211–219. [Google Scholar] [CrossRef]
- Chao, T.K.; Ke, F.Y.; Liao, Y.P.; Wang, H.C.; Yu, C.P.; Lai, H.C. Triage of cervical cytological diagnoses of atypical squamous cells by DNA methylation of paired boxed gene 1 (PAX1). Diagn. Cytopathol. 2013, 41, 41–46. [Google Scholar] [CrossRef]
- Mackay Memorial Hospital. The Biomarker Analysis in Locally Advanced (ClinicalTrials.gov Identifier: NCT03635216). Available online: https://clinicaltrials.gov/study/NCT03635216 (accessed on 22 June 2025).
- Wuhan University. Molecular Markers in Cervical Cancer Screening in the Feasibility of the Mathematical Markov Model Analysis (ClinicalTrials.gov Identifier: NCT00889902). Available online: https://clinicaltrials.gov/study/NCT00889902 (accessed on 22 June 2025).
- International Agency for Research on Cancer. Effectiveness and Impact of the Lived Experience Cancer Awareness Campaign on Screening Participation (ClinicalTrials.gov Identifier: NCT06874985). Available online: https://clinicaltrials.gov/study/NCT06874985 (accessed on 22 June 2025).
- Gül Öztaş, H.; Işik, K. The Effect of Cervical Cancer Education Given to Women in Turkey on Knowledge, Attitudes, and Health Beliefs: A Randomized Controlled Study. Public Health Nurs. 2025, 42, 363–373. [Google Scholar] [CrossRef]
- Istanbul University—Cerrahpasa. Web-Based Education Program and Educational Booklet Developed on Cervical Cancer (ClinicalTrials.gov Identifier: NCT06705309). Available online: https://clinicaltrials.gov/study/NCT06705309 (accessed on 22 June 2025).
- Hunt, B.; Fregnani, J.H.T.G.; Brenes, D.; Schwarz, R.A.; Salcedo, M.P.; Possati-Resende, J.C.; Antoniazzi, M.; de Oliveira Fonseca, B.; Santana, I.V.V.; de Macêdo Matsushita, G.; et al. Cervical lesion assessment using real-time microendoscopy image analysis in Brazil: The CLARA study. Int. J. Cancer 2021, 149, 431–441. [Google Scholar] [CrossRef]
- Brookdale University Hospital Medical Center. Diagnostic Imaging Aid for Management of Cervical Lesions (FFC) (ClinicalTrials.gov Identifier: NCT02406365). Available online: https://clinicaltrials.gov/study/NCT02406365 (accessed on 22 June 2025).
- Aaron, J.; Nitin, N.; Travis, K.; Kumar, S.; Collier, T.; Park, S.Y.; José-Yacamán, M.; Coghlan, L.; Follen, M.; Richards-Kortum, R.; et al. Plasmon resonance coupling of metal nanoparticles for molecular imaging of carcinogenesis in vivo. J. Biomed. Opt. 2007, 12, 034007. [Google Scholar] [CrossRef]
- Fabian, D. Clinical Trial of Molecular Biomarkers in Women with Uterine Cervix Cancer (ClinicalTrials.gov Identifier: NCT05462951). Available online: https://clinicaltrials.gov/study/NCT05462951 (accessed on 22 June 2025).
- Universiteit Antwerpen. First-Void Urine Samples for the Follow-Up of Women Treated for High-Grade Cervical Intraepithelial Neoplasia (CIN) (ClinicalTrials.gov Identifier: NCT03542513). Available online: https://clinicaltrials.gov/study/NCT03542513 (accessed on 22 June 2025).
- UNC Lineberger Comprehensive Cancer Center. Application of Plasma Circulating HPV DNA Testing to Management of Cervical Intraepithelial Neoplasia (ClinicalTrials.gov Identifier: NCT04274465). Available online: https://clinicaltrials.gov/study/NCT04274465 (accessed on 22 June 2025).
- University of Mississippi Medical Center Clinical. Translational and Biomarker-Based Female Genital HPV Induced Dysplasia and Cancer Screening Study Using Cf-HPV-DNA Blood Tests (TTMV HPV DNA) (ClinicalTrials.gov Identifier: NCT05536843). Available online: https://clinicaltrials.gov/study/NCT05536843 (accessed on 22 June 2025).
- Yuan, L. Study on AUNIP as a Novel Tumor Marker for Cervical Cancer (ClinicalTrials.gov Identifier: NCT06118463). Available online: https://clinicaltrials.gov/study/NCT06118463 (accessed on 22 June 2025).
- Universiteit Antwerpen. Dry Run of the ScreenUrSelf Trial (ClinicalTrials.gov Identifier: NCT05996796). Available online: https://clinicaltrials.gov/study/NCT05996796 (accessed on 22 June 2025).
- Hendrickx, J.O.; Van Keer, S.; Donders, G.; Weyers, S.; Doyen, J.; Beyers, K.C.L.; Rios-Cortes, A.; Meers, N.; Téblick, L.; Vankerckhoven, V.V.J. Home-based urinary HPV self-sampling for the detection of cervical cancer precursor lesions: Attitudes and preferences from Belgian females participating in the CASUS study. Arch. Public Health 2025, 83, 32. [Google Scholar] [CrossRef]
- Universiteit Antwerpen. Developing a Combined Molecular Screening and Triage Test for Cervical Cancer in Self-Samples (COMBISCREEN) (ClinicalTrials.gov Identifier: NCT06598176). Available online: https://clinicaltrials.gov/study/NCT06598176 (accessed on 22 June 2025).
- Universiteit Antwerpen. Cervical Cancer Screening Based on First-Void Urine Self-Sampling to Reach Un(Der)-Screened Women: ScreenUrSelf Trial (ScreenUrSelf) (ClinicalTrials.gov Identifier: NCT05996783). Available online: https://clinicaltrials.gov/study/NCT05996783 (accessed on 22 June 2025).
- Buelens, C.; Stabel, M.; Wildiers, A.; Peremans, L.; Van Hal, G.; Van Doorsselaere, L.; Lievens, A.; Vorsters, A.; Van Keer, S.; Verhoeven, V. Experiences and Perceptions of Cervical Cancer Screening Using Self-Sampling among Under-Screened Women in Flanders. Healthcare 2024, 12, 1704. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).