Drosophila melanogaster: How and Why It Became a Model Organism
Abstract
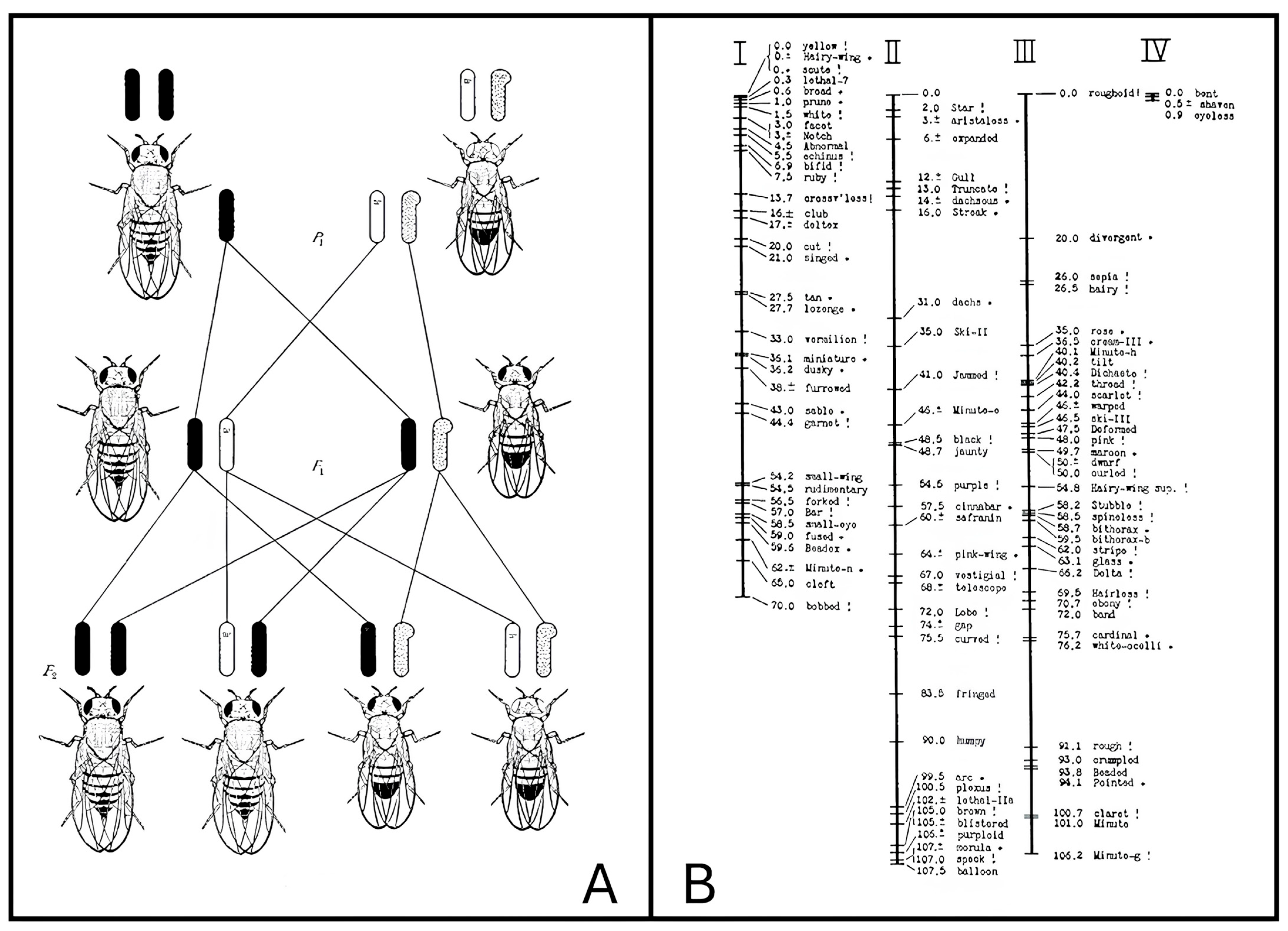
1. Introduction: A Brief Historical Background
2. People Who Contributed to Making the Fruit Fly a Reference Model Organism
- Walter Jakob Gehring discovered the homeobox in 1983 [21] and was also involved in the development and application of enhancer trapping methods.
- Seymour Benzer used forward genetics to investigate the genetic basis of various behaviors such as phototaxis and learning and discovered the first circadian rhythm mutants in Drosophila [24,25,26,27]. Concerning this insect, he also studied neurodegeneration and aging and isolated the first long-life mutant called Methuselah [28].
- Leslie Birgit Vosshall studied the genetic and behavioral mechanisms involved in the olfaction and feeding behavior of fruit flies, mosquitoes, and humans [43].
- Michael Ashburner identified a cascade of genetic controls in the post-larval development triggered by ecdysone (polytene chromosome puffs) [46]; he was also a member of the consortium involved in the sequencing and annotation of the Drosophila genome and actively participated in setting up the FlyBase, Gene Ontology, and ChEBI databases [47,48].
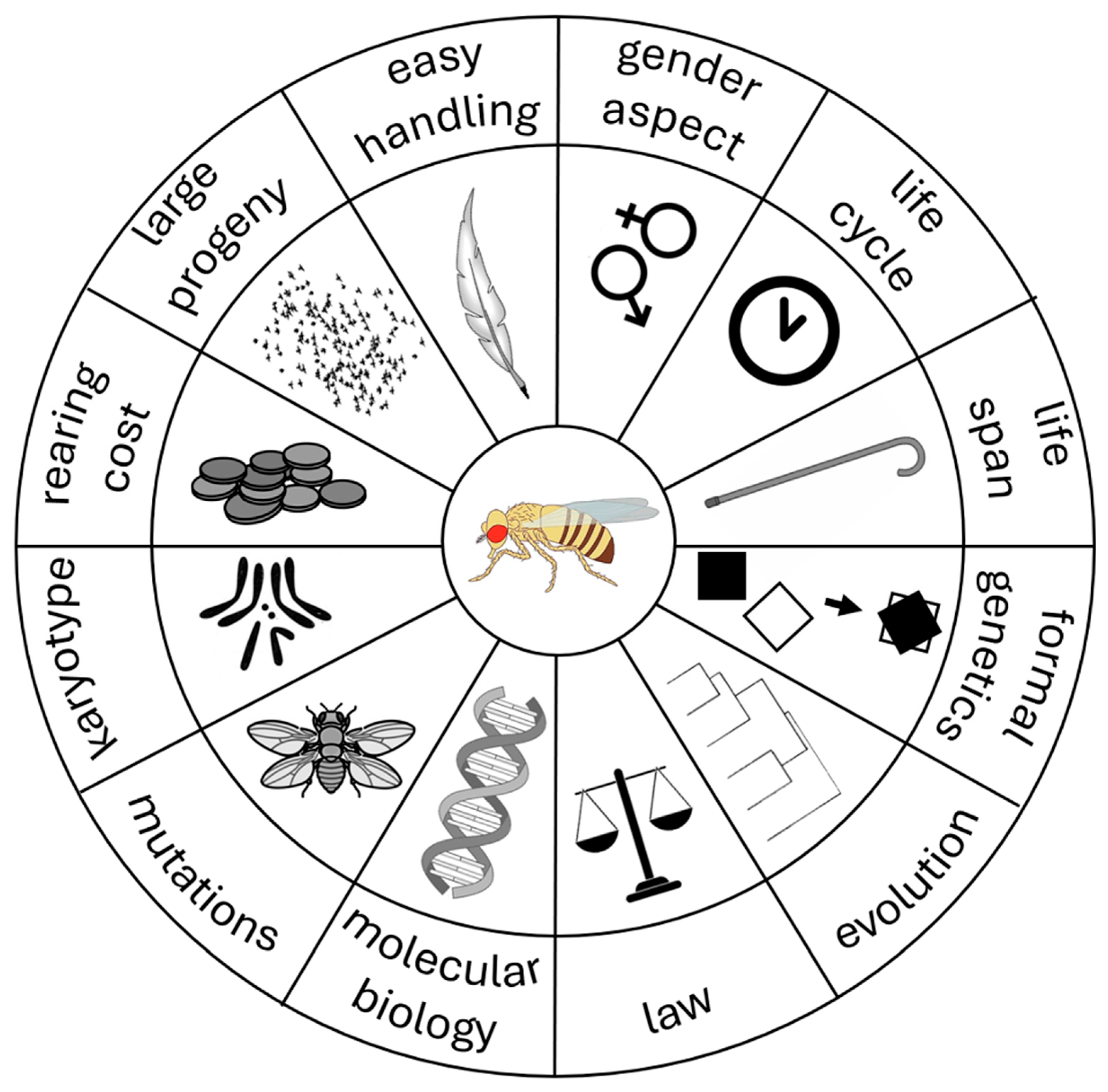
3. Why Use Drosophila melanogaster?
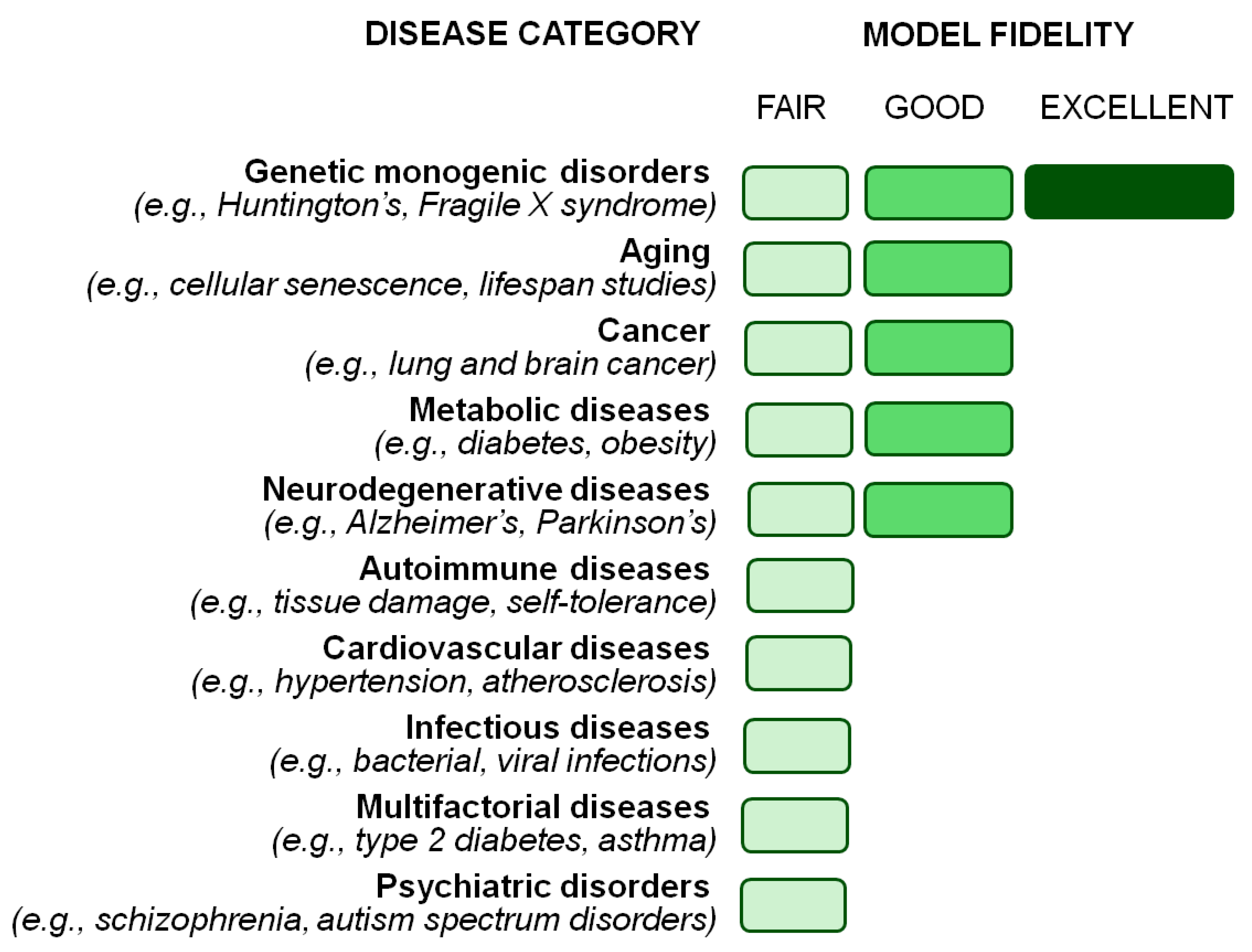
4. Using Drosophila melanogaster to Study Human Conditions
4.1. Drosophila melanogaster as a Model in Cancer Research
4.1.1. Drosophila Model for Colorectal Cancer
4.1.2. Drosophila Model for Lung Cancer
4.1.3. Drosophila Model for Glioblastoma Multiforme
4.2. Drosophila melanogaster as a Model for Neurodegenerative and Neurodevelopmental Diseases
4.3. Drosophila as a Model for Other Human Pathologies
4.4. Drosophila as a Model for Human Infectious Diseases
4.5. Drosophila as a Model for Drug Identification and Testing
4.6. Limits of Drosophila melanogaster When Modeling Human Diseases
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baudry, E.; Viginier, B.; Veuille, M. Non-African Populations of Drosophila melanogaster Have a Unique Origin. Mol. Biol. Evol. 2004, 21, 1482–1491. [Google Scholar] [CrossRef][Green Version]
- Morgan, T.H. Biographical. Available online: https://www.nobelprize.org/prizes/medicine/1933/morgan/biographical/ (accessed on 12 February 2025).
- Morgan, T.H. Sex Limited Inheritance in Drosophila. Science (1979) 1910, 32, 120–122. [Google Scholar] [CrossRef]
- Sturtevant, A. The Linear Arrangement of Six Sex-Linked Factors in Drosophila, as Shown by Their Mode of Association. J. Exp. Zool. 1913, 14, 43–59. [Google Scholar] [CrossRef]
- Morgan, T.H. The Theory of the Gene; Yale University Press: New Haven, CT, USA, 1926. [Google Scholar]
- Hirtle, P.B. Copyright Services: Copyright Term and the Public Domain. Available online: https://guides.library.cornell.edu/copyright/publicdomain (accessed on 13 February 2025).
- Carlson, E.A. Genes, Radiation, and Society: The Life and Work of H.J. Muller; Cornell University Press: Ithaca, NY, USA, 1981. [Google Scholar]
- Muller, H.J. The Production of Mutations by X-Rays. Proc. Natl. Acad. Sci. USA 1928, 14, 714–726. [Google Scholar] [CrossRef]
- Muller, H.J. Artificial Transmutation of the Gene. Science (1979) 1927, 66, 84–87. [Google Scholar] [CrossRef]
- Lewis, E.B. A Gene Complex Controlling Segmentation in Drosophila. Nature 1978, 276, 565–570. [Google Scholar] [CrossRef]
- Nüsslein-Volhard, C.; Wieschaus, E. Mutations Affecting Segment Number and Polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Schuler, G.; Steinman, R.M. Murine Epidermal Langerhans Cells Mature into Potent Immunostimulatory Dendritic Cells in Vitro. J. Exp. Med. 1985, 161, 526–546. [Google Scholar] [CrossRef] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.-Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene. Science (1979) 1998, 282, 2085–2088. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.-M.; Hoffmann, J.A. The Dorsoventral Regulatory Gene Cassette Spätzle/Toll/Cactus Controls the Potent Antifungal Response in Drosophila Adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Zehring, W.A.; Wheeler, D.A.; Reddy, P.; Konopka, R.J.; Kyriacou, C.P.; Rosbash, M.; Hall, J.C. P-Element Transformation with Period Locus DNA Restores Rhythmicity to Mutant, Arrhythmic Drosophila melanogaster. Cell 1984, 39, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Zehring, W.A.; Wheeler, D.A.; Pirrotta, V.; Hadfield, C.; Hall, J.C.; Rosbash, M. Molecular Analysis of the Period Locus in Drosophila melanogaster and Identification of a Transcript Involved in Biological Rhythms. Cell 1984, 38, 701–710. [Google Scholar] [CrossRef]
- Bargiello, T.A.; Jackson, F.R.; Young, M.W. Restoration of Circadian Behavioural Rhythms by Gene Transfer in Drosophila. Nature 1984, 312, 752–754. [Google Scholar] [CrossRef]
- Bargiello, T.A.; Young, M.W. Molecular Genetics of a Biological Clock in Drosophila. Proc. Natl. Acad. Sci. USA 1984, 81, 2142–2146. [Google Scholar] [CrossRef]
- Allocca, M.; Zola, S.; Bellosta, P. The Fruit Fly, Drosophila melanogaster: The Making of a Model (Part I). In Drosophila melanogaster Model for Recent Advances in Genetics and Therapeutics; InTech: Shenzhen, China, 2018. [Google Scholar]
- Hales, K.G.; Korey, C.A.; Larracuente, A.M.; Roberts, D.M. Genetics on the Fly: A Primer on the Drosophila Model System. Genetics 2015, 201, 815–842. [Google Scholar] [CrossRef]
- McGinnis, W.; Levine, M.S.; Hafen, E.; Kuroiwa, A.; Gehring, W.J. A Conserved DNA Sequence in Homoeotic Genes of the Drosophila Antennapedia and Bithorax Complexes. Nature 1984, 308, 428–433. [Google Scholar] [CrossRef]
- Levine, M.; Hafen, E.; Garber, R.L.; Gehring, W.J. Spatial distribution of Antennapedia transcripts during Drosophila development. EMBO J. 1983, 2, 2037–2046. [Google Scholar] [CrossRef]
- Levine, M.; Rubin, G.M.; Tjian, R. Human DNA sequences homologous to a protein coding region conserved between homeotic genes of Drosophila. Cell 1984, 38, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Benzer, S. Genetic dissection of behavior. Sci. Am. 1973, 229, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Benzer, S. Behavioral mutants of drosophila isolated by countercurrent distribution. Proc. Natl. Acad. Sci. USA 1967, 58, 1112–1119. [Google Scholar] [CrossRef]
- Quinn, W.G.; Harris, W.A.; Benzer, S. Conditioned behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1974, 71, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Konopka, R.J.; Benzer, S. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1971, 68, 2112–2116. [Google Scholar] [CrossRef]
- Lin, Y.J.; Seroude, L.; Benzer, S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science 1998, 282, 943–946. [Google Scholar] [CrossRef]
- Zemelman, B.V.; Lee, G.A.; Ng, M.; Miesenböck, G. Selective photostimulation of genetically chARGed neurons. Neuron 2002, 33, 15–22. [Google Scholar] [CrossRef]
- Lima, S.Q.; Miesenböck, G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 2005, 121, 141–152. [Google Scholar] [CrossRef]
- Spradling, A.C.; Rubin, G.M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science 1982, 218, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Celniker, S.E.; Rubin, G.M. The Drosophila melanogaster genome. Annu. Rev Genom. Hum Genet. 2003, 4, 89–117. [Google Scholar] [CrossRef]
- Parks, S.; Wakimoto, B.; Spradling, A. Replication and expression of an X-linked cluster of Drosophila chorion genes. Dev. Biol. 1986, 117, 294–305. [Google Scholar] [CrossRef]
- Lin, H.; Spradling, A.C. Fusome asymmetry and oocyte determination in Drosophila. Dev. Genet. 1995, 16, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Margolis, J.; Spradling, A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 1995, 121, 3797–3807. [Google Scholar] [CrossRef]
- Lin, H.; Spradling, A.C. Germline stem cell division and egg chamber development in transplanted Drosophila germaria. Dev. Biol. 1993, 159, 140–152. [Google Scholar] [CrossRef]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef]
- Egger, B.; Chell, J.M.; Brand, A.H. Insights into neural stem cell biology from flies. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 39–56. [Google Scholar] [CrossRef]
- Zirin, J.; Jusiak, B.; Lopes, R.; Ewen-Campen, B.; Bosch, J.A.; Risbeck, A.; Forman, C.; Villalta, C.; Hu, Y.; Perrimon, N. Expanding the Drosophila toolkit for dual control of gene expression. Elife 2024, 12, RP94073. [Google Scholar] [CrossRef]
- Ravindran, S. Profile of Norbert Perrimon. Proc. Natl. Acad. Sci. USA 2014, 111, 7501–7502. [Google Scholar] [CrossRef]
- Robertson, H.M.; Warr, C.G.; Carlson, J.R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2003, 100, 14537–14542. [Google Scholar] [CrossRef]
- Hallem, E.A.; Carlson, J.R. Coding of odors by a receptor repertoire. Cell 2006, 125, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Vosshall, L.B.; Wong, A.M.; Axel, R. An olfactory sensory map in the fly brain. Cell 2000, 102, 147–159. [Google Scholar] [CrossRef]
- Suzuki, D.T.; Procunier, D. Temperature-sensitive mutations in Drosophila melanogaster. 3. Dominant lethals and semilethals on chromosome 2. Proc. Natl. Acad. Sci. USA 1969, 62, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Holden, J.J.; Suzuki, D.T. Temperature-sensitive mutations in Drosophila melanogaster. XII. The genetic and developmental effects of dominant lethals on chromosome 3. Genetics 1973, 73, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M. Induction of puffs in polytene chromosomes of in vitro cultured salivary glands of Drosophila melanogaster by ecdysone and echysone analogues. Nat. New Biol. 1971, 230, 222–224. [Google Scholar] [CrossRef]
- Ashburner, M. Won for all how the Drosophila genome Was Sequenced; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 2006; ISBN 978-0-87969-802-7. [Google Scholar]
- Rubin, G.M. Michael Ashburner (1942–2023). Curr. Biol. 2023, 33, R881–R883. [Google Scholar] [CrossRef]
- Perveen, F.K. Introduction to Drosophila. In Drosophila melanogaster-Model for Recent Advances in Genetics and Therapeutics; InTech: Shenzhen, China, 2018. [Google Scholar]
- Yamaguchi, M.; Yoshida, H. Drosophila as a Model Organism. Adv. Exp. Med. Biol. 2018, 1067, 1–10. [Google Scholar]
- Kaufman, T.C. A Short History and Description of Drosophila melanogaster Classical Genetics: Chromosome Aberrations, Forward Genetic Screens, and the Nature of Mutations. Genetics 2017, 206, 665–689. [Google Scholar] [CrossRef] [PubMed]
- Lemeunier, F.; David, J.; Tsacas, L. The Melanogaster Species Group. In The Genetics and Biology of Drosophila; Ashburner, M., Carson, H., Thompson, J., Eds.; Academic Press: London, UK, 1986; Volume 3, pp. 147–188. [Google Scholar]
- Bridges, C.B. SALIVARY CHROMOSOME MAPS With a Key to the Banding of the Chromosomes of Drosophila melanogaster. J. Hered. 1935, 26, 60–64. [Google Scholar] [CrossRef]
- Korge, G. Chromosome Puff Activity and Protein Synthesis in Larval Salivary Glands of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1975, 72, 4550–4554. [Google Scholar] [CrossRef] [PubMed]
- Walker, V.K.; Ashburner, M. The control of ecdysterone-regulated puffs in Drosophila salivary glands. Cell 1981, 26, 269–277. [Google Scholar] [CrossRef]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The Genome Sequence of Drosophila melanogaster. Science (1979) 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A Systematic Analysis of Human Disease-Associated Gene Sequences In Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef]
- Su, T.T. Drug Screening in Drosophila; Why, When, and When Not? Wiley Interdiscip. Rev. Dev. Biol. 2019, 8, e346. [Google Scholar] [CrossRef]
- Naz, F.; Siddique, Y.H. Drosophila melanogaster a Versatile Model of Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2021, 20, 487–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor initiation and early tumorigenesis: Molecular mechanisms and interventional targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef]
- Parvy, J.P.; Hodgson, J.A.; Cordero, J.B. Drosophila as a Model System to Study Nonautonomous Mechanisms Affecting Tumour Growth and Cell Death. Biomed. Res. Int. 2018, 7152962. [Google Scholar] [CrossRef] [PubMed]
- Sonoshita, M.; Cagan, R.L. Modeling Human Cancers in Drosophila. Curr. Top. Dev. Biol. 2017, 121, 287–309. [Google Scholar] [CrossRef]
- Watson, K.L.; Justice, R.W.; Bryant, P.J. Drosophila in Cancer Research: The First Fifty Tumor Suppressor Genes. J. Cell Sci. Suppl. 1994, 18, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Victor Atoki, A.; Aja, P.M.; Shinkafi, T.S.; Ondari, E.N.; Adeniyi, A.I.; Fasogbon, I.V.; Dangana, R.S.; Shehu, U.U.; Akin-Adewumi, A. Exploring the Versatility of Drosophila melanogaster as a Model Organism in Biomedical Research: A Comprehensive Review. Fly 2025, 19, 2420453. [Google Scholar] [CrossRef]
- Hadorn, E. Die Degeneration Der Imaginalscheiben Bei Letalen Drosophila-Larven Der Mutation Lethal-Giant. Rev. Suisse. Zool. 1938, 45, 425–429. [Google Scholar]
- Gateff, E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 1978, 200, 1448–1459. [Google Scholar] [CrossRef]
- Bilder, D.; Li, M.; Perrimon, N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 2000, 289, 113–116. [Google Scholar] [CrossRef]
- Humbert, P.O.; Grzeschik, N.A.; Brumby, A.M.; Galea, R.; Elsum, I.; Richardson, H.E. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 2008, 27, 6888–6907. [Google Scholar] [CrossRef]
- Huang, L.; Muthuswamy, S.K. Polarity protein alterations in carcinoma: A focus on emerging roles for polarity regulators. Curr. Opin. Genet. Dev. 2010, 20, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Frappaolo, A.; Karimpour-Ghahnavieh, A.; Cesare, G.; Fraschini, R.; Vaccari, T.; Giansanti, M.G. GOLPH3 protein controls organ growth by interacting with TOR signaling proteins in Drosophila. Cell. Death Dis. 2022, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Frappaolo, A.; Giansanti, M.G. Using Drosophila melanogaster to Dissect the Roles of the mTOR Signaling Pathway in Cell Growth. Cells 2023, 12, 2622. [Google Scholar] [CrossRef]
- Khan, C.; Rusan, N.M. Using Drosophila to Uncover the Role of Organismal Physiology and the Tumor Microenvironment in Cancer. Trends Cancer 2024, 10, 289–311. [Google Scholar] [CrossRef]
- Ying, L.; Saavedra, P.; Perrimon, N. Cancer cachexia: Lessons from Drosophila. Dis. Model Mech. 2022, 15, dmm049298. [Google Scholar] [CrossRef]
- Munnik, C.; Xaba, M.P.; Malindisa, S.T.; Russell, B.L.; Sooklal, S.A. Drosophila melanogaster: A platform for anticancer drug discovery and personalized therapies. Front. Genet. 2022, 13, 949241. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Raskov, H.; Søby, J.H.; Troelsen, J.; Bojesen, R.D.; Gögenur, I. Driver gene mutations and epigenetics in colorectal cancer. Ann. Surg. 2020, 271, 75–85. [Google Scholar] [CrossRef]
- Itatani, Y.; Sonoshita, M.; Kakizaki, F.; Okawa, K.; Stifani, S.; Itoh, H.; Sakai, Y.; Taketo, M.M. Characterization of Aes nuclear foci in colorectal cancer cells. J. Biochem. 2016, 159, 133–140. [Google Scholar] [CrossRef][Green Version]
- Sonoshita, M.; Itatani, Y.; Kakizaki, F.; Sakimura, K.; Terashima, T.; Katsuyama, Y.; Sakai, Y.; Taketo, M.M. Promotion of colorectal cancer invasion and metastasis through activation of NOTCH-DAB1-ABL-RHOGEF protein TRIO. Cancer Discov. 2015, 5, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Villegas, S.N. One hundred years of Drosophila cancer research: No longer in solitude. Dis. Model Mech. 2019, 12, dmm039032. [Google Scholar] [CrossRef]
- Casali, A.; Batlle, E. Intestinal stem cells in mammals and Drosophila. Cell Stem Cell 2009, 4, 124–127. [Google Scholar] [CrossRef]
- Bangi, E.; Murgia, C.; Teague, A.G.; Sansom, O.J.; Cagan, R.L. Functional exploration of colorectal cancer genomes using Drosophila. Nat. Commun. 2016, 7, 13615. [Google Scholar] [CrossRef]
- Smolarz, B.; Łukasiewicz, H.; Samulak, D.; Piekarska, E.; Kołaciński, R.; Romanowicz, H. Lung Cancer-Epidemiology, Pathogenesis, Treatment and Molecular Aspect (Review of Literature). Int. J. Mol. Sci. 2025, 26, 2049. [Google Scholar] [CrossRef]
- Andrew, D.J.; Ewald, A.J. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev. Biol. 2010, 341, 34–55. [Google Scholar] [CrossRef]
- Ghabrial, A.; Luschnig, S.; Metzstein, M.M.; Krasnow, M.A. Branching morphogenesis of the Drosophila tracheal system. Annu. Rev. Cell Dev. Biol. 2003, 19, 623–647. [Google Scholar] [CrossRef] [PubMed]
- Park, W.Y.; Miranda, B.; Lebeche, D.; Hashimoto, G.; Cardoso, W.V. FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Dev. Biol. 1998, 201, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.D.; Cagan, R.L. Drosophila Lung Cancer Models Identify Trametinib plus Statin as Candidate Therapeutic. Cell Rep. 2016, 14, 1477–1487. [Google Scholar] [CrossRef]
- Das, T.K.; Cagan, R.L. KIF5B-RET Oncoprotein Signals through a Multi-kinase Signaling Hub. Cell Rep. 2017, 20, 2368–2383. [Google Scholar] [CrossRef] [PubMed]
- Shergalis, A.; Bankhead, A., 3rd; Luesakul, U.; Muangsin, N.; Neamati, N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef]
- Boockvar, J.A.; Kapitonov, D.; Kapoor, G.; Schouten, J.; Counelis, G.J.; Bogler, O.; Snyder, E.Y.; McIntosh, T.K.; O’Rourke, D.M. Constitutive EGFR signaling confers a motile phenotype to neural stem cells. Mol. Cell Neurosci. 2003, 24, 1116–1130. [Google Scholar] [CrossRef]
- Maher, E.A.; Furnari, F.B.; Bachoo, R.M.; Rowitch, D.H.; Louis, D.N.; Cavenee, W.K.; DePinho, R.A. Malignant glioma: Genetics and biology of a grave matter. Genes Dev. 2001, 15, 1311–1333. [Google Scholar] [CrossRef]
- Furnari, F.B.; Fenton, T.; Bachoo, R.M.; Mukasa, A.; Stommel, J.M.; Stegh, A.; Hahn, W.C.; Ligon, K.L.; Louis, D.N.; Brennan, C.; et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007, 21, 2683–2710. [Google Scholar] [CrossRef]
- Read, R.D.; Cavenee, W.K.; Furnari, F.B.; Thomas, J.B. A drosophila model for EGFR-Ras and PI3K-dependent human glioma. PLoS Genet. 2009, 5, e1000374. [Google Scholar] [CrossRef]
- GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Tomchik, S.M. Modeling neurodegenerative and neurodevelopmental disorders in the Drosophila mushroom body. Learn. Mem. 2024, 31, a053816. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Parenti, I.; Rabaneda, L.G.; Schoen, H.; Novarino, G. Neurodevelopmental Disorders: From Genetics to Functional Pathways. Trends Neurosci. 2020, 43, 608–621. [Google Scholar] [CrossRef]
- O’Kane, C.J. Drosophila as a model organism for the study of neuropsychiatric disorders. Curr. Top Behav. Neurosci. 2011, 7, 37–60. [Google Scholar] [CrossRef] [PubMed]
- McGurk, L.; Berson, A.; Bonini, N.M. Drosophila as an In Vivo Model for Human Neurodegenerative Disease. Genetics 2015, 201, 377–402. [Google Scholar] [CrossRef]
- Nitta, Y.; Sugie, A. Studies of neurodegenerative diseases using Drosophila and the development of novel approaches for their analysis. Fly 2022, 16, 275–298. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Turner, G.C. The mushroom body. Curr. Biol. 2010, 20, R11–R12. [Google Scholar] [CrossRef] [PubMed]
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila tools and assays for the study of human diseases. Dis. Model Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Branson, K.; Robie, A.A.; Bender, J.; Perona, P.; Dickinson, M.H. High-throughput ethomics in large groups of Drosophila. Nat. Methods 2009, 6, 451–457. [Google Scholar] [CrossRef]
- Inagaki, H.K.; Kamikouchi, A.; Ito, K. Protocol for quantifying sound-sensing ability of Drosophila melanogaster. Nat. Protoc. 2010, 5, 26–30. [Google Scholar] [CrossRef]
- McGuire, S.E.; Deshazer, M.; Davis, R.L. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog. Neurobiol. 2005, 76, 328–347. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.C.; Dickinson, M.H. A new chamber for studying the behavior of Drosophila. PLoS ONE 2010, 5, e8793. [Google Scholar] [CrossRef]
- Bolus, H.; Crocker, K.; Boekhoff-Falk, G.; Chtarbanova, S. Modeling Neurodegenerative Disorders in Drosophila melanogaster. Int. J. Mol. Sci. 2020, 21, 3055. [Google Scholar] [CrossRef]
- Ma, M.; Moulton, M.J.; Lu, S.; Bellen, H.J. ‘Fly-ing’ from rare to common neurodegenerative disease mechanisms. Trends Genet. 2022, 38, 972–984. [Google Scholar] [CrossRef]
- Shweta; Sharma, K.; Shakarad, M.; Agrawal, N.; Maurya, S.K. Drosophila glial system: An approach towards understanding molecular complexity of neurodegenerative diseases. Mol. Biol. Rep. 2024, 51, 1146. [Google Scholar] [CrossRef]
- Gatto, C.L.; Broadie, K. Drosophila modeling of heritable neurodevelopmental disorders. Curr. Opin. Neurobiol. 2011, 21, 834–841. [Google Scholar] [CrossRef]
- Trajković, J.; Makevic, V.; Pesic, M.; Pavković-Lučić, S.; Milojevic, S.; Cvjetkovic, S.; Hagerman, R.; Budimirovic, D.B.; Protic, D. Drosophila melanogaster as a Model to Study Fragile X-Associated Disorders. Genes 2022, 14, 87. [Google Scholar] [CrossRef]
- Atsoniou, K.; Giannopoulou, E.; Georganta, E.M.; Skoulakis, E.M.C. Drosophila Contributions towards Understanding Neurofibromatosis 1. Cells 2024, 13, 721. [Google Scholar] [CrossRef]
- Johnson, R.; Cagan, R. Drosophila as a Model for Human Disease. In Vogel and Motulsky’s Human Genetics; Springer: Berlin/Heidelberg, Germany, 2010; pp. 795–811. [Google Scholar]
- Wan, L.; Molloy, S.S.; Thomas, L.; Liu, G.; Xiang, Y.; Rybak, S.L.; Thomas, G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 1998, 94, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell. Biol. 2002, 3, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Aslan, J.E.; Thomas, L.; Shinde, P.; Shinde, U.; Simmen, T. Caught in the act: Protein adaptation and the expanding roles of the PACS proteins in tissue homeostasis and disease. J. Cell Sci. 2017, 130, 1865–1876. [Google Scholar] [CrossRef]
- Schuurs-Hoeijmakers, J.H.; Oh, E.C.; Vissers, L.E.; Swinkels, M.E.; Gilissen, C.; Willemsen, M.A.; Holvoet, M.; Steehouwer, M.; Veltman, J.A.; de Vries, B.B.; et al. Recurrent de novo mutations cause defective cranial-neural-crest migration and define a recognizable intellectual-disability syndrome. Am. J. Hum. Genet. 2012, 91, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.E.; Jean-Marçais, N.; Yang, E.; Heron, D.; Tatton-Brown, K.; van der Zwaag, P.A.; Bijlsma, E.K.; Krock, B.L.; Backer, E.; Kamsteeg, E.J.; et al. A recurrent de novo PACS2 heterozygous missense variant causes neonatal-onset developmental epileptic encephalopathy, facial dysmorphism, and cerebellar dysgenesis. Am. J. Hum. Genet. 2018, 102, 995–1007. [Google Scholar] [CrossRef]
- Youker, R.T.; Shinde, U.; Day, R.; Thomas, G. At the crossroads of homoeostasis and disease: Roles of the PACS proteins in membrane traffic and apoptosis. Biochem. J. 2009, 421, 1–15. [Google Scholar] [CrossRef]
- Lusk, L.; Smith, S.; Martin, C.; Taylor, C.; Chung, W. PACS1 Neurodevelopmental Disorder. In GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 1993. [Google Scholar] [PubMed]
- Köttgen, M.; Benzing, T.; Simmen, T.; Tauber, R.; Buchholz, B.; Feliciangeli, S.; Huber, T.B.; Schermer, B.; Kramer-Zucker, A.; Höpker, K.; et al. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J. 2005, 24, 705–716. [Google Scholar] [CrossRef]
- Dombernowsky, S.L.; Samsøe-Petersen, J.; Petersen, C.H.; Instrell, R.; Hedegaard, A.M.; Thomas, L.; Atkins, K.M.; Auclair, S.; Albrechtsen, R.; Mygind, K.J.; et al. The sorting protein PACS-2 promotes ErbB signalling by regulating recycling of the metalloproteinase ADAM17. Nat. Commun. 2015, 6, 7518. [Google Scholar] [CrossRef]
- Villar-Pazos, S.; Thomas, L.; Yang, Y.; Chen, K.; Lyles, J.B.; Deitch, B.J.; Ochaba, J.; Ling, K.; Powers, B.; Gingras, S.; et al. Neural deficits in a mouse model of PACS1 syndrome are corrected with PACS1- or HDAC6-targeting therapy. Nat. Commun. 2023, 14, 6547. [Google Scholar] [CrossRef]
- Frappaolo, A.; Zaccagnini, G.; Riparbelli, M.G.; Colotti, G.; Callaini, G.; Giansanti, M.G. PACS deficiency disrupts Golgi architecture and causes cytokinesis failures and seizure-like phenotype in Drosophila melanogaster. Open Biol. 2025, 15, 240267. [Google Scholar] [CrossRef] [PubMed]
- Kanca, O.; Andrews, J.C.; Lee, P.T.; Patel, C.; Braddock, S.R.; Slavotinek, A.M.; Cohen, J.S.; Gubbels, C.S.; Aldinger, K.A.; Williams, J.; et al. De novo variants in WDR37 are associated with epilepsy, colobomas, dysmorphism, developmental delay, intellectual disability, and cerebellar hypoplasia. Am. J. Hum. Genet. 2019, 105, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Armstrong, C.M.; Bertin, N.; Ge, H.; Milstein, S.; Boxem, M.; Vidalain, P.O.; Han, J.D.; Chesneau, A.; Hao, T.; et al. A map of the interactome network of the metazoan C. elegans. Science 2004, 303, 540–543. [Google Scholar] [CrossRef]
- Malovannaya, A.; Lanz, R.B.; Jung, S.Y.; Bulynko, Y.; Le, N.T.; Chan, D.W.; Ding, C.; Shi, Y.; Yucer, N.; Krenciute, G.; et al. Analysis of the human endogenous coregulator complexome. Cell 2011, 145, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Nair-Gill, E.; Bonora, M.; Zhong, X.; Liu, A.; Miranda, A.; Stewart, N.; Ludwig, S.; Russell, J.; Gallagher, T.; Pinton, P.; et al. Calcium flux control by Pacs1-Wdr37 promotes lymphocyte quiescence and lymphoproliferative diseases. EMBO J. 2021, 40, e104888. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Yoshihashi, H.; Uehara, T.; Miyama, S.; Kosaki, K.; Takenouchi, T. Coloboma may be a shared feature in a spectrum of disorders caused by mutations in the WDR37-PACS1-PACS2 axis. Am. J. Med. Genet. A 2021, 185, 884–888. [Google Scholar] [CrossRef]
- Simmen, T.; Aslan, J.E.; Blagoveshchenskaya, A.D.; Thomas, L.; Wan, L.; Xiang, Y.; Feliciangeli, S.F.; Hung, C.H.; Crump, C.M.; Thomas, G. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005, 24, 717–729. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, H.; Yan, T.; Liu, L.; Yu, L.; Huang, Y.; Li, F.; Zeng, Y.; Huang, W.; Zhang, Y.; et al. A novel multi-exon deletion of PACS1 in a three-generation pedigree: Supplements to PACS1 neurodevelopmental disorder Spectrum. Front. Genet. 2021, 12, 690216. [Google Scholar] [CrossRef]
- Arnedo, M.; Ascaso, Á.; Latorre-Pellicer, A.; Lucia-Campos, C.; Gil-Salvador, M.; Ayerza-Casas, A.; Pablo, M.J.; Gómez-Puertas, P.; Ramos, F.J.; Bueno-Lozano, G.; et al. Molecular Basis of the Schuurs-Hoeijmakers Syndrome: What We Know about the Gene and the PACS-1 Protein and Novel Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 9649. [Google Scholar] [CrossRef]
- Lee, S.-H.; Min, K.-J. Drosophila melanogaster as a Model System in the Study of Pharmacological Interventions in Aging. Transl. Med. Aging 2019, 3, 98–103. [Google Scholar] [CrossRef]
- Xia, B.; de Belle, J.S. Non-Genetic Transgenerational Inheritance of Acquired Traits in Drosophila. In Drosophila melanogaster—Model for Recent Advances in Genetics and Therapeutics; InTech: Shenzhen, China, 2018. [Google Scholar]
- Schneider, D. Using Drosophila as a Model Insect. Nat. Rev. Genet. 2000, 1, 218–226. [Google Scholar] [CrossRef]
- Leulier, F.; Marchal, C.; Miletich, I.; Limbourg-Bouchon, B.; Benarous, R.; Lemaitre, B. Directed Expression of the HIV-1 Accessory Protein Vpu in Drosophila Fat-body Cells Inhibits Toll-dependent Immune Responses. EMBO Rep. 2003, 4, 976–981. [Google Scholar] [CrossRef]
- Marchal, C.; Vinatier, G.; Sanial, M.; Plessis, A.; Pret, A.-M.; Limbourg-Bouchon, B.; Théodore, L.; Netter, S. The HIV-1 Vpu Protein Induces Apoptosis in Drosophila via Activation of JNK Signaling. PLoS ONE 2012, 7, e34310. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hanley, K.A. RNA Interference Modulates Replication of Dengue Virus in Drosophila melanogaster Cells. BMC Microbiol. 2010, 10, 127. [Google Scholar] [CrossRef]
- Sessions, O.M.; Barrows, N.J.; Souza-Neto, J.A.; Robinson, T.J.; Hershey, C.L.; Rodgers, M.A.; Ramirez, J.L.; Dimopoulos, G.; Yang, P.L.; Pearson, J.L.; et al. Discovery of Insect and Human Dengue Virus Host Factors. Nature 2009, 458, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.A.; Chen, Y.; Chan, C.M.; Chan, C.S.M.; Chan, P.K.S.; Chui, Y.L.; Fung, K.P.; Waye, M.M.Y.; Tsui, S.K.W.; Chan, H.Y.E. In Vivo Functional Characterization of the SARS-Coronavirus 3a Protein in Drosophila. Biochem. Biophys. Res. Commun. 2005, 337, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-M.; Ma, C.-W.; Chan, W.-Y.; Chan, H.Y.E. The SARS-Coronavirus Membrane Protein Induces Apoptosis through Modulating the Akt Survival Pathway. Arch. Biochem. Biophys. 2007, 459, 197–207. [Google Scholar] [CrossRef]
- Rose, P.P.; Hanna, S.L.; Spiridigliozzi, A.; Wannissorn, N.; Beiting, D.P.; Ross, S.R.; Hardy, R.W.; Bambina, S.A.; Heise, M.T.; Cherry, S. Natural Resistance-Associated Macrophage Protein Is a Cellular Receptor for Sindbis Virus in Both Insect and Mammalian Hosts. Cell Host Microbe 2011, 10, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hopkins, K.; Sabin, L.; Yasunaga, A.; Subramanian, H.; Lamborn, I.; Gordesky-Gold, B.; Cherry, S. ERK Signaling Couples Nutrient Status to Antiviral Defense in the Insect Gut. Proc. Natl. Acad. Sci. USA 2013, 110, 15025–15030. [Google Scholar] [CrossRef]
- Chotkowski, H.L.; Ciota, A.T.; Jia, Y.; Puig-Basagoiti, F.; Kramer, L.D.; Shi, P.-Y.; Glaser, R.L. West Nile Virus Infection of Drosophila melanogaster Induces a Protective RNAi Response. Virology 2008, 377, 197–206. [Google Scholar] [CrossRef]
- Schnettler, E.; Sterken, M.G.; Leung, J.Y.; Metz, S.W.; Geertsema, C.; Goldbach, R.W.; Vlak, J.M.; Kohl, A.; Khromykh, A.A.; Pijlman, G.P. Noncoding Flavivirus RNA Displays RNA Interference Suppressor Activity in Insect and Mammalian Cells. J. Virol. 2012, 86, 13486–13500. [Google Scholar] [CrossRef]
- Hao, L.; Sakurai, A.; Watanabe, T.; Sorensen, E.; Nidom, C.A.; Newton, M.A.; Ahlquist, P.; Kawaoka, Y. Drosophila RNAi Screen Identifies Host Genes Important for Influenza Virus Replication. Nature 2008, 454, 890–893. [Google Scholar] [CrossRef]
- Nakamoto, M.; Moy, R.H.; Xu, J.; Bambina, S.; Yasunaga, A.; Shelly, S.S.; Gold, B.; Cherry, S. Virus Recognition by Toll-7 Activates Antiviral Autophagy in Drosophila. Immunity 2012, 36, 658–667. [Google Scholar] [CrossRef]
- Adamson, A.; LaJeunesse, D. A Study of Epstein-Barr Virus BRLF1 Activity in a Drosophila Model System. Sci. World J. 2012, 2012, 347597. [Google Scholar] [CrossRef]
- Adamson, A.L.; Wright, N.; LaJeunesse, D.R. Modeling Early Epstein-Barr Virus Infection in Drosophila melanogaster: The BZLF1 Protein. Genetics 2005, 171, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, R.; Shemer-Avni, Y.; Adler, N.; Neuman-Silberberg, S. Human Cytomegalovirus Immediate-Early-Gene Expression Disrupts Embryogenesis in Transgenic Drosophila. Transgenic Res. 2008, 17, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Kotadia, S.; Kao, L.R.; Comerford, S.A.; Jones, R.T.; Hammer, R.E.; Megraw, T.L. PP2A-Dependent Disruption of Centrosome Replication and Cytoskeleton Organization in Drosophila by SV40 Small Tumor Antigen. Oncogene 2008, 27, 6334–6346. [Google Scholar] [CrossRef]
- Moser, T.S.; Sabin, L.R.; Cherry, S. RNAi Screening for Host Factors Involved in Vaccinia Virus Infection Using Drosophila Cells. J. Vis. Exp. 2010, 35, 2137. [Google Scholar] [CrossRef]
- Nainu, F.; Rahmatika, D.; Emran, T.B.; Harapan, H. Potential Application of Drosophila melanogaster as a Model Organism in COVID-19-Related Research. Front. Pharmacol. 2020, 11, 588561. [Google Scholar] [CrossRef]
- van de Leemput, J.; Han, Z. Drosophila, a Powerful Model to Study Virus-Host Interactions and Pathogenicity in the Fight against SARS-CoV-2. Cell Biosci. 2021, 11, 110. [Google Scholar] [CrossRef]
- Guichard, A.; Lu, S.; Kanca, O.; Bressan, D.; Huang, Y.; Ma, M.; Sanz Juste, S.; Andrews, J.C.; Jay, K.L.; Sneider, M.; et al. A Comprehensive Drosophila Resource to Identify Key Functional Interactions between SARS-CoV-2 Factors and Host Proteins. Cell Rep. 2023, 42, 112842. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, G.; Huang, X.; Lee, H.; Lee, J.-G.; Yang, P.; van de Leemput, J.; Huang, W.; Kane, M.A.; Yang, P.; et al. SARS-CoV-2 Nsp6 Damages Drosophila Heart and Mouse Cardiomyocytes through MGA/MAX Complex-Mediated Increased Glycolysis. Commun. Biol. 2022, 5, 1039. [Google Scholar] [CrossRef] [PubMed]
- Herrera, P.; Cauchi, R.J. Functional Characterisation of the ACE2 Orthologues in Drosophila Provides Insights into the Neuromuscular Complications of COVID-19. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166818. [Google Scholar] [CrossRef]
- Bangi, E. A Drosophila Based Cancer Drug Discovery Framework. Adv. Exp. Med. Biol. 2019, 1167, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Arch, M.; Fuentes, E.; Cardona, P.-J. Drosophila melanogaster Experimental Model to Test New Antimicrobials: A Methodological Approach. Front. Microbiol. 2024, 15, 1478263. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.P.; Karge, R.A.; Koch, H.; Voigt, A.; Weber, Y.G.; Wolking, S. The Fruit Fly Drosophila melanogaster as a Screening Model for Antiseizure Medications. Front. Pharmacol. 2024, 15, 1489888. [Google Scholar] [CrossRef] [PubMed]
- Maitra, U.; Ciesla, L. Using Drosophila as a Platform for Drug Discovery from Natural Products in Parkinson’s Disease. Medchemcomm 2019, 10, 867–879. [Google Scholar] [CrossRef]
- Narayanan, A.S.; Rothenfluh, A. I Believe I Can Fly!: Use of Drosophila as a Model Organism in Neuropsychopharmacology Research. Neuropsychopharmacology 2016, 41, 1439–1446. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [PubMed]
- Bier, E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005, 6, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Wangler, M.F.; Yamamoto, S.; Bellen, H.J. Fruit flies in biomedical research. Genetics 2015, 199, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Casas-Tintó, S. Drosophila as a Model for Human Disease: Insights into Rare and Ultra-Rare Diseases. Insects 2024, 15, 870. [Google Scholar] [CrossRef]
- Chang, R.B.; Beatty, G.L. The interplay between innate and adaptive immunity in cancer shapes the productivity of cancer immunosurveillance. J. Leukoc. Biol. 2020, 108, 363–376. [Google Scholar] [CrossRef]
- Liu, D.; Liu, L.; Zhao, X.; Zhang, X.; Chen, X.; Che, X.; Wu, G. A comprehensive review on targeting diverse immune cells for anticancer therapy: Beyond immune checkpoint inhibitors. Crit. Rev. Oncol. Hematol. 2025, 210, 104702. [Google Scholar] [CrossRef]
- Verheyen, E.M. The power of Drosophila in modeling human disease mechanisms. Dis. Model Mech. 2022, 15, dmm049549. [Google Scholar] [CrossRef]
- Tue, N.T.; Dat, T.Q.; Ly, L.L.; Anh, V.D.; Yoshida, H. Insights from Drosophila melanogaster model of Alzheimer’s disease. Front. Biosci. 2020, 25, 134–146. [Google Scholar] [CrossRef]
- Cesur, M.F.; Basile, A.; Patil, K.R.; Çakır, T. A new metabolic model of Drosophila melanogaster and the integrative analysis of Parkinson’s disease. Life Sci. Alliance 2023, 6, e202201695. [Google Scholar] [CrossRef]
- Jensen, R.L.; Pedersen, K.S.; Loeschcke, V.; Ingmer, H.; Leisner, J.J. Limitations in the use of Drosophila melanogaster as a model host for gram-positive bacterial infection. Lett. Appl. Microbiol. 2007, 44, 218–223. [Google Scholar] [CrossRef]
- Nisha; Raj, K.; Pragati; Tandon, S.; Chanu, S.I..; Sarkar, S. Aging: Reading, Reasoning, and Resolving Using Drosophila as a Model System. In Models, Molecules and Mechanisms in Biogerontology; Rath, P., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
| Cancer Type | Genetic Mutations | Why Drosophila | Ref. |
|---|---|---|---|
| Colorectal cancer | RasG12V, p53, Pten apc | Similarly to human CRC, Drosophila CRC models display altered cell differentiation and cell growth associated with the disruption of intestinal homeostasis | [81] |
| Lung cancer | RasG12V Pten |
| [86] |
| Lung cancer | KIF5B-RET | Drosophila KIF5B-RET model suggests novel therapeutic strategies for targeting KIF5B-RET fusions | [87] |
| Glioblastoma multiforme | Glial specific expression of activated EGFR and dp110 (repo>dEGFRλ; dp110CAAX) |
| [92] |
| NDD/#MIM | Human/Drosophila Gene | The Drosophila Disease Model | Ref. |
|---|---|---|---|
| PACS1-NDD MIM#615009 | PACS1/dPACS | Loss of dPACS leads to defects in tubulin acetylation and severe bang sensitivity, a phenotype associated with seizures | [123] |
| PACS2-NDD MIM#618067 | PACS2/dPACS | Loss of dPACS leads to defects in tubulin acetylation and severe bang sensitivity, a phenotype associated with seizures | [123] |
| WDR37-NDD MIM #618652 | wdr37 | Loss of wdr37 causes bang sensitivity and a defect in grip strength | [124] |
| Virus Full Name | Virus Acronym | Associated Disease | Why Drosophila | Ref. |
|---|---|---|---|---|
| Human immune-deficiency virus 1 | HIV1 | Acquired immune deficiency syndrome (AIDS) | it allowed us to understand the role of Toll and JNK pathways during infection | [135,136] |
| Dengue virus | DENV | Dengue hemorrhagic fever (DHF) | it allowed for a better understanding of the role of RNA interference (RNAi) in infection control | [137,138] |
| Severe acute respiratory syndrome coronavirus | SARS-CoV | Severe acute respiratory syndrome (SARS) | it allowed for better understanding the protein–protein interactions between viral and host proteins | [139,140] |
| Sindbis virus | SINV | Sindbis fever | it allowed for better understanding the entry mechanism of the virus inside cells and the role of the ERK pathway in Drosophila and mosquito (the natural virus carrier) intestinal immunity | [141,142] |
| West Nile virus | WNV | West Nile fever | it allowed for exploring the possibility to control the infection via RNAi | [143,144] |
| Influenza A virus | IAV | Pandemic flu | it allowed for identifying several conserved host factors important for virus replication | [145] |
| Vesicular stomatitis virus | VSV | Oncolytic virus causing a flu-like condition | it allowed for studying of the role of Toll-7 in controlling virus infection | [146] |
| Epstein–Barr virus | EBV | Mononucleosis; also involved in cancer and multiple sclerosis | it allowed for the identification of human EBV-targeted tumor suppressors | [147,148] |
| Human cytomegalovirus | HCMV | Birth defects | it provided a model to study how HCMV impairs embryonic development | [149] |
| Simian virus 40 | SV40 | Debated role in oncogenesis | possible oncogenetic routes have been disclosed in the fly | [150] |
| Vaccinia virus | VACV | Rash and fever; also used as a vaccine for smallpox | it allowed for the identification of host factors required for viral entry | [151] |
| Severe acute respiratory syndrome coronavirus 2 | SARS-CoV-2 | Coronavirus disease (COVID)-19 | it allowed for the identification of key functional interactions between viral factors and host proteins and its relationship with cardiovascular and neuromuscular complications in humans | [152,153,154,155,156] |
| Category | Drosophila melanogaster | Homo sapiens |
|---|---|---|
| Organs Involved | Fat body, midgut, Malpighian tubules (analogous to liver and kidney functions) | Liver, kidneys, intestines, lungs |
| Enzyme Systems | Cytochrome P450 monooxygenases (less diverse), esterases, glutathione S-transferases | Extensive cytochrome P450 families (CYP1, CYP2, CYP3), UGTs, SULTs, esterases |
| Blood–Brain Barrier | Present but structurally and functionally simpler; lacks tight junctions of vertebrate BBB | Complex structure with tight junctions, astrocytic end-feet, and selective permeability |
| Absorption | Primarily through ingestion; limited oral bioavailability studies | Oral, intravenous, subcutaneous, transdermal, etc. |
| Distribution | Open circulatory system; hemolymph distributes compounds | Closed circulatory system; plasma protein binding and tissue perfusion |
| Metabolism | Simplified metabolic pathways; limited phase I and II reactions | Complex phase I (oxidation, reduction, hydrolysis) and phase II (conjugation) metabolism |
| Excretion | Malpighian tubules and hindgut; excretion into feces | Renal (urine), biliary (feces), pulmonary, and sweat excretion routes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giansanti, M.G.; Frappaolo, A.; Piergentili, R. Drosophila melanogaster: How and Why It Became a Model Organism. Int. J. Mol. Sci. 2025, 26, 7485. https://doi.org/10.3390/ijms26157485
Giansanti MG, Frappaolo A, Piergentili R. Drosophila melanogaster: How and Why It Became a Model Organism. International Journal of Molecular Sciences. 2025; 26(15):7485. https://doi.org/10.3390/ijms26157485
Chicago/Turabian StyleGiansanti, Maria Grazia, Anna Frappaolo, and Roberto Piergentili. 2025. "Drosophila melanogaster: How and Why It Became a Model Organism" International Journal of Molecular Sciences 26, no. 15: 7485. https://doi.org/10.3390/ijms26157485
APA StyleGiansanti, M. G., Frappaolo, A., & Piergentili, R. (2025). Drosophila melanogaster: How and Why It Became a Model Organism. International Journal of Molecular Sciences, 26(15), 7485. https://doi.org/10.3390/ijms26157485








