Trace Elements—Role in Joint Function and Impact on Joint Diseases
Abstract
1. Introduction
2. Effects of Trace Elements on Joint Function
3. Beneficial and Detrimental Interactions Between Trace Elements for the Human Body
4. The Role of Trace Elements in Joint Diseases
4.1. The Role of Trace Elements in Rheumatoid Arthritis
4.1.1. The Role of Fe in Rheumatoid Arthritis
4.1.2. The Role of Cu in Rheumatoid Arthritis
4.1.3. The Role of Co in Rheumatoid Arthritis
4.1.4. The Role of Mn in Rheumatoid Arthritis
4.1.5. The Role of Zn in Rheumatoid Arthritis
4.1.6. The Role of Ag in Rheumatoid Arthritis
4.1.7. The Role of Cd in Rheumatoid Arthritis
4.1.8. The Role of Hg in Rheumatoid Arthritis
4.1.9. The Role of Pb in Rheumatoid Arthritis
4.1.10. The Role of Ni in Rheumatoid Arthritis
4.1.11. The Role of Se in Rheumatoid Arthritis
4.1.12. The Role of B in Rheumatoid Arthritis
4.1.13. The Role of Si in Rheumatoid Arthritis
| Trace Element | Level in Disease | Impact on the Disorder | Additional Information |
|---|---|---|---|
| Iron (Fe) [97,98,99,100,101,102,103,104,105] | Decreased in serum; elevated in the synovial membrane | Patients have Fe deficiency anaemia; Fe deposits may indicate a link between their presence and the pathophysiology of chronic inflammation in people with RA; | Due to the potential involvement of ferroptosis in the pathogenesis of RA, it appears to be a potential therapeutic target; |
| Copper (Cu) [98,107,108,109,110,111,112] | Elevated | Elevated Cu concentration may be related to chronic inflammation, in which there is increased synthesis of interleukin (IL)-1), IL-6, and tumor necrosis factor-alpha (TNF-α), stimulating hepatocytes to synthesise ceruloplasmin; | Cuproptosis may be associated with RA progression, and research is ongoing into the use of drugs that affect cuproptosis to limit the progression of RA; |
| Cobalt (Co) [113,114] | - | Co can generate reactive oxygen species (ROS), leading to oxidative tissue damage; Co (II) nanocomplexes with potential therapeutic applications due to their anti-inflammatory properties; | - |
| Manganese (Mn) [99,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130] | Elevated/unchanged/decreased in serum; decreased in hair and synovial fluid; | Mn superoxide dismutase (MnSOD) reduces swelling; Mn-containing SOD mimetics reduce swelling, decrease damage to joint cartilage and bone, reduce inflammatory cell infiltration, and alleviate joint pain; Mn nanoparticles remove excess ROS, promote the polarisation of macrophages from the M1 to M2 phenotype; Mn nanoparticles can also serve as effective drug carriers; | - |
| Zinc (Zn) [99,107,108,120,121,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148] | Decreased | Zn supplementation reduces the risk of developing osteopenia or osteoporosis; Zn aspartate and Zn citrate lower the levels of rheumatoid factor (RF), antibodies against citrullinated proteins (ACPA) and C-reactive protein (CRP), and reduce joint erosion and osteophyte formation; Zn nanoparticles reduce M1 macrophage infiltration into the synovial membrane, levels of IL-1 and TNF-α, and ACPA; | - |
| Silver (Ag) [149,150,151,152] | - | Ag nanoparticles exhibit therapeutic effects: anti-inflammatory and antioxidant. Reduction of swelling and inflammatory cell infiltration; reduction of pro-inflammatory cytokines such as TNF-α, IL-1β and interleukin-6; shift of macrophage polarisation from M1 to M2 phenotype; reduction in ROS and increase in superoxide dismutase (SOD) and catalase (CAT) activity; inhibition of osteoclast formation; | - |
| Cadmium (Cd) [153,154,155,156,158] | Elevated | Cd increases the production of ACPA and RF; Cd increases ROS production; by inhibiting the growth and inducing apoptosis of hypertrophied inflammatory synoviocytes and inflammatory effector cells Cd shows a potential role in RA treatment; | Cd is strongly associated with the formation of nodules in the lungs of patients with RA; |
| Mercury (Hg) [131,159,160,161,162] | Elevated | Hg disrupts the DNA function of these cells and inhibits collagen synthesis of synovial membrane cells; Hg can cause autoimmune dysfunction and systemic inflammation; | Hg sulphide nanoparticles show a potential role in the RA treatment; |
| Lead (Pb) [99,137,153,159,163] | Elevated | Pb causes oxidative damage, and has inflammatory and immunological properties; Pb also disrupts the function of synoviocytes by inhibiting collagen synthesis and disrupting the DNA function of these cells; | - |
| Nickel (Ni) [99,164,165] | Elevated | Pb disrupts antioxidant function, depletes glutathione, increases ROS production and causes inflammation. | - |
| Selenium (Se) [97,108,118,167,168,169,170,177,178,179,180,181] | Decreased | Se is responsible for the activity of the glutathione peroxidase (GSH-Px) enzyme; Se may also inhibit osteoclastogenesis by suppressing receptor activator of nuclear factor kappa-Β ligand (RANKL) expression on CD4+ T lymphocytes; Se nanoparticles (SeNPs) show potential usefulness in the treatment of RA because of their antioxidant and anti-inflammatory properties; | Excessive Se supplementation can cause symptoms of Se overload, which include hair and nail loss and brittleness, digestive problems, skin rash, reduced haemoglobin levels, garlic breath and nervous system abnormalities; |
| Boron (B) [56,183,184] | Decreased | Reduces inflammatory markers (TNF-α, IL-1α, IL-6, CRP, ESR); improves clinical scores (DAS28, CDAI, SDAI); acts as an adjuvant to biological drugs like etanercept in RA therapy. | Significantly lower B levels were found in serum, bone, and synovial fluid of RA patients; B supplements (e.g., calcium fructoborate, sodium tetraborate) improved symptoms in clinical and preclinical studies. |
| Silicon (Si) [185,186,187,188,189,190,191,192,193,194] | Elevated | Silicon inhibits inflammation and oxidative stress | Chronic exposure to silicon may increase the risk of developing RA |
4.2. The Role of Trace Elements in Osteoarthritis
4.2.1. The Role of Fe in Osteoarthritis
4.2.2. The Role of Cu in Osteoarthritis
4.2.3. The Role of Co in Osteoarthritis
4.2.4. The Role of I in Osteoarthritis
4.2.5. The Role of Mn in Osteoarthritis
4.2.6. The Role of Zn in Osteoarthritis
4.2.7. The Role of Cd in Osteoarthritis
4.2.8. The Role of Hg in Osteoarthritis
4.2.9. The Role of Pb in Osteoarthritis
4.2.10. The Role of Ni in Osteoarthritis
4.2.11. The Role of Se in Osteoarthritis
4.2.12. The Role of B in Osteoarthritis
4.3. The Role of Trace Elements in Psoriatic Arthritis
4.3.1. The Role of Fe and Cu in Psoriatic Arthritis
4.3.2. The Role of Mn in Psoriatic Arthritis
4.3.3. The Role of Zn in Psoriatic Arthritis
4.3.4. The Role of Cd in Psoriatic Arthritis
4.3.5. The Role of Se in Psoriatic Arthritis
| Trace Element | Level in Disease | Impact on the Disorder | Additional Information |
|---|---|---|---|
| Iron (Fe) [282] | Elevated | - | Elevated Fe concentrations were observed only in the form of polyarticular disease; |
| Copper (Cu) [282,283,284,285] | Elevated [282,284,285]/decreased [283] | Possible impact on the pathogenesis of imflammation; potentially useful in monitoring disease activity and treatment progress; | High Cu concentrations were observed in both polyarticular and mono- or oligoarticular forms; Higher Cu concentrations were observed in patients with positive inflammatory markers: erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP); Intravenous administration of methotrexate (MTX) reduces Cu concentrations; |
| Zinc (Zn) [283,284,287,288] | Decreased | The possible therapeutic effect of Zn sulphate-administered orally, it reduces clinical symptoms. However, its role is unclear; | Intravenous administration of MTX causes an increase in Zn concentration; |
| Cadmium (Cd) [289] | Elevated | Possible influense on the pathogenesis of inflammation; | Cd levels were positively linked to serum inflammatory markers like ESR, CRP and cyclooxygenase-3 (COX-2); |
| Selenium (Se) [268,283,285,290] | Decreased | Possible impact on the pathogenesis of inflammation. Potentially useful in monitoring patients during treatment; | Reduced levels of selenoprotein P (SELENOP) and low activity of glutathione peroxidise 3 (GPx3) were also observed; MTX therpy causes an increase in Se concentration. Increased Se levels result in a decrease in CRP and ESR; |
4.4. The Role of Trace Elements in Ankylosing Spondylitis
4.4.1. The Role of Fe in Ankylosing Spondylitis
4.4.2. The Role of Cu in Ankylosing Spondylitis
4.4.3. The Role of Mn in Ankylosing Spondylitis
4.4.4. The Role of Zn in Ankylosing Spondylitis
4.4.5. The Role of Cd in Ankylosing Spondylitis
4.4.6. The Role of Se in Ankylosing Spondylitis
4.5. The Role of Trace Elements in Systemic Lupus Erythematosus
4.5.1. The Role of Fe in Systemic Lupus Erythematosus
4.5.2. The Role of Cu in Systemic Lupus Erythematosus
4.5.3. The Role of Zn in Systemic Lupus Erythematosus
4.5.4. The Role of Cd in Systemic Lupus Erythematosus
4.5.5. The Role of Hg in Systemic Lupus Erythematosus
4.5.6. The Role of Ni in Systemic Lupus Erythematosus
4.5.7. The Role of Se in Systemic Lupus Erythematosus
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| 8-OHdG | 8-hydroxy-2’-deoxyguanosine |
| ACAN | aggrecan core protein |
| ACD | anemia of chronic disease |
| ACPA | anti-citrullinated peptide antibodies |
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| AgNP | Ag nanoparticles |
| ALP | alkaline phosphatase |
| AS | ankylosing spondylitis |
| ASDAS | Ankylosing Spondylitis Disease Activity Score |
| BAK1 | Bcl-2 antagonist/killer 1 |
| BASDAI | Bath Ankylosing Spondylitis Disease Activity Index |
| BAX | bcl-2-associated X protein |
| BAX1 | bcl-2 Associated X-protein 1 |
| BMI | body mass index |
| BMP2 | bone morphogenetic protein 2 |
| BMSCs | bone marrow mesenchymal stem cells |
| B | boron |
| CDAI | clinical disease activity index |
| COX-2 | cyclooxygenase-2 |
| CRGs | cuproptosis-related genes |
| CTDs | connective tissue diseases |
| DAMPs | damage-associated molecular patterns |
| DAS28 | disease activity score 28 |
| DIO2 | iodothyronine deiodinase-2 |
| DMARDs | disease-modifying anti-rheumatic drugs |
| DMT1 | divalent metal transporter 1 |
| DTH | delayed-type hypersensitivity |
| ERAP1 | endoplasmic reticulum aminopeptidase 1 |
| ERK | extracellular signal-regulated kinase |
| ESR | erythrocyte sedimentation rate |
| FGF-1 | fibroblast growth factor 1 |
| FLSs | fibroblast-like synovial cells |
| GPx | glutathione peroxidase |
| GTF | glycosyltransferase |
| HIF-1α | hypoxia-inducible factor 1 |
| HLA-B27 | human leukocyte antigen B27 |
| hs-CRP | high-sensitivity C-reactive protein |
| IA | inflammatory arthritis |
| IDA | iron deficiency anemia |
| IGF-1 | insulin-like growth factor 1 |
| IL | interleukin; |
| IL23R | interleukin-23 receptor |
| IRP1 | iron regulatory protein 1 |
| ISCA2 | iron-sulfur cluster assembly 2 |
| JAK | Janus kinase |
| KBD | Kashin–Beck disease |
| LNT-Se | lentinan-Se |
| MAPK | Mitogen-Activated Protein Kinase; |
| MCP-1 | monocyte chemotactic protein-1 |
| MMP | matrix metalloproteinases |
| MnSOD | manganese superoxide dismutase |
| MR | Mendelian randomization |
| MSCs | mesenchymal stem cells |
| MTs | metallothioneins |
| MTF-1 | metal transcription factor-1 |
| NAMPT | nicotinamide phosphoribosyltransferase |
| NFATc1 | nuclear factor of activated T cells 1 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | natural killer |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| OA | osteoarthritis |
| PsA | psoriatic arthritis |
| RA | rheumatoid arthritis |
| RANKL | receptor activator of nuclear factor kappa-Β ligand; |
| RANKL/RANK/OPG | receptor activator of nuclear factor-κB ligand/receptor activator of nuclear factor-κB/osteoprotegerin |
| r-axSpA | radiographic axial spondyloarthritis |
| RF | rheumatoid factor |
| ROS | reactive oxygen species |
| RUNX2 | runt-related transcription factor 2 |
| SDAI | simple disease activity index |
| SELENOP | selenoprotein P |
| SeMetFa NPs | selenium-methionine-folic acid nanoparticles |
| SeNPs | selenium nanoparticles |
| Si | silicon |
| SIIS | silicone implant incompatibility syndrome |
| SII | systemic immune-inflammation index |
| SLE | systemic lupus erythematosus |
| SOD | superoxide dismutase |
| sTfR | soluble transferrin receptor |
| T3 | triiodothyronine |
| T4 | thyroxine |
| TCA | tricarboxylic acid |
| TGF-β | transforming growth factor β |
| TH | thyroid hormones; |
| TIBC | total iron binding capacity |
| TJR | total joint replacement |
| TR1 | thioredoxin reductase 1 |
| TRAP | tartrate-resistant acid phosphatase |
| TSAT | transferrin saturation |
| Wnt | wingless-related integration site |
| ZIP8 | Zrt- and Irt-like Protein 8 |
| ZNF345 | zinc finger protein 345 |
| NLRP3 | NLR family pyrin domain containing 3 |
| PI3K/Akt | phosphoinositide 3-kinase/Protein kinase B |
| Zip14 | zinc-regulated transporter, iron-regulated transporter-like protein 14 |
References
- Leafblad, N.; Mizels, J.; Tashjian, R.; Chalmers, P. Adhesive Capsulitis. Phys. Med. Rehabil. Clin. N. Am. 2023, 34, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Cardoneanu, A.; Macovei, L.A.; Burlui, A.M.; Mihai, I.R.; Bratoiu, I.; Rezus, I.I.; Richter, P.; Tamba, B.I.; Rezus, E. Temporomandibular Joint Osteoarthritis: Pathogenic Mechanisms Involving the Cartilage and Subchondral Bone, and Potential Therapeutic Strategies for Joint Regeneration. Int. J. Mol. Sci. 2023, 24, 171. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lin, Y.; Jiang, Y.; Yao, Q.; Chen, R.; Zhao, Y.Z.; Kou, L. Recombinant protein drugs-based intra articular drug delivery systems for osteoarthritis therapy. Eur. J. Pharm. Biopharm. 2023, 183, 33–46. [Google Scholar] [CrossRef]
- Hwang, M.C.; Ridley, L.; Reveille, J.D. Ankylosing spondylitis risk factors: A systematic literature review. Clin. Rheumatol. 2021, 40, 3079. [Google Scholar] [CrossRef]
- Semenistaja, S.; Skuja, S.; Kadisa, A.; Groma, V. Healthy and Osteoarthritis-Affected Joints Facing the Cellular Crosstalk. Int. J. Mol. Sci. 2023, 24, 4120. [Google Scholar] [CrossRef]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef]
- Lubrano, E.; Perrotta, F.M. Sex-related differences in psoriatic arthritis. Lancet Rheumatol. 2023, 5, e699–e701. [Google Scholar] [CrossRef]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022, 13, 1007165. [Google Scholar] [CrossRef]
- Gottlieb, A.; Merola, J.F. Psoriatic arthritis for dermatologists. J. Dermatolog. Treat. 2020, 31, 662–679. [Google Scholar] [CrossRef]
- Radu, A.F.; Bungau, S.G. Management of rheumatoid arthritis: An overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Mertz, W. The essential trace elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [PubMed]
- Perrelli, M.; Wu, R.; Liu, D.J.; Lucchini, R.G.; Del Bosque-Plata, L.; Vergare, M.J.; Akhter, M.P.; Ott, J.; Gragnoli, C. Heavy metals as risk factors for human diseases—A Bayesian network approach. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 9275–9310. [Google Scholar] [CrossRef] [PubMed]
- Jannetto, P.J.; Cowl, C.T. Elementary Overview of Heavy Metals. Clin. Chem. 2023, 69, 336–349. [Google Scholar] [CrossRef]
- Karim, A.; Bajbouj, K.; Qaisar, R.; Hall, A.C.; Hamad, M. The role of disrupted iron homeostasis in the development and progression of arthropathy. J. Orthop. Res. 2022, 40, 1243–1250. [Google Scholar] [CrossRef]
- He, Q.; Yang, J.; Pan, Z.; Zhang, G.; Chen, B.; Li, S.; Xiao, J.; Tan, F.; Wang, Z.; Chen, P.; et al. Biochanin A protects against iron overload associated knee osteoarthritis via regulating iron levels and NRF2/System xc-/GPX4 axis. Biomed. Pharmacother. 2023, 157, 113915. [Google Scholar] [CrossRef]
- Sun, K.; Guo, Z.; Hou, L.; Xu, J.; Du, T.; Xu, T.; Guo, F. Iron homeostasis in arthropathies: From pathogenesis to therapeutic potential. Ageing Res. Rev. 2021, 72, 101481. [Google Scholar] [CrossRef]
- Pang, N.; Ding, M.; Yang, H.; Zhong, Q.; Zheng, L.; Luo, D.; Yao, Y. Iron overload causes macrophages to produce a pro-inflammatory phenotype in the synovium of hemophiliac arthritis via the acetyl-p53 pathway. Haemophilia 2024, 30, 195–203. [Google Scholar] [CrossRef]
- Griffith, D.P.; Liff, D.A.; Ziegler, T.R.; Esper, G.J.; Winton, E.F. Acquired copper deficiency: A potentially serious and preventable complication following gastric bypass surgery. Obesity 2009, 17, 827–831. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Xu, L.; Wang, B.; Lin, W.; Luo, Y. Copper regulation of immune response and potential implications for treating orthopedic disorders. Front. Mol. Biosci. 2022, 9, 1065265. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Queally, J.M.; Devitt, B.M.; Butler, J.S.; Malizia, A.P.; Murray, D.; Doran, P.P.; O’Byrne, J.M. Cobalt ions induce chemokine secretion in primary human osteoblasts. J. Orthop. Res. 2009, 27, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Drynda, A.; Drynda, S.; Kekow, J.; Lohmann, C.H.; Bertrand, J. Differential effect of cobalt and chromium ions as well as cocr particles on the expression of osteogenic markers and osteoblast function. Int. J. Mol. Sci. 2018, 19, 3034. [Google Scholar] [CrossRef]
- McCarthy, E.M.; Floyd, H.; Addison, O.; Zhang, Z.J.; Oppenheimer, P.G.; Grover, L.M. Influence of Cobalt Ions on Collagen Gel Formation and Their Interaction with Osteoblasts. ACS Omega 2018, 3, 10129–10138. [Google Scholar] [CrossRef]
- Eltit, F.; Noble, J.; Sharma, M.; Benam, N.; Haegert, A.; Bell, R.H.; Simon, F.; Duncan, C.P.; Garbuz, D.S.; Greidanus, N.V.; et al. Cobalt ions induce metabolic stress in synovial fibroblasts and secretion of cytokines/chemokines that may be diagnostic markers for adverse local tissue reactions to hip implants. Acta Biomater. 2021, 131, 581–594. [Google Scholar] [CrossRef]
- Gunnarsdottir, I.; Dahl, L. Iodine intake in human nutrition: A systematic literature review. Food Nutr. Res. 2012, 56, 19731. [Google Scholar] [CrossRef]
- Kim, H.Y.; Mohan, S. Role and Mechanisms of Actions of Thyroid Hormone on the Skeletal Development. Bone Res. 2013, 1, 146–161. [Google Scholar] [CrossRef]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef]
- Taskozhina, G.; Batyrova, G.; Umarova, G.; Issanguzhina, Z.; Kereyeva, N. The Manganese–Bone Connection: Investigating the Role of Manganese in Bone Health. J. Clin. Med. 2024, 13, 4679. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a therapeutic agent in bone regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Jeyaraman, N.; Nallakumarasamy, A.; Potty, A.G.; Gupta, A.; Iyengar, K.P.; Jain, V.K. Silver nanoparticle technology in orthopaedic infections. World J. Orthop. 2023, 14, 662–668. [Google Scholar] [CrossRef]
- Aurore, V.; Caldana, F.; Blanchard, M.; Kharoubi Hess, S.; Lannes, N.; Mantel, P.Y.; Filgueira, L.; Walch, M. Silver-nanoparticles increase bactericidal activity and radical oxygen responses against bacterial pathogens in human osteoclasts. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 601–607. [Google Scholar] [CrossRef]
- Engström, A.; Skerving, S.; Lidfeldt, J.; Burgaz, A.; Lundh, T.; Samsioe, G.; Vahter, M.; Åkesson, A. Cadmium-induced bone effect is not mediated via low serum 1,25-dihydroxy vitamin D. Environ. Res. 2009, 109, 188–192. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, D.; Shi, X.; Zhao, H.; Liu, Z. Cadmium toxicity: A role in bone cell function and teeth development. Sci. Total Environ. 2021, 769, 144646. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Zhu, G.; Chen, X. Low levels of cadmium exposure affect bone by inhibiting Lgr4 expression in osteoblasts and osteoclasts. J. Trace Elem. Med. Biol. 2022, 73, 127025. [Google Scholar] [CrossRef]
- Fernández-Torres, J.; Plata-Rodríguez, R.; Zamudio-Cuevas, Y.; Martínez-Nava, G.A.; Landa-Solís, C.; Mendoza Soto, L.; Olivos-Meza, A.; Suárez-Ahedo, C.; Barbier, O.C.; Narváez-Morales, J.; et al. Effect of cadmium on the viability on monolayer cultures of synoviocytes, chondrocytes, and Hoffa: A preliminary study. Toxicol. Ind. Health 2020, 36, 940–945. [Google Scholar] [CrossRef]
- Rodríguez, J.; Mandalunis, P.M. A review of metal exposure and its effects on bone health. J. Toxicol. 2018, 2018, 4854152. [Google Scholar] [CrossRef]
- Vianna, A.D.S.; Matos, E.P.D.; Jesus, I.M.D.; Asmus, C.I.R.F.; Câmara, V.D.M. Human exposure to mercury and its hematological effects: A systematic revie. Cad. Saude Publica 2019, 35, e00091618. [Google Scholar] [CrossRef]
- Pamphlett, R.; Jew, S.K. Mercury is taken up selectively by cells involved in joint, bone, and connective tissue disorders. Front. Med. 2019, 6, 168. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Backstrand, J.R. Lead toxicity and pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xiong, C.; Xu, W.; Mei, R.; Cheng, T.; Yu, X. Factors Affecting the Aluminum, Arsenic, Cadmium and Lead Concentrations in the Knee Joint Structures. Front. Public Health 2021, 9, 758074. [Google Scholar] [CrossRef]
- Beier, E.E.; Sheu, T.J.; Dang, D.; Holz, J.D.; Ubayawardena, R.; Babij, P.; Puzas, J.E. Heavy metal ion regulation of gene expression: Mechanisms by which lead inhibits osteoblastic bone-forming activity through modulation of the Wnt/β-catenin signaling pathway. J. Biol. Chem. 2015, 290, 18216–18226. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Aschner, M.; Zhang, F.; Guo, X.; Buha Djordevic, A.; Sotnikova, T.I.; Korobeinikova, T.V.; Domingo, J.L.; Farsky, S.H.P.; Tinkov, A.A. Molecular mechanisms of environmental pollutant-induced cartilage damage: From developmental disorders to osteoarthritis. Arch. Toxicol. 2024, 98, 2763–2796. [Google Scholar] [CrossRef] [PubMed]
- Denkhaus, E.; Salnikow, K. Nickel essentiality, toxicity, and carcinogenicity. Crit. Rev. Oncol. Hematol. 2002, 42, 35–56. [Google Scholar] [CrossRef]
- Kanaji, A.; Orhue, V.; Caicedo, M.S.; Virdi, A.S.; Sumner, D.R.; Hallab, N.J.; Yoshiaki, T.; Sena, K. Cytotoxic effects of cobalt and nickel ions on osteocytes in vitro. J. Orthop. Surg. Res. 2014, 9, 91. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Deng, H.; Liu, H.; Yang, Z.; Bao, M.; Lin, X.; Han, J.; Qu, C. Progress of Selenium Deficiency in the Pathogenesis of Arthropathies and Selenium Supplement for Their Treatment. Biol. Trace Elem. Res. 2022, 200, 4238–4249. [Google Scholar] [CrossRef]
- Honkanen, V.E.A. The Factors Affecting Plasma Glutathione Peroxidase And Selenium In Rheumatoid Arthritis: A multiple linear regression analysis. Scand. J. Rheumatol. 1991, 20, 385–391. [Google Scholar] [CrossRef]
- Zeng, H.; Cao, J.J.; Combs, G.F. Selenium in bone health: Roles in antioxidant protection and cell proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Yang, T.; Lee, S.Y.; Park, K.C.; Park, S.H.; Chung, J.; Lee, S. The Effects of Selenium on Bone Health: From Element to Therapeutics. Molecules 2022, 27, 392. [Google Scholar] [CrossRef]
- Cheng, A.W.M.; Bolognesi, M.; Kraus, V.B. DIO2 modifies inflammatory responses in chondrocytes. Osteoarthr. Cartil. 2012, 20, 440–445. [Google Scholar] [CrossRef]
- Cheng, H.L.; Yen, C.C.; Huang, L.W.; Hu, Y.C.; Huang, T.C.; Hsieh, B.S.; Chang, K.L. Selenium Lessens Osteoarthritis by Protecting Articular Chondrocytes from Oxidative Damage through Nrf2 and NF-κB Pathways. Int. J. Mol. Sci. 2024, 25, 2511. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A. Pivotal role of boron supplementation on bone health: A narrative review. J. Trace Elem. Med. Biol. 2020, 62, 126577. [Google Scholar] [CrossRef] [PubMed]
- Newnham, R.E. Essentiality of boron for healthy bones and joints. Environ. Health Perspect. 1994, 102, 83–85. [Google Scholar] [CrossRef]
- Hunt, C.D. The biochemical effects of physiologic amounts of dietary boron in animal nutrition models. Environ. Health Perspect. 1994, 102, 35–43. [Google Scholar] [CrossRef]
- Li, G.; Cheng, T.; Yu, X. The Impact of Trace Elements on Osteoarthritis. Front. Med. 2021, 8, 771297. [Google Scholar] [CrossRef]
- Korkmaz, M.; Turkmen, R.; Demirel, H.H.; Saritas, Z.K. Effect of Boron on the Repair of Osteochondral Defect and Oxidative Stress in Rats: An Experimental Study. Biol. Trace Elem. Res. 2019, 187, 425–433. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on human health effects of boron. J. Trace Elem. Med. Biol. 2014, 28, 383–387. [Google Scholar] [CrossRef]
- Mladenović, Ž.; Johansson, A.; Willman, B.; Shahabi, K.; Björn, E.; Ransjö, M. Soluble silica inhibits osteoclast formation and bone resorption in vitro. Acta Biomater. 2014, 10, 406–418. [Google Scholar] [CrossRef]
- Kim, E.J.; Bu, S.Y.; Sung, M.K.; Choi, M.K. Effects of silicon on osteoblast activity and bone mineralization of MC3T3-E1 cells. Biol. Trace Elem. Res. 2013, 152, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.R.; Ha, S.W.; Camalier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, C.; Ransjö, M. Orthosilicic acid inhibits human osteoclast differentiation and bone resorption. PLoS ONE 2024, 19, e0312169. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.C.; Wang, X.H.; Wiens, M.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Müller, W.E.G. Silicate modulates the cross-talk between osteoblasts (SaOS-2) and osteoclasts (RAW 264.7 cells): Inhibition of osteoclast growth and differentiation. J. Cell. Biochem. 2012, 113, 3197–3206. [Google Scholar] [CrossRef]
- Carlisle, E.M. In vivo requirement for silicon in articular cartilage and connective tissue formation in the chick. J. Nutr. 1976, 106, 478–484. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon as an essential trace element in animal nutrition. Silicon Biochem. 2007, 121, 123–139. [Google Scholar] [CrossRef]
- Kitala, K.; Tanski, D.; Godlewski, J.; Krajewska-Włodarczyk, M.; Gromadziński, L.; Majewski, M. Copper and Zinc Particles as Regulators of Cardiovascular System Function—A Review. Nutrients 2023, 15, 3040. [Google Scholar] [CrossRef]
- Doguer, C.; Ha, J.H.; Collins, J.F. Intersection of Iron and Copper Metabolism in the Mammalian Intestine and Liver. Compr. Physiol. 2018, 8, 1433. [Google Scholar] [CrossRef]
- Bodiga, S.; Krishnapillai, M.N. Concurrent repletion of iron and zinc reduces intestinal oxidative damage in iron- and zinc-deficient rats. World J. Gastroenterol. 2007, 13, 5707. [Google Scholar] [CrossRef]
- Olivares, M.; Pizarro, F.; Ruz, M.; De Romaña, D.L. Acute inhibition of iron bioavailability by zinc: Studies in humans. BioMetals 2012, 25, 657–664. [Google Scholar] [CrossRef]
- Lönnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef]
- Sandstrom, B.; Davidsson, L.; Cederblad, A.; Lonnerdal, B. Oral iron, dietary ligands and zinc absorption. J. Nutr. 1985, 115, 411–414. [Google Scholar] [CrossRef]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68, 442S–446S. [Google Scholar] [CrossRef]
- Donangelo, C.M.; Woodhouse, L.R.; King, S.M.; Viteri, F.E.; King, J.C. Supplemental zinc lowers measures of iron status in young women with low iron reserves. J. Nutr. 2002, 132, 1860–1864. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Miyakoshi, Y.; Kobayashi, K.; Sakae, K.; Kawasaki, I.; Suzuki, Y.; Tamura, J. Long-term intake of a high zinc diet causes iron deficiency anemia accompanied by reticulocytosis and extra-medullary erythropoiesis. Toxicol. Lett. 2009, 191, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.C.G.; Morgan, E.H. Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol. Trace Elem. Res. 1996, 55, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Karaaslan, O. The Relationship between Iodine and Selenium Levels with Anxiety and Depression in Patients with Euthyroid Nodular Goiter. Oman Med. J. 2020, 35, e161. [Google Scholar] [CrossRef]
- Ćwiertnia, A.; Kozłowski, M.; Cymbaluk-Płoska, A. The Role of Iron and Cobalt in Gynecological Diseases. Cells 2022, 12, 117. [Google Scholar] [CrossRef]
- Czarnek, K.; Terpilowska, S.; Siwicki, A.K. Selected aspects of the action of cobalt ions in the human body. Cent. J. Immunol. 2015, 40, 236. [Google Scholar] [CrossRef] [PubMed]
- Nemec, A.A.; Leikauf, G.D.; Pitt, B.R.; Wasserloos, K.J.; Barchowsky, A. Nickel Mobilizes Intracellular Zinc to Induce Metallothionein in Human Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2008, 41, 69. [Google Scholar] [CrossRef] [PubMed]
- Spiller, H.A. Rethinking mercury: The role of selenium in the pathophysiology of mercury toxicity. Clin. Toxicol. 2018, 56, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.G. A Pharmacological and Toxicological Profile of Silver as an Antimicrobial Agent in Medical Devices. Adv. Pharmacol. Sci. 2010, 2010, 910686. [Google Scholar] [CrossRef]
- Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Rău, G.; Ciocîlteu, M.V.; Mogoşanu, G.D. Zinc-Boron Complex-Based Dietary Supplements for Longevity and Healthy Life. Curr. Health Sci. J. 2023, 49, 381. [Google Scholar] [CrossRef]
- Ghio, A.J.; Tong, H.; Soukup, J.M.; Dailey, L.A.; Cheng, W.Y.; Samet, J.M.; Kesic, M.J.; Bromberg, P.A.; Turi, J.L.; Upadhyay, D.; et al. Sequestration of mitochondrial iron by silica particle initiates a biological effect. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2013, 305, 712–724. [Google Scholar] [CrossRef][Green Version]
- Mercadante, C.J.; Herrera, C.; Pettiglio, M.A.; Foster, M.L.; Johnson, L.C.; Dorman, D.C.; Bartnikas, T.B. The effect of high dose oral manganese exposure on copper, iron and zinc levels in rats. Biometals 2016, 29, 417. [Google Scholar] [CrossRef]
- Rozenberg, J.M.; Kamynina, M.; Sorokin, M.; Zolotovskaia, M.; Koroleva, E.; Kremenchutckaya, K.; Gudkov, A.; Buzdin, A.; Borisov, N. The Role of the Metabolism of Zinc and Manganese Ions in Human Cancerogenesis. Biomedicines 2022, 10, 1072. [Google Scholar] [CrossRef]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef]
- Venetsanopoulou, A.I.; Alamanos, Y.; Voulgari, P.V.; Drosos, A.A. Epidemiology and Risk Factors for Rheumatoid Arthritis Development. Mediterr. J. Rheumatol. 2023, 34, 404–413. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.A. In the Clinic® rheumatoid arthritis. Ann. Intern. Med. 2019, 170, ITC1–ITC15. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid arthritis: A brief overview of the treatment. Med. Princ. Pract. 2019, 27, 501–507. [Google Scholar] [CrossRef]
- Wu, D.; Luo, Y.; Li, T.; Zhao, X.; Lv, T.; Fang, G.; Ou, P.; Li, H.; Luo, X.; Huang, A.; et al. Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment. Front. Immunol. 2022, 13, 1051082. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef]
- Yazar, M.; Sarban, S.; Kocyigit, A.; Isikan, U.E. Synovial Fluid and Plasma Selenium, Copper, Zinc, and Iron Concentrations in Patients with Rheumatoid Arthritis and Osteoarthritis. Biol. Trace Elem. Res. 2005, 106, 123–132. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Shen, J.; Jin, Y.; Chang, C.; Hong, M.; Guo, S.; He, D. Circulating Level of Blood Iron and Copper Associated with Inflammation and Disease Activity of Rheumatoid Arthritis. Biol. Trace Elem. Res. 2023, 201, 90–97. [Google Scholar] [CrossRef]
- Khadim, R.M.; Al-Fartusie, F.S. Evaluation of some trace elements and antioxidants in sera of patients with rheumatoid arthritis: A case–control study. Clin. Rheumatol. 2023, 42, 55–65. [Google Scholar] [CrossRef]
- Khalaf, W.; Al-Rubaie, H.A.; Shihab, S. Studying anemia of chronic disease and iron deficiency in patients with rheumatoid arthritis by iron status and circulating hepcidin. Hematol. Rep. 2019, 11, 16–19. [Google Scholar] [CrossRef]
- Majhi, T. Iron deficiency in rheumatoid arthritic patients especially with in the middle age. Int. J. Syst. Biol. 2010, 2, 1. [Google Scholar]
- Stefanova, K.I.; Delcheva, G.T.; Maneva, A.I.; Batalov, A.Z.; Geneva-Popova, M.G.; Karalilova, R.V.; Simitchiev, K.K. Pathobiochemical Mechanisms Relating Iron Homeostasis with Parameters of Inflammatory Activity and Autoimmune Disorders in Rheumatoid Arthritis. Folia Med. 2018, 60, 124–132. [Google Scholar] [CrossRef]
- Fritz, P.; Saal, J.G.; Wicherek, C.; Kiinig, A.; Laschner, W.; Rautenstrauch, H. Quantitative photometrical assessment of iron deposits in synovial membranes in different joint diseases. Rheumatol. Int. 1996, 15, 211–216. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, Q.; Xi, Y.; Xie, Z.; Shen, J.; Li, Z.; Li, Z.; Qin, D. Ferroptosis in Rheumatoid Arthritis: A Potential Therapeutic Strategy. Front. Immunol. 2022, 13, 779585. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Chen, Y.; Dang, J.; Zeng, D.; Guo, X.; Weng, W.; Zhao, J.; Shi, X.; Chen, J.; et al. Heterogeneous ferroptosis susceptibility of macrophages caused by focal iron overload exacerbates rheumatoid arthritis. Redox Biol. 2024, 69, 103008. [Google Scholar] [CrossRef] [PubMed]
- Vera, E.; Vallvé, J.C.; Linares, V.; Paredes, S.; Ibarretxe, D.; Bellés, M. Serum Levels of Trace Elements (Magnesium, Iron, Zinc, Selenium, and Strontium) are Differentially Associated with Surrogate Markers of Cardiovascular Disease Risk in Patients with Rheumatoid Arthritis. Biol. Trace Elem. Res. 2024, 203, 3570–3584. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Yang, X.; Cai, G.; Fan, D.; Xia, Q.; Liu, L.; Hu, Y.; Ding, N.; Xu, S.; Wang, L.; et al. Serum Levels of Copper and Zinc in Patients with Rheumatoid Arthritis: A Meta-analysis. Biol. Trace Elem. Res. 2015, 168, 1–10. [Google Scholar] [CrossRef]
- Sahebari, M.; Ayati, R.; Mirzaei, H.; Sahebkar, A.; Hejazi, S.; Saghafi, M.; Saadati, N.; Ferns, G.A.; Ghayour-Mobarhan, M. Serum Trace Element Concentrations in Rheumatoid Arthritis. Biol. Trace Elem. Res. 2016, 171, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Strecker, D.; Mierzecki, A.; Radomska, K. Copper levels in patients with rheumatoid arthritis. Ann. Agric. Environ. Med. 2013, 20, 312–316. [Google Scholar]
- Önal, S.; Nazıroğlu, M.; Çolak, M.; Bulut, V.; Flores-Arce, M.F. Effects of different medical treatments on serum copper, selenium and zinc levels in patients with rheumatoid arthritis. Biol. Trace Elem. Res. 2011, 142, 447–455. [Google Scholar] [CrossRef]
- Ullah, Z.; Ullah, M.I.; Hussain, S.; Kaul, H.; Lone, K.P. Determination of Serum Trace Elements (Zn, Cu, and Fe) in Pakistani Patients with Rheumatoid Arthritis. Biol. Trace Elem. Res. 2017, 175, 10–16. [Google Scholar] [CrossRef]
- Han, J.; Luo, J.; Wang, C.; Kapilevich, L.; Zhang, X. Roles and mechanisms of copper homeostasis and cuproptosis in osteoarticular diseases. Biomed. Pharmacother. 2024, 174, 116570. [Google Scholar] [CrossRef] [PubMed]
- Irfan, S.; Rani, A.; Riaz, N.; Arshad, M.; Kashif Nawaz, S. Comparative Evaluation of Heavy Metals in Patients with Rheumatoid Arthritis and Healthy Control in Pakistani Population. Iran. J. Public Health 2017, 46, 626. [Google Scholar] [PubMed]
- El-Sharkawy, R.G.; Taha, R.H.; Ghanem, H.B. Immobilization of novel inorganic nano-complexes onto MWCNT nanomaterials as a novel adsorbent and anti-inflammatory therapy in an induced model of rheumatoid arthritis. Nanotechnology 2020, 31, 305706. [Google Scholar] [CrossRef] [PubMed]
- Hällgren, R.; Svenson, K.; Johansson, E.; Lindh, U. Elevated granulocyte manganese in rheumatoid arthritis and other connective tissue diseases. J. Rheumatol. 1985, 12, 876–880. Available online: https://pubmed.ncbi.nlm.nih.gov/4087268/ (accessed on 7 June 2025).
- Cotzias, G.C.; Papavasiliou, P.S.; Hughes, E.R.; Tang, L.; Borg, D.C. Slow turnover of manganese in active rheumatoid arthritis accelerated by prednisone. J. Clin. Investig. 1968, 47, 992–1001. [Google Scholar] [CrossRef]
- Sarban, S.; Isikan, U.E.; Kocabey, Y.; Kocyigit, A. Relationship between synovial fluid and plasma manganese, arginase, and nitric oxide in patients with rheumatoid arthritis. Biol. Trace Elem. Res. 2007, 115, 97–106. [Google Scholar] [CrossRef]
- Guan, T.; Wu, Z.; Xu, C.; Su, G. The association of trace elements with arthritis in US adults: NHANES 2013–2016. J. Trace Elem. Med. Biol. 2023, 76, 127122. [Google Scholar] [CrossRef]
- Joo, S.H.; Lee, J.; Hutchinson, D.; Song, Y.W. Prevalence of rheumatoid arthritis in relation to serum cadmium concentrations: Cross-sectional study using Korean National Health and Nutrition Examination Survey (KNHANES) data. BMJ Open 2019, 9, e023233. [Google Scholar] [CrossRef]
- Das, D.C.; Jahan, I.; Uddin, M.G.; Hossain, M.M.; Chowdhury, M.A.Z.; Fardous, Z.; Rahman, M.M.; Kabir, A.K.M.H.; Deb, S.R.; Siddique, M.A.B.; et al. Serum CRP, MDA, Vitamin C, and Trace Elements in Bangladeshi Patients with Rheumatoid Arthritis. Biol. Trace Elem. Res. 2021, 199, 76–84. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Brabazon, D.; Naher, S. Association between essential trace and toxic elements in scalp hair samples of smokers rheumatoid arthritis subjects. Sci. Total Environ. 2011, 412–413, 93–100. [Google Scholar] [CrossRef]
- Pasquier, C.; Mach, P.S.; Raichvarg, D.; Sarfati, G.; Amor, B.; Delbarre, F. Manganese-containing superoxide-dismutase deficiency in polymorphonuclear leukocytes of adults with rheumatoid arthritis. Inflammation 1984, 8, 27–32. [Google Scholar] [CrossRef]
- Parizada, B.; Werber, M.M.; Nimrod, A. Protective effects of human recombinant mnsod in adjuvant arthritis and bleomycin-induced lung fibrosis. Free Radic. Res. 1991, 15, 297–301. [Google Scholar] [CrossRef]
- Salvemini, D.; Mazzon, E.; Dugo, L.; Serraino, I.; De Sarro, A.; Caputi, A.P.; Cuzzocrea, S. Amelioration of Joint Disease in a Rat Model of Collagen-Induced Arthritis by M40403, a Superoxide Dismutase Mimetic. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2001, 44, 2909–2921. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Bani, D.; Bencini, A.; Brandi, M.L.; Calosi, L.; Cantore, M.; Carossino, A.M.; Ghelardini, C.; Valtancoli, B.; Failli, P. Therapeutic effects of the superoxide dismutase mimetic compound MnII MeO2A on experimental articular pain in rats. Mediat. Inflamm. 2013, 2013, 905360. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Liu, M.; Yang, H.; Li, X.F.; Liu, S.; Li, K.; Zhang, J.; Zhao, X. Manganese Dioxide-Based pH-Responsive Multifunctional Nanoparticles Deliver Methotrexate for Targeted Rheumatoid Arthritis Treatment. Biomater. Res. 2025, 29, 0187. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.; Zeng, H.; Meng, W.; Liu, G.; Zhang, W.; Zhao, P.; Zhang, Q.; Chen, M.; Chen, J. Manganese-Based Immunomodulatory Nanocomposite with Catalase-Like Activity and Microwave-Enhanced ROS Elimination Ability for Efficient Rheumatoid Arthritis Therapy. Small 2023, 19, 2304610. [Google Scholar] [CrossRef]
- Xia, T.; Zhu, Y.; Li, K.; Hao, K.; Chai, Y.; Jiang, H.; Lou, C.; Yu, J.; Yang, W.; Wang, J.; et al. Microneedles loaded with cerium-manganese oxide nanoparticles for targeting macrophages in the treatment of rheumatoid arthritis. J. Nanobiotechnol. 2024, 22, 103. [Google Scholar] [CrossRef]
- Li, H.; Jin, X.; Chu, B.; Zhang, K.; Qin, X.; Pan, S.; Zhao, Y.; Shi, H.; Zhang, J.; Wang, H.; et al. Inflammation Targeting and Responsive Multifunctional Drug-Delivery Nanoplatforms for Combined Therapy of Rheumatoid Arthritis. Small 2025, 21, 2500113. [Google Scholar] [CrossRef]
- Yang, B.; Yao, H.; Yang, J.; Chen, C.; Guo, Y.; Fu, H.; Shi, J. In Situ Synthesis of Natural Antioxidase Mimics for Catalytic Anti-Inflammatory Treatments: Rheumatoid Arthritis as an Example. J. Am. Chem. Soc. 2022, 144, 314–330. [Google Scholar] [CrossRef]
- Afridi, H.I.; Talpur, F.N.; Kazi, T.G.; Brabazon, D. Estimation of toxic elements in the samples of different cigarettes and their effect on the essential elemental status in the biological samples of Irish smoker rheumatoid arthritis consumers. Environ. Monit. Assess. 2015, 187, 157. [Google Scholar] [CrossRef]
- Duarte, G.B.S.; Callou, K.R.D.A.; Almondes, K.G.D.S.; Rogero, M.M.; Pollak, D.F.; Cozzolino, S.M.F. Evaluation of biomarkers related to zinc nutritional status, antioxidant activity and oxidative stress in rheumatoid arthritis patients. Nutr. Health 2022, 28, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Ala, S.; Shokrzadeh, M.; Pur Shoja, A.M.; Saeedi Saravi, S.S. Zinc and copper plasma concentrations in Rheumatoid arthritis patients from a selected population in Iran. Pak. J. Biol. Sci. 2009, 12, 1041–1044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hassan, W.M. Oxidative DNA Damage and Zinc Status in Patients With Rheumatoid Arthritis in Duhok, Iraq. Cureus 2024, 16, e52860. [Google Scholar] [CrossRef] [PubMed]
- Zoli, A.; Altomonte, L.; Caricchio, R.; Galossi, A.; Mirone, L.; Ruffini, M.P.; Magaró, M. Serum zinc and copper in active rheumatoid arthritis: Correlation with interleukin 1β and tumour necrosis factor α. Clin. Rheumatol. 1998, 17, 378–382. [Google Scholar] [CrossRef]
- Mierzecki, A.; Strecker, D.; Radomska, K. A pilot study on zinc levels in patients with rheumatoid arthritis. Biol. Trace Elem. Res. 2011, 143, 854–862. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Brabazon, D.; Naher, S. Interaction between zinc, cadmium, and lead in scalp hair samples of pakistani and irish smokers rheumatoid arthritis subjects in relation to controls. Biol. Trace Elem. Res. 2012, 148, 139–147. [Google Scholar] [CrossRef]
- Naveh, Y.; Schapira, D.; Ravel, Y.; Geller, E.; Scharf, Y. Zinc metabolism in rheumatoid arthritis: Plasma and urinary zinc and relationship to disease activity. J. Rheumatol. 1997, 24, 643–646. [Google Scholar]
- Yang, M.; Su, Y.; Xu, K.; Wan, X.; Xie, J.; Liu, L.; Yang, Z.; Xu, P. Iron, copper, zinc and magnesium on rheumatoid arthritis: A two-sample Mendelian randomization study. Int. J. Environ. Health Res. 2024, 34, 2776–2789. [Google Scholar] [CrossRef]
- Fang, D.; Jiang, D.; Shi, G.; Song, Y. The association between dietary zinc intake and osteopenia, osteoporosis in patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 2024, 25, 710. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Dong, S.; Yin, H.; Yang, Y.; Xiong, G. Regulatory effect of zinc finger protein A20 on rheumatoid arthritis through NLRP3/ Caspase-1 signaling axis mediating pyroptosis of HFLS- RA cells. Cell. Mol. Biol. 2023, 69, 179–184. [Google Scholar] [CrossRef]
- Hasan, M.; Yadav, P.; Ansari, M.A.; Ali, S.; Khan, H.A. Therapeutic Dose of Zinc Aspartate and Zinc Citrate Attenuates Disease Activity Indices in Rheumatoid Arthritis. Biol. Trace Elem. Res. 2024, 203, 3742–3753. [Google Scholar] [CrossRef]
- Simkin, P.A. Oral Zinc Sulphate in Rheumatoid Arthritis. Lancet 1976, 308, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, P.C.; Mowat, A.G. Zinc sulphate in rheumatoid arthritis. Ann. Rheum. Dis. 1982, 41, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Rasker, J.J.; Kardaun, S.H. Lack of beneficial effect of zinc sulphate in rheumatoid arthritis. Scand. J. Rheumatol. 1982, 11, 168–170. [Google Scholar] [CrossRef]
- Qi, W.; Jin, L.; Wu, C.; Liao, H.; Zhang, M.; Zhu, Z.; Han, W.; Chen, Q.; Ding, C. Treatment with FAP-targeted zinc ferrite nanoparticles for rheumatoid arthritis by inducing endoplasmic reticulum stress and mitochondrial damage. Mater. Today Bio 2023, 21, 100702. [Google Scholar] [CrossRef]
- Gad, S.S.; Fayez, A.M.; Abdelaziz, M.; Abou El-ezz, D. Amelioration of autoimmunity and inflammation by zinc oxide nanoparticles in experimental rheumatoid arthritis. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 1975–1981. [Google Scholar] [CrossRef]
- Ansari, M.M.; Ahmad, A.; Mishra, R.K.; Raza, S.S.; Khan, R. Zinc Gluconate-Loaded Chitosan Nanoparticles Reduce Severity of Collagen-Induced Arthritis in Wistar Rats. ACS Biomater. Sci. Eng. 2019, 5, 3380–3397. [Google Scholar] [CrossRef]
- Singh, A.; Boregowda, S.S.; Moin, A.; Abu Lila, A.S.; Aldawsari, M.F.; Khafagy, E.S.; Alotaibi, H.F.; Jayaramu, R.A. Biosynthesis of Silver Nanoparticles Using Commiphora mukul Extract: Evaluation of Anti-Arthritic Activity in Adjuvant-Induced Arthritis Rat Model. Pharmaceutics 2022, 14, 2318. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, X.; Chen, W.; Chen, P.; Zhu, H.; Yang, B.; Liang, J.; Zeng, F. Amelioration of the rheumatoid arthritis microenvironment using celastrol-loaded silver-modified ceria nanoparticles for enhanced treatment. J. Nanobiotechnol. 2025, 23, 372. [Google Scholar] [CrossRef]
- He, Z.H.; Zou, J.T.; Chen, X.; Gong, J.S.; Chen, Y.; Jin, L.; Liu, Y.W.; Rao, S.S.; Yin, H.; Tan, Y.J.; et al. Ångstrom-scale silver particles ameliorate collagen-induced and K/BxN-transfer arthritis in mice via the suppression of inflammation and osteoclastogenesis. Inflamm. Res. 2023, 72, 2053–2072. [Google Scholar] [CrossRef] [PubMed]
- Disaanayake, D.M.B.T.; Faoagali, J.; Laroo, H.; Hancock, G.; Whitehouse, M. Efficacy of some colloidal silver preparations and silver salts against Proteus bacteria, one possible cause of rheumatoid arthritis. Inflammopharmacology 2014, 22, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, Q.; Peng, S.; Tan, T.; Mei, G.; Chen, H.; Zhao, Y.; Yao, P.; Tang, Y. Associations of blood and urinary heavy metals with rheumatoid arthritis risk among adults in NHANES, 1999–2018. Chemosphere 2022, 289, 133147. [Google Scholar] [CrossRef] [PubMed]
- Frangos, T.; Maret, W. Zinc and cadmium in the aetiology and pathogenesis of osteoarthritis and rheumatoid arthritis. Nutrients 2021, 13, 53. [Google Scholar] [CrossRef]
- Murphy, D.; Sinha-Royle, E.; Bellis, K.; Harrington, C.; Hutchinson, D. Nodular rheumatoid arthritis (RA): A distinct disease subtype, initiated by cadmium inhalation inducing pulmonary nodule formation and subsequent RA–associated autoantibody generation. Med. Hypotheses 2019, 122, 48–55. [Google Scholar] [CrossRef]
- Castañeda, C.R.; García-Martínez, B.; Zamudio-Cuevas, Y.; Fernández-Torres, J.; Flores, K.M. Cadmium exposure and its role in joint disease: A brief review of experimental and population-based evidence. J. Trace Elem. Med. Biol. 2025, 89, 127651. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Qiao, L.; Yang, Z.; He, Y.; Bao, M.; Lin, X.; Han, J. Association of blood cadmium levels and all-cause mortality among adults with rheumatoid arthritis: The NHANES cohort study. J. Trace Elem. Med. Biol. 2024, 83, 127406. [Google Scholar] [CrossRef]
- Bonaventura, P.; Courbon, G.; Lamboux, A.; Lavocat, F.; Marotte, H.; Albarède, F.; Miossec, P. Protective effect of low dose intra-articular cadmium on inflammation and joint destruction in arthritis. Sci. Rep. 2017, 7, 2415. [Google Scholar] [CrossRef]
- Goldberg, R.L.; Kaplan, S.R.; Fuller, G.C. Effect of heavy metals on human rheumatoid synovial cell proliferation and collagen synthesis. Biochem. Pharmacol. 1983, 32, 2763–2766. [Google Scholar] [CrossRef]
- Motts, J.A.; Shirley, D.L.; Silbergeld, E.K.; Nyland, J.F. Novel biomarkers of mercury-induced autoimmune dysfunction: A cross-sectional study in Amazonian Brazil. Environ. Res. 2014, 132, 12–18. [Google Scholar] [CrossRef]
- Gardner, R.M.; Nyland, J.F.; Silva, I.A.; Maria Ventura, A.; Maria de Souza, J.; Silbergeld, E.K. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil: A cross-sectional study. Environ. Res. 2010, 110, 345–354. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Liang, R.; Wu, S.; Xuan, C.; Lv, W.; Li, J. Preparation and anti-inflammatory effect of mercury sulphide nanoparticle-loaded hydrogels. J. Drug Target. 2024, 32, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Bamonti, F.; Fulgenzi, A.; Novembrino, C.; Ferrero, M.E. Metal chelation therapy in rheumathoid arthritis: A case report: Successful management of rheumathoid arthritis by metal chelation therapy. BioMetals 2011, 24, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.M.; Christensen, J.M. Chromium, Nickel and Cadmium in Biological Fluids in Patients with Rheumatoid Arthritis Compared to Healthy Controls. Acta Pharmacol. Toxicol. 1986, 59, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Afridi, H.I.; Kazi, T.G.; Talpur, F.N.; Naher, S.; Brabazon, D. Relationship between toxic metals exposure via cigarette smoking and rheumatoid arthritis. Clin. Lab. 2014, 60, 1735–1745. [Google Scholar] [CrossRef]
- Niedermeier, W.; Creitz, E.E.; Holley, H.L. Trace metal composition of synovial fluid from patients with rheumatoid arthritis. Arthritis Rheum. 1962, 5, 439–444. [Google Scholar] [CrossRef]
- Knekt, P.; Heliövaara, M.; Aho, K.; Alfthan, G.; Marniemi, J.; Aromaa, A. Serum selenium, serum alpha-tocopherol, and the risk of rheumatoid arthritis. Epidemiology 2000, 11, 402–405. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Fan, D.; Xia, Q.; Wang, M.; Pan, F. Common trace metals in rheumatoid arthritis: A systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2019, 56, 81–89. [Google Scholar] [CrossRef]
- Tarp, U.; Hansen, J.C.; Overvad, K.; Thorling, E.B.; Tarp, B.D.; Graudal, H. Glutathione peroxidase activity in patients with rheumatoid arthritis and in normal subjects: Effects of long-term selenium supplementation. Arthritis Rheum. 1987, 30, 1162–1166. [Google Scholar] [CrossRef]
- Qin, J.; Huang, X.; Wang, N.; Zhou, P.; Zhang, H.; Chen, Z.; Liang, K.; Gong, D.; Zeng, Q.; Niu, P.; et al. Supranutritional selenium suppresses ROS-induced generation of RANKL-expressing osteoclastogenic CD4+ T cells and ameliorates rheumatoid arthritis. Clin. Transl. Immunol. 2021, 10, e1338. [Google Scholar] [CrossRef]
- Turk, M.A.; Liu, Y.; Pope, J.E. Non-pharmacological interventions in the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Autoimmun. Rev. 2023, 22, 103323. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Majmasanaye, M.; Faramarzi, F.; Eshraghi, A.; Faress, F. Investigation of the Effect of Oral Selenium on the Reduction of Clinical Symptoms and Joint Pain in Patients With Rheumatoid Arthritis in the Iranian Population. J. Clin. Pharmacol. 2023, 63, 1197–1204. [Google Scholar] [CrossRef]
- Zamani, B.; Taghvaee, F.; Akbari, H.; Mohtashamian, A.; Sharifi, N. Effects of Selenium Supplementation on the Indices of Disease Activity, Inflammation and Oxidative Stress in Patients with Rheumatoid Arthritis: A Randomized Clinical Trial. Biol. Trace Elem. Res. 2024, 202, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Uysal, B.; Sahin, N.; Kara, H. Effects of Nutritional Status and Foods Consumed on Inflammation and Disease Activity in Patients with Rheumatoid Arthritis. Medicina 2024, 60, 1197. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Siderova, V.; Neve, J. Selenium supplementation in rheumatoid arthritis investigated in a double blind, placebo-controlled trial. Scand. J. Rheumatol. 2001, 30, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Doube, A.; Dudson, D.; Wallace, J. Inadequate calcium, folic acid, vitamin E, zinc, and selenium intake in rheumatoid arthritis patients: Results of a dietary survey. Semin. Arthritis Rheum. 1997, 27, 180–185. [Google Scholar] [CrossRef]
- Rehman, A.; John, P.; Bhatti, A. Biogenic selenium nanoparticles: Potential solution to oxidative stress mediated inflammation in rheumatoid arthritis and associated complications. Nanomaterials 2021, 11, 2005. [Google Scholar] [CrossRef]
- Ren, S.X.; Zhang, B.; Lin, Y.; Ma, D.S.; Yan, H. Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 gene expression in a rheumatoid arthritis rat model. Med. Sci. Monit. 2019, 25, 991–1000. [Google Scholar] [CrossRef]
- Zou, B.; Xiong, Z.; Yu, Y.; Shi, S.; Li, X.; Chen, T. Rapid Selenoprotein Activation by Selenium Nanoparticles to Suppresses Osteoclastogenesis and Pathological Bone Loss. Adv. Mater. 2024, 36, 2401620. [Google Scholar] [CrossRef]
- Shinde, V.; Desai, K. Selenium-Methionine-Folic Acid Nanoparticles (SeMetFa NPs) and Its In Vivo Efficacy Against Rheumatoid Arthritis. Biol. Trace Elem. Res. 2024, 202, 2184–2198. [Google Scholar] [CrossRef]
- Vieira, A.T.; Silveira, K.D.; Arruda, M.C.C.; Fagundes, C.T.; Gonçalves, J.L.; Silva, T.A.; Neves, M.J.; Menezes, M.A.B.C.; Nicoli, J.R.; Teixeira, M.M.; et al. Treatment with Selemax®, a selenium-enriched yeast, ameliorates experimental arthritis in rats and mice. Br. J. Nutr. 2012, 108, 1829–1838. [Google Scholar] [CrossRef][Green Version]
- Goldhaber, S.B. Trace element risk assessment: Essentiality vs. toxicity. Regul. Toxicol. Pharmacol. 2003, 38, 232–242. [Google Scholar] [CrossRef]
- Hussain, S.A.; Abood, S.J.; Gorial, F.I. The adjuvant use of calcium fructoborate and borax with etanercept in patients with rheumatoid arthritis: Pilot study. J. Intercult. Ethnopharmacol. 2017, 6, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Trivillin, V.A.; Abramson, D.B.; Bumaguin, G.E.; Bruno, L.J.; Garabalino, M.A.; Monti Hughes, A.; Heber, E.M.; Feldman, S.; Schwint, A.E. Boron neutron capture synovectomy (BNCS) as a potential therapy for rheumatoid arthritis: Boron biodistribution study in a model of antigen-induced arthritis in rabbits. Radiat. Environ. Biophys. 2014, 53, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Prescha, A.; Zabłocka-Słowińska, K.A.; Płaczkowska, S.; Gorczyca, D.; Łuczak, A.; Grajeta, H. Silicon intake and plasma level and their relationships with systemic redox and inflammatory markers in rheumatoid arthritis patients. Adv. Clin. Exp. Med. 2019, 28, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Boudigaard, S.H.; Schlünssen, V.; Vestergaard, J.M.; Søndergaard, K.; Torén, K.; Peters, S.; Kromhout, H.; Kolstad, H.A. Occupational exposure to respirable crystalline silica and risk of autoimmune rheumatic diseases: A nationwide cohort study. Int. J. Epidemiol. 2021, 50, 1213–1226. [Google Scholar] [CrossRef]
- Fireman, E.M.; Fireman Klein, E. Association between silicosis and autoimmune disease. Curr. Opin. Allergy Clin. Immunol. 2024, 24, 45–50. [Google Scholar] [CrossRef]
- Otsuki, T.; Maeda, M.; Murakami, S.; Hayashi, H.; Miura, Y.; Kusaka, M.; Nakano, T.; Fukuoka, K.; Kishimoto, T.; Hyodoh, F.; et al. Immunological Effects of Silica and Asbestos. Cell. Mol. Immunol. 2007, 4, 261–268. [Google Scholar]
- Morotti, A.; Sollaku, I.; Franceschini, F.; Cavazzana, I.; Fredi, M.; Sala, E.; De Palma, G. Systematic Review and Meta-analysis on the Association of Occupational Exposure to Free Crystalline Silica and Rheumatoid Arthritis. Clin. Rev. Allergy Immunol. 2022, 62, 333–345. [Google Scholar] [CrossRef]
- Min, Y.S.; Kim, M.G.; Ahn, Y.S. Rheumatoid arthritis in silica-exposed workers. Int. J. Environ. Res. Public Health 2021, 18, 12776. [Google Scholar] [CrossRef]
- Speck-Hernandez, C.A.; Montoya-Ortiz, G. Silicon, a Possible Link between Environmental Exposure and Autoimmune Diseases: The Case of Rheumatoid Arthritis. Arthritis 2012, 2012, 604187. [Google Scholar] [CrossRef] [PubMed]
- Ilar, A.; Klareskog, L.; Saevarsdottir, S.; Wiebert, P.; Askling, J.; Gustavsson, P.; Alfredsson, L. Occupational exposure to asbestos and silica and risk of developing rheumatoid arthritis: Findings from a Swedish population-based case-control study. RMD Open 2019, 5, e000978. [Google Scholar] [CrossRef] [PubMed]
- Blanc, P.D.; Trupin, L.; Yelin, E.H.; Schmajuk, G. Assessment of Risk of Rheumatoid Arthritis Among Underground Hard Rock and Other Mining Industry Workers in Colorado, New Mexico, and Utah. JAMA Netw. Open 2022, 5, e2236738. [Google Scholar] [CrossRef] [PubMed]
- Cohen Tervaert, J.W.; Kappel, R.M. Silicone implant incompatibility syndrome (SIIS): A frequent cause of ASIA (Shoenfeld’s syndrome). Immunol. Res. 2013, 56, 293–298. [Google Scholar] [CrossRef]
- Fadil, A.; Muaidi, Q.I.; Alayat, M.S.; AlMatrafi, N.A.; Subahi, M.S.; Alshehri, M.A. The Effectiveness of closed kinetic chain exercises in individuals with knee osteoarthritis: A systematic review and meta-analysis. PLoS ONE 2025, 20, e0322475. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Mauri, C.; Cerulli, C.; Grazioli, E.; Minganti, C.; Tranchita, E.; Scotto di Palumbo, A.; Parisi, A. Role of exercise on pain, functional capacity, and inflammatory biomarkers in osteoarthritis: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2025, 68, 101909. [Google Scholar] [CrossRef]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- Wei, W.; Qi, X.; Cheng, B.; He, D.; Qin, X.; Zhang, N.; Zhao, Y.; Chu, X.; Shi, S.; Cai, Q.; et al. An atlas of causal association between micronutrients and osteoarthritis. Prev. Med. 2024, 185, 108063. [Google Scholar] [CrossRef]
- Carroll, G.J.; Breidahl, W.H.; Olynyk, J.K. Characteristics of the arthropathy described in hereditary hemochromatosis. Arthritis Care Res. 2012, 64, 9–14. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S.; Tian, Y.; Si, H.; Zeng, Y.; Wu, Y.; Liu, Y.; Li, M.; Sun, K.; Wu, L.; et al. Genetic Causal Association between Iron Status and Osteoarthritis: A Two-Sample Mendelian Randomization. Nutrients 2022, 14, 3683. [Google Scholar] [CrossRef]
- Radakovich, L.B.; Burton, L.H.; Culver, L.A.; Afzali, M.F.; Marolf, A.J.; Olver, C.S.; Santangelo, K.S. Systemic iron reduction via an iron deficient diet decreases the severity of knee cartilage lesions in the Dunkin-Hartley guinea pig model of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1482–1494. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, J.; Si, H.; Wu, Y.; Zhou, S.; Shen, B. The Role Played by Ferroptosis in Osteoarthritis: Evidence Based on Iron Dyshomeostasis and Lipid Peroxidation. Antioxidants 2022, 11, 1668. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.; Xiao, Y.; Zhang, Y.; Zhang, W.; Doherty, M.; Nestor, J.; Li, C.; Ye, J.; Sha, T.; et al. Combining single-cell RNA sequencing and population-based studies reveals hand osteoarthritis-associated chondrocyte subpopulations and pathways. Bone Res. 2023, 11, 58. [Google Scholar] [CrossRef]
- Pan, Z.; He, Q.; Zeng, J.; Li, S.; Li, M.; Chen, B.; Yang, J.; Xiao, J.; Zeng, C.; Luo, H.; et al. Naringenin protects against iron overload-induced osteoarthritis by suppressing oxidative stress. Phytomedicine 2022, 105, 154330. [Google Scholar] [CrossRef]
- Prasadam, I.; Schrobback, K.; Kranz-Rudolph, B.; Fischer, N.; Sonar, Y.; Sun, A.R.J.; Secondes, E.; Klein, T.; Crawford, R.; Subramaniam, V.N.; et al. Effects of iron overload in human joint tissue explant cultures and animal models. J. Mol. Med. 2024, 103, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Simão, M.; Ea, H.K.; Cohen-Solal, M.; Richette, P.; Branco, J.; Cancela, M.L. Iron overload in a murine model of hereditary hemochromatosis is associated with accelerated progression of osteoarthritis under mechanical stress. Osteoarthr. Cartil. 2016, 24, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wang, C.; Zhu, J.; Gou, Y. Iron overload promotes hemochromatosis-associated osteoarthritis via the mTORC1-p70S6K/4E-BP1 pathway. Int. Immunopharmacol. 2024, 131, 111848. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhang, H.; Bai, L.; Yue, L.; Zhang, W.; Liang, J.; Chang, B.; Yang, Y.; Hu, Z.; Chen, L.; et al. Synovium is a sensitive tissue for mapping the negative effects of systemic iron overload in osteoarthritis: Identification and validation of two potential targets. J. Transl. Med. 2023, 21, 661. [Google Scholar] [CrossRef]
- Xiang, Z.; Mei, H.; Wang, H.; Yao, X.; Rao, J.; Zhang, W.; Xu, A.; Lu, L. Cuproptosis and its potential role in musculoskeletal disease. Front. Cell Dev. Biol. 2025, 13, 1570131. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Jie, T.; Wang, M.; Zhang, S.; Yang, H.; Jian, W.; Dai, D.; Xu, R.; Yue, B.; et al. Association between serum Copper-Zinc-Selenium mixture and multiple health outcomes. Bioact. Mater. 2025, 50, 432–442. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, C.; Sun, Y.; Francis, M.; Ryu, M.S.; Grider, A.; Ye, K. Genetically predicted circulating levels of copper and zinc are associated with osteoarthritis but not with rheumatoid arthritis. Osteoarthr. Cartil. 2021, 29, 1029–1035. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Tian, T.; Wang, B.; Pan, Y. Meta-analysis of the Relationship Between Zinc and Copper in Patients with Osteoarthritis. Biol. Trace Elem. Res. 2024, 203, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhang, Y.; Meng, C.; Li, C.; Jia, D.; Xu, Y. The effect of copper and vitamin D on osteoarthritis outcomes: A Mendelian randomization study. Medicine 2024, 103, e39828. [Google Scholar] [CrossRef] [PubMed]
- Nam, E.; Han, J.; Suh, J.M.; Yi, Y.; Lim, M.H. Link of impaired metal ion homeostasis to mitochondrial dysfunction in neurons. Curr. Opin. Chem. Biol. 2018, 43, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, D.; Gallo, A.; Piccioli, M.; Novellino, E.; Ciofi-Baffoni, S.; Banci, L. [4Fe-4S] cluster assembly in mitochondria and its impairment by copper. J. Am. Chem. Soc. 2017, 139, 719–730. [Google Scholar] [CrossRef]
- Urrutia, P.J.; Aguirre, P.; Tapia, V.; Carrasco, C.M.; Mena, N.P.; Núñez, M.T. Cell death induced by mitochondrial complex I inhibition is mediated by Iron Regulatory Protein 1. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 2202–2209. [Google Scholar] [CrossRef]
- Tandon, M.; Chetla, N.; Hodges, J.; Koul, A.; Dharia, S.; Shah, D.; Samayamanthula, S.; Raghuwanshi, J.S.; Sitsabeshon, A.; Oommen, N.; et al. Mechanical Considerations and Clinical Implications of Joint Arthroplasty Metallosis. Cureus 2024, 16, e76592. [Google Scholar] [CrossRef]
- Matusiewicz, H.; Richter, M. Metal ions release from metallic orthopedic implants exposed to tribocorrosion and electrochemical corrosion conditions in simulated body fluids: Clinical context and in vitro experimental investigations. World J. Adv. Res. Rev. 2022, 14, 261–283. [Google Scholar] [CrossRef]
- Dutta, A.; Nutt, J.; Slater, G.; Ahmed, S. Review: Trunnionosis leading to modular femoral head dissociation. J. Orthop. 2021, 23, 199–202. [Google Scholar] [CrossRef]
- Vendittoli, P.A.; Amzica, T.; Roy, A.G.; Lusignan, D.; Girard, J.; Lavigne, M. Metal Ion Release With Large-Diameter Metal-on-Metal Hip Arthroplasty. J. Arthroplast. 2011, 26, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Stołtny, T.; Dobrakowski, M.; Augustyn, A.; Rokicka, D.; Kasperczyk, S. The concentration of chromium and cobalt ions and parameters of oxidative stress in serum and their impact on clinical outcomes after metaphyseal hip arthroplasty with modular metal heads. J. Orthop. Surg. Res. 2023, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Tower, S.S.; Cho, C.S.; Bridges, R.L.; Gessner, B.D. Prevalence of Cobalturia among Adults with Joint Replacements. JAMA Netw. Open 2021, 4, e2121758. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Meng, H.; Freer, F.; Kwon, J.; Shelton, J.C.; Knight, M.M. Sub-toxic levels of Co2+ are anti-inflammatory and protect cartilage from degradation caused by IL-1β. Clin. Biomech. 2020, 79, 104924. [Google Scholar] [CrossRef]
- Díez-Tercero, L.; Delgado, L.M.; Bosch-Rué, E.; Perez, R.A. Evaluation of the immunomodulatory effects of cobalt, copper and magnesium ions in a pro inflammatory environment. Sci. Rep. 2021, 11, 11707. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Mathieu, F.; Boelaert, M.; Begaux, F.; Suetens, C.; Rivera, M.T.; Nève, J.; Perlmutter, N.; Vanderpas, J. Selenium and iodine supplementation of rural Tibetan children affected by Kashin-Beck osteoarthropathy. Am. J. Clin. Nutr. 2003, 78, 137–144. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Suetens, C.; Mathieu, F.; Begaux, F.; Zhu, D.; Rivera, M.T.; Boelaert, M.; Nève, J.; Perlmutter, N.; Vanderpas, J. Kashin-Beck osteoarthropathy in rural Tibet in relation to selenium and iodine status (abstract). N. Engl. J. Med. 1998, 339, 1112–1120. [Google Scholar] [CrossRef]
- Ren, F.L.; Guo, X.; Zhang, R.J.; Wang, S.J.; Zuo, H.; Zhang, Z.T.; Geng, D.; Yu, Y.; Su, M. Effects of selenium and iodine deficiency on bone, cartilage growth plate and chondrocyte differentiation in two generations of rats. Osteoarthr. Cartil. 2007, 15, 1171–1177. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Shan, L.; Liu, M.; Zhang, Z.; Wang, Z.; Zhang, X.; Meng, H.; Song, Y.; Zhang, W.; et al. Chronic Iodine Intake Excess Damages the Structure of Articular Cartilage and Epiphyseal Growth Plate. Biol. Trace Elem. Res. 2024, 202, 4078–4086. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; He, Y.; Wu, J.; Xia, F.; Li, Q.; Luo, X. Identification for heavy metals exposure on osteoarthritis among aging people and Machine learning for prediction: A study based on NHANES 2011-2020. Front. Public Health 2022, 10, 906774. [Google Scholar]
- Farì, G.; Santagati, D.; Pignatelli, G.; Scacco, V.; Renna, D.; Cascarano, G.; Vendola, F.; Bianchi, F.P.; Fiore, P.; Ranieri, M.; et al. Collagen Peptides, in Association with Vitamin C, Sodium Hyaluronate, Manganese and Copper, as Part of the Rehabilitation Project in the Treatment of Chronic Low Back Pain. Endocr. Metab. Immune Disord.-Drug Targets 2021, 22, 108–115. [Google Scholar] [CrossRef]
- Shi, H.; Wang, H.; Yu, M.; Su, J.; Zhao, Z.; Gao, T.; Zhang, Q.; Wei, Y. Serum trace elements and osteoarthritis: A meta-analysis and Mendelian randomization study. J. Trace Elem. Med. Biol. 2024, 86, 127520. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Nava, G.A.; Mendoza-Soto, L.; Fernández-Torres, J.; Zamudio-Cuevas, Y.; Reyes-Hinojosa, D.; Plata-Rodríguez, R.; Olivos-Meza, A.; Ruíz-Huerta, E.A.; Armienta-Hernández, M.A.; Hernández-Álvarez, E.; et al. Effect of cadmium on the concentration of essential metals in a human chondrocyte micromass culture. J. Trace Elem. Med. Biol. 2020, 62, 126614. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moreno, M.; Soto-Hermida, A.; Pertega, S.; Oreiro, N.; Fernandez-Lopez, C.; Rego-Perez, I.; Blanco, F.J. Mitochondrial DNA (mtDNA) haplogroups and serum levels of anti-oxidant enzymes in patients with osteoarthritis. BMC Musculoskelet. Disord. 2011, 12, 264. [Google Scholar] [CrossRef]
- Ostalowska, A.; Birkner, E.; Wiecha, M.; Kasperczyk, S.; Kasperczyk, A.; Kapolka, D.; Zon-Giebel, A. Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthr. Cartil. 2006, 14, 139–145. [Google Scholar] [CrossRef]
- Zang, Z.S.; Xu, Y.M.; Lau, A.T.Y. Molecular and pathophysiological aspects of metal ion uptake by the zinc transporter ZIP8 (SLC39A8). Toxicol. Res. 2016, 5, 987–1002. [Google Scholar] [CrossRef]
- Yang, W.M.; Lv, J.F.; Wang, Y.Y.; Xu, Y.M.; Lin, J.; Liu, J.; Chen, J.J.; Wang, X.Z. The Daily Intake Levels of Copper, Selenium, and Zinc Are Associated with Osteoarthritis but Not with Rheumatoid Arthritis in a Cross-sectional Study. Biol. Trace Elem. Res. 2023, 201, 5662–5670. [Google Scholar] [CrossRef]
- Zeng, W.; Hong, E.; Ye, W.; Ma, L.; Cun, D.; Huang, F.; Jiang, Z. Mendelian randomization of serum micronutrients and osteoarthritis risk: Focus on zinc. Nutr. J. 2025, 24, 38. [Google Scholar] [CrossRef]
- Qu, X.; Yang, H.; Yu, Z.; Jia, B.; Qiao, H.; Zheng, Y.; Dai, K. Serum zinc levels and multiple health outcomes: Implications for zinc-based biomaterials. Bioact. Mater. 2020, 5, 410–422. [Google Scholar] [CrossRef]
- Jakoniuk, M.; Biegaj, M.; Kochanowicz, J.; Łysoń, T.; Lankau, A.; Wilkiel, M.; Socha, K. Relationship between Selected Micronutrient Concentrations, Total Antioxidant Status, Pain Severity, and the Image of 1H MR Spectroscopy in Degenerative Spine Disease: A Case-Control Study. J. Clin. Med. 2022, 11, 5586. [Google Scholar] [CrossRef]
- Choi, W.S.; Chun, J.S. Upregulation of lipocalin-2 (LCN2) in osteoarthritic cartilage is not necessary for cartilage destruction in mice. Osteoarthr. Cartil. 2017, 25, 401–405. [Google Scholar] [CrossRef]
- Lee, M.; Won, Y.; Shin, Y.; Kim, J.H.; Chun, J.S. Reciprocal activation of hypoxia-inducible factor (HIF)-2α and the zinc-ZIP8-MTF1 axis amplifies catabolic signaling in osteoarthritis. Osteoarthr. Cartil. 2016, 24, 134–145. [Google Scholar] [CrossRef]
- Li, L.; Cao, J.; Li, L.; Wu, G.; Xiao, J. Associations of Blood Cadmium Levels With Osteoarthritis Among US Adults in NHANES 2013-2018. J. Occup. Environ. Med. 2024, 66, e333–e337. [Google Scholar] [CrossRef] [PubMed]
- Jakoniuk, M.; Kochanowicz, J.; Lankau, A.; Wilkiel, M.; Socha, K. Concentration of Selected Macronutrients and Toxic Elements in the Blood in Relation to Pain Severity and Hydrogen Magnetic Resonance Spectroscopy in People with Osteoarthritis of the Spine. Int. J. Environ. Res. Public Health 2022, 19, 11377. [Google Scholar] [CrossRef] [PubMed]
- Eduviges, Z.C.Y.; Martínez-Nava, G.; Reyes-Hinojosa, D.; Mendoza-Soto, L.; Fernández-Torres, J.; López-Reyes, A.; Olivos-Meza, A.; Armienta-Hernández, M.A.; Ruíz-Huerta, E.A.; de Jesús González-Guadarrama, M.; et al. Impact of cadmium toxicity on cartilage loss in a 3D in vitro model. Environ. Toxicol. Pharmacol. 2020, 74, 103307. [Google Scholar] [CrossRef] [PubMed]
- Bodo, M.; Balloni, S.; Lumare, E.; Bacci, M.; Calvitti, M.; Dell’Omo, M.; Murgia, N.; Marinucci, L. Effects of sub-toxic Cadmium concentrations on bone gene expression program: Results of an in vitro study. Toxicol. In Vitro 2010, 24, 1670–1680. [Google Scholar] [CrossRef]
- Chen, X.; Wang, G.; Li, X.; Gan, C.; Zhu, G.; Jin, T.; Wang, Z. Environmental level of cadmium exposure stimulates osteoclasts formation in male rats. Food Chem. Toxicol. 2013, 60, 530–535. [Google Scholar] [CrossRef]
- Urzì Brancati, V.; Aliquò, F.; Freni, J.; Pantano, A.; Galipò, E.; Puzzolo, D.; Minutoli, L.; Marini, H.R.; Campo, G.M.; D’Ascola, A. The Effects of Seleno-Methionine in Cadmium-Challenged Human Primary Chondrocytes. Pharmaceuticals 2024, 17, 936. [Google Scholar] [CrossRef]
- Marsit, C.J. Influence of environmental exposure on human epigenetic regulation. J. Exp. Biol. 2015, 218, 71–79. [Google Scholar] [CrossRef]
- Chauhan, S.; Dunlap, K.; Duffy, L.K. Effects of methylmercury and theaflavin digallate on adipokines in mature 3T3-L1 adipocytes. Int. J. Mol. Sci. 2019, 20, 2755. [Google Scholar] [CrossRef]
- Zioła-Frankowska, A.; Dąbrowski, M.; Kubaszewski, Ł.; Rogala, P.; Kowalski, A.; Frankowski, M. An analysis of factors affecting the mercury content in the human femoral bone. Environ. Sci. Pollut. Res. 2017, 24, 547–557. [Google Scholar] [CrossRef]
- Kosik-Bogacka, D.I.; Lanocha-Arendarczyk, N.; Kot, K.; Ciosek, Z.; Zietek, P.; Karaczun, M.; Pilarczyk, B.; Tomza-Marciniak, A.; Podlasinska, J.; Kalisinska, E.; et al. Effects of biological factors and health condition on mercury and selenium concentrations in the cartilage, meniscus and anterior cruciate ligament. J. Trace Elem. Med. Biol. 2017, 44, 201–208. [Google Scholar] [CrossRef]
- DeMartini, J.; Wilson, A.; Powell, J.S.; Powell, C.S. Lead Arthropathy and Systemic Lead Poisoning from an Intraarticular Bullet. Am. J. Roentgenol. 2001, 176, 1103–1104. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.E.; Shi, X.A.; Schwartz, T.A.; Chen, J.C.; Renner, J.B.; Caldwell, K.L.; Helmick, C.G.; Jordan, J.M. Whole blood lead levels are associated with radiographic and symptomatic knee osteoarthritis: A cross-sectional analysis in the Johnston County Osteoarthritis Project. Arthritis Res. Ther. 2011, 13, R37. [Google Scholar] [CrossRef] [PubMed]
- Khaled Abo-Elmaaty, R. The correlation between blood lead levels and knee osteoarthritis: A preliminary Egyptian study. Int. J. Clin. Rheumatol. 2017, 12, 83. [Google Scholar]
- Carmouche, J.J.; Puzas, J.E.; Zhang, X.; Tiyapatanaputi, P.; Cory-Slechta, D.A.; Gelein, R.; Zuscik, M.; Rosier, R.N.; Boyce, B.F.; O’Keefe, R.J.; et al. Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency. Environ. Health Perspect. 2005, 113, 749–755. [Google Scholar] [CrossRef]
- Holz, J.D.; Beier, E.; Sheu, T.J.; Ubayawardena, R.; Wang, M.; Sampson, E.R.; Rosier, R.N.; Zuscik, M.; Puzas, J.E. Lead induces an osteoarthritis-like phenotype in articular chondrocytes through disruption of TGF-β signaling. J. Orthop. Res. 2012, 30, 1760–1766. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Khan, M. Interplay of reactive oxygen species and nitric oxide in the pathogenesis of experimental lead-induced hypertension. Clin. Exp. Pharmacol. Physiol. 2007, 34, 920–925. [Google Scholar] [CrossRef]
- Yan, R.; Ding, J.; Yang, Q.; Zhang, X.; Han, J.; Jin, T.; Shi, S.; Wang, X.; Zheng, Y.; Li, H.; et al. Lead acetate induces cartilage defects and bone loss in zebrafish embryos by disrupting the GH/IGF-1 axis. Ecotoxicol. Environ. Saf. 2023, 253, 114666. [Google Scholar] [CrossRef]
- Zoeger, N.; Roschger, P.; Hofstaetter, J.G.; Jokubonis, C.; Pepponi, G.; Falkenberg, G.; Fratzl, P.; Berzlanovich, A.; Osterode, W.; Streli, C.; et al. Lead accumulation in tidemark of articular cartilage. Osteoarthr. Cartil. 2006, 14, 906–913. [Google Scholar] [CrossRef]
- Dahlstrand, H.; Stark, A.; Anissian, L.; Hailer, N.P. Elevated Serum Concentrations of Cobalt, Chromium, Nickel, and Manganese After Metal-On-Metal Alloarthroplasty of the Hip: A Prospective Randomized Study. J. Arthroplast. 2009, 24, 837–845. [Google Scholar] [CrossRef]
- Cracchiolo, A.; Revell, P. Metal Concentration in Synovial Fluids of Patients with Prosthetic Knee Arthroplasty. Clin. Orthop. Relat. Res. 1982, 170, 169–174. [Google Scholar] [CrossRef]
- Savarino, L.; Cadossi, M.; Chiarello, E.; Fotia, C.; Greco, M.; Baldini, N.; Giannini, S. How do metal ion levels change over time in hip resurfacing patients? A cohort study. Sci. World J. 2014, 2014, 291925. [Google Scholar] [CrossRef] [PubMed]
- Brodziak-Dopierała, B.; Kwapuliński, J.; Sobczyk, K.; Kowol, J. The occurrence of nickel and other elements in tissues of the hip joint. Ecotoxicol. Environ. Saf. 2011, 74, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, P.; Vejvodova, M.; Vaculovic, T.; Slaninova, I.; Emmer, J.; Tomas, T.; Ryba, L.; Burda, J.; Pavkova Goldbergova, M. Cytotoxic effects and comparative analysis of Ni ion uptake by osteoarthritic and physiological osteoblasts. Sci. Rep. 2024, 14, 16133. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Jeyaraman, N.; Ramasubramanian, S.; Hari, R.; Jeyaraman, M. Predictive Modeling of Osteoarthritis Using Biochemical Markers: A Cross-Sectional Analysis. J. Orthop. Case Rep. 2024, 14, 237–245. [Google Scholar] [CrossRef]
- Wahl, L.; Samson Chillon, T.; Seemann, P.; Ohrndorf, S.; Ochwadt, R.; Becker, W.; Schomburg, L.; Hoff, P. Serum selenium, selenoprotein P and glutathione peroxidase 3 in rheumatoid, psoriatic, juvenile idiopathic arthritis, and osteoarthritis. J. Nutr. Biochem. 2025, 135, 109776. [Google Scholar] [CrossRef]
- Ba, Y.; Sun, L.; Zuo, J.; Yu, S.Y.; Yang, S.; Ding, L.M.; Feng, Z.C.; Li, Z.Y.; Zhou, G.Y.; Yu, F.F. Association of oxidative stress and Kashin–Beck disease integrated Meta and Bioinformatics analysis. Osteoarthr. Cartil. 2022, 30, 1606–1615. [Google Scholar] [CrossRef]
- Cao, J.J.; Gregoire, B.R.; Zeng, H. Selenium deficiency decreases antioxidative capacity and is detrimental to bone microarchitecture in mice. J. Nutr. 2012, 142, 1526–1531. [Google Scholar] [CrossRef]
- Mcclung, J.P.; Roneker, C.A.; Mu, W.; Lisk, D.J.; Langlais, P.; Liu, F.; Lei, X.G. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc. Natl. Acad. Sci. USA 2004, 101, 8852–8857. [Google Scholar] [CrossRef]
- Masuko, K.; Schomburg, L.; Aleemuddin Quamri, M.; Tan, Y. A national cross-sectional analysis of selenium intake and risk of osteoarthritis: NHANES 2003–2016. Front. Public Health 2023, 10, 1047605. [Google Scholar]
- Pizzorno, L. Nothing Boring About Boron. Integr. Med. 2015, 14, 35. [Google Scholar]
- Nielsen, F.H.; Eckhert, C.D. Boron. Adv Nutr. 2020, 11, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Gaby, A.R. Natural treatments for osteoarthritis. Altern. Med. Rev. 1999, 4, 330–341. [Google Scholar] [PubMed]
- Helliwell, T.R.; Kelly, S.A.; Walsh, H.P.J.; Klenerman, L.; Haines, J.; Clark, R.; Roberts, N.B. Elemental Analysis of Femoral Bone From Patients With Fractured Neck of Femur or Osteoarthrosis. Bone 1996, 18, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.U.; Lingxia, Z.; Schrauzer, G.N.; Xiong, G. Selenium, Boron, and Germanium Deficiency in the Etiology of Kashin-Beck Disease. Biol. Trace Elem. Res. 2000, 77, 193–197. [Google Scholar] [CrossRef]
- Fang, W.; Wu, P.; Hu, R.; Huang, Z. Environmental Se-Mo-B Deficiency and its possible effects on crops and Keshan-Beck disease (KBD) in the Chousang area, Yao County, Shaanxi Province, China. Environ. Geochem. Health 2003, 25, 267–280. [Google Scholar] [CrossRef]
- Karmacharya, P.; Chakradhar, R.; Ogdie, A. The epidemiology of psoriatic arthritis: A literature review. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101692. [Google Scholar] [CrossRef]
- Kishimoto, M.; Deshpande, G.A.; Fukuoka, K.; Kawakami, T.; Ikegaya, N.; Kawashima, S.; Komagata, Y.; Kaname, S. Clinical features of psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 2021, 35, 101670. [Google Scholar] [CrossRef]
- Azuaga, A.B.; Ramírez, J.; Cañete, J.D. Psoriatic Arthritis: Pathogenesis and Targeted Therapies. Int. J. Mol. Sci. 2023, 24, 4901. [Google Scholar] [CrossRef]
- Oriente, P.; Scarpa, R.; Pucino, A.; Torella, M.; Riccio, A.; Oriente, C.B. Supportive laboratory findings in psoriatic arthritis. Clin. Rheumatol. 1984, 3, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Tsiami, S.; Chillon, T.S.; Rousis, E.; Kisters, K.; Karmeli, S.; Kiltz, U.; Schomburg, L.; Baraliakos, X. Trace Element Deficiency in Axial Spondyloarthritis and Psoriatic Arthritis in Relation to Markers of Inflammation and Remission. Int. J. Mol. Sci. 2025, 26, 4924. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, X.; Liu, T.; Liu, Z. The Effect of Methotrexate on Serum Levels of Trace/Mineral Elements in Patients with Psoriatic Arthritis. Biol. Trace Elem. Res. 2021, 199, 4498–4503. [Google Scholar] [CrossRef] [PubMed]
- Fatty acids and antioxidant micronutrients in psoriatic arthritis. J. Reumathol. 1995, 22, 103–108. Available online: https://pubmed.ncbi.nlm.nih.gov/7699656/ (accessed on 2 June 2025).
- Yen, J.H.; Tsai, W.C.; Lin, C.H.; Ou, T.T.; Hu, C.J.; Liu, H.W. Manganese Superoxide Dismutase Gene Polymorphisms in Psoriatic Arthritis. Dis. Markers 2004, 19, 263. [Google Scholar] [CrossRef][Green Version]
- Clemmensen, O.J.; Siggaard-Andersen, J.; Worm, A.M.; Stahl, D.; Frost, F.; Bloch, I. Psoriatic arthritis treated with oral zinc sulphate. Br. J. Dermatol. 1980, 103, 411–415. [Google Scholar] [CrossRef]
- Leibovici, V.; Statter, M.; Weinrauch, L.; Tzfoni, E.; Matzner, Y. Effect of zinc therapy on neutrophil chemotaxis in psoriasis. Isr. J. Med. Sci. 1990, 26, 306–309. Available online: https://pubmed.ncbi.nlm.nih.gov/2380030/ (accessed on 29 May 2025).
- Markiewicz-Górka, I.; Chowaniec, M.; Martynowicz, H.; Wojakowska, A.; Jaremków, A.; Mazur, G.; Wiland, P.; Pawlas, K.; Poręba, R.; Gać, P. Cadmium Body Burden and Inflammatory Arthritis: A Pilot Study in Patients from Lower Silesia, Poland. Int. J. Environ. Res. Public Health 2022, 19, 3099. [Google Scholar] [CrossRef]
- Deyab, G.; Hokstad, I.; Aaseth, J.; Småstuen, M.C.; Whist, J.E.; Agewall, S.; Lyberg, T.; Tveiten, D.; Hjeltnes, G.; Zibara, K.; et al. Effect of anti-rheumatic treatment on selenium levels in inflammatory arthritis. J. Trace Elem. Med. Biol. 2018, 49, 91–97. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, S.; Shao, F.; Sun, Y. Ankylosing spondylitis: From pathogenesis to therapy. Int. Immunopharmacol. 2025, 145, 113709. [Google Scholar] [CrossRef]
- Givian, A.; Azizan, A.; Jamshidi, A.; Mahmoudi, M.; Farhadi, E. Iron metabolism in rheumatic diseases. J. Transl. Autoimmun. 2025, 10, 100267. [Google Scholar] [CrossRef]
- Chang, S.; Tang, M.; Zhang, B.; Xiang, D.; Li, F. Ferroptosis in inflammatory arthritis: A promising future. Front. Immunol. 2022, 13, 955069. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, L.; Yang, Z.; Wu, C.; Xie, W.; Zhang, J.; Li, W.; Li, W.; Gao, Y.; Zhang, T. Association between serum iron status and the risk of five bone and joint-related diseases: A Mendelian randomization analysis. Front. Endocrinol. 2024, 15, 1364375. [Google Scholar] [CrossRef]
- Yang, M.; Yu, H.; Xu, K.; Xie, J.; Zheng, H.; Feng, R.; Wang, J.; Xu, P. No evidence of a genetic causal relationship between ankylosing spondylitis and iron homeostasis: A two-sample Mendelian randomization study. Front. Nutr. 2023, 10, 1047640. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, H.; Zhang, Y.; Liu, Y.; Zhu, G.; Zhao, W.; Shang, Q.; He, J.; Zhou, Z.; Shen, G.; et al. Dry and wet experiments reveal diagnostic clustering and immune landscapes of cuproptosis patterns in patients with ankylosing spondylitis. Int. Immunopharmacol. 2024, 127, 111326. [Google Scholar] [CrossRef]
- Wei, B.; Wang, S.; Li, S.; Gu, Q.; Yue, Q.; Tang, Z.; Zhang, J.; Liu, W. Unveiling Cuproptosis-Driven Molecular Clusters and Immune Dysregulation in Ankylosing Spondylitis. J. Inflamm. Res. 2025, 18, 863–882. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Deng, Y.; Ma, Y.; Shao, M.; Ni, M.; Zhang, T.; Wang, X.; Xu, S.; Chen, Y.; Xu, S.; et al. Common mineral nutrients in ankylosing spondylitis: A 2-sample Mendelian randomization study. Int. J. Rheum. Dis. 2022, 25, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.H.; Tsai, W.C.; Chen, C.J.; Lin, C.H.; Ou, T.T.; Hu, C.J.; Liu, H.W. Cytochrome P450 1A1 and manganese superoxide dismutase genes polymorphisms in ankylosing spondylitis. Immunol. Lett. 2003, 88, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Shiue, I. Relationship of environmental exposures and ankylosing spondylitis and spinal mobility: US NHAENS, 2009-2010. Int. J. Environ. Health Res. 2015, 25, 322–329. [Google Scholar] [CrossRef]
- Hulander, E.; Zverkova Sandström, T.; Beckman Rehnman, J.; Law, L.; Söderberg, S.; Forsblad-d’Elia, H. Patients with radiographic axial spondylarthritis have an impaired dietary intake—A cross-sectional study with matched controls from northern Sweden. Arthritis Res. Ther. 2023, 25, 142. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, J.C.; Mantilla, M.J.; Pulido, S.; Isaza, J.R.; Tuta, E.; Agudelo, C.A.; Londono, J. A Practical Overview of the Articular Manifestations of Systemic Lupus Erythematosus. Cureus 2023, 15, e44964. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, F.; Perricone, C.; Cipriano, E.; Massaro, L.; Natalucci, F.; Capalbo, G.; Leccese, I.; Bogdanos, D.; Spinelli, F.R.; Alessandri, C.; et al. Joint involvement in systemic lupus erythematosus: From pathogenesis to clinical assessment. Semin. Arthritis Rheum. 2017, 47, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Carini, M.; Fredi, M.; Cavazzana, I.; Bresciani, R.; Ferrari, F.; Monti, E.; Franceschini, F.; Biasiotto, G. Frequency Evaluation of the Interleukin-6 −174G>C Polymorphism and Homeostatic Iron Regulator (HFE) Mutations as Disease Modifiers in Patients Affected by Systemic Lupus Erythematosus and Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 16300. [Google Scholar] [CrossRef]
- Wincup, C.; Sawford, N.; Rahman, A. Pathological mechanisms of abnormal iron metabolism and mitochondrial dysfunction in systemic lupus erythematosus. Expert Rev. Clin. Immunol. 2021, 17, 957–967. [Google Scholar] [CrossRef]
- Dong, W.; Xu, H.; Wei, W.; Ning, R.; Chang, Y. Advances in the study of ferroptosis and its relationship to autoimmune diseases. Int. Immunopharmacol. 2024, 140, 112819. [Google Scholar] [CrossRef]
- Zheng, Y.; Yan, F.; He, S.; Luo, L. Targeting ferroptosis in autoimmune diseases: Mechanisms and therapeutic prospects. Autoimmun. Rev. 2024, 23, 103640. [Google Scholar] [CrossRef]
- Lai, B.; Wu, C.H.; Wu, C.Y.; Luo, S.F.; Lai, J.H. Ferroptosis and Autoimmune Diseases. Front. Immunol. 2022, 13, 916664. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Y.; Wang, Y.; Shen, H. Disrupting the immune homeostasis: The emerging role of macrophage ferroptosis in autoimmune diseases. Int. Immunopharmacol. 2025, 157, 114745. [Google Scholar] [CrossRef]
- Chang, J.; Wu, Q.; Wang, G. Research advancements in the association between prevalent trace metals and connective tissue diseases. Environ. Geochem. Health 2025, 47, 16. [Google Scholar] [CrossRef]
- Tóth, C.N.; Baranyai, E.; Csípő, I.; Tarr, T.; Zeher, M.; Posta, J.; Fábián, I. Elemental Analysis of Whole and Protein Separated Blood Serum of Patients with Systemic Lupus Erythematosus and Sjögren’s Syndrome. Biol. Trace Elem. Res. 2017, 179, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V.; Reynolds, T.; Bjørklund, G. Increased frequency of delayed type hypersensitivity to metals in patients with connective tissue disease. J. Trace Elem. Med. Biol. 2015, 31, 230–236. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Aaseth, J. Delayed-type hypersensitivity to metals in connective tissue diseases and fibromyalgia. Environ. Res. 2018, 161, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Sun, X.; Guo, Y.; Shao, K.; Qian, Y.; Huang, H.; Liu, B.; Wen, C.; Mao, Y. Genetically determined selenium concentrations and risk for autoimmune diseases. Nutrition 2021, 91–92, 111391. [Google Scholar] [CrossRef] [PubMed]
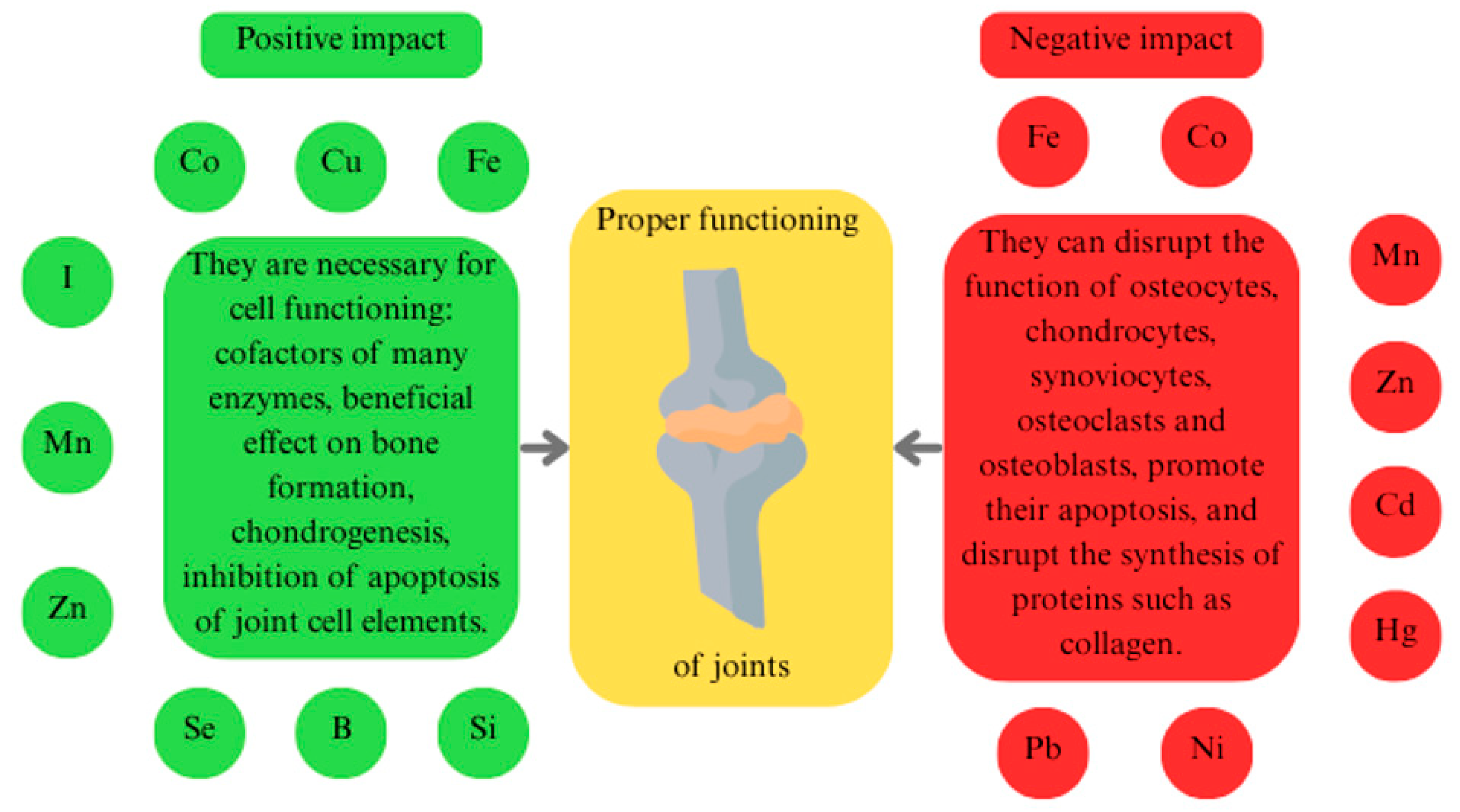
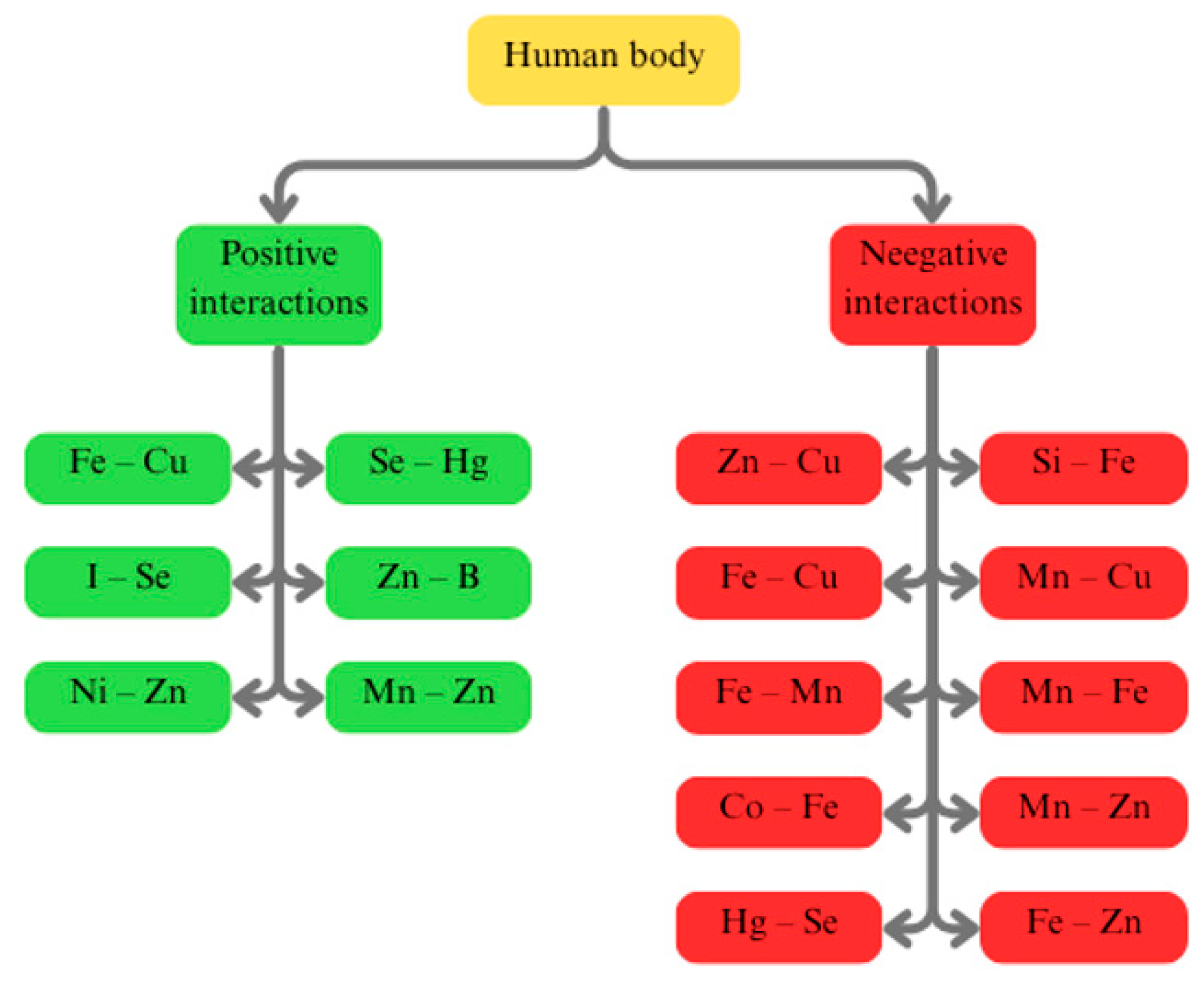
| Trace Element | Level in Disease | Impact on the Disorder | Additional Information |
|---|---|---|---|
| Iron (Fe) [199,200,201,202,203,204,205,206,207,208,209,210] | Elevated | Fe affects ferroptosis of chondrocytes, lipid peroxidation, overexpression of metalloproteinases, and increased release of proinflammatory cytokines; | - |
| Copper (Cu) [97,211,212,213,214,215,216,217,218] | Elevated | Cu affects cuproptosis of chondrocytes, increased reactive oxygen species (ROS) production, disruption in the tricarboxylic acid cycle, maturation of Fe-sulfur clusters, and changes in pro-inflammatory cytokine release; | - |
| Cobalt (Co) [219,220,221,222,223,224,225,226] | Normal | In low concentrations, Co has anti-inflammatory effects; in high concentrations, Co can cause local and systemic metallosis; | Co accumulates as a result of joint replacement; |
| Iodine (I) [227,228,229,230] | Normal | I participates in the formation of bones and cartilage; | Dietary I deficiency is involved in the development of KBD; |
| Manganese (Mn) [58,118,231,232,233,234,235,236,237] | Decreased | Mn is a cofactor of superoxide dismutase 2 (SOD2), which neutralises ROS, and a cofactor of glycosyltransferase (GTF), which participates in the formation of cartilage components; | - |
| Zinc (Zn) [154,214,233,238,239,240,241,242,243] | Elevated | Zn participates in the Zrt- and Irt-like Protein 8 (zinc-ZIP8)-MTF1- hypoxia-inducible factor 2 alpha (HIF-2α) pathway, is a cofactor of enzymes, and mediates the inflammatory response; | - |
| Cadmium (Cd) [58,137,231,234,244,245,246,247,248,249] | Elevated | Cd stimulates cytokine production, metalloproteinase activity, integrin dysregulation, and bone demineralisation, therefore accelerating cartilage degradation; | Smoking cigarettes increases the concentration of Cd in the body; |
| Mercury (Hg) [41,231,250,251,252,253] | Normal | - | The effect of Hg on OA has not been confirmed; |
| Lead (Pb) [254,255,256,257,258,259,260,261] | Elevated | Pb promotes the upregulation of matrix metalloproteinases, reduces ROS production, and disrupts normal collagen synthesis; | - |
| Nickel (Ni) [156,262,263,264,265,266] | Normal | Ni may exacerbate inflammation, oxidative stress, and cellular apoptosis; | Ni accumulates as a result of joint replacement; |
| Selenium (Se) [49,54,58,231,238,241,267,268,269,270,271,272] | Decreased | Se enhances antioxidant defences, suppresses inflammation, and reduces the activity of cartilage-degrading enzymes; | There is research suggesting that increased dietary Se intake accelerates the progression of OA; |
| Boron (B) [56,58,59,273,274,275,276,277,278] | Decreased | B supports osteogenesis, acts as an antioxidant, reduces pro-inflammatory cytokines, and participates in hormonal balance; | - |
| Trace Element | Level in Disease | Impact on the Disorder | Additional Information |
|---|---|---|---|
| Iron (Fe) [292,293,294,295] | Elevated intracellularly (in granulocytes, platelets); dysregulated systemically | Fe overload amplifies oxidative stress and inflammation; ferroptosis contributes to joint tissue damage; Fe affects macrophage polarisation, T-cell response, and cytokine production; | MR studies report conflicting results: one suggests a genetic association, another finds no causality; potential therapeutic targets include Fe chelators and ferroptosis inhibitors; |
| Copper (Cu) [296,297,298] | Elevated | Cu may induce cuproptosis—a novel mitochondrial-dependent cell death linked to inflammation; promotes oxidative stress and immune activation; | Cuproptosis-related genes (MTF1, ATP7A, SLC31A1) are upregulated in AS; no significant causal link found in MR study |
| Manganese (Mn) [299] | Not significantly altered | Mn is a cofactor for Mn superoxide dismutase (MnSOD), which detoxifies ROS in mitochondria; its deficiency could theoretically exacerbate oxidative stress, but no genetic link with AS was found; | A genetic study (Yen et al. [250]) found no association between MnSOD polymorphisms and AS susceptibility; |
| Zinc (Zn) [298] | Possibly decreased; data inconclusive; | Zn is crucial for immune regulation and inflammation, but MR analysis does not support a definitive causal role in AS; | Slight association in basic models, but not supported by MR-Egger or CAUSE, suggesting a false-positive; |
| Cadmium (Cd) [289,300] | Elevated (in environmentally exposed groups) | Cd promotes oxidative stress, mitochondrial dysfunction, and proinflammatory cytokine release ↑ cyclooxygenase 2 (COX-2), ↓ interleukin (IL)-10), contributing to joint inflammation and structural damage; | Polish and NHANES studies: Cd positively correlates with BASDAI, ESR, CRP, and spine stiffness; |
| Selenium (Se) [298,301] | Decreased (dietary) | Se deficiency may enhance oxidative stress and impair immune regulation in AS, although MR data do not support causality; | Intake below Nordic recommendations in r-axSpA patients; further trials needed to assess effects of supplementation; |
| Trace Element | Level in Disease | Impact on the Disorder | Additional Information |
|---|---|---|---|
| Iron (Fe) [304,305,306,307,308,309] | Dysregulated: often functionally deficient; intracellular overload in immune cells; | Functional Fe deficiency due to IL–6–driven hepcidin ↑ leads to mitochondrial dysfunction and immune dysregulation. Ferroptosis (from Fe overload) causes ROS ↑ and chronic inflammation in joint tissues; | Genetic factors (e.g., IL-6 −174G>C, HFE mutations) influence Fe metabolism and may affect SLE susceptibility; |
| Copper (Cu) [310,311] | Elevated | Elevated Cu is linked to inflammation, oxidative stress, and possibly cuproptosis, which disrupts mitochondrial function and immune gene expression; | In patients with SLE, Cu shifts from ceruloplasmin to albumin. Cuproptosis-related genes (e.g., LIAS) are differentially expressed; |
| Zinc (Zn) [310,311] | Decreased | Zn deficiency impairs immune function and promotes joint inflammation. Zn-binding to abnormal proteins in serum reflects inflammatory changes; | Redistribution of Zn from albumin to IgG, ceruloplasmin, and transferrin in SLE serum samples; |
| Cadmium (Cd) [156] | Elevated (in high exposure groups) | Cd increases ROS production, activates NF-κB/Mitogen-Activated Protein Kinase (MAPK), impairs antioxidant enzymes (via Zn/Cu displacement), and promotes proinflammatory cytokines and autoimmunity; | Blood Cd levels in women with SLE were 3–4x higher than controls; positively correlated with CRP, negatively with IL-10; |
| Mercury (Hg) [312,313] | Elevated (in sensitive individuals) | Hg induces type IV hypersensitivity, alters protein structure (neoantigens), and promotes autoreactive T-cell responses and joint inflammation; | LTT-MELISA tested positive in 47% of patients with SLE vs. 10% of controls; symptom improvement reported after amalgam removal; |
| Nickel (Ni) [312,313] | Hypersensitivity common | Ni acts as a hapten, binding to self-proteins and inducing chronic inflammation via Th1/Th2 imbalance; may aggravate joint inflammation in SLE; | MELISA: 52% of CTD patients (incl. SLE) Ni-sensitive vs. 27% of controls; symptom relief observed after Ni-containing dental material removal; |
| Selenium (Se) [314] | Decreased (dietary); genetically protective | Se regulates T/NK cell activation and reduces oxidative stress. Low Se may worsen inflammation and joint damage in SLE; | MR study: genetically higher Se levels are associated with reduced SLE risk. Se supplementation improved outcomes in lupus-prone mice; |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryliński, Ł.; Brylińska, K.; Woliński, F.; Sado, J.; Smyk, M.; Komar, O.; Karpiński, R.; Prządka, M.; Baj, J. Trace Elements—Role in Joint Function and Impact on Joint Diseases. Int. J. Mol. Sci. 2025, 26, 7493. https://doi.org/10.3390/ijms26157493
Bryliński Ł, Brylińska K, Woliński F, Sado J, Smyk M, Komar O, Karpiński R, Prządka M, Baj J. Trace Elements—Role in Joint Function and Impact on Joint Diseases. International Journal of Molecular Sciences. 2025; 26(15):7493. https://doi.org/10.3390/ijms26157493
Chicago/Turabian StyleBryliński, Łukasz, Katarzyna Brylińska, Filip Woliński, Jolanta Sado, Miłosz Smyk, Olga Komar, Robert Karpiński, Marcin Prządka, and Jacek Baj. 2025. "Trace Elements—Role in Joint Function and Impact on Joint Diseases" International Journal of Molecular Sciences 26, no. 15: 7493. https://doi.org/10.3390/ijms26157493
APA StyleBryliński, Ł., Brylińska, K., Woliński, F., Sado, J., Smyk, M., Komar, O., Karpiński, R., Prządka, M., & Baj, J. (2025). Trace Elements—Role in Joint Function and Impact on Joint Diseases. International Journal of Molecular Sciences, 26(15), 7493. https://doi.org/10.3390/ijms26157493







