Targeting Cellular Senescence with Liposome-Encapsulated Fisetin: Evidence of Senomorphic Effect
Abstract
1. Introduction
2. Results
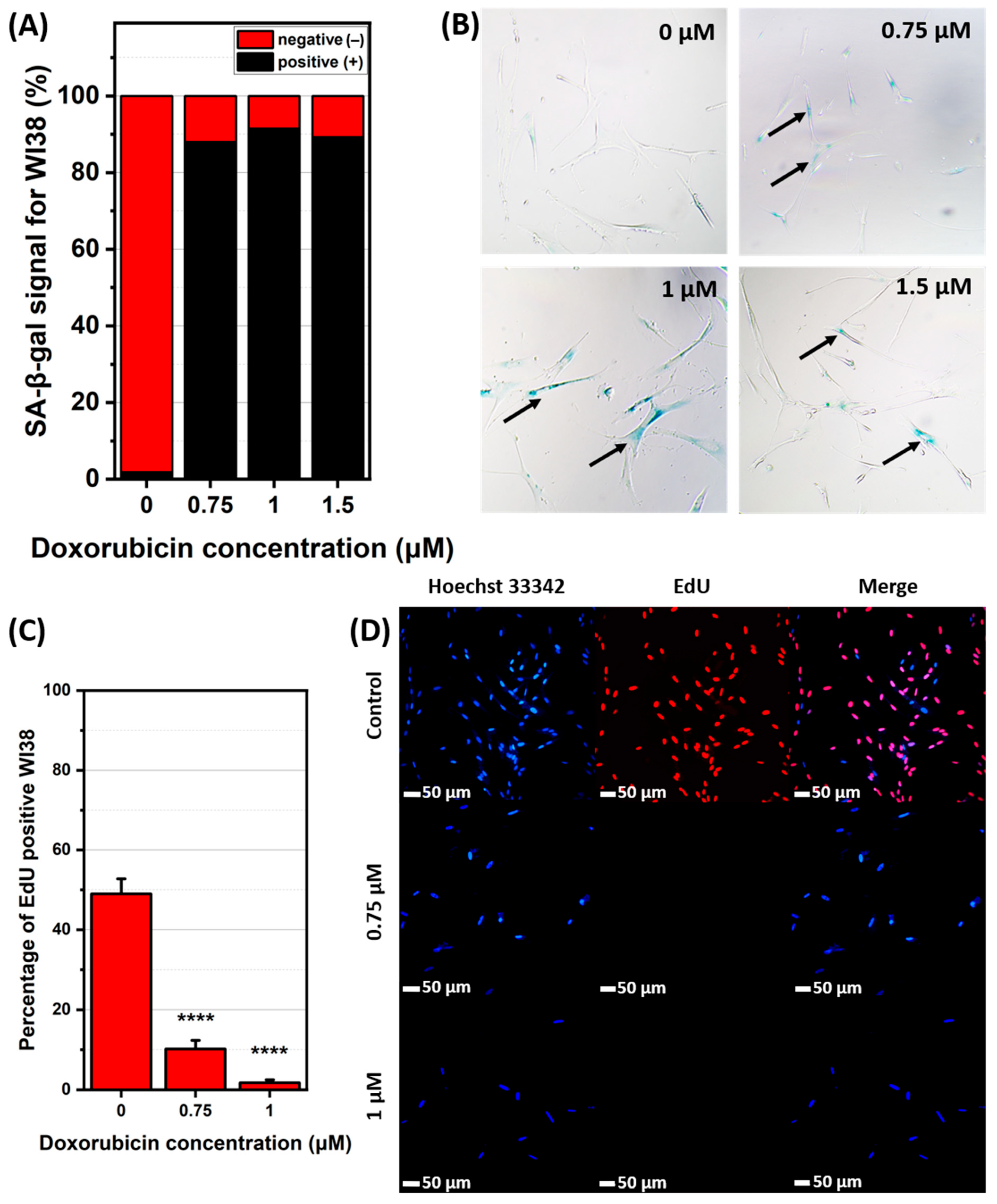
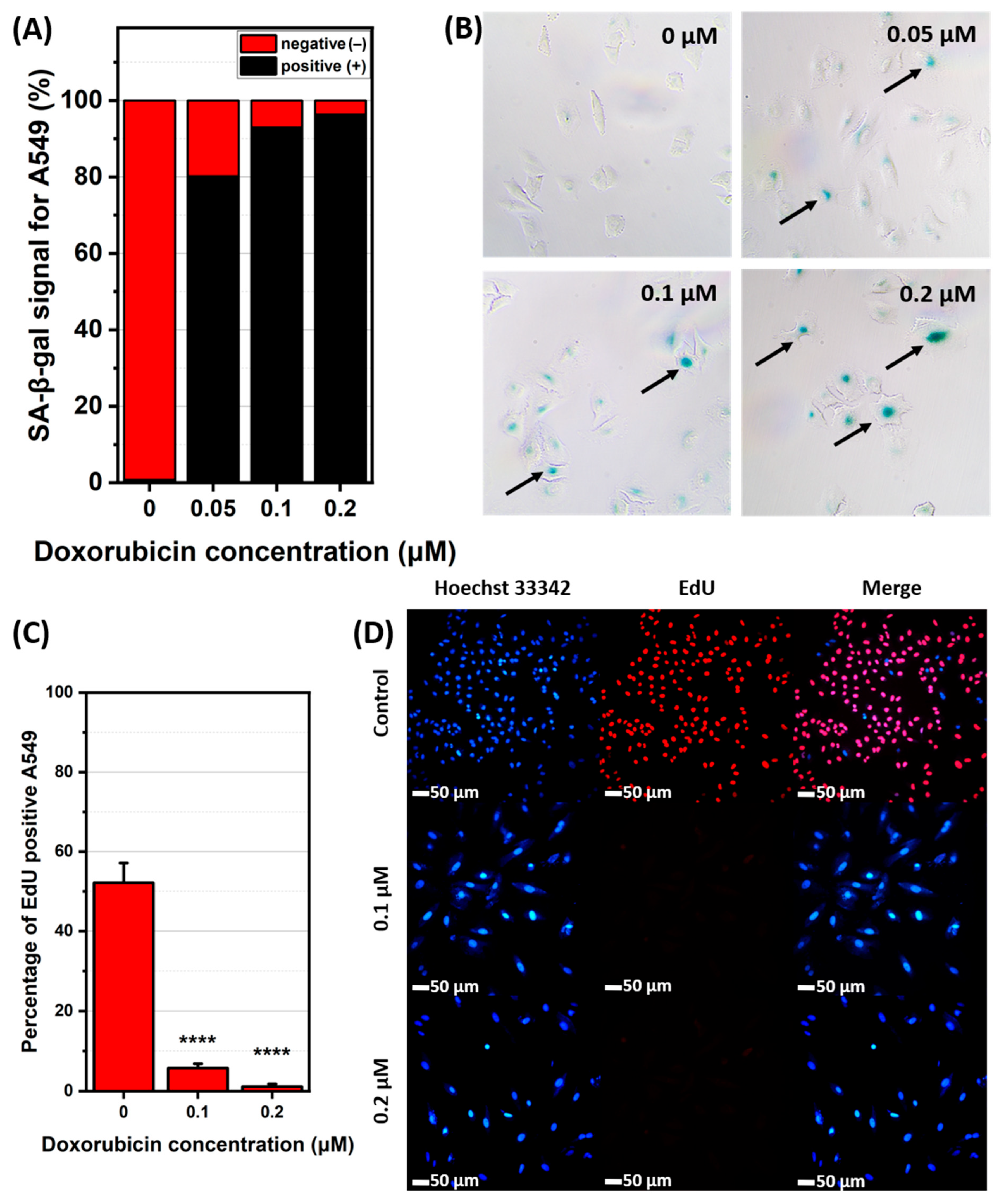
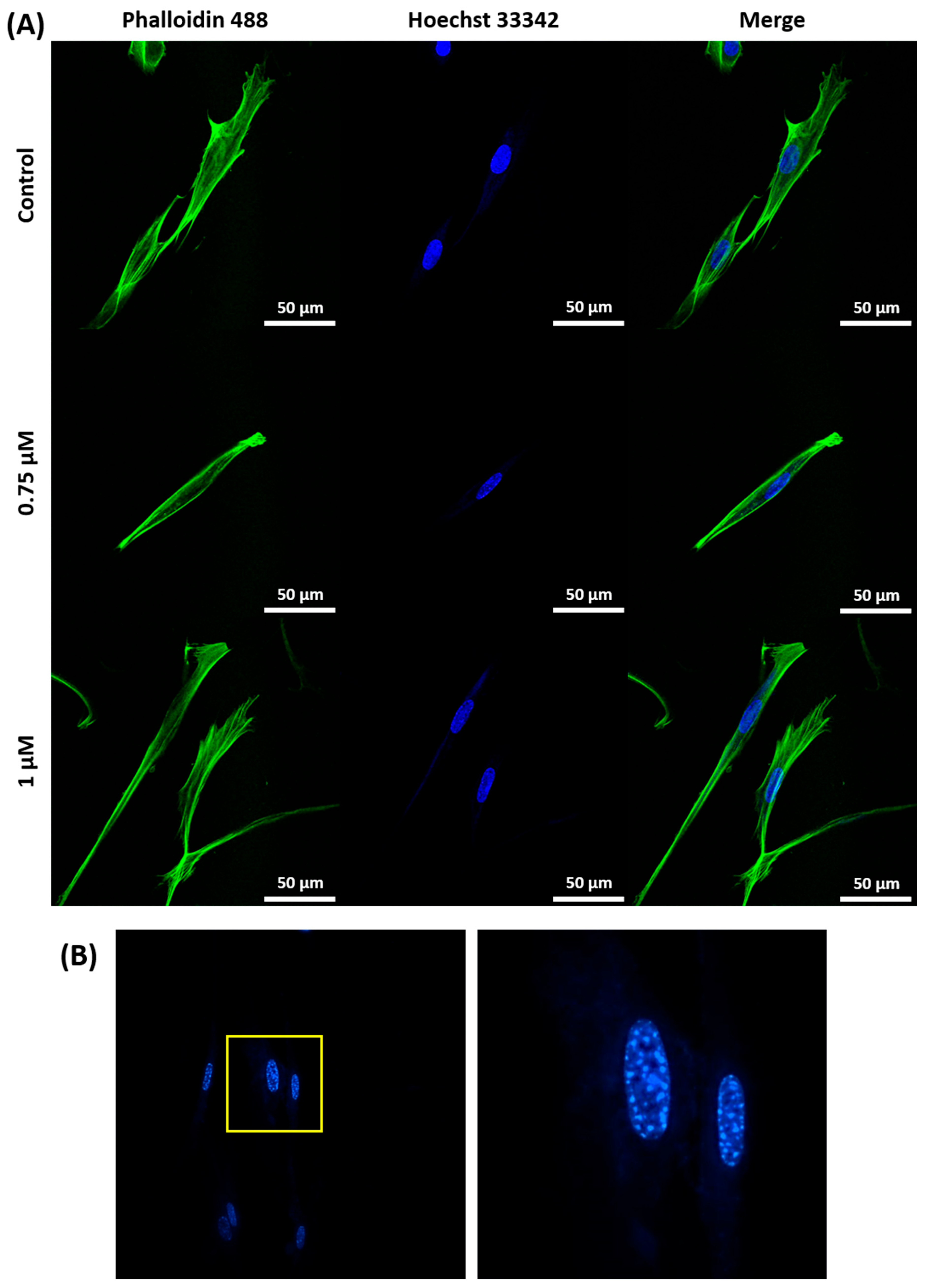
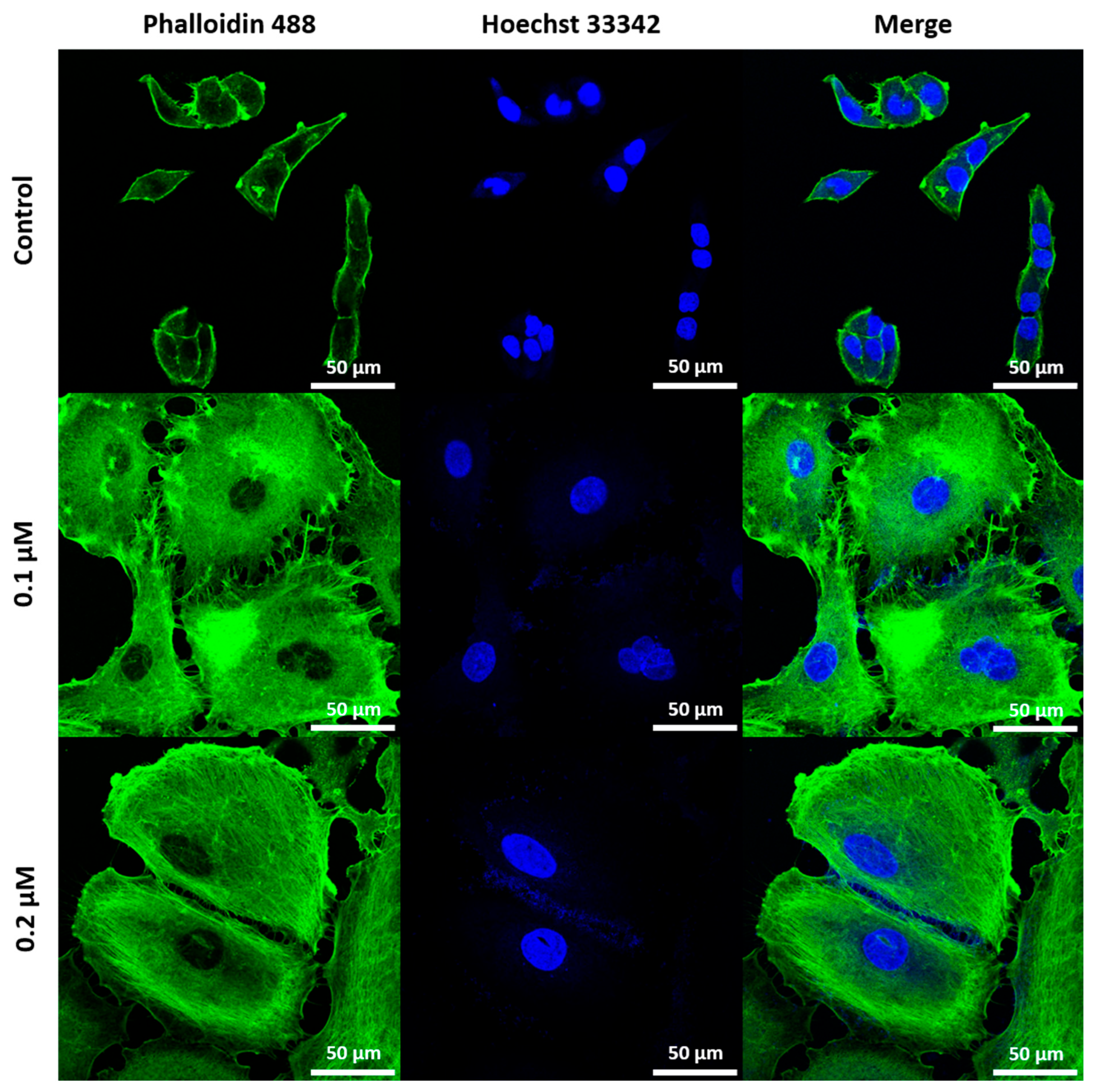
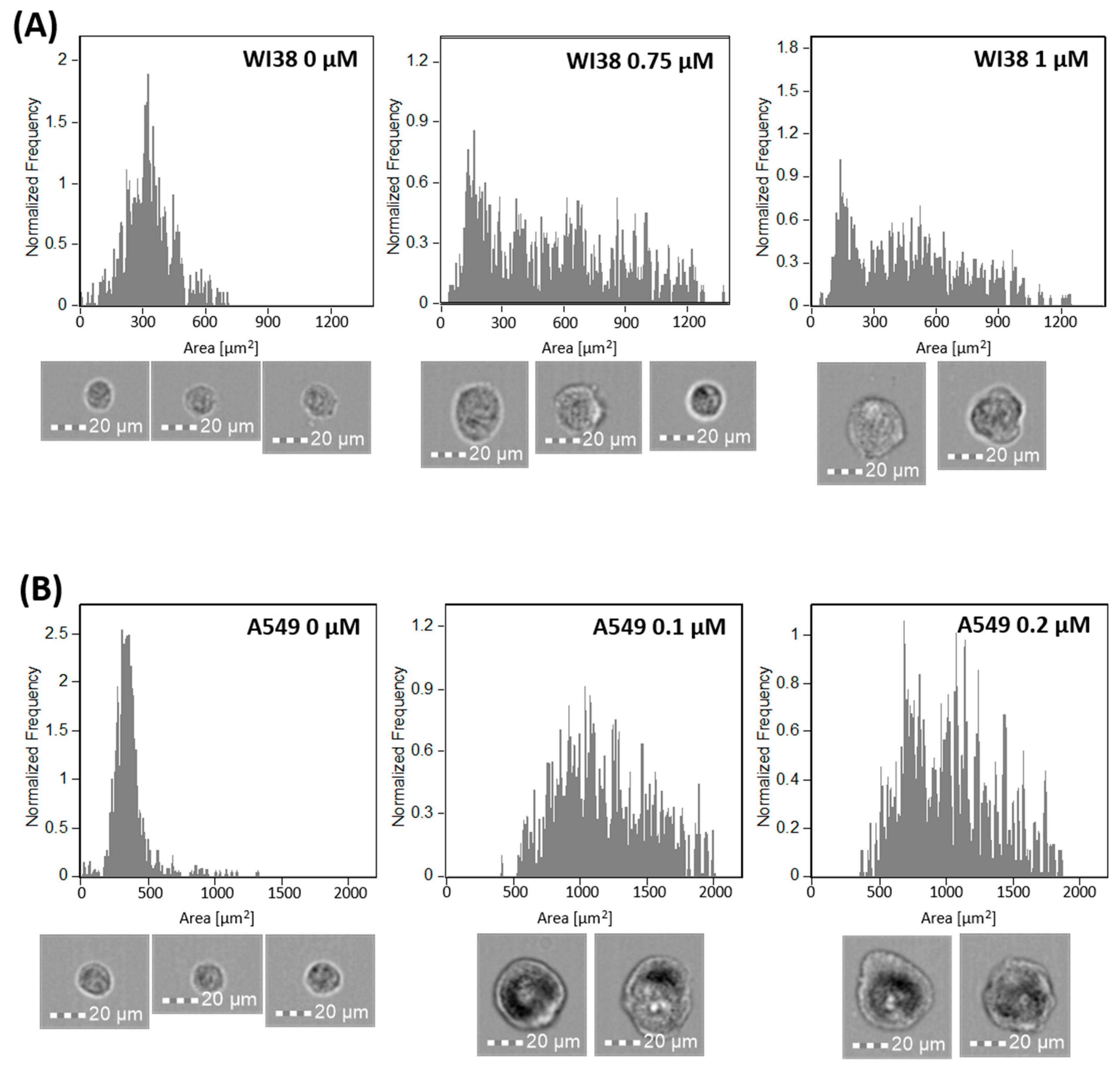
2.1. Induction and Identification of Cellular Senescence
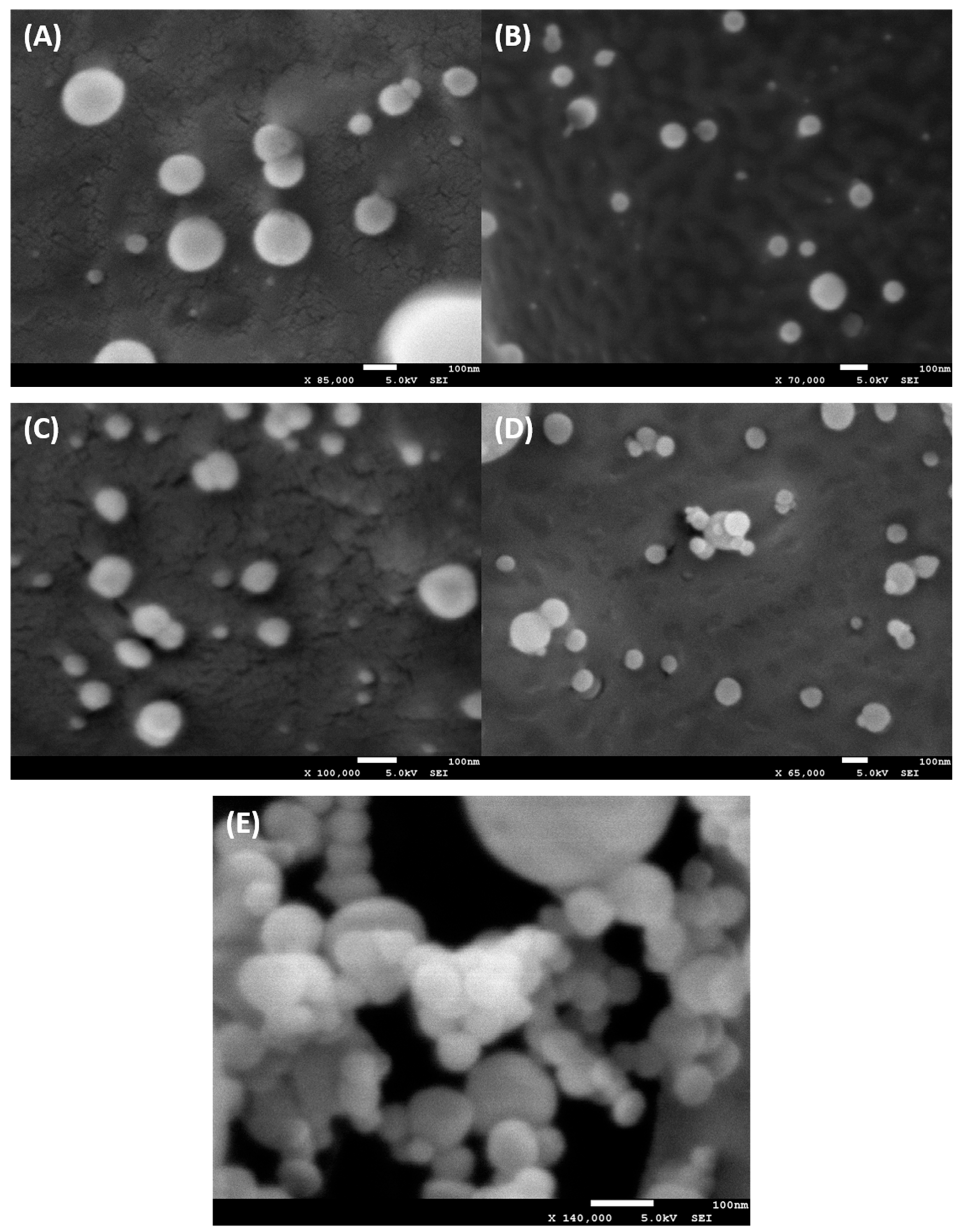
2.2. Characterization of Liposomes
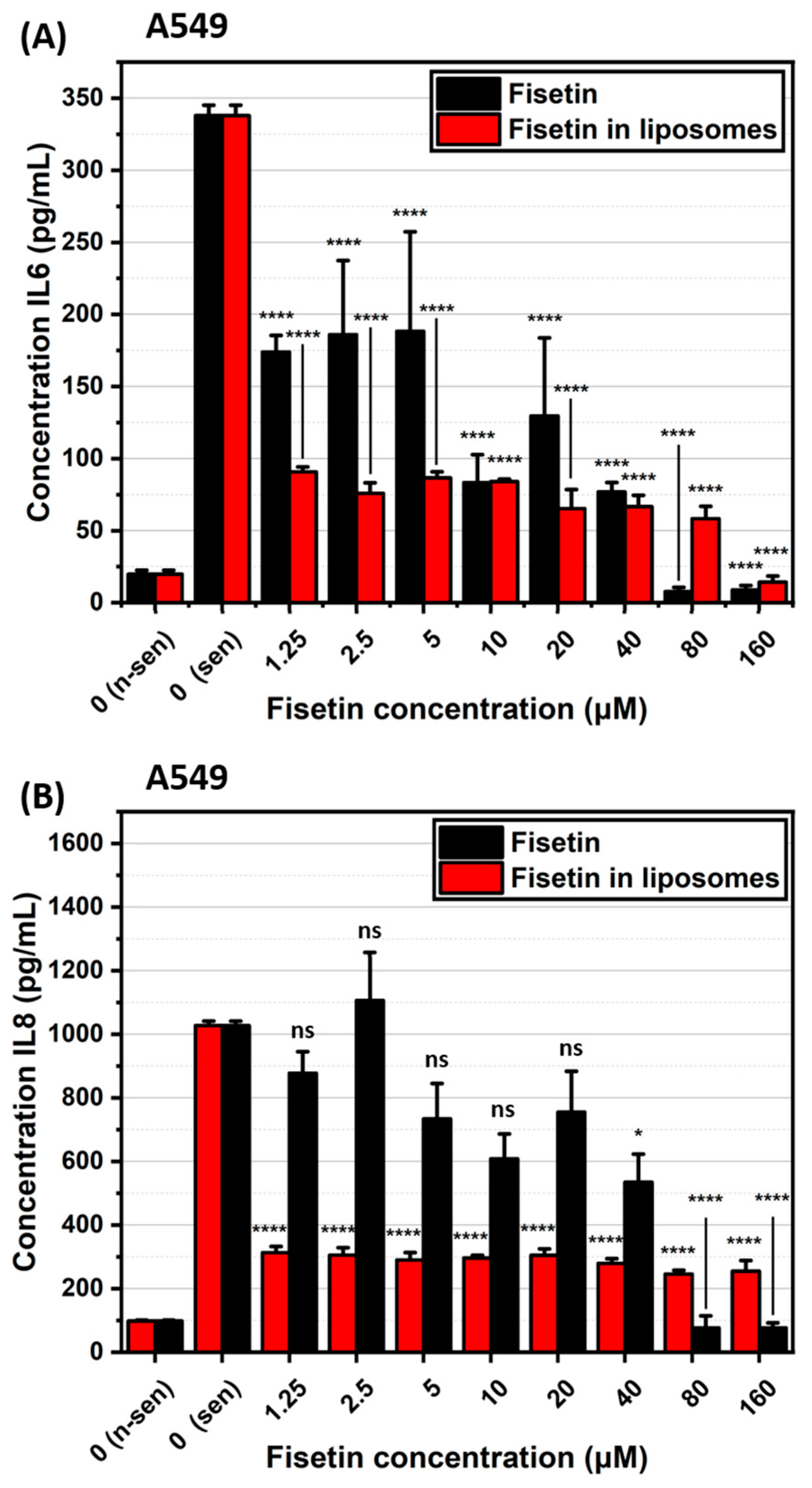
2.3. Impact of Fisetin-Encapsulated Liposomes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures
4.3. Senescence Induction
4.4. Live/Dead Cell Viability Assay
4.5. SA-β-Galactosidase Staining
4.6. EdU Cell Proliferation Assay
4.7. Morphology Evaluation
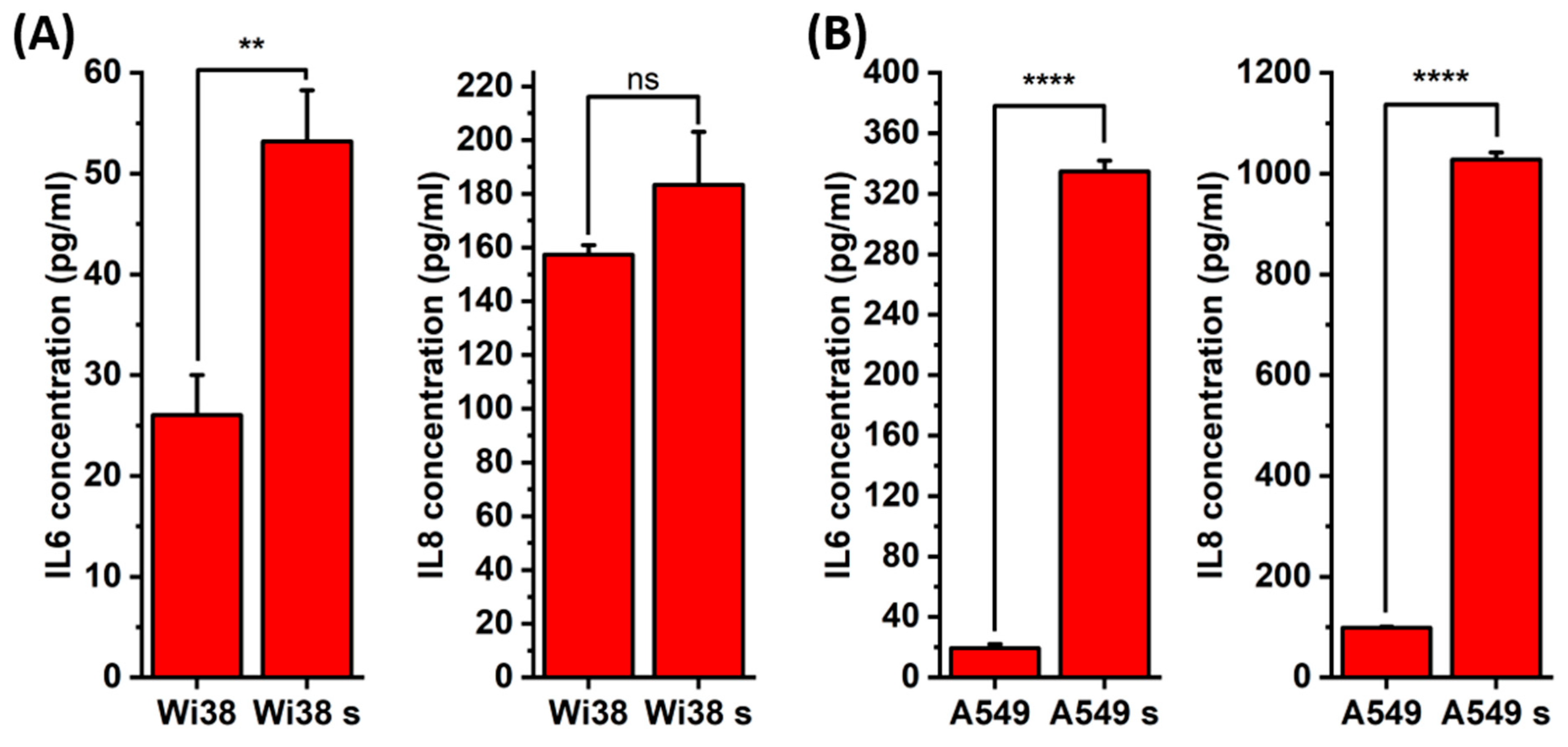
4.8. Interleukin Level Evaluation
4.9. Liposomes Formulation
4.10. Liposome Characterization
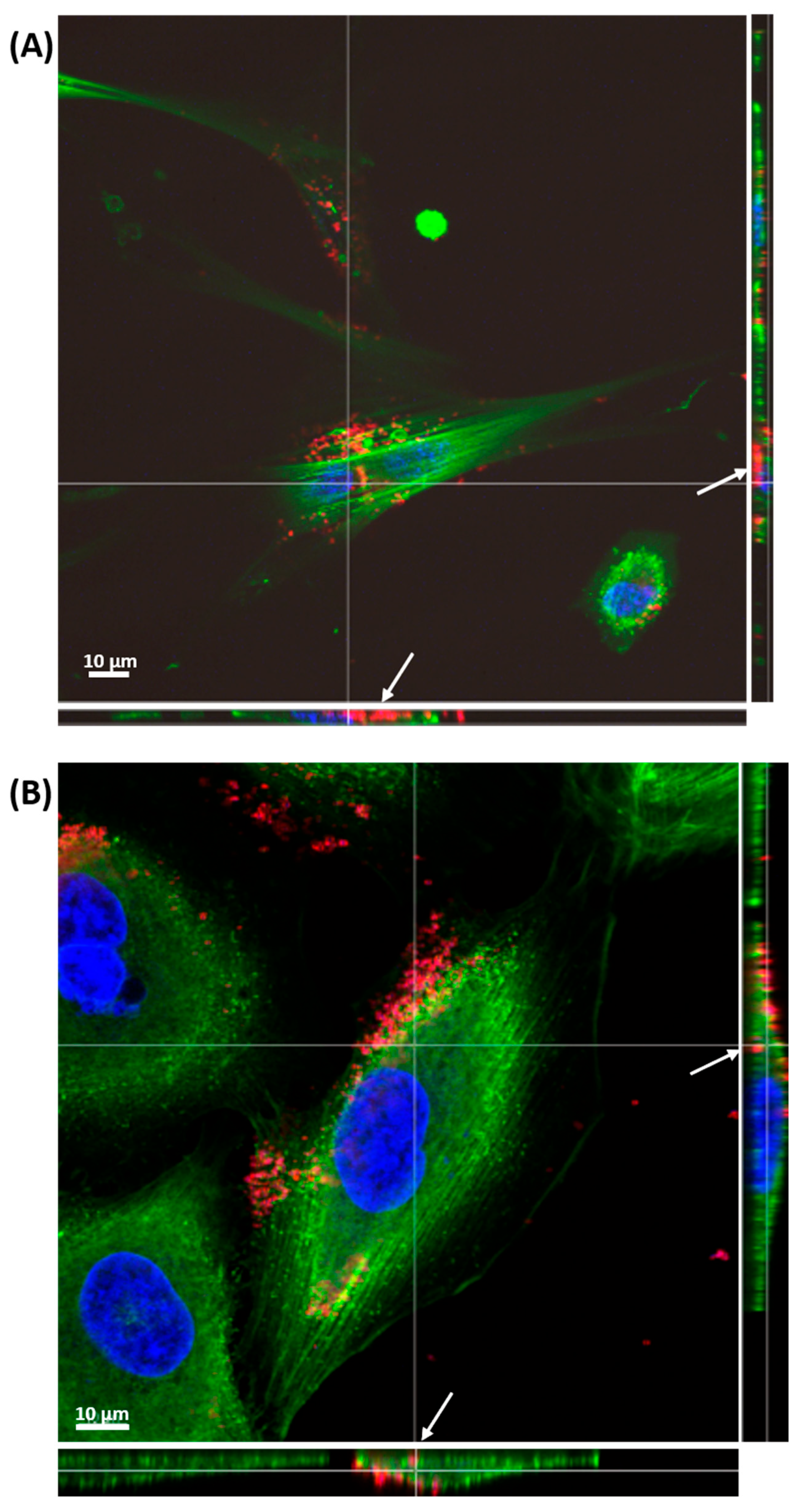
4.11. Cellular Uptake of Liposomes
4.12. Cell Viability Assay After Fisetin Treatment
4.13. Interleukin Evaluation After Fisetin Treatment
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayflick, L.; Moorhead, P.S. The Serial Cultivation of Human Diploid Cell Strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Hayflick, L. The Limited In Vitro Lifetime of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Sager, R. Senescence As a Mode of Tumor Suppression. Environ. Health Perspect. 1991, 93, 59–62. [Google Scholar] [CrossRef]
- Yasuda, T.; Baba, H.; Ishimoto, T. Cellular senescence in the tumor microenvironment and context-specific cancer treatment strategies. FEBS J. 2021, 290, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Petit, C.; Moreau, M.; Toutain, B.; Henry, C.; Roché, H.; Bonichon-Lamichhane, N.; Salmon, J.P.; Lemonnier, J.; Campone, M.; et al. Regulation of senescence escape by TSP1 and CD47 following chemotherapy treatment. Cell Death Dis. 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Roberson, R.S.; Kussick, S.J.; Vallieres, E.; Chen, S.-Y.J.; Wu, D.Y. Escape from Therapy-Induced Accelerated Cellular Senescence in p53-Null Lung Cancer Cells and in Human Lung Cancers. Cancer Res. 2005, 65, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fang, J.; Chen, J. Tumor cell senescence response produces aggressive variants. Cell Death Discov. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- Brenner, E.; Schörg, B.F.; Ahmetlić, F.; Wieder, T.; Hilke, F.J.; Simon, N.; Schroeder, C.; Demidov, G.; Riedel, T.; Fehrenbacher, B.; et al. Cancer immune control needs senescence induction by interferon-dependent cell cycle regulator pathways in tumours. Nat. Commun. 2020, 11, 1335. [Google Scholar] [CrossRef]
- Okuma, A.; Hanyu, A.; Watanabe, S.; Hara, E. p16Ink4a and p21Cip1/Waf1 promote tumour growth by enhancing myeloid-derived suppressor cells chemotaxis. Nat. Commun. 2017, 8, 2050. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New agents that target senescent cells: The flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging 2017, 9, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Dong, X.; Li, J.; Tai, G.; Qiu, S.; Wei, W.; Silumbwe, C.W.; Damdinjav, D.; Otieno, J.N.; Li, X.; et al. Fisetin Clears Senescent Cells Through the Pi3k-Akt-Bcl-2/Bcl-xl Pathway to Alleviate Diabetic Aortic Aging. Phytother. Res. 2025, 39, 2757–2775. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Mecocci, P. Senotherapeutics: Targeting senescent cells for the main age-related diseases. Mech. Ageing Dev. 2021, 197, 111526. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Zhai, Z.; Fu, Y.; Zhang, X.; Zhang, Y.; Zhou, C.; Huang, X.; Deng, L. Liposomes loaded with quercetin for resolution of lung inflammation in a lipopolysaccharide-induced mouse model of sepsis. Biomed. Mater. 2023, 18, 035004. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Rovira, M.; Galiana, I.; Giménez, C.; Lozano-Torres, B.; Paez-Ribes, M.; Llanos, S.; Chaib, S.; Muñoz-Martín, M.; Ucero, A.C.; et al. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med. 2018, 10, e9355. [Google Scholar] [CrossRef]
- Hou, S.; Lai, C.; Song, Y.; Wang, H.; Ni, J.; Tan, M. A food-grade and senescent cell-targeted fisetin delivery system based on whey protein isolate-galactooligosaccharides Maillard conjugate. Food Sci. Hum. Wellness 2024, 13, 688–697. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Thapa, R.K.; Shin, B.S.; Jeong, J.-H.; Kim, J.-R.; Yong, C.S.; Kim, J.O. CD9 monoclonal antibody-conjugated PEGylated liposomes for targeted delivery of rapamycin in the treatment of cellular senescence. Nanotechnology 2017, 28, 095101. [Google Scholar] [CrossRef]
- Thapa, R.K.; Nguyen, H.T.; Jeong, J.-H.; Kim, J.R.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Progressive slowdown/prevention of cellular senescence by CD9-targeted delivery of rapamycin using lactose-wrapped calcium carbonate nanoparticles. Sci. Rep. 2017, 7, 43299. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cerviño, M.C.; Fuioaga, C.P.; Atanase, L.I.; Abraham, G.A.; Rivero, G. Electrohydrodynamic Techniques for the Manufacture and/or Immobilization of Vesicles. Polymers 2023, 15, 795. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Mignet, N.; Seguin, J.; Romano, M.R.; Brullé, L.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Chabot, G.G. Development of a liposomal formulation of the natural flavonoid fisetin. Int. J. Pharm. 2012, 423, 69–76. [Google Scholar] [CrossRef]
- Prasanna, P.L.; Renu, K.; Gopalakrishnan, A.V. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020, 250, 117599. [Google Scholar] [CrossRef]
- Linders, A.N.; Dias, I.B.; Fernández, T.L.; Tocchetti, C.G.; Bomer, N.; Van der Meer, P. A review of the pathophysiological mechanisms of doxorubicin-induced cardiotoxicity and aging. npj Aging 2024, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; Van Willigenburg, H.; Feijtel, D.A.; et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132–147.e16. [Google Scholar] [CrossRef]
- Casella, G.; Munk, R.; Kim, K.M.; Piao, Y.; De, S.; Abdelmohsen, K.; Gorospe, M. Transcriptome signature of cellular senescence. Nucleic Acids Res. 2019, 47, 7294–7305. [Google Scholar] [CrossRef]
- Pacifico, F.; Magni, F.; Leonardi, A.; Crescenzi, E. Therapy-Induced Senescence: Novel Approaches for Markers Identification. Int. J. Mol. Sci. 2024, 25, 8448. [Google Scholar] [CrossRef]
- Bojko, A.; Czarnecka-Herok, J.; Charzynska, A.; Dabrowski, M.; Sikora, E. Diversity of the Senescence Phenotype of Cancer Cells Treated with Chemotherapeutic Agents. Cells 2019, 8, 1501. [Google Scholar] [CrossRef]
- Kosar, M.; Bartkova, J.; Hubackova, S.; Hodny, Z.; Lukas, J.; Bartek, J. Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16ink4a. Cell Cycle 2011, 10, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Heckenbach, I.; Mkrtchyan, G.V.; Ben Ezra, M.; Bakula, D.; Madsen, J.S.; Nielsen, M.H.; Oró, D.; Osborne, B.; Covarrubias, A.J.; Idda, M.L.; et al. Nuclear morphology is a deep learning biomarker of cellular senescence. Nat. Aging 2022, 2, 742–755. [Google Scholar] [CrossRef]
- He, L.; Li, M.; Wang, X.; Wu, X.; Yue, G.; Wang, T.; Zhou, Y.; Lei, B.; Zhou, G. Morphology-based deep learning enables accurate detection of senescence in mesenchymal stem cell cultures. BMC Biol. 2024, 22, 1. [Google Scholar] [CrossRef]
- Kamat, P.; Macaluso, N.; Li, Y.; Agrawal, A.; Winston, A.; Pan, L.; Stewart, T.; Starich, B.; Milcik, N.; Min, C.; et al. Single-cell morphology encodes functional subtypes of senescence in aging human dermal fibroblasts. Sci. Adv. 2025, 11, eads1875. [Google Scholar] [CrossRef]
- Zhao, H.; Halicka, H.D.; Traganos, F.; Jorgensen, E.; Darzynkiewicz, Z. New biomarkers probing depth of cell senescence assessed by laser scanning cytometry. Cytom. Part A 2010, 77, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; Brandenburg, S.; Demaria, M. Induction and Validation of Cellular Senescence in Primary Human Cells. J. Vis. Exp. 2018, 136, e57782. [Google Scholar] [CrossRef] [PubMed]
- Tato-Costa, J.; Casimiro, S.; Pacheco, T.; Pires, R.; Fernandes, A.; Alho, I.; Pereira, P.; Costa, P.; Castelo, H.B.; Ferreira, J.; et al. Therapy-Induced Cellular Senescence Induces Epithelial-to-Mesenchymal Transition and Increases Invasiveness in Rectal Cancer. Clin. Color. Cancer 2015, 15, 170–178. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Khusro, F.H.; Mustafa Adhami, V.; Suh, Y.; Mukhtar, H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int. J. Cancer 2012, 130, 1695–1705. [Google Scholar] [CrossRef]
- Alekseeva, A.A.; Moiseeva, E.V.; Onishchenko, N.; Boldyrev, I.A.; Singin, A.S.; Budko, A.P.; Shprakh, Z.S.; Molotkovsky, J.G.; Vodovozova, E.L. Liposomal formulation of a methotrexate lipophilic prodrug: Assessment in tumor cells and mouse T-cell leukemic lymphoma. Int. J. Nanomed. 2017, 12, 3735–3749. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, W.Y.; Ko, Y.T. The Effect of Surface Charges on the Cellular Uptake of Liposomes Investigated by Live Cell Imaging. Pharm. Res. 2017, 34, 704–717. [Google Scholar] [CrossRef]
- Mateos-Maroto, A.; Gai, M.; Brückner, M.; Marques, R.d.C.; Harley, I.; Simon, J.; Mailänder, V.; Morsbach, S.; Landfester, K. Systematic modulation of the lipid composition enables the tuning of liposome cellular uptake. Acta Biomater. 2023, 158, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Quantitative Assessment of the Comparative Nanoparticle-Uptake Efficiency of a Range of Cell Lines. Small 2011, 7, 3341–3349. [Google Scholar] [CrossRef] [PubMed]



| Day 1 | Day 30 | |||||
|---|---|---|---|---|---|---|
| Z-Average nm | PDI | ζ-Potential mV | Z-Average nm | PDI | ζ-Potential mV | |
| Liposomes | 115.9 ± 0.9 | 0.155 ± 0.004 | −20.3 ± 0.6 | 116.5 ± 1.0 | 0.181 ± 0.017 | −8.3 ± 0.5 |
| Liposomes + Fisetin | 95.1 ± 0.9 | 0.178 ± 0.008 | −11.6 ± 1.2 | 92.5 ± 0.3 | 0.184 ± 0.017 | −7.0 ± 0.3 |
| Fisetin Encapsulated Liposomes | Empty Liposomes | |
|---|---|---|
| DOPC | 78 | 80.4 |
| DSPE | 13 | 13.4 |
| Cholesterol | 6 | 6.2 |
| Fisetin | 3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henschke, A.; Grześkowiak, B.; Ivashchenko, O.; Sánchez-Cerviño, M.C.; Coy, E.; Moya, S. Targeting Cellular Senescence with Liposome-Encapsulated Fisetin: Evidence of Senomorphic Effect. Int. J. Mol. Sci. 2025, 26, 7489. https://doi.org/10.3390/ijms26157489
Henschke A, Grześkowiak B, Ivashchenko O, Sánchez-Cerviño MC, Coy E, Moya S. Targeting Cellular Senescence with Liposome-Encapsulated Fisetin: Evidence of Senomorphic Effect. International Journal of Molecular Sciences. 2025; 26(15):7489. https://doi.org/10.3390/ijms26157489
Chicago/Turabian StyleHenschke, Agata, Bartosz Grześkowiak, Olena Ivashchenko, María Celina Sánchez-Cerviño, Emerson Coy, and Sergio Moya. 2025. "Targeting Cellular Senescence with Liposome-Encapsulated Fisetin: Evidence of Senomorphic Effect" International Journal of Molecular Sciences 26, no. 15: 7489. https://doi.org/10.3390/ijms26157489
APA StyleHenschke, A., Grześkowiak, B., Ivashchenko, O., Sánchez-Cerviño, M. C., Coy, E., & Moya, S. (2025). Targeting Cellular Senescence with Liposome-Encapsulated Fisetin: Evidence of Senomorphic Effect. International Journal of Molecular Sciences, 26(15), 7489. https://doi.org/10.3390/ijms26157489







