A Naturally Occurring Urinary Collagen Type I Alpha 1-Derived Peptide Inhibits Collagen Type I-Induced Endothelial Cell Migration at Physiological Concentrations
Abstract
1. Introduction
2. Results
2.1. Shortlisting of Peptides Likely to Have Biological Activity Based on Abundance
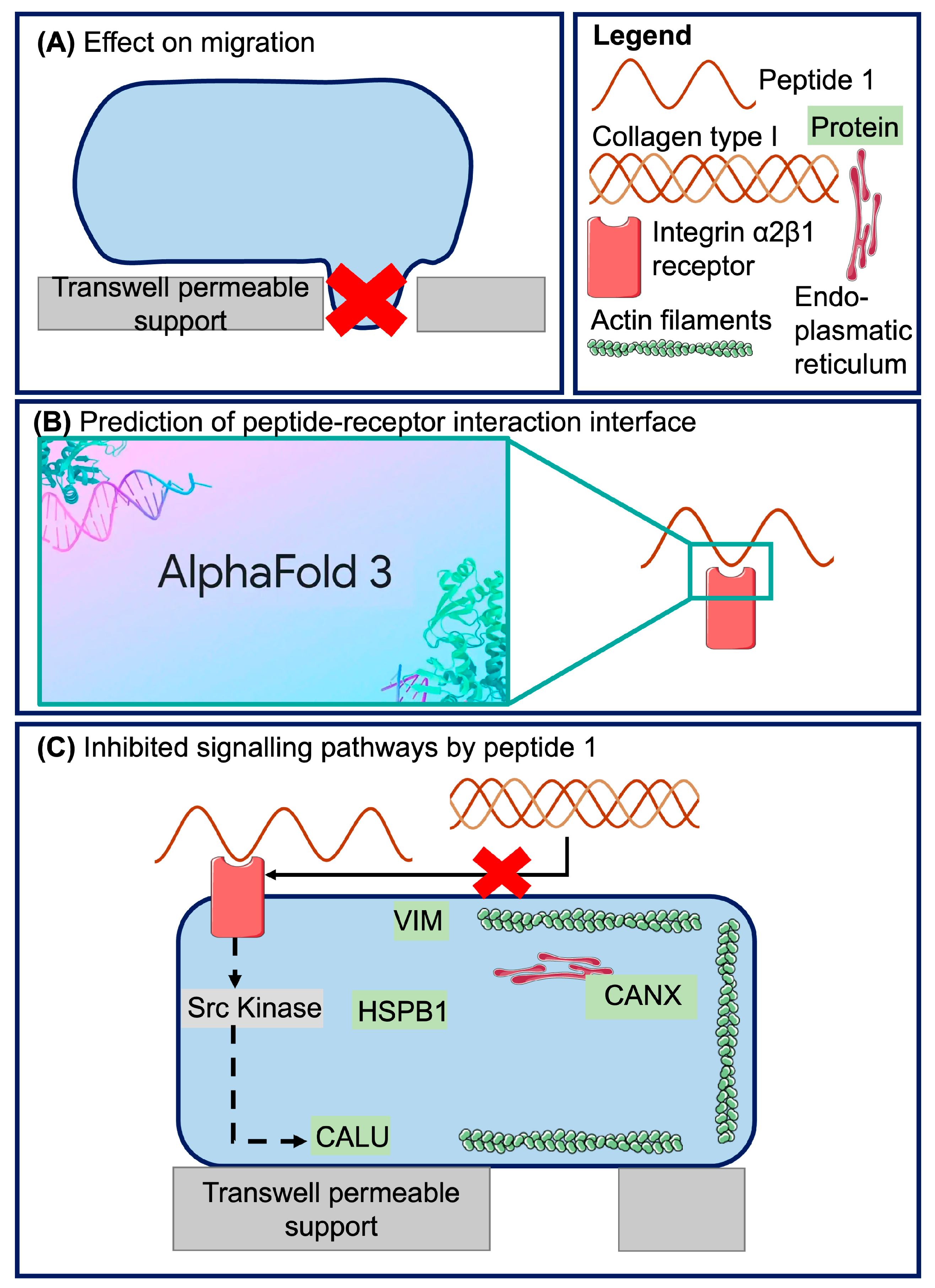
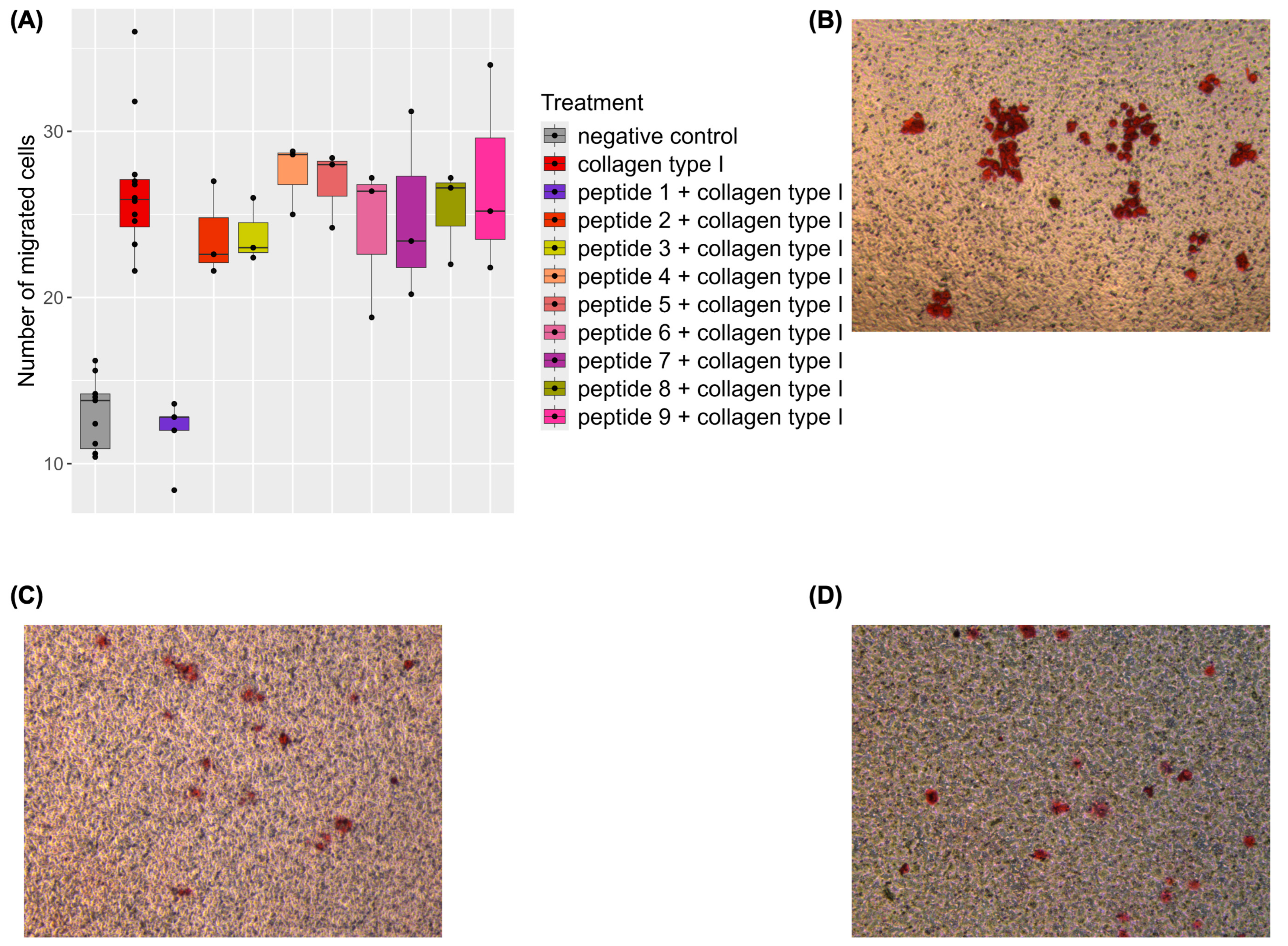
2.2. Inhibition of Collagen-Induced Migration by a Naturally Occurring Urinary Peptide
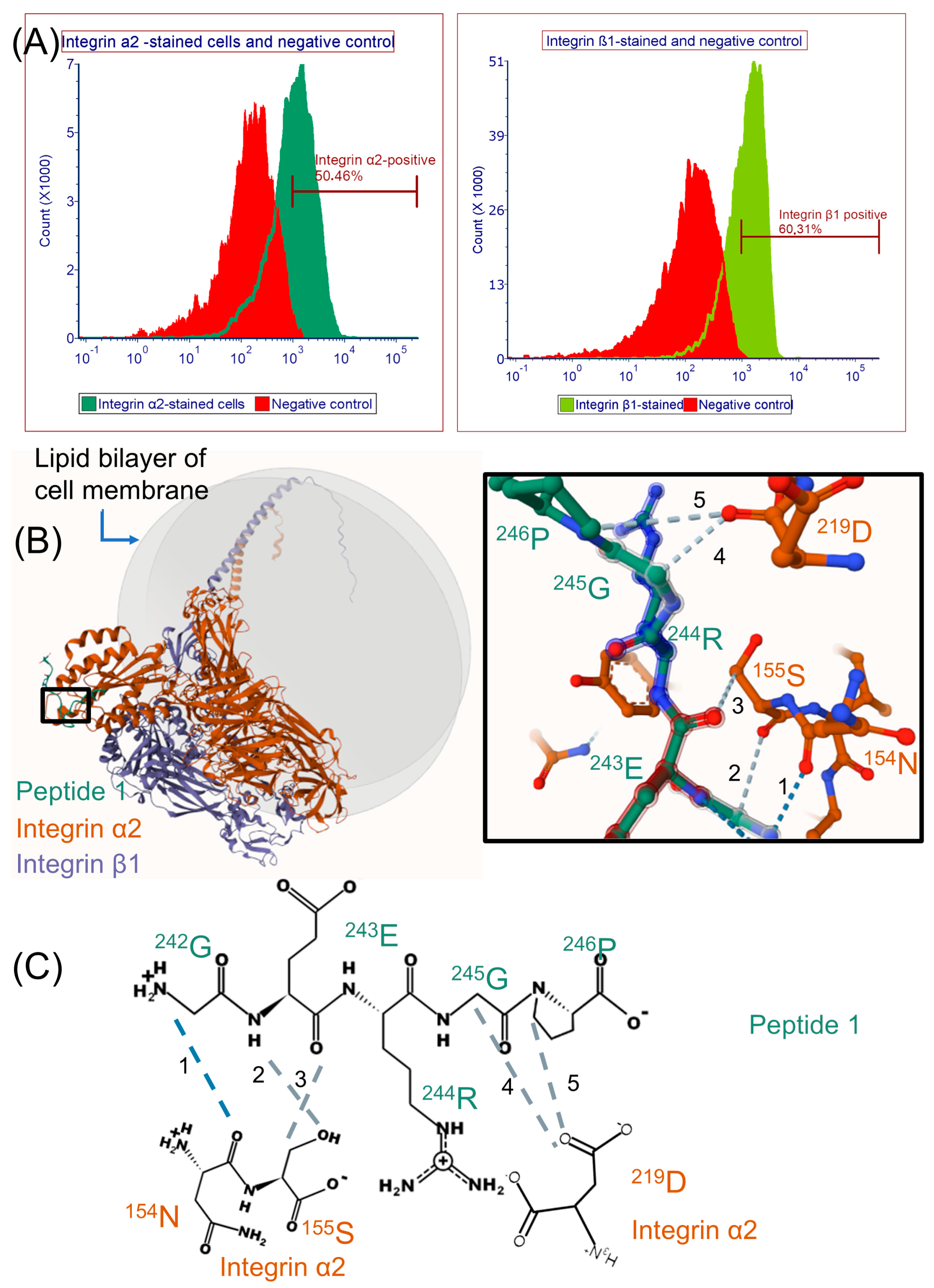
2.3. Modeling Peptide 1 and Integrin α2β1 Receptor Interaction with AlphaFold 3
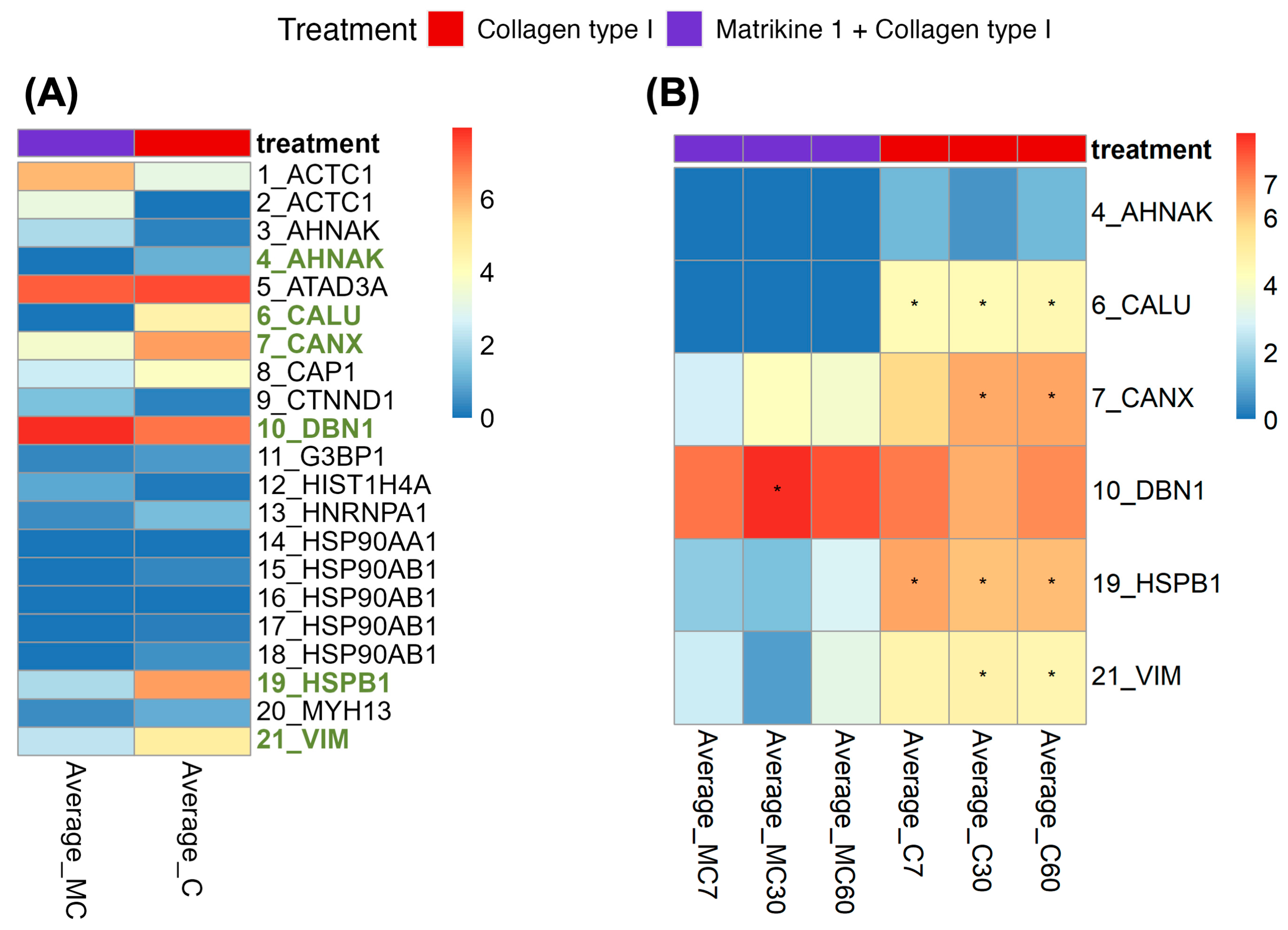
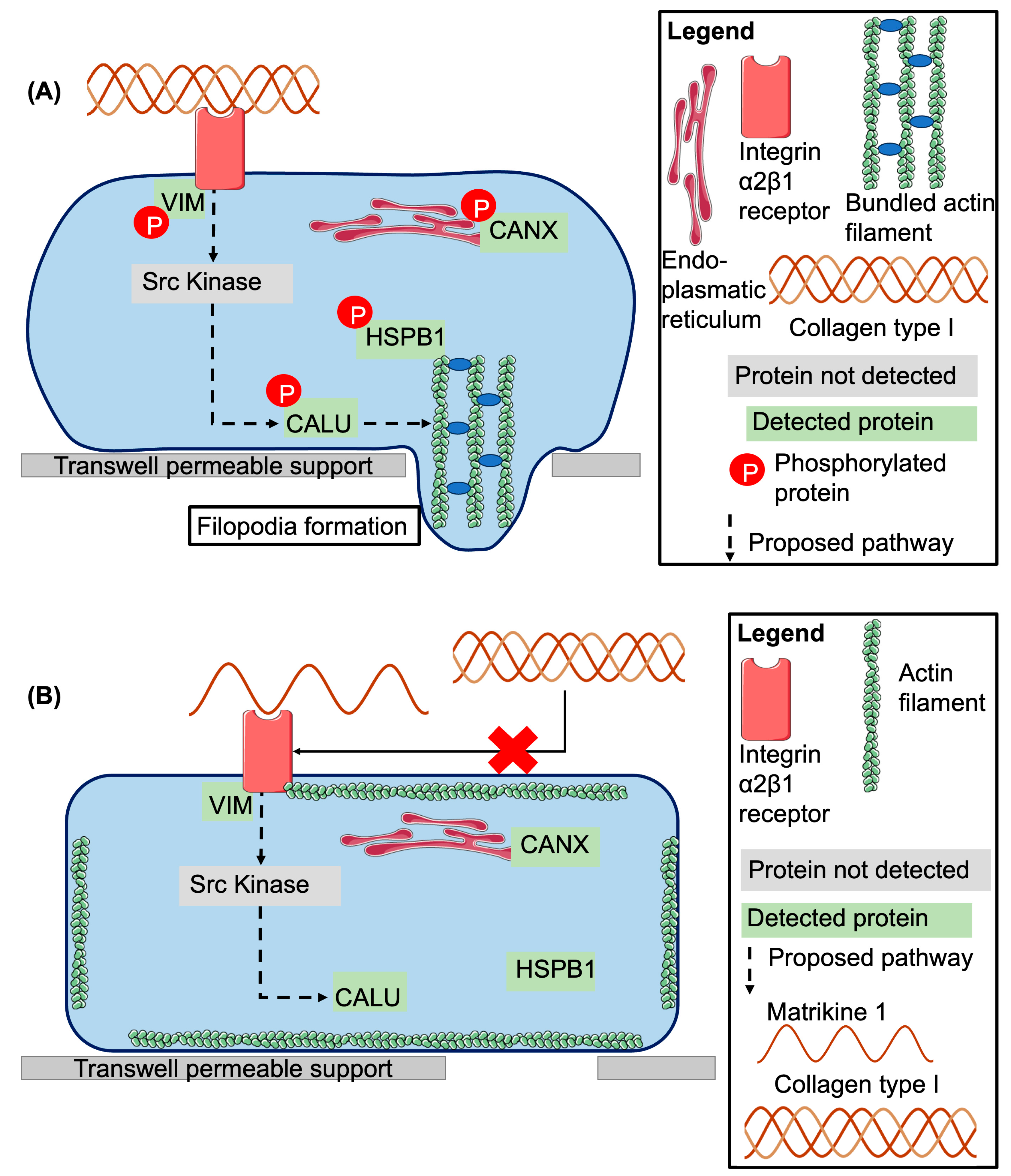
2.4. Investigation of Potentially Affected Signaling Pathways
3. Discussion
3.1. Biological Activity of Peptide 1 and Suggested Interaction with Integrin α2β1
3.2. Investigation of Signaling Pathways
3.3. Proposed Model of Peptide 1 Action
4. Materials and Methods
4.1. Cell Culture
4.2. Peptides and Collagen Type I
4.3. Migration Assay
4.4. Flow Cytometry Analysis
4.5. Alphafold 3 Predictions
4.6. Sample Collection
4.7. Western Blotting
4.8. LC–MS/MS Analysis and MS Data Processing
4.9. Statistical Analysis of Proteomics Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 7-AAD | 7-Aminoactinomycin D |
| BSA | Bovine Serum Albumin |
| CE–MS/MS | capillary electrophoresis combined with mass spectrometry |
| CKD | Chronic kidney disease |
| COL(I) | Collagen type I |
| COLα1 (I) | Collagen type I, alpha 1 chain |
| COLα2 (I) | Collagen type I, alpha 2 chain |
| COPD | chronic obstructive pulmonary disease |
| CXCL | C-X-C Ligand |
| CXCR | C-X-C Chemokine receptor |
| EBM2 | Endothelial Basal Medium |
| ECM | Extracellular Matrix |
| EDTA | Ethylenediaminetetraacetic acid |
| EGM2 | Endothelial Growth Medium |
| ERK | Extracellular Regulated Kinase |
| FAK | Focal Adhesion Kinase |
| FBS | Fetal Bovine Serum |
| FDR | False Discovery Rate |
| FITC | Fluorescein isothiocyanate |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| IP3 | 1,4,5-triphosphate |
| JNK | C-jun N-terminal kinase |
| LC–MS/MS | Liquid Chromatography—Mass spectrometry |
| MMP | Matrix-metalloproteinases |
| PAK | P21 (Rac1) Activated Kinase |
| PE | phycoerythrin |
| PFA | Paraformaldehyde |
| PBS | Phosphate-Buffered Saline |
| PKC | Protein Kinase C |
| PLC | Phospholipase C |
| PRAK | P38-regulated/activated protein kinase |
| PSM | Peptide-spectral match |
| STAT | Signal Transducer and Activator of Transcription |
| TBS | Tris-buffered Saline |
| VEGF | Vascular Endothelial Growth Factor |
Appendix A
Appendix A.1
Appendix A.2
Appendix B
| Treatment | Mean Number of Migrated Cells | Standard Deviation | Number of Replicates | Number of Independent Experiments |
|---|---|---|---|---|
| Peptide 1 | 18.7 | 4.3 | 15 | 2 |
| Peptide 2 | 20.5 | 7.14 | 10 | 1 |
| Peptide 3 | 14.6 | 3.78 | 10 | 1 |
| Peptide 4 | 18.9 | 4.25 | 10 | 1 |
| Peptide 5 | 20 | 5.08 | 10 | 1 |
| Peptide 6 | 14.2 | 5.55 | 10 | 1 |
| Peptide 7 | 16.9 | 7.75 | 10 | 1 |
| Peptide 8 | 16.9 | 4.72 | 10 | 1 |
| Peptide 9 | 16.3 | 4.83 | 10 | 1 |
| Negative control | 17.02 | 5.93 | 55 | 7 |
| Collagen type I | 30.58 | 7.41 | 60 | 7 |
| Peptide 1 + collagen type I | 15.92 | 4.44 | 25 | 2 |
| Peptide 2 + collagen type I | 27.73 | 7.73 | 15 | 2 |
| Peptide 3 + collagen type I | 27.8 | 8.66 | 15 | 2 |
| Peptide 4 + collagen type I | 31.53 | 7.53 | 15 | 2 |
| Peptide 5 + collagen type I | 30.87 | 3.48 | 15 | 2 |
| Peptide 6 + collagen type I | 28.13 | 8.71 | 15 | 2 |
| Peptide 7 + collagen type I | 31.13 | 16.75 | 15 | 2 |
| Peptide 8 + collagen type I | 29.27 | 6 | 15 | 2 |
| Peptide 9 + collagen type I | 31 | 7.72 | 15 | 2 |
References
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The Extracellular Matrix as a Multitasking Player in Disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef] [PubMed]
- Devos, H.; Zoidakis, J.; Roubelakis, M.G.; Latosinska, A.; Vlahou, A. Reviewing the Regulators of COL1A1. Int. J. Mol. Sci. 2023, 24, 10004. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhang, Z.; Zhu, A.; Xiong, X.; Zhang, J.; Xu, J.; Sy, M.-S.; Li, C. Targeting Type I Collagen for Cancer Treatment. Int. J. Cancer 2022, 151, 665–683. [Google Scholar] [CrossRef]
- Di Lullo, G.A.; Sweeney, S.M.; Korkko, J.; Ala-Kokko, L.; San Antonio, J.D. Mapping the Ligand-Binding Sites and Disease-Associated Mutations on the Most Abundant Protein in the Human, Type I Collagen. J. Biol. Chem. 2002, 277, 4223–4231. [Google Scholar] [CrossRef]
- Li, S.; Sampson, C.; Liu, C.; Piao, H.; Liu, H.-X. Integrin Signaling in Cancer: Bidirectional Mechanisms and Therapeutic Opportunities. Cell Commun. Signal. 2023, 21, 266. [Google Scholar] [CrossRef]
- Sun, L.; Guo, S.; Xie, Y.; Yao, Y. The Characteristics and the Multiple Functions of Integrin Β1 in Human Cancers. J. Transl. Med. 2023, 21, 787. [Google Scholar] [CrossRef] [PubMed]
- Aman, J.; Margadant, C. Integrin-Dependent Cell–Matrix Adhesion in Endothelial Health and Disease. Circ. Res. 2023, 132, 355–378. [Google Scholar] [CrossRef]
- Godoy, P.; Hengstler, J.G.; Ilkavets, I.; Meyer, C.; Bachmann, A.; Müller, A.; Tuschl, G.; Mueller, S.O.; Dooley, S. Extracellular Matrix Modulates Sensitivity of Hepatocytes to Fibroblastoid Dedifferentiation and Transforming Growth Factor Beta-Induced Apoptosis. Hepatology 2009, 49, 2031–2043. [Google Scholar] [CrossRef]
- Viale-Bouroncle, S.; Gosau, M.; Morsczeck, C. Collagen I Induces the Expression of Alkaline Phosphatase and Osteopontin via Independent Activations of FAK and ERK Signalling Pathways. Arch. Oral Biol. 2014, 59, 1249–1255. [Google Scholar] [CrossRef]
- Krasny, L.; Shimony, N.; Tzukert, K.; Gorodetsky, R.; Lecht, S.; Nettelbeck, D.M.; Haviv, Y.S. An In-Vitro Tumour Microenvironment Model Using Adhesion to Type I Collagen Reveals Akt-Dependent Radiation Resistance in Renal Cancer Cells. Nephrol. Dial. Transplant. 2010, 25, 373–380. [Google Scholar] [CrossRef]
- Jarvis, G.E.; Best, D.; Watson, S.P. Glycoprotein VI/Fc Receptor γ Chain-Independent Tyrosine Phosphorylation and Activation of Murine Platelets by Collagen. Biochem. J. 2004, 383, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Suzawa, M.; Kikuchi, T.; Nishida, E.; Fujita, T.; Matsumoto, T. Differentiation and Transforming Growth Factor-β Receptor Down-Regulation by Collagen-A2β1 Integrin Interaction Is Mediated by Focal Adhesion Kinase and Its Downstream Signals in Murine Osteoblastic Cells. J. Biol. Chem. 1997, 272, 29309–29316. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, S.; Chung, L.W. Induction of Integrin A2 in a Highly Bone Metastatic Human Prostate Cancer Cell Line: Roles of RANKL and AR under Three-Dimensional Suspension Culture. Mol. Cancer 2014, 13, 208. [Google Scholar] [CrossRef]
- Stejskalová, A.; Fincke, V.; Nowak, M.; Schmidt, Y.; Borrmann, K.; von Wahlde, M.-K.; Schäfer, S.D.; Kiesel, L.; Greve, B.; Götte, M. Collagen I Triggers Directional Migration, Invasion and Matrix Remodeling of Stroma Cells in a 3D Spheroid Model of Endometriosis. Sci. Rep. 2021, 11, 4115. [Google Scholar] [CrossRef] [PubMed]
- Goetsch, K.P.; Kallmeyer, K.; Niesler, C.U. Decorin Modulates Collagen I-Stimulated, but Not Fibronectin-Stimulated, Migration of C2C12 Myoblasts. Matrix Biol. 2011, 30, 109–117. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix Metalloproteinases (MMPs), the Main Extracellular Matrix (ECM) Enzymes in Collagen Degradation, as a Target for Anticancer Drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Vizovišek, M.; Fonović, M.; Turk, B. Cysteine Cathepsins in Extracellular Matrix Remodeling: Extracellular Matrix Degradation and Beyond. Matrix Biol. 2019, 75, 141–159. [Google Scholar] [CrossRef]
- Jariwala, N.; Ozols, M.; Bell, M.; Bradley, E.; Gilmore, A.; Debelle, L.; Sherratt, M.J. Matrikines as Mediators of Tissue Remodelling. Adv. Drug Deliv. Rev. 2022, 185, 114240. [Google Scholar] [CrossRef]
- Latosinska, A.; Siwy, J.; Faguer, S.; Beige, J.; Mischak, H.; Schanstra, J.P. Value of Urine Peptides in Assessing Kidney and Cardiovascular Disease. Proteom. —Clin. Appl. 2021, 15, 2000027. [Google Scholar] [CrossRef]
- Latosinska, A.; Siwy, J.; Mischak, H.; Frantzi, M. Peptidomics and Proteomics Based on CE-MS as a Robust Tool in Clinical Application: The Past, the Present, and the Future. Electrophoresis 2019, 40, 2294–2308. [Google Scholar] [CrossRef]
- Staatz, W.D.; Fok, K.F.; Zutter, M.M.; Adams, S.P.; Rodriguez, B.A.; Santoro, S.A. Identification of a Tetrapeptide Recognition Sequence for the Alpha 2 Beta 1 Integrin in Collagen. J. Biol. Chem. 1991, 266, 7363–7367. [Google Scholar] [CrossRef]
- Cha, B.-H.; Shin, S.R.; Leijten, J.; Li, Y.-C.; Singh, S.; Liu, J.C.; Annabi, N.; Abdi, R.; Dokmeci, M.R.; Vrana, N.E.; et al. Integrin-Mediated Interactions Control Macrophage Polarization in 3D Hydrogels. Adv. Healthc. Mater. 2017, 6, 1700289. [Google Scholar] [CrossRef]
- Mehta, M.; Madl, C.M.; Lee, S.; Duda, G.N.; Mooney, D.J. The Collagen I Mimetic Peptide DGEA Enhances an Osteogenic Phenotype in Mesenchymal Stem Cells When Presented from Cell-Encapsulating Hydrogels. J. Biomed. Mater. Res. A 2015, 103, 3516–3525. [Google Scholar] [CrossRef]
- Madamanchi, A.; Santoro, S.A.; Zutter, M.M. A2β1 Integrin. Adv. Exp. Med. Biol. 2014, 819, 41–60. [Google Scholar] [CrossRef]
- Mineur, P.; Guignandon, A.; Lambert, C.A.; Amblard, M.; Lapière, C.M.; Nusgens, B.V. RGDS and DGEA-Induced [Ca2+]i Signalling in Human Dermal Fibroblasts. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2005, 1746, 28–37. [Google Scholar] [CrossRef]
- McCann, T.J.; Terranova, G.; Keyte, J.W.; Papaioannou, S.S.; Mason, W.T.; Meikle, M.C.; McDonald, F. An Analysis of Ca2+ Release by DGEA: Mobilization of Two Functionally Distinct Internal Stores in Saos-2 Cells. Am. J. Physiol. Cell Physiol. 1998, 275, C33–C41. [Google Scholar] [CrossRef]
- McCann, T.J.; Mason, W.T.; Meikle, M.C.; McDonald, F. A Collagen Peptide Motif Activates Tyrosine Kinase-Dependent Calcium Signalling Pathways in Human Osteoblast-like Cells. Matrix Biol. 1997, 16, 273–283. [Google Scholar] [CrossRef]
- Kim, H.-K.; Joe, Y.A. DGDA, a Local Sequence of the Kringle 2 Domain, Is a Functional Motif of the Tissue-Type Plasminogen Activator’s Antiangiogenic Kringle Domain. Biochem. Biophys. Res. Commun. 2010, 391, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chakrabarti, R.; Keats, E.C.; Chen, M.; Chakrabarti, S.; Khan, Z.A. Regulation of Vascular Endothelial Growth Factor Expression by Extra Domain B Segment of Fibronectin in Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8333–8343. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Moore, E. Collagen-Derived Peptide, DGEA, Inhibits pro-Inflammatory Macrophages in Biofunctional Hydrogels. J. Mater. Res. 2022, 37, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Vines, J.B.; Patterson, J.L.; Chen, H.; Javed, A.; Jun, H.-W. Osteogenic Differentiation of Human Mesenchymal Stem Cells Synergistically Enhanced by Biomimetic Peptide Amphiphiles Combined with Conditioned Medium. Acta Biomater. 2011, 7, 675–682. [Google Scholar] [CrossRef]
- Chen, G.; Kong, P.; Jiang, A.; Wang, X.; Sun, Y.; Yu, T.; Chi, H.; Song, C.; Zhang, H.; Subedi, D.; et al. A Modular Programmed Biphasic Dual-Delivery System on 3D Ceramic Scaffolds for Osteogenesis in Vitro and in Vivo. J. Mater. Chem. B 2020, 8, 9697–9717. [Google Scholar] [CrossRef]
- Hamaia, S.; Farndale, R.W. Integrin Recognition Motifs in the Human Collagens. Adv. Exp. Med. Biol. 2014, 819, 127–142. [Google Scholar] [CrossRef]
- Knight, C.G.; Morton, L.F.; Peachey, A.R.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. The Collagen-Binding A-Domains of Integrins A1β1 and α2β1Recognize the Same Specific Amino Acid Sequence, GFOGER, in Native (Triple-Helical) Collagens. J. Biol. Chem. 2000, 275, 35–40. [Google Scholar] [CrossRef]
- Reyes, C.D.; García, A.J. A2β1 Integrin-Specific Collagen-Mimetic Surfaces Supporting Osteoblastic Differentiation. J. Biomed. Mater. Res. A 2004, 69A, 591–600. [Google Scholar] [CrossRef]
- Fraser, D.; Benoit, D. Dual Peptide-Functionalized Hydrogels Differentially Control Periodontal Cell Function and Promote Tissue Regeneration. Biomater. Adv. 2022, 141, 213093. [Google Scholar] [CrossRef]
- Clark, A.Y.; Martin, K.E.; García, J.R.; Johnson, C.T.; Theriault, H.S.; Han, W.M.; Zhou, D.W.; Botchwey, E.A.; García, A.J. Integrin-Specific Hydrogels Modulate Transplanted Human Bone Marrow-Derived Mesenchymal Stem Cell Survival, Engraftment, and Reparative Activities. Nat. Commun. 2020, 11, 114. [Google Scholar] [CrossRef]
- Consonni, A.; Cipolla, L.; Guidetti, G.; Canobbio, I.; Ciraolo, E.; Hirsch, E.; Falasca, M.; Okigaki, M.; Balduini, C.; Torti, M. Role and Regulation of Phosphatidylinositol 3-Kinase β in Platelet Integrin A2β1 Signaling. Blood 2012, 119, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.M.; DiLullo, G.; Slater, S.J.; Martinez, J.; Iozzo, R.V.; Lauer-Fields, J.L.; Fields, G.B.; Antonio, J.D.S. Angiogenesis in Collagen I Requires A2β1 Ligation of a GFP*GER Sequence and Possibly P38 MAPK Activation and Focal Adhesion Disassembly. J. Biol. Chem. 2003, 278, 30516–30524. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, A.V.; Bray, L.J.; Haller, B.; Shaposhnykov, A.; Binner, M.; Freudenberg, U.; Guck, J.; Werner, C. 3D Extracellular Matrix Interactions Modulate Tumour Cell Growth, Invasion and Angiogenesis in Engineered Tumour Microenvironments. Acta Biomater. 2016, 36, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.F.; Snelgrove, R.J. The Multifaceted Roles of the Matrikine Pro-Gly-Pro in Pulmonary Health and Disease. Eur. Respir. Rev. 2018, 27, 180017. [Google Scholar] [CrossRef]
- Misiura, M.; Miltyk, W. Proline—Containing Peptides-New Insight and Implications: A Review. BioFactors 2019, 45, 857–866. [Google Scholar] [CrossRef]
- Weathington, N.M.; van Houwelingen, A.H.; Noerager, B.D.; Jackson, P.L.; Kraneveld, A.D.; Galin, F.S.; Folkerts, G.; Nijkamp, F.P.; Blalock, J.E. A Novel Peptide CXCR Ligand Derived from Extracellular Matrix Degradation during Airway Inflammation. Nat. Med. 2006, 12, 317–323. [Google Scholar] [CrossRef]
- Hahn, C.S.; Scott, D.W.; Xu, X.; Roda, M.A.; Payne, G.A.; Wells, J.M.; Viera, L.; Winstead, C.J.; Bratcher, P.; Sparidans, R.W.; et al. The Matrikine N-α-PGP Couples Extracellular Matrix Fragmentation to Endothelial Permeability. Sci. Adv. 2015, 1, e1500175. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Yu, F.; Sun, Y.; Huang, F.; Chen, Y.; Yang, Z.; Ding, G. Inhibition of Prostate Cancer DU-145 Cells Proliferation by Anthopleura Anjunae Oligopeptide (YVPGP) via PI3K/AKT/mTOR Signaling Pathway. Mar. Drugs 2018, 16, 325. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhang, Y.; Yang, M.; Huang, B.; Zhou, Y. Collagen-Derived N-Acetylated Proline-Glycine-Proline in Intervertebral Discs Modulates CXCR1/2 Expression and Activation in Cartilage Endplate Stem Cells to Induce Migration and Differentiation Toward a Pro-Inflammatory Phenotype. Stem Cells 2015, 33, 3558–3568. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.W.; Heo, S.C.; Lee, T.W.; Park, G.T.; Yoon, J.W.; Jang, I.H.; Kim, S.-C.; Ko, H.-C.; Ryu, Y.; Kang, H.; et al. N-Acetylated Proline-Glycine-Proline Accelerates Cutaneous Wound Healing and Neovascularization by Human Endothelial Progenitor Cells. Sci. Rep. 2017, 7, 43057. [Google Scholar] [CrossRef]
- Thaler, R.; Zwerina, J.; Rumpler, M.; Spitzer, S.; Gamsjaeger, S.; Paschalis, E.P.; Klaushofer, K.; Varga, F. Homocysteine Induces Serum Amyloid A3 in Osteoblasts via Unlocking RGD-Motifs in Collagen. FASEB J. 2013, 27, 446–463. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, A.V.; Woodruff, M.A.; Bai, H.; Muller, D.J.; Hutmacher, D.W. The Effect of Unlocking RGD-Motifs in Collagen I on Pre-Osteoblast Adhesion and Differentiation. Biomaterials 2010, 31, 2827–2835. [Google Scholar] [CrossRef]
- Davis, G.E. Affinity of Integrins for Damaged Extracellular Matrix: Avβ3 Binds to Denatured Collagen Type I through RGD Sites. Biochem. Biophys. Res. Commun. 1992, 182, 1025–1031. [Google Scholar] [CrossRef]
- Davis, G.E.; Bayless, K.J.; Davis, M.J.; Meininger, G.A. Regulation of Tissue Injury Responses by the Exposure of Matricryptic Sites within Extracellular Matrix Molecules. Am. J. Pathol. 2000, 156, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Mao, C.; Jia, Y.; Fu, Y.; Kong, W. Extracellular Matrix Dynamics in Vascular Remodeling. Am. J. Physiol.—Cell Physiol. 2020, 319, C481–C499. [Google Scholar] [CrossRef] [PubMed]
- Argilés, Á.; Siwy, J.; Duranton, F.; Gayrard, N.; Dakna, M.; Lundin, U.; Osaba, L.; Delles, C.; Mourad, G.; Weinberger, K.M.; et al. CKD273, a New Proteomics Classifier Assessing CKD and Its Prognosis. PLoS ONE 2013, 8, e62837. [Google Scholar] [CrossRef]
- Hobson, S.; Mavrogeorgis, E.; He, T.; Siwy, J.; Ebert, T.; Kublickiene, K.; Stenvinkel, P.; Mischak, H. Urine Peptidome Analysis Identifies Common and Stage-Specific Markers in Early Versus Advanced CKD. Proteomes 2023, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.T.; Jasilek, A.; Mischak, H.; Nkuipou-Kenfack, E.; Latosinska, A.; Welsh, P.I.; Jackson, C.E.; Cannon, J.; McConnachie, A.; Delles, C.; et al. The Novel Urinary Proteomic Classifier HF1 Has Similar Diagnostic and Prognostic Utility to BNP in Heart Failure. ESC Heart Fail. 2020, 7, 1595–1604. [Google Scholar] [CrossRef]
- Bannaga, A.S.; Metzger, J.; Kyrou, I.; Voigtländer, T.; Book, T.; Melgarejo, J.; Latosinska, A.; Pejchinovski, M.; Staessen, J.A.; Mischak, H.; et al. Discovery, Validation and Sequencing of Urinary Peptides for Diagnosis of Liver Fibrosis-A Multicentre Study. EBioMedicine 2020, 62, 103083. [Google Scholar] [CrossRef]
- Diaz-Ricart, M.; Torramade-Moix, S.; Pascual, G.; Palomo, M.; Moreno-Castaño, A.B.; Martinez-Sanchez, J.; Vera, M.; Cases, A.; Escolar, G. Endothelial Damage, Inflammation and Immunity in Chronic Kidney Disease. Toxins 2020, 12, 361. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Magalhães, P.; Pontillo, C.; Pejchinovski, M.; Siwy, J.; Krochmal, M.; Makridakis, M.; Carrick, E.; Klein, J.; Mullen, W.; Jankowski, J.; et al. Comparison of Urine and Plasma Peptidome Indicates Selectivity in Renal Peptide Handling. Proteom. Clin. Appl. 2018, 12, e1700163. [Google Scholar] [CrossRef]
- Ganapathy, V.; Leibach, F.H. Carrier-Mediated Reabsorption of Small Peptides in Renal Proximal Tubule. Am. J. Physiol.-Ren. Physiol. 1986, 251, F945–F953. [Google Scholar] [CrossRef]
- Fiedler, L.R.; Schönherr, E.; Waddington, R.; Niland, S.; Seidler, D.G.; Aeschlimann, D.; Eble, J.A. Decorin Regulates Endothelial Cell Motility on Collagen I through Activation of Insulin-like Growth Factor I Receptor and Modulation of Alpha2beta1 Integrin Activity. J. Biol. Chem. 2008, 283, 17406–17415. [Google Scholar] [CrossRef]
- Nakamura, J.; Shigematsu, S.; Yamauchi, K.; Takeda, T.; Yamazaki, M.; Kakizawa, T.; Hashizume, K. Biphasic Function of Focal Adhesion Kinase in Endothelial Tube Formation Induced by Fibril-Forming Collagens. Biochem. Biophys. Res. Commun. 2008, 374, 699–703. [Google Scholar] [CrossRef]
- Senger, D.R.; Perruzzi, C.A.; Streit, M.; Koteliansky, V.E.; de Fougerolles, A.R.; Detmar, M. The A1β1 and A2β1 Integrins Provide Critical Support for Vascular Endothelial Growth Factor Signaling, Endothelial Cell Migration, and Tumor Angiogenesis. Am. J. Pathol. 2002, 160, 195–204. [Google Scholar] [CrossRef]
- de Kruijf, P.; Lim, H.D.; Overbeek, S.A.; Zaman, G.J.R.; Kraneveld, A.D.; Folkerts, G.; Leurs, R.; Smit, M.J. The Collagen-Breakdown Product N-Acetyl-Proline-Glycine-Proline (N-α-PGP) Does Not Interact Directly with Human CXCR1 and CXCR2. Eur. J. Pharmacol. 2010, 643, 29–33. [Google Scholar] [CrossRef]
- Knight, C.G.; Morton, L.F.; Onley, D.J.; Peachey, A.R.; Messent, A.J.; Smethurst, P.A.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. Identification in Collagen Type I of an Integrin A2β1-Binding Site Containing an Essential GER Sequence. J. Biol. Chem. 1998, 273, 33287–33294. [Google Scholar] [CrossRef]
- Siwy, J.; Mullen, W.; Golovko, I.; Franke, J.; Zürbig, P. Human Urinary Peptide Database for Multiple Disease Biomarker Discovery. Proteom.—Clin. Appl. 2011, 5, 367–374. [Google Scholar] [CrossRef]
- COL1A1—Collagen Alpha-1(I) Chain—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P02452/entry (accessed on 9 January 2023).
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- ITGA2—Integrin Alpha-2—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P17301/entry (accessed on 19 September 2024).
- ITGB1—Integrin Beta-1—Homo Sapiens (Human)|UniProtKB|UniProt. Available online: https://www.uniprot.org/uniprotkb/P05556/entry (accessed on 19 September 2024).
- Harmalkar, A.; Lyskov, S.; Gray, J.J. Reliable Protein—Protein Docking with AlphaFold, Rosetta, and Replica Exchange. eLife 2025, 13, RP94029. [Google Scholar] [CrossRef]
- Burke, D.F.; Bryant, P.; Barrio-Hernandez, I.; Memon, D.; Pozzati, G.; Shenoy, A.; Zhu, W.; Dunham, A.S.; Albanese, P.; Keller, A.; et al. Towards a Structurally Resolved Human Protein Interaction Network. Nat. Struct. Mol. Biol. 2023, 30, 216–225. [Google Scholar] [CrossRef]
- Chiang, Y.; Hui, W.-H.; Chang, S.-W. Encoding Protein Dynamic Information in Graph Representation for Functional Residue Identification. Cell Rep. Phys. Sci. 2022, 3, 100975. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, C.; Zhao, Y.; Ji, Y.; Wang, X.; Liu, Y. LINC00926 Is Involved in Hypoxia-Induced Vascular Endothelial Cell Dysfunction via miR-3194-5p Regulating JAK1/STAT3 Signaling Pathway. Eur. J. Histochem. 2023, 67. [Google Scholar] [CrossRef]
- He, Q.; He, W.; Dong, H.; Guo, Y.; Yuan, G.; Shi, X.; Wang, D.; Lu, F. Role of Liver Sinusoidal Endothelial Cell in Metabolic Dysfunction-Associated Fatty Liver Disease. Cell Commun. Signal. CCS 2024, 22, 346. [Google Scholar] [CrossRef]
- Sgarioto, M.; Vigneron, P.; Patterson, J.; Malherbe, F.; Nagel, M.-D.; Egles, C. Collagen Type I Together with Fibronectin Provide a Better Support for Endothelialization. Comptes Rendus Biol. 2012, 335, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Iyer, R.P.; Zamilpa, R.; Yabluchanskiy, A.; DeLeon-Pennell, K.Y.; Hall, M.E.; Kaplan, A.; Zouein, F.A.; Bratton, D.; Flynn, E.R.; et al. A Novel Collagen Matricryptin Reduces Left Ventricular Dilation Post-Myocardial Infarction by Promoting Scar Formation and Angiogenesis. J. Am. Coll. Cardiol. 2015, 66, 1364–1374. [Google Scholar] [CrossRef]
- Murdoch, C.; Monk, P.N.; Finn, A. Cxc Chemokine Receptor Expression On Human Endothelial Cells. Cytokine 1999, 11, 704–712. [Google Scholar] [CrossRef]
- Emsley, J.; Knight, C.G.; Farndale, R.W.; Barnes, M.J. Structure of the Integrin A2β1-Binding Collagen Peptide. J. Mol. Biol. 2004, 335, 1019–1028. [Google Scholar] [CrossRef]
- Emsley, J.; Knight, C.G.; Farndale, R.W.; Barnes, M.J.; Liddington, R.C. Structural Basis of Collagen Recognition by Integrin A2β1. Cell 2000, 101, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kamata, T.; Liddington, R.C.; Takada, Y. Interaction between Collagen and the A2 I-Domain of Integrin A2β1: Critical Role Of Conserved Residues In The Metal Ion-Dependent Adhesion Site (Midas) Region. J. Biol. Chem. 1999, 274, 32108–32111. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Estavillo, D.; Emsley, J.; Bankston, L.A.; Liddington, R.C.; Cruz, M.A. Mapping the Collagen-Binding Site in the I Domain of the Glycoprotein Ia/IIa (Integrin A2β1). J. Biol. Chem. 2000, 275, 4205–4209. [Google Scholar] [CrossRef]
- Potel, C.M.; Lemeer, S.; Heck, A.J.R. Phosphopeptide Fragmentation and Site Localization by Mass Spectrometry: An Update. Anal. Chem. 2019, 91, 126–141. [Google Scholar] [CrossRef]
- Martin, D.M.A.; Nett, I.R.E.; Vandermoere, F.; Barber, J.D.; Morrice, N.A.; Ferguson, M.A.J. Prophossi: Automating Expert Validation of Phosphopeptide-Spectrum Matches from Tandem Mass Spectrometry. Bioinformatics 2010, 26, 2153–2159. [Google Scholar] [CrossRef]
- Ostrowska-Podhorodecka, Z.; Ding, I.; Lee, W.; Tanic, J.; Abbasi, S.; Arora, P.D.; Liu, R.S.; Patteson, A.E.; Janmey, P.A.; McCulloch, C.A. Vimentin Tunes Cell Migration on Collagen by Controlling Β1 Integrin Activation and Clustering. J. Cell Sci. 2021, 134, jcs254359. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, A.; Wilson, N.A.; Djamali, A. Heat Shock Protein 27 (HSP27): Biomarker of Disease and Therapeutic Target. Fibrogenes. Tissue Repair 2012, 5, 7. [Google Scholar] [CrossRef]
- Kostenko, S.; Moens, U. Heat Shock Protein 27 Phosphorylation: Kinases, Phosphatases, Functions and Pathology. Cell. Mol. Life Sci. 2009, 66, 3289–3307. [Google Scholar] [CrossRef]
- Evans, I.M.; Britton, G.; Zachary, I.C. Vascular Endothelial Growth Factor Induces Heat Shock Protein (HSP) 27 Serine 82 Phosphorylation and Endothelial Tubulogenesis via Protein Kinase D and Independent of P38 Kinase. Cell. Signal. 2008, 20, 1375–1384. [Google Scholar] [CrossRef]
- Shah, K.; Shokat, K.M. A Chemical Genetic Screen for Direct V-Src Substrates Reveals Ordered Assembly of a Retrograde Signaling Pathway. Chem. Biol. 2002, 9, 35–47. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S.; Zhang, H.; Jing, Y.; Ji, X.; Wan, Q.; Liu, Y. CALU Promotes Lung Adenocarcinoma Progression by Enhancing Cell Proliferation, Migration and Invasion. Respir. Res. 2024, 25, 267. [Google Scholar] [CrossRef]
- Chevet, E.; Wong, H.N.; Gerber, D.; Cochet, C.; Fazel, A.; Cameron, P.H.; Gushue, J.N.; Thomas, D.Y.; Bergeron, J.J.M. Phosphorylation by CK2 and MAPK Enhances Calnexin Association with Ribosomes. EMBO J. 1999, 18, 3655–3666. [Google Scholar] [CrossRef] [PubMed]
- Augustin-Voss, H.G.; Pauli, B.U. Migrating Endothelial Cells Are Distinctly Hyperglycosylated and Express Specific Migration-Associated Cell Surface Glycoproteins. J. Cell Biol. 1992, 119, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Worth, D.C.; Daly, C.N.; Geraldo, S.; Oozeer, F.; Gordon-Weeks, P.R. Drebrin Contains a Cryptic F-Actin–Bundling Activity Regulated by Cdk5 Phosphorylation. J. Cell Biol. 2013, 202, 793–806. [Google Scholar] [CrossRef]
- Connors, W.L.; Jokinen, J.; White, D.J.; Puranen, J.S.; Kankaanpaöaö, P.; Upla, P.; Tulla, M.; Johnson, M.S.; Heino, J. Two Synergistic Activation Mechanisms of A2β1 Integrin-Mediated Collagen Binding. J. Biol. Chem. 2007, 282, 14675–14683. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Domínguez-Pérez, M.; Mercado, I.; Villarreal-Molina, M.T.; Jacobo-Albavera, L. Use of Human Umbilical Vein Endothelial Cells (HUVEC) as a Model to Study Cardiovascular Disease: A Review. Appl. Sci. 2020, 10, 938. [Google Scholar] [CrossRef]
- Mischak, H.; Kolch, W.; Aivaliotis, M.; Bouyssié, D.; Court, M.; Dihazi, H.; Dihazi, G.H.; Franke, J.; Garin, J.; de Peredo, A.G.; et al. Comprehensive Human Urine Standards for Comparability and Standardization in Clinical Proteome Analysis. Proteom.—Clin. Appl. 2010, 4, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Mokou, M.; Klein, J.; Makridakis, M.; Bitsika, V.; Bascands, J.-L.; Saulnier-Blache, J.S.; Mullen, W.; Sacherer, M.; Zoidakis, J.; Pieske, B.; et al. Proteomics Based Identification of KDM5 Histone Demethylases Associated with Cardiovascular Disease. EBioMedicine 2019, 41, 91–104. [Google Scholar] [CrossRef]
- Megarioti, A.H.; Primo, C.; Kapetanakis, G.C.; Athanasopoulos, A.; Sophianopoulou, V.; André, B.; Gournas, C. The Bul1/2 Alpha-Arrestins Promote Ubiquitylation and Endocytosis of the Can1 Permease upon Cycloheximide-Induced TORC1-Hyperactivation. Int. J. Mol. Sci. 2021, 22, 10208. [Google Scholar] [CrossRef]
- Megarioti, A.H.; Esch, B.M.; Athanasopoulos, A.; Koulouris, D.; Makridakis, M.; Lygirou, V.; Samiotaki, M.; Zoidakis, J.; Sophianopoulou, V.; André, B.; et al. Ferroptosis-Protective Membrane Domains in Quiescence. Cell Rep. 2023, 42, 113561. [Google Scholar] [CrossRef]
- Makridakis, M.; Vlahou, A. GeLC-MS: A Sample Preparation Method for Proteomics Analysis of Minimal Amount of Tissue. Methods Mol. Biol. 2018, 1788, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Claeys, T.; Van Den Bossche, T.; Perez-Riverol, Y.; Gevaert, K.; Vizcaíno, J.A.; Martens, L. lesSDRF Is More: Maximizing the Value of Proteomics Data through Streamlined Metadata Annotation. Nat. Commun. 2023, 14, 6743. [Google Scholar] [CrossRef] [PubMed]
| Peptide Number | Peptide Sequence | Start AA Position- Stop AA Position | Rel. Abundance Plasma | Rel. Abundance Urine | Fold Change Plasma/Urine |
|---|---|---|---|---|---|
| 1 | NGDDGEAGKP GRPGERGPOGO | 229-249 | 0.16 | 7.72 | 0.02 |
| 2 | NSGEOGAOGS KGDTGAKGEO GPVG | 432-455 | 0.44 | 6.87 | 0.06 |
| 3 | AOGDRGEOGP OGPA | 798-811 | 0.34 | 5.60 | 0.06 |
| 4 | ADGQPGAKGE OGDAGAKGDA GPPGO | 819-843 | 0.81 | 4.29 | 0.19 |
| 5 | ANGAOGNDGA KGDAGAOGAO GSQGAOG | 699-725 | 0.19 | 2.73 | 0.07 |
| 6 | SOGSPGPDGK TGPOGP | 543-558 | 0.79 | 1.71 | 0.46 |
| 7 | ESGREGAOGA EGSOGRDGSO GAKGDRGETGP | 1011-1041 | 0.24 | 1.70 | 0.14 |
| 8 | AGPOGEAGKO GEQGVOGDLG AOGP | 649-672 | 0.68 | 1.15 | 0.59 |
| 9 | VGPOGPOGPO GPPGPPS | 177-1193 | 0.03 | 1.41 | 0.02 |
| Peptide | Protein the Peptide Is Derived from | Abundance (Peptide 1 + COL(I) vs. COL(I) | Abundance (Peptide 1 + COL(I) vs. COL(I)) per Time Point |
|---|---|---|---|
| LPSGSGAASPTGSAVDIR | AHNAK (Q09666) | LFC: −1.08 p-value: 0.04 | N.S. |
| VHNDAQSFDYDHDAFLGAEEAK | CALU (O43852) | LFC: −4.47 p-value: 0.01 | At 7 min: LFC: −4.43, p-value: 0.02 At 30 min: LFC: −4.43, p-value: 0.02 At 60 min: LFC: −4.55, p-value: 0.02 |
| AEEDEILNRSPR | CANX (P27824) | LFC: −2.68 p-value: 0.01 | At 30 min: LFC: −2.34, p-value: 0.03 At 60 min: LFC: −2.79, p-value: 0.03 |
| LSSPVLHR | DBN1 (Q16643) | LFC: 1 p-value: 0.01 | At 30 min: LFC: 2.01, p-value: 0.03 |
| QLSSGVSEIR | HSPB1 (P04792) | LFC: −4.31 p-value: 0.01 | At 7 min: LFC: −4.95, p-value: 0.03 At 30 min: LFC: −4.68, p-value: 0.03 At 60 min: LFC: −3.3, p-value: 0.03 |
| SLYASSPGGVYATR | VIM (P08670) | LFC: −2.46 p-value: 0.01 | At 30 min: LFC: −3.97, p-value: 0.03 At 60 min: LFC: −1.36, p-value: 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devos, H.; Mina, I.K.; Paradeisi, F.; Makridakis, M.; Tserga, A.; Mokou, M.; Zoidakis, J.; Mischak, H.; Vlahou, A.; Latosinska, A.; et al. A Naturally Occurring Urinary Collagen Type I Alpha 1-Derived Peptide Inhibits Collagen Type I-Induced Endothelial Cell Migration at Physiological Concentrations. Int. J. Mol. Sci. 2025, 26, 7480. https://doi.org/10.3390/ijms26157480
Devos H, Mina IK, Paradeisi F, Makridakis M, Tserga A, Mokou M, Zoidakis J, Mischak H, Vlahou A, Latosinska A, et al. A Naturally Occurring Urinary Collagen Type I Alpha 1-Derived Peptide Inhibits Collagen Type I-Induced Endothelial Cell Migration at Physiological Concentrations. International Journal of Molecular Sciences. 2025; 26(15):7480. https://doi.org/10.3390/ijms26157480
Chicago/Turabian StyleDevos, Hanne, Ioanna K. Mina, Foteini Paradeisi, Manousos Makridakis, Aggeliki Tserga, Marika Mokou, Jerome Zoidakis, Harald Mischak, Antonia Vlahou, Agnieszka Latosinska, and et al. 2025. "A Naturally Occurring Urinary Collagen Type I Alpha 1-Derived Peptide Inhibits Collagen Type I-Induced Endothelial Cell Migration at Physiological Concentrations" International Journal of Molecular Sciences 26, no. 15: 7480. https://doi.org/10.3390/ijms26157480
APA StyleDevos, H., Mina, I. K., Paradeisi, F., Makridakis, M., Tserga, A., Mokou, M., Zoidakis, J., Mischak, H., Vlahou, A., Latosinska, A., & Roubelakis, M. G. (2025). A Naturally Occurring Urinary Collagen Type I Alpha 1-Derived Peptide Inhibits Collagen Type I-Induced Endothelial Cell Migration at Physiological Concentrations. International Journal of Molecular Sciences, 26(15), 7480. https://doi.org/10.3390/ijms26157480







