Enhancing Tissue Integration and Reducing Inflammation in Silicone and Human Acellular Dermal Matrix Implants via Vacuum Plasma Treatment
Abstract
1. Introduction
2. Results
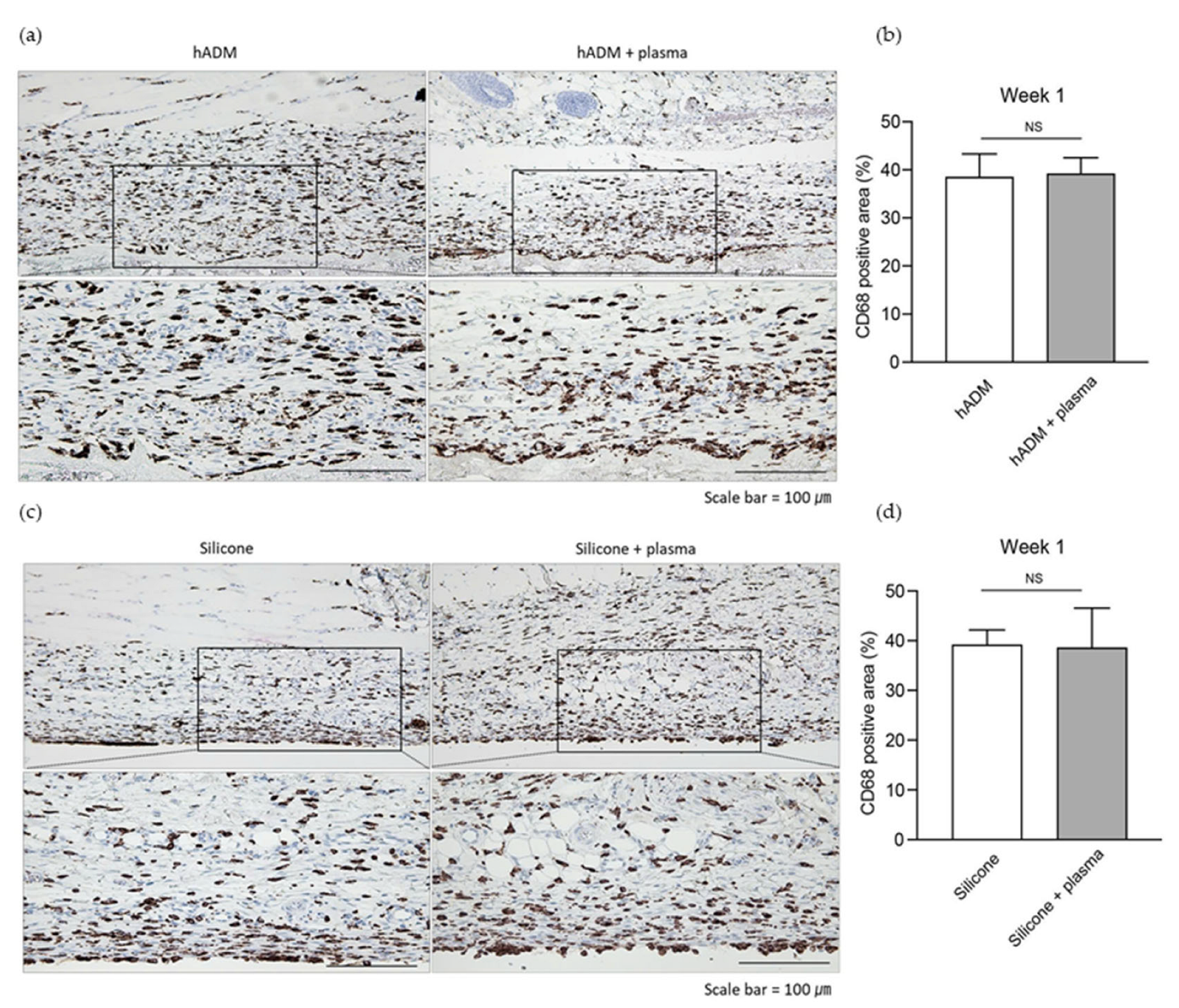
2.1. Plasma Does Not Alter the Acute Immune Response Post-Implantation
2.2. Capsule Thickness Around Implanted Silicone and hADM
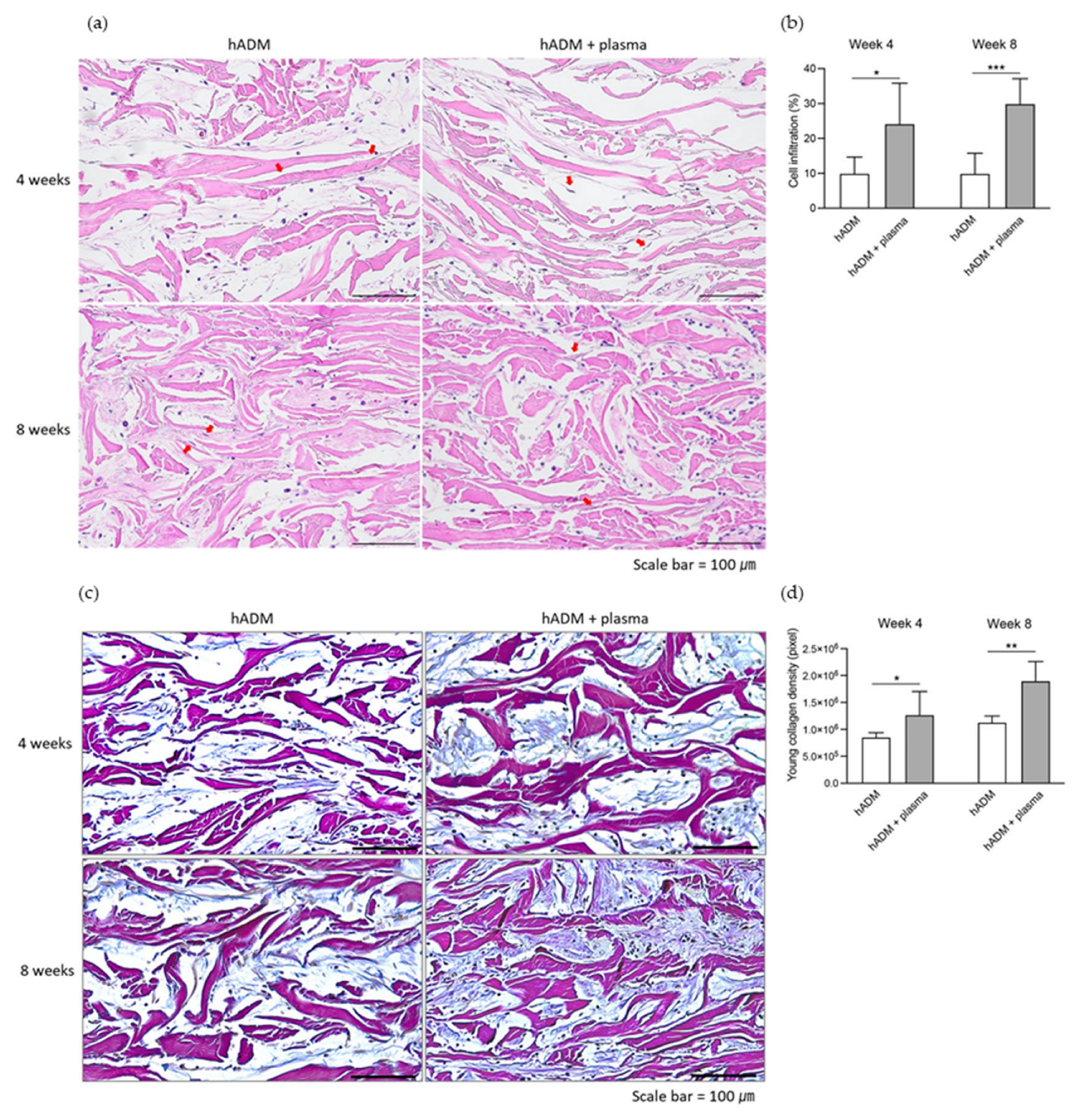
2.3. Fibroblast Infiltration and Neocollagenesis in hADM
2.4. Neovascularization Within and Around hADM
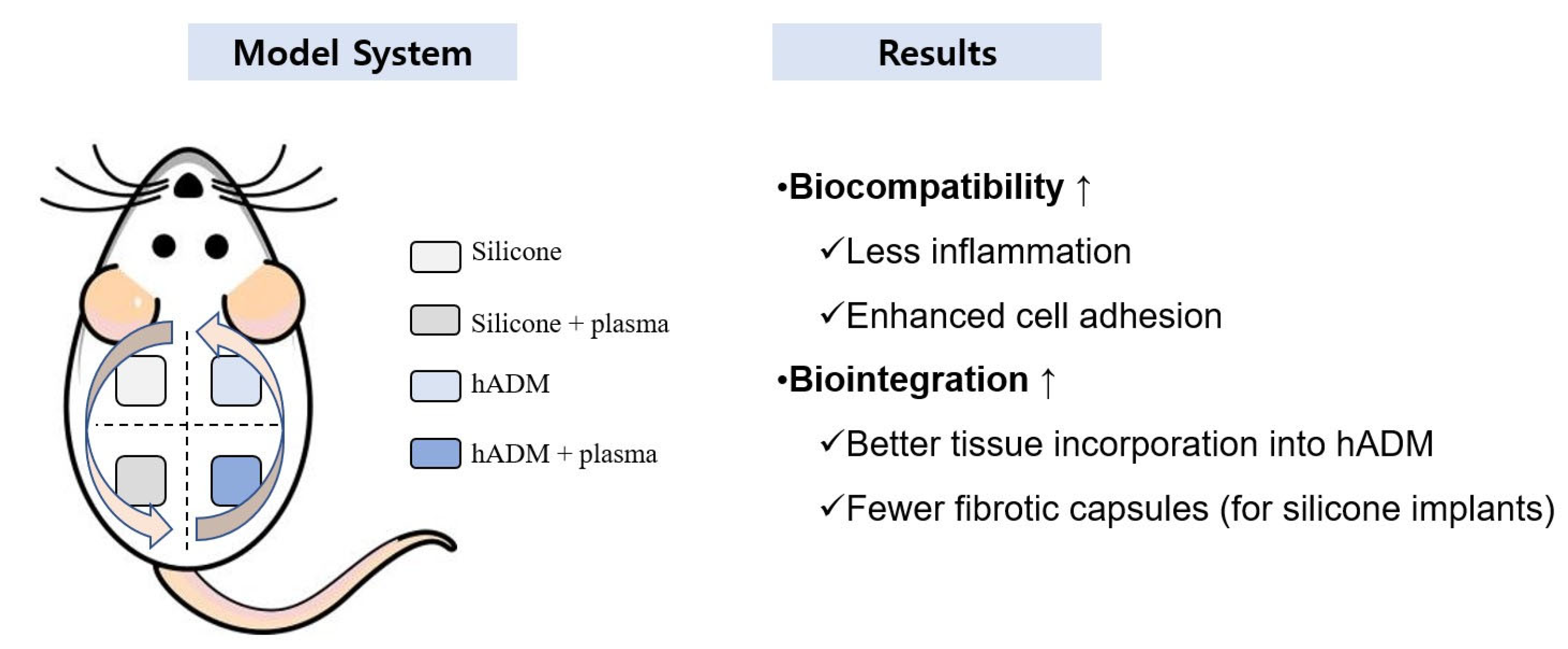
3. Discussion
4. Materials and Methods
4.1. Device Setup and Plasma Treatment
4.2. In Vivo Study
4.2.1. Experimental Animals
4.2.2. Experimental Design
4.2.3. Tissue Section Preparation
4.2.4. Histological Analysis
4.2.5. Immunohistochemistry
4.2.6. Herovici Staining
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hADM | Human acellular dermal matrix |
| CD68 | Cluster of Differentiation 68 (macrophage marker) |
| CD31 | Cluster of Differentiation 31 (endothelial cell marker) |
| H&E | Hematoxylin and eosin |
| NIH | National Institutes of Health |
| SPSS | Statistical Package for the Social Sciences |
| SME | Small and medium enterprises |
| KOICA | Korea International Cooperation Agency |
| MEF | Medical Education and Fellowship (MEF Fellowship Program) |
| MSS | Ministry of SMEs and Startups (Korea) |
| IACUC | Institutional Animal Care and Use Committee |
References
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Med. Gas Res. 2013, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Karthik, C.; Sarngadharan, S.C.; Thomas, V. Low-Temperature Plasma Techniques in Biomedical Applications and Therapeutics: An Overview. Int. J. Mol. Sci. 2024, 25, 524. [Google Scholar] [CrossRef]
- Fathollah, S.; Mirpour, S.; Mansouri, P.; Dehpour, A.R.; Ghoranneviss, M.; Rahimi, N.; Safaie Naraghi, Z.; Chalangari, R.; Chalangari, K.M. Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci. Rep. 2016, 6, 19144. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, M.J.; Chernets, N.; Zhang, J.; Kurpad, D.S.; Fridman, G.; Fridman, A.; Freeman, T.A. Skeletal cell differentiation is enhanced by atmospheric dielectric barrier discharge plasma treatment. PLoS ONE 2013, 8, e82143. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Clyne, A.M. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 2011, 2447–2450. [Google Scholar]
- Park, J.; Lee, H.; Lee, H.J.; Kim, G.C.; Kim, D.Y.; Han, S.; Song, K. Non-Thermal Atmospheric Pressure Plasma Efficiently Promotes the Proliferation of Adipose Tissue-Derived Stem Cells by Activating NO-Response Pathways. Sci. Rep. 2016, 6, 39298. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Lee, H.J.; Kim, G.C.; Kim, S.S.; Han, S.; Song, K. Non-thermal atmospheric pressure plasma is an excellent tool to activate proliferation in various mesoderm-derived human adult stem cells. Free Radic. Biol. Med. 2019, 134, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Bolgeo, T.; Maconi, A.; Gardalini, M.; Gatti, D.; Di Matteo, R.; Lapidari, M.; Longhitano, Y.; Savioli, G.; Piccioni, A.; Zanza, C. The Role of Cold Atmospheric Plasma in Wound Healing Processes in Critically Ill Patients. J. Pers. Med. 2023, 13, 736. [Google Scholar] [CrossRef]
- Shibata, Y.; Hosaka, M.; Kawai, H.; Miyazaki, T. Glow discharge plasma treatment of titanium plates enhances adhesion of osteoblast-like cells to the plates through the integrin-mediated mechanism. Int. J. Oral Maxillofac. Implants 2002, 17, 771–777. [Google Scholar]
- Kawai, H.; Shibata, Y.; Miyazaki, T. Glow discharge plasma pretreatment enhances osteoclast differentiation and survival on titanium plates. Biomaterials 2004, 25, 1805–1811. [Google Scholar] [CrossRef]
- Giro, G.; Tovar, N.; Witek, L.; Marin, C.; Silva, N.R.; Bonfante, E.A.; Coelho, P.G. Osseointegration assessment of chairside argon-based nonthermal plasma-treated Ca-P coated dental implants. J. Biomed. Mater. Res. A 2013, 101, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Verboket, R.D.; Henrich, D.; Janko, M.; Sommer, K.; Neijhoft, J.; Sohling, N.; Weber, B.; Frank, J.; Marzi, I.; Nau, C. Human Acellular Collagen Matrices-Clinical Opportunities in Tissue Replacement. Int. J. Mol. Sci. 2024, 25, 7088. [Google Scholar] [CrossRef]
- Xu, H.; Wan, H.; Sandor, M.; Qi, S.; Ervin, F.; Harper, J.R.; Silverman, R.P.; McQuillan, D.J. Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair. Tissue Eng. Part A 2008, 14, 2009–2019. [Google Scholar] [CrossRef]
- Liu, J.; Hou, J.; Li, Z.; Wang, B.; Sun, J. Efficacy of Acellular Dermal Matrix in Capsular Contracture of Implant-Based Breast Reconstruction: A Single-Arm Meta-analysis. Aesthetic Plast. Surg. 2020, 44, 735–742. [Google Scholar] [CrossRef]
- Chopra, K.; Buckingham, B.; Matthews, J.; Sabino, J.; Tadisina, K.K.; Silverman, R.P.; Goldberg, N.H.; Slezak, S.; Singh, D.P. Acellular dermal matrix reduces capsule formation in two-stage breast reconstruction. Int. Wound J. 2017, 14, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Lee, B.; Shin, C.; You, B.; Oh, H.S.; Lee, J.; Lee, J.; Oh, S.K.; Oh, S.H. Improvement in Biocompatibility and Biointegration of Human Acellular Dermal Matrix through Vacuum Plasma Surface Treatment. Bioengineering 2024, 11, 359. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- DeLisser, H.M.; Christofidou-Solomidou, M.; Strieter, R.M.; Burdick, M.D.; Robinson, C.S.; Wexler, R.S.; Kerr, J.S.; Garlanda, C.; Merwin, J.R.; Madri, J.A.; et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am. J. Pathol. 1997, 151, 671–677. [Google Scholar]
- Staton, C.A.; Reed, M.W.; Brown, N.J. A critical analysis of current in vitro and in vivo angiogenesis assays. Int. J. Exp. Pathol. 2009, 90, 195–221. [Google Scholar] [CrossRef]
- Stefanelli, V.; Lombardi, J.; Ferrer, J.; Gardocki-Sandor, M. Vascularization of Human Acellular Dermal Matrices: A Comparative Study in a Nonhuman Primate Model. Tissue Eng. Part A 2025, 31, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Bayston, R. Capsule formation around breast implants. JPRAS Open 2022, 31, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.; Ozturk, S.; Deveci, M.; Ural, A.U.; Onguru, O.; Isik, S. Experimental assessment of the neo-vascularisation of acellular dermal matrix in the wound bed pretreated with mesenchymal stem cell under subatmospheric pressure. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 107–114. [Google Scholar] [CrossRef]
- Lee, J.M.; Seo, Y.J.; Shim, D.B.; Lee, H.J.; Kim, S.H. Surgical outcomes of tympanoplasty using a sterile acellular dermal allograft: A prospective randomised controlled study. Acta Otorhinolaryngol. Ital. 2018, 38, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Schnarrs, R.H.; Carman, C.M.; Tobin, C.; Chase, S.A.; Rossmeier, K.A. Complication Rates With Human Acellular Dermal Matrices: Retrospective Review of 211 Consecutive Breast Reconstructions. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1118. [Google Scholar] [CrossRef]
- Kim, D.Y.; An, S.H.; Woo, J.H.; Baek, M.K.; Kang, I.G. Cosmetic outcome of implantation of cross-linked human acellular dermal matrix after parotidectomy. Br. J. Oral Maxillofac. Surg. 2019, 57, 301–305. [Google Scholar] [CrossRef]
- Fitzgerald, A.M.; Kirkpatrick, J.J.; Foo, I.T.; Naylor, I.L. Human skin histology as demonstrated by Herovici's stain: A guide for the improvement of dermal substitutes for use with cultured keratinocytes? Burns 1996, 22, 200–202. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, K.B.; Lee, Y.I.; Kim, J.; Nguyen, N.H.; Kim, Y.J.; Jung, I.; Lee, J.; Jeon, H.-J.; Lim, Y.; Lee, S.J.; et al. Enhancing Tissue Integration and Reducing Inflammation in Silicone and Human Acellular Dermal Matrix Implants via Vacuum Plasma Treatment. Int. J. Mol. Sci. 2025, 26, 5854. https://doi.org/10.3390/ijms26125854
Chung KB, Lee YI, Kim J, Nguyen NH, Kim YJ, Jung I, Lee J, Jeon H-J, Lim Y, Lee SJ, et al. Enhancing Tissue Integration and Reducing Inflammation in Silicone and Human Acellular Dermal Matrix Implants via Vacuum Plasma Treatment. International Journal of Molecular Sciences. 2025; 26(12):5854. https://doi.org/10.3390/ijms26125854
Chicago/Turabian StyleChung, Kyung Bae, Young In Lee, Jihee Kim, Ngoc Ha Nguyen, Yoo Jin Kim, Inhee Jung, Jeonghoon Lee, Hyun-Jeong Jeon, Youbong Lim, Sung Jun Lee, and et al. 2025. "Enhancing Tissue Integration and Reducing Inflammation in Silicone and Human Acellular Dermal Matrix Implants via Vacuum Plasma Treatment" International Journal of Molecular Sciences 26, no. 12: 5854. https://doi.org/10.3390/ijms26125854
APA StyleChung, K. B., Lee, Y. I., Kim, J., Nguyen, N. H., Kim, Y. J., Jung, I., Lee, J., Jeon, H.-J., Lim, Y., Lee, S. J., & Lee, J. H. (2025). Enhancing Tissue Integration and Reducing Inflammation in Silicone and Human Acellular Dermal Matrix Implants via Vacuum Plasma Treatment. International Journal of Molecular Sciences, 26(12), 5854. https://doi.org/10.3390/ijms26125854





